Abstract
Animal-derived RNA viruses frequently generate viral factories in infected cells. However, the details of how RNA viruses build such intracellular structures are poorly understood. In this study, we examined the structure and formation of the viral factories, called viral speckle of transcripts (vSPOTs), that are produced in the nuclei of host cells by Borna disease virus (BDV). Super-resolution microscopic analysis showed that BDV assembled vSPOTs as intranuclear cage-like structures with 59–180-nm pores. The viral nucleoprotein formed the exoskeletons of vSPOTs, whereas the other viral proteins appeared to be mainly localized within these structures. In addition, stochastic optical reconstruction microscopy revealed that filamentous structures resembling viral ribonucleoprotein complexes (RNPs) appeared to protrude from the outer surfaces of the vSPOTs. We also found that vSPOTs disintegrated into RNPs concurrently with the breakdown of the nuclear envelope during mitosis. These observations demonstrated that BDV generates viral replication factories whose shape and formation are regulated, suggesting the mechanism of the integrity of RNA virus persistent infection in the nucleus.
Keywords: chromosomes, microscopy, negative-strand RNA virus, nucleus, ribonuclear protein (RNP)
Introduction
All viruses seek locations within their host cells in which to safely replicate and assemble. The viral factories that are assembled after viral infection serve as such sites. The virus-specific intracellular compartments contain viral replication complexes and play essential roles in the viral life cycle in host cells. Animal-derived RNA viruses frequently form viral factories called viral inclusion bodies. Positive-strand RNA viruses assemble replication factories, which are often surrounded by membranes, within the host cytoplasm (1). For example, dengue virus infection induces the formation of invaginated vesicles ∼90 nm in diameter within the rough endoplasmic reticulum (ER)2 that are connected to the cytoplasm via paths that have a ∼10 nm width (2). Furthermore, severe acute respiratory syndrome coronavirus and arteriviruses are known to form interconnected double-membraned vesicles that are connected with the ER during the early stages of infection (3, 4).
Negative-strand RNA viruses are known to generate inclusion bodies containing viral ribonucleoprotein complexes (RNPs) in infected cells. Recently, the structural details of the viral replication factories of some negative-strand RNA viruses have been determined through electron microscopic (EM) analysis. Respiratory syncytial virus infection induces the formation of non-membrane-bound inclusion bodies within the cytoplasm, which contain amorphous materials (5). The inclusion bodies of vesicular stomatitis virus are produced in the cytoplasm and do not appear to be associated with specific organelles, such as the ER or mitochondria (6, 7). Rabies virus (RV) forms cytoplasmic spherical inclusion bodies containing a cavity during the early stages of infection and membranous inclusion bodies during later stages (8). These observations demonstrate that although many animal RNA viruses form specific types of viral factories or inclusion bodies, mainly in the cytoplasm, their structural and spatial features are highly polymorphic. The viral factories play important roles in the viral life cycle. However, the mechanisms by which these viral factories are produced in infected host cells and the roles of viral components in their construction during viral replication are poorly understood.
Borna disease virus (BDV) is a non-segmented negative-strand (NNS) RNA virus that belongs to the family Bornaviridae of the order Mononegavirales (9, 10). Although almost all eukaryotic RNA viruses replicate in the cytoplasm of infected cells, BDV uniquely conducts replication in the nuclei of infected cells (11). In addition, BDV noncytolytically establishes a persistent infection. Therefore, this virus is believed to be the only animal RNA virus that can establish a parasitic infection within the nucleus. BDV infection is known to be associated with the production of inclusion bodies, called Joest-Degen bodies, in the nuclei of the brain cells of infected horses (12). Our recent study has revealed that BDV generates nuclear bodies similar to Joest-Degen bodies in the nuclei of cultured cells. These bodies, called viral speckle of transcripts (vSPOTs), are membrane-free structures that are associated with the host chromatin and appear to be an assemblage of viral RNPs (13). Although vSPOTs are predicted to be essential for the BDV life cycle, it is not known how BDV stably maintains its viral factories in the nucleus or how viral RNPs are arranged within the viral factories, whether as disorderly aggregates or as regularly arranged units.
In this study, using super-resolution microscopic techniques, we examined the fine structure of the viral factories of BDV that form in the nuclei of infected cells. Our observations indicated that BDV RNPs, which consisted of genomic RNA enveloped by the viral nucleoprotein (N), assemble intranuclear cage-like spherical structures with pores of ∼100 nm in diameter. Furthermore, we demonstrated that, concomitantly with the breakdown of the nuclear envelope during mitosis, the vSPOTs are deconstructed, and the viral RNPs rapidly relocate to condensed mitotic chromosomes. These results suggest that BDV generates intranuclear viral factories whose shape and formation are highly regulated by nuclear dynamics. The regulated dynamics of the viral factories may be critical to maintaining the unique persistent infection of BDV in the host cell nucleus.
Results
vSPOTs Are the Viral Replication Factories of BDV
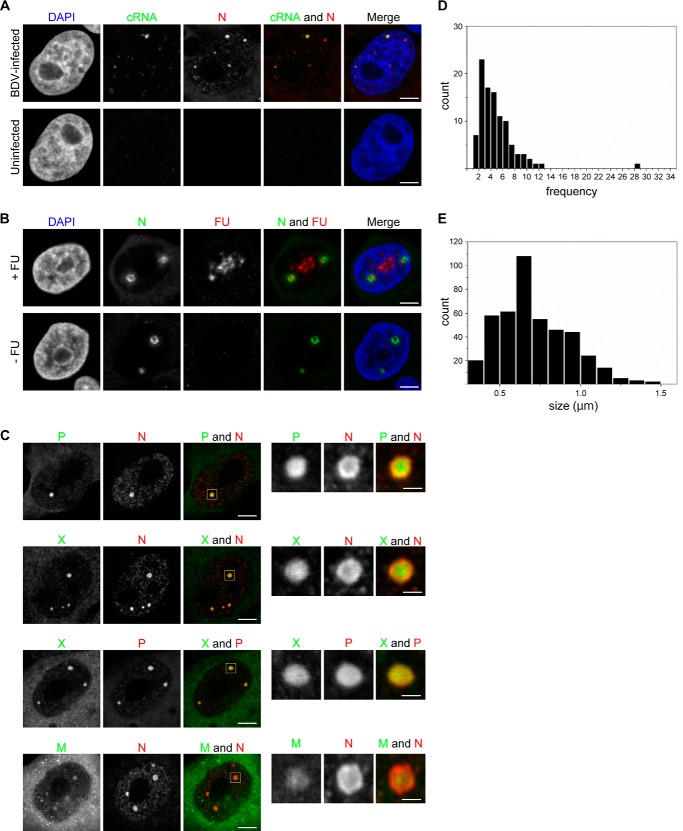
Previous studies have revealed that the expression of N or phosphoprotein (P) alone is insufficient for the formation of vSPOT-like structures in the nuclei of transfected cells (14, 15), thus indicating that vSPOTs require viral replication for their formation and contain viral RNAs. In fact, fluorescence in situ hybridization (FISH) analysis has demonstrated that vSPOTs contain genome-sense RNA (13). Using FISH analysis with antisense riboprobes, we also observed that BDV complementary RNA (cRNA) was localized within vSPOTs (Fig. 1A). The 56% of vSPOTs were FISH signal positive (n = 300). In addition, signals from newly synthesized RNAs were detected in vSPOTs by using the uridine analog 5′-fluorouridine (FU), which is incorporated into nascent RNAs (Fig. 1B). The 49% of vSPOTs were FU signal positive (n = 272). These results indicate that vSPOTs contain BDV RNPs and that the replication or transcription of BDV may occur within vSPOTs.
FIGURE 1.
vSPOTs serve as replication sites for BDV. A, BDV cRNA was detected in BDV-infected OL cells by using FISH. B, the uridine analog FU was added to the culture medium of BDV-infected OL cells and incubated for 2 h, after which immunofluorescence staining was performed with an anti-N monoclonal antibody. DNA was counterstained with DAPI. The specific signals were observed by using CLSM. C, BDV-infected OL cells were subjected to immunofluorescence analysis using anti-P polyclonal/anti-N monoclonal antibodies, anti-X polyclonal/anti-N monoclonal antibodies, anti-X polyclonal/anti-N monoclonal antibodies, and anti-M polyclonal/anti-N monoclonal antibodies and were observed by using CLSM. The right panels shows enlarged images of vSPOTs shown in the left panel. D and E, the histograms of the number of vSPOTs per cell (n = 101) (D) and the size of vSPOTs (n = 444) (E) are shown, respectively. Scale bars: 5 μm in A, B, and the left panel of C; 1 μm in the right panel of C.
Confocal laser scanning microscopy (CLSM) revealed that vSPOTs contain both structural and non-structural proteins of BDV, including N, P, and X proteins (X) and matrix protein (M) (13, 16) (Fig. 1C). The average number of vSPOTs per cell was 4.6 (S.D. 5.0), and the average diameter of vSPOTs was 0.66 μm (S.D. 0.2) (Fig. 1, D and E). CLSM also showed that N was distributed mainly in the outer layer of the vSPOTs, whereas other viral proteins were detected within these structures (Fig. 1C, enlarged panels). However, it is not known whether these viral proteins assemble into isomorphic vSPOTs within the nucleus.
BDV Produces Cage-like Viral Factories with a Nucleoprotein Exoskeleton
Recently, new microscopic imaging techniques, called super-resolution microscopic techniques, have been developed to overcome the resolution limit of light microscopy (17). Structured illumination microscopy (SIM), one type of super-resolution microscopy, has a spatial resolution that is twice that of conventional microscopy (18, 19). We therefore applied SIM to the structural analysis of the vSPOTs in BDV-infected nuclei to investigate the locations of the viral proteins in the vSPOTs. SIM revealed that N formed a thin ring constituting the rim of each vSPOT (Fig. 2, A–C). Furthermore, the N-containing ring appeared to have small gaps with widths of ∼100 nm (Fig. 2B, arrowheads), which were not detected with conventional CLSM (Fig. 1C). In contrast, P associated with the rims of the vSPOTs formed by N (Fig. 2B, arrows) as well as formed a web-like structure within the vSPOTs (Fig. 2, A–C). The structures formed by N and P were reproducibly observed in most of the vSPOTs (Fig. 2B) and in the independently immunostained cells (Fig. 2C). X was randomly distributed within vSPOTs (Fig. 2, D–G). M was also found within vSPOTs but had a very sparse distribution (Fig. 2, H and I).
FIGURE 2.
Distribution of BDV proteins in vSPOTs. A–I, BDV-infected OL cells were subjected to immunofluorescence staining using anti-P polyclonal/anti-N monoclonal antibodies (A–C), anti-X polyclonal/anti-N monoclonal antibodies (D and E), anti-X polyclonal/anti-P monoclonal antibodies (F and G), and anti-M polyclonal/anti-N monoclonal antibodies (H and I). DNA was counterstained with DAPI. The immunofluorescence signals were observed by using SIM. Maximum intensity projection images are shown in A, D, F, and H. The arrowheads in B indicate the gaps in the rims of the vSPOTs. The arrows in B indicate regions on the edges of the vSPOTs in which N and P are colocalized. Scale bars: 5 μm in A, D, F, and H; 500 nm in B, C, E, G, and I.
vSPOTs were observed in the form of variably sized structures in infected nuclei (Fig. 1E). SIM analysis clearly demonstrated that the viral materials were similarly distributed in the structures regardless of their size, with N forming the outer layer of the structures and the other viral proteins being localized within the N-containing shells (Fig. 2). These observations suggest that vSPOTs are isomorphic structures whose size might change depending on viral replication and/or the conditions of the intranuclear environment.
We next used SIM to obtain Z-stack images compiled from nine 0.125-μm thick optical sections showing the distribution of antibodies specific for each viral protein. Consistently with the above described results, N was mainly localized to the shells of the vSPOTs, which had small gaps (Fig. 3, A, B, and D). Notably, the sections near the tops and bottoms of the structures showed that N is woven into a mesh-like pattern. The mesh-like structure was also confirmed by another kind of super-resolution microscopy (see below). The diameters of the pores within this lattice were ∼110–120 nm. This finding is consistent with the observation that the ring formed by N contained small gaps of apparently 100 nm in diameter (Fig. 2B) and suggests that BDV N formed a cage-like exoskeleton surrounding each vSPOT in the nucleus. The Z-stack image also revealed that the web-like P-containing structure was distributed throughout the vSPOT interior and connected with the N-containing outer ring (Fig. 3A). X and M were also found within the vSPOTs, but they were more randomly distributed than P (Fig. 3, B–D). Together, these observations demonstrated that vSPOTs are assembled intranuclear porous structures formed by N.
FIGURE 3.
Series of optical sections of vSPOTs. A–D, Z-stack images compiled from nine vertical optical sections of a vSPOT that were subjected to immunofluorescence staining using anti-P polyclonal/anti-N monoclonal antibodies (A), anti-X polyclonal/anti-N monoclonal antibodies (B), anti-X polyclonal/anti-P monoclonal antibodies (C) and anti-M polyclonal/anti-N monoclonal antibodies (D). Scale bars: 500 nm.
The Outer Layers of vSPOTs Contain Viral RNPs
The results described above indicated that N forms the outer shells of vSPOTs. Whether the N in the outer shell exists in the form of RNPs was still unclear. To investigate this issue, we used direct stochastic optical reconstruction microscopy (dSTORM), a super-resolution microscopic technique that enables visualization of biological samples at a resolution of tens of nanometers (20, 21). Using dSTORM imaging, we reconstructed higher resolution images demonstrating the locations of N and P in vSPOTs. As shown in Fig. 4A, N was mainly localized on the edges of vSPOTs, as was observed with SIM. The same images reconstructed with a 120-nm resolution were very similar in appearance to the images obtained by SIM (data not shown). As observed with SIM, the mesh-like structure and gaps in the structure formed by N were also observed with dSTORM (Fig. 4, A and B). The sizes of gaps (59–180 nm) were large enough compared with the resolution limit of dSTORM (here we conservatively used 30-nm Gaussian spots for reconstruction), showing that N forms the porous cage-like structure. Notably, dSTORM of N revealed that the outer layers of vSPOTs seemed to be composed of filamentous structures. Many rosary-like thin filaments were observed protruding from the outer frames of the vSPOTs (Fig. 4A, panels 1 and 2). In contrast, P appeared to form a net-like structure composed of thin fibers within the vSPOTs, as observed with SIM (Fig. 4C, panels 1 and 2).
FIGURE 4.
The outer layers of vSPOTs contain RNP-like filamentous structures. A–C, dSTORM analyses of BDV-infected OL cells separately conducted using an anti-N antibody (A and B) and an anti-P antibody (C). Two images derived from different vSPOTs are shown for each protein (left and right panels). Magnified images of the regions in the squares in the top panels are shown in the bottom panels (A and C). The arrowheads in A indicate the gaps observed in SIM. The sizes of gaps are indicated in B. Scale bars: 500 nm in B, and the top panels of A and C, 100 nm in the bottom panels of A and C.
BDV RNPs Disperse from vSPOTs during Mitosis
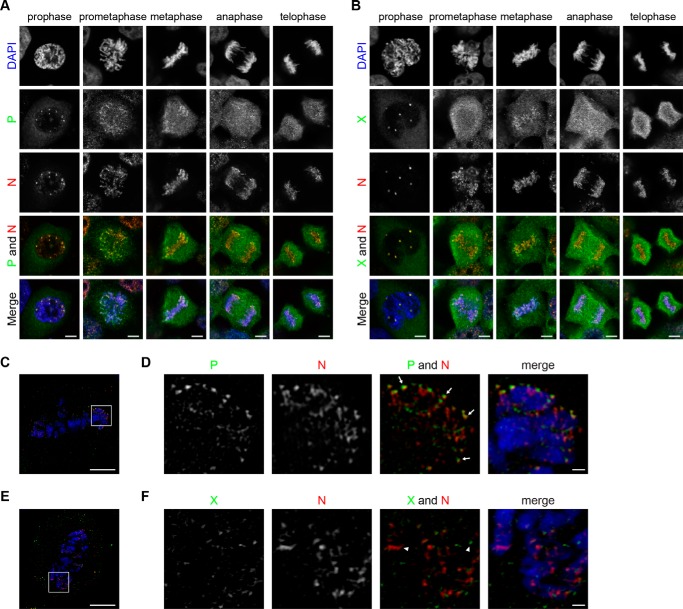
As shown in Fig. 5, A and B, viral components are relocated to condensed chromosomes concurrently with the disruption of the nuclear envelope at prometaphase. To understand whether viral RNPs relocated to the condensed chromosomes in the form of vSPOT-like structures, we investigated the distribution of viral proteins on metaphase chromosomes. As shown in Fig. 5, C–F, SIM revealed that N existed as filamentous structures, not ring-like structures, in association with metaphase chromosomes. Furthermore, intense N and P signals stayed very closely on mitotic chromosomes (Fig. 5D, arrows), whereas X seldom colocalized with N (Fig. 5F, arrowheads). Given that N and P signals partially overlapped only at the rims of the vSPOTs in non-mitotic cells (Fig. 2), these observations indicated that BDV RNPs rapidly dispersed from vSPOT structures and were redistributed on condensed chromosomes simultaneously with nuclear envelope breakdown.
FIGURE 5.
Deconstruction of vSPOTs and distribution of viral RNPs during the mitosis of BDV-infected cells. A and B, immunofluorescence analyses of BDV-infected OL cells in various mitotic phases. Prophase, prometaphase, metaphase, anaphase, and telophase cells were stained by using anti-P polyclonal/anti-N monoclonal antibodies (A) or anti-X polyclonal/anti-N monoclonal antibodies (B). DNA was counterstained with DAPI. The specific signals were observed by using CLSM. C–F, SIM analyses of the metaphase chromosomes of BDV-infected OL cells. The cells were stained with anti-P polyclonal/anti-N monoclonal antibodies (C and D) or with anti-X polyclonal/anti-N monoclonal antibodies (E and F). Magnified images of the areas in the squares in C and E are shown in D and F, respectively. The arrows in D indicate co-localized N and P. The arrowheads in F indicate noncolocalization of N and X. Scale bars: 5 μm in A-C and E; 500 nm in D and F.
Discussion
Recently, structural analyses of the inclusion bodies of some NNS viruses have been conducted by using CLSM and EM techniques. However, the precise distribution, as well as the interaction of each viral protein in these structures, has not been demonstrated. This lack of information is due in large part to the limitations of conventional methods, such as the resolution of microscopy and the difficulties of immunostaining. Isolating viral factories from infected cells while preserving their structures is also difficult because they are unstable. Thus, super-resolution microscopy might be a good method for investigating the construction of viral factories in infected cells in detail.
Our previous study (13) using CLSM and immuno-EM analyses has shown that BDV N is localized to the edges of vSPOTs in infected cells. In this study, we demonstrated the distribution of not only N but also P, X, and M within vSPOT in more detail. Our observations revealed that N assembled into the cage-like structures comprising the outer layers of vSPOTs and that P and X formed random web-like structures within vSPOTs. Recently, the morphogenesis of RV cytoplasmic inclusion bodies has been investigated by using CLSM and EM (8), and RV inclusion bodies were found to be spherical structures composed mainly of viral N and P proteins. Intriguingly, the N protein of RV was also localized to the rims of inclusion bodies and formed an apparently shell-like structure. Furthermore, Nanbo et al. (22) have shown that the NP protein of Ebola virus localizes to the rim of the cytoplasmic viral inclusion bodies. These observations suggest that the nucleoproteins of NNS viruses play a fundamental role in forming the exoskeleton of their inclusion bodies in infected cells.
In previous studies, newly synthesized RNAs of NNS viruses have been detected within cages composed of N protein (8, 22). Given that inclusion bodies have been identified as the replication sites of NNS viruses, an exoskeleton consisting of N might play a role in protecting nascent viral RNAs against degradation and/or the host-sensing of these RNAs. Indeed, RNase A treatment of RV-infected cells does not eliminate the signals from the viral RNAs within inclusion bodies (8), thus indicating that the NNS viral RNAs that accumulate in inclusion bodies are protected from the host environment by the cages formed by N protein.
In this study, we observed that ∼100-nm diameter pores in the N-containing cages of the vSPOTs through SIM. Note that the pore sizes are likely to be smaller because P seems to penetrate the pores observed by N. Importantly, the signal-to-noise ratio of our SIM images were high enough, enabling to distinguish the structure from the noise pattern derived from deconvolution. Moreover, the structure was observed with two different super-resolution imaging methods strongly suggested that the structure is not an artifact of a specific microscopy. It was possible that the porous structure was due to insufficient antibody staining that often resulted in punctuated appearance in super-resolution microscopy of, for example, microtubules (23).3 However, our observation was hard to be explained by such a technical reason because the thickness of the cage formed by N was ∼250 nm (measured from the images with dSTORM), which should contain enough number of antigens unlikely to exclude a 100-nm region. The same results with two super-resolution microscopies further supports our conclusion that the cage structure had a mesh-like shell. Such mesh-like shells might play an important role in the shuttling of viral and host factors in and out of vSPOTs. Indeed, BDV P was shown to shuttle in and out of vSPOTs, whereas N was immobilized within them (13, 24). Furthermore, we demonstrated that certain host factors, which advantageously play a role in viral replication, such as high mobility group box protein 1 (HMGB1), can enter vSPOTs, thus indicating that the pores might allow the selective permeability of these viral factories. Our previous studies have shown that HMGB1 directly interacts with P in infected cells. Interestingly, HMGB1 does not colocalize with the vSPOTs of cells infected with a recombinant BDV with a mutated P, which fails to bind to HMGB1 yet displays unimpaired polymerase-cofactor activity (13). These observations suggest that interaction with P might facilitate entry into vSPOTs through their pores. Further studies are required to understand the roles of the pores in the selective permeability of vSPOTs by host factors and in viral replication.
EM analysis has shown that the rims of the vSPOTs contain RNP-like helical structures (13). Using dSTORM, we also found that anti-N antibodies labeled filamentous structures located at the surfaces of vSPOTs. Given that vSPOTs are the replication sites of BDV, the helical structures observed near their surface by dSTORM may be BDV RNPs, thus suggesting that the RNPs in the vSPOTs might have at least two different functions: a barrier function in the form of the cage surrounding each vSPOT and a template function in viral replication. P, which is an L polymerase cofactor and is necessary to initiate viral transcription and replication, was detected mainly within the vSPOTs, thus suggesting that the RNPs on the interior surface of the cage might contribute to transcribing nascent viral RNAs near that location. The detection of X, a negative regulator of BDV polymerase (25, 26), only within vSPOTs may support this hypothesis. However, whether the RNPs on the outer surface of the cage participate in viral replication in addition to functioning as a frame is unknown. Because N interacts with the host chromatin, it is possible that the RNPs on the outer surface of cages anchor vSPOTs within the nucleus by interacting with the chromosomes. Although our super-resolution microscopic analyses suggested the existence of RNPs on the outer surface of vSPOTs, our technics did not show the interaction between the host chromatin and RNPs on the surface of vSPOTs. Further analyses may reveal the precise functions of viral RNPs in different vSPOT regions.
Here, we also demonstrated that vSPOTs are rapidly deconstructed during prometaphase and that BDV RNPs relocate to the condensed chromosomes. These observations suggest that vSPOT formation is controlled by host factor(s) whose expression or association with BDV RNPs may change concurrently with the disruption of the nuclear envelope at prometaphase. Recently, we have reported that X-linked RNA-binding motif protein (RBMX), a nuclear factor that participates in maintaining the proper cohesion of sister chromatids, is involved in the formation of vSPOTs in the nucleus (27). Interestingly, we found that knocking down RBMX expression by using siRNA disrupted vSPOT formation and the diffusion of RNP components onto heterochromatin, even in interphase nuclei. RBMX is known to associate with chromatin in interphase cells (28), thus suggesting that proper chromatin structure is necessary for vSPOT formation. The formation of the porous cage-like structure of vSPOTs by RNPs may conceivably be related to the chromosomal dynamics of infected cells. However, the mechanism by which vSPOTs are rapidly deconstructed coordinately with the disruption of the nuclear envelope is still unknown. Identification of the host factor that controls the deconstruction of vSPOTs would be a breakthrough to reveal a molecular mechanism of this event.
In this study, we showed that BDV RNP assembles a virus-specific porous cage-like viral factory. Structural analyses of the inclusion bodies formed by other animal RNA viruses will facilitate the elucidation of the common mechanism through which these viruses efficiently replicate in their specific host environments.
Experimental Procedures
Cell Lines and Viruses
The OL cell line was cultured in Dulbecco's modified Eagle's medium (Life Technologies) supplemented with 5% fetal bovine serum. BDV-infected OL cells, a cell line persistently infected with BDV strain huP2br (29), were cultured under the same conditions as the parental cell line.
Immunostaining
Cells cultured on coverslips were fixed with 4% paraformaldehyde for 10 min, blocked with 10% normal goat serum containing 0.5% Triton X-100 for 15 min, and then probed with specific anti-BDV antibodies (anti-N antibody and anti-P antibody (30), anti-X antibody (14), and anti-M antibody (16)) for 2 h at room temperature. After cells were washed twice with PBS, they were incubated with secondary antibodies (Alexa Fluor® 488-conjugated goat anti-rabbit IgG and Alexa Fluor® 555-conjugated goat anti-mouse IgG for confocal microscopy and SIM and an Alexa Fluor® 647-conjugated F(ab′)2 fragment of goat anti-mouse IgG (Life Technologies) for dSTORM) as well as with 4′,6′-diamidino-2-phenylindole (DAPI) for 1 h at room temperature. After the cells were washed three times with PBS, they were mounted in ProLong® Gold Antifade Reagent (Life Technologies) for confocal microscopy or in 2,2′-thiodiethanol-containing (31) antifading reagents (5% 1,4-diazabicyclo[2.2.2]octane and 1% propyl gallate) for SIM.
Fluorescence Imaging
Confocal microscopy was performed using an A1 confocal laser-scanning microscope (Nikon) with a CFI Apo Lambda S ×60 objective lens (N.A. = 1.4).
Raw SIM images were obtained with a DeltaVision OMX imaging system (GE Healthcare) equipped with a UPlanSApo ×100 oil-immersion objective lens (N.A. = 1.4, Olympus). Super-resolution images were reconstructed by using the built-in software program softWoRx. Chromatic aberration, magnification, and the geometric shifting of each EM-CCD cameras were corrected by using software developed in-house, with an alignment precision level of 4–8 nm in the lateral axis and ∼30 nm in the vertical axis over the entire field of view.4 The DAPI signals that bled through the green and red channels were used as references to correct these aberrations (32).
For dSTORM analysis, immunostained samples labeled with Alexa 647 on 8-well Lab-Tek II chamber slides (Thermo Scientific) were incubated in 10 mm aqueous sodium borohydride for 5 min at room temperature (33), washed once with PBS, and then mounted in an oxygen-scavenging buffer (100 mm Tris (pH 8.0), 5% (w/v) glucose, 40 μg/ml of catalase, and 0.5 mg/ml of glucose oxidase). dSTORM images were obtained in wide-field mode with a DeltaVision OMX imaging system. The samples were illuminated with a 633-nm laser at maximum power, and 20,000–30,000 images (256 × 256 pixels) were recorded at a frame rate of 33 Hz. The Alexa 647 molecules were photoactivated by a weak illumination of 405 nm and images of DAPI-stained nuclei were simultaneously collected for drift correction by using a weak pulse from a 405-nm laser. The first 1,000–3,000 images were not used for reconstruction because too many fluorescent molecules were observed in them. Image reconstruction was performed as described previously (34), with slight modifications. After image pre-processing via drift correction, de-noising, and deconvolution, the fluorescent molecules were localized by using the DAOSTORM program (35). Each peak was rendered as a Gaussian peak with its full width at a half-maximum of 30 nm.
Fluorescence in Situ Hybridization
cDNAs representing the 5′ of the BDV genome were amplified by PCR and inserted into the pcDNA3 vector. Using linearized plasmid DNA as a template, in vitro transcription using T7 RNA polymerase was performed to synthesize digoxigenin-labeled RNA probes according to the manufacturer's instructions (Roche Applied Science). BDV-infected or uninfected OL cells cultured on coverslips were fixed with 4% paraformaldehyde for 10 min at room temperature and then treated with 0.1 n HCl for 15 min. After permeabilization of the cells with 0.25% Triton X-100 for 5 min, the endogenous peroxidase was inactivated with 0.5% H5IO6 in PBS for 10 min. The cells were then washed with 2× SSC (1× SSC: 0.15 m NaCl, 0.015 m sodium citrate, pH 7.0) and incubated in hybridization buffer (50% formamide, 3× SSC, 5× Denhardt's solution (Nacalai Tesque) and 100 μg/ml of salmon sperm DNA) for 30 min at 65 °C, and this was followed by probe hybridization in hybridization buffer overnight at 37 °C. The cells were washed sequentially with 4× SSC, 2× SSC containing 2 μg/ml of RNase A, and 0.2× SSC. Fluorescence signals were detected by using an anti-digoxigenin-POD antibody (Roche Applied Science) and a TSATM-Plus Fluorescein System (PerkinElmer Life Sciences) according to the manufacturer's instructions.
Immunodetection of Nascent RNA
FU was added to the culture medium to a final concentration of 5 mm, and the cells were incubated for 2 h in a CO2-containing incubator. The cells were fixed with methanol/acetic acid for 20 min at −20 °C and immunostained. An anti-BrdU antibody (B8434: Sigma) was used to detect the nascent RNA signals.
Author Contributions
Y. Hirai and Y. Hirano carried out the experiments. Y. Hirai, Y. Hirano, A. M., T. H., and K. T. analyzed the data sets. Y. Hirai, Y. Hirano, A. M., Y. Hiraoka, and K. T. prepared the manuscript.
This work was supported in part by Japan Society for the Promotion of Science (JSPS)/The Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant Numbers JP26253027, JP15H01259, JP16H06429, JP16K21723, JP16H06430 (to K. T.), JP26870286 (to Y. Hirai), JP25840008 (to Y. Hirano), JP16H01440, JP15K14500 (to A. M.), and JP26116511 (to Y. Hiraoka), and JSPS Core-to-Core Program A, the Advanced Research Networks (to K. T.), the Basic Science and Platform Technology Program for Innovative Biological Medicine (to K. T.) from the Japanese Agency for Medical Research and Development (AMED), and a grant from the Takeda Science Foundation (to K. T.). The authors declare that they have no conflicts of interest with the contents of this article.
Y. Hirano, A. Matsuda, and Y. Hiraoka, unpublished results.
A. Matsuda, H. Asakawa, T. Kojidani, C. Ohtsuki, L. Schermelleh, Y. Hirano, T. Haraguchi, and Y. Hiraoka, manuscript in preparation.
- ER
- endoplasmic reticulum
- RV
- rabies virus
- RNP
- ribonucleoprotein
- BDV
- Borna disease virus
- NNS
- non-segmented negative-strand
- FU
- 5′-fluorouridine
- vSPOTs
- viral speckle of transcripts
- SIM
- structured illumination microscopy
- dSTORM
- direct stochastic optical reconstruction microscopy
- HMGB1
- high mobility group box protein 1
- RBMX
- X-linked RNA-binding motif protein
- CLSM
- confocal laser scanning microscopy.
References
- 1. Paul D., and Bartenschlager R. (2013) Architecture and biogenesis of plus-strand RNA virus replication factories. World J. Virol. 2, 32–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Welsch S., Miller S., Romero-Brey I., Merz A., Bleck C. K., Walther P., Fuller S. D., Antony C., Krijnse-Locker J., and Bartenschlager R. (2009) Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knoops K., Kikkert M., Worm S. H., Zevenhoven-Dobbe J. C., van der Meer Y., Koster A. J., Mommaas A. M., and Snijder E. J. (2008) SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLos Biol. 6, e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knoops K., Bárcena M., Limpens R. W., Koster A. J., Mommaas A. M., and Snijder E. J. (2012) Ultrastructural characterization of arterivirus replication structures: reshaping the endoplasmic reticulum to accommodate viral RNA synthesis. J. Virol. 86, 2474–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. García J., García-Barreno B., Vivo A., and Melero J. A. (1993) Cytoplasmic inclusions of respiratory syncytial virus-infected cells: formation of inclusion bodies in transfected cells that coexpress the nucleoprotein, the phosphoprotein, and the 22K protein. Virology 195, 243–247 [DOI] [PubMed] [Google Scholar]
- 6. Heinrich B. S., Cureton D. K., Rahmeh A. A., and Whelan S. P. (2010) Protein expression redirects vesicular stomatitis virus RNA synthesis to cytoplasmic inclusions. PLoS Pathog. 6, e1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zajac B. A., and Hummeler K. (1970) Morphogenesis of the nucleoprotein of vesicular stomatitis virus. J. Virol. 6, 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lahaye X., Vidy A., Pomier C., Obiang L., Harper F., Gaudin Y., and Blondel D. (2009) Functional characterization of Negri bodies (NBs) in rabies virus-infected cells: evidence that NBs are sites of viral transcription and replication. J. Virol. 83, 7948–7958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider U. (2005) Novel insights into the regulation of the viral polymerase complex of neurotropic Borna disease virus. Virus Res. 111, 148–160 [DOI] [PubMed] [Google Scholar]
- 10. Tomonaga K., Kobayashi T., and Ikuta K. (2002) Molecular and cellular biology of Borna disease virus infection. Microbes Infect. 4, 491–500 [DOI] [PubMed] [Google Scholar]
- 11. Briese T., de la Torre J. C., Lewis A., Ludwig H., and Lipkin W. I. (1992) Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc. Natl. Acad. Sci. U.S.A. 89, 11486–11489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ludwig H., and Bode L. (2000) Borna disease virus: new aspects on infection, disease, diagnosis and epidemiology. Rev. Sci. Tech. 19, 259–288 [DOI] [PubMed] [Google Scholar]
- 13. Matsumoto Y., Hayashi Y., Omori H., Honda T., Daito T., Horie M., Ikuta K., Fujino K., Nakamura S., Schneider U., Chase G., Yoshimori T., Schwemmle M., and Tomonaga K. (2012) Bornavirus closely associates and segregates with host chromosomes to ensure persistent intranuclear infection. Cell Host Microbe 11, 492–503 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi T., Zhang G., Lee B. J., Baba S., Yamashita M., Kamitani W., Yanai H., Tomonaga K., and Ikuta K. (2003) Modulation of Borna disease virus phosphoprotein nuclear localization by the viral protein X encoded in the overlapping open reading frame. J. Virol. 77, 8099–8107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobayashi T., Kamitani W., Zhang G., Watanabe M., Tomonaga K., and Ikuta K. (2001) Borna disease virus nucleoprotein requires both nuclear localization and export activities for viral nucleocytoplasmic shuttling. J. Virol. 75, 3404–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chase G., Mayer D., Hildebrand A., Frank R., Hayashi Y., Tomonaga K., and Schwemmle M. (2007) Borna disease virus matrix protein is an integral component of the viral ribonucleoprotein complex that does not interfere with polymerase activity. J. Virol. 81, 743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hell S. W. (2009) Microscopy and its focal switch. Nat. Methods 6, 24–32 [DOI] [PubMed] [Google Scholar]
- 18. Gustafsson M. G. (2000) Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 [DOI] [PubMed] [Google Scholar]
- 19. Gustafsson M. G. (2005) Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. U.S.A. 102, 13081–13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heilemann M., van de Linde S., Schüttpelz M., Kasper R., Seefeldt B., Mukherjee A., Tinnefeld P., and Sauer M. (2008) Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. Engl. 47, 6172–6176 [DOI] [PubMed] [Google Scholar]
- 21. Rust M. J., Bates M., and Zhuang X. (2006) Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nanbo A., Watanabe S., Halfmann P., and Kawaoka Y. (2013) The spatio-temporal distribution dynamics of Ebola virus proteins and RNA in infected cells. Sci. Rep. 3, 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lang T., and Rizzoli S. O. (2010) Membrane protein clusters at nanoscale resolution: more than pretty pictures. Physiology 25, 116–124 [DOI] [PubMed] [Google Scholar]
- 24. Charlier C. M., Wu Y. J., Allart S., Malnou C. E., Schwemmle M., and Gonzalez-Dunia D. (2013) Analysis of borna disease virus trafficking in live infected cells by using a virus encoding a tetracysteine-tagged p protein. J. Virol. 87, 12339–12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schneider U., Naegele M., Staeheli P., and Schwemmle M. (2003) Active Borna disease virus polymerase complex requires a distinct nucleoprotein-to-phosphoprotein ratio but no viral X protein. J. Virol. 77, 11781–11789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez M., Sanchez A., Cubitt B., Rosario D., and de la Torre J. C. (2003) A reverse genetics system for Borna disease virus. J. Gen. Virol. 84, 3099–3104 [DOI] [PubMed] [Google Scholar]
- 27. Hirai Y., Honda T., Makino A., Watanabe Y., and Tomonaga K. (2015) X-linked RNA-binding motif protein (RBMX) is required for the maintenance of Borna disease virus nuclear viral factories. J. Gen. Virol. 96, 3198–3203 [DOI] [PubMed] [Google Scholar]
- 28. Matsunaga S., Takata H., Morimoto A., Hayashihara K., Higashi T., Akatsuchi K., Mizusawa E., Yamakawa M., Ashida M., Matsunaga T. M., Azuma T., Uchiyama S., and Fukui K. (2012) RBMX: a regulator for maintenance and centromeric protection of sister chromatid cohesion. Cell Rep. 1, 299–308 [DOI] [PubMed] [Google Scholar]
- 29. Nakamura Y., Takahashi H., Shoya Y., Nakaya T., Watanabe M., Tomonaga K., Iwahashi K., Ameno K., Momiyama N., Taniyama H., Sata T., Kurata T., de la Torre J. C., and Ikuta K. (2000) Isolation of Borna disease virus from human brain tissue. J. Virol. 74, 4601–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe M., Zhong Q., Kobayashi T., Kamitani W., Tomonaga K., and Ikuta K. (2000) Molecular ratio between borna disease viral-p40 and -p24 proteins in infected cells determined by quantitative antigen capture ELISA. Microbiol. Immunol. 44, 765–772 [DOI] [PubMed] [Google Scholar]
- 31. Staudt T., Lang M. C., Medda R., Engelhardt J., and Hell S. W. (2007) 2,2′-Thiodiethanol: a new water soluble mounting medium for high resolution optical microscopy. Microsc. Res. Tech. 70, 1–9 [DOI] [PubMed] [Google Scholar]
- 32. Matsuda A., Chikashige Y., Ding D.-Q., Ohtsuki C., Mori C., Asakawa H., Kimura H., Haraguchi T., and Hiraoka Y. (2015) Highly condensed chromatins are formed adjacent to subtelomeric and decondensed silent chromatin in fission yeast. Nat. Commun. 6, 7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaughan J. C., Jia S., and Zhuang X. (2012) Ultrabright photoactivatable fluorophores created by reductive caging. Nat. Methods 9, 1181–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuda A., Shao L., Boulanger J., Kervrann C., Carlton P. M., Kner P., Agard D., and Sedat J. W. (2010) Condensed mitotic chromosome structure at nanometer resolution using PALM and EGFP-histones. PLoS ONE 5, e12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holden S. J., Uphoff S., and Kapanidis A. N. (2011) DAOSTORM: an algorithm for high-density super-resolution microscopy. Nat. Methods 8, 279–280 [DOI] [PubMed] [Google Scholar]