Abstract
Neutrophils constitute the first line of cellular defense in response to bacterial and fungal infections and rely on granular proteins to kill microorganisms, but uncontrolled secretion of neutrophil cargos is injurious to the host and should be closely regulated. Thus, increased plasma levels of neutrophil secretory proteins, including myeloperoxidase and elastase, are associated with tissue damage and are hallmarks of systemic inflammation. Here, we describe a novel high-throughput screening approach to identify small molecule inhibitors of the interaction between the small GTPase Rab27a and its effector JFC1, two central regulators of neutrophil exocytosis. Using this assay, we have identified small molecule inhibitors of Rab27a-JFC1 binding that were also active in cell-based neutrophil-specific exocytosis assays, demonstrating the druggability of Rab GTPases and their effectors. These compounds, named Nexinhibs (neutrophil exocytosis inhibitors), inhibit exocytosis of azurophilic granules in human neutrophils without affecting other important innate immune responses, including phagocytosis and neutrophil extracellular trap production. Furthermore, the compounds are reversible and potent inhibitors of the extracellular production of superoxide anion by preventing the up-regulation of the granule membrane-associated subunit of the NADPH oxidase at the plasma membrane. Nexinhibs also inhibit the up-regulation of activation signature molecules, including the adhesion molecules CD11b and CD66b. Importantly, by using a mouse model of endotoxin-induced systemic inflammation, we show that these inhibitors have significant activity in vivo manifested by decreased plasma levels of neutrophil secretory proteins and significantly decreased tissue infiltration by inflammatory neutrophils. Altogether, our data present the first neutrophil exocytosis-specific inhibitor with in vivo anti-inflammatory activity, supporting its potential use as an inhibitor of systemic inflammation.
Keywords: exocytosis, inflammation, inhibitor, innate immunity, lipopolysaccharide (LPS), neutrophil, JFC1, Rab27a GTPases and effectors, druggability of GTPases, systemic inflammation
Introduction
Neutrophils constitute the first line of cellular defense in response to bacterial and fungal infections (1) and set the pace for the subsequent innate and adaptive immune responses (2). At the infection site, neutrophils kill pathogens through intracellular and extracellular mechanisms. These include phagocytosis (3), the formation of extracellular traps (4), the secretion of microbicidal cargos from intracellular stores (5), and the production of reactive oxygen species (ROS)3 (6) that altogether generate a hostile environment for the pathogens at the extracellular milieu.
The four secretory organelles present in neutrophils engage sequentially in exocytosis depending on stimuli strength (7, 8). Ideally, the exocytosis of specific and azurophilic granules, which contain the most toxic neutrophil cargos, takes place at the infection site where high concentrations of pathogens are present. However, under pathological conditions, neutrophils encounter a variety of stimuli capable of inducing exocytosis in circulation. Therefore, high levels of neutrophil proteases and pro-oxidative factors are present in plasma in many clinical and experimental conditions associated with systemic inflammation. Increased plasma levels of neutrophil secretory proteins are hallmarks of endotoxemia and sepsis and are also observed in sterile trauma, leading to systemic inflammatory response syndrome. In particular, neutrophil-derived plasma myeloperoxidase (MPO) predicts endothelial dysfunction (9) and is an indicator of the onset of sepsis (10). MPO is also involved in the pathogenesis of cardiovascular disease (11, 12) and arthritis (13). Similarly, secreted neutrophil elastase is important for the development of acute respiratory distress syndrome (14), and tissue damage through the release of proteolytic enzymes and oxygen radicals has largely been involved in the development of sepsis (15, 16). Altogether, these studies highlight that uncontrolled neutrophil secretion represents a major hazard and emphasize the need for new strategies for the treatment of systemic inflammation mediated by neutrophil dysfunction.
The small GTPase Rab27a is an essential regulator of neutrophil exocytosis (17). This process also requires JFC1 (synaptotagmin-like protein1, Slp1) (18), a 562-amino acid protein characterized as a Rab27a effector by three independent groups (19–21). Rab27a and JFC1 regulate intracellular trafficking, docking, fusion, and exocytosis of specific and azurophilic granules (18, 19, 22, 23), but they are dispensable for other innate immune-related neutrophil functions, including phagocytosis (24) and NET production (25). JFC1 contains two tandem C2 domains at its carboxyl terminus and a Rab-binding domain (RBD) at the amino terminus (21, 26). The RBD of JFC1 is formed by two synaptotagmin-like protein-homology domains and contains a TGDWF motif (27). Secretion is entirely dependent on JFC1 binding to Rab27a because mutation of tryptophan 83 of the TGDWF domain, which is conserved in humans and mice, abolishes JFC1-Rab27a binding and inhibits secretion (27).
JFC1 regulates neutrophil exocytosis by two independent mechanisms. First, JFC1 interacts with the Gem-interacting protein through its C2B domain to regulate actin depolymerization around secretory granules by inhibiting RhoA-dependent mechanisms and allowing JFC1-containing secretory organelles to move toward the plasma membrane by maintaining an actin-free environment in their surroundings (18). Second, JFC1 regulates docking of secretory granules by bridging the Rab27a-containing vesicles and the plasma membrane through interaction with both Rab27a, through its RBD, and with plasma membrane phosphatidylinositol 1,4,5-trisphosphate through its C2A domain (19, 22). Based on these properties, down-regulation of JFC1 decreases vesicular docking at the plasma membrane leading to a net decrease in secretion (19).
Rab27a and its effector molecules play fundamental roles in the modulation of the neutrophil inflammatory response by controlling cellular release of inflammatory proteinases and oxidative factors, including myeloperoxidase (19, 28). Importantly, knocking out either Rab27a or JFC1 decreases plasma levels of neutrophil secretory proteins, reduces tissue infiltration by neutrophils, and increases survival in a mouse model of endotoxin-induced systemic inflammation (29). Because neither JFC1 nor Rab27a regulate trafficking of azurophilic granules to the phagosome (23, 24), it would be expected that small molecules interfering with the Rab27a-JFC1 binding will have similar anti-inflammatory properties without affecting other neutrophil innate immune functions.
Here, using a novel high-throughput screening approach, we identify the first small molecule inhibitors of Rab27a-JFC1 binding and neutrophil exocytosis. We show that these compounds are non-toxic and reversible neutrophil inhibitors that decrease the extracellular release of proteases and the production of reactive oxidative species, without affecting NET production or phagosomal maturation. Finally, one of these inhibitors shows significant activity in vivo supporting its potential use as a systemic inflammation modulator.
Results
High-throughput Screening (HTS) for Inhibitors of Rab27a and JFC1 Interaction
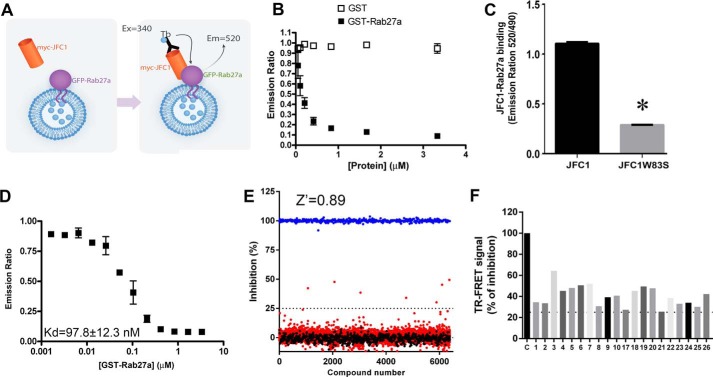
The uncontrolled release of pro-inflammatory secretory factors is a major clinical problem associated with several human diseases. Neutrophils store and secrete some of the most potent pro-inflammatory factors, and their secretory proteins have been associated with the development of several human diseases and syndromes, including sepsis, arthritis, cardiovascular disease, and autoimmune disorders. Pharmacological inhibitors targeting specific molecular interactions that control neutrophil exocytosis are much needed. Here, we focused on the small GTPase Rab27a and its effector JFC1. The interaction between Rab27a and JFC1 is essential for the engagement of neutrophil azurophilic granules in exocytosis for the release of the most toxic neutrophil cargoes. To identify inhibitors of neutrophil exocytosis, we developed an assay to screen molecular libraries to identify novel small molecule inhibitors of the specific binding between the small GTPase Rab27a and its effector JFC1, two modulators of neutrophil exocytosis and neutrophil-induced inflammation (19, 28). The assay is based on the principle of time-resolved FRET (TR-FRET) using a highly stable fluorescence donor, terbium cryptate, and green fluorescent protein (EGFP) as the acceptor. The assay is performed using cell lysates expressing JFC1 with a Myc tag in its amino-terminal domain and EGFP-Rab27a. We next use a terbium-conjugated anti-Myc antibody, which specifically binds to the tag moiety in Myc-JFC1 (Fig. 1A). FRET signal is triggered by the close proximity between the donor (terbium) and acceptor (EGFP) in response to the specific binding of Myc-JFC1 to EGFP-Rab27a. Because the reaction is performed using lysates obtained by non-denaturing methods, it measures the binding of JFC1 to Rab27a on intact secretory vesicles, i.e. in their natural environment, an approach that has many advantages over alternative methods that measure protein-protein interactions in solution. In competitive binding assays using recombinant GST-Rab27a, we show that the specific signal from the Myc-JFC1-EGFP-Rab27a interaction decreases to basal levels in response to increasing concentrations of GST-Rab27a but not when control GST is used (Fig. 1B). To further demonstrate that the TR-FRET signal is specific for JFC1-Rab27a binding, we utilized JFC1-W83S, a JFC1 molecule with a point mutation in tryptophan 83 that impairs the binding of JFC1 to Rab27a (27) by interfering with the Rab-binding domain in JFC1. Here, we show that the expression of the Rab27a-binding-deficient mutant of JFC1 (JFC1-W83S) instead of wild type JFC1 decreases TR-FRET signal (Fig. 1C), further indicating that this assay specifically measures the interaction between Rab27a and JFC1. Finally, homologous competition binding assays using recombinant Rab27a yield an estimated Kd ∼97 nm (Fig. 1D). This dissociation constant is in the same order of the known dissociation constants for Rab27a binding to other effectors (30).
FIGURE 1.
High-throughput screening for the identification of inhibitors of the JFC1-Rab27a interaction. A, schematic representation of the TR-FRET binding reaction. Cell lysates expressing Myc-JFC1 or EGFP-Rab27a were mixed and incubated with terbium-conjugated anti-Myc antibody. The samples were excited at 340 nm. The emission peak of terbium (centered at 490 nm) overlaps with the excitation spectrum of GFP. FRET signal was measured by detecting GFP emission at 520 nm, and results are expressed as the emission ratio of the acceptor (GFP, 520 nm)/donor (terbium, 490 nm, used as internal control). An increased emission ratio is indicative of specific binding. B, specific signal of the Myc-JFC1/EGFP-Rab27a TR-FRET reaction was inhibited by recombinant-purified GST-Rab27a but not GST. The baseline reading for the reaction in the absence of GST or GST-Rab27a was 1.050 ± 0.005. C, single amino acid mutant JFC1-W83S has decreased signal in the TR-FRET assay. Mean ± S.E. from triplicates of one experiment representative of three experiments. *, p < 0.001. D, homologous competitive binding experiments for Rab27a using the TR-FRET assay. Specific binding of a constant concentration of EGFP-Rab27a in the presence of various concentrations of GST-Rab27a was measured. IC50 values were determined using appropriate concentrations (12.5, 25, or 50 nm) of EGFP-Rab27a, so the concentration of EGFP-Rab27a was less than half the IC50. Kd value was then calculated using the homologous competitive binding curve fitted to a built-in equation of one-site competition (GraphPad Prism). The assay assumes that GST-Rab27a and EGFP-Rab27a have similar affinity for JFC1. Error bars correspond to S.E. of three replicates. E, HTS for small molecule inhibitors of the JFC1-Rab27a interaction were performed using the Maybridge HitFinder library. Compounds (red circles) or DMSO (black circles) were added by pin tool into 384-well plates containing lysates expressing Myc-JFC1 and incubated for 15 min at 20 °C. Next, EGFP-Rab27a or EGFP (negative control, blue circles) expressing lysates were loaded using a liquid handling device, and samples were further incubated for 15 min. Reactions were started by addition of terbium-conjugated anti-Myc antibody, and TR-FRET signal was measured by detecting the ratio of the acceptor (GFP, 520 nm)/donor (terbium, 490 nm). Compounds found to inhibit binding exceeding the 3-σ statistical limit (dotted line) were considered primary positive hits. F, inhibitory activity of 20 compounds chosen for follow-up experiments. Compounds 1, 4, and 20 correspond to Nexinhib1, -4, and -20.
We have performed screening assays using two Maybridge HitFinder libraries (MH4 and MH12) for a total of 32,000 compounds. A representative result for a screening performed with a discrete set of compounds is presented in Fig. 1E. The assay has a large signal-to-background ratio, with a calculated Z-factor >0.8 (Fig. 1E). Compound efficacy was estimated after data conversion to percent inhibition as described previously (31), and 20 compounds exhibiting inhibition of Rab27a-JFC1 binding in the primary assay that exceeded 3 S.D. were defined as primary hits (Fig. 1F). The inhibitory activity of these compounds was further tested in triplicate at the 10 μm concentration used in the primary screening.
Identification of Inhibitors of Neutrophil Exocytosis
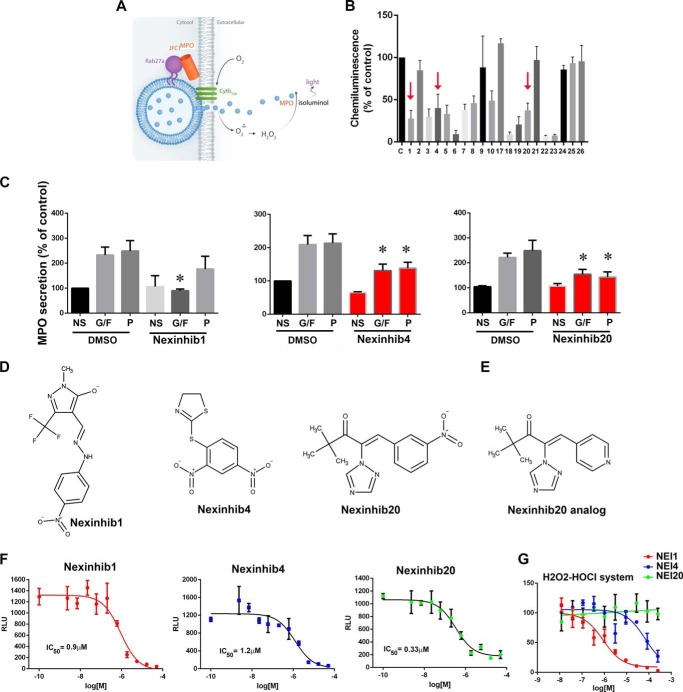
Next, the compounds identified in the primary screening were tested in an HTS-compatible cell-based assay designed to analyze the potency of Rab27a-JFC1 inhibitors for their ability to decrease exocytosis of intact human neutrophil. This assay allowed us to identify and eliminate compounds that, based on their intrinsic characteristics, may not conserve inhibitory activity in intact cells due to decreased permeability, for example,. This allowed as to prioritize cell-active probes. This cell-based assay measures the secretion of myeloperoxidase by human neutrophils using cell-impermeant isoluminol-dependent chemiluminescence in the absence of exogenous peroxidases (Fig. 2A). We tested all 20 compounds that were positive in the primary HTS assay against human neutrophils by using triplicate reactions. These studies identified a structurally non-redundant set of 13 compounds that were positive in both the primary TR-FRET assay and the exocytosis secondary assay (Fig. 2B). For subsequent experiments, “solids” were obtained from Maybridge HitFinder library, resuspended in DMSO at 10 mm, and stored at −20 °C.
FIGURE 2.
Identification of neutrophil exocytosis-specific inhibitors. A, schematic representation of the cell-based secondary screening used for the identification of cell-active inhibitors of MPO secretion. B, 13 compounds, inhibitors in the parent TR-FRET assay, were identified as cell actives in the PMA-induced MPO secretion assay. Mean ± S.E. from three independent experiments using neutrophils from independent donors. The three compounds that were further followed up based on their inhibitory effect on MPO secretion assays (C) are indicated with arrows. C, analysis of MPO secretion. Human neutrophils were treated with the indicated compounds for 1 h at 10 μm and subsequently stimulated with GM-CSF and fMLP (G/F), PMA (P), or left untreated (NS). MPO secretion was analyzed by ELISA. Three inhibitors were identified. Mean ± S.E. from 4 to 11 independent experiments using neutrophil from different healthy donors. *, p < 0.05. D, molecular structures of Nexinhibs. E, molecular structure of Nexinhib20 analog. F, dose-response inhibitory activity of Nexinhibs using the chemiluminescence-based MPO secretion assay. Mean ± S.E. of three biological replicates. G, cell-free luminescence assay showing scavenger activity of Nexinhib (NEI) 1, low scavenger activity of Nexinhib4, and no scavenger activity of Nexinhib20.
To directly measure the effect of the selected compounds on cargo release, the 20 primary hits were vetted against a functional assay that analyzes the exocytosis of azurophilic granules. Because of the large number of compounds tested, dose-response assays were impractical at this point, and so in this assay, human neutrophils were treated with potential inhibitors at 10 μm (dose-response MPO secretion assays for selected compounds are included below). The cells were then stimulated with the physiological stimuli GM-CSF, for priming, followed by exposure to the bacterially derived peptide fMLP or treated with the PKC agonist phorbol ester (PMA). The concentration of myeloperoxidase in the conditioned medium was determined immunologically by ELISA. Two compounds that were positive in the primary and secondary assays were found to inhibit the secretion of myeloperoxidase by human neutrophils in response to both physiological stimuli and PKC agonists (Fig. 2C, for the complete panel of compounds please refer to supplemental Fig. S1). Because Rab27a is essential for the regulation of azurophilic granule exocytosis in neutrophils in response to both GM-CSF/fMLP and PKC agonists, we considered these two compounds to have potential physiological significance. These compounds were named Nexinhib (neutrophil exocytosis inhibitor) 4 and 20. One additional compound, Nexinhib1, was found to inhibit neutrophil secretion in response to physiological stimuli but not PMA and was also included in follow-up assays. The molecular structures of these cell actives are presented in Fig. 2D, and their molecular signatures were confirmed by mass spectrometry (supplemental Fig. S2). The structure of one additional compound used in downstream assays, Nexinhib20 analog, is presented in Fig. 2E. Structural similarities between Nexinhibs was calculated by 3D alignment using MarvinSketch software and are presented in supplemental Fig. S3.
To better understand differential inhibitory efficacies of Nexinhibs, the compounds were tested in dose-response assays by the chemiluminescence-based exocytosis assays described in Fig. 2A, using a 10-point 3-fold serial dilution of the hit compounds in DMSO (0.5% final concentration). All three inhibitors decreased the isoluminol-dependent signal with calculated IC50 values of 0.9, 1.2, and 0.33 μm for Nexinhib1, 4, and 20, respectively (Fig. 2F).
Next, to investigate whether these compounds may interfere with the chemiluminescence reaction by scavenging reactive oxidative species rather than inhibiting myeloperoxidase exocytosis, selected compounds were vetted against cell-free, isoluminol-dependent, chemiluminescence assays triggered by exogenous H2O2 and HOCl, two neutrophil-derived compounds known to oxidize isoluminol. We show that compound Nexinhib20 does not inhibit the cell-free chemiluminescence reaction (Fig. 2G). We also show that Nexinhib4 has little effect on the cell-free reaction at concentrations that were effective in inhibiting neutrophil-induced chemiluminescence (Fig. 2G). In contrast, compound Nexinhib1 showed inhibitory effects on this cell-free assay suggesting that it may act as ROS scavenger as well as secretion inhibitor (Fig. 2G). These results highlight Nexinhib20 as the most potent cell-active probe with no scavenger activity.
Inhibitory Effect of Nexinhib20 Is Specific for the Rab27a-JFC1 Interaction
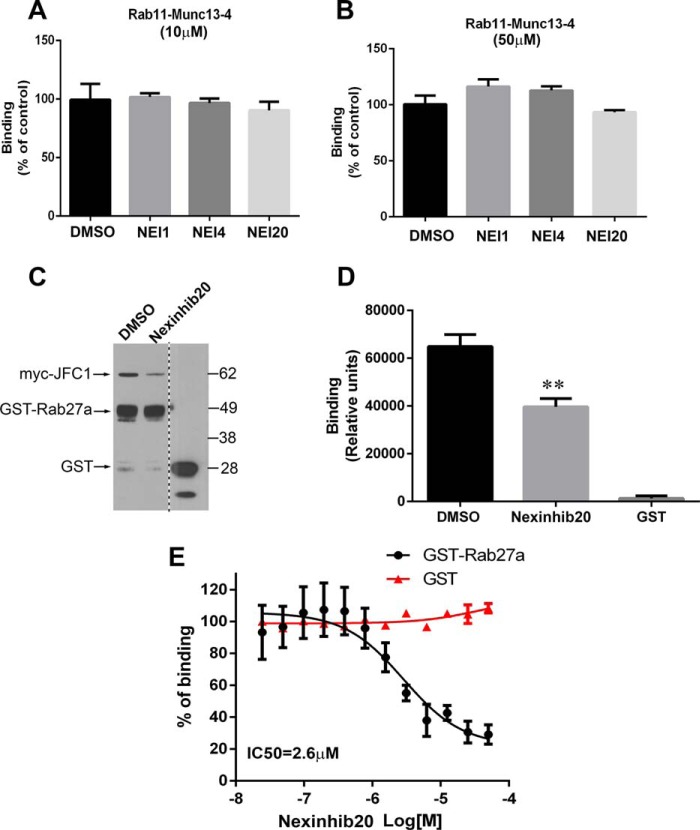
All small Rab GTPases share a common mechanism of GTP-dependent binding to their respective effectors. However, each pair is characterized by highly specific binding properties, and therefore, it is unlikely that Rab27a-JFC1 inhibitors would have high affinity for other Rab GTPase-effector complexes. Nonetheless, we have designed counterscreening assays to further establish the specificity of the identified Rab27a-JFC1 effectors. The tests consist of analyzing the effect of the identified compounds in TR-FRET assays to evaluate the binding of the small GTPase Rab11 to its effector Munc13-4 (32). Also, this assay was instrumental for identifying compounds that may interfere with EGFP emission. Compounds also active in these counterscreenings were considered nonspecific and/or false-positive. No significant inhibitory effects on Rab11-Munc13-4 binding were observed for any of the primary hits either at 10 μm (Fig. 3A) or at 50 μm (Fig. 3B). These compounds were also negative in counterscreening assays for the analysis of Rab27a-Munc13-4 binding (data not shown). These data further support the specificity of these compounds for the JFC1-Rab27a pair and rule out putative fluorescence interference during the primary screening.
FIGURE 3.
Nexinhibs specifically inhibit Rab27a-JFC1 binding but do not interfere with the interaction of other Rab GTPases and effectors. A and B, counterscreening TR-FRET assay for Rab11 and Munc13-4 shows lack of activity for Nexinhibs (NEI) on Rab11-Munc13-4 binding either at 10 μm (A) or at 50 μm (B). C, orthogonal validation pulldown assay showing significant inhibition of Rab27a-JFC1 binding by Nexinhib20 (50 μm). D, quantification of pulldown assay shown in C. Mean ± S.E. from three independent experiments. E, ELISA approach to determine the potency of Nexinhib20 to inhibit the Rab27a-JFC1 interaction performed in dose-response format using recombinant GST-Rab27a or GST (negative control) to bind to JFC1. The experiments were performed in triplicate using a 12-point 2-fold serial dilutions of the hit compounds in DMSO. Mean ± S.E. of three independent experiments.
The inhibitory effect of Nexinhib20 for the Rab27a-JFC1 pair was confirmed by orthogonal validation assays. First, we show that Nexinhib20 inhibits Rab27a-JFC1 co-precipitation (Fig. 3, C and D) in pulldown assays. Nexinhib4 was found to partially inhibit binding in this assay (supplemental Fig. S4). Next, we used a recombinant protein ELISA approach to determine the potency of Nexinhib20. Nexinhib20 demonstrated a dose-dependent inhibitory activity on the binding of Rab27a to JFC1 with a calculated IC50 = 2.6 μm (Fig. 3E). Although Nexinhib1 and -4 inhibited the reaction when tested at high concentrations (>10 μm), lack of a dose-dependent response precluded us from calculating IC50 values for these compounds. Because Nexinhib4 and -20 were proven to be active against the Rab27a-JFC1 complex, in dose-response mode in secondary cell-based exocytosis screenings, also active in validation assays, soluble in the range relevant to its potency, and inactive against counterscreenings, these compounds were prioritized for follow-up experiments, cell-based assays, and in vivo experiments, whereas Nexinhib1 was included as an additional control.
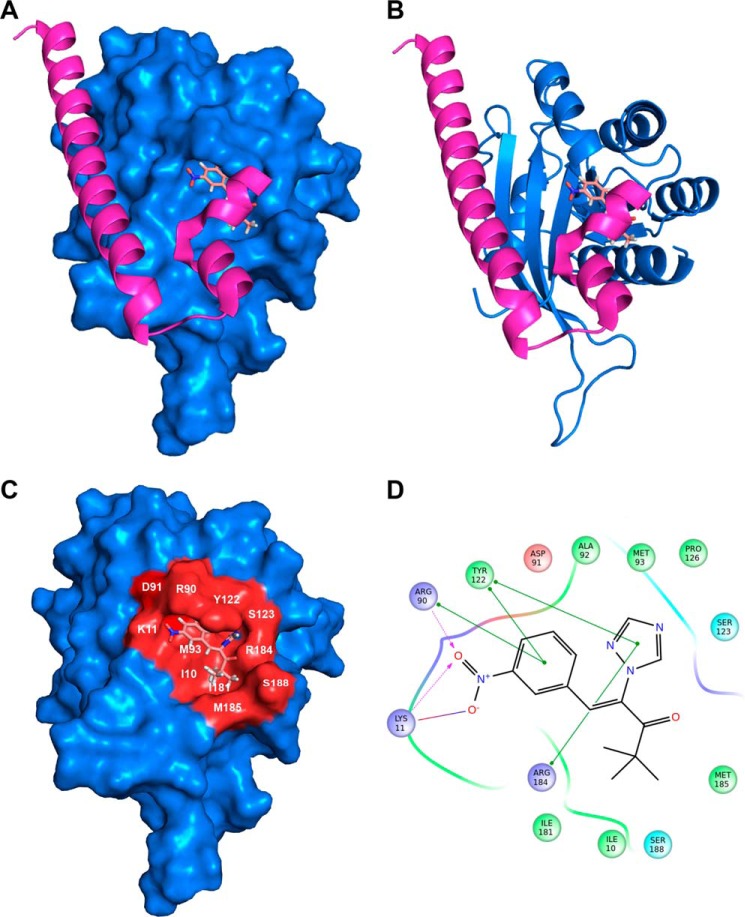
To identify possible small molecule-binding sites within the Rab27a structure, molecular docking studies for the pair Nexinhib20-Rab27a were performed using Sitemap and Glide (Schrödinger, LLC, New York) as described under “Materials and Methods.” A binding pocket for Nexinhib20 with the highest druggability score was identified within a Rab27a domain previously associated with the binding to the synaptotagmin-like homology domain of Slp2a (Fig. 4, A–D). Thus, we have identified Tyr-122 in Rab27a as an important residue present in this pocket that mediates π-π stacking interactions with Nexinhib20 (Fig. 4D). Importantly, structural studies have previously identified Tyr-122 as a key residue for the Rab27a effector selectivity (33). In this way, point mutation of Tyr-122 disrupts the complex between Rab27a and Slp2a (33), which contains a Rab-binding domain highly similar to Slp1 (JFC1) (20). Altogether, our studies support a mechanism of action of Nexinhib20 through the occupation of a druggable pocket in Rab27a and the consequent disruption of the effector binding to this small GTPase.
FIGURE 4.
Molecular docking analysis of Nexinhib20 on Rab27a suggests its mechanism of Rab27a/JFC1 disruption. A, surface representation of Rab27a (blue) in complex with Nexinhib20 shown as sticks in the best scoring pose compared with the binding of Exophilin4 (Slp2a) (magenta, Protein Data Bank code 3BC1). B, schematic representation of RAb27a bound to Nexinhib20 as in A. C, Rab27a binding surface of Nexinhib20 is identified in red. The Rab27a residues interacting with the Nexinhib20 compound are indicated. D, two-dimensional schematic representation of the Rab27a and Nexinhib20 complex interactions. Green lines indicate π-π stacking interactions; purple dashed arrows represent side-chain hydrogen bond interactions. Positively charged, negatively charged, polar, and hydrophobic residues are depicted with blue, red, cyan, and green circles, respectively.
Nexinhibs Do Not Induce Apoptosis or Cell Death
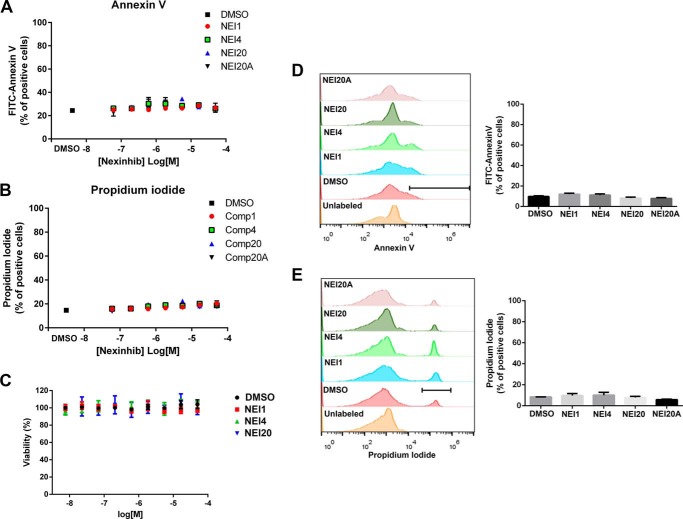
It is expected that compounds with potential biological activity do not induce cellular damage. To analyze the possible detrimental effects of the compounds under study, the hits were tested for their ability to induce apoptosis, interfere with metabolic activity, or decrease cellular viability. First, the toxicity of Nexinhibs was tested in dose-response assays using flow cytometry analyses for the detection of markers of apoptosis and cell death. In these experiments, human granulocytes were incubated with the indicated concentrations of compounds or DMSO (1%) for 1 h. The occurrence of early apoptosis was determined by the analysis of the expression of phosphatidylserine at the outer leaflet of the plasma membrane using FITC annexin V staining. Cell death was tested by the analysis of propidium iodide incorporation into cells due to membrane permeabilization. Our data show that the selected exocytosis inhibitors neither induce apoptosis (Fig. 5A) nor do they trigger cell death (Fig. 5B) even at high compound concentrations. Second, the effect of Nexinhibs on neutrophil metabolic activity was measured in cells after their exposure to increasing concentrations of compounds in a dose-response format, using a luminescence-based ATP detection system, as described previously (35). This assay is based on the analysis of the number of viable cells in culture by the quantification of the ATP present in the lysates, an indicator of metabolically active cells. The assay utilizes a thermostable luciferase-catalyzed reaction to convert luciferin to oxyluciferin and light in the presence of ATP. In Fig. 5C, we show that Nexinhibs do not affect neutrophil metabolic activity even when tested at concentrations as high as 50 μm. In additional experiments, we found that a longer exposure time (4 h) to 10 μm Nexinhibs, a time point at which neutrophils undergo apoptosis when induced by other agents (36, 37), did not induce apoptosis or cell death (Fig. 5, D and E). Altogether, our studies indicate that Nexinhibs do not induce cell damage at 1- or 4-h incubation times even at high compound concentrations.
FIGURE 5.
Nexinhibs do not induce apoptosis or cell death. A and B, dose-response analyses of the effect of compounds on apoptosis and cell viability, respectively. Cells were incubated in the presence of Nexinhibs (NEI) at the indicated concentrations for 1 h. A, early signs of apoptosis were established by staining phosphatidylserine at the outer leaflet of the plasma membrane using FITC annexin V after treatment with compounds or DMSO. B, cell death in compound- and DMSO-treated groups was analyzed by incorporation of propidium iodide (PI) as described under “Materials and Methods.” C, metabolic activity was measured in a dose-response format 1 h after treatment of human neutrophils with compounds or DMSO using a luminescent cell viability assay for the quantification of ATP, as described under “Materials and Methods.” D and E, analysis of early apoptosis and cell death was performed as in A and B, respectively, 4 h after treatment with the indicated compounds at 10 μm. Left panels, representative histograms from flow cytometry analyses of annexin V (D) or propidium iodide (E) are shown. Right panels, the number of FITC annexin V-positive (D) or propidium iodide-positive cells (E) after compound or DMSO treatment was quantified, and results were expressed as % of positive cells for each experimental condition. A–E, mean ± S.E., n = 3.
Nexinhib4 and -20 Regulate Exocytosis-dependent Neutrophil Functions
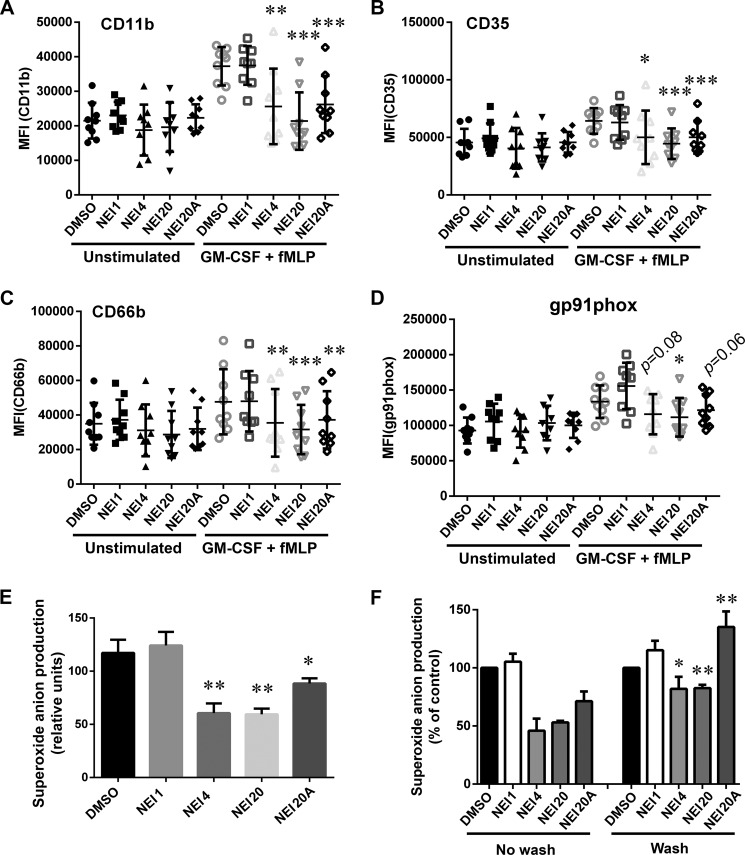
Neutrophil granule membranes contain a large variety of adhesion molecules, receptors, and the NADPH oxidase membrane-associated subunits that are up-regulated at the plasma membrane in response to stimuli-induced exocytosis. To better understand the extent of the inhibitory activity of Nexinhibs, and because Rab27a and JFC1 regulate exocytosis of not only primary granules but also other neutrophil secretory organelles, we analyzed the effect of these compounds on the plasma membrane presentation of a series of endogenous granule markers that are important regulators of neutrophil function, inflammation, and immunity. Human neutrophils were stimulated using the physiological agonists GM-CSF and fMLP, and the plasma membrane expression levels of the adhesion molecule CD11b, the galectin 3 receptor (38) and adhesion molecule CD66b (39), and the complement receptor CR1 (CD35) were evaluated by flow cytometry using specific antibodies that detect extracellular epitopes of the indicated markers (Fig. 6, A–C). Treatment of neutrophils with Nexinhibs4 and -20 for 1 h prevented the up-regulation of both adhesion molecules and receptors (Fig. 6, A–C). Equally effective was the Nexinhib20 analog (Nexinhib20A), a small molecule that shares >70% of the molecular structure with Nexinhib20 (Figs. 6, A–C, and 2D, respectively) and that also significantly inhibits MPO secretion from human neutrophils (supplemental Fig. S5). No inhibitory effect was detected in neutrophils treated with Nexinhib1 highlighting the regulatory activity of the molecular moieties that are exclusively present in Nexinhibs4, -20, and -20A.
FIGURE 6.
Functional analyses identify Nexinhibs as efficient inhibitors of neutrophil exocytosis and extracellular superoxide anion production. A–D, up-regulation of the neutrophil adhesion molecule CD11b, the receptors CD66b and CD35, and the NADPH oxidase membrane-associated subunit gp91phox at the plasma membrane was evaluated by flow cytometry using specific antibodies that detect extracellular epitopes of the indicated markers. Human neutrophils were incubated with the indicated Nexinhibs (NEI) at 10 μm or DMSO and subsequently treated with GM-CSF and fMLP or vehicle (Unstimulated). Symbols correspond to individual donors, and error bars indicate mean ± S.E. of nine independent donors. *, p < 0.02; **, p < 0.003; ***, p < 0.0003, versus DMSO, stimulated. Paired Student's t test. E, production of extracellular superoxide anion by phorbol ester-stimulated human neutrophils treated either with the indicated compounds at 10 μm or vehicle for 1 h was analyzed using the cytochrome c reduction assay. Mean ± S.E. from 7 to 10 independent donors. *, p < 0.04; **, p < 0.001, versus DMSO. Unpaired Student's t test. F, superoxide anion production was measured as in E except that, where indicated, the Nexinhib or DMSO-treated cells were washed twice in PBS before the addition of stimuli. Mean ± S.E. (n = 4). *, p < 0.02; **, p < 0.002, versus same compound, no wash condition.
Nexinhibs Inhibit the Up-regulation of gp91phox at the Plasma Membrane and Are Reversible Inhibitors of the Extracellular Production of Superoxide Anion
Superoxide anion production by the enzymatic complex NADPH oxidase is one of the hallmarks of neutrophil activation and an important defense mechanisms against bacteria and fungi (40). The activation of the oxidase by soluble stimuli involves the up-regulation of the number of cytochrome b558 subunits at the plasma membrane from intracellular stores (7), a Rab27a-dependent and exocytosis-mediated mechanism (28, 41). To analyze whether Nexinhibs prevent cytochrome b558 up-regulation at the cell surface, human neutrophils were stimulated with GM-CSF and fMLP, and cytochrome b558 was detected using the antibody 7D5, which interacts with the extracellular domain of the cytochrome b558 subunit gp91phox (42). In Fig. 6D, we show that Nexinhib20 significantly inhibits cytochrome b558 plasma membrane expression. Although both Nexinhib4 and -20A also decreased the plasma membrane up-regulation of gp91phox, differences did not reach statistical significance. On the contrary, compound Nexinhib1 did not affect gp91phox plasma membrane expression.
Next, to establish the effect of Nexinhibs on the activation of the oxidase, we used the cytochrome c reduction assay, a method that specifically detects extracellular superoxide anion production (43). Here, we show that Nexinhib4 and Nexinhib20 but not Nexinhib1 significantly inhibit superoxide anion production (Fig. 6E) further supporting their ability to inhibit the activation of the NADPH oxidase in human neutrophils. In addition, although Nexinhib20A significantly inhibited superoxide anion, its effect was less pronounced than that observed for Nexinhib-4 and -20. Importantly, the inhibitory activity of Nexinhibs was greatly reduced or lost after washing, indicating that their effects are reversible and not regulated by covalent interactions with the target proteins (Fig. 6F).
Nexinhibs Do Not Interfere with Exocytosis-independent Bacterium-induced Innate Immune Responses
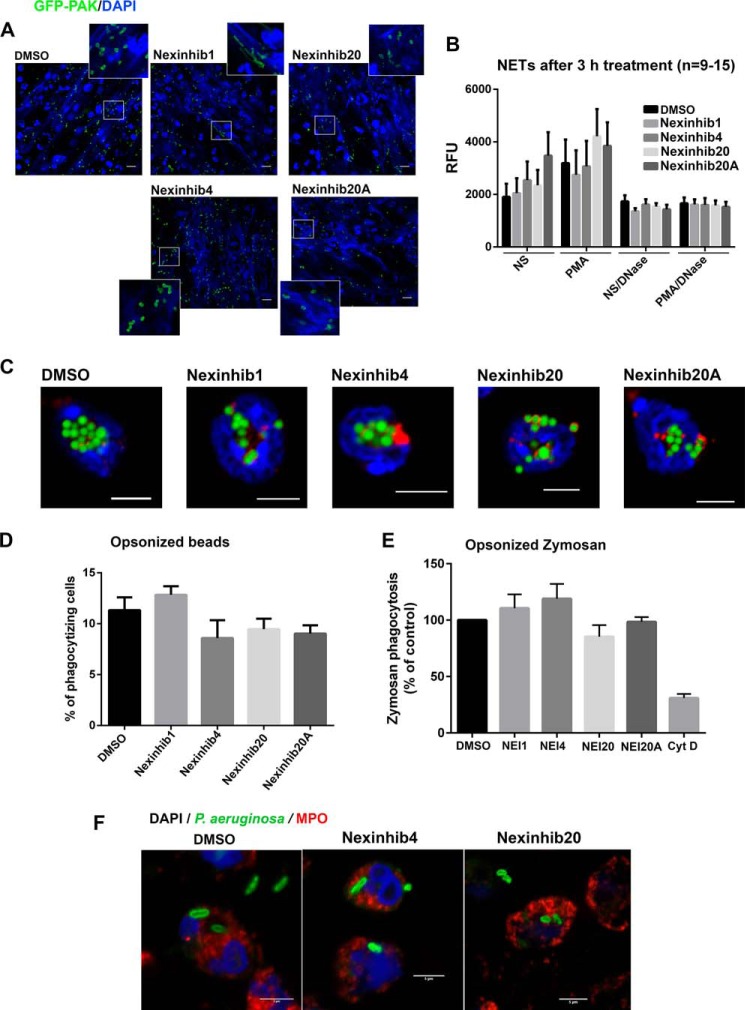
The production of neutrophil extracellular traps (NETs) is an important mechanism of cellular defense against bacterial infections (4). NET production involves the excretion of bactericidal protein-covered chromatin by neutrophils to trap and kill bacteria extracellularly (44). The production of NETs is exocytosis-independent and is not regulated by Rab27a in neutrophils (25). In Fig. 7, we show that Nexinhibs do not inhibit the formation of NETs induced by either live Pseudomonas aeruginosa (Fig. 7A) or by the PKC agonist PMA (Fig. 7B), as analyzed by confocal microscopy or by fluorometry using the cell-impermeant DNA-binding fluorescent probe SYTOX Green, respectively. In addition, Nexinhib treatment did not induce NET formation, with the exception of Nexinhib20A that significantly increased NET production in the absence of additional treatment (Fig. 7B). Altogether, the data presented in Fig. 7 indicate that Nexinhibs do not inhibit NET production. Furthermore, fluorescence microscopy analysis of particle internalization using opsonized latex beads suggests that Nexinhibs do not interfere with the process of phagocytosis (Fig. 7C). This was further confirmed by quantitative flow cytometry analysis of fluorescent particle internalization (Fig. 7D). Additional quantitative experiments using vehicle- or compound-treated human neutrophils to phagocytose opsonized zymosan show that Nexinhibs do not interfere with the process of phagocytosis (Fig. 7E). Instead, we observed a slight increase in phagocytosis with Nexinhib1 and -4 and a not significant mild decrease with Nexinhib20. Furthermore, phagocytosis assays using live P. aeruginosa (Fig. 7F) show bacterial internalization in compound-treated neutrophils. Altogether, these data suggest that Nexinhib4, -20, and -20A regulate exocytosis-dependent mechanisms but do not interfere with other important neutrophil-mediated innate immune responses.
FIGURE 7.
Nexinhibs do not interfere with NET production or phagocytosis. A, Nexinhib-treated human neutrophils produce NETs (DNA staining, blue, DAPI) and trap bacteria (P. aeruginosa, red). Scale bar, 10 μm. B, compound-treated neutrophils respond to PMA by producing NETs. Human neutrophils were stimulated with PMA for 3 h in the presence of the indicated compound or vehicle. Where indicated, the reactions were performed in the presence of DNase to dismantle NETs as a negative control. NETs were analyzed using the cell-impermeant DNA-staining probe SYTOX Green and quantified by fluorometry. Mean ± S.E. (n = 9–15). C, confocal microscopy analysis of the phagocytosis of opsonized fluorescent latex beads by mouse neutrophils after treatment with the indicated compounds at 10 μm or vehicle for 1 h. Green, beads; red, MPO; blue, DAPI. Scale bar, 5 μm. D, quantification of phagocytosis by flow cytometry. Experiments were performed as in C. Mean ± S.E. of neutrophil samples from six independent mice. E, phagocytosis of opsonized zymosan by human neutrophils after treatment with the indicated compounds at 10 μm or vehicle for 1 h. The data represent the mean ± S.E. from five independent donors. Cyt D, cytochalasin D. F, confocal microscopy analysis of the phagocytosis of opsonized live bacteria by human neutrophils after treatment with the indicated compounds at 10 μm or vehicle for 1 h. Scale bar, 5 μm.
Mechanisms Mediating Nexinhib20 Inhibition of Neutrophil Exocytosis
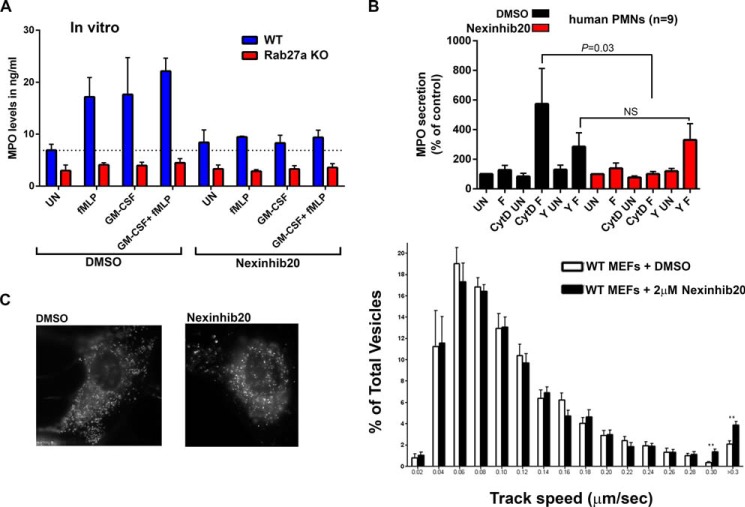
The inhibitory effect of Nexinhib20 on neutrophil exocytosis was confirmed using bone marrow-derived neutrophils from mice. In Fig. 8A, we show that Nexinhib20 efficiently decreases exocytosis of azurophilic granules in neutrophils stimulated with fMLP, GM-CSF, or both. Furthermore, genetic validation of these results is provided by experiments run in parallel using neutrophils from Rab27a-KO mice, showing undetectable levels of myeloperoxidase secretion either in the presence or absence of Nexinhib20. The lack of negative effect of Nexinhib20 on exocytosis of unstimulated neutrophils is more likely explained by the participation of a Rab27a effector different from JFC1 in the regulation of the minimal basal secretion detected in wild type murine neutrophils compared with Rab27a-KO neutrophils.
FIGURE 8.
Mechanism of Nexinhib20 regulation of neutrophil exocytosis. A, Nexinhib20 inhibits the exocytosis of azurophilic granule to basal levels similar to those observed for Rab27a-KO neutrophils. Neutrophils from wild type or Rab27a-KO mice were incubated with 10 μm Nexinhib20 or vehicle for 1 h; the cells were stimulated with the indicated stimuli, and secreted MPO was quantified by ELISA. Mean ± S.E. (n = 3). B, Nexinhibs inhibit MPO secretion upstream of JFC1-dependent inactivation of the RhoA pathway. Human neutrophils were treated with 10 μm Nexinhib20 or vehicle for 1 h. Subsequently, the cells were incubated in the presence of 10 μg/ml cytochalasin D (CytD), with the ROCK kinase inhibitor Y27632 (Y) or DMSO and stimulated with fMLP (F) or left untreated (UN). Mean ± S.E. (n = 9). C, Nexinhib20 interferes with vesicular docking at the plasma membrane. Mouse embryonic fibroblasts were transfected for the expression of the lysosomal marker LysoTracker. Vesicular trafficking in the plane parallel to the plasma membrane (exocytic active zone) was analyzed by TIRFM. Left panel, representative images showing that Nexinhib20 does not alter the overall lysosomal distribution. Right panel, quantitative analysis of vesicular dynamics in DMSO or Nexinhib20-treated cells. Histograms representing the speeds of lysosomes in DMSO- (white bars) or compound (black bars)-treated cells are shown. The speeds for the independent vesicles were binned in 0.02-μm/s increments and plotted as a percentage of total vesicles for a given cell. Results are represented as mean ± S.E. from 40 DMSO- and 40 compound-treated cells. **, p < 0.01. n = 3.
We have previously shown that JFC1 recruits the RhoA-GAP Gem-interacting protein to mediate RhoA inhibition in areas surrounding the secretory granule (18). Thus, in the absence of JFC1 function, RhoA activity increases and secretion is impaired, whereas inhibition of the RhoA downstream kinase ROCK mimics JFC1 function and increases secretion (18). To better understand how the Nexinhibs inhibit exocytosis, we next analyzed the ability of Nexinhib20 to modulate secretion in cells treated with the ROCK kinase inhibitor, Y27632. In Fig. 8B, we show that the inhibitory effect of Nexinhib20 is bypassed when ROCK is also inhibited. Similar results were observed in neutrophils treated with Nexinhib4 (supplemental Fig. S6). These results support our previous data showing that JFC1-mediated RhoA inhibition is important for the Rab27a effector to mediate secretion. These data also suggest that Nexinhibs act upstream of JFC1-mediated RhoA/ROCK inactivation (18).
Actin depolymerization facilitates secretion by allowing access of secretory granules to the plasma membrane, and JFC1 was demonstrated to depolymerize actin in the granule-surrounding areas to facilitate trafficking (18). Here, we show that Nexinhib20 inhibits secretion even in cytochalasin D-treated neutrophils, an agent known to facilitate exocytosis by depolymerizing actin (Fig. 8B) (46). Similar results were observed for Nexinhib4 (supplemental Fig. S6). Because neutrophil granules are free to access the docking points at the plasma membrane under these assay conditions, our data support the idea that JFC1 is important for additional steps downstream of actin polymerization. This is also in agreement with our previous results that JFC1 binds to phosphatidylinositol 1,4,5-trisphosphate through its C2A domain to mediate vesicular docking (22). To test this in further detail, we next analyzed the possible regulation of vesicular tethering/docking by Nexinhib20. In this study we quantified vesicular trafficking in the exocytic active zone in the plane parallel to the plasma membrane. In Fig. 8C, we show that Nexinhib20-treated cells have a significant increase in the number of high motility vesicles. These data confirm the phenotype we have previously observed in JFC1 down-regulated cells that JFC1 modulates speed and displacement of long distant movement of LAMP-positive vesicles (19) and suggest that Nexinhib20 inhibits docking. Altogether, our data highlight that Nexinhib20 regulates vesicular trafficking and neutrophil function through mechanisms that are upstream of ROCK kinase inhibition and vesicular docking mediated by JFC1.
Nexinhib20 Decreases Neutrophil-mediated Systemic Inflammation
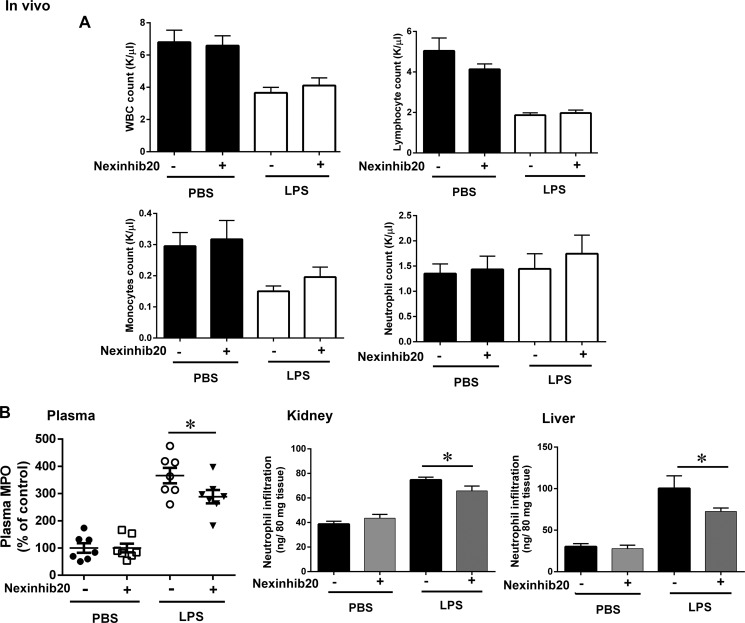
Neutrophil secretory proteins are involved in the development of systemic inflammatory response syndromes triggered both by physical trauma (47) and infection (15). Next, to analyze a possible role for neutrophil exocytosis inhibitors in preventing systemic inflammation, we used a model of LPS-induced systemic inflammation in mice. In this model, a peak of cytokines and neutrophil secretory protein release can be detected 4 h after the insult of a single intraperitoneal administration of LPS (29). In Fig. 9, we show that Nexinhib20 has no effect on LPS-induced mobilization of either total leukocytes or individual cell groups, including monocytes, lymphocytes, and neutrophils as determined by the quantification of these cell populations in circulation 4 h post-LPS insult (Fig. 9A). Importantly, a significant decrease in the level of neutrophil secretory proteins in plasma was observed in mice treated with Nexinhib20 prior to the administration of LPS, suggesting that the inhibitor effectively prevents LPS-induced neutrophil exocytosis in vivo (Fig. 9B, left panel). Furthermore, Nexinhib20-treated mice showed a significant decrease in tissue infiltration by inflammatory neutrophils that was particularly manifested in kidneys and liver (Fig. 9B, center and right panels). Altogether, our data support that Nexinhib20 has a significant anti-inflammatory activity in vivo as demonstrated here by attenuated neutrophil secretion and infiltration.
FIGURE 9.
Nexinhib20 has a protective effect in a mouse model of endotoxin-induced systemic inflammation. Mice were injected (i.p.) with Nexinhib20 (30 mg/kg) or vehicle 3 h before insult with a single intraperitoneal dose of LPS (E. coli 0111:B4 Alexis Biochemicals) (7.5 mg/kg), and blood and tissues were collected 4 h post-LPS administration. A, blood cell counts, mean ± S.E. (n = 7). B, effect of Nexinhib20 on LPS-induced systemic neutrophil secretion and tissue infiltration. Left panel, quantification of plasma levels of neutrophil-secreted MPO was performed by ELISA and expressed as percentage of control (PBS). Mean ± S.E. *, p < 0.05 (n = 7). Center and right panels, Nexinhib20 decreases neutrophil infiltration into tissues in a model of endotoxemia. Mice were treated as above, and the level of neutrophil infiltration into the indicated tissues was analyzed by the quantification of total tissue myeloperoxidase. Significant decrease in neutrophil infiltration was observed in kidneys (p < 0.05) and liver (p = 0.05). Mean ± S.E. (n = 7).
Discussion
Neutrophil secretory proteins are pro-inflammatory factors associated with several human pathologies, including vascular disease, autoimmunity, cancer progression, cardiovascular disease, arthritis, and sepsis. Rab27a and JFC1 play essential roles in the regulation of exocytosis of pro-inflammatory neutrophil cargo. Here, we have identified a small group of molecularly diverse inhibitors of Rab27a-JFC1 binding that to varying degrees can specifically counteract exocytosis-dependent mechanisms and thus interfere with neutrophil-dependent inflammation. Importantly, although these neutrophil inhibitors decrease exocytosis both in vitro and in vivo, they do not interfere with the important innate immune functions of neutrophils, including NET production and phagocytosis. Our findings support the idea that inhibitors of neutrophil exocytosis are effective anti-inflammatory molecules with potential applications in clinical conditions in which neutrophil secretion is abnormally up-regulated.
To the best of our knowledge, this is the first identification of small molecules that specifically inhibit a Rab-effector binding interaction thus demonstrating that Rab GTPases and effectors are potentially druggable. In this study, we have created a new screening technology for Rab GTPases and effectors that is expandable to similar pathways associated with other clinical disorders caused by defects in the function of Rab GTPases or their interacting proteins. This approach led us to the identification of small molecules that selectively and specifically inhibit Rab27a and JFC1, not only providing new tools to study a novel pathway directly associated with human diseases but also leading to the development of pre-therapeutic leads for neutrophil exocytosis inhibitors and the treatment of inflammatory processes.
Myeloperoxidase and neutrophil proteases are involved in neutrophil-induced inflammation. Although MPO acts as a nitric oxide oxidase impairing normal vascular function, elastase is able to degrade multiple substrates, including extracellular matrix components, leading to tissue invasion during inflammation and cancer progression. Cathepsin G and proteinase 3, serine proteases released by neutrophils upon activation, are also associated with pro-inflammatory processes (48, 49). These proteins are stored in azurophilic granules together with other proteases and membrane permeability-inducing peptides (8). Thus, azurophilic granules function as the reservoir for one of the most toxic cocktails stored in any cell in the human body. Because the process of exocytosis is not selective for the cargoes stored in those granules, unregulated neutrophil activation would result in the secretion of a large pool of pro-inflammatory components. In this sense, targeting neutrophil exocytosis constitutes a much more effective approach than utilizing inhibitors for specific proteases, a method that although partially successful in some clinical conditions is ultimately ineffective in systemic inflammation (50) because of the large battery of co-secreted toxicity mediators. Supporting our approach, McLeish and co-workers (51) demonstrated that inhibition of neutrophil exocytosis by means of cell-permeable neutrophil exocytosis inhibitory peptides prevents tissue damage in an animal model of infection. In our approach not only do Nexinhibs inhibit secretion but they also significantly decreased tissue infiltration, a phenotype that recapitulates that observed in the Rab27a-KO mice (29) and is explained by the inhibitory effects that Rab27a deficiency or Nexinhib20 exerts on secretion and on the up-regulation of several adhesion molecules at the neutrophil plasma membrane (29). At a mechanistic level, our data suggest that pharmacological interference with the binding between Rab27a and JFC1 in vivo directly decreases the secretion of toxic cargoes by circulating neutrophils (Fig. 10). Moreover, Nexinhibs do not seem to inhibit the release of leukocytes from the bone marrow as the number of circulating leukocytes is not different between treated and non-treated groups. This is supported by the observations that the compound decreases plasma levels of secretory proteins but not neutrophil counts (Fig. 9). In addition to its inhibitory effects on exocytosis, Nexinhib20 decreased neutrophil infiltration into liver and kidneys. Because neutrophil migration is mediated by adhesion molecules that are up-regulated at the plasma membrane through exocytosis, and a possible role for azurophilic granule secretion on neutrophil migration has also been previously proposed (52), it is likely that Nexinhibs inhibit tissue infiltration by interfering with both the up-regulation of important adhesion molecules at the plasma membrane and azurophilic granule exocytosis in vivo. Altogether, our observation that Nexinhibs prevent systemic inflammation supports the idea that small molecules that inhibit neutrophil granule mobilization and secretion are potentially useful to pharmacologically targeting inflammation in several clinical conditions.
FIGURE 10.
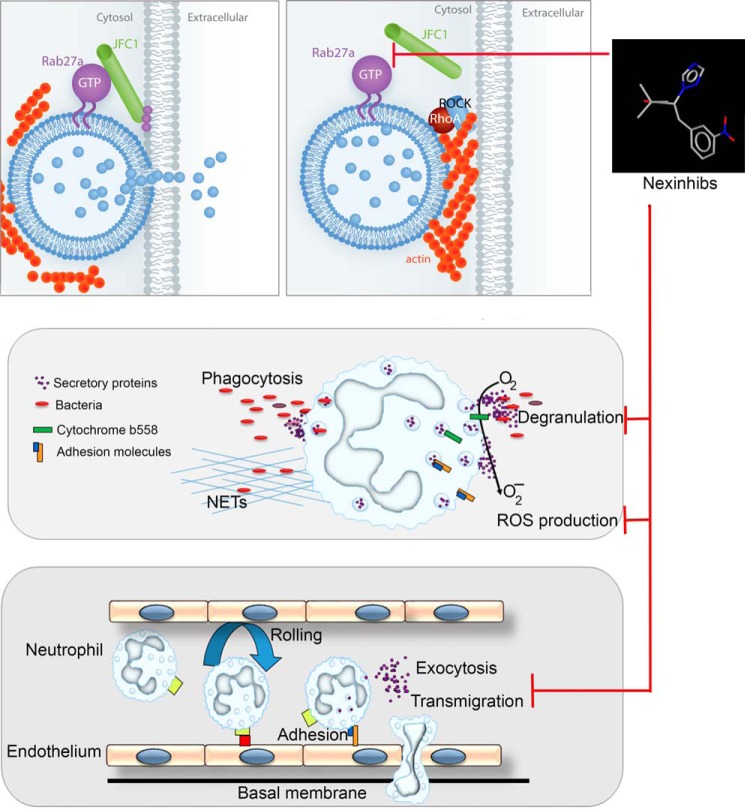
Mechanism of action of neutrophil exocytosis inhibitors, Nexinhibs. Upper panels, left, the small GTPase Rab27a interacts with its effector JFC1 to facilitate vesicular trafficking and cargo secretion. Right, Nexinhib20 inhibits the Rab27a-JFC1 binding and impairs exocytosis possibly by an indirect increase in RhoA signaling, a process up-regulated in JFC1-deficient cells (18). Middle, inhibition of neutrophil exocytosis impairs both neutrophil cargo release and the up-regulation of important granule membrane-associated regulatory proteins, including the cytochrome b558 of the NADPH oxidase, with the consequent decrease of extracellular ROS production. Lower panel, in vivo inhibition of neutrophil exocytosis decreases plasma levels of neutrophil toxic proteins during systemic inflammation and decreases transmigration into tissues, most likely by inhibition of the up-regulation of adhesion molecules.
In addition to controlling azurophilic granule exocytosis and tissue infiltration, Rab27a regulates the production of extracellular ROS by the NADPH oxidase (28), a function associated with pro-inflammatory processes (53). Although inhibitors of the NADPH oxidase NOX2 have been largely pursued, specific potent inhibitors have not been found so far. Here, we have identified compounds that exploit the necessary up-regulation of the membrane-associated subunit of the oxidase, the cytochrome b558, from the granule membrane to the plasma membrane. Because Rab27a and JFC1 are dispensable for phagosomal maturation, these compounds inhibit extracellular superoxide anion production without interfering with the important role played by ROS in innate immunity. Thus, the small molecules identified here have triple anti-inflammatory action by preventing protease secretion, tissue infiltration, and extracellular ROS generation.
Importantly, although some small Rab GTPases, including Rab27a, are expressed in several tissues, the specificity of cellular mechanisms associated with a Rab protein is determined by its effectors, which are differentially expressed in different cell types. In this regard, down-regulation of JFC1, which controls Rab27a-dependent exocytosis in neutrophils (19), does not significantly affect secretion of cytotoxic T lymphocytes, for example (54). Therefore, inhibitors of the Rab27a-JFC1 interaction, although potentially good candidates for therapeutic intervention in inflammation, are not expected to negatively affect the adaptive immune response.
Two of the compounds discovered in our screen for neutrophil inhibitors contain a nitro-aromatic group. Although nitro groups are thought to bias compounds for bioactivation risk by nitro reduction (55), our data suggest that these molecules are reversible inhibitors, do not decrease cell viability, and in the case of Nexinhib20 do not interfere with cell-free redox reactions. Furthermore, similar structural moieties are present in previously reported Food and Drug Administration-approved drugs, in which steric hindrance of the nitro group by the presence of other structural moieties that favor metabolic pathways is proposed to minimize the bioactivation risk of nitro reduction (55). This is the case for the calcium-channel blocker Nifedipine. Although inhibitors of Rab-Rab effectors pairs have not been identified previously, two compounds that inhibit binding of Ras to the effector c-Raf-1 were identified, Kobe0065 and Kobe2602, that coincidentally contain nitrobenzene groups predicted to cause steric hindrance with the effector surface residues (56). Different from the Kobe family compounds, the nitro group present in Nexinhib20 appears not to be essential for its inhibitory effect as the initial structure-activity relationship using a close molecule, Nexinhib20 analog, lacking this group, maintained many of the inhibitory effects observed in the original molecule albeit with less potency. Thus, Nexinhib20 analog may serve as a framework for the development of Rab27a exocytosis inhibitors with higher potency and specificity.
In conclusion, we present the identification of the first specific inhibitors of Rab27a-dependent secretory functions of innate immune cells. We believe that the discovery of these molecules has enormous potential implication for the development of novel therapeutic approaches for the control of several diseases, including vascular inflammation, arthritis, and sepsis in which the secretion of neutrophil secretory proteins is involved (57–59).
Materials and Methods
HTS and Counterscreens
For HTS we designed a TR-FRET utilizing lysates obtained by non-denaturing methods thus conserving intracellular organelles. To this end, cell lysates expressing Myc-JFC1 or EGFP-Rab27a are mixed and incubated with a terbium-conjugated anti-Myc antibody. The samples are excited at 340 nm. The emission peak of terbium (centered at 490 nm) overlaps with the excitation spectrum of GFP. FRET signal is measured by detecting GFP emission at 520 nm, and results are expressed as the emission ratio of the acceptor (GFP, 520 nm)/donor (terbium, 490 nm, used as internal control) (60). An increased emission ratio is indicative of specific binding. Lysates were prepared using Jump-InTM TITM 293 cells, which contain two stable R4 integrase-specific recognition sites and an integration-activated blasticidin resistant gene. Jump-InTM TITM 293 cells were transfected with retargeting vectors expressing EGFP-Rab27a, Myc-JFC1, or empty vectors; blasticidin-selected cells were tested for protein expression, and clones expressing similar levels of EGFP-Rab27a, Myc-JFC1, or control proteins were selected for lysate preparation. For screenings, the individual components of the reaction were expressed in independent cell lines. The calculated concentration of the tagged proteins in each lysate was ∼25 nm. The cells were resuspended in relaxation buffer (100 mm KCl, 3 mm NaCl, 3.5 mm MgCl2, 1 mm ATP, and 10 mm PIPES (pH 7.3)) containing protease inhibitors (cOmplete EDTA-free, Roche Applied Science). For lysis, we use nitrogen cavitation, a method that disrupts the plasma membrane but preserves intracellular organelles and minimizes lysosomal protease release (61). Lysates were spun at 14,000 rpm for 1 min at 4 °C to remove nuclei and tested in small scale TR-FRET reactions, flash-frozen using liquid nitrogen, and stored at −80 °C. HTS for small molecule inhibitors of the JFC1-Rab27a interaction were performed using the Maybridge HitFinder (MBHF) libraries (MH4 and MH12) for a total of 32,000 compounds. Compounds (10 μm) or DMSO (0.5% final concentration) was added by pin tool into 384-well plates containing lysates expressing Myc-JFC1 and incubated for 15 min at 20 °C. Next, EGFP-Rab27a or EGFP-(negative control) expressing lysates were loaded using a liquid handling device, and samples were further incubated for 15 min. Reactions were started by addition of the terbium-conjugated anti-Myc antibody (Cisbio, Bedford, MA), and TR-FRET signal was measured by detecting the ratio of the acceptor (GFP, 520 nm)/donor (terbium, 490 nm). The reactions were followed for up to 30 min after the addition of antibody using a 2104 EnVision multilabel plate reader. For subsequent experiments, solids were obtained from Maybridge HitFinder library, resuspended in DMSO at 10 mm, and stored at −20 °C.
Neutrophil Isolation
Human neutrophils were isolated from normal donor's blood by Ficoll density centrifugation, as described previously (62). Murine bone marrow-derived neutrophils were isolated using a Percoll gradient fractionation system as described (18). A three-layer Percoll gradient was used (52, 64, and 72%), and neutrophils were isolated from the 64–72% interface, washed, and used in the assays.
Secondary Cell-based Chemiluminescence Assay and Neutrophil Secretion Assays
For cell-based secondary screenings, extracellular MPO-dependent ROS production was measured using the chemiluminescence reactions mediated by isoluminol (28). To this end, 3 × 105 neutrophils were resuspended in serum-free RPMI 1640 medium in the presence of cell-impermeant isoluminol, and reactions were carried out in the absence of exogenous peroxidase. Neutrophils were incubated in the presence of compounds or DMSO (0.5%) pin-tooled into 384-well plates in a 40-μl volume. Control experiments were run in parallel in the presence of sodium azide (0.5 mm) to inhibit endogenous myeloperoxidase. After addition of stimuli (PMA) or vehicle using liquid handling devices (BioRaptor FRD, Beckman Coulter), MPO-dependent chemiluminescence was continuously monitored for 30 min at 37 °C using a 2104 EnVision multilabel plate reader. For secretion assays, 0.5 × 106 neutrophils were incubated in RPMI 1640 medium in polystyrene 96-well plates in the presence of compounds or vehicle for 1 h and stimulated for the indicated times at 37 °C with PMA (0.1 μg/ml), GM-CSF (10 ng/ml), and fMLP (1 μm) or vehicle. The cells were spun down, and supernatants were transferred to clean plates using plate filters to avoid contamination from cellular secretory proteins. MPO was measured by ELISA (R&D Systems) as we showed before (18, 19).
Superoxide Anion Detection
Superoxide anion production was continuously monitored using the superoxide dismutase-inhibitable cytochrome c reduction assay at 37 °C (62). Human neutrophils (1 × 106) were washed twice with phosphate-buffered saline (PBS), resuspended in RPMI 1640 medium, and incubated in the presence of the indicated Nexinhib or vehicle (DMSO, 0.1%) for 1 h. The cells were stimulated with phorbol ester (0.1 mg/ml). Superoxide anion production was continuously monitored using the superoxide dismutase-inhibitable cytochrome c reduction assay at 37 °C as described previously (62).
Production of NETs
NET production was analyzed using cell-impermeant nucleic acid staining, as described previously (4, 25). Briefly, human neutrophils treated with Nexinhibs or DMSO (0.1%) were seeded into 96-well plates (1 × 105/well) and stimulated with PMA in the presence or absence of DNase I (100 units/ml) for 3 h at 37 °C. Then, the cell-impermeable nucleic acid stain SYTOX Green (Invitrogen) was added to a final concentration of 5 μm. Non-stimulated neutrophils were used as controls. The samples were analyzed for fluorescence intensity (485 nm excitation and 527 nm emission) using a SpectraMax Gemini EM spectrofluorometer (Molecular Devices) as described previously (4, 25).
In some experiments, NET production was analyzed by confocal microscopy as described previously (24). To this end, neutrophils treated with Nexinhibs or DMSO were incubated in the presence of live P. aeruginosa (GFP), a generous gift from Dr. Arne Rietsch, Case Western Reserve University, Cleveland, OH. Neutrophils were seeded on untreated coverglass dishes, treated with inhibitors, stimulated with bacteria, fixed, and incubated with DAPI for 15 min at 21 °C. Samples were analyzed by confocal microscopy using a Zeiss LSM 710 laser-scanning confocal microscope attached to a Zeiss Observer Z1 microscope using the ×40 oil Plan Apo 1.4 numerical aperture infinity-corrected optics at 21 °C. Images were processed using ImageJ.
Phagocytosis
For the quantitative analysis of phagocytosis, 1 × 106 neutrophils were treated with either Nexinhibs (10 μm), cytochalasin D (10 μg/ml), or vehicle (0.1% DMSO) for 1 h and subsequently incubated with either serum-opsonized amine-modified polystyrene fluorescent beads (1 μm average diameter, Sigma) or with fluorophore-conjugated opsonized Zymosan (Life Technologies, Inc.) for 30 min at 37 °C. A trypan blue quenching solution (1.2 mg/ml final concentration) was added to the samples, and the number of phagocytizing cells was immediately quantified by flow cytometry using a NovoCyte flow cytometer (ACEA Biosciences) and analyzed using FlowJo. In some experiments phagocytosis was measured using tetramethylrhodamine-labeled live P. aeruginosa. Briefly, neutrophils were treated either with DMSO or Nexinhibs for 1 h. Serum-opsonized fluorescently labeled live bacteria were added to 2 × 106 neutrophils at a ratio of 3:1 (bacteria/neutrophil) in a final volume of 0.3 ml of PBS. The samples were incubated for 1 h at 4 °C for synchronization and subsequently incubated for 45 min at 37 °C. A trypan blue quenching solution was added to the samples. Samples were washed with PBS, fixed with 4% paraformaldehyde, used for immunofluorescence, and analyzed by confocal microscopy.
Up-regulation of Plasma Membrane Adhesion Molecules
The up-regulation of granule membrane-associated neutrophil markers at the plasma membrane was analyzed by flow cytometry. In these studies, 1 × 106 human neutrophils were resuspended in phenol red-free RPMI 1640 medium, treated with Nexinhibs or vehicle for 1 h, and stimulated with GM-CSF (10 ng/ml) for 30 min followed by the addition of fMLP (1 μm) or left untreated at 37 °C. The reactions were stopped by transferring the samples to ice and immediately spinning down the cells to initiate blocking and staining. To analyze the plasma membrane expression of plasma membrane markers, the cells were blocked in ice-cold PBS containing 1% BSA and stained with phycoerythrin (PE)-, FITC-, or Alexa647-conjugated anti-human CD11b, CD35, CD66b, and gp91phox antibodies. The cells were then washed and fixed in 1% paraformaldehyde in PBS. Samples were analyzed using a NovoCyte flow cytometer, and the data were processed using FlowJo software.
Apoptosis, Cell Death, and Metabolic Assays
For apoptosis and cell death assays, we used the FITC Annexin V Apoptosis Detection kit I (BD Biosciences). Human granulocytes (1 × 106 cells) were incubated in the presence of the indicated Nexinhibs for 1 or 4 h. The cells were stained according to manufacturer's instructions and analyzed by flow cytometry exactly as described by the manufacturer. For metabolic assays, neutrophils were treated with the indicated compounds and analyzed using CellTiter-Glo® luminescent cell viability assay (Promega) that measures ATP availability in the cell lysates, an indicator of metabolically active cells. This assay uses a thermostable luciferase-catalyzed reaction to convert luciferin to oxyluciferin and light, in the presence of ATP.
TIRF Microscopy
The analysis of vesicular trafficking in LysoTracker-labeled fibroblasts treated with Nexinhib20 or DMSO was performed by TIRF microscopy using a ×100 1.45 numerical aperture TIRF objective (Nikon) on a Nikon TE2000U microscope custom-modified with a TIRF illumination module as described (34). Images were acquired on a 14-bit cooled charge-coupled device camera (Hamamatsu) controlled through NIS-Elements software. For live experiments, the images were recorded using 300–500-ms exposures depending on the fluorescence intensity of the sample. The images were then analyzed using ImageJ software.
Animal Model of LPS-induced Systemic Inflammation
Our experiments utilize ashen mice (C57BL/6-Rab27aash/ash) (45) and their parental strain C57BL/6 (WT). Mice (6–8 weeks old) were maintained in a pathogen-free environment and had access to food and water ad libitum. All animal studies were performed in compliance with the United States Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and according to National Institutes of Health and institutional guidelines.
For in vivo studies of myeloperoxidase secretion and neutrophil infiltration, mice were injected (i.p.) with compounds (30 mg/kg) dissolved in 500 μl of 5% DMSO in PBS or vehicle 3 h before LPS insult. Next, the mice were injected intraperitoneally with a sublethal dose of LPS (Escherichia coli 0111:B4 Alexis Biochemicals) (7.5 mg/kg) diluted in pyrogen-free sterile PBS in a total volume of 500 μl or vehicle, at the opposite abdominal site. Blood samples were collected by intraorbital bleeding at 4 h post-injection. Mice were sacrificed, and tissues were immediately collected, transferred to ice-cold PBS, and processed for myeloperoxidase determination by ELISA as described before (18).
Molecular Docking
The coordinates for Rab27a were obtained from the RCSB Protein Data Bank (code 3BC1), and the structure was prepared using the Protein Preparation module in Maestro software (Maestro, Version 10.6, Schrödinger, LLC). The ligand Nexinhib20 (JFD00787) was converted to a 3D structure with LIGPREP (LigPrep, version 3.8, Schrödinger, LLC) and assigned partial charges with EPIK (Epik, version 3.6, Schrödinger, LLC). SiteMap (SiteMap 3.6, Schrödinger, LLC) was used to identify small molecule-binding sites within the Rab27a structure. A binding site with the highest druggability score was identified, which also mediates binding between Rab27a and Slp2 around residue Tyr-122. This site was utilized to dock Nexinhib20 with Induced Fit Docking using GLIDE (Glide version 7.1, Schrödinger, LLC) for all docking calculations and PRIME (Prime version 4.4, Schrödinger, LLC) for the side-chain prediction of residues within a 5-Å distance of any ligand pose. The extended sampling protocol returned similar poses within the binding site, and the best-scored pose was selected for further analysis. PyMOL (The PyMOL Molecular Graphics System. Version 1.8; Schrödinger, LLC) was used for preparing the figures.
Pulldown and ELISA Binding Assays
Pulldown assays were performed as described previously (18). Briefly, 5 μg of GST-Rab27a or GST (control) were bound to 30 μl of prewashed glutathione-Sepharose 4B (Amersham Biosciences). Beads were washed with PBS and utilized to pull down JFC1 by incubation with 293T cell lysates expressing Myc-JFC1 (100 μl). Recombinant proteins and lysates were rotated overnight at 4 °C in binding buffer, containing protease inhibitors (cOmplete EDTA-free; Roche Applied Science). Beads were washed three times with wash buffer (PBS containing 0.05% Tween 20) and once with ice-cold PBS. The proteins in the pulldown were resuspended in sample buffer; samples were resolved by NuPAGE gel electrophoresis, and JFC1 and GST were detected by Western blotting. For ELISA binding assays, GST or GST-Rab27a (2 μg/ml) in PBS, 0.05% Tween 20, 5% blotto were bound to glutathione-coated 96-well plates (Pierce) by incubation for 1 h at room temperature. The wells were washed three times with PBS followed by the addition of Myc-JFC1 lysates in the presence of compounds or DMSO, and plates were incubated for 1 h. After a further three washes, anti-Myc-HRP antibody (Invitrogen, catalog no. 46-0709, 1:4000 in PBS, 0.05% Tween 20, 5% blotto) was added, and plates were incubated for 2 h at room temperature. The wells were washed three times; the HRP signal was developed by standard procedures, and the colorimetric reaction was quantified using a spectrophotometer (Molecular Devices).
Small Molecules Used in This Study
The following small molecules were used: SMILES: Nexinhib1, 5-hydroxy-1-methyl-3-(trifluoromethyl)-1H-pyrazole-4-carbaldehyde N-(4-nitrophenyl)hydrazine; Nexinhib4, 2-[(2,4-dinitrophenyl)sulfanyl]-4,5-dihydro-1,3-thiazole; Nexinhib20, 4,4-dimethyl-1-(3-nitrophenyl)-2-(1H-1,2,4-triazol-1-yl)pent-1-en-3-one; Nexinhib20A, 4,4-dimethyl-1-(4-pyridyl)-2-(1,2,4-triazol-1-yl)pent-1-en-3-one. For Smiles, the following were used: Nexinhib1, Cn1c(c(c(n1)C(F)(F)F)/C=N–c2ccc(cc2)[N+](=O)[O-])O; Nexinhib4, c1cc(c(cc1[N+](=O)[O-])[N+](=O)[O-])SC2=NCCS2; Nexinhib20, CC(C)(C)C(=O)/C(=C/c1cccc(c1)[N+](=O)[O-])/n2cncn2; Nexinhib20A, CC(C)(C)C(=O)C(=CC1=CC=NC=C1)N2C=NC=N2.
Statistical Analysis
Data are presented as means, and error bars correspond to standard errors of the means (S.E.) unless otherwise indicated. Statistical significance was determined using either unpaired or paired Student's t test or the analysis of variance test using GraphPad InStat (version 3) or Excel software, and graphs were made using GraphPad Prism (version 4) software.
Author Contributions
J. L. J. performed experiments, analyzed data, contributed ideas and comments, and contributed to writing the manuscript. M. R. performed experiments, analyzed data, and contributed ideas and comments. J. H. performed experiments and analyzed data. J. Z. and S. J. B. performed experiments and contributed ideas and comments. L. A. performed experiments. N. B. and E. G. performed molecular docking experiments and analyzed data. H. R. contributed ideas and comments. S. D. C. conceived the idea, designed the manuscript and the experimental approach, performed experiments, analyzed data, and wrote the manuscript with input from J. L. J.
Supplementary Material
This work was supported by National Institutes of Health Grants HL088256 and GM105894 from USPHS (to S. D. C.) and by American Heart Association fellowships (to M. R.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Figs. S1–S6.
- ROS
- reactive oxygen species
- HTS
- high-throughput screening
- RBD
- Rab-binding domain
- MPO
- myeloperoxidase
- fMLP
- formyl-methionyl-leucyl-phenylalanine
- TR-FRET
- time-resolved FRET
- EGFP
- enhanced GFP
- PMA
- phorbol 12-myristate 13-acetate
- TIRF
- total internal reflection fluorescence
- NET
- neutrophil extracellular trap.
References
- 1. Segal A. W. (2005) How neutrophils kill microbes. Annu. Rev. Immunol. 23, 197–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mantovani A., Cassatella M. A., Costantini C., and Jaillon S. (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 [DOI] [PubMed] [Google Scholar]
- 3. Lee W. L., Harrison R. E., and Grinstein S. (2003) Phagocytosis by neutrophils. Microbes Infect. 5, 1299–1306 [DOI] [PubMed] [Google Scholar]
- 4. Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., and Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 [DOI] [PubMed] [Google Scholar]
- 5. Amulic B., Cazalet C., Hayes G. L., Metzler K. D., and Zychlinsky A. (2012) Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489 [DOI] [PubMed] [Google Scholar]
- 6. Babior B. M. (1999) NADPH oxidase: an update. Blood 93, 1464–1476 [PubMed] [Google Scholar]
- 7. Borregaard N. (1988) Subcellular localization and dynamics of components of the respiratory burst oxidase. J. Bioenerg. Biomembr. 20, 637–651 [DOI] [PubMed] [Google Scholar]
- 8. Borregaard N., and Cowland J. B. (1997) Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89, 3503–3521 [PubMed] [Google Scholar]
- 9. Vita J. A., Brennan M. L., Gokce N., Mann S. A., Goormastic M., Shishehbor M. H., Penn M. S., Keaney J. F. Jr., and Hazen S. L. (2004) Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 110, 1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kothari N., Keshari R. S., Bogra J., Kohli M., Abbas H., Malik A., Dikshit M., and Barthwal M. K. (2011) Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J. Crit. Care 26, 435.e1–7 [DOI] [PubMed] [Google Scholar]
- 11. Zheng L., Nukuna B., Brennan M. L., Sun M., Goormastic M., Settle M., Schmitt D., Fu X., Thomson L., Fox P. L., Ischiropoulos H., Smith J. D., Kinter M., and Hazen S. L. (2004) Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baldus S., Heeschen C., Meinertz T., Zeiher A. M., Eiserich J. P., Münzel T., Simoons M. L., Hamm C. W., and CAPTURE Investigators (2003) Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 108, 1440–1445 [DOI] [PubMed] [Google Scholar]
- 13. Stamp L. K., Khalilova I., Tarr J. M., Senthilmohan R., Turner R., Haigh R. C., Winyard P. G., and Kettle A. J. (2012) Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology 51, 1796–1803 [DOI] [PubMed] [Google Scholar]
- 14. Moraes T. J., Chow C. W., and Downey G. P. (2003) Proteases and lung injury. Crit. Care Med. 31, S189–S194 [DOI] [PubMed] [Google Scholar]
- 15. Brown K. A., Brain S. D., Pearson J. D., Edgeworth J. D., Lewis S. M., and Treacher D. F. (2006) Neutrophils in development of multiple organ failure in sepsis. Lancet 368, 157–169 [DOI] [PubMed] [Google Scholar]
- 16. Nuijens J. H., Abbink J. J., Wachtfogel Y. T., Colman R. W., Eerenberg A. J., Dors D., Kamp A. J., Strack van Schijndel R. J., Thijs L. G., and Hack C. E. (1992) Plasma elastase α1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J. Lab. Clin. Med. 119, 159–168 [PubMed] [Google Scholar]
- 17. Catz S. D. (2014) The role of Rab27a in the regulation of neutrophil function. Cell. Microbiol. 16, 1301–1310 [DOI] [PubMed] [Google Scholar]
- 18. Johnson J. L., Monfregola J., Napolitano G., Kiosses W. B., and Catz S. D. (2012) Vesicular trafficking through cortical actin during exocytosis is regulated by the Rab27a effector JFC1/Slp1 and the RhoA-GTPase-activating protein Gem-interacting protein. Mol. Biol. Cell 23, 1902–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brzezinska A. A., Johnson J. L., Munafo D. B., Crozat K., Beutler B., Kiosses W. B., Ellis B. A., and Catz S. D. (2008) The Rab27a effectors JFC1/Slp1 and Munc13-4 regulate exocytosis of neutrophil granules. Traffic 9, 2151–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuroda T. S., Fukuda M., Ariga H., and Mikoshiba K. (2002) The Slp homology domain of synaptotagmin-like proteins 1–4 and Slac2 functions as a novel Rab27A binding domain. J. Biol. Chem. 277, 9212–9218 [DOI] [PubMed] [Google Scholar]
- 21. Strom M., Hume A. N., Tarafder A. K., Barkagianni E., and Seabra M. C. (2002) A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 277, 25423–25430 [DOI] [PubMed] [Google Scholar]
- 22. Catz S. D., Johnson J. L., and Babior B. M. (2002) The C2A domain of JFC1 binds to 3′-phosphorylated phosphoinositides and directs plasma membrane association in living cells. Proc. Natl. Acad. Sci. U.S.A. 99, 11652–11657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Munafó D. B., Johnson J. L., Ellis B. A., Rutschmann S., Beutler B., and Catz S. D. (2007) Rab27a is a key component of the secretory machinery of azurophilic granules in granulocytes. Biochem. J. 402, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monfregola J., Johnson J. L., Meijler M. M., Napolitano G., and Catz S. D. (2012) MUNC13–4 protein regulates the oxidative response and is essential for phagosomal maturation and bacterial killing in neutrophils. J. Biol. Chem. 287, 44603–44618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munafo D. B., Johnson J. L., Brzezinska A. A., Ellis B. A., Wood M. R., and Catz S. D. (2009) DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J. Innate Immun. 1, 527–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McAdara Berkowitz J. K., Catz S. D., Johnson J. L., Ruedi J. M., Thon V., and Babior B. M. (2001) JFC1, a novel tandem C2 domain-containing protein associated with the leukocyte NADPH oxidase. J. Biol. Chem. 276, 18855–18862 [DOI] [PubMed] [Google Scholar]
- 27. Johnson J. L., Pacquelet S., Lane W. S., Eam B., and Catz S. D. (2005) Akt regulates the subcellular localization of the Rab27a-binding protein JFC1 by phosphorylation. Traffic 6, 667–681 [DOI] [PubMed] [Google Scholar]
- 28. Johnson J. L., Brzezinska A. A., Tolmachova T., Munafo D. B., Ellis B. A., Seabra M. C., Hong H., and Catz S. D. (2010) Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic 11, 533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson J. L., Hong H., Monfregola J., and Catz S. D. (2011) Increased survival and reduced neutrophil infiltration of the liver in Rab27a- but not Munc13-4-deficient mice in lipopolysaccharide-induced systemic inflammation. Infect. Immun. 79, 3607–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kimura T., Kaneko Y., Yamada S., Ishihara H., Senda T., Iwamatsu A., and Niki I. (2008) The GDP-dependent Rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines. J. Cell Sci. 121, 3092–3098 [DOI] [PubMed] [Google Scholar]
- 31. Sergienko E., Su Y., Chan X., Brown B., Hurder A., Narisawa S., and Millán J. L. (2009) Identification and characterization of novel tissue-nonspecific alkaline phosphatase inhibitors with diverse modes of action. J. Biomol. Screen. 14, 824–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson J. L., He J., Ramadass M., Pestonjamasp K., Kiosses W. B., Zhang J., and Catz S. D. (2016) Munc13-4 is a Rab11-binding protein that regulates Rab11-positive vesicle trafficking and docking at the plasma membrane. J. Biol. Chem. 291, 3423–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chavas L. M., Ihara K., Kawasaki M., Torii S., Uejima T., Kato R., Izumi T., and Wakatsuki S. (2008) Elucidation of Rab27 recruitment by its effectors: structure of Rab27a bound to exophilin4/Slp2-a. Structure 16, 1468–1477 [DOI] [PubMed] [Google Scholar]
- 34. Johnson J. L., Napolitano G., Monfregola J., Rocca C. J., Cherqui S., and Catz S. (2013) Upregulation of the Rab27a-dependent trafficking and secretory mechanisms improves lysosomal transport, alleviates endoplasmic reticulum stress, and reduces lysosome overload in cystinosis. Mol. Cell. Biol. 33, 2950–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madoux F., Li X., Chase P., Zastrow G., Cameron M. D., Conkright J. J., Griffin P. R., Thacher S., and Hodder P. (2008) Potent, selective and cell penetrant inhibitors of SF-1 by functional ultra-high-throughput screening. Mol. Pharmacol. 73, 1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Croker B. A., O'Donnell J. A., Nowell C. J., Metcalf D., Dewson G., Campbell K. J., Rogers K. L., Hu Y., Smyth G. K., Zhang J. G., White M., Lackovic K., Cengia L. H., O'Reilly L. A., Bouillet P., et al. (2011) Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc. Natl. Acad. Sci. U.S.A. 108, 13135–13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watson R. W., O'Neill A., Brannigan A. E., Brannigen A. E., Coffey R., Marshall J. C., Brady H. R., and Fitzpatrick J. M. (1999) Regulation of Fas antibody induced neutrophil apoptosis is both caspase and mitochondrial dependent. FEBS Lett. 453, 67–71 [DOI] [PubMed] [Google Scholar]
- 38. Feuk-Lagerstedt E., Jordan E. T., Leffler H., Dahlgren C., and Karlsson A. (1999) Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J. Immunol. 163, 5592–5598 [PubMed] [Google Scholar]
- 39. Yoon J., Terada A., and Kita H. (2007) CD66b regulates adhesion and activation of human eosinophils. J. Immunol. 179, 8454–8462 [DOI] [PubMed] [Google Scholar]
- 40. Babior B. M., and Woodman R. C. (1990) Chronic granulomatous disease. Semin. Hematol. 27, 247–259 [PubMed] [Google Scholar]
- 41. Uriarte S. M., Rane M. J., Luerman G. C., Barati M. T., Ward R. A., Nauseef W. M., and McLeish K. R. (2011) Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J. Immunol. 187, 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamauchi A., Yu L., Pötgens A. J., Kuribayashi F., Nunoi H., Kanegasaki S., Roos D., Malech H. L., Dinauer M. C., and Nakamura M. (2001) Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b558, to the extracellular peptide portion of primate gp91phox. Microbiol. Immunol. 45, 249–257 [DOI] [PubMed] [Google Scholar]
- 43. Babior B. M., Kipnes R. S., and Curnutte J. T. (1973) Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 52, 741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Papayannopoulos V., Metzler K. D., Hakkim A., and Zychlinsky A. (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson S. M., Yip R., Swing D. A., O'Sullivan T. N., Zhang Y., Novak E. K., Swank R. T., Russell L. B., Copeland N. G., and Jenkins N. A. (2000) A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. U.S.A. 97, 7933–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jog N. R., Rane M. J., Lominadze G., Luerman G. C., Ward R. A., and McLeish K. R. (2007) The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am. J. Physiol. Cell Physiol 292, C1690–C1700 [DOI] [PubMed] [Google Scholar]
- 47. Johnson G. B., Brunn G. J., and Platt J. L. (2004) Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J. Immunol. 172, 20–24 [DOI] [PubMed] [Google Scholar]
- 48. Shimoda N., Fukazawa N., Nonomura K., and Fairchild R. L. (2007) Cathepsin G is required for sustained inflammation and tissue injury after reperfusion of ischemic kidneys. Am. J. Pathol. 170, 930–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coeshott C., Ohnemus C., Pilyavskaya A., Ross S., Wieczorek M., Kroona H., Leimer A. H., and Cheronis J. (1999) Converting enzyme-independent release of tumor necrosis factor α and IL-1β from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc. Natl. Acad. Sci. U.S.A. 96, 6261–6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iwata K., Doi A., Ohji G., Oka H., Oba Y., Takimoto K., Igarashi W., Gremillion D. H., and Shimada T. (2010) Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Intern. Med. 49, 2423–2432 [DOI] [PubMed] [Google Scholar]
- 51. Uriarte S. M., Rane M. J., Merchant M. L., Jin S., Lentsch A. B., Ward R. A., and McLeish K. R. (2013) Inhibition of neutrophil exocytosis ameliorates acute lung injury in rats. Shock 39, 286–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colvin R. A., Means T. K., Diefenbach T. J., Moita L. F., Friday R. P., Sever S., Campanella G. S., Abrazinski T., Manice L. A., Moita C., Andrews N. W., Wu D., Hacohen N., and Luster A. D. (2010) Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat. Immunol. 11, 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dang P. M., Stensballe A., Boussetta T., Raad H., Dewas C., Kroviarski Y., Hayem G., Jensen O. N., Gougerot-Pocidalo M. A., and El-Benna J. (2006) A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J. Clin. Invest. 116, 2033–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Holt O., Kanno E., Bossi G., Booth S., Daniele T., Santoro A., Arico M., Saegusa C., Fukuda M., and Griffiths G. M. (2008) Slp1 and Slp2-a localize to the plasma membrane of CTL and contribute to secretion from the immunological synapse. Traffic 9, 446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walsh J. S., and Miwa G. T. (2011) Bioactivation of drugs: risk and drug design. Annu. Rev. Pharmacol. Toxicol. 51, 145–167 [DOI] [PubMed] [Google Scholar]
- 56. Cox A. D., Fesik S. W., Kimmelman A. C., Luo J., and Der C. J. (2014) Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Dis. 13, 828–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Babior B. M. (2000) Phagocytes and oxidative stress. Am. J. Med. 109, 33–44 [DOI] [PubMed] [Google Scholar]
- 58. Zhang C., Yang J., Jacobs J. D., and Jennings L. K. (2003) Interaction of myeloperoxidase with vascular NAD(P)H oxidase-derived reactive oxygen species in vasculature: implications for vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 285, H2563–H2572 [DOI] [PubMed] [Google Scholar]
- 59. Brovkovych V., Gao X. P., Ong E., Brovkovych S., Brennan M. L., Su X., Hazen S. L., Malik A. B., and Skidgel R. A. (2008) Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am. J. Physiol. Lung Cell Mol. Physiol. 295, L96–L103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Riddle S. M., Vedvik K. L., Hanson G. T., and Vogel K. W. (2006) Time-resolved fluorescence resonance energy transfer kinase assays using physiological protein substrates: applications of terbium-fluorescein and terbium-green fluorescent protein fluorescence resonance energy transfer pairs. Anal. Biochem. 356, 108–116 [DOI] [PubMed] [Google Scholar]
- 61. Klempner M. S., Mikkelsen R. B., Corfman D. H., and André-Schwartz J. (1980) Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J. Cell Biol. 86, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Markert M., Andrews P. C., and Babior B. M. (1984) Measurement of O2− production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 105, 358–365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.