Abstract
Halomonas sp. Y2 is a halotolerant alkaliphilic strain from Na+-rich pulp mill wastewater with high alkalinity (pH >11.0). Transcriptome analysis of this isolate revealed this strain may use various transport systems for pH homeostasis. In particular, the genes encoding four putative Na+/H+ antiporters were differentially expressed upon acidic or alkaline conditions. Further evidence, from heterologous expression and mutant studies, suggested that Halomonas sp. Y2 employs its Na+/H+ antiporters in a labor division way to deal with saline and alkaline environments. Ha-NhaD2 displayed robust Na+(Li+) resistance and high transport activities in Escherichia coli; a ΔHa-nhaD2 mutant exhibited growth inhibition at high Na+(Li+) concentrations at pH values of 6.2, 8.0, and 10.0, suggesting its physiological role in osmotic homeostasis. In contrast, Ha-NhaD1 showed much weaker activities in ion exporting and pH homeostasis. Ha-Mrp displayed a combination of properties similar to those of Mrp transporters from some Bacillus alkaliphiles and neutrophiles. This conferred obvious Na+(Li+, K+) resistance in E. coli-deficient strains, as those ion transport spectra of some neutrophil Mrp antiporters. Conversely, similar to the Bacillus alkaliphiles, Ha-Mrp showed central roles in the pH homeostasis of Halomonas sp. Y2. An Ha-mrp-disrupted mutant was seriously inhibited by high concentrations of Na+(Li+, K+) but only under alkaline conditions. Ha-NhaP was determined to be a K+/H+ antiporter and shown to confer strong K+ resistance both at acidic and alkaline stresses.
Keywords: membrane protein, pH regulation, potassium transport, sodium-proton exchange, stress response, transcriptomics
Introduction
It is well known that microbial cells have evolved mechanisms to regulate ion and pH homeostasis in response to developmental cues and to adapt to constantly changing environments (1). Considerable efforts have been dedicated to understand the adaptive mechanisms of osmotic and pH homeostasis in different microorganisms (2, 3). Prokaryotes have developed multilevel adaptive strategies in response to alkaline and saline stress, including regulated adjustments of cell wall structure, plasma membrane lipid composition, membrane transport systems, bioenergetics, and osmoregulation (4). To adapt to environmental stresses, common strategies are shared among diverse organisms, whereas specific adaptive mechanisms have also been developed by some bacteria to deal with extreme environments (5). For example, by up-regulating deaminases, ATP synthase, and the microaerophilic cytochrome d oxidoreductase, Escherichia coli alters its metabolism to generate acids in the cytoplasm in response to high external pH (3). In alkaliphilic Bacillus sp., a Na+ transport cycle is coupled to cytoplasmic proton accumulation, which has an indispensable role in pH homeostasis (6).
A major strategy for bacterial pH homeostasis is the use of transporters that catalyze active proton transport (7), resulting in the efflux of intracellular monovalent cations (such as Na+, K+, and Li+) in exchange for external protons. This process has an essential role in reducing the cytoplasmic concentration of toxic cations and in supporting Na+/K+-dependent cytoplasmic pH homeostasis under alkaline conditions (6, 8). Most of Na+(K+)/H+ exchangers are monovalent antiporters and are part of the large cation/proton antiporters (CPA)3 superfamily, which includes CPA1 and CPA2 (TC 2.A.36 and 2.A.37), and the Nha (Na+/H+) family, which includes NhaA, NhaB, NhaC, and NhaD (9). Mrp-type antiporters, classified as the CPA3 subfamily of the CPA family, are produced by operons consisting of six or seven genes (10). Bacteria generally have several cation/proton antiporters that can be used in response to environmental stress (9). It has been proposed that different antiporters have different functions in ion and pH homeostasis, such as the NhaA and NhaB antiporters of E. coli. Ec-NhaA functions best in an alkaline environment, whereas Ec-NhaB is more active in neutral environments (11). It was previously demonstrated that NhaA is the key Na+/H+ antiporter of E. coli and many other enterobacteria (12), whereas the hetero-oligomeric Mrp antiporter, present in some alkaliphilic Bacillus sp., has an indispensable role at high pH values (7). Membrane transport systems have been extensively studied in neutrophilic, halophilic, and alkaliphilic bacteria, but less is known about these systems in obligate polyextremophiles. Mesbah et al. (13) investigated 12 monovalent antiporters in the halophilic alkalithermophile Natranaerobius thermophilus draft genome, including eight NhaC type, two CPA1 antiporters, one CPA2, and one novel Nha antiporter. Eight proteins were found to complement the Na+-sensitive phenotype of the E. coli KNabc strain functionally, and the other four were able to complement K+-uptake deficiency of E. coli TK2420.
Halophilic and alkaliphilic bacteria have been isolated from many different environments, including soda lakes, soils, and environments contaminated by human activity (6). As alkaline environments often have a high salt content, especially soda lakes (14), double extremophiles, specifically haloalkaliphilic bacteria, are mainly distributed in saline niches. A wealth of taxonomic and ecological information has been obtained on haloalkaliphiles from soda lakes. However, the adaptations required for growth in these conditions is less clear (4).
Halomonas sp. Y2, which was deposited at the China Center for Type Culture Collection (CCTCC M208188), is a halotolerant and alkaliphilic strain that was isolated from alkaline pulp mill wastewater of high sodium content (15). It grows in broad NaCl concentrations (0–200 g liter−1) and pH range (5.0–11.0), with an optimal NaCl range of 20–30 g liter−1 and an optimal pH of 10.0. The strain exhibits excellent ability in alkaline pulp mill wastewater treatment (pH 11.0). Because of the extreme alkaline environment from which Halomonas sp. Y2 was isolated, transcriptome analysis was conducted to investigate the adaptive mechanism of osmotic and pH homeostasis. Furthermore, all four putative monovalent transporters that were identified from its genome and exhibiting different transcriptional expression patterns were physiologically characterized for their contributions to the survival of Halomonas sp. Y2 in saline and alkaline environments.
Results
Transcriptome Changes in Response to Alkaline Stress
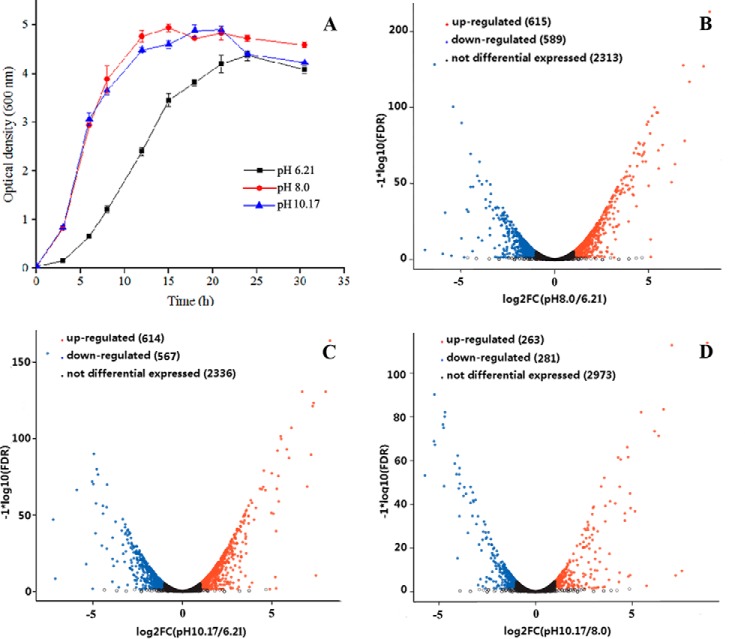
The growth of Halomonas sp. Y2 displayed different growth profiles under acidic or alkaline conditions as shown in Fig. 1A. Compared with growth at alkaline conditions, the growth in acid medium (pH 6.21) was apparently delayed, with a 12-h incubation time to mid-exponential growth phase. To investigate the adaptive mechanism of Halomonas sp. Y2 to alkaline environment, Illumina PE libraries were constructed for transcriptional sequencing, using extracted RNA from these three pH samples, which were collected during mid-exponential growth phase.
FIGURE 1.
Growth curves and volcano plot of differentially expressed consensus sequences of Halomonas sp. Y2. A, growth curves of the strain in LBK medium at three pH values of 6.21, 8.0, and 10.17. B, differentially expressed genes between pH 8.0 and 6.21. C, differentially expressed genes between pH 10.17 and 6.21. D, differentially expressed genes between pH 10.17 and 8.0. The x axis shows the fold-change values between the two compared pH values based on a log2 scale, and the y axis shows the FDR value of differentially expressed genes based on a log10 scale.
Transcriptome sequencing revealed various transcriptional responses to alkaline stress (supplemental Table S1–S3). To account for multiple hypothesis testing, false discovery rate (FDR) q-values <0.005 and |logged fold change (logFC)| ≥1 were used to identify differentially expressed genes (DEGs). As a result, numerous DEGs were identified from bacteria grown under alkaline stress when compared with transcript levels in acidic medium. The volcano diagrams shown in Fig. 1, B–D, indicate that significant transcriptional differences occurred between acidic and alkaline stresses, with ∼1200 DEGs detected when comparing pH 10.17 with 6.21 or pH 8.0 with 6.21. In contrast, when comparing the transcript levels at two alkaline pH values, only 544 DEGs were observed. In total, 782 DEGs were shared between pH 10.17 and 8.0 samples, whereas only ∼380 DEGs were found during both acid and alkaline stresses.
As shown in Table 1, the DEGs to 21 predicated pathways were assigned with analysis based on Clusters of Orthologous Groups of proteins (COG). Upon comparison of DEGs at pH 10.17 with those of 6.21, the genes encoding proteins involved in cell motility, energy production, and conversion and the transport and metabolism of amino acids, lipids, and inorganic ions were predominantly up-regulated in response to alkaline stress. In particular, the up-regulated genes (DUGs) related to amino acid transport and metabolism represented the largest proportion (62 genes, 18.0% of total DUGs) followed by genes encoding energy production and conversion (32 genes, 9.3% of total DUGs). Among the up-regulated genes related to amino acid transport and metabolism, the genes encoding ABC type and branched-chain amino acid transporters showed obviously up-regulation upon alkaline stress. In addition, inorganic ion transport and metabolism-related genes, including many TRAP and ABC type transporting proteins, were up-regulated upon alkaline stress (supplemental Table S1–S3).
TABLE 1.
Number of genes that were differentially expressed in alkaline conditions
The functional categories were assigned with the STRING database (String version 9.0). FDR values less than 0.005 and |logFC| ≥1 were used as the threshold to judge differentially up-regulated genes (DUGs) and differentially down-regulated genes (DDGs).
| Functional category | 8.0 vs. 6.21 |
10.17 vs. 6.21 |
10.17 vs. 8.0 |
|||
|---|---|---|---|---|---|---|
| DDGs | DUGs | DDGs | DUGs | DDGs | DUGs | |
| Chromatin structure and dynamics | 0 | 1 | 0 | 1 | 0 | 0 |
| Translation, ribosomal structure, and biogenesis | 7 | 4 | 50 | 10 | 3 | 6 |
| Transcription | 2 | 22 | 16 | 25 | 6 | 8 |
| Replication, recombination, and repair | 1 | 13 | 17 | 7 | 7 | 6 |
| Cell cycle control, cell division, chromosome partitioning | 0 | 2 | 6 | 0 | 1 | 1 |
| Cell wall/membrane/envelope biogenesis | 3 | 8 | 25 | 11 | 4 | 7 |
| Cell motility | 0 | 24 | 3 | 20 | 12 | 1 |
| Post-translational modification, protein turnover, chaperones | 4 | 27 | 15 | 27 | 10 | 6 |
| Signal transduction mechanisms | 5 | 16 | 9 | 18 | 5 | 2 |
| Intracellular trafficking, secretion, and vesicular transport | 2 | 2 | 8 | 4 | 0 | 3 |
| Defense mechanisms | 2 | 2 | 3 | 3 | 0 | 1 |
| Energy production and conversion | 1 | 37 | 18 | 32 | 22 | 10 |
| Amino acid transport and metabolism | 3 | 50 | 25 | 62 | 14 | 20 |
| Nucleotide transport and metabolism | 4 | 5 | 15 | 3 | 0 | 0 |
| Carbohydrate transport and metabolism | 2 | 16 | 24 | 13 | 14 | 5 |
| Coenzyme transport and metabolism | 4 | 4 | 18 | 5 | 4 | 4 |
| Lipid transport and metabolism | 1 | 25 | 7 | 24 | 9 | 3 |
| Inorganic ion transport and metabolism | 12 | 20 | 33 | 24 | 15 | 20 |
| Secondary metabolites biosynthesis, transport, and catabolism | 4 | 15 | 12 | 11 | 9 | 0 |
| General function prediction only | 5 | 21 | 23 | 20 | 8 | 4 |
| Function unknown | 6 | 26 | 27 | 24 | 12 | 16 |
Transcriptional Analysis of Four Putative Na+/H+ Antiporters
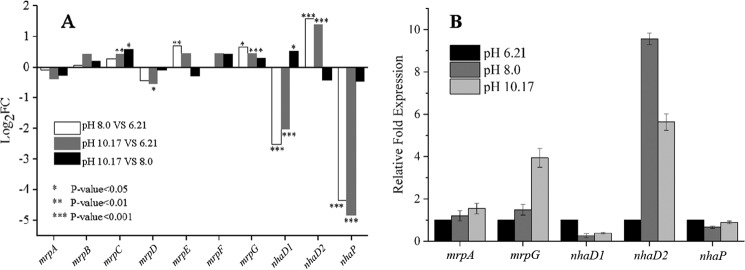
Based on the importance of Na+/H+ antiporters in pH homeostasis and Na+ enrichment in alkaline pulp mill wastewater, from which Halomonas sp. Y2 was isolated, four putative Na+/H+ antiporters identified in the draft genome were further investigated. These antiporters designated as Ha-NhaD1, Ha-NhaD2, Ha-NhaP, and Ha-Mrp exhibited different transcriptional profiles in response to alkaline stress (Fig. 2A). Using fragments per kilobase per million reads (FPKM) and log2FC as the criteria for transcriptional differences, the transcript levels of four antiporters under acidic and alkaline conditions were compared. As the Ha-mrp gene encodes a seven-subunit complex, the transcript levels of these seven subunit-encoding genes were analyzed separately. Among these subunits, mrpA and mrpD are both large subunits, whereas the other five are much smaller. These seven subunit-encoding genes displayed two different transcriptional responses to alkaline stress; however, there was no significant difference among the three pH samples, with a p value generally higher than 0.05 (Fig. 2A). Among three pH values, the five small subunits B, C, and E–G, exhibited the highest transcript levels at pH 10.17. However, both mrpA and mrpD subunits showed a small decrease at higher pH values.
FIGURE 2.
Differential expression of four Na+/H+ antiporter encoding genes in Halomonas sp. Y2. A, expressions in LBK medium of pH 6.21, 8.0, and 10.17 determined by transcriptome analysis. B, expressions in LBK medium of pH 6.21, 8.0, and 10.17 determined by RT-PCR. The 2−ΔΔCT values were calculated from quadruplicate runs of two independent experiments.
The transcript levels of both Ha-nhaD1 and Ha-nhaP exhibited a surprisingly high response to acid stress (pH 6.21). In comparison with other genes, Ha-nhaD1 had a markedly high FPKM value of 391.6 at pH 6.21, with more than a 10- and 5-fold down-regulation at pH 8.0 and 10.17, respectively (data not shown). The normalized log2FC values of pH 8.0 versus 6.21 and pH 10.17 versus 6.21 were −2.5 and −2.0, respectively (Fig. 2A). Similarly, Ha-nhaP was significantly down-regulated in response to alkaline conditions of pH 8.0 and 10.17. In contrast, Ha-nhaD2 showed obvious up-regulation when comparing alkaline environments to acidic stress. As shown in Fig. 2A, the lowest transcript level was at pH 6.21, whereas a higher level was detected under alkaline stress.
To verify the pH responses of these four putative antiporters, transcript levels were further confirmed by real time quantitative reverse transcription-PCR (RT-PCR) analysis, with the 16S rRNA gene used as an internal control; the reaction buffer without template DNA was used as a negative control. The relative expressions of the tested genes in the samples were calculated using the 2−ΔΔCT method, by using the average expression levels at pH 6.21 as 1. In some species of Bacillus pseudofirmus OF4 and B. subtilis, mrpA is an essential subunit for the transport activity of Mrp. Therefore, mrpA was used to investigate the transcript expression of Ha-mrp at different pH values; in addition, the small subunit mrpG gene was also used for comparison. In agreement with transcriptome results, these four antiporters presented differential transcription in response to alkaline stress (Fig. 2B). The transcript levels of mrpA varied marginally between acidic and alkaline conditions, whereas mrpG levels increased upon transition from acidic to alkaline stress. It is notable that Ha-nhaD2 showed a significant up-regulation at both tested alkaline pH values, with a 9- and 5.6-fold increase at pH 8.0 and 10.17, respectively. In agreement with the acid-induced up-regulation of Ha-nhaD1 observed in transcriptome analysis, the highest expression of Ha-nhaD1 was also observed at pH 6.21 by RT-PCR. Regarding Ha-nhaP levels, variations at the three tested pH values were modest, with somewhat reduced expression upon alkaline stress.
Sequence Analysis of Four Putative Na+/H+ Antiporters
Among these four antiporters, BLASTP analysis suggested that Ha-NhaD1 and Ha-NhaD2 were closely related with a 72% amino acid sequence identity. They are both homologues of the NhaD antiporters from Alkalimonas amylolytica and Vibrio parahemeolyticus, with ∼74 and 63% sequence identity, respectively (16, 17). Another putative Na+/H+ antiporter was annotated as unknown but showed ∼43% sequence identity to the K+ (Na+)/H+ NhaP-type antiporters from V. parahemeolyticus and Vibrio cholerae (18, 19) and ∼31% identity to those Na+/H+ antiporters of moderately halophilic bacteria, Halobacillus dabanensis D-8T and Halobacillus aidingensis AD-6T (20, 21). The predicted seven subunits of the mrp operon were phylogenetically related to the recently reported Mrp protein from Halomonas zhaodongensis, with ∼85% identity (22). In addition, Ha-Mrp was closely related to Mrp from B. pseudofirmus OF4, with 37% identity when comparing MrpA sequences and 48% identity with MrpD sequences (23). In addition, MrpA and MrpD had a 35 and 48% sequence identity with corresponding proteins from B. subtilis (8).
Complementation Assay in E. coli KNabc and TK2420
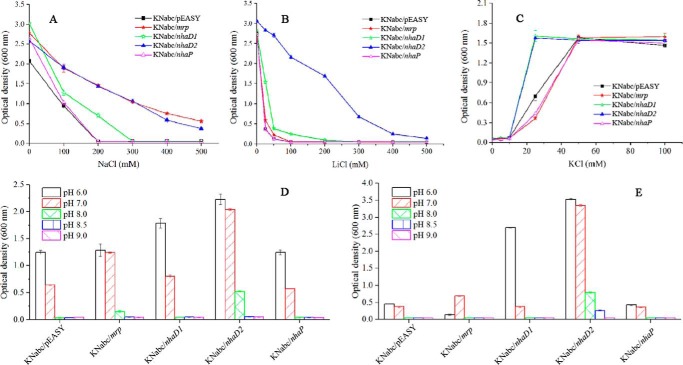
To confirm the functions of four putative Na+/H+ antiporters in cation transport, heterologous expression was carried out in the Na+(K+)/H+ antiporter-deficient E. coli KNabc (ΔnhaA, ΔnhaB, and ΔchaA). Complementary growth of E. coli KNabc recombinants were tested in LBK medium with different NaCl and LiCl concentrations. Recombinant E. coli KNabc harboring only the vector (pEASY-blunt) was used as a negative control for comparison. As shown in Fig. 3A, KNabc/pEASY control strain showed no growth in the presence of 200 mm NaCl. However, the expression of Ha-mrp and Ha-nhaD2 in E. coli KNabc conferred significant Na+ tolerance to this mutant, with apparent growth in the presence of 500 mm NaCl. In contrast, these two antiporters displayed different complementary abilities for Li+ tolerance. As shown in Fig. 3B, Ha-NhaD2 conferred significant growth tolerance, whereas Ha-Mrp only exhibited very weak growth at concentrations of Li+ below 50 mm. In contrast to the robust complementation of Ha-NhaD2, Ha-NhaD1 exhibited very weak growth complementation under Na+ or Li+ pressures at pH 7.0. Regarding KNabc/nhaP, no difference was observed between the growth of KNabc/nhaP and the control at any of the tested Na+ and Li+ concentrations.
FIGURE 3.
Growth of E. coli KNabc or E. coli TK2420 recombinants at different concentrations of NaCl, KCl, and LiCl or different pH values. A, growth of E. coli KNabc recombinants in LBK medium at various NaCl concentrations at pH 7.0. B, growth of E. coli KNabc recombinants in LBK medium at various LiCl concentrations at pH 7.0. C, growth of E. coli TK2420 recombinants in MM at various KCl concentrations at pH 7.0. D, E. coli KNabc transformants in the presence of 200 mm NaCl at different pH values. E, E. coli KNabc transformants in the presence of 20 mm LiCl (for KNabc/pEASY, KNabc/mrp, and KNabc/nhaP) or 50 mm LiCl (for KNabc/nhaD1 and KNabc/nhaD2) at different pH values.
To test K+ transport abilities, these four antiporters were also expressed in the K+ uptake-deficient E. coli mutant TK2420 (Δkdp, Δkup, and Δtrk). In comparison with the control E. coli TK2420 transformant (carrying only an empty vector), notable complementation was observed in TK2420/nhaD2 and especially TK2420/nhaD1 clones. As shown in Fig. 3C, significantly enhanced growth was detected in the presence of K+ at concentrations lower than 30 mm. In contrast, the expression of Ha-mrp and Ha-nhaP obviously exacerbated the requirement for high levels of K+, as growth was weak when compared with that of the empty vector control at low K+ concentrations (Fig. 3C). Such growth differences imply that Ha-Mrp and Ha-NhaP possess K+ exporting abilities, whereas the other two proteins contribute to K+ import, as the K+ efflux ability observed in the KefB from A. amylolytica (24).
To investigate Na+ resistance mediated by these antiporters, growth complementation was tested in Na+-enriched LBK medium (200 mm Na+). As expected, growth inhibition by Na+ was more pronounced as the pH increased. As shown in Fig. 3D, KNabc/pEASY (carrying an empty vector) showed weak growth at pH 7.0, and KNabc/nhaP exhibited a similar growth profile. KNabc/nhaD2 showed modest growth complementation from pH 6.0 to 8.0, whereas KNabc/nhaD1 displayed remarkable enhanced growth at pH 6.0; no complementation was detected at other tested pH conditions. In contrast, Ha-Mrp only conferred Na+ resistance under neutral or alkaline stress, as no growth complementation was detected at pH 6.0.
Based on the result shown in Fig. 3B, 50 mm Li+ was used to investigate the alkaline resistance of KNabc/nhaD1 and KNabc/nhaD2 in LBK medium at different pH values. Based on the weak Li+ exporting ability of Ha-Mrp and Ha-NhaP, a lower concentration (20 mm Li+) was used for KNabc/mrp, KNabc/nhaP, and KNabc/pEASY for growth at different pH values. Li+ growth complementation was also observed to be pH-dependent in the KNabc/nhaD1 assays, with this strain exhibiting a growth preference of acidic stress (Fig. 3E). Compared with Ha-NhaD1, Ha-NhaD2 strongly impaired the growth of E. coli KNabc at a wide range of pH values (from pH 6.0 to 8.5). In agreement with the poor complementation ability of Ha-Mrp for Li+ resistance, only weak complementation was observed in neutral medium of KNabc/mrp.
Antiport Activity in Everted Membrane Vesicles of E. coli KNabc
A Na+ (Li+)(K+)/H+ antiport activity assay was then performed using everted membrane vesicles isolated from E. coli KNabc carrying one of the four antiporters of interest. Vesicles isolated from E. coli KNabc/pEASY were used as a negative control, whereas KNabc/nhaA from E. coli was used as positive control. As expected, no activity was observed in KNabc/pEASY, whereas high activity was detected in KNabc/nhaA (66.9 and 82.3% dequenchings for 10 mm Na+ and Li+ at pH 8.0, respectively). As shown in Fig. 4, despite the phenotypic differences between Ha-NhaD1 and Ha-NhaD2 in terms of Na+ and Li+ resistance in E. coli KNabc, these antiporters both exhibited similar and strong Na+ and Li+ transporting abilities at pH 8.0 but were devoid of K+ transport activity. Ha-Mrp showed relatively lower Na+ and Li+ transport activities compared with those of Ha-NhaD1 and Ha-NhaD2. In addition, weak K+ transport activity was observed in Ha-Mrp vesicles at pH 8.0. In accordance with growth complementation results, no dequenching signal was detected in vesicles of KNabc/nhaP after the addition of 10 mm Na+ or Li+, and minor K+ export activity was detected. Such K+ transport activity was much lower than that of its reported homologues, such as Vc-NhaP2 from V. cholerae and Aa-NhaP from A. amylolytica (19, 24).
FIGURE 4.

Antiport activities of antiporters in sub-bacterial vesicles. Fluorescence-based assays were monitored in a buffer containing 10 mm Tris-MES (pH 8.0), 140 mm choline chloride, 5 mm MgCl2, 1 μm acridine orange, and 60 μg of vesicle protein were used in the assay. At the onset of the experiment, 5 mm Tris-l-lactate was added, and the resulting fluorescence quenching was recorded. The antiporter activity was measured from the dequenching of fluorescence upon the subsequent addition of 10 mm Na+, K+, or Li+. KNabc/pEASY carrying the empty vector was used as negative control, and KNabc/nhaA harboring Ec-NhaA was used as positive control. AU, arbitrary units.
Contribution of Na+/H+ Antiporters to the Survival of Halomonas sp. Y2 in Acidic or Alkaline Environments
Obvious differences among the four Na+/H+ antiporters based on transcriptional analyses and transport activity prompted us to further investigate physiological roles of these antiporters in the native membrane. We constructed deletion mutants and compared their growth in LB medium with indicated Na+, Li+, or K+ concentrations and at three pH values as shown in Table 2.
TABLE 2.
Growth of Halomonas sp. Y2 and antiporter mutants in LB-based medium with different cations and pH values (A600 nm)
As no growth was observed of Halomonas sp. Y2 when growing in the presence of 15% NaCl at pH 6.2, 10% NaCl was supplemented into the pH 6.2 medium.
| Strain | Y2 | ΔHa-mrp | ΔHa-nhaD1 | ΔHa-nhaD2 | ΔHa-nhaP | ||
|---|---|---|---|---|---|---|---|
| NaCl | pH 6.2 | 2% | 4.07 | 4.02 | 4.15 | 4.11 | 4.16 |
| 10% | 4.01 | 4.06 | 3.91 | 2.10 | 4.09 | ||
| pH 8.0 | 2% | 4.53 | 4.67 | 4.69 | 4.73 | 4.54 | |
| 15% | 2.81 | 0.21 | 2.83 | 2.36 | 2.91 | ||
| pH 10.0 | 2% | 4.33 | 3.19 | 4.18 | 4.27 | 4.24 | |
| 15% | 0.09 | 1.12 | 0.71 | 2.22 | |||
| LiCl | pH 6.2 | 2% | 0.99 | 0.89 | 0.92 | 0.97 | 0.92 |
| 6% | 0.92 | 0.93 | 0.89 | 0.86 | 0.89 | ||
| pH 8.0 | 2% | 3.48 | 3.05 | 3.55 | 3.57 | 3.32 | |
| 6% | 0.93 | 0.071 | 0.95 | 0.76 | 0.89 | ||
| pH 10.0 | 2% | 0.09 | 0.1 | 0.09 | 0.09 | 0.12 | |
| 6% | 0.09 | 0.11 | 0.09 | 0.12 | 0.09 | ||
| KCl | pH 6.2 | 2% | 1.05 | 0.99 | 1.02 | 1.12 | 0.21 |
| 8% | 0.26 | 0.26 | 0.27 | 0.25 | 0.19 | ||
| pH 8.0 | 2% | 3.5 | 3.67 | 3.61 | 3.71 | 1.54 | |
| 8% | 0.11 | 0.08 | 0.12 | 0.11 | 0.21 | ||
| pH 10.0 | 2% | 4.25 | 1.77 | 4.27 | 4.41 | 3.99 | |
| 8% | 4.48 | 0.05 | 4.31 | 4.26 | 1.12 | ||
ΔHa-mrp was more sensitive to high pH environments when in the presence of 15% NaCl; the significant growth inhibition was observed at pH 8.0 and complete imbibition at pH 10.0. In contrast, at high NaCl concentrations the deletion of Ha-nhaD2 resulted in strong growth inhibition at the three pH values tested, especially at pH 10.0. Conversely, the cation sensitivity of strain ΔHa-nhaD1 was lower than that of ΔHa-nhaD2, which is in agreement with its weak complementation ability for E. coli KNabc.
The four mutants at different pH values exhibited much weaker sensitivity to Li+ than for Na+. As shown in Table 2, only the deletion of Ha-mrp resulted in significant growth inhibition in the presence of 6% LiCl at pH 8.0, although slight inhibition was observed in the ΔHa-nhaD2 mutant. Growth at pH 10.0 could not be assessed because Halomonas sp. Y2 was repressed by the presence of Li+; no growth was observed even in the presence of 0.1% LiCl (data not shown).
In the presence of 2 and 8% KCl, Halomonas sp. Y2 displayed an interesting growth profile at the three pH values. Much better growth was observed at pH 10.0. In agreement with the K+ complementation study, the absence of Ha-Mrp and Ha-NhaP resulted in obvious K+ sensitivity in Halomonas sp. Y2. As shown in Table 2, the K+ sensitivity of the mrp-disrupted strain was detected only at pH 10.0, implying that K+ export by Ha-Mrp is also dependent on alkaline pH. In contrast to Ha-Mrp, the deletion of Ha-nhaP inhibited the growth at all three pH values and with significant growth inhibition observed in the presence of 2% KCl at pH 6.2. Coinciding with an observed absence of K+/H+ exporting activity for Ha-NhaD1 and Ha-NhaD2, both ΔHa-nhaD1 and ΔHa-nhaD2 mutants exhibited similar growth profiles at all three tested pH values.
Discussion
Soda lakes and soda deserts represent the most stable and naturally occurring alkaline environments found worldwide (25). Different from these natural saline/alkaline environments, pulp mill wastewater generated from NaOH extraction of wheat straw is a special saline and alkaline environment derived from human activity and possessing a very high chemical oxygen demand value. Compared with the soda environments, the wastewater has some similar properties such as high pH (pH >11.0) and high Na+ content (20 g liter−1). However, it is a unique extreme environment with a very high pollution load and more stressful conditions for living organisms (26). The halotolerant and alkaliphilic Halomonas sp. Y2 isolate was dominant in this harsh environment (27) and attracted our attention for its superior adaptive abilities. As expected, Halomonas sp. Y2 displayed significant transcriptional differences when comparing growth during acidic and alkaline stresses. Based on the transcriptional analyses, Halomonas sp. Y2 was supposed to develop some special mechanism to deal with the alkaline environment, especially expressions of many other transport systems during adaptation to alkaline environments. Based on genomic analysis of Oceanobacillus and Bacillus strains, these transport systems were also proposed to be important for growth in alkaline environments (28–30).
It is well established that growth of extremely alkaliphilic Bacillus species at high pH is dependent on the presence of Na+ and Na+/H+ antiporters. Such transporters have an essential and even dominant role in pH homeostasis in many bacteria (6). Sodium enrichment and high alkaline conditions of the pulp mill wastewater prompted us to address the transcriptional response and physiological functions of four putative Na+/H+ antiporters in Halomonas sp. Y2. These four antiporters displayed differential and sometimes opposite transcriptional profiles upon acidic and alkaline stresses, which was further confirmed by RT-PCR. At the transcript level, the up-regulation of each Ha-mrp subunit was modest, and even minor down-regulation was detected. In combination with the modest cation transport activities in E. coli KNabc, we first hypothesized that Mrp was not essential for pH homeostasis in the wild type strain. Unexpectedly, in the presence of high concentrations of Na+, Li+, or K+, deletion of mrpF significantly inhibited growth in conditions of high alkalinity, demonstrating that this system has indispensable roles in Na+-, Li+-, and K+-dependent pH homeostasis. In some extremely alkaliphilic Bacillus sp., the unusual hetero-oligomeric Mrp antiporter was found to be indispensable for survival in alkaline environments (5, 6). In contrast, the dominant antiporter for pH homeostasis in neutrophilic B. subtilis is TetL but not Mrp. Instead, the Mrp system has a dominant role in Na+ resistance in this organism (31). The central role of Ha-Mrp for pH homeostasis is similar to Mrp antiporters in other alkaliphiles (32, 33). However, Ha-Mrp exhibited a different cation export profile; other Mrp systems from alkaliphiles function as an electrogenic Na+(Li+)/H+ antiporter with no detectable K+/H+ antiport ability. Differently, by catalyzing Na+, Li+, and K+ efflux for exchanging protons, Ha-Mrp exhibited a similar substrate spectrum to the Mrp system of B. subtilis and the recently reported Hz-Mrp from H. zhaodongensis (8, 22). We suspected that these combined properties mediate the ability to survive in environments with high salinity and alkalinity in Halomonas sp. Y2.
In contrast to the toxicity conferred by Na+ and Li+, it is accepted that K+ is essential for bacterial growth. However, it has also been demonstrated that excess K+ is detrimental to organisms (34). Consequently, K+ efflux systems are essential for cell growth under conditions of high extracellular K+ pressure (18). Halomonas sp. Y2 exhibited a remarkable and interesting pH-dependent K+ efflux activity. In the presence of high concentrations of K+, better growth was observed under high alkalinity. The deletion of Ha-mrp significantly inhibited growth under alkaline stress at pH 10.0, suggesting that Ha-Mrp also has an essential role in K+ resistance during pH homeostasis. Ha-NhaP exhibited a relatively weak effect but possessed a broader pH profile, in preventing excessive accumulation of potassium in the cytoplasm. This result was different from the physiological role of Vc-NhaP2 (from V. cholerae), which has a protective effect against K+ in acidic but not in alkaline conditions (19).
The NhaD-type antiporter is widely distributed in many organisms. However, to our knowledge, its transport abilities have so far only been verified in very few halophilic or haloalkaliphilic Proteobacteria. In this study, among the four putative Na+/H+ antiporters, the gene encoding Ha-NhaD2 was of particular interest as it was up-regulated during alkaline stress, based on transcriptome and RT-PCR analysis. Furthermore, heterologous expression demonstrated that Ha-NhaD2 exhibits high Na+(Li+)/H+ antiport activities. Kurz et al. (35) have proposed that NhaD-type antiporters have a unique role in conferring resistance to high salinity. Combined with the high cation sensitivity of ΔHa-nhaD2 mutant in the presence of high salinity, Ha-NhaD2 in Halomonas sp. Y2 was further evidence for the functions of NhaD-type antiporters in resistance to high salinity. As a homologue of Ha-NhaD2, Ha-NhaD1 exhibited a very similar and high dequenching activity to that of Ha-NhaD2 in the E. coli membrane. Conversely, its ability to confer cation resistance and pH homeostasis to E. coli KNabc and wild type Halomonas sp. Y2 was minimal. As these two NhaD homologues locate or are heterologously expressed in the same strain, but display different in vivo responses to the environmental stress, we suspect that lower expression levels, lower protein stability, or higher hydrophobicity might be explained by sequence variations in Ha-NhaD1.
In summary, as a special double extremophile isolated from alkaline pulp mill wastewater, the halotolerant and alkaliphilic strain Halomonas sp. Y2 may have developed some special strategies in coupling with harsh environments. Especially, as shown in Fig. 5, the four investigated Na+(K+)/H+ antiporters play important but different physiological roles in cation resistance and pH homeostasis. Most bacterial genomes have multiple genes and operons predicted to encode Na+(K+)/H+ antiporters (9). We propose that the reason for this could be due to different functions in coupling various environmental stresses, such as the case for the four antiporters in Halomonas sp. Y2. Some transporters were active in monovalent resistance during high pH conditions, whereas others were activated upon acidic stress by exporting different monovalent cations.
FIGURE 5.
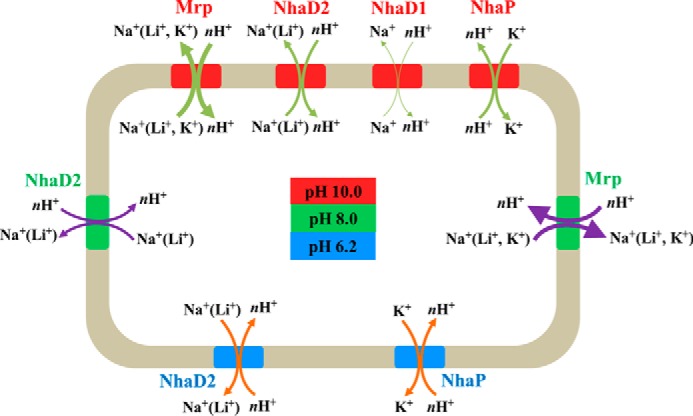
Schematic diagram of Na+(K+)/H+ antiporters in Halomonas sp. Y2 in response to acid and alkaline stresses. Blue represents the environmental stress and functional antiporters of pH 6.2. Green represents the environmental stress and functional antiporters of pH 8.0. Red represents the environmental stress and functional antiporters of pH 10.0. The line thickness of arrows represents the ability of antiporters in monovalent cations transport.
Experimental Procedures
Bacterial Strains, Plasmids, and Growth Conditions
The bacterial strains and plasmids used in this study are listed in Table 3. Halomonas sp. Y2 was isolated from alkaline pulp mill wastewater previously (15). To assess the function of the putative Na+/H+ antiporters predicted from Halomonas sp. Y2, heterologous expression was carried out in strain E. coli KNabc, an E. coli strain in which the genes encoding the specific Na+/H+ antiporters, Ec-nhaA and Ec-nhaB, and the nonspecific Ec-chaA had been inactivated. The triple antiporter-deficient strain is sensitive to 200 mm NaCl and pH values above 8.0 at 37 °C (36). It was used as a host for the complementary assay and membrane vesicle preparation. The K+ uptake-deficient E. coli mutant TK2420 (Δkdp, Δkup, and Δtrk) was also used to test the K+ transport ability.
TABLE 3.
Bacterial strains and plasmids
| Strain or plasmid | Relevant phenotype or genotype | Ref. |
|---|---|---|
| Strain | ||
| Halomonas sp. Y2 | Halotolerant alkaliphilic strain | 15 |
| E. coli DH5α | Competent cell for cloning | |
| E. coli KNabc | ΔnhaA, ΔnhaB, and ΔchaA | 42 |
| E. coli TK2420 | Δkdp, Δkup, and Δtrk | 43 |
| E. coli S17–1 | Competent cell for gene knockout | |
| Plasmid | ||
| pEASYBlunt | Cloning vector | TransGen Biotech |
| pEASYBlunt-nhaD1 | pEASYBlunt with Ha-nhaD1 | This study |
| pEASYBlunt-nhaD2 | pEASYBlunt with Ha-nhaD2 | This study |
| pEASYBlunt-nhaP | pEASYBlunt with Ha-nhaP | This study |
| pEASYBlunt-mrp | pEASYBlunt with Ha-mrp | This study |
| pK18mobSacB | Kmr mobsacB | |
| pK18mobSacB-ΔnhaD1 | pK18mobSacB with ΔHa-nhaD1 | This study |
| pK18mobSacB-ΔnhaD2 | pK18mobSacB with ΔHa-nhaD2 | This study |
| pK18mobSacB-ΔnhaP | pK18mobSacB with ΔHa-nhaP | This study |
| pK18mobSacB-Δmrp | pK18mobSacB with ΔHa-mrp | This study |
| pBBR1MCS-5 | Carrier for gene anaplerosis | |
| pBBR1MCS-5-nhaD1 | pBBR1MCS-5 with Ha-nhaD1 | This study |
| pBBR1MCS-5-nhaD2 | pBBR1MCS-5 with Ha-nhaD2 | This study |
| pBBR1MCS-5-nhaP | pBBR1MCS-5 with Ha-nhaP | This study |
| pBBR1MCS-5-mrp | pBBR1MCS-5 with Ha-mrp | This study |
Growth Curves of Halomonas sp. Y2 under Acid or Alkaline Stress
Halomonas sp. Y2 was cultured overnight in LBK medium with 2% NaCl, adjusted to pH 8.0. Bacteria were diluted 100-fold in pH 6.21 (50 mm KH2PO4-NaOH buffered), pH 8.0 (50 mm Tris-HCl buffered), and pH 10.17 (50 mm NaHCO3-NaOH) in LBK medium with 2% NaCl addition and cultured at 37 °C by shaking at 180 rpm. Cultures were sampled at intervals for absorbance (A600 nm) measurement.
RNA Preparation and Transcriptome Response of Halomonas sp. Y2 to Alkaline Stress
Halomonas sp. Y2 was cultured to its late exponential phase in LBK medium (pH 6.21, 8.0, and 10.17) and used for RNA extraction. Total RNA was extracted utilizing TRIzol® reagent according to the manufacturer's instructions (Invitrogen), and genomic DNA was removed using DNase I (TaKaRa). RNA quality was determined by 2100 BioAnalyzer (Agilent) and quantified using the ND-2000 (NanoDrop Technologies). High quality RNA samples (A260/280 nm = 1.8–2.0 and A260/230 nm ≥2.0) was used for illumina sequencing.
For illumina HiSeq2500 sequencing, RNA samples were sent to Majorbio (Shanghai, China), and at least 5 μg of total RNA was used for Illumina PE library construction (200–300 bp). The paired end raw reads were trimmed and quality-controlled by Seq PreP and Sickle using default parameters. Then clean reads were separately aligned to the draft genome of Halomonas sp. Y2 with orientation mode using bowtie2 software (37), and mapping criteria of bowtie were used as follows: sequencing reads should be uniquely matched to the genome allowing up to two mismatches, without insertions or deletions. RSEM was used to quantify gene and isoform abundances (38). The DEGs between the three pH values samples were identified using EdgeR (39). The trimmed mean of M-values method was selected to compute normalization factors and DEGs between two samples. Normalized FPKM and log2FC were used to present the expression difference of proteins under acid or alkaline stress. FDR value less than 0.005 and |logFC| ≥1 were used as the threshold to judge the significance of gene expression difference. Deduced proteins with homologues in other organisms were used to determine the clusters of COG item; the COG functional categories were assigned with the STRING database (String version 9.0).
RT-PCR Analysis
RNA of Halomonas sp. Y2 was extracted and purified as described under “Transcriptional Analysis of Four Putative Na+/H+ Antiporters.” For each growth condition, three independent biological replicate cultures were tested. First strand cDNA synthesis was performed with 100 ng of total RNA in a reaction mixture of 20 μl using a 5× All-In-One RT MasterMix with AccuRT genomic DNA removal kit (ABM). Quantitative PCR was carried out in a MyiQTM2 iCycler (two-color real-time PCR detection system) (Bio-Rad) with a Real Master mix kit (SYBR Green, Tiangen). Primers concerned in this part were designed with the Beacon Designer software and are shown in supplemental Table S4. Data were analyzed by the 2−ΔΔCT method, and 16S rRNA gene from Halomonas sp. Y2 was used as a reference gene in the process. RT-PCR assay was done at least four times for each sample.
DNA Extraction, Cloning, and Plasmids
Genomic DNA of Halomonas sp. Y2 was extracted using the bacterial genomic DNA extraction kit (Promega). The antiporter encoding genes (Ha-mrp, Ha-nhaD1, Ha-nhaD2, and Ha-nhaP) were obtained by PCR using the genomic DNA of Halomonas sp. Y2 as a template. Phusion high-fidelity DNA polymerase (New England Biolabs) and primers listed in supplemental Table S4 were used for cloning by following the proposed protocols.
Four recombinants in E. coli KNabc were designated as KNabc/mrp, KNabc/nhaD1, KNabc/nhaD2, and KNabc/nhaP; recombinants in E. coli TK2420 were designated as TK2420/mrp, TK2420/nhaD1, TK2420/nhaD2, and TK2420/nhaP, respectively. Strain E. coli DH5α, E. coli KNabc, E. coli TK2420, Halomonas sp. Y2, and corresponding recombinants were routinely cultured at 37 °C in LBK medium in which Na+ was replaced by 87 mm K+ (pH 7.0), with 100 μg ml−1 ampicillin addition if needed (36).
Complementation Assays of Transformants of E. coli KNabc or TK2420
Complementation assay with E. coli KNabc and TK2420 transformants were carried out in LBK liquid medium as described below. To investigate the Na+ resistance of four E. coli KNabc recombinants, LBK with defined NaCl concentrations (0, 100, 200, 300, 400, and 500 mm) were used for growth tests. For K+ complementary assay, growth of E. coli TK2420 transformants was measured in minimal medium (MM) with different K+ concentrations (0, 10, 15, 20, 25, 30, 35, 40, and 50 mm) at pH 7.0. The MM was prepared following the method described by Epstein and Kim (40). For Li+ resistance analysis, experiments were also performed in LBK medium with supplemented LiCl (0, 5, 10, 50, 100, 150, 200, and 300 mm). To investigate pH adaptation, LBK medium with 200 mm Na+ was used for Na+ complementation, as well as the supplementation of 20 or 50 mm Li+ for complementation at defined pH conditions. Tris-HCl buffer (50 mm) was used for the adjustment to the indicated pH values of 6.0, 7.0, 8.0, 8.5, and 9.0. Recombinant carrying empty vector pEASY-blunt in E. coli KNabc was used as a control.
Everted Membrane Vesicle Preparation and ΔpH-dependent Antiport Activity Measurement
Transformants of E. coli KNabc were grown in LBK medium and incubated to 0.6 (A600 nm) at 37 °C, and then 0.5 mm isopropyl 1-thio-β-d-galactopyranoside (final concentration) was added and cultured to stationary phase. Everted membrane vesicle was prepared as described previously with slight modifications (41). In brief, the harvested cells were washed three times with 10 mm Tris-HCl buffer (pH 7.6), containing 140 mm choline chloride, 0.5 mm dithiothreitol, 5 mm MgCl2, and 10% glycerol, and then suspended into the same buffer with 10 μg ml−1 DNase I and 0.1 mm phenylmethylsulfonyl fluoride addition. The bacterial suspension was then passed through a French press (Aminco). Unbroken cells were removed by centrifugations at 7000 × g for 15 min. The membrane fraction was collected by ultracentrifugation at 184,000 × g for 90 min at 4 °C and then suspended in fresh buffer. The concentration of extracted protein was measured by Bradford reagent using bovine serum albumin (BSA) as a standard. The resulting proteins were stored at −70 °C.
The monocation antiporter activity was measured by acridine orange fluorescence dequenching (36). Cation addition (Na+, K+, and Li+) results in dequenching of fluorescence that reflects cation-dependent proton movement out of the inverted membrane vesicles. At the end of the assay, 25 mm ammonium chloride was added to dissipate the remaining protonmotive force (PMF) and bring fluorescence back to baseline. In brief, 2 ml of assay mixture containing 10 mm Tris-MES (pH 7.0–9.0), 140 mm choline chloride, 5 mm MgCl2, 1 μm acridine orange, and 60 μg of vesicle protein were used in the assay. Respiration-dependent formation of the transmembrane pH gradient was initiated by the addition of 5 mm Tris-l-lactate, and the resulting quenching of acridine orange fluorescence was monitored using an F-4600 spectrofluorophotometer with excitation at 480 nm (5-nm slit) and emission at 525 nm (5-nm slit). The antiporter activity was measured from the dequenching of fluorescence upon the subsequent addition of 10 mm Na+, K+, or Li+. The percent dequenching was calculated relative to the initial respiration-dependent quench (17).
Construction of In-frame Deletion Mutants and Complementation Strains
For gene knock-out, DNA sequences upstream and downstream from the desired genes were joined by recombinant PCR that contained three PCRs to form the defective gene fragments. All the primers used for gene knock-out and complementation are listed in supplemental Table S4. The final PCR fragments were ligated into the shuttle vector pK18mobsacB and transferred into strain E. coli S17-1. The 489-, 493-, 575-, and 270-bp (the subunit of mrpF) fragments in Ha-nhaD1, Ha-nhaD2, Ha-nhaP, and Ha-mrp, respectively, were in-frame deleted. To promote the homology recombination, the recombinant E. coli S17-1 strains that contained pK18mobsacB with four deletion fragments were cultivated with Halomonas sp. Y2. Then the recombinant strains were selected by LB medium with kanamycin (50 μg ml−1) and ampicillin (100 μg ml−1). Finally, the resultant mutants were selected by LB medium with 20% sucrose at 37 °C.
The intact DNA sequences with the shuttle vector pBBRAMCS-5 were transferred into the deletion strains by the same method as described for the gene knockouts. The resultant complementary mutants were selected by LB medium with gentamicin (50 μg ml−1) and ampicillin (100 μg ml−1). All mutants were verified by PCR and DNA sequencing.
Growth of Deletion and Complementary Mutants under Different Saline and Alkaline Stress
To test the sensitivity of constructed mutants to alkalinity, these constructed mutants and their parental strain Halomonas sp. Y2 were cultured in LB-based medium at three different pH values (6.2, 8.0, and 10.0). Various concentrations of Na+, Li+, and K+ were supplemented as indicated in Table 2. After 18 h of incubation at 37 °C, the optical density (A600 nm) was measured. As for the growth of complementary strains, growth under the same conditions was tested.
Nucleotide Sequence Accession Numbers
The draft genome sequence of Halomonas sp. Y2 and the RNA sequencing data have been deposited in NCBI database under accession numbers SRP073747 (DNA sequence) and SRP075842 (RNA sequence). The nucleotide sequences encoding Ha-NhaD1, Ha-NhaD2, Ha-NhaP, and seven subunits encoding genes of Ha-Mrp from Halomonas sp. Y2 have been deposited into the GenBankTM nucleotide sequence database under the accession numbers from KT995460 to KT995469.
Author Contributions
C. Y. Y. and F. T. designed the experiments and analyzed the data. C. Y. Y. and P. X. prepared the manuscript. B. C., Y. W. M., Y. B. C., C. F. L., and H. J. Y. conducted the experiments.
Supplementary Material
Acknowledgments
We thank Dr. T. A. Krulwich (Icahn School of Medicine at Mount Sinai, New York) and Prof. S. S. Yang (China Agricultural University) for kindly providing E. coli TK2420 and E. coli KNabc.
This work was supported by National Natural Science Foundation of China Grant 31370153. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Tables S1–S4.
- CPA
- cation/proton antiporters
- DEG
- differentially expressed gene
- COG
- clusters of orthologous groups of protein
- FPKM
- fragments per kilobase per million reads
- FDR
- false discovery rate
- FC
- fold change.
References
- 1. Chanroj S., Wang G., Venema K., Zhang M. W., Delwiche C. F., and Sze H. (2012) Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front. Plant Sci. 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stolyar S., He Q., Joachimiak M. P., He Z., Yang Z. K., Borglin S. E., Joyner D. C., Huang K., Alm E., Hazen T. C., Zhou J., Wall J. D., Arkin A. P., and Stahl D. A. (2007) Response of Desulfovibrio vulgaris to alkaline stress. J. Bacteriol. 189, 8944–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilks J. C., Kitko R. D., Cleeton S. H., Lee G. E., Ugwu C. S., Jones B. D., BonDurant S. S., and Slonczewski J. L. (2009) Acid and base stress and transcriptomic responses in Bacillus subtilis. Appl. Environ. Microbiol. 75, 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banciu H. L., and Muntyan M. S. (2015) Adaptive strategies in the double-extremophilic prokaryotes inhabiting soda lakes. Curr. Opin. Microbiol. 25, 73–79 [DOI] [PubMed] [Google Scholar]
- 5. Slonczewski J. L., Fujisawa M., Dopson M., and Krulwich T. A. (2009) Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 55, 1–79 [DOI] [PubMed] [Google Scholar]
- 6. Padan E., Bibi E., Ito M., and Krulwich T. A. (2005) Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta 1717, 67–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krulwich T. A., Sachs G., and Padan E. (2011) Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 9, 330–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito M., Guffanti A. A., Oudega B., and Krulwich T. A. (1999) Mrp, a multigene, multifunctional locus in Bacillus subtilis with roles in resistance to cholate and to Na+ and in pH homeostasis. J. Bacteriol. 181, 2394–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krulwich T. A., Hicks D. B., and Ito M. (2009) Cation/proton antiporter complements of bacteria: why so large and diverse? Mol. Microbiol. 74, 257–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saier M. H. Jr., Eng B. H., Fard S., Garg J., Haggerty D. A., Hutchinson W. J., Jack D. L., Lai E. C., Liu H. J., Nusinew D. P., Omar A. M., Pao S. S., Paulsen I. T., Quan J. A., Sliwinski M., et al. (1999) Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim. Biophys. Acta 1422, 1–56 [DOI] [PubMed] [Google Scholar]
- 11. Pinner E., Padan E., and Schuldiner S. (1992) Cloning, sequencing, and expression of the nhuB Gene, encoding a Na+/H+ antiporter in Escherichia coli. J. Biol. Chem. 267, 11064–11068 [PubMed] [Google Scholar]
- 12. Padan E., Tzubery T., Herz K., Kozachkov L., Rimon A., and Galili L. (2004) NhaA of Escherichia coli, as a model of a pH regulated Na+/H+ antiporter. Biochim. Biophys. Acta 1658, 2–13 [DOI] [PubMed] [Google Scholar]
- 13. Mesbah N. M., Cook G. M., and Wiegel J. (2009) The halophilic alkalithermophile Natranaerobius thermophiles adapts to multiple environmental extremes using a large repertoire of Na+(K+)/H+ antiporters. Mol. Microbiol. 74, 270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grant W. D., and Sorokin D. Y. (2011) in Extremophiles Handbook (Horikoshi K., ed) pp. 27–54, Springer, Tokyo [Google Scholar]
- 15. Yang C., Wang Z., Li Y., Niu Y., Du M., He X., Ma C., Tang H., and Xu P. (2010) Metabolic versatility of halotolerant and alkaliphilic strains of Halomonas isolated from alkaline black liquor. Bioresour. Technol. 101, 6778–6784 [DOI] [PubMed] [Google Scholar]
- 16. Dzioba J., Ostroumov E., Winogrodzki A., and Dibrov P. (2002) Cloning, functional expression in Escherichia coli and primary characterization of a new Na+/H+ antiporter, NhaD, of Vibrio cholerae. Mol. Cell. Biochem. 229, 119–124 [DOI] [PubMed] [Google Scholar]
- 17. Liu J., Xue Y., Wang Q., Wei Y., Swartz T. H., Hicks D. B., Ito M., Ma Y., and Krulwich T. A. (2005) The activity profile of the NhaD-type Na+(Li+)/H+ antiporter from the soda lake haloalkaliphile Alkalimonas amylolytica is adaptive for the extreme environment. J. Bacteriol. 187, 7589–7595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radchenko M. V., Waditee R., Oshimi S., Fukuhara M., Takabe T., and Nakamura T. (2006) Cloning, functional expression and primary characterization of Vibrio parahaemolyticus K+/H+ antiporter genes in Escherichia coli. Mol. Microbiol. 59, 651–663 [DOI] [PubMed] [Google Scholar]
- 19. Resch C. T., Winogrodzki J. L., Patterson C. T., Lind E. J., Quinn M. J., Dibrov P., and Häse C. C. (2010) The putative Na+/H+ antiporter of Vibrio cholerae, Vc-NhaP2, mediates the specific K+/H+ exchange in vivo. Biochemistry 49, 2520–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang L. F., Jiang J. Q., Zhao B. S., Zhang B., Feng D. Q., Lu W. D., Wang L., and Yang S. S. (2006) A Na+/H+ antiporter gene of the moderately halophilic bacterium Halobacillus dabanensis D-8T: cloning and molecular characterization. FEMS Microbiol. Lett. 255, 89–95 [DOI] [PubMed] [Google Scholar]
- 21. Zou Y. J., Yang L. F., Wang L., and Yang S. S. (2008) Cloning and characterization of a Na+/H+ antiporter gene of the moderately halophilic bacterium Halobacillus aidingensis AD-6T. J. Microbiol. 46, 415–421 [DOI] [PubMed] [Google Scholar]
- 22. Meng L., Hong S., Liu H., Huang H., Sun H., Xu T., and Jiang J. (2014) Cloning and identification of Group 1 mrp operon encoding a novel monovalent cation/proton antiporter system from the moderate halophile Halomonas zhaodongensis. Extremophiles 18, 963–972 [DOI] [PubMed] [Google Scholar]
- 23. Ito M., Guffanti A. A., and Krulwich T. A. (2001) Mrp-dependent Na+/H+ antiporters of Bacillus exhibit characteristics that are unanticipated for completely secondary active transporters. FEBS Lett. 496, 117–120 [DOI] [PubMed] [Google Scholar]
- 24. Wei Y., Liu J., Ma Y., and Krulwich T. A. (2007) Three putative cation/proton antiporters from the soda lake alkaliphile Alkalimonas amylolytica N10 complement an alkali-sensitive Escherichia coli mutant. Microbiology 153, 2168–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horikoshi K. (1999) Alkaliphiles: Some applications of their products for biotechnology. Microbiol. Mol. Biol. Rev. 63, 735–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang G., Shi J. X., and Langrish T. A. (2007) A new pulping process for wheat straw to reduce problems with the discharge of black liquor. Bioresour. Technol. 98, 2829–2835 [DOI] [PubMed] [Google Scholar]
- 27. Yang C., Niu Y., Su H., Wang Z., Tao F., Wang X., Tang H., Ma C., and Xu P. (2010) A novel microbial habitat of alkaline black liquor with very high pollution load: microbial diversity and the key members in application potentials. Bioresour. Technol. 101, 1737–1744 [DOI] [PubMed] [Google Scholar]
- 28. Takami H., Takaki Y., and Uchiyama I. (2002) Genome sequence of Oceanobacillus iheyensis isolated from the Iheya Ridge and its unexpected adaptive capabilities to extremely environments. Nucleic Acids Res. 30, 3927–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janto B., Ahmed A., Ito M., Liu J., Hicks D. B., Pagni S., Fackelmayer O. J., Smith T. A., Earl J., Elbourne L. D., Hassan K., Paulsen I. T., Kolstø A. B., Tourasse N. J., Ehrlich G. D., et al. (2011) Genome of alkaliphilic Bacillus pseudofirmus OF4 reveals adaptations that support the ability to grow in an external pH range from 7.5 to 11.4. Environ. Microbiol. 13, 3289–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takami H. (2011) in Extremophiles Handbook (Horikoshi K., ed) pp. 184–211, Springer, Tokyo [Google Scholar]
- 31. Swartz T. H., Ikewada S., Ishikawa O., Ito M., and Krulwich T. A. (2005) The Mrp system: a giant among monovalent cation/proton antiporters? Extremophiles 9, 345–354 [DOI] [PubMed] [Google Scholar]
- 32. Hamamoto T., Hashimoto M., Hino M., Kitada M., Seto Y., Kudo T., and Horikoshi K. (1994) Characterization of a gene responsible for the Na+/H+ antiporter system of alkalophilic Bacillus species strain C-125. Mol. Microbiol. 14, 939–946 [DOI] [PubMed] [Google Scholar]
- 33. Ito M., Guffanti A. A., Wang W., and Krulwich T. A. (2000) Effects of nonpolar mutations in each of the seven Bacillus subtilis mrp genes suggest complex interactions among the gene products in support of Na+ and alkali but not cholate resistance. J. Bacteriol. 182, 5663–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Putnoky P., Kereszt A., Nakamura T., Endre G., Grosskopf E., Kiss P., and Kondorosi A. (1998) The pha gene cluster of Rhizobium meliloti involved in pH adaptation and symbiosis encodes a novel type of K+-efflux system. Mol. Microbiol. 28, 1091–1101 [DOI] [PubMed] [Google Scholar]
- 35. Kurz M., Brünig A. N., and Galinski E. A. (2006) NhaD type sodium/proton antiporter of Halomonas elongata: a salt stress response mechanism in marine habitats? Saline Syst. 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldberg E. B., Arbel T., Chen J., Karpel R., Mackie G. A., Schuldiner S., and Padan E. (1987) Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 84, 2615–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langmead B., and Salzberg S. L. (2012) Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li B., and Dewey C. N. (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robinson M. D., McCarthy D. J., and Smyth G. K. (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Epstein W., and Kim B. S. (1971) Potassium loci in Escherichia coli K-12. J. Bacterial. 108, 639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosen B. P. (1986) Ion extrusion systems in Escherichia coli. Methods Enzymol. 125, 328–336 [DOI] [PubMed] [Google Scholar]
- 42. Nozaki K., Kuroda T., Mizushima T., and Tsuchiya T. (1998) A new Na+/H+ antiporter, NhaD, of Vibrio parahaemolyticus. Biochim. Biophys. Acta 1369, 213–220 [DOI] [PubMed] [Google Scholar]
- 43. Epstein W., Buurman E., McLaggan D., and Naprstek J. (1993) Multiple mechanisms, roles and controls of K+ transport in Escherichia coli. Biochem. Soc. Trans. 21, 1006–1010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.