Abstract
Inorganic arsenic (iAs) is ubiquitous in the environment as arsenite (AsIII) and arsenate (AsV) compounds and biotransformation of these toxic chemicals leads to the extraordinary variety of organoarsenic species found in nature. Despite classification as a human carcinogen based on data from populations exposed through contaminated drinking water, only recently has a need for regulatory limits on iAs in food been recognized. The delay was due to the difficulty in risk assessment of dietary iAs, which critically relies on speciation analysis providing occurrence data for iAs in food – and not simply for total arsenic.
In the present review the state of knowledge regarding arsenic speciation in food and diet is evaluated with focus on iAs and human exposure assessment through different dietary approaches including duplicate diet studies, market basket surveys, and total diet studies. The analytical requirements for obtaining reliable data for iAs in food are discussed and iAs levels in foods and beverages are summarized, along with information on other (potentially) toxic co-occurring organoarsenic compounds.
Quantitative exposure assessment of iAs in food is addressed, focusing on the need of capturing variability and extent of exposure and identifying what dietary items drive very high exposure for certain population groups. Finally, gaps and uncertainties are discussed, including effect of processing and cooking, and iAs bioavailability.
Keywords: inorganic arsenic, arsenic speciation, food, dietary exposure, risk assessment, human health
Graphical abstract
1 Introduction
Arsenic is widely distributed in the earth’s crust as elemental arsenic and as the inorganic ions arsenite (AsIII) and arsenate (AsV). Inorganic arsenic (iAs), a collective name for different naturally occurring chemical species of the two oxyanions, is thus ubiquitous in the environment. Dissolved forms of arsenic in water are essentially inorganic, arsenite being the dominant species under reducing conditions, and arsenate the most stable species in oxygenated environments (WHO, 2001). Since iAs compounds are toxic to biota, biotransformation of these toxic chemicals leads to a variety of organoarsenic species, which in turn enter biosynthetic pathways leading to the about one hundred compounds found in nature (Taylor et al., this issue). Common iAs metabolites produced by mammals (including humans) are the pentavalent methylated species MMA (monomethylarsonic acid) and DMA (dimethylarsinic acid).1 In the aquatic environment, and especially in marine biota, a number of organic arsenocompounds belonging to different chemical classes (e.g. arsenosugars, arsenolipids) are found leading to total arsenic levels on the order of 1-100 μg/g.
Of the organic forms of arsenic, arsenobetaine, which is the major species in fish and most seafood, is generally assumed to be of no toxicological concern (FAO/WHO, 2011). DMA – and in traces MMA – are present in various foods, including rice, other plant-derived food and seafood. In vivo oral studies have shown adverse effects on the urinary bladder, kidneys, thyroid, and fetal development for DMA, whereas the gastrointestinal tract is the primary target organ of MMA, followed by kidney, thyroid, and reproductive system (US FDA, 2016). The studies in animals showed a carcinogenic potential for DMA; however the data regarding human carcinogenicity are inconclusive, hence IARC classified these methylated forms as possibly carcinogenic to humans (Group 2B) (IARC, 2012). Arsenosugars and arsenolipids are mainly metabolized in humans to DMA, and limited albeit growing information is available regarding their toxicity (Taylor et al., this issue). Along with MMA and DMA, these compounds have been proposed to be classified as ‘potentially toxic’ from a food safety perspective, in contrast to the innocuous arsenobetaine (Feldmann and Krupp, 2011).
In contrast to organic arsenic, iAs is extremely toxic and current risk assessments of dietary exposure to arsenic are entirely based on the inorganic forms. The general population is exposed to iAs almost exclusively via the diet, with food being the major contributor to intake when arsenic concentrations in water are <10 μg/L (the WHO guideline value for drinking water), while drinking water becomes the major source of exposure to iAs when water with arsenic concentrations well above 10 μg/L is used for drinking and cooking (EFSA, 2009; FAO/WHO, 2011). The IARC has established a causal role for oral exposure to iAs on skin, lung, and bladder cancers, and has shown suggestive evidence for liver, kidney, and prostate cancers (IARC, 2012). Apart from cancer – and skin lesions (EFSA, 2009) – a wide range of other adverse health effects such as cardiovascular diseases, developmental toxicity, abnormal glucose metabolism, type II diabetes and neurotoxicity are likely related to chronic ingestion of iAs (FAO/WHO, 2011). Susceptibility to the toxic effects of iAs varies considerably between individuals and populations depending on variations in iAs metabolism related to such factors as age, gender, life stage (e.g. pregnancy, lactation), nutritional status, and genetic polymorphisms in the regulation of enzymes responsible for iAs biotransformation (EFSA, 2009); evidence that the gut microbiota could also play a role is emerging (Carlin et al., 2016).
Key epidemiologic evidence for risk assessment of dietary iAs comes from populations chronically exposed to high arsenic levels in drinking water (>50 μg/L) in several countries, including southwestern Taiwan (Chen et al., 2010), Bangladesh (Kurokawa et al., 2001), northern Chile (Smith et al., 1998), and Argentina (Hopenhayn-Rich et al., 1998). Even though threshold mechanisms can be postulated for the adverse health effects of iAs (in particular, carcinogenicity) (Cohen et al., 2013), owing to existing uncertainties on dose-response relationships iAs is dealt with as a non-threshold toxicant (EFSA, 2009). As a result, exposure levels for iAs with no appreciable health risk, i.e. a tolerable daily or weekly intake, cannot be identified. Instead, reference points for health protection are currently based on benchmark responses of a given percentage of extra risk from human data. A benchmark dose lower confidence limit (BMDL) for 0.5% excess risk of lung cancer has been established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (BMDL0.5 = 3 μg/kg bw/day) (FAO/WHO, 2011), whereas the European Food Safety Authority (EFSA) identified a range of BMDL values for 1% excess risk of cancers of the lung, skin and bladder, as well as skin lesions (BMDL01 = 0.3-8 μg/kg bw/day) (EFSA, 2009). Therefore, for risk characterization an assessment of the margins of exposure (MOEs) between the identified reference points and the estimated daily dietary exposure to iAs is required, since there are no exposure levels associated with the absence of appreciable health risk on long-term (lifetime) basis.
Although the EFSA and JECFA assessments are relatively recent, new evidence of adverse effects for populations chronically exposed to iAs via drinking water at moderate to low levels, such as those resulting from water arsenic concentrations below 50 μg/L, continues to emerge (D'Ippoliti et al., 2015; Leonardi et al., 2012; Garcia-Esquinas et al., 2013; Moon et al., 2013; Zheng et al., 2013). Such evidence has not fed into a new risk assessment yet. Evidence also exists of negative impacts on foetal and infant development (Gilbert-Diamond et al., 2016; Rahman et al., 2007; Rahman et al., 2009; Raqib et al., 2009; Vahter, 2008) and impaired cognitive function in pre-school-aged children (Hamadani et al., 2011), and there is a need for further data supporting identification of dose-response relationships and critical exposure times (including in utero exposure) for these outcomes.
Despite classification as a human carcinogen following chronic dietary exposure, only recently has a need for regulatory limits of iAs in food been recognized in the U.S. and Europe, much later than for other toxic dietary elements such as cadmium, lead and (methyl)mercury. The delay was due to the difficulty in risk assessment of dietary iAs, which critically relies on chemical speciation analysis providing occurrence data for iAs in food – and not simply for the sum of inorganic and organic arsenic species (i.e. ‘total arsenic’). Accurate assessment of dietary exposure to iAs is challenging since, as mentioned earlier, arsenic occurs in food also as various organic species with lower to negligible toxicity. In some commodities, especially in fish and seafood, organoarsenic species occur at very high levels and thus speciation analysis for the selective determination of iAs is key. Considering arsenic in food as being present exclusively as iAs would lead to a considerable overestimation of the health risk related to dietary arsenic exposure.
In the last several years, robust analytical methods for selective determination of iAs have become widely available and international standards or regulatory limits have been proposed for iAs in polished rice (at the Codex Alimentarius level) (FAO/WHO, 2014), and adopted for iAs in rice and rice-based products (e.g., at the European Union level) (EU, 2015), respectively. In the present review the state of knowledge regarding arsenic speciation in food and diet is evaluated with focus on iAs and human exposure assessment through different dietary approaches (including duplicate diet studies, market basket surveys, and total diet studies). The analytical requirements for obtaining reliable data for iAs in food are discussed and iAs levels in foods and beverages are summarized, along with information on other (potentially) toxic co-occurring organoarsenic compounds.
Quantitative exposure assessment of iAs in food is addressed, focusing on the need for capturing variability and extent of exposure and identifying what dietary items drive very high exposure for certain population groups. Finally, gaps and uncertainties are discussed, including the effects of processing and cooking, and iAs bioavailability.
2 Common analytical techniques for determination of arsenic species in food
The area of analytical methods for iAs has been the subject of recent reviews (Jackson et al., 2015; Maher et al., 2015; Petursdottir et al., 2015) and will be only briefly summarized here.
Historically, food monitoring and official control targeted total arsenic and thus there is much more data for total arsenic than for iAs in foods. Until recently, iAs measurements (speciation analysis) were not routine in most laboratories and the necessary instrumentation and expertise to perform the analysis were lacking. This landscape is rapidly changing as the demand for iAs measurements increases and standard validated methods become available.
For speciation analysis it is necessary to extract arsenic from the sample without changing the chemical speciation. Owing to their different chemical nature, water-soluble arsenic species (including iAs) require different extraction strategies than fat-soluble arsenolipids. Water, methanol, acid, base and enzymatic extractions have all been used to extract water-soluble arsenic species from plant and animal tissues (Conklin et al., 2012; Foster et al., 2007; Kirby and Maher, 2002; Liu et al., 2015; Maher et al., 2012; Nookabkaew et al., 2013; Pétursdóttir et al., 2014b; Raab et al., 2005; Sadee et al., 2016). Arsenobetaine, DMA and MMA are relatively stable in dilute acids and bases and do not readily decompose to iAs; however As(III) and As(V) can readily interconvert. Even though it is generally considered that trivalent arsenic compounds are more toxic than the pentavalent forms at least at high doses (EFSA, 2009), human risk assessment is based on iAs and does not make distinction between the easily interconvertible trivalent and pentavalent inorganic species. Therefore it has become common practice to add an oxidant (usually H2O2) (Raber et al., 2012) to convert all As(III) to AsV and to express the AsV concentration as iAs. This approach also removes the need to quantify As(III) in the presence of other closely eluting arsenic species when using anion exchange chromatography coupled to inductively coupled plasma-mass spectrometry (ICP-MS). The most common method for species extraction uses dilute (1-2%) HNO3 and this has been shown to be an effective extractant for plant (Amaral et al., 2013; Huang et al., 2010; Maher et al., 2013) and fish tissue (Petursdottir et al., 2014b), as well as a wide variety of other food matrices (Cubadda et al, 2016).
The most widely used analytical technique for arsenic speciation is high performance liquid chromatography (HPLC) coupled to ICP-MS or atomic florescence spectroscopy (AFS). For iAs and other water-soluble species, the most common chromatographic method employed is anion exchange chromatography and a number of different eluents have been used, e.g., carbonate, phosphate or malonic acid. For more complex arsenic speciation analyses, such as determination of arsenosugars in marine algae, additional chromatographic separations such as cation exchange are necessary, whereas arsenolipids in fish oil or fatty fish require reversed phase chromatography and molecular mass spectrometry for structural identification (Taylor et al., this issue).
For iAs determination in rice – a staple food with relatively high arsenic concentrations – it has been shown that the exact analytical method does not unduly influence the quantification of iAs and should not be an impediment to regulation (de la Calle et al., 2011). Because only quantification of iAs is currently essential from a food safety standpoint, a more pragmatic, simple and cost-effective approach is to use hydride generation (HG) techniques coupled to atomic absorption spectrophotometry (AAS), AFS, or ICP-MS, which require only adding a reducing agent and HCl to effectively target and quantify iAs (Musil et al., 2014; Petursdottir et al., 2014a; Rasmussen et al., 2013). Use of HG also results in increased sensitivityand separates the analyte from the matrix (Musil et al., 2014). However, operating conditions have to be tuned carefully to achieve selective iAs determination and for most food matrices the HG-based approaches can only be viewed as screening methods.
A number of inter-laboratory studies have been conducted that included other foodstuffs in addition to rice. The quantitation of iAs in seafood remains problematic (Baer et al., 2011; Petursdottir et al., 2012; Petursdottir et al, 2014b), likely because iAs is usually a small proportion of total arsenic and extraction efficiencies, species interconversion and other methodological issues (e.g., peak tailing from closely eluting arsenic species, and matrix-induced shifts in retention time and baseline stability) appear to affect the result. In the European proficiency test IMEP-112, 74 laboratories - including seven expert in arsenic speciation - determined iAs in wheat, spinach and seaweed (Fucus vesiculosus). Of these, 85% produced satisfactory results for wheat and 60% for spinach, but only 20% for seaweed (de la Calle et al., 2012). Similarly a US proficiency test of rice, apple juice and kelp involving 41 participating laboratories demonstrated acceptable performance for speciation of rice and juice, but not kelp (Briscoe et al., 2016). Some arsenosugars are unstable to acid hydrolysis and this might be the cause of extreme over-estimation of As(V) if acid extraction is used (Chávez-Capilla et al., 2016).
IMEP-41 was a collaborative trial that aimed to demonstrate the applicability of a standardized extraction and analysis procedure to determine iAs in a variety of food matrices (Fiamegkos et al., 2016). The extraction used concentrated HCl followed by extraction to chloroform and back extraction into water and analysis by HG-AAS. Five expert laboratories and 13 other participating laboratories provided results for iAs for seven different food types (all Reference Materials, RMs) and in general the results were encouraging in that the required accuracy and precision were met, suggesting that this relatively simple and cost effective method could be employed by many laboratories to achieve acceptable iAs data. However, problems were again encountered with one sample of marine origin (mussels) and the use of chloroform solvent as extractant was considered a drawback to the method.
The availability of suitable food-based Certified (Standard) Reference Materials (CRM/SRM) with certified values for speciated arsenic (or even just for iAs) is lagging behind demand for speciation analysis. For rice, the US National Institute of Standards and Technology (NIST) produces a rice flour 1568b, the EU Joint Research Centre-Institute for Reference Materials and Measurements (JRC-IRMM) produces ERM-BC211, and the National Metrology Institute of Japan produces NMIJ CRM 7503-a, all having certified values for iAs and other species including DMA. As described above, IMEP-112 used NIST 1570a (spinach leaves) and therefore good consensus values for iAs exist for this CRM. Similarly, good consensus values are available for other CRM used in IMEP interlaboratory studies (e.g. ERM CE278k mussel tissue, DORM-4 fish protein). NIST intend to issue new SRMs or certify existing SRMs for arsenic species, e.g., 2986 (geoduck), 1974 (muscle tissue) and 3035 (apple juice). Additionally many existing reference materials from EU JRCIRMM, NIST, NRCC, IAEA, and other national and international standard reference agencies have been analysed by single and multiple investigators and the relevant data have been recently summarized (see table 7 in Maher et al., 2015).
Even though the total arsenic concentration does not provide useful information for risk assessment, it may still be worthwhile for regulatory purposes. Since it is relatively easy to measure total arsenic compared with iAs, if the former is below the regulatory threshold there is no need to perform the more time-consuming speciation analysis. Also, it is very informative to compare the total arsenic measurement to the sum of arsenic species from speciation analysis, since if the sum of species is much less (or more) than the total arsenic analysis then the speciation analysis should be viewed as questionable. An exception is the case where other compounds that need different extractants (e.g., arsenolipids) are present, since in this case total arsenic is by definition greater than the sum of iAs and the other water-soluble species.
3 Summary of arsenic species levels in foods and beverages
In terrestrial environments, iAs enters the food chain through plant crops, which absorb it through their roots according to phytoavailable levels in soils. The nature of the soil parent material is a major factor determining the iAs concentration in soils, even though soils are enriched in iAs compared with their parent rocks (Mandal and Suzuki, 2002). Apart from arsenic levels, soil physicochemical properties are important in determining the fraction of the element that is available to plants, such as ability to adsorb iAs and prevent its migration in the soil solution (Punshon et al., this issue). Whereas plants grown in arsenic-rich environments can take up substantial amounts of iAs in their edible portions (Signes-Pastor et al., 2008; Williams et al., 2007), animals metabolize and excrete excess iAs and hence foods of animal origin generally do not incorporate sizeable iAs quantities.
In aquatic environments - especially in the marine environment - iAs is taken up by biota at the bottom of the food-chain and extensively biotransformed to a wide range of organoarsenic metabolites with lower toxicities, which are then found in tissues (Taylor et al., this issue). Concentrations of iAs in marine and freshwater fish are normally very low (<5 ng/g), although some freshwater fish species have been shown to accumulate higher iAs levels if grown in arsenic contaminated waters (Jankong et al., 2007). Shellfish (e.g., mussels) and some seaweeds may contain moderate and very high levels of iAs, respectively (EFSA, 2009).
Based on available data on dietary intake (see Section 5), the foods that contribute the most to iAs exposure are rice and rice derived products, non rice-based cereals and cereal products (typically wheat-based), certain vegetables, fruit and fruit juices, shellfish, seaweeds, water, and other non-alcoholic and alcoholic beverages (Cubadda et al., 2016; EFSA, 2009; Oguri et al., 2014; Wong et al., 2013). Understanding iAs levels and the ratios of iAs to total arsenic in each of these food groups is important from a risk assessment perspective and to identify potential means for reducing exposure.
These foods can be categorized in two main classes. The first is represented by foods that normally have relatively high to very high levels (i.e., tens to thousands of ng/g) of iAs, such as rice, most shellfish and seaweeds. Rice is an exception among cereals in terms of iAs content owing to the ability of the plant to take up and translocate iAs to the grain, and the semiaquatic anaerobic growing environment (paddy field), which favours root uptake of iAs (Punshon et al., this issue). Levels of iAs in rice vary widely within and among world regions, but values around 100 ng/g are common and 130 ng/g has been recently reported as the mean of published data (Lynch et al., 2014). The significance of rice as a source of iAs in the diet is the fact that it is the most important grain with regard to human nutrition and caloric intake for a large proportion of the world's human population, especially in South Asia (Schmidt, 2015). Furthermore, it is the staple food in many areas with endemic arsenicosis in South-East Asia, where iAs levels in rice are further increased due to use of contaminated water in food preparation and cooking as well as in crop irrigation (Carbonell-Barrachina et al., 2009). High levels of iAs have also been found in a number of rice-based products such as rice waffles, rice wafers, rice crackers and rice cakes, which can make an important contribution to the dietary exposure of children (Carbonell-Barrachina et al., 2012; Signes-Pastor et al., 2016; Sun et al., 2009; Karagas et al., 2016). In the EU for these commodities a maximum limit of 300 ng/g has been set, compared to 200 ng/g for white rice; on the other hand, the maximum iAs level for rice specifically destined for production of food for infants and young children is 100 ng/g (EU, 2015) and the same limit is under consideration in the USA (see FDA, 2016). Crustaceans and, especially, molluscs, may also contain substantial amounts of iAs (mean of published data 130 ng/g; Lynch et al., 2014). Levels in edible seaweeds can be even higher, on the order of thousands of ng/g in some commercial species (EFSA, 2009).
The second class of foods is represented by wheat along with other non rice-based cereal grains (and derived products), vegetables and fruit (and derived products), water and beverages. In the absence of arsenic environmental contamination, these commodities generally have relatively low iAs concentrations (<20 ng/g, and in many cases <10 ng/g for solid foods, ≤5 ng/g beverages, and ≤1 μg/L for water)2 but contribute to the dietary exposure of iAs owing to their high consumption levels. The most important items in terms of iAs intake normally are wheat and other non rice-based cereals, and water (see e.g. EFSA, 2014). Wheat is the main cereal staple at the basis of the diet in many world regions. Levels of iAs in wheat are one order of magnitude lower than in rice (Williams et al., 2007); as an example, in a nation-wide study in Italy a mean content of 9 ng/g (dry weight basis) was found in wheat grain (Cubadda et al., 2010). However, this staple may attain substantially higher concentrations of iAs when grown in arsenic-contaminated soils. For example, the average iAs content of wheat grain was 55 ng/g in the presence of higher phytoavailable iAs levels in soil (Cubadda et al., 2010), with individual grain samples up to about 400 ng/g (Cubadda et al., unpublished results). This sensitivity to growing conditions can be particularly important in arsenic-endemic areas, where it is increasingly recognized that exposure from food in addition to that from drinking water increases iAs exposure and plays a significant role in aggregate exposure (Kile et al., 2007; Signes-Pastor et al., 2008). In such exposure scenarios, an accurate estimation of the total dietary intake of iAs, via both water and food, is important in order to obtain reliable dose-response data for the observed health effects; these data were mainly lacking in early epidemiological studies and had to be estimated in order to perform dose-response assessment (EFSA, 2009).
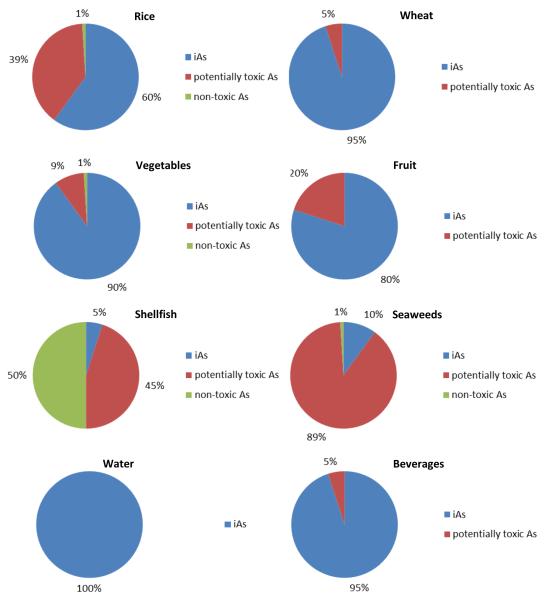
Since the vast majority of arsenic occurrence data in food refer to total arsenic and not to iAs specifically, based on an assessment of published data and expert judgment, we have estimated the expected proportions of iAs to total arsenic in the food groups that contribute the majority of iAs exposure (Figure 1). As suggested by Feldmann and Krupp (2011), the remaining species making up the total arsenic content of each food group were categorized in two different fractions, i.e. the ‘potentially toxic fraction’, made up of organoarsenicals other than arsenobetaine (e.g., arsenolipids, arsenosugars, methylated species such as DMA), and the ‘non-toxic fraction’, i.e., arsenobetaine. These two fractions are not toxicologically equivalent and, even though to date it has not been possible to thoroughly assess the risks posed by all the different organoarsenic compounds belonging to the ‘potentially toxic fraction’, it appears sensible to distinguish them in order to highlight existing uncertainties related to the unknown toxicity of part of the arsenic species present in the food commodities considered.
Figure 1.
Estimated proportions of iAs and other arsenic species belonging to the ‘potentially toxic fraction’ and the ‘non-toxic fraction’ (arsenobetaine) in important contributors to iAs dietary exposure (for references see Table S1, Supplementary Material).
It should be noted that the proportion of iAs and other arsenic species shown in Figure 1 are averages and considerable variability is observed for some items. In particular, in the case of rice the proportion of iAs varies between ca. 30% and 90%, with the remaining fraction dominated by DMA (EFSA, 2009; Lynch et al., 2014). In the case of vegetables and fruit, the iAs content of the edible portion of many individual plant species is averaged. The same holds true for seaweeds, where the iAs proportion varies from about 1% in most commercial species to >50% in some brown algae, to ≥70% in hijiki (EFSA, 2009). Less variability is observed in wheat (taken as the most representative item of the ‘non rice-based cereals and cereal products’ group) as well as wheat products such as bread and pasta, where iAs generally represents ≥95% of the total arsenic (Cubadda et al., 2010, D’Amato et al., 2011). Other ingredients in more complex processed wheat-based products may contribute other arsenic species, and 90% appears to be a sound general estimate for the entire category.
Infant food is a special food category that merits attention because it is aimed at a vulnerable population. Many products for infants and young children are rice-based. Pre-cooked, milled rice is a dominant carbohydrate source for weaning infants up to one year of age, due to the bland taste, material properties, low allergen potential and nutritional value (EFSA, 2009). For children who suffer from cow's milk allergy or lactose intolerance, rice-based formulas or rice drinks are sometimes substituted for infant formula or cow’s milk. A number of studies investigated infant food and found that iAs was the dominant arsenic species, sometimes with levels of concern in baby rice (Carbonell-Barrachina et al., 2012; Carignan et al., 2016; Jackson et al., 2012; Juskelis et al., 2013; Karagas et al., 2016; Meharg et al., 2008; Signes-Pastor et al., 2016; USFDA, 2016; Vela and Heitkemper, 2004).
Many products popular among older children are also largely rice-based (e.g. breakfast cereals, rice crackers). Investigations that have focused on these products and rice products targeted at the macrobiotic, vegan, lactose intolerant and gluten-free food market also found substantial amounts of iAs in a significant proportion of samples, showing that they represent an important exposure source for the relevant population groups (Carbonell-Barrachina et al., 2012; Signes-Pastor et al., 2016; Sun et al., 2009). Infants with celiac disease appear to be a particularly vulnerable subgroup of the population (Munera-Picazo et al., 2014).
4 Effect of food processing and preparation
Both processing of raw food into food products and the common practices of food preparation and cooking in the household may affect iAs levels in food. As this topic has been reviewed previously (e.g., Bundschuh et al., 2012; Del Razo, et al., 2002; Diaz et al., 2004), here we focus on key take-home points relevant to processes significantly impacting human exposure to iAs and ways to reduce it.
Food processing techniques can have contrasting effects on iAs concentrations. For example, milling reduces total and iAs concentrations in cereal grains, as arsenic is enriched in the outer layers of the kernel. As such, polishing leads to a substantial decrease in iAs concentration in rice and thus white rice contains lower iAs concentrations than the corresponding brown rice (Torres-Escribano et al., 2008). On the other hand, this means that bran or wholegrain flour and derived products have higher iAs contents, even though bioaccessibility of this arsenic compared to the nonwholegrain products has yet to be established. Another process that appears to result in higher iAs concentration is parboiling, which is done on rice before milling (in the husk); it is likely that the iAs contained in the bran is solubilized by hot water and the subsequent steaming transports iAs within the endosperm (He et al., 2012).
As far as food preparation and cooking in the household is concerned, the concentration of iAs in the food, the concentration of iAs in the cooking medium (if any) and the characteristics of the preparation process are key in determining the iAs levels in the final product. In fact, cooking may be the most important process affecting both total arsenic concentrations and arsenic speciation (Bundschuh et al., 2012).
For foods prepared with water, the concentration of iAs in the water is critical. Preparation of dough with water containing higher than background arsenic concentrations detectably increases the iAs content of bread (Cubadda et al., 2015). In boiling food, use of water with negligible arsenic levels may extract iAs from the food matrix. At the (low) iAs concentration normally found in pasta, for instance, boiling with water containing very small amounts of arsenic decreases the original levels in the raw food (Cubadda et al., 2003), whereas an increase in iAs in the final product is usually observed with water arsenic >2-3 μg/L (Cubadda et al., unpublished results). Salt addition is also important as it affects aspects such as osmotic pressure and the extent of the loss of the water-soluble iAs into cooking water.
Most foods prepared with arsenic-contaminated water tend to absorb arsenic from the water and thus have higher total arsenic and iAs concentrations than the raw food items. For example, arsenic concentrations in prepared soups, beans/pulses (Del Razo et al., 2002), breads (Vahter, 1995), rice (Ackerman et al., 2005; Bae et al., 2002; Laparra et al., 2005; Torres-Escribano et al., 2008) and vegetables such as corn and cauliflower (Diaz et al., 2004) can be much higher than in the raw foods when cooked in arsenic-contaminated water (or when compared to similar food items prepared in low-arsenic water). With some exceptions, foods that take up more water or are cooked longer appear to absorb more arsenic (Del Razo et al., 2002; Laparra et al., 2005), while boiling vegetables in low-arsenic water can indeed be a means to decrease the iAs content of the cooked product (reviewed in Bundschuh et al., 2012).
The most intensive studies of the effects of food preparation have been conducted with rice. In particular, procedures for cooking arsenic-rich rice are of special importance in arsenic-endemic areas where rice plays a vital role as the main source of energy and protein intake. Rinsing rice with water until it runs clear, then boiling the rinsed rice in excess water that is discarded after cooking substantially reduces arsenic exposure (Sengupta et al., 2006). Even if the water itself is not entirely arsenic-free, this two-step process provides an easily realized strategy for reducing arsenic exposure via cooked rice (Carbonell-Barrachina et al., 2009; Mihucz et al., 2007; Mihucz et al., 2010; Raab et al., 2009; Rahman et al., 2006; Sengupta et al., 2006). Steaming also reduces exposure slightly relative to common procedures (Raab et al., 2009), while a more complex procedure involving cooking in an off-the-shelf coffee percolator reduces iAs even more than rinsing and cooking in excess water, especially if the perfused water is discarded (Carey et al., 2015).
For foods other than rice, the effects of food processing vary with food item and cooking procedure. Some items lose moisture during cooking and thus the concentration of arsenic (and iAs) per unit mass increases, including microwaved and fried sea bass (Ersoy et al., 2006), salted cod and bivalves (Devesa et al., 2001b, Devesa et al., 2001c). On the other hand, steaming decreased total arsenic concentrations in cooked mussels, apparently by removing some arsenic species (Lai et al., 2004). Although early studies suggested that sustained temperatures >150 °C are needed to change arsenic species from organic forms to more toxic inorganic forms (van Elteren and Slejkovec, 1997), baking, frying and grilling alter speciation of organic arsenocompounds only; this was found in fish (Devesa et al., 2001a; Hanaoka et al., 2001), but not red algae (Shibata et al., 1990; Wei et al., 2003, Almela et al., 2005). Boiling, however, did sometimes reduce iAs in algae (Laparra et al., 2003; Ichikawa et al., 2006). For the iAs-rich seaweed Hizikia fusiforme, traditional washing and soaking effectively reduces iAs content (Laparra et al., 2003; Rose et al., 2007). Finally, for preserved food, arsenic losses (solubilization) to the preservation solution have also been reported, which may result in a (slight) decrease of iAs levels (EFSA, 2009).
5 Fate of ingested inorganic arsenic and biomarkers of exposure
Several studies in rodents and humans indicate that As(III) and As(V) present in drinking water are rapidly and nearly completely (about 95%) absorbed after ingestion (ATSDR, 2007). In food commodities, iAs is likely to be bound to thiol groups in peptides and proteins and it might to be less absorbable compared to soluble inorganic forms in water. Therefore the question arises whether the absorption and toxicity of iAs through food is as high as through water.
Overall, the absorption of ingested arsenic species is expected to vary depending on the solubility of the arsenical compounds (the more water soluble the compound, the greater its absorption), the presence of other food constituents and nutrients in the gastrointestinal tract, and on the food matrix itself (EFSA, 2009). As an example, Juhasz et al. (2008) demonstrated that whereas the bioavailability of iAs in mung beans was almost 100% in a swine model, it was only 50% for lettuce and chard, suggesting an influence of the non-digestible polysaccharide component of the vegetable on gastrointestinal absorption. Using the same animal model, the bioavailability of arsenic in cooked Basmati white rice containing entirely iAs was 89% (Juhasz et al., 2006). In these experiments, the bioavailability of DMA and MMA was found to be much lower.
He and Zheng (2010) conducted a pilot, preliminary mass balance study with two human subjects, in which the amount of speciated arsenic excreted in the urine (see hereunder in Section 5 for urinary arsenic excretion) was compared with the amount ingested from rice consumption. Taking into account that 24% of the arsenic in rice was iAs and assuming that 33% of the DMA in rice was absorbed (value from Juhasz et al., 2006), the estimated absorption of iAs for each of the two subjects was 66% and 80%, respectively. However, the authors noted that actual bioavailability is likely to be somewhat higher, as some of the arsenic entering systemic circulation may be eliminated through hair and skin.
Several studies have been conducted on the bioaccessibility of arsenic species from various food in vitro (see Yager et al., 2015, for a detailed overview) and those referring specifically to iAs are summarized in Table 1. Although these studies are not useful for estimating the amount of iAs that enters systemic circulation, they do provide information about the release of bound arsenic from food under various conditions and factors that can modulate the amount of iAs available for intestinal absorption, such as Fe3+ concentration (Alava et al., 2013). Overall, these studies indicate that iAs in rice, seaweed and food composites appears to be largely solubilized after simulated GI digestion becoming potentially available for absorption in the intestine, whereas bioaccessibility was lower for, e.g., berries and clams.
Table 1.
Studies on in vitro bioaccessibility of iAs contained in different food items.
| Food type | Specific items | Condition | % iAs solubilizeda | Ref |
|---|---|---|---|---|
| Rice | Eight different rice varieties | Cooked | >90 (63-99)b | Laparra et al., 2005 |
| Rice | Long Grain (3), Extra Long Grain (2), Long Grain Parboiled (6), Brown rice (7) | Cooked | 80 (53-102)c | He et al., 2012 |
| Rice | Parbolided and non-parboiled white long-grain rice | Cooked | 82 (64-99)d | Signes-Pastor et al., 2012 |
| Rice | Polished, Basmati, parboiled | Cooked | 80 (78-81) | Alava et al., 2013 |
| Food composites | Fifteen duplicate diets of Mexican schoolchildren | Cooked | 71 (33-97) | Garcıa-Rico et al., 2012 |
| Country foods | Berries | Raw, unwashed | 45 (18-79)e | Koch et al., 2013 |
| Clams | Mya arenaria | Raw | 44f | Koch et al., 2007 |
| Edible seaweed | Hizikia fusiforme | Cooked | 88 | Laparra et al., 2003 |
| Edible seaweed | Hizikia fusiforme | Soaked | 85 | Brandon et al., 2014 |
Mean (min-max)
The percentage of iAs was 80% in cooked rice
Total arsenic (iAs is 52% of total solubilized arsenic on average). Arsenic in brown rice was in general less bioaccessible
Result for the two highest concentration of arsenic in cooking water summarized (As in rice expected to be mainly iAs)
Contrary to berries that predominantly contained iAs, other food collected (hares, edible mushrooms) had a more complex speciation and results are not easily interpreted. Therefore, only berries have been considered here.
Only clams from a contaminated site where iAs was the dominating species were considered in this summary. In seaweed (Fucus sp.) containing half of total arsenic as iAs the bioaccessibility was 77% on average
From this appraisal of the literature in the field, it appears that the bioavailability of iAs in foods importantly contributing to exposure is high and similar to that from drinking water. Up-to-date risk assessments address iAs from both food and water as sources of exposure that contribute in a qualitatively identical way (EFSA 2009; FAO/WHO 2011). Nevertheless, more research in this area would be welcome, with specific focus on iAs and studies should be performed on ready-to-eat food, since preparation and cooking can significantly affect bioaccessibility. For instance, cooking seaweeds was found to increase the bioaccessibility of iAs in H. fusiforme to levels of concern (Laparra et al., 2003).
Apart from absorption, others aspects of the fate of ingested arsenic in the gastrointestinal tract are matter for further research since they may have important implications for human health.
One important issue is that of the potential conversion of organic species to iAs. This had been generally not believed to occur, but recently demethylation of MMA and DMA has been demonstrated in rice during simulated GI digestion (Chávez-Capilla et al., 2016). The consequences of such a finding for a staple like rice are substantial and thus in vivo studies are needed to find out whether this may happen in the human gut. Interestingly, degradation and demethylation of arsenosugars with iAs formation was also observed in the same study, but for standard solutions only and not when ingested within the corresponding foodstuff, which highlights the relevance of the food matrix in GI processes.
Another relevant topic is the possible interaction of iAs with gut microbiota. Evidence is emerging that gut microbiome compositions might play a role in the individual response to exposure and that such interaction could affect iAs metabolism and toxicity (Lu et al., 2014b; Yu et al., 2016). In turn, iAs appears capable of perturbing the gut microbiome and its metabolic profile in mice (Lu et al., 2014a).
Ingested iAs is largely biotransformed and excreted in urine with a short half-time of a few days mainly as MMA and DMA, with typical ratios of 10–30% iAs, 10–20% MMA, and 60–80% DMA (EFSA, 2009). Urinary DMA percentage is regarded as an indicator of methylation efficiency and in many studies the primary methylation index, defined as the ratio between MMA and iAs concentrations, and secondary methylation index, the ratio between DMA and MMA concentrations, are calculated to assess the methylation capacity of the first and second methylation step, respectively (Cubadda et al., 2012). Marked inter-individual variations in iAs metabolism have been observed depending on such factors as age, life stage, gender, nutritional status, and genetic polymorphisms in the regulation of enzymes responsible for iAs biotransformation (EFSA, 2009; Vahter et al., 1995).
Several studies from Europe, the United States, South America, and southeast Asia have indicated a roughly 1:1 ratio between the sum of the concentrations of iAs and related metabolites in urine and the concentrations of iAs in water in cases where the As intake from water exceeds that from food (FAO/WHO, 2011). However, when levels of As in drinking water are lower, the ratio of the sum of urinary iAs and related metabolites in urine to iAs in water is higher than 1. The sum of iAs and its methylated metabolites in urine reflects exposure from all sources, not only dietary intake, but if the non-dietary sources of exposure (e.g. air, cigarette smoke) are negligible it can be used as a helpful biomarker of dietary exposure (Hamadani et al., 2011; García-Esquinas et al., 2013; Gilbert-Diamond et al., 2016; Leonardi et al., 2012; Moon et al., 2013; Rahman et al., 2009; Raqib et al., 2009; Vahter et al., 1995; Zheng et al., 2013). The main limitations are that urinary arsenic reflects only recent exposure (few days) and is affected by the ingestion of DMA contained in food (e.g. rice) (Cascio et al., 2011) or produced in the body as the consequence of the metabolism of arsenosugars and arsenolipids present in seaweed and seafood (Lai et al., 2004; Taylor et al., this issue). In populations consuming significant quantities of these food items, the higher dietary intake of DMA or its production as metabolite of organoarsenic species limit the usefulness of the speciated urinary arsenic as biomarker of iAs exposure. As an alternative, nail arsenic (i.e. total arsenic in nails) can be used as longer-term biomarker, since it reflects chronic exposure to iAs over several months and is marginally influenced by ingestion of other arsenic species (Cubadda et al., 2015). Hair can also be used since, as nails, they accumulate iAs because of their high keratin content containing sulfhydryl groups that bind arsenite, but they have some limitations including a greater potential for contamination by exogenous iAs (EFSA, 2009; Orloff et al., 2009).
The passage of iAs over the mammary gland is limited and little arsenic is excreted in breast milk (Vahter, 2008). This has important consequences since, as long as breast milk is the only food, the infant is protected against iAs exposure during the breastfeeding period even if the mother’s exposure is significant (Vahter, 2008; Carignan et al. 2015). On the other hand, formula prepared from drinking water with moderate arsenic levels may cause considerable postnatal iAs exposure (Carignan et al. 2015).
6 Human exposure to dietary inorganic arsenic
Until very recently, the occurrence data for arsenic in food collected in the framework of monitoring schemes and official food control across the world regarded total arsenic without differentiating the various arsenic species. In recent years, increasing awareness of the threat to human health represented by iAs led national and international bodies to assess human exposure to dietary iAs in order to characterize the risks related to the presence of arsenic in food. However, due to the paucity of data for iAs, such assessments estimated human dietary exposure by (1) assuming that a defined percentage of total arsenic is present as iAs, (2) selecting fixed occurrence figures for some commodities, or (3) combining (few) actually measured data with estimates based on different assumptions (Arnich et al., 2012; EFSA, 2009, 2014; FAO/WHO, 2011; Yost et al., 1998, 2004). All three of these approaches have significant limitations, mainly because the limited amount of arsenic speciation data for most food commodities did not allow researchers to characterize or model ‘typical’ iAs concentrations for specific food items or to calculate reliable iAs to total arsenic ratios. In addition, a high degree of variability has been observed across regions for both absolute iAs concentrations and ratios of iAs to total arsenic in a number of important food items contributing to iAs exposure, notably rice. In order to prevent an underestimation of the health risk for the population, these exposure assessments often made conservative assumptions – which, however, were affected by a consistent degree of uncertainty and might have actually led to an overestimation of exposure (EFSA, 2014). Having noted these drawbacks, a brief overview of key assessments using these approaches is given below; a detailed review is found in FAO/WHO (2011). Our focus is specifically on iAs, since human exposure to organic arsenic species from seafood is dealt with in another paper in this issue (Taylor et al., this issue). Moreover, a specific, more detailed focus on rice is found in Davis et al. (this issue).
Using a small dataset of iAs occurrence values, various assumptions and modeling (Yost et al., 1998), and subsequently a more refined approach (Yost et al., 2004) based on a market basket survey (Schoof et al., 1999), mean intakes in the range 0.07-0.2 μg/kg bw/day for US (all population groups) and 3.2 μg/day for Canadian children aged 1-6 years (equal to 0.21 μg/kg bw/day assuming a body weight of 15 kg) were estimated. Mean iAs dietary exposures approximately within this range for the US general population have been estimated by four other studies (Meacher et al., 2002; Meliker et al., 2006; Tsuji et al., 2007; Xue et al., 2010), each giving exposure ranges related to the inclusion of different sources of iAs. For example, Xue et al. (2010) using probabilistic modeling found a mean iAs exposure from food for the general US population of 0.05 μg/kg bw/day, approximately two times higher than the mean iAs exposures from drinking water, and a 95th-percentile exposure of 0.19 μg/kg bw/day. Meliker et al. (2006) reported 95th-percentile exposures of 0.34 μg/kg bw/day, with a maximum of 1.80 μg/kg bw/day, from an area in south-east Michigan where drinking water was contaminated. In a further study aimed at comparing the contribution of food, drinking and cooking water to estimated iAs exposure in several U.S. study populations with varying background tap water arsenic levels, Kurzius-Spencer et al. (2014) found intakes of 24.5–26.1 μg/day, with approximately 30% of intake from food in subjects with tap water arsenic >10 μg/L. In subjects with tap water arsenic ≤10 μg/L, aggregate iAs exposure was 9.4–11.8 μg/day, with 54–75% of intake from food, highlighting that the majority of iAs exposure is attributable to solid food in subjects with tap water arsenic ≤10 μg/L.
In 2009, EFSA estimated the iAs dietary intake in Europe assuming that the proportion of iAs varied from 50 to 100% of the total arsenic in food commodities other than fish and seafood, with 70% considered as best reflecting an overall average (EFSA, 2009). Fixed values for iAs of 30 ng/g in fish and 100 ng/g in seafood were considered realistic for calculating human dietary exposure. With these assumptions, the iAs dietary intake across 19 European countries was estimated to range from 0.13 to 0.56 μg/kg bw/day for average consumers, and from 0.37 to 1.22 μg/kg bw/day for 95th percentile consumers. High consumers of rice in Europe, such as certain ethnic groups, were estimated to have a daily dietary exposure of about 1 μg/kg bw/day, whereas exposures of about 4 μg/kg bw/day were estimated for high consumers of algae-based products. The limited available evidence did not indicate a different dietary exposure for vegetarians from that of the general population, unless they consume a large amount of algae-based products.
In 2014, EFSA refined previous estimates and calculated the mean dietary exposure among infants, toddlers and other children to range from 0.20 to 1.37 μg/kg bw/day, while the 95th percentile exposure ranged from 0.36 to 2.09 μg/kg bw/day (EFSA, 2014). Mean dietary exposure among the adult population in Europe (including adults, elderly and very elderly) ranged from 0.09 to 0.38 μg/kg bw/day, and 95th percentile exposure ranged from 0.14 to 0.64 μg/kg bw/day. For all the age classes except infants and toddlers, the main contributor to dietary exposure to iAs was the food group ‘Grain-based processed products (non rice-based)’, in particular, wheat bread and rolls. Other food groups that were important contributors to iAs exposure were rice, milk and dairy products (main contributor in infants and toddlers), and drinking water.
The high inherent uncertainty of exposure estimates based on assumptions and modeling calls for approaches based on actual (i.e., measured) iAs concentrations as occurrence data. Only in a few cases have attempts to actually measure iAs in food been made in national or regional surveys aiming to assess dietary exposure at the population level using market-basket approaches or actual total diet studies; the methodology used in these studies and the resulting mean estimated iAs exposures are summarized in Table 2. Overall, the estimated iAs mean exposures range from 0.02 to 0.26 μg/kg bw/day, with the exception of the Japanese study where the estimated exposure for the adult population is higher (Table 2). Given the relevance of food preparation and cooking on iAs levels in food, it is important to note that in one market-basket study food items were prepared and/or cooked before analysis (Oguri et al., 2014) – as it is done in total diet studies – whereas in the other one they were not (Fontcuberta et al., 2011).
Table 2.
Studies assessing dietary exposure to iAs at the population or individual level on the basis of measured iAs concentrations as occurrence data. Dietary background exposures only are addressed (data for arsenic-endemic areas are not included).
| iAs in fooda |
Analytical technique | Mean exposure | Study design | Study level |
Country | Year | Population of interest |
Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| μg/day | μg/kg bw/day | ||||||||
| Population exposure | |||||||||
| M | HPLC-ICP-MS | 2.1-5.6 | 0.067-0.215 | TDS | National | Italy | 2012-14 | All age groups | Cubadda et al., 2016 |
| M | HPLC-HEPO-HG-ICP-MS | 24 | 0.40c | Market-basket survey | Regional | Japan | 2011 | Adults | Oguri et al., 2014 |
| M, ns | HG-ICP-MSb | 13.4 | 0.22 | TDS | National | Hong Kong | 2010-11 | Adults | Wong et al., 2013 |
| M, ns | HR-ICP-MSb | 1.4-7 | 0.024-0.246 | TDS | National | UK | 2006 | All age groups | Rose et al., 2010 |
| M, ns | ICP-MSb | 6.1-18.5 | 0.09-0.26 | Market-basket survey | Regional | Spain | 2002-03 | Adults | Fontcuberta et al., 2011 |
| Individual exposure | |||||||||
| M | HPLC-HEPO-HG-ICP-MS | 6.5 | 0.13 | Duplicate diet (n=25) | Regional | Japan | 2009 | Adults, females | Oguri et al., 2012 |
| M | HPLC-HEPO-HG-ICP-MS | 27 | 0.45c | Duplicate diet (n=29) | National | Japan | 1997-98 | Adults | Oguri et al., 2012 |
| M, ns | HG-AAS | 10 | 0.17c | Duplicate diet (n=4) | Local | Japan | 1989 | Adults | Mohri et al., 1990 |
M = measured, ns = non-specific analytical method
After HCl/CHCl3 extraction
Estimated on a basis of a body weight of 60 kg
A drawback of most of these studies is that results generally suffered from high limits of detection for iAs, resulting in substantial percentages of samples (50-90%) with non-detectable levels, i.e. ‘left-censored data’. This, in turn, resulted in a high uncertainty of exposure estimates (e.g., see the wide lower bound-upper bound estimate ranges, when computed). In a single study iAs was quantified in all samples (51 food groups) except one (the ‘oils and fats’ category) (Cubadda et al. 2016). According to this study, the mean exposure of infants and toddlers (< 3 y), children (≥ 3 to < 10 y), and adolescents (≥ 10 to < 18 y), was 2.8, 2.4, and 1.4 times that of adults, respectively, whereas it was almost identical to that of adults for the elderly (≥ 65 y). The 95th percentile exposure of adults was 0.249-0.284 μg/kg bw/day, whereas it was 2.4, 1.4, and 0.9 times that value for children, adolescents, and the elderly, respectively. The foods contributing the most to iAs dietary exposure of the total population were cereals and cereal products (35%, especially rice, bread and pasta) and water and other non-alcoholic beverages (26%, especially bottled water), followed by vegetables (11%), fruit (7%), crustaceans and molluscs (4%), and alcoholic beverages (4%). When data for consumers only were considered, the main contributors to the dietary intake were found to be rice and bottled water, followed by fruit and fruit juices for infants and toddlers, and by alcoholic beverages and vegetables for adults. Compared to this European total diet study, rice makes an even higher contribution to total iAs exposure in the Hong Kong total diet study and in the Japanese market-basket study, as expected: 45% and 62%, respectively. In the latter study, hijiki is the second major contributor (28%) to the estimated mean iAs exposure via the diet (Oguri et al., 2014; Wong et al., 2013).
In Table 2, studies addressing individual exposure to iAs as assessed by means of duplicate diets in Japan are also summarized (Mohri et al., 1990; Oguri et al., 2012). The mean exposures estimated in these studies are within the range of exposures assessed at the population level in Japan and in other countries by means of TDS or market basket studies.
Dietary exposures to iAs in arsenic endemic areas are at least one order of magnitude higher compared to the background exposures discussed so far. This is especially true for people living in rural areas that consume foods produced locally and rely on a single source of contaminated groundwater for drinking, irrigating crops and preparing foods. As an example, in a rural village of Northern Chile, in which the water used by the population for drinking and cooking purposes contained 572 μg/L of arsenic, Diaz et al. (2004) estimated iAs mean intake at 1444 μg/day (i.e., 24 μg/kg bw/day, if a body weight of 60 kg is assumed). After the water arsenic was reduced to 41 μg/L, the dietary exposure decreased ten times and the contribution of water to exposure decreased from 96% to 75% (Diaz et al., 2004).
Apart from arsenic-endemic areas that clearly represent public health emergencies, current mean and high-level dietary exposure estimates in a number of countries show that, for a large part of the population, the margins of exposure with the current benchmark dose lower confidence limits (BMDLs) from EFSA and JECFA are narrow or even non-existent. Therefore, iAs exposure clearly represents a food safety priority worldwide. How to mitigate iAs dietary exposure is the subject of another paper in this issue (Nachman et al., this issue).
7 Conclusions
Inorganic arsenic is the critical arsenic species3 for human risk assessment of dietary arsenic. It is the dominant arsenic species in food of terrestrial origin, where it represents 60% (rice) to almost 100% of total arsenic. The food category contributing the most to iAs dietary exposure of the general population is cereals and cereal products, with rice playing a dominant role especially where it is a staple diet item. Other commonly reported contributors to dietary iAs intake include – besides water –vegetables, fruit, and beverages.
Although arsenic speciation in fish and seafood has attracted a lot of attention because of the high total arsenic levels, these commodities are not generally important contributors to iAs dietary exposure, except for shellfish and seaweed in the regions where consumption levels of these items are appreciable.
Arsenic in drinking water4 is present as iAs. When the arsenic concentration in water is at background levels, food is the major contributor to the intake of iAs in the general population. When the arsenic concentration in water is high, drinking water tends to become the major source of exposure to iAs and the latter may increase to levels that represent a major public health concern; this is currently the case for millions of people living around the large deltas and along major rivers in poor regions of South and East-Asia where geogenic arsenic is an important groundwater contaminant.
Exposure to dietary iAs is higher in younger age groups, due to their greater food consumption on a body weight basis and also to specific dietary habits (e.g., higher consumption of rice-based products in infants and toddlers). This does not necessarily indicate greater risk of adverse health effects to children, because iAs effects are generally a result of long term exposure, but those exposed to higher iAs intakes regularly since early childhood would be expected to be prone to long-term health effects. For infants that are not breastfed, exposure is dependent on the arsenic content in the water used to reconstitute infant formulas, whereas substitution of breastmilk with rice-based infant formula considerably increases iAs intake. In terms of hazard identification and characterization, more information on critical windows of iAs exposure is needed. In particular, this is true for prenatal and early life exposure and potential health effects later in life.
Duplicate diet studies and total diet studies with actually measured iAs concentrations are the most accurate means to estimate dietary exposure at the individual and population level, respectively, but they require robust and sensitive analytical methods for iAs detection in complex food matrices. They are also expensive and other approaches - such as modeling, also comprising data from human biomonitoring (Cubadda et al., 2012, 2015; Carignan et al., 2015) - have been used. For instance, duplicate diets and modeled arsenic were compared in a population-based study and the pros and cons of the methods discussed by Kurzius-Spencer et al. (2013).
Gaps and uncertainties in exposure assessment of dietary iAs are primarily a result of limited data of iAs occurrence in the different food commodities, which is relevant when the methods used to estimate exposure do not rely on actually measured iAs concentrations but on available databases. Existing data do not allow reliable characterization of either ‘typical’ iAs concentrations for specific food items or the fraction of iAs relative to total arsenic. From a risk assessment perspective, despite evidence that iAs in foods importantly contributing to exposure (e.g. rice) is absorbed to a similar degree to that in drinking water, limited knowledge exists on iAs bioavailability from a range of other food sources. The fate and transformation of iAs and other arsenic species during human digestion appears to be an even more crucial knowledge gap, with evidence only recently emerging on, e.g., the interaction of iAs with gut microbiome.
Finally, although iAs is clearly the top priority in risk assessment and risk management of dietary arsenic, it has to be remarked that a large proportion of organic arsenic in some fish and fish products (fish oil) and, especially, in other seafood is represented by compounds (primarily arsenolipids and arsenosugars) that are not thoroughly characterized in terms of their toxicological properties and potential risks for human health (Taylor et al., this issue). Information is also insufficient for methylated arsenicals, especially DMA, which is the most abundant species of this group in food.
Supplementary Material
Highlights.
Inorganic arsenic is the critical species for risk assessment of dietary arsenic
It dominates in food of terrestrial origin whereas it is a minor species in seafood
The most important contributors to exposure are cereals and cereal products
If contaminated water is consumed, it becomes the major source of exposure
Important organoarsenicals in the diet are not well characterized toxicologically
Acknowledgements
This paper is a product of the Collaborative on Food with Arsenic and Associated Risk and Regulation (C-FARR), a two year effort led by the Dartmouth Superfund Research Program and Children’s Environmental Health and Disease Prevention Research Center. C-FARR is supported by the Dartmouth College Toxic Metals Superfund Research Program through funds from the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) under Award Number 1R13ES026493-01 to C. Chen and Award Number P42ES007373 to B. Stanton, as well as the Children's Environmental Health and Disease Prevention Research Center at Dartmouth through funds from NIEHS Award Number P01ES022832 and the U.S. Environmental Protection Agency (EPA) under Award Number RD83544201, both to M.R. Karagas. The goal of C-FARR is to synthesize the current information pertaining to arsenic from soil to plate, based on key questions and knowledge gaps identified by policy stakeholders and scientists from interdisciplinary backgrounds, to inform future regulatory and policy decisions affecting dietary arsenic exposure. Kathryn Cottingham was supported in part by NIEHS award number P01ES022832 and EPA award number RD83544201. Margaret Kurzius-Spencer received partial support from a career development grant through the Southwest Environmental Health Sciences Center, NIEHS grant number P30 ES006694 (N. Cherrington, Director) and from the Center for Indigenous Environmental Health Research, NIH/EPA grant number P50 ES-14-010 (J. Burgess, Director). Yoshira Ornelas Van Horne received support from an NIH training grant, number T32 ES007091.
Footnotes
Monomethylarsonic acid, MMA(V), and dimethylarsinic acid, DMA(V), are found in a range of food of animal origin as products of iAs metabolism (the former generally as a minor compound). In vivo, monomethylarsonous acid, MMA(III), and dimethylarsinous acid, DMA(III), are labile metabolites of iAs and are highly toxic species of importance to iAs’s mode of toxic action (EFSA et al., 2009, Cohen et al., 2013). These two trivalent methylated arsenicals are reactive, unstable species and generally do not occur in food. In this review, MMA(V) and DMA(V) will be indicated simply as MMA and DMA, respectively.
Mushrooms are often included in the food group ‘vegetables’ in dietary intake studies. Some species can have substantial iAs concentrations (see Table S1 for references), but their contribution to exposure is generally minor due to limited consumption levels.
Here ‘species’ is used as synonymous of ‘form’, in a distinction pointing to inorganic versus organic forms of arsenic. Strictly speaking, As(III) and As(V) (i.e., the oxoanions arsenite and arsenate and the related thio-complexes) are the actual chemical species, whereas iAs is the sum of the two. However, no distinction between As(III) and As(V) is made in risk assessment, also because they are readily interconvertible in the conditions of the human GI tract.
Drinking water means here whatever type of water used for drinking purposes (including tap water, well-water, natural mineral water, and bottled water).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman AH, Creed PA, Parks AN, Fricke MW, Schwegel CA, Creed JT, Heitkemper DT, Vela NP. Comparison of a chemical and enzymatic extraction of arsenic from rice and an assessment of the arsenic absorption from contaminated water by cooked rice. Environ. Sci. Technol. 2005;39:5241–5246. doi: 10.1021/es048150n. [DOI] [PubMed] [Google Scholar]
- Alava P, Du Laing G, Odhiambo M, Verliefde A, Tack F, Van de Wiele TR. Arsenic bioaccessibility upon gastrointestinal digestion is highly determined by its speciation and lipid-bile salt interactions. J. Environ. Sci. Health A. 2013;48:656–665. doi: 10.1080/10934529.2013.732367. [DOI] [PubMed] [Google Scholar]
- Almela C, Laparra JM, Velez D, Barbera R, Farre R, Montoro R. Arsenosugars in raw and cooked edible seaweed: characterization and bioaccessibility. J. Agric. Food Chem. 2005;53:7344–7351. doi: 10.1021/jf050503u. [DOI] [PubMed] [Google Scholar]
- Amaral CDB, Nobrega JA, Nogueira ARA. Sample preparation for arsenic speciation in terrestrial plants-A review. Talanta. 2013;115:291–299. doi: 10.1016/j.talanta.2013.04.072. [DOI] [PubMed] [Google Scholar]
- Arnich N, Sirot V, Rivière G, Jean J, Noël L, Guérin T, Leblanc JC. Dietary exposure to trace elements and health risk assessment in the 2nd French Total Diet Study. Food. Chem. Toxicol. 2012;50:2432–2449. doi: 10.1016/j.fct.2012.04.016. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological profile for arsenic. U. S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2007. [Google Scholar]
- Bae M, Watanabe C, Inaoka T, Sekiyama M, Sudo N, Bokul MH, Ohtsuka R. Arsenic in cooked rice in Bangladesh. Lancet. 2002;360:1839–1840. doi: 10.1016/S0140-6736(02)11738-7. [DOI] [PubMed] [Google Scholar]
- Baer I, Baxter M, Devesa V, Velez D, Raber G, Rubio R, Llorente-Mirandes T, Sloth JJ, Robouch P, de la Calle B. Performance of laboratories in speciation analysis in seafood - Case of methylmercury and inorganic arsenic. Food Control. 2011;22:1928–1934. [Google Scholar]
- Brandon EF, Janssen PJ, de Wit-Bos L. Arsenic: bioaccessibility from seaweed and rice, dietary exposure calculations and risk assessment. Food Addit Contam A. 2014;31:1993–2003. doi: 10.1080/19440049.2014.974687. [DOI] [PubMed] [Google Scholar]
- Briscoe ML, Ugrai TM, Creswell J, Carter AT. An interlaboratory comparison study for the determination of arsenic and arsenic species in rice, kelp, and apple juice. Spectroscopy. 2016;30:20–30. [Google Scholar]
- Bundschuh J, Nath B, Bhattacharya P, Liu CW, Armienta MA, Lopez MVM, Lopez DL, Jean JS, Cornejo L, Macedo LFL, Tenuta A. Arsenic in the human food chain: the Latin American perspective. Sci. Total Environ. 2012;429:92–106. doi: 10.1016/j.scitotenv.2011.09.069. [DOI] [PubMed] [Google Scholar]
- Carbonell-Barrachina AA, Signes-Pastor AJ, Vazquez-Araujo L, Burio F, Sengupta B. Presence of arsenic in agricultural products from arsenic-endemic areas and strategies to reduce arsenic intake in rural villages. Mol. Nutr. Food Res. 2009;53:531–541. doi: 10.1002/mnfr.200900038. [DOI] [PubMed] [Google Scholar]
- Carbonell-Barrachina AA, Wu X, Ramírez-Gandolfo A, Norton GJ, Burló F, Deacon C, Meharg AA. Inorganic arsenic contents in rice-based infant foods from Spain, UK, China and USA. Environ Pollut. 2012;163:77–83. doi: 10.1016/j.envpol.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Carey M, Jiujin X, Gomes Farias J, Meharg AA. Rethinking rice preparation for highly efficient removal of inorganic arsenic using percolating cooking water. PLoS One. 2015;10:e0131608. doi: 10.1371/journal.pone.0131608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, Cottingham KL, Jackson BP, Farzan SF, Gandolfi AJ, Punshon T, Folt CL, Karagas MR. Estimated exposure to arsenic in breastfed and formula-fed infants in a United States cohort. Environ. Health Persp. 2015;123:500–506. doi: 10.1289/ehp.1408789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, Punshon T, Karagas MR, Cottingham KL. Potential exposure to arsenic from infant rice cereal. Ann. Glob. Health. 2016;82:221–224. doi: 10.1016/j.aogh.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin DJ, Naujokas MF, Bradham KD, Cowden J, Heacock M, Henry HF, Lee JS, Thomas DJ, Thompson C, Tokar EJ, Waalkes MP, Birnbaum LS, Suk WA. Arsenic and environmental health: state of the science and future research opportunities. Environ. Health Persp. 2016;124:890–899. doi: 10.1289/ehp.1510209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C, Raab A, Jenkins RO, Feldmann J, Meharg A.A, Haris, P.I. The impact of a rice based diet on urinary arsenic. J. Environ. Monit. 2011;13:257–65. doi: 10.1039/c0em00482k. [DOI] [PubMed] [Google Scholar]
- Chávez-Capilla T, Beshai M, Maher W, Kelly T, Foster S. Bioaccessibility and degradation of naturally occurring arsenic species from food in the human gastrointestinal tract. Food Chem. 2016;212:189–197. doi: 10.1016/j.foodchem.2016.05.163. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chiou HY, Hsu LI, Hsueh YM, Wu MM, Chen CJ. Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan. Environ Res. 2010;110:455–462. doi: 10.1016/j.envres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Arnold LL, Beck BD, Lewis AS, Eldan M. Evaluation of the carcinogenicity of inorganic arsenic. Crit. Rev. Toxicol. 2013;43:711–752. doi: 10.3109/10408444.2013.827152. [DOI] [PubMed] [Google Scholar]
- Conklin SD, Shockey N, Kubachka K, Howard KD, Carson MC. Development of an ion chromatography-inductively coupled plasma-mass spectrometry method to determine inorganic arsenic in liver from chickens treated with roxarsone. J. Agr. Food Chem. 2012;60:9394–9404. doi: 10.1021/jf302366a. [DOI] [PubMed] [Google Scholar]
- Cubadda F, Aureli F, D’Amato M, Raggi A, Turco AC, Mantovani A. Speciated urinary arsenic as a biomarker of dietary exposure to inorganic arsenic in residents living in high-arsenic areas in Latium, Italy. Pure Appl. Chem. 2012;84:203–214. [Google Scholar]
- Cubadda F, Ciardullo S, D’Amato M, Raggi A, Aureli F, Carcea M. Arsenic contamination of the environment-food chain: a survey on wheat as a test plant to investigate phytoavailable arsenic in Italian agricultural soils and as a source of inorganic arsenic in the diet. J. Agric. Food Chem. 2010;58:10176–10183. doi: 10.1021/jf102084p. [DOI] [PubMed] [Google Scholar]
- Cubadda F, D’Amato M, Aureli F, Raggi A, Mantovani A. Dietary exposure of the Italian population to inorganic arsenic: the 2012-2014 Total Diet Study. Food Chem. Toxicol. 2016;98:148–158. doi: 10.1016/j.fct.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Cubadda F, D’Amato M, Mancini FR, Aureli F, Raggi A, Busani L, Mantovani A. Assessing human exposure to inorganic arsenic in high-arsenic areas of Latium: a biomonitoring study integrated with indicators of dietary intake. Ann. Ig. 2015;27:39–51. doi: 10.7416/ai.2015.2021. [DOI] [PubMed] [Google Scholar]
- Cubadda F, Raggi A, Zanasi F, Carcea M. From durum wheat to pasta: effect of technological processing on the levels of arsenic, cadmium, lead and nickel – a pilot study. Food Addit. Contam. 2003;20:353–360. doi: 10.1080/0265203031000121996. [DOI] [PubMed] [Google Scholar]
- D’Amato M, Aureli F, Ciardullo S, Raggi A, Cubadda F. Arsenic speciation in wheat and wheat products using ultrasound- and microwave-assisted extraction and anion exchange chromatography-inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 2011;26:207–213. [Google Scholar]
- D'Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, Michelozzi P. Arsenic in drinking water and mortality for cancer and chronic diseases in central Italy, 1990-2010. PLoS One. 2015;10:e0138182. doi: 10.1371/journal.pone.0138182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Argos J, Slaughter F, Gossai A, Ahsan H, Karagas M. Assessment of human dietary exposure to arsenic through rice. Sci. Total Environ. 2017 doi: 10.1016/j.scitotenv.2017.02.119. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Calle MB, Baer I, Robouch P, Cordeiro F, Emteborg H, Baxter MJ, Brereton N, Raber G, Velez D, Devesa V, Rubio R, Llorente-Mirandes T, Raab A, Feldmann J, Sloth JJ, Rasmussen RR, D'Amato M, Cubadda F. Is it possible to agree on a value for inorganic arsenic in food? The outcome of IMEP-112. Anal. Bioanal. Chem. 2012;404:2475–2488. doi: 10.1007/s00216-012-6398-4. [DOI] [PubMed] [Google Scholar]
- de la Calle MB, Emteborg H, Linsinger TPJ, Montoro R, Sloth JJ, Rubio R, Baxter MJ, Feldmann J, Vermaercke P, Raber G. Does the determination of inorganic arsenic in rice depend on the method? Trac-Trend. Anal. Chem. 2011;30:641–651. [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Garcia-Salcedo J, Sanmiguel MF, Rivera M, Hernandez MC, Cebrian ME. Arsenic levels in cooked food and assessment of adult dietary intake of arsenic in the Region Lagunera, Mexico. Food Chem. Toxicol. 2002;40:1423–1431. doi: 10.1016/s0278-6915(02)00074-1. [DOI] [PubMed] [Google Scholar]
- Devesa V, Macho ML, Jalon M, Urieta I, Munoz O, Suner MA, Lopez F, Velez D, Montoro R. Arsenic in cooked seafood products: Study on the effect of cooking on total and inorganic arsenic contents. J. Agric. Food Chem. 2001a;49:4132–4140. doi: 10.1021/jf010274l. [DOI] [PubMed] [Google Scholar]
- Devesa V, Martinez A, Suner MA, Benito V, Velez D, Montoro R. Kinetic study of transformations of arsenic species during heat treatment. J. Agric. Food Chem. 2001b;49:2267–2271. doi: 10.1021/jf001328e. [DOI] [PubMed] [Google Scholar]
- Devesa V, Martinez A, Suner MA, Velez D, Almela C, Montoro R. Effect of cooking temperatures on chemical changes in species of organic arsenic in seafood. J. Agric. Food Chem. 2001c;49:2272–2276. doi: 10.1021/jf0013297. [DOI] [PubMed] [Google Scholar]
- Diaz OP, Leyton I, Muñoz O, Núñez N, Devesa V, Súñer MA, Velez D, Montoro R. Contribution of water, bread, and vegetables (raw and cooked) to dietary intake of inorganic arsenic in a rural village of Northern Chile. J. Agric. Food Chem. 2004;52:1773–1779. doi: 10.1021/jf035168t. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Panel on Contaminants in the Food Chain (CONTAM): Scientific opinion on arsenic in food. EFSA J. 2009;7(10):1351. [Google Scholar]
- EFSA (European Food Safety Authority) Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014;12(3):3597. [Google Scholar]
- Ersoy B, Yanar Y, Kucukgulmez A, Celik M. Effects of four cooking methods on the heavy metal concentrations of sea bass fillets (Dicentrarchus labrax Linne, 1785) Food Chem. 2006;99:748–751. [Google Scholar]
- EU (European Union) Commission Regulation (EU) 2015/1006 of 25 June 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels of inorganic arsenic in foodstuffs. Off. J. E.U. 2015;L161:14–16. [Google Scholar]
- FAO (Food and Agriculture Organization) WHO (World Health Organization) Safety evaluation of certain contaminants in food, prepared by the seventy-second meeting of the Joint FAO/WHO Expert Committee on food additives. WHO Food Additives Series. 2011;63:153–316. [Google Scholar]
- FAO (Food and Agriculture Organization) WHO (World Health Organization) Joint FAO/WHO Food Standards Programme Codex Alimentarius Commission 37th Session Geneva. Switzerland: Jul 14-18, 2014. Report of the eighth session of the Codex Committee on Contaminants in Foods, The Hague, The Netherlands 31 March–4 April 2014. 2014. [Google Scholar]
- Feldmann J, Krupp EM. Critical review or scientific opinion paper: arsenosugars - a class of benign arsenic species or justification for developing partly speciated arsenic fractionation in foodstuffs? Anal. Bioanal. Chem. 2011;399:1735–1741. doi: 10.1007/s00216-010-4303-6. [DOI] [PubMed] [Google Scholar]
- Fiamegkos I, Cordeiro F, Robouch P, Vélez D, Devesa V, Raber G, Sloth JJ, Rasmussen RR, Llorente-Mirandes T, Lopez-Sanchez JF, Rubio R, Cubadda F, D’Amato M, Feldmann J, Raab A, Emteborg H, de la Calle MB. Accuracy of a method based on atomic absorption spectrometry to determine inorganic arsenic in food: Outcome of the collaborative trial IMEP-41. Food Chem. 2016;213:169–179. doi: 10.1016/j.foodchem.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Fontcuberta M, Calderon J, Villalbì JR, Centrich F, Portaña S, Espelt AA, Duran J, Nebot M. Total and inorganic arsenic in marketed food and associated health risks for the Catalan (Spain) population. J. Agric. Food Chem. 2011;59:10013–10022. doi: 10.1021/jf2013502. [DOI] [PubMed] [Google Scholar]
- Foster S, Maher W, Krikowa F, Apte S. A microwave-assisted sequential extraction of water and dilute acid soluble arsenic species from marine plant and animal tissues. Talanta. 2007;71:537–549. doi: 10.1016/j.talanta.2006.04.027. [DOI] [PubMed] [Google Scholar]
- García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, Howard B, Farley J, Best LG, Navas-Acien A. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidem. Biomar. 2013;22:1944–1953. doi: 10.1158/1055-9965.EPI-13-0234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcıa-Rico L, Tejeda-Valenzuela L, Velez D, Montoro R. Content of selenium, total and inorganic arsenic and bioaccessibility of arsenic in children diets of Mexico. J. Sci. Food Agric. 2012;92:1725–1731. doi: 10.1002/jsfa.5538. [DOI] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, Karagas MR. Relation between in utero arsenic exposure and birth outcomes in a cohort of mothers and their newborns from New Hampshire. Environ. Health Persp. 2016;124:1299–1307. doi: 10.1289/ehp.1510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen SE, Huda SN, Vahter M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int. J. Epidemiol. 2011;40:1593–604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- Hanaoka K, Goessler W, Ohno H, Irgolic KJ, Kaise T. Formation of toxic arsenical in roasted muscles of marine animals. Appl. Organometal. Chem. 2001;15:61–66. [Google Scholar]
- He Y, Pedigo CE, Lam B, Cheng Z, Zheng Y. Bioaccessibility of arsenic in various types of rice in an in vitro gastrointestinal fluid system. J. Environ. Sci. Health B. 2012;47:74–80. doi: 10.1080/03601234.2012.611431. [DOI] [PubMed] [Google Scholar]
- He Y, Zheng Y. Assessment of in vivo bioaccessibility of arsenic in dietary rice by a mass approach. Sci. Total Environ. 2010;408:1430–1436. doi: 10.1016/j.scitotenv.2009.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int. J. Epidemiol. 1998;27:561–569. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- Huang JH, Ilgen G, Fecher P. Quantitative chemical extraction for arsenic speciation in rice grains. J. Anal. Atom. Spectrom. 2010;25:800–802. [Google Scholar]
- Ichikawa S, Kamoshida M, Hanaoka K, Hamano M, Maitani T, Kaise T. Decrease of arsenic in edible brown algae Hijikia fusiforme by the cooking process. Appl. Organometal. Chem. 2006;20:585–590. [Google Scholar]
- International Agency for Research on Cancer . Arsenic, Metals, Fibres and Dusts. Volume 100C. IARC; Lyon: 2012. (Monographs on the evaluation of carcinogenic risks to humans). Available at: http://monographs.iarc.fr/ENG/Monographs/vol100C/ [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Punshon T. Recent Advances in the measurement of arsenic, cadmium, and mercury in rice and other foods. Curr. Environ. Health Rep. 2015;2:15–24. doi: 10.1007/s40572-014-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Taylor VF, Punshon T, Cottingham KL. Arsenic concentration and speciation in infant formulas and first foods. Pure Appl. Chem. 2012;84:215–223. doi: 10.1351/PAC-CON-11-09-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankong P, Chalhoud C, Kienzl N, Goessler W, Francesconi KA, Visoottiviseth P. Arsenic accumulation and speciation in freshwater fish living in arsenic-contaminated waters. Environ. Chem. 2007;4:11–17. [Google Scholar]
- Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, Sansom L, Naidu R. Application of an in vivo swine model for the determination of arsenic bioavailability in hydroponically-grown vegetables. Chemosphere. 2008;71:1963–1969. doi: 10.1016/j.chemosphere.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, Sansom L, Naidu R. In vivo assessment of arsenic bioavailability in rice and its significance for human health risk assessment. Environ. Health Persp. 2006;114:1826–1831. doi: 10.1289/ehp.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juskelis R, Li W, Nelson J, Cappozzo JC. Arsenic speciation in rice cereals for infants. J. Agric. Food Chem. 2013;61:10670–10676. doi: 10.1021/jf401873z. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Punshon T, Sayarath V, Jackson BP, Folt CL, Cottingham KL. Association of rice and rice product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609–16. doi: 10.1001/jamapediatrics.2016.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman Q, Rahman M, Mahiuddin G, Christiani DC. Dietary Arsenic Exposure in Bangladesh. Environ. Health Persp. 2007;115:889–893. doi: 10.1289/ehp.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J, Maher W. Measurement of water-soluble arsenic species in freeze-dried marine animal tissues by microwave-assisted extraction and HPLC-ICP-MS. J. Anal. Atom. Spectrom. 2002;17:838–843. [Google Scholar]
- Koch I, Dee J, House K, Sui J, Zhang J, McKnight-Whitford A, Reimer KJ. Bioaccessibility and speciation of arsenic in country foods from contaminated sites in Canada. Sci. Total Environ. 2013;449:1–8. doi: 10.1016/j.scitotenv.2013.01.047. [DOI] [PubMed] [Google Scholar]
- Koch I, McPherson K, Smith P, Easton L, Doe KG, Reimer KJ. Arsenic bioaccessibility and speciation in clams and seaweed from a contaminated marine environment. Mar. Pollut. Bull. 2007;54:586–594. doi: 10.1016/j.marpolbul.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Ogata K, Idemori M, Tsumori S, Miyaguni H, Inoue S, Hotta N. Investigation of skin manifestations of arsenicism due to intake of arsenic-contaminated groundwater in residents of Samta, Jessore, Bangladesh. Arch. Dermatol. 2001;137:102–103. [PubMed] [Google Scholar]
- Kurzius-Spencer M, Burgess JL, Harris RB, Hartz V, Roberge J, Huang S, Hsu CH, O'Rourke MK. Contribution of diet to aggregate arsenic exposures-an analysis across populations. J. Expo. Sci. Environ. Epidemiol. 2014;24:156–162. doi: 10.1038/jes.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M, O'Rourke MK, Hsu CH, Hartz V, Harris RB, Burgess JL. Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. J. Expo. Sci. Environ. Epidemiol. 2013;23:442–449. doi: 10.1038/jes.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai VWM, Sun Y, Ting E, Cullen WR, Reimer KJ. Arsenic speciation in human urine: are we all the same? Toxicol. Appl. Pharmacol. 2004;198:297–306. doi: 10.1016/j.taap.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Laparra JM, Velez D, Barbera R, Farre R, Montoro R. Bioavailability of inorganic arsenic in cooked rice: Practical aspects for human health risk assessments. J. Agric. Food Chem. 2005;53:8829–8833. doi: 10.1021/jf051365b. [DOI] [PubMed] [Google Scholar]
- Laparra JM, Velez D, Montoro R, Barbera R, Farre R. Estimation of arsenic bioaccessibility in edible seaweed by an in vitro digestion method. J. Agric. Food Chem. 2003;51:6080–6085. doi: 10.1021/jf034537i. [DOI] [PubMed] [Google Scholar]
- Leonardi G, Vahter M, Clemens F, Goessler W, Gurzau E, Hemminki K, Hough R, Koppova K, Kumar R, Rudnai P, Surdu S, Fletcher T. Inorganic arsenic and basal cell carcinoma in areas of Hungary, Romania, and Slovakia: a case-control study. Environ. Health Persp. 2012;120:721–726. doi: 10.1289/ehp.1103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Peng H, Lu X, Le XC. Enzyme-assisted extraction and liquid chromatography mass spectrometry for the determination of arsenic species in chicken meat. Anal. Chimica Acta. 2015;888:1–9. doi: 10.1016/j.aca.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Lu K, Abo RP, Schlieper KA, Graffam ME, Levine S, Wishnok JS, Swenberg JA, Tannenbaum SR, Fox JG. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ. Health Persp. 2014a;122:284–291. doi: 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Mahbub R, Cable PH, Ru H, Parry NMA, Bodnar WM, Wishnok JS, Styblo M, Swenberg JA, Fox JG, Tannenbaum SR. Gut microbiome phenotypes driven by host genetics affect arsenic metabolism. Chem. Res. Toxicol. 2014b;27:172–174. doi: 10.1021/tx400454z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HN, Greenberg GI, Pollock MC, Lewis AS. A comprehensive evaluation of inorganic arsenic in food and considerations for dietary intake analyses. Sci Total Environ. 2014;496:299–313. doi: 10.1016/j.scitotenv.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Maher M, Krikowa F, Ellwood M, Foster S, Jagtap R, Raber G. Overview of hyphenated techniques using an ICP-MS detector with an emphasis on extraction techniques for measurement of metalloids by HPLC–ICPMS. Microchem. J. 2012;105:15–31. [Google Scholar]
- Maher W, Foster S, Krikowa F, Donner E, Lombi E. Measurement of inorganic arsenic species in rice after nitric acid extraction by HPLC-ICPMS: verification using XANES. Environ. Sci. Technol. 2013;47:5821–5827. doi: 10.1021/es304299v. [DOI] [PubMed] [Google Scholar]
- Maher WA, Ellwood MJ, Krikowa F, Raber G, Foster S. Measurement of arsenic species in environmental, biological fluids and food samples by HPLC-ICPMS and HPLC-HG-AFS. J. Anal. Atom. Spectrom. 2015;30:2129–2183. [Google Scholar]
- Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta. 2002;58:201–235. [PubMed] [Google Scholar]
- Meacher DM, Menzel DB, Dillencourt MD, Bic LF, Schoof RA, Yost LJ, Eickhoff JC, Farr CH. Estimation of multimedia inorganic arsenic intake in the US population. Hum. Ecol. Risk Assess. 2002;8:1697–1721. [Google Scholar]
- Meharg AA, Sun G, Williams PN, Adomako E, Deacon C, Zhu YG, Feldmann J, Raab A. Inorganic arsenic levels in baby rice are of concern. Environ. Pollut. 2008;152:746–749. doi: 10.1016/j.envpol.2008.01.043. [DOI] [PubMed] [Google Scholar]
- Meliker JR, Franzblau A, Slotnick MJ, Nriagu JO. Major contributors to inorganic arsenic intake in southeastern Michigan. Int. J Hyg. Envir. Heal. 2006;209:399–411. doi: 10.1016/j.ijheh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Mihucz VG, Silversmit G, Szalóki I, Samber B. d., Schoonjans T, Tatár E, Vincze L, Virág I, Yao J, Záray G. Removal of some elements from washed and cooked rice studied by inductively coupled plasma mass spectrometry and synchrotron based confocal micro-X-ray fluorescence. Food Chem. 2010;121:290–297. [Google Scholar]
- Mihucz VG, Tatár E, Virág I, Zang C, Jao Y, Záray G. Arsenic removal from rice by washing and cooking with water. Food Chem. 2007;105:1718–1725. [Google Scholar]
- Mohri T, Hisanaga A, Ishinishi N. Arsenic intake and excretion by Japanese adults: a 7-day duplicate diet study. Food Chem. Toxicol. 1990;28:521–9. doi: 10.1016/0278-6915(90)90123-5. [DOI] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, Goessler W, Pollak J, Silbergeld EK, Howard BV, Navas-Acien A. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann. Intern. Med. 2013;159:649–59. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munera-Picazo S, Ramirez-Gandolfo A, Burlo F, Carbonell-Barrachina AA. Inorganic and total arsenic contents in rice-based foods for children with celiac disease. J. Food Sci. 2014;79:T122–128. doi: 10.1111/1750-3841.12310. [DOI] [PubMed] [Google Scholar]
- Musil S, Petursdottir AH, Raab A, Gunnlaugsdottir H, Knipp E, Feldmann J. Speciation without chromatography using selective hydride generation: inorganic arsenic in rice and samples of marine origin. Anal. Chem. 2014;86:993–999. doi: 10.1021/ac403438c. [DOI] [PubMed] [Google Scholar]
- Nachman K, Ginsberg G, Miller M, Murray C, Nigra A, Pendergrast C. Mitigating dietary arsenic exposure: current status and recommendations for an improved path forward. Sci. Total Environ. 2017 doi: 10.1016/j.scitotenv.2016.12.112. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nookabkaew S, Rangkadilok N, Mahidol C, Promsuk G, Satayavivad J. Determination of arsenic species in rice from Thailand and other Asian countries using simple extraction and HPLC-ICPMS analysis. J. Agr. Food Chem. 2013;61:6991–6998. doi: 10.1021/jf4014873. [DOI] [PubMed] [Google Scholar]
- Oguri T, Yoshinaga J, Tao H, Nakazato T. Daily intake of inorganic arsenic and some organic arsenic species of Japanese subjects. Food Chem. Toxicol. 2012;50:2663–2667. doi: 10.1016/j.fct.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Oguri T, Yoshinaga J, Tao H, Nakazato T. Inorganic arsenic in the Japanese diet: daily intake and source. Arch. Environ. Contam. Toxicol. 2014;66:100–112. doi: 10.1007/s00244-013-9947-8. [DOI] [PubMed] [Google Scholar]
- Orloff K, Mistry K, Metcalf S. Biomonitoring for environmental exposures to arsenic. J. Toxicol. Environ. Health B. 2009;12:509–524. doi: 10.1080/10937400903358934. [DOI] [PubMed] [Google Scholar]
- Petursdottir AH, Friedrich N, Musil S, Raab A, Gunnlaugsdottir H, Krupp EM, Feldmann J. Hydride generation ICP-MS as a simple method for determination of inorganic arsenic in rice for routine biomonitoring. Anal. Methods. 2014a;6:5392–5396. [Google Scholar]
- Petursdottir AH, Gunnlaudsdottir H, Jorundsdottir H, Raab A, Krupp EM, Feldmann J. Determination of inorganic arsenic in seafood: Emphasizing the need for certified reference materials. Pure Appl. Chem. 2012;84:191–202. [Google Scholar]
- Pétursdóttir ÁH, Gunnlaugsdóttir H, Krupp EM, Feldmann J. Inorganic arsenic in seafood: Does the extraction method matter? Food Chem. 2014b;150:353–359. doi: 10.1016/j.foodchem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Petursdottir AH, Sloth JJ, Feldmann J. Introduction of regulations for arsenic in feed and food with emphasis on inorganic arsenic, and implications for analytical chemistry. Anal. Bioanal. Chem. 2015;407:8385–8396. doi: 10.1007/s00216-015-9019-1. [DOI] [PubMed] [Google Scholar]
- Punshon T, Guerinot ML, Jackson B, Scheckel K, Warczak T, Meharg A. Understanding arsenic dynamics in arable systems to decrease human dietary arsenic exposure. Sci. Total Environ. 2017 doi: 10.1016/j.scitotenv.2016.12.111. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab A, Baskaran C, Feldmann J, Meharg AA. Cooking rice in a high water to rice ratio reduces inorganic arsenic content. J. Environ. Monit. 2009;11:41–44. doi: 10.1039/b816906c. [DOI] [PubMed] [Google Scholar]
- Raab A, Fecher P, Feldmann J. Determination of arsenic in algae - Results of an interlaboratory trial: Determination of arsenic species in the water-soluble fraction. Microchim. Acta. 2005;151:153–166. [Google Scholar]
- Raber G, Stock N, Hanel P, Murko M, Navratilova J, Francesconi KA. An improved HPLC-ICPMS method for determining inorganic arsenic in food: Application to rice, wheat and tuna fish. Food Chem. 2012;134:524–532. [Google Scholar]
- Rahman A, Vahter M, Ekström EC, Rahman M, Golam Mustafa AH, Wahed MA, Yunus M, Persson LA. Association of arsenic exposure during pregnancy with fetal loss and infant death: a cohort study in Bangladesh. Am. J. Epidemiol. 2007;165:1389–1396. doi: 10.1093/aje/kwm025. [DOI] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, Persson LA, Ekström EC. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169:304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Hasegawa H, Rahman MM, Miah MAM. Influence of cooking method on arsenic retention in cooked rice related to dietary exposure. Sci. Total Environ. 2006;370:51–60. doi: 10.1016/j.scitotenv.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque AM, Nermell B, Yunus M, Roy S, Persson LA, Arifeen SE, Moore S, Vahter M. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol. Lett. 2009;185:197–202. doi: 10.1016/j.toxlet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Rasmussen RR, Qian Y, Sloth JJ. SPE HG-AAS method for the determination of inorganic arsenic in rice-results from method validation studies and a survey on rice products. Anal. Bioanal. Chem. 2013;405:7851–7857. doi: 10.1007/s00216-013-6936-8. [DOI] [PubMed] [Google Scholar]
- Rose M, Baxter M, Brereton N, Baskaran C. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit. Contam. A. 2010;27:1380–1404. doi: 10.1080/19440049.2010.496794. [DOI] [PubMed] [Google Scholar]
- Rose M, Lewis J, Langford N, Baxter M, Origgi S, Barber M, MacBain H, Thomas K. Arsenic in seaweed–forms, concentration and dietary exposure. Food Chem. Toxicol. 2007;45:1263–1267. doi: 10.1016/j.fct.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Sadee BA, Foulkes ME, Hill SJ. An evaluation of extraction techniques for arsenic in staple diets (fish and rice) utilising both classical and enzymatic extraction methods. Food Addit. Contam. A. 2016;33:433–41. doi: 10.1080/19440049.2015.1132479. [DOI] [PubMed] [Google Scholar]
- Schmidt CW. The challenge of regulating arsenic in rice. Environ. Health Persp. 2015;123:A18–19. doi: 10.1289/ehp.123-A16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof RA, Yost LJ, Eickhoff J, Crecelius EA, Cragin DW, Meacher DM, Menzel DB. A market basket survey of inorganic arsenic in food. Food Chem. Toxicol. 1999;37:839–846. doi: 10.1016/s0278-6915(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Sengupta MK, Hossain MA, Mukherjee A, Ahamed S, Das B, Nayak B, Pal A, Chakraborti D. Arsenic burden of cooked rice: Traditional and modern methods. Food Chem. Toxicol. 2006;44:1823–1829. doi: 10.1016/j.fct.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Jin K, Morita M. Arsenic compounds in the edible red alga, Porphyra tenera, and in nori and yakinori, food items produced from red algae. Appl. Organometal. Chem. 1990;4:255–260. [Google Scholar]
- Signes-Pastor AJ, Al-Rmalli SW, Jenkins RO, Carbonell-Barrachina AA, Haris PI. Arsenic bioaccessibility in cooked rice as affected by arsenic in cooking water. J. Food Sci. 2012;77:T201–206. doi: 10.1111/j.1750-3841.2012.02948.x. [DOI] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Carey M, Meharg AA. Inorganic arsenic in rice-based products for infants and young children. Food Chem. 2016;191:128–134. doi: 10.1016/j.foodchem.2014.11.078. [DOI] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Mitra K, Sarkhel S, Hobbes M, Burló F, de Groot WT, Carbonell-Barrachina AA. Arsenic speciation in food and estimation of the dietary intake of inorganic arsenic in a rural village of West Bengal, India. J. Agric. Food Chem. 2008;56:9469–9474. doi: 10.1021/jf801600j. [DOI] [PubMed] [Google Scholar]
- Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am. J. Epidemiol. 1998;147:660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- Sun GX, Williams PN, Zhu YG, Deacon C, Carey AM, Raab A, Feldmann J, Meharg AA. Survey of arsenic and its speciation in rice products such as breakfast cereals, rice crackers and Japanese rice condiments. Environ Int. 2009;35:473–475. doi: 10.1016/j.envint.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, Francesconi K, Karagas M. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017 doi: 10.1016/j.scitotenv.2016.12.113. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Escribano S, Leal M, Velez D, Montoro R. Total and inorganic arsenic concentrations in rice sold in Spain, effect of cooking, and risk assessments. Environ. Sci. Technol. 2008;42:3867–3872. doi: 10.1021/es071516m. [DOI] [PubMed] [Google Scholar]
- Tsuji JS, Yost LJ, Barraj LM, Scrafford CG, Mink PJ. Use of background inorganic arsenic exposures to provide perspective on risk assessment results. Regul. Toxicol. Pharm. 2007;48:59–68. doi: 10.1016/j.yrtph.2007.01.004. [DOI] [PubMed] [Google Scholar]
- US FDA (U.S. Food and Drug Administration) Arsenic in Rice and Rice Products Risk Assessment Report. 2016 Available at http://www.fda.gov/Food/FoodScienceResearch/RiskSafetyAssessment/default.htm.
- Vahter M. Health effects of early life exposure to arsenic. Basic Clin. Pharmacol. Toxicol. 2008;102:204–11. doi: 10.1111/j.1742-7843.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- Vahter M, Concha G, Nermell B, Nilsson R, Dulout F, Natarajan AT. A unique metabolism of inorganic arsenic in native Andean women. Eur. J. Pharmacol. 1995;293:455–462. doi: 10.1016/0926-6917(95)90066-7. [DOI] [PubMed] [Google Scholar]
- van Elteren JT, Slejkovec Z. Ion-exchange separation of eight arsenic compounds by high-performance liquid chromatography UV decomposition hydride generation atomic fluorescence spectrometry and stability tests for food treatment procedures. J. Chromatogr. A. 1997;789:339–348. doi: 10.1016/s0021-9673(97)00703-6. [DOI] [PubMed] [Google Scholar]
- Vela NP, Heitkemper DT. Total arsenic determination and speciation in infant food products by ion chromatography-inductively coupled plasma-mass spectrometry. J. AOAC Int. 2004;87:244–252. [PubMed] [Google Scholar]
- Wei C, Li WH, Zhang C, Van Hulle M, Cornelis R, Zhang XR. Safety evaluation of organoarsenical species in edible Porphyra from the China Sea. J. Agric. Food Chem. 2003;51:5176–5182. doi: 10.1021/jf026117j. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Environmental Health Criteria 224: Arsenic and arsenic compounds. Second edition Geneva: 2001. [Google Scholar]
- Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007;41:6854–6859. doi: 10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- Wong WW, Chung SW, Chan BT, Ho YY, Xiao Y. Dietary exposure to inorganic arsenic of the Hong Kong population: results of the first Hong Kong total diet study. Food Chem. Toxicol. 2013;51:379–385. doi: 10.1016/j.fct.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003-2004 NHANES Data. Environ. Health Persp. 2010;118:345–50. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JW, Greene T, Schoof RA. Arsenic relative bioavailability from diet and airborne exposures: Implications for risk assessment. Sci. Total Environ. 2015;536:368–381. doi: 10.1016/j.scitotenv.2015.05.141. [DOI] [PubMed] [Google Scholar]
- Yost LJ, Schoof RA, Aucoin R. Intake of inorganic arsenic in the North American diet. Hum. Ecol. Risk Assess. 1998;4:137–152. [Google Scholar]
- Yost LJ, Tao SH, Egan SK, Barraj LM, Smith KM, Tsuji JS, Lowney YW, Schoof RA, Rachman NJ. Estimation of dietary intake of inorganic arsenic in US children. Hum. Ecol. Risk Assess. 2004;10:473–483. [Google Scholar]
- Yu H, Wu B, Zhang XX, Liu S, Yu J, Cheng S, Ren HQ, Ye L. Arsenic metabolism and toxicity influenced by ferric iron in simulated gastrointestinal tract and the roles of gut microbiota. Environ. Sci. Technol. 2016;50:7189–7197. doi: 10.1021/acs.est.6b01533. [DOI] [PubMed] [Google Scholar]
- Zheng LY, Umans JG, Tellez-Plaza M, Yeh F, Francesconi KA, Goessler W, Silbergeld EK, Guallar E, Howard BV, Weaver VM, Navas-Acien A. Urine arsenic and prevalent albuminuria: evidence from a population-based study. Am. J. Kidney Dis. 2013;61:385–394. doi: 10.1053/j.ajkd.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.