Summary
Objective
Posttraumatic epilepsy (PTE) accounts for 20% of acquired epilepsies. Experimental models are important for studying epileptogenesis. We previously reported that repetitive high-frequency oscillations with spikes (rHFOSs) occur early after lateral fluid percussion injury (FPI) and may be a biomarker for PTE. The objective of this study was to use multiple electrodes in rat hippocampal and neocortical regions to describe the long-term electroencephalographic and behavioral evolution of rHFOSs and epileptic seizures after traumatic brain injury (TBI).
Methods
Adult male rats underwent mild, moderate, or severe FPI or sham injury followed by video–electroencephalography (EEG) recordings with a combination of 16 neocortical and hippocampal electrodes at an early, intermediate, or late time-point after injury, up to 52 weeks. Recordings were analyzed for the presence of rHFOSs and seizures.
Results
Analysis was done on 28 rats with FPI and 7 shams. Perilesional rHFOSs were recorded in significantly more rats after severe (70.3%) than mild (20%) injury or shams (14.3%). Frequency of occurrence was significantly highest in the early (10.8/h) versus late group (3.2/h). Late focal seizures originating from the same electrodes were recorded in significantly more rats in the late (87.5%) versus early period (22.2%), occurring almost exclusively in injured rats. Seizure duration increased significantly over time, averaging 19 s at the beginning of the early period and 27 s at the end of the late period. Seizure frequency also increased significantly over time, from 4.4 per week in the early group to 26.4 per week in the late group. Rarely, rats displayed early seizures or generalized seizures.
Significance
FPI results in early rHFOSs and later spontaneous focal seizures arising from peri-lesional neocortex, supporting its use as a model for PTE. Epilepsy severity increased over time and was related to injury severity. The association between early rHFOSs and later focal seizures suggests that rHFOSs may be a potential noninvasive biomarker of PTE.
Keywords: Lateral fluid percussion injury, Seizure, Electroencephalography, Posttraumatic epilepsy, Biomarker
Epilepsy accounts for 1% of the global burden of disease and is one of the most common serious neurologic disorders.1 Epileptogenesis, the process by which normal brain becomes capable of generating recurrent, spontaneous seizures, is not well understood. Further characterization of this process is crucial to the development of strategies aimed at preventing epilepsy. The latent period, defined as the time between a potential epileptogenic injury and the first clinical seizure, is generally thought to be the time period when cellular and molecular mechanisms responsible for the development of spontaneous seizures occur.2 Posttraumatic epilepsy (PTE), accounting for 20% of acquired epilepsies and 4% of all epilepsy,3 is one of the few types of human epilepsy where timing of the insulting event is known, and therefore the subsequent period of potential epileptogenesis can be investigated and possibly treated.4 Consequently animal studies of PTE are not only relevant as they pertain to an important clinical condition, but they may be one of the best models for studying epileptogenesis as it occurs in humans.
A commonly used animal model of human traumatic brain injury (TBI) is fluid percussion injury (FPI),5 which can lead to the development of spontaneous seizures, or PTE.6–8 Different groups using this model have employed variabilities including age, injury location, and location and timing of electroencephalography (EEG) recording, making results difficult to interpret and compare. Discrepancies as to where seizures arise7,9–11 are dependent in part on these experimental variables. There is support for both neocortical8 and hippocampal7,10 onset of seizures after FPI. However, there is controversy as to whether some events after FPI truly represent seizures, or are age-dependent spike-wave discharges (SWDs).12 We recently demonstrated the presence of repetitive high-frequency oscillations with spikes (rHFOSs) after FPI, which are localized to the injured area.13 The current study extends that work to demonstrate the later occurrence of focal seizures in this model of TBI.
The objective of this study was to use multiple electrodes positioned in rat hippocampal and neocortical regions within, adjacent, and remote from the site of injury to describe the EEG and behavioral evolution of epileptic seizures after FPI. The use of more extensive electrode coverage allows for better characterization of EEG events, such as location of onset, spread, frequency, and waveform morphology, in order to determine whether these events have characteristics of seizures versus age-dependent SWDs and to further characterize the time-course of EEG and behavioral features during the development of experimental PTE. These are important issues to clarify if further work on epileptogenesis after FPI is to be pursued.
Methods
Overview
Experiments were conducted on adult male Sprague-Dawley rats (300–350 g, approximately 65 ± 16 days old at time of FPI) (Charles River, Hollister, CA, U.S.A.) as approved by the University of California Los Angeles Institutional Animal Care and Use Committee. Animals were housed in standard environmental conditions on a 12 h light/dark cycle at 22°C with food and water available ad libitum.
Rats underwent induction of TBI or sham surgery, and later had depth electrodes implanted in the neocortex and hippocampus for continuous video and EEG monitoring for detection of rHFOSs and seizures. Data pertaining to rHFOSs, which included a subset of these rats, have been reported previously13; however, the present study also includes new data about seizures and data from additional animals. After recording was complete, animals were sacrificed and histological examination was performed to confirm electrode placement.
Induction of TBI
TBI was induced using lateral FPI.14 Detailed information can be found in the Supplementary Methods section. Briefly, 34 rats had a plastic injury cap affixed over a 3-mm craniotomy centered 3 mm posterior to bregma and 6 mm lateral to midline overlying the left cerebral hemisphere. The level of anesthesia was lowered immediately prior to impact, and FPI was delivered with a pressure pulse duration of 10 msec, at 3.2–3.5 atm. The pressure pulse amplitude and waveform were measured within the charging chamber, before the pressure valve, and displayed on the connected laptop after each injury. The pressure was delivered just at the point when the paw reflex could again be elicited. Despite the lowered level of anesthesia, impact resulted in loss of the paw reflex, and rats were monitored for the duration of paw reflex suppression in order to classify injury severity as described later. Rats were excluded from further experiments if dura had been breached.
Sham TBI
Seven rats were anesthetized and underwent craniotomy as described previously but did not undergo FPI.
Assignment to injury severity group
Despite the use of uniform injury parameters, there is inherent variability in acute injury severity using this model. Rats were assigned to an injury severity group based on previously published a priori classification criteria,15,16 with higher impact injuries resulting in a longer duration of paw reflex suppression and more severe histopathologic changes.16,17 Criteria for mild injury were set as duration of paw reflex suppression between 60 and 120 s, moderate between 120 and 300 s, and severe >300 s.
Electrode implantation
The early group was implanted with neocortical and hippocampal electrodes immediately after FPI, the intermediate group between 9 and 13 weeks, and the late group at least 26 weeks after FPI. Screw electrodes were implanted in the ipsilateral skull lateral to the craniotomy, the contralateral skull in the frontal bone, and over the cerebellum bilaterally as reference and ground electrodes. Small burr holes were made for implantation of electrodes consisting of pairs of 60-μm diameter tungsten wires with a 0.5-mm vertical tip separation. These were placed in ipsilateral frontal neocortex, parietal neocortex anterior to the craniotomy, within the craniotomy, and posterior to the craniotomy, and in contralateral parietal neocortex in the area homotopic to the craniotomy. Pairs of neocortical electrodes were implanted at a depth of 2.0 and 0.5 mm, corresponding to placement within layers V–VI and layers I–II, respectively. Two pairs of electrodes were implanted in ipsilateral CA3 of the posterior hippocampus (coordinates: anterior-posterior [AP] = −5.6; medial-lateral [ML] = 3.5; dorsal-ventral [DV] = 5.0); and the anterior hippocampus (coordinates: AP = −3.5; ML = 2.0; DV = 4.5; Fig. 1A). Electrode amphenol pins were connected to a plug at the center of the skull, and fixed in place with dental cement.
Figure 1.
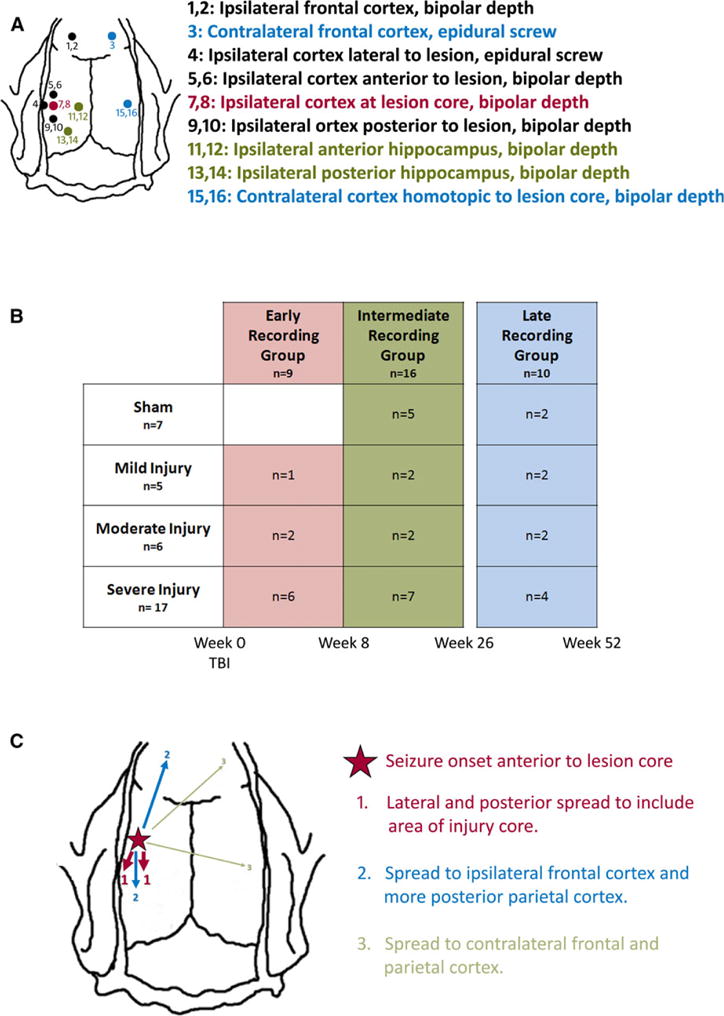
(A) Schematic representation of depth micro-electrode and epidural screw electrode placement for continuous EEG recordings after lateral fluid percussion injury. Electrodes denoted in red are within the lesion core, electrodes denoted in black are within the neocortex ipsilateral to the injury, electrodes denoted in green are within the ipsilateral hippocampus, and electrodes denoted in blue are in the hemisphere contralateral to the injury. (B) Schematic of the experimental design showing number of animals within each injury severity and each recording time. (C) Schematic showing the average spread pattern of a focal seizure. The seizure was first recorded by electrodes in the neocortex just anterior to the lesion site (red star), and then would usually spread first to neocortical electrodes in the lesion core and the area just lateral to it (areas marked 1). Next the seizure would usually spread to electrodes in the ipsilateral frontal neocortex and more posterior parietal neocortex (areas marked 2), and finally in some cases it would also spread to the contralateral frontal neocortex and area homotopic to the lesion (areas marked 3).
Epilepsia © ILAE
Electrophysiologic and video recordings
Epidural and depth EEG data were recorded wide-band 0.1 Hz to 5.0 kHz, sampled at 10 kHz/channel (16 channels) using DataPac software, and stored on external hard drives. Parallel video recordings were carried out with an Alibi 4-Camera 700 TVL 65′ IR system (Super Circuits, Austin, TX, U.S.A.), which included infrared recordings of rat behavior during the dark phase. Once the EEG recording was started during the designated postinjury interval (early, intermediate, or late), the recording continued for at least 1 month, or in some cases until the headcap was lost.
EEG data were reviewed daily. Files containing rHFOSs or seizures were saved for further analysis. rHFOSs have been described previously in this model,13 consisting of arcuate waveforms with a frequency of 10–16 Hz localized to electrodes recording from within and directly surrounding the lesion core. EEG data from both FPI and sham rats were reviewed to detect the presence of events with similar morphology, whether localized to the injury area or seen elsewhere. For the purpose of this particular study, a seizure was defined as an electrographic event lasting a minimum of 10 s with a buildup of rhythmic activity with evolution in at least two of location, amplitude, or frequency, and accompanying change in behavior. Video recordings were reviewed for each seizure to determine any ictal behavioral change.
Histology
Upon completion of EEG recording, rats were deeply anesthetized with pentobarbital and transcardially perfused with phosphate-buffered saline and 4% paraformaldehyde. Brains were removed and gross inspection was performed for possible non–TBI-related causes of seizures such as abscess or infection. Brains were then postfixed in 4% paraformaldehyde, cryoprotected in 30% sucrose at 4°C overnight, and then stored at −80°C until being cut in 40-μm coronal sections on a cryostat. Slices were Nissl stained with thionine and examined with use of light microscopy.
Statistical analyses
The proportion of rats with different events was compared between groups using the chi-square test. For normally distributed data, t-tests or one-way analysis of variance (ANOVA) were used as appropriate, followed by Tukey’s post hoc test. Statistical significance was accepted as p < 0.05.
Results
Thirty-four rats underwent FPI. Four died during the procedure and two were excluded because of dural breech, leaving 28 for analysis. Five rats were classified with mild injury, six with moderate, and 17 with severe16 (Table 1). Seven additional rats underwent sham FPI surgery and were used as controls. Nine of 28 FPI rats were recorded in the early period, 11 in the intermediate period, and 8 in the late period. Recordings started the same day as FPI in the early group, on average 74.5 days after FPI in the intermediate group (range 64–88 days after FPI), and on average 225.9 days after FPI in the late group (range 182–298 days after FPI). Of the seven sham-injured rats, five were recorded in the intermediate period and two in the late period (Fig. 1B).
Table 1.
Characteristics of lateral fluid percussion injury in rats
| Sham (n = 7) | Mild (n = 5) | Moderate (n = 6) | Severe (n = 17) | |
|---|---|---|---|---|
| Duration of paw reflex suppression in seconds (mean ± SE) | n/a | 103.8 ± 10.5 | 248.5 ± 14.6 | 408.1 ± 33.3 |
| Duration of isoflurane exposure in mins (mean ± SE) | 28.7 ± 2.1 | 51.2 ± 3.5 | 51.2 ± 5.0 | 49.5 ± 2.3 |
Four additional rats died and two were excluded due to subdural bleeding.
Characteristics of electrophysiologic events and accompanying behavioral states
There were four distinct types of electroclinical events recorded after TBI: rHFOSs, early seizures, late focal seizures, and generalized seizures. These are described below.
Repetitive high-frequency oscillations with spikes
rHFOSs, which we have described previously,13 were characterized by an abrupt change from background with increased amplitude and arcuate morphology, lasting on average 2.02 (±0.98) seconds, followed by abrupt offset and return to baseline (Fig. 2A). rHFOSs were always localized to the injury site; 100% were recorded from electrodes within parietal neocortex adjacent to and/or including the craniectomy site. None were localized to electrodes in frontal neocortex, and they never occurred synchronously in both hemispheres. There was no behavioral correlate to these events on video monitoring and they occurred during both active and quiet behavioral states.
Figure 2.
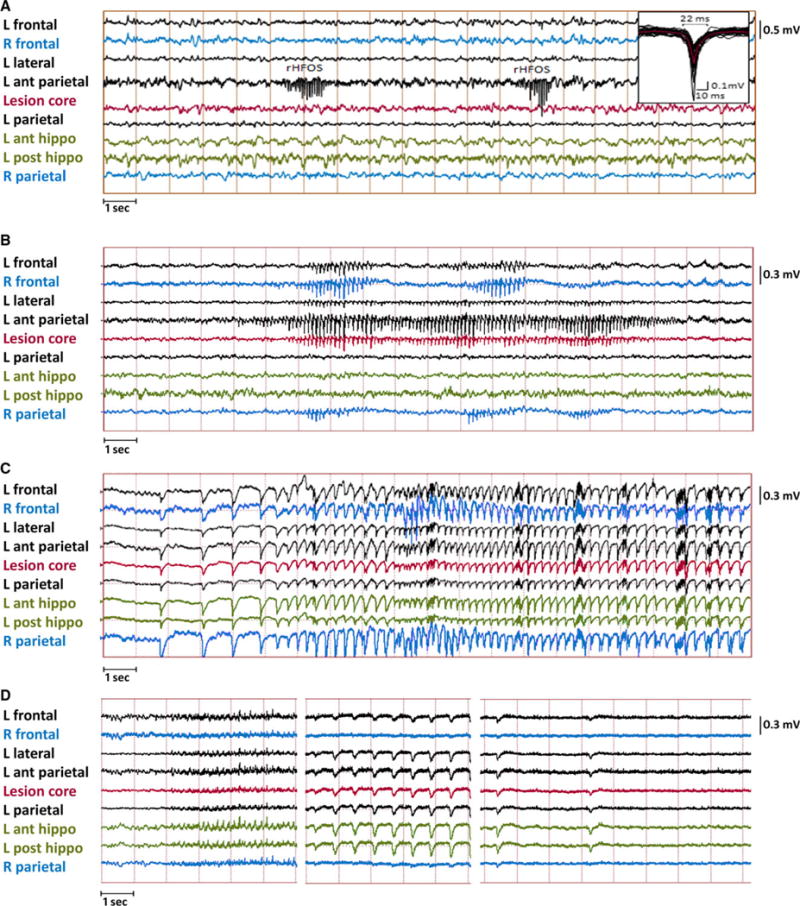
Examples of electrophysiologic events captured on continuous EEG recording from rats after lateral fluid percussion injury. (A) Example of two repetitive high-frequency oscillation with spikes (rHFOSs) recorded by neocortical depth electrodes implanted just anterior to the injured area. The first rHFOS had a duration of 1.2 s and the second rHFOS 0.75 s, whereas both were ~14 Hz. Inset shows the individual waves (black traces, n = 26) from each rHFOS and the averaged wave (red trace) with a duration of 22 msec measured from baseline. (B) Example of a focal seizure starting in the contacts anterior to and within the injury core (onset marked by black arrow), then spreading to more frontal electrodes both ipsilaterally and contralaterally. Postictally there is focal slowing. (C) Example of generalized ictal discharges recorded during a wet-dog shake in one rat early after injury; and (D) example of a generalized seizure recorded in a rat within 24 h of dying from status epilepticus. Postictally there is generalized suppression.
Epilepsia © ILAE
rHFOSs occurred in 17 (61%) of 28 injured rats, and in a significantly greater proportion of rats after moderate or severe injury than after sham or mild injury (χ2 = 8.8991, p = 0.031; Fig. 3A), with a trend for a higher frequency of occurrence with greater injury severity (F = 2.87, p = 0.052; Fig. 3C). There was a trend for rHFOSs to occur in a higher percentage of rats in the early group compared with intermediate or late groups (χ2 = 4.9782, p = 0.081; Fig. 3B), and the frequency of occurrence decreased significantly with later recording periods (F = 7.09, p = 0.004), with differences between the early and both the intermediate and late groups (Tukey honest significant difference (HSD) = 5.62, p < 0.01; Fig. 3D). The duration of rHFOSs remained quite stable and did not differ between recording groups (F = 2.49, p = 0.119) or injury severities (F = 1.26, p = 0.326). The average onset of rHFOSs was 14.2 (±4.8) days postinjury in the early group. There were no differences in onset time between injury severities within the early group (p > 0.05). Onset was not assessed in the intermediate and late groups, as the first occurrence of these events was already missed.
Figure 3.
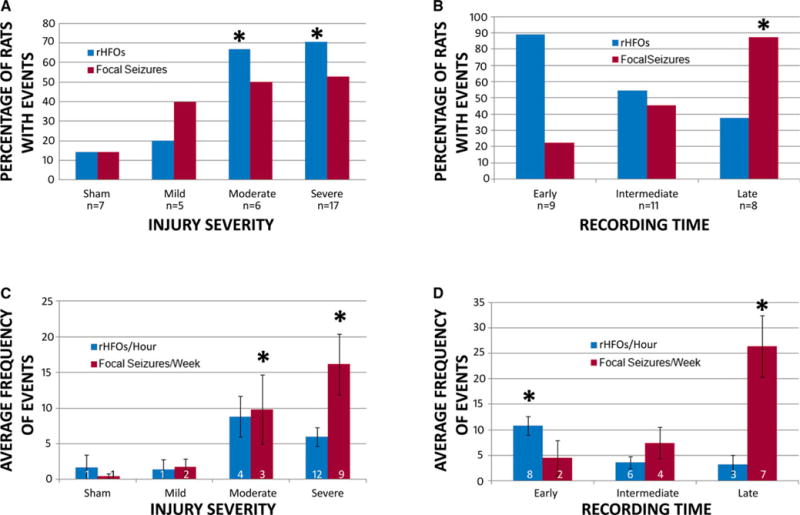
Rates of abnormal EEG events captured after lateral fluid percussion injury. (A) Percentage of rats within each group categorized by injury severity that had repetitive high-frequency oscillations with spikes (rHFOSs) (blue bars) and focal seizures (red bars) on EEG during monitoring. There was a significantly greater proportion of rats with rHFOSs in the moderate and severe injury groups compared to the sham and mild injury groups, and a trend for a greater proportion of rats in these groups to have focal seizures. (B) Percentage of rats within each group categorized by recording time that had rHFOSs (blue bars) and focal seizures (red bars). There was a trend for there to be a greater proportion of rats with rHFOSs in the early group compared with the late group, while there were significantly more rats with focal seizures in the late group compared with the early group. (C) The average frequency of rHFOSs per hour (blue bars) and focal seizures per week (red bars) in rats within each injury severity group. There was no difference in the frequency of rHFOSs between groups. However, there were more frequent focal seizures in the moderate and severe injury groups compared with the sham and mild injury groups (D) The average frequency of rHFOSs per hour (blue bars), and focal seizures per week (red bars), in rats within each recording time group. rHFOSs were more frequent in the early group compared with later groups, and focal seizures were more frequent in the late group compared with earlier groups. (*p < 0.05)
Epilepsia © ILAE
Early seizures
One rat had seizures within the first week after injury. Four days after severe FPI this rat had seizures associated with large-amplitude generalized discharges recorded from neocortical and hippocampal electrodes, with a frequency of approximately 0.5–1 Hz at the beginning of the seizure, evolving to 6 Hz (Fig. 2C). The duration of discharges was approximately 30 s, during which time the rat exhibited freezing behavior. At electrographic offset there was a brief wet-dog shake. These seizures occurred repeatedly, one to six times an hour, for 2 days. This rat did not have any further seizures until day 74, when it developed generalized seizures and died from status epilepticus as described below.
Late focal seizures
Spontaneous focal seizures occurred in 14 (50%) of 28 injured rats and one of 7 sham-injured rats (14%). Focal seizures were always associated with behavioral arrest but no other appreciable clinical signs. Electrographically they consisted of a buildup in amplitude of rhythmic spike-wave discharges with an initial central frequency of 9 Hz, later slowing to 8 Hz toward the end of the seizure (Fig. 2B). Ninety-four percent originated in electrode 5 or 6, in parietal neocortex just anterior to the injury site, with another 6% at electrodes 7 and 8, at the lesion core. The next group of electrodes involved were consistently 4 (parietal lobe lateral to the injury), 7, and 8 (at center of original craniotomy), later spreading to electrodes 1 and 2 (ipsilateral frontal), 3 (contralateral frontal), and 15 and 16 (homotopic to lesion core). Less frequently (35%), the spread involved electrodes 11–14 in the ipsilateral hippocampus. A typical seizure spread pattern can be seen in Figure 1C. Late focal seizures never originated in frontal cortex, hippocampus, or contralateral to FPI.
Seizures occurred most commonly in rats in the late period, then the intermediate period, and least in the early period (χ2 = 10.0354, p = 0.007; Fig. 3B). The frequency of seizure occurrence also increased with later recording times (F = 7.6, p = 0.003; Fig. 3D). Although we could detect no effect of injury severity on the proportion of rats in each group with seizures (χ2 = 3.1809, p = 0.365; Fig. 3A), the average frequency of seizure occurrence did increase with greater injury severity (F = 2.96, p = 0.0475; Fig. 3C). Of 14 rats with focal seizures, 10 rats (71%) also had rHFOSs. The four rats with PTE without preceding rHFOSs were all from the late group. Of 17 rats with rHFOSs, 10 later developed seizures (59%). The seven rats with rHFOSs that did not develop seizures were all from the early or intermediate group. The average onset of focal seizures was 62 (±9.6) days postinjury in the early group and 64.2 (±13.2) days postinjury in the intermediate group. All rats in the late group had focal seizures recorded within 3 days after electrode implantation. Two injured rats from the early group and five injured rats from the intermediate group had focal seizures, and in all cases the first rHFOSs were recorded before the onset of seizures. Seven injured rats from the late group had focal seizures, but rHFOSs were only recorded in three of these animals. The one sham animal with focal seizures was also from the late group, and rHFOSs were not recorded. The seizures were identical to those seen in injured animals, with onset in electrodes just anterior to and within the craniotomy site.
Duration of focal seizures was assessed as a way of determining whether the severity of these events changed over time. The duration of the first seizure in the late group, which was unlikely to be the first seizure the animal experienced, was significantly longer than the first seizures recorded in the earlier groups (T = 2.65, p = 0.033). The average duration of the last three seizures recorded in the early group was not significantly different from the first seizures in this group (p > 0.05). The average duration of the last three seizures in the intermediate group was longer than the average of the first seizures (T = 2.72, p = 0.026). There was no difference between the average duration of the first seizure and the last three seizures in the late group (p > 0.05; Fig. 4A).
Figure 4.
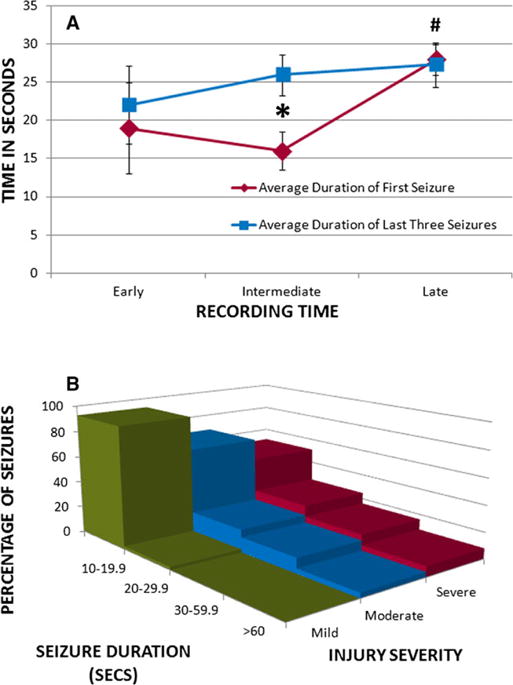
Seizure duration in relationship to recording time and injury severity. (A) Graph showing the average duration of the first seizure (red line) and the last three recorded seizures (blue line) for the injured rats in the early, intermediate, and late recording groups. The average duration of the first seizure recorded was significantly longer in the late group compared with the early group (#p < 0.05). The average duration of the last three seizures recorded was significantly longer than the first seizures recorded in the intermediate group (*p < 0.05), an effect not seen in the early or late groups. (B) Histogram showing the proportion of seizures of different durations in the mild (green), moderate (blue), and severe (red) injury groups. Rats had longer duration seizures after a more severe head injury. In the mild injury group the majority of seizures were of short duration, lasting <20 s. In the moderate injury group there was a larger proportion of seizures lasting >20 s, although there were still few seizures lasting >1 min. In the severe injury group there were even more seizures lasting longer than 20, 30, and even 60 s.
Epilepsia © ILAE
Seizure duration not only increased over time, but longer seizure duration was associated with severity of TBI. More than 90% of focal seizures in rats with mild FPI were between 10 and 20 s in duration (Fig. 4B), whereas in rats with moderate FPI, 65% of seizures lasted between 10 and 20 s, and the rest lasted >20 s. In rats with severe FPI, approximately 50% of seizures lasted <20 s, whereas the remaining seizures were longer, with some >60 s.
Late generalized-onset seizures
Three rats with severe injury (17.6%) had seizures with generalized discharges on EEG (Fig. 2D). Seizures began at days 74 and 128 post-FPI in two of these animals; both died of status epilepticus 1 day later, and neither rat had focal seizures recorded prior to generalized seizures. In the 24 h before their death they had repetitive seizures with generalized discharges at onset, starting with a frequency of approximately 1 Hz and then building up to a frequency of 3–6 Hz. The EEG would often show cessation of seizure activity for only less than a minute before it would build up again. The behavioral correlate was sustained tonus. Of the two rats that died of status epilepticus, one was the same rat that had exhibited early wet-dog shakes described earlier. The third rat, which had generalized seizures but did not have status epilepticus, had infrequent seizures beginning on day 62 that started with a buildup of low-amplitude rhythmic slowing in perilesional neocortical electrodes before spreading to adjacent electrodes and eventually involving all electrodes. The seizures lasted between 40 and 85 s. This rat also had more typical rHFOSs and focal seizures as described earlier, which had started on day 55.
Histology
Gross inspection of brains after perfusion did not reveal any non–TBI-related causes of seizures such as abscess or infection, including in rats with status epilepticus. Representative thionine-stained coronal sections, at a plane including the injury and the posterior hippocampus, were reviewed and showed that more severe injury, as classified by toe-pinch response, was associated with greater tissue loss and cortical thinning, ventricular enlargement, and deformation of the hippocampus ipsilateral to the side of injury (Fig. S1). No lesions were noted in any sham-injured animals, including the sham rat with seizures.
Discussion
Long-term EEG monitoring after lateral FPI demonstrated the occurrence of focal-onset seizures, which differ from previously described age-dependent SWDs.12,18 Rats were implanted with electrodes either early after TBI, in the intermediate period (8–13 weeks), or in the late period (>26 weeks after TBI). Because there is a limitation on how long implanted electrodes and head-caps permit continuous recordings from awake, freely moving animals, this design allowed us to identify early electrophysiologic changes after FPI, follow the progression of EEG abnormalities, and characterize the time course of development of seizures and PTE. Overall, 50% of rats developed PTE; however, that partly reflects different timings of EEG recordings. The proportion of rats with PTE increased with later EEG recordings, with 87.5% of rats in the late group having developed seizures. In comparison, other groups have found PTE rates of 100%,8 50%,7 or as low as 30%.19 These recordings show progression of seizures during the continuing process of epileptogenesis, with increasing seizure duration and frequency of occurrence at later periods. As well, more severe injury was associated with longer and more frequent seizures, and with the occurrence of generalized-onset seizures with more severe behavioral changes. We also found that previously described rHFOSs13 are associated with more severe TBI, and occur early after TBI before the onset of focal seizures.
Seizures
Seizures in this FPI model originated in perilesional neocortex, consistent with previous reports, although other groups have found secondary epileptogenesis with seizures originating from limbic structures.6,10 One rat with severe injury had early generalized-onset seizures with wet-dog shakes, which are thought to be hippocampal in origin,20 while the same rat plus one other with severe injury also had late generalized-onset seizures, suggesting severe TBI can damage limbic structures leading to epileptogenesis in those areas. However, no focal seizures originated from hippocampus in our experiments. Others have also demonstrated focal seizures originating from frontal neocortex after parietal injury,6 a finding we did not replicate. Different experimental parameters such as age, and site of injury within the parietal lobe, may account for this discrepancy. The extensive EEG coverage used in this study, including the frontal lobes bilaterally, should have identified frontal-onset seizures if they occurred. Montages consisting of four electrodes versus two have been shown to more reliably detect seizures after FPI21; therefore, the 16 electrodes used here should be extremely reliable for detecting seizure onset. A 10 s cutoff was used to define ictal events because of concerns voiced in the animal literature about whether shorter events are epileptic seizures,22,23 even though we are well aware that seizures in patients and animal studies can last <10 s.6,8,24 The average latency to first seizure that we report for the various recording groups is dependent on this practical definition of seizures, and may actually have been smaller if shorter duration seizures had been included.25
The focal seizures that develop after TBI in this FPI model have features different from spontaneous SWDs that have been reported in older rats, including Sprague-Dawleys.12,18 In particular, Rodgers et al.12 have indicated that seizure-like SWDs are common after both sham injury and rostral parasagittal FPI in adult Sprague-Dawley rats. They argue that these discharges do not reflect PTE after FPI. There are many important differences between the age-dependent SWDs they describe and the focal seizures found in our model, which are summarized in Table 2. It may also be important that rats in the Rodgers et al. study were Sprague-Dawley rats from Harlan laboratories, whereas the present study used Sprague-Dawley rats from Charles River. Substrain differences in epileptogenicity have previously been described between Sprague-Dawley rats from these two vendors.26 We have not observed age-dependent SWDs in our sham or injured rats. Our findings of focal neocortical seizures after FPI are consistent with the findings of others, such as the work of D’Ambrosio et al.,6,9,10 supporting the use of FPI as a model of PTE.
Table 2.
Differences between focal seizures described in this study and age-dependent spike-wave discharges (SWDs) previously described by Rodgers et al.12
| Focal seizures | Age-dependent SWDs12 | |
|---|---|---|
| Relationship to injury severity | Occurred more often in injured rats than shams, also occurred more often and with longer duration after more severe injury | No difference between sham and TBI rats |
| Location | Consistent onset in perilesional neocortex, at parietal electrodes | Lack of consistent focal onset, majority had synchronous bihemispheric onsets |
| Duration | 10 to >60 s | >6 s in older animals |
| Spread | Often showed spread from perilesional parietal neocortex to frontal neocortex and contralateral areas | No consistent spread pattern |
Repetitive high-frequency oscillations with spikes
rHFOSs were the first EEG change seen and were localized to the same neocortical electrodes from which focal seizures later originated. Fifty-nine percent of rats with rHFOSs later had focal seizures. It is important to note that rHFOSs were seen more often early after FPI than at later times when focal seizures were becoming more frequent, suggesting that rHFOSs may be temporarily expressed before evolving into late focal seizures. The only rats with rHFOSs that did not have later seizures were from the early and intermediate groups. It is possible that seizures would have been detected if they had been recorded for longer periods. Seventy-one percent of rats with focal seizures had preceding rHFOSs. The rats with PTE without rHFOSs were all from the late group, and may have actually had earlier rHFOSs that resolved by the time of late recordings. These findings indicate that rHFOSs could be a noninvasive biomarker of epileptogenesis after TBI.
TBI severity
The development of PTE was linked to TBI severity, similar to what is seen clinically4,27 and in other animal models.28 rHFOSs and focal seizures were recorded earlier in rats with more severe FPI compared to mild FPI, and by the late period, the highest proportion of rats with PTE had had either a moderate or severe injury. Seizure duration, a marker of epilepsy severity, was progressively longer with more severe brain injury. Consistent with other groups studying experimental PTE, we found that spontaneous seizures after TBI increase in duration as epilepsy progresses.5,7 As well, generalized seizures and even status epilepticus leading to death, were seen only in animals with the most severe FPI. However, wet-dog shakes and generalized seizures that occurred as part of status epilepticus likely represent a different seizure type, as those animals did not have any focal seizures. The injury resulting from FPI in these particular animals may have had different cellular and molecular consequences compared to rats with focal seizures.
One limitation of this study is that a detailed histopathologic analysis was not performed, as the focus of this paper was on electrophysiologic changes during epileptogenesis. Acute injury severity was defined by established criteria that are related to the degree of impact.15–17 Thionine staining was done on brain slices to assess the extent of the injury, and in this context no lesion was detectable in any of the sham rats, including the one with seizures. However, it is possible that more sensitive indicators of neuropathologic processes, such as inflammation and glial reactivity, may be involved in epileptogenesis and cannot be ruled out in these animals. Future studies of experimental PTE should include more detailed histopathologic examination.
Conclusions
PTE is not only a clinically relevant issue, but may also be an excellent model for studying the process of epileptogenesis as it relates to other forms of epilepsy. Significant studies performed by various groups in recent years support the development of PTE after FPI with a variety of different parameters. Although conflicting reports suggest that some seizures described in this model are age-dependent SWDs rather than PTE, our findings are in support of other groups reporting focal neocortical seizures after FPI.6,9,10 We have used more extensive EEG monitoring to demonstrate the presence of EEG events meeting criteria for spontaneous seizures and conclude that the lateral FPI model of TBI described herein leads to the development of PTE after a latent period. In addition, we have further characterized the temporal evolution of previously described rHFOSs seen early after FPI, which decrease in frequency over time postinjury when spontaneous seizures start to develop. The time course or evolution of epilepsy that we have illustrated, and the link between epilepsy and injury severity, make this a clinically relevant model for studying not only posttraumatic epilepsy but the process of epileptogenesis in general. The latent period in this model can be well defined, allowing for future studies of molecular and structural changes responsible for development of a network capable of generating spontaneous seizures. The association between early rHFOSs and late focal-onset seizures needs to be assessed further to determine whether rHFOSs could be a noninvasive biomarker for epileptogenesis.
Supplementary Material
Figure S1. Representative coronal sections Nissl-stained with thionine through the level of the hippocampus in rats with sham, mild, moderate, or severe lateral fluid percussion injury.
Figure S2. Further examples of focal-onset seizures recorded after lateral fluid percussion injury.
Data S1. Methods.
Key Points.
Perilesional repetitive high-frequency oscillations with spikes (rHFOSs) are recorded early after FPI, with later development of spontaneous focal seizures from the same electrodes
The severity of PTE increases over time, and is related to TBI severity
rHFOSs may be a noninvasive biomarker for the development of PTE
Focal seizures after FPI have distinct characteristics differentiating them from age-dependent spike-wave discharges
Acknowledgments
The authors would like to thank Mr. Joyel Almajano and Ms. Yan Cai for their technical support. AYR was supported by a Clinical Fellowship award from Alberta Innovates-Health Solutions. AB was supported by NS065877. CCG was supported by UCLA BIRC, NS05489, NS027544, NS057420, Child Neurology Foundation/Winokur Family Foundation, Today’s and Tomorrow’s Children Fund, and the Thrasher Research Foundation. RJS was supported by NS071048 and Citizens United for Research in Epilepsy. JE was supported by NS033310 and NS080181.
Biography

Aylin Reid is assistant professor of neurology at University Health Network/University of Toronto.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.White HS. Animal models of epileptogenesis. Neurology. 2002;59:S7–S14. doi: 10.1212/wnl.59.9_suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Timothy J, Pandit L, et al. Post-traumatic epilepsy: an overview. Clin Neurol Neurosurg. 2006;110:421–422. doi: 10.1016/j.clineuro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Herman S. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 5.Thompson HJ, Lifshitz J, Marklund N, et al. Lateral fluid percussion brain injury: a 15 year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 6.Curia G, Levitt M, Fender JS, et al. Impact of injury location and severity on posttraumatic epilepsy in the rat: role of frontal neocortex. Cereb Cortex. 2011;21:1574–1592. doi: 10.1093/cercor/bhq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharatishvili I, Nissinen JP, McIntosh TK, et al. A model of posttraumatic epilepsy induced by lateral fluid percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 8.D’Ambrosio R, Hakimian S, Stewart T, et al. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132:2805–2821. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Ambrosio R, Fairbanks JP, Fender JS, et al. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Ambrosio R, Fender JS, Fairbanks JP, et al. Progression from fronto-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolkadze T, Pitkänen A. Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma. 2012;20:789–812. doi: 10.1089/neu.2011.1954. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers KM, Dudek FE, Barth DS. Progressive, seizure-like, spike-wave discharges are common in both injured and uninjured Sprague-Dawley rats: implications for the fluid percussion injury model of post-traumatic epilepsy. J Neurosci. 2015;35:9194–9204. doi: 10.1523/JNEUROSCI.0919-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bragin A, Li L, Almajano J, et al. Pathologicelectrographic changes after experimental traumatic brain injury. Epilepsia. 2016;57(5):735–745. doi: 10.1111/epi.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanier ER, Lee SM, Vespa PM, et al. Increased hippocampal CA3 vulnerability to low-level kainic acid following lateral fluid percussion injury. J Neurotrauma. 2003;20:409–420. doi: 10.1089/089771503765355496. [DOI] [PubMed] [Google Scholar]
- 15.Giza CC, Prins ML, Hovda DA, et al. Genes preferentially induced by depolarization after concussive brain injury: effects of age and injury severity. J Neurotrauma. 2002;19:387–402. doi: 10.1089/08977150252932352. [DOI] [PubMed] [Google Scholar]
- 16.Prins ML, Lee SM, Cheng CL, et al. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res Dev Brain Res. 2006;95:272–282. doi: 10.1016/0165-3806(96)00098-3. [DOI] [PubMed] [Google Scholar]
- 17.Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 18.Pearce PS, Friedman D, Lafrancois JJ, et al. Spike-wave discharges in adult Sprague-Dawley rats and their implications for animal models of temporal lobe epilepsy. Epilepsy Behav. 2014;32:121–131. doi: 10.1016/j.yebeh.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shultz SR, Cardamone L, Liu YR, et al. Can structural or functional changes following traumatic brain injury in the rat predeict epileptic outcome? Epilepsia. 2013;54:1240–1250. doi: 10.1111/epi.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerner-Natoli M, Rondouin G, Baldy-Moulinier M. Evolution of wet dog shakes during kindling in rats: comparison between hippocampal and amygdala kindling. Exp Neurol. 1984;83:1–12. doi: 10.1016/0014-4886(84)90040-2. [DOI] [PubMed] [Google Scholar]
- 21.Eastman CL, Fender JS, Temkin NR, et al. Optimized methods for epilepsy therapy development using an etiologically realistic model of focal epilepsy in the rat. Exp Neurol. 2014;264C:150–162. doi: 10.1016/j.expneurol.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Ambrosio R, Miller JW. What is an epileptic seizure? Unifying definitions in clinical practice and animal research to develop novel treatments. Epilepsy Curr. 2010;10:61–66. doi: 10.1111/j.1535-7511.2010.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudek FE, Bertram EH. Counterpoint to “What is an epileptic seizure” by D’Ambrosio and Miller. Epilepsy Curr. 2010;10:91–94. doi: 10.1111/j.1535-7511.2010.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakhade SN, Klein PM, Huynh T, et al. Development of later life spontaneous seizures in a rodent model of hypoxia-induced neonatal seizures. Epilepsia. 2011;52:753–765. doi: 10.1111/j.1528-1167.2011.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Löscher W, Hirsch LJ, Schmidt D. The enigma of the latent period in the development of symptomatic acquired epilepsy – traditional view versus new concepts. Epilepsy Behav. 2015;52(Pt A):78–92. doi: 10.1016/j.yebeh.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Langer M, Brandt C, Lӧscher W. Therapeutic window of opportunity for the neuroprotective effect of valproate versus the competitive AMPA receptor antagonist NS1209 following status epilepticus in rats. Neuropharmacology. 2011;61:1033–1047. doi: 10.1016/j.neuropharm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44:S11–S17. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- 28.Kharatishvili I, Pitkänen A. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 2010;90:47–59. doi: 10.1016/j.eplepsyres.2010.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative coronal sections Nissl-stained with thionine through the level of the hippocampus in rats with sham, mild, moderate, or severe lateral fluid percussion injury.
Figure S2. Further examples of focal-onset seizures recorded after lateral fluid percussion injury.
Data S1. Methods.


