Abstract
Purpose
Several organizations are developing clinical trials to evaluate adjuvant radiotherapy (RT) for bladder cancer patients at elevated risk of locoregional failure after radical cystectomy. Clinical target volumes (CTVs) and organs at risk (OARs) for this treatment have not been defined in detail. Our purpose was to develop multi-institutional consensus CTVs and OARs for male and female bladder cancer patients undergoing adjuvant RT in clinical trials.
Methods and Materials
We convened a multi-disciplinary group of bladder cancer specialists from fifteen centers and five countries. Six radiation oncologists and seven urologists participated in the development of the initial contours. The group proposed initial language for the CTVs and OARs, and each radiation oncologist contoured them on CT scans of a male and female cystectomy patient with input from ≥1 urologist. Based on the initial contouring, the group updated its CTV and OAR descriptions. The cystectomy bed, the area of greatest controversy, was contoured by another six radiation oncologists, and the cystectomy bed contouring language was again updated. To determine if the revised language produced consistent contours, CTVs and OARs were redrawn by six additional radiation oncologists. We evaluated their contours for level of agreement using the Landis-Koch interpretation of the κ-statistic.
Results
The group proposed that patients at elevated risk for local-regional failure with negative margins should be treated to the pelvic nodes alone (internal/external iliac, distal common iliac, obturator, and presacral) whereas patients with positive margins should be treated to the pelvic nodes and cystectomy bed. Proposed OARs included the rectum, bowel space, bone marrow, and urinary diversion. Consensus language describing the CTVs and OARs was developed and externally validated. The revised instructions were found to produce consistent contours.
Conclusions
Consensus descriptions of CTVs and OARs were successfully developed and can be employed in clinical trials of adjuvant RT for bladder cancer.
INTRODUCTION
Radical cystectomy with or without chemotherapy has a 5-year overall survival of approximately 60% for patients with pathologic T2 disease confined to the bladder but only 10–40% for stage ≥pT3 when disease extends into the extravesicular tissues (1). Pelvic failures after radical cystectomy are common, especially for ≥pT3 urothelial carcinoma with a cumulative incidence of locoregional failure of 32% at 5 years in the SWOG 8710 cohort (1). Adjuvant radiation therapy (RT) can reduce locoregional failure and may even improve overall survival (2), but currently has no defined role, in part because of toxicity reported in older series using 1980s radiation techniques. An externally validated risk stratification to identify patients at highest risk for local-regional failure who are most likely to benefit from adjuvant RT has been developed based on pathologic T-stage, surgical margin status, and extent of the lymph node dissection (1, 3–5). Recently, there has been growing interest in adjuvant RT, and several clinical trial organizations, including the NRG and groups in France (GETUG-AFU), the United Kingdom (NCRI) and India (Tata Memorial Hospital) have already opened or are in the process of developing clinical trials of adjuvant radiation (6). However, the clinical target volumes (CTVs) and organs at risk (OARs) for adjuvant radiation for bladder cancer are not well-defined. The purpose of this study was to achieve a multi-disciplinary, multi-institutional, international consensus defining CTVs and OARs for bladder cancer patients undergoing adjuvant radiation in clinical trials and to describe those targets and OARs in a way that generates consistent contours.
METHODS AND MATERIALS
A multi-disciplinary group of bladder cancer specialists representing 15 institutions from five countries were involved in this study. Six radiation oncologists and seven urologists participated in the development of the initial proposed contours via teleconferences and electronic mail. After initial language was developed, a sample set of computed tomography (CT) images of a representative male and female post-cystectomy patient (both pT3N0) were distributed to members of the panel. Both patients were simulated supine with IV and oral contrast. Each radiation oncologist in collaboration with ≥1 urologist submitted contours for the CTVs and OARs for both cases. The panel identified OARs as the bowel (contoured as a bowel bag), rectum, bony pelvis, urinary diversion, and the ostomy bag (when applicable).
Based on the first round of contouring, the initial development group updated its descriptions of the CTVs and OARs. As the area of greatest controversy was the cystectomy bed, an additional six radiation oncologists contoured the cystectomy bed on the same CT datasets using the new updated contouring language to externally validate the new contouring instructions. Their cystectomy bed contours were evaluated for level of agreement, and the description for the cystectomy bed contours was adjusted to address feedback from the second development group. To evaluate whether the updated language produced consistent contours, all of the CTVs and OARs were redrawn on new male and female CT sets by 6 additional radiation oncologists who were blinded to the previous CTV and OAR descriptions. The validation CTVs and OARs were evaluated for level of agreement. Feedback from the validation group were aggregated and summarized. Dose and fractionation schemes were also discussed with a consensus recommendation of 4500 – 5040 cGy in 180 cGy fractions.
Statistical Methods
A MATLAB (Mathworks, Natick, MA) software program evaluated the individual expert’s contours for level of agreement using an imputation method with expected maximum (EM) algorithms for simultaneous truth and performance level estimation (STAPLE)(7, 8). In this approach, the true contouring decisions at each voxel are formulated as maximum likelihood estimates from the observed contours by optimizing sensitivity and specificity parameters of each expert’s performance using the EM algorithm, assuming a binomial distribution. We estimated overall agreement using the generalized κ-statistic. The goodness of agreement was evaluated according to Landis and Koch criteria (9). κ-statistic level of agreement is perfect if the κ-statistic is 1, almost perfect (0.8<κ<1.0), substantial (0.6<κ≤ 0.8), moderate (0.4<κ≤0.6), fair (0.2<κ≤0.4), slight (0<κ≤0.2), or poor (−1<κ≤0).
RESULTS
The primary concern of the initial development group was excessive small bowel exposure and the subsequent risk of bowel obstruction. Based on a prior patterns of failure analysis after cystectomy (10), the group proposed that CTVs for patients at high risk for local-regional failure after cystectomy be based on surgical margin status. For patients with negative margins, the predominant pattern of failure was in the pelvic sidewall nodal region (10), and the panel recommended targeting the pelvic sidewall nodal region and excluding the cystectomy bed. For patients with positive margins, the predominant pattern of failure was again in the pelvic sidewall nodal region, but there were significantly more failures in the cystectomy bed (10). The panel felt that for positive margin patients, the CTV should include the pelvic nodal region and the cystectomy bed. While the patterns of failure study suggested that failure in the pre-sacral region is rare in negative margin patients, the group felt that nonetheless this region should be targeted in both negative and positive margin patients because the cost in terms of normal tissue exposure was felt to be low with modern techniques (11).
The CTVs included a pelvic lymph node CTV (CTVn) which included the presacral nodes and the bilateral distal common iliac, external iliac, internal iliac, and obturator nodes. The other CTV was a cystectomy bed CTV (CTVcb) that included the tissue in the pelvis that surrounded the intact empty bladder and proximal vagina or prostate preoperatively as well as the surgical cystectomy bed, including the anterolateral para-rectal tissues.
Initial contours from the development group were generated. There was some divergence of opinion on the initial contouring that resulted in discussions to identify a consensus description for the CTVs and OARs. The area of greatest discussion was the cystectomy bed CTV. Six additional genito-urinary radiation oncologists re-contoured the cystectomy bed on the same CT image sets using the updated consensus language. A STAPLE analysis was again performed to facilitate discussion. Feedback on the cystectomy bed CTV was used to again update the contouring language.
The updated consensus contouring descriptions for all of the CTVs and OARs were evaluated by six additional radiation oncologists (the validation group) from four institutions in the US, Canada, France, and India who had not participated in the initial or second development groups. These physicians drew the CTVs and OARs based on the revised contouring descriptions on CT scans from different patients. The level of agreement achieved within the validation group was felt to be adequate (Table 1). The final consensus contouring guidelines are presented below followed by a summary of the feedback from the validation group.
Table 1.
Analysis of agreement for the final consensus CTVs and OARs contoured by the validation cohort on male and female cystectomy cases
| CTV Combined | CTVn | CTVcb | Bowel Space | Rectum | Pelvic Bone | Urinary Diversion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| Sensitivity | 74% | 75% | 65% | 63% | 84% | 82% | 75% | 78% | 81% | 78% | 76% | 70% | 71% | 56% |
| Specificity | 97% | 98% | 99% | 99% | 93% | 94% | 98% | 98% | 99% | 99% | 99% | 100% | 96% | 99% |
| Vol. Mean (cc) | 378 | 462 | 227 | 229 | 170 | 227 | 1,034 | 1,362 | 43 | 88 | 861 | 822 | 26 | 26 |
| Vol. Min (cc) | 280 | 389 | 142 | 165 | 98 | 149 | 631 | 827 | 18 | 47 | 553 | 715 | 14 | 12 |
| Vol. Max (cc) | 525 | 579 | 292 | 281 | 373 | 427 | 1,447 | 1,946 | 53 | 125 | 1,281 | 1,103 | 42 | 51 |
| Vol. SD | −96 | −75 | −59 | −41 | −103 | −101 | −354 | −451 | −13 | −31 | −260 | −167 | −11 | −16 |
| STAPLE (cc) | 376 | 454 | 239 | 264 | 132 | 198 | 1,084 | 1,514 | 49 | 103 | 998 | 1,138 | 20 | 23 |
| Intersection (cc) | 140 | 187 | 63 | 55 | 67 | 106 | 442 | 708 | 16 | 43 | 410 | 376 | 6 | 4 |
| Union Vol. (cc) | 748 | 833 | 462 | 457 | 418 | 504 | 1,945 | 2,105 | 62 | 136 | 1,348 | 1,258 | 62 | 69 |
| Kappa (κ) | 0.60 | 0.62 | 0.56 | 0.56 | 0.51 | 0.58 | 0.63 | 0.68 | 0.72 | 0.70 | 0.72 | 0.72 | 0.48 | 0.41 |
CTV
The CTV will only include the pelvic lymph nodes (CTVn) without the cystectomy bed in patients with negative surgical margins and will include the pelvic lymph nodes (CTVn) plus the cystectomy bed (CTVcb) in patients with positive margins (10). Adjuvant RT to the pelvic nodes is intended to complement the thorough pelvic lymph node dissection that is an important component of radical cystectomy.
Cystectomy Bed CTV (CTVcb)
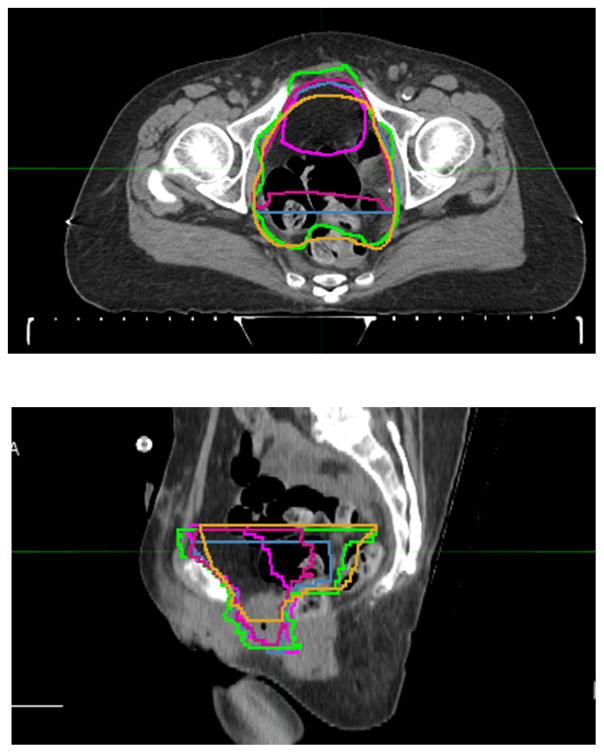
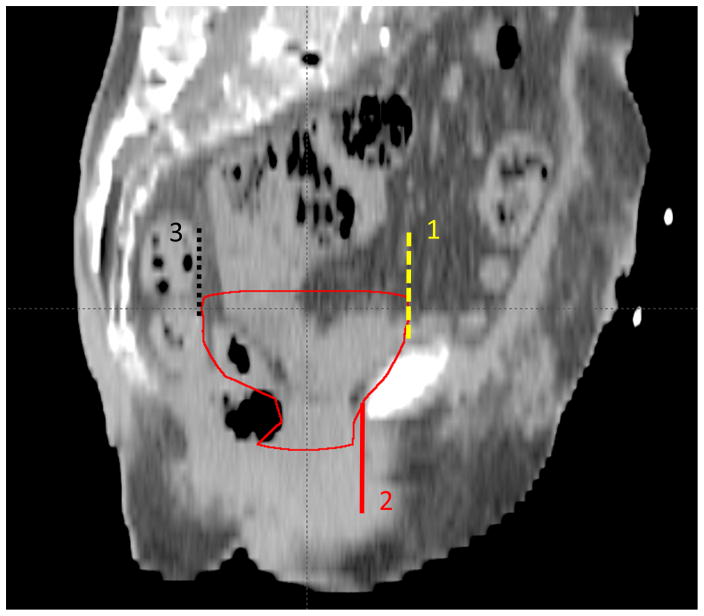
The initial cystectomy bed CTV contours submitted by each expert are shown in Figure 1. The consensus cystectomy bed CTV is intended to include tissue in the pelvis that surrounded the intact empty bladder and proximal vagina or prostate preoperatively, and the surgical cystectomy bed including the anterolateral para-rectal tissues. These contours do not intentionally include or exclude surgical clips. In addition, small bowel should not be carved out of the CTV. The consensus anatomic borders of the cystectomy CTV are shown in Table 2. Sagittal and axial views of the cystectomy bed in a female patient are illustrated (Figures 2–3).
Figure 1.
Initial investigator contours for the cystectomy bed superimposed. Each panel member’s contours are assigned a different color.
Table 2.
Anatomical borders of the cystectomy bed CTV for patients with positive margins
| Superior | The contour will extend 2 cm superior to the superior aspect of the pubic symphysis. |
| Anterior | The contour will extend to the posterior aspect of the pubic rami/symphysis. Above and below the pubic symphysis, the contour will stop anteriorly at the planes defined by extending lines superiorly (Plane 1 in Figures 2 and 3) and inferiorly (Plane 2 in Figures 3 and 4) from the posterior aspect of the symphysis. |
| Posterior | The contours will abut the anterior one-third of the external ano-rectal circumference without extending into the ano-rectum. Above the level of the rectum, the contour will stop posteriorly at the plane defined by extending a line superiorly from the anterior border of the rectum (Plane 3 in Figures 2 and 3). |
| Lateral | The contour will extend to the medial border of the obturator internus muscles bilaterally. Inferior to the obturator internus muscles, the lateral border of the contour will extend to vaginal wall or the prostate bed. |
| Inferior | The contour will stop 2–3mm (1 CT slice on axial images) above the penile bulb for males and 1 cm below the lower pole of the obturator foramen for females. |
Figure 2.
Representative sagittal slice showing the cystectomy bed on a female patient. Above and below the pubic symphysis, the cystectomy bed contour will stop anteriorly at the planes defined by extending lines superiorly (Plane 1) and inferiorly from the anterior border (Plane 2). Above the level of the rectum, the contour will stop posteriorly at the plane defined by extending a line superiorly from the anterior border of the rectum (Plane 3).
Figure 3.
A) Representative axial slices showing the cystectomy bed in the upper pelvis and B) lower pelvis of a female patient.
Pelvic Lymph Node CTV (CTVn)
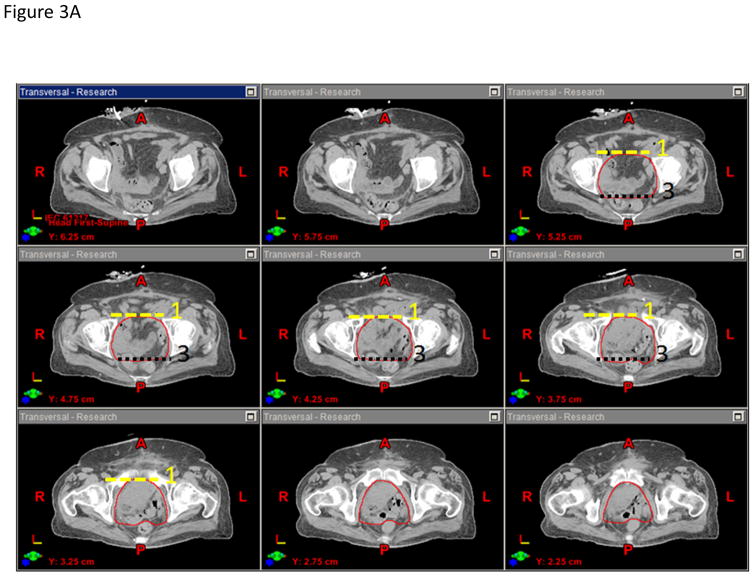
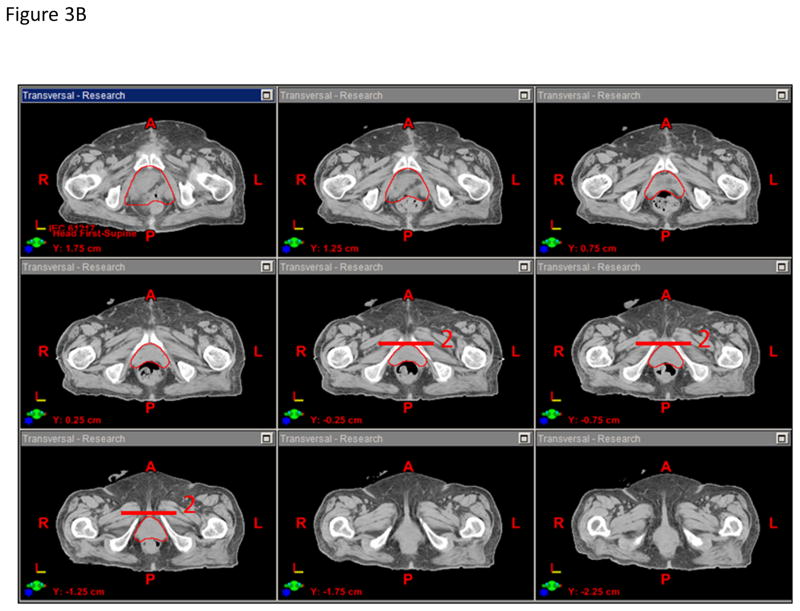
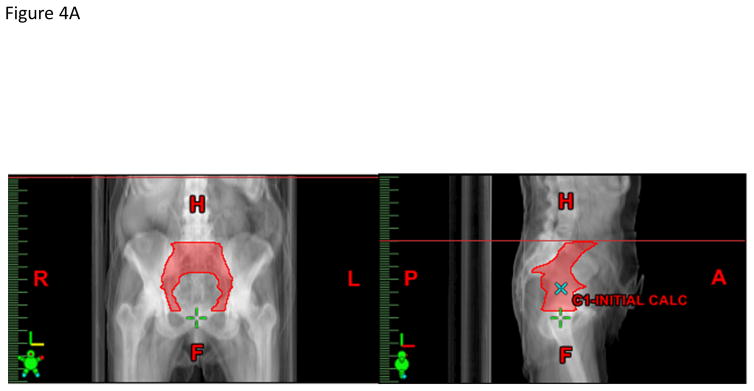
The panel felt that the CTVn should include the presacral nodes and the bilateral distal common iliac, external iliac, internal iliac, and obturator nodes and can be created from the union of three sub-structures: sacral nodes, iliac nodes, and obturator nodes (Figures 4A–B). The presacral nodes should extend from L5-S1 to the top of S3 and will include 1.0–1.5 cm of tissue anterior to the sacrum and between the vessel contours. The iliac nodes will be derived by contouring the common iliac, external and internal iliac vessels starting superiorly at L5-S1. To better visualize the pelvic vasculature, IV contrast is recommended but not required since some patients may have renal insufficiency. The external iliac vessels will be contoured inferiorly to the top of the femoral heads, and the internal iliac vessels will be contoured inferiorly until they are no longer visible on the CT scan or exit through the true pelvis via the greater sciatic notch. The iliac nodes will be created by expanding the iliac vessels by 7 mm in the anterior, posterior and lateral dimensions, but not the superior and inferior dimensions. The obturator nodes will encompass 1.0 cm width of tissue medial to the obturator internus muscles extending from the anterior border of the ilium to the posterior border of the ilium. The obturator nodes will be contoured starting superiorly where the internal and external iliac vessel contours stop and extend inferiorly to the top of the symphysis pubis. The CTVn should be created from the union of the sacral nodes, iliac nodes, and obturator nodes, but should be trimmed so as not to extend outside the true pelvis, nor into muscle or bone. The panel felt that the CTVn should not be cropped out of bowel to reduce the risk of a marginal miss as the primary site of failure is in the pelvic sidewall region. The CTVn mirrors the pelvic nodal CTV described in the RTOG atlas for high-risk prostate cancer with the exception that bowel is not cropped out of the CTVn (12).
Figure 4.
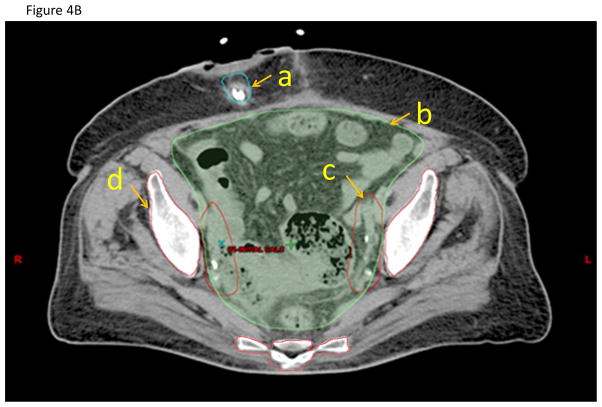
A) Anterior-posterior and lateral projections of the pelvic node CTV. B) Representative axial CT slice showing the (a) urinary diversion, (b) bowel space, (c) nodal CTV, and (d) pelvic bone contours.
PTV
The appropriate CTV-to-PTV expansion will vary by institution; however, the panel suggested a uniform 0.5–0.7 cm expansion.
Bowel contours
The bowel contour should include the entire small bowel, cecum, ascending, transverse, and sigmoid colon in one bowel bag contour. The panel felt that distinguishing between small and large bowel is difficult in the post-cystectomy setting even with oral contrast. Superiorly, the bowel contour will start 3 cm above the superior extent of the CTVn. Inferiorly, the contour will be discontinued on the CT slice where no portion of bowel is visible. The contours will include the volume surrounding loops of bowel out to the edge of the peritoneum as bowel may occupy this space at any time during the course of treatment (Figure 4B).
Rectum
The rectum contour will include the rectum and anal canal and will be contoured on every slice from the recto-sigmoid junction superiorly to the level of the ischial tuberosities inferiorly.
Pelvic Bones
The pelvic bones should be contoured to track radiation plan parameters that may be associated with changes in bone marrow reserve or insufficiency fractures. It is recommended that hot spots be avoided in this organ at risk when possible. Contour the pelvic bones starting superiorly 1 cm above the superior extent of the CTVn extending inferiorly to 1 cm inferior to the inferior limit of the CTVcb. The pelvic bones can be auto-contoured using a CT density-based algorithm (Figure 4B).
Urinary Diversion
The panel recommended that the urinary diversion be contoured so treatment planners can avoid bringing beams directly through this structure as long as CTV/PTV coverage is not compromised. Determining which tissues are included in the urinary diversion contour depends on the type of diversion that the patient has had. If the patient has had a non-continent diversion with a bowel conduit (e.g. ileal conduit), the contour will include the stoma and the visible portion of the bowel conduit (Figure 4B). If the patient has had a continent non-orthotopic catheterizable diversion (e.g. Indiana pouch), the contour will include the stoma, bowel conduit and internal urine reservoir. If the patient has had a continent orthotopic diversion (e.g. Studer pouch), the contour will include the bowel reservoir. To better delineate the urinary diversion, oral or rectal contrast may be injected into the diversion, but this was not felt to be mandatory. For some patients, some of the tissues of the urinary diversion may not be fully captured in the CT simulation so only a portion will be contoured. The panel recommended discussion with the surgeon regarding the type of urinary diversion and the sites of anastomosis. Reducing dose to the urinary diversion is likely to lower the risk of complications at the anastomosis. Since there are no consensus dose limits for the urinary diversion, measuring the dose to the urinary diversion and associated toxicities will be important for future studies.
Ostomy Bag
For patients who have a non-continent urinary diversion, it is recommended that the external ostomy bag be contoured so treatment planners can avoid bringing beams directly through this structure when possible. This structure should be created by contouring the entire external urine reservoir that is visible on the CT simulation. Simulating and treating with an empty ostomy bag is preferred.
Feedback from the validation group and updated recommendations
The importance of IV contrast to better delineate the nodal CTV was emphasized because anatomic changes after cystectomy make identifying the vascular structures in the pelvis challenging. The instructions to begin the nodal CTV at the L5/S1 interface was criticized as not sufficiently detailed since the anterior and posterior aspects of the L5/S1 interface are frequently at different levels in the cranio-caudal dimension. In addition, some felt that the L5/S1 interface may be too inferior to capture adequately the distal common iliac nodes for the female sample case. The panel recommended that if the distal common iliac nodal region is not well-covered, the pelvic nodal CTV could be expanded superiorly to the posterior-inferior edge of the L5 vertebral body and potentially higher based on the patient’s anatomy, the clinical scenario, and OAR tolerances. The panel recommended adequate coverage of the distal common iliac region as the 5-year cumulative incidence of common iliac nodal failure was 10% for patients with ≥pT3 disease, with most failures in the distal common iliac region (10). Some thought that the cystectomy bed contouring instructions for the lateral border inferior to the obturator internus muscles was not sufficiently detailed. The panel recommended that inferior to the obturator internus muscles, the lateral border should extend to the lateral edge of the vaginal wall or the prostate bed.
DISCUSSION
Detailed consensus clinical target volumes that define the pelvic lymph nodes and the cystectomy bed as well as organs at risk for adjuvant RT for bladder cancer have been successfully defined and validated by a multidisciplinary, international committee of bladder cancer experts. This contouring guide has been adapted by the new NRG-GU001 prospective trial of adjuvant RT versus observation for patients at high-risk of locoregional failure following radical cystectomy. Based on a previous dosimetric analysis of adjuvant RT for bladder cancer that found that IMRT resulted in significantly reduced bowel and rectal dose compared to 3-D conformal radiotherapy (11), the NRG trial requires IMRT to reduce the toxicity to these organs at risk and to minimize the significant GI toxicities that were reported in the past with adjuvant RT delivered using techniques from the 1970s and 1980s. With the adoption of highly conformal radiation delivery techniques comes a greater emphasis on careful delineation of the clinical target volumes to prevent a marginal miss. We believe that this contouring guide addresses this need.
The area of greatest discussion in the development of the consensus contours was the cystectomy bed CTV, as this target has not been well-defined in the radiation oncology literature. The superior border was felt to be a major contributor to the dose received by normal bowel. It was felt that a 2 cm margin above the pubic symphysis was appropriate as the superior border based on the typical extension of the bladder above the pubic symphysis. If bowel OARs cannot be met, the cystectomy bed CTV could be trimmed superiorly if needed. The appropriate posterior border was also discussed, with some recommending that the CTV be expanded to include the anterior one-half of the external ano-rectal circumference. The consensus was that including the anterior one-third of the external ano-rectal circumference would be sufficient since the anterior one-half of the rectum would be included within the PTV. For the lateral borders, the panel felt it was not necessary to include the full width of the obturator internus muscles, although the full width is likely to be included in the PTV. Limiting the lateral expansion of the CTV was favored to reduce toxicity, especially to the femoral heads. It was felt that normal bowel should not be carved out of the cystectomy bed CTV as the position of loops of bowel is variable and carving out bowel could result in inadequate coverage of the target.
There was less debate about the contouring of the pelvic lymph node region. The superior border was set at the traditional RTOG L5/S1 interface used for whole pelvis prostate radiation (12, 13). The decision on whether to set the L5/S1 interface at the anterior or posterior interface which can affect the superior extent of the field was left to the discretion of the treating physician. Coverage of the more proximal iliac nodal region was not felt to be necessary in most cases because of the increased risk of bowel toxicity and the increased competing risk of distant metastases for patients with proximal common iliac nodal involvement. Standard expansions from the vessels were employed. The panel recommended that the pelvic lymph node CTV should be carved out of bone and muscle but not bowel, again because of concerns about marginal miss given daily variations in the position of bowel.
In our opinion, there are several benefits to achieving consensus contours for adjuvant RT for bladder cancer and developing language to clearly describe those contours so that they can be reproducibly generated. Such consensus is likely to reduce the heterogeneity of contouring in a given protocol. Deviations from the protocol-specified contours could potentially obscure the benefits or harms of the experimental treatment, which was a major concern in the initial enrollment for RTOG 0529, a phase II study evaluating the use of IMRT in the treatment of anal cancer, and the impetus for the creation of the RTOG ano-rectal contouring atlas. An additional benefit of achieving international consensus contours is to facilitate comparisons across different adjuvant RT trials to generate more meaningful meta-analyses of the outcomes associated with adjuvant RT.
The present study has limitations. The contours are based on a selected panel of experts and highly dependent on the results of a single patterns of failure study (10) which has not been externally validated, specifically our decision to exclude the cystectomy bed for patients with negative margins. The size of the validation group that tested the consensus contouring language to ensure that it results in consistent contours was relatively small (6 radiation oncologists) and only 4 CT datasets were used, so how the recommended contours will perform in the broader radiation oncology community is still unclear. The ultimate validation of the consensus contouring instructions will be the consistency of the contouring and the patterns of failure in the NRG-GU001 trial and other adjuvant RT trials.
CONCLUSIONS
CTVs and OARs for adjuvant radiation for bladder cancer have been successfully developed and the language validated to ensure that they produce consistently uniform contours. The results will be applicable to clinical trials of adjuvant radiation in bladder cancer.
SUMMARY.
Several organizations are developing clinical trials of adjuvant radiotherapy (RT) for bladder cancer patients at elevated risk of locoregional failure. Clinical target volumes and organs at risk for this treatment have not been defined in detail. We developed a multi-institutional, multidisciplinary, international consensus contouring guide for bladder cancer adjuvant RT. Negative margin patients should be treated to the pelvic nodes alone, and positive margin patients should be treated to the pelvic nodes and cystectomy bed.
Acknowledgments
Funding sources: Supported by grants U24CA180803 and U24CA081647 from the National Cancer Institute
Footnotes
Conflict of interest: J.C. discloses employment at Elekta AB. The authors have nothing else to disclose.
BIBLIOGRAPHY
- 1.Christodouleas JP, Baumann BC, He J, et al. Optimizing bladder cancer locoregional failure risk stratification after radical cystectomy using SWOG 8710. Cancer. 2014;120:1272–1280. doi: 10.1002/cncr.28544. [DOI] [PubMed] [Google Scholar]
- 2.Zaghloul MS, Awwad HK, Akoush HH, et al. Postoperative radiotherapy of carcinoma in bilharzial bladder: improved disease free survival through improving local control. Int J Radiat Oncol Biol Phys. 1992;23:511–517. doi: 10.1016/0360-3016(92)90005-3. [DOI] [PubMed] [Google Scholar]
- 3.Froehner M, Novotny V, Wirth MP, et al. External validation of a model to predict locoregional failure after radical cystectomy. Cancer. 2014 doi: 10.1002/cncr.28876. [DOI] [PubMed] [Google Scholar]
- 4.Ku JH, Kim M, Jeong CW, et al. Risk prediction models of locoregional failure after radical cystectomy for urothelial carcinoma: external validation in a cohort of korean patients. Int J Radiat Oncol Biol Phys. 2014;89:1032–1037. doi: 10.1016/j.ijrobp.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Baumann BC, He J, Hwang WT, et al. Validating a Local Failure Risk Stratification for Use in Prospective Studies of Adjuvant Radiation Therapy for Bladder Cancer. Int J Radiat Oncol Biol Phys. 2016;95:703–706. doi: 10.1016/j.ijrobp.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christodouleas JP, Hwang WT, Baumann BC. Adjuvant Radiation for Locally Advanced Bladder Cancer? A Question Worth Asking. Int J Radiat Oncol Biol Phys. 2016;94:1040–1042. doi: 10.1016/j.ijrobp.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 10.Baumann BC, Guzzo TJ, He J, et al. Bladder cancer patterns of pelvic failure: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:363–369. doi: 10.1016/j.ijrobp.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 11.Baumann BC, Noa K, Wileyto EP, et al. Adjuvant radiation therapy for bladder cancer: A dosimetric comparison of techniques. Med Dosim. 2015;40:372–377. doi: 10.1016/j.meddos.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–387. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sargos P, Guerif S, Latorzeff I, et al. Definition of lymph node areas for radiotherapy of prostate cancer: A critical literature review by the French Genito-Urinary Group and the French Association of Urology (GETUG-AFU) Cancer Treat Rev. 2015;41:814–820. doi: 10.1016/j.ctrv.2015.10.005. [DOI] [PubMed] [Google Scholar]