Abstract
Background:
Prevalence estimates of Posttraumatic Stress Disorder (PTSD) among breast cancer patients varied widely in existing studies. This study aimed to provide an overall prevalence estimate of PTSD among breast cancer patients, and the prevalence estimates related to specific PTSD diagnosis tools.
Methods:
Systematic search of relevant articles was made from seven databases. Freeman-Tukey Double Arcsine Transformation was used to estimate the overall prevalence of PTSD. Sub-group and meta-regression analyses were used to investigate the between-study sources of heterogeneity. Publication bias was examined using Egger’s funnel plot and Begg test.
Results:
The pooled prevalence of PTSD among breast cancer patients was [9.6%, 95% confidence intervals (95%CI)=7.9–11.5%]. Studies that used Clinician Administered PTSD Scale-Form (CAPS) method alone yielded much higher prevalence (19.0%, 95%CI=13.1–25.5%, n=5) than three or fourth edition Structured Clinical Interview for Diagnostic and Statistical Manual (SCID) method alone (3.0%, 95%CI= 2.2–3.9%, n=11). Prevalence estimates for studies that used the methods: PTSD Checklist—Civilian Version (PCL-C) cut-off, PCL-C cluster, and Impact of Event Scale (IES) cut-off were (7.0%, 95%CI= 3.9–10.8%, n=10), (11.5%, 95%CI= 8.6–15.6%, n=11) and (15.1%, 95%CI= 12.3–18.2%, n=4), respectively. Heterogeneity between-study was substantial (I2=44.9–92.3%).
Conclusion:
About 9.6% of the breast cancer patients would develop the PTSD symptoms. Those who were younger, non-Caucasian and recently completed treatment would be at a greater risk of developing PTSD.
Keywords: Posttraumatic stress disorder, Breast cancer, Prevalence, Meta-analysis
Introduction
The publication of the Diagnostic and Statistical Manual of Mental Disorders, ver. IV (DSM-IV) in 1994, made it feasible to acknowledge cancer as a possible traumatic stressor that might induce PTSD (1). According to the DSM-IV, PTSD is a psychiatric disorder that may occur when an individual experiences, or witnesses, or encounters some traumatic events such as threatened death, serious injury, and anything that puts a person’s life under threat. Stress symptoms of PTSD include re-experiencing the trauma, avoiding trauma reminders, numbness, and hyper-arousal.
Interestingly, women suffer PTSD more than men, and this may be due to gender differences in the role of social life or level of resistance, and neurobiological response to the trauma (2). Females are the most affected by breast cancer in the world. The global breast cancer cases in 1980 were 641000, which increased to 1643000 in 2010, and the annual increase rate of breast cancer in the world was about 3.1% (3). Many breast cancer patients might experience psychological problems, for example, anxiety, depressive disorders and PTSD (4, 5). The prevalence of PTSD among patients with breast cancer varied widely between literature, with estimates ranging from 0% (6) to 32.3% (7). This kind of variation could be attributed to age at the diagnosis (8–11), education (12), social economic status (8), race (13), cancer stage (9) and time since treatment (14). In addition, the different reported prevalence of PTSD among breast cancer patients could partially be ascribed to the diversity of diagnostic instruments and study populations (15). Although a previous meta-analysis estimated pooled prevalence of PTSD diagnosed by the SCID (16) and the PCL-C (17) among breast cancer patients, no comparison was “performed between interview methods, or between screening questionnaire methods, because only the PCL-C and the SCID were used more than once” (15).
Knowing the burden and probable predictors of PTSD, this study, therefore, we conducted a comprehensive research to include more studies that used a variety of diagnosis tools as possible. Firstly, data from eligible studies were combined to obtain a pooled estimate of prevalence of PTSD among breast cancer patients, and then the effects of some variables on the prevalence estimate variation were examined. Finally, the possible predictors of PTSD induced by breast cancer were explored.
Methods
Data collection
The seven Databases: PubMed, Web of Science, Embase, Chinese National Knowledge Infrastructure (CNKI), Chinese Biomedicine (CBM), Wanfang and Weipu were searched for all relevant articles on PTSD and breast cancer. The following search terms were used, without restriction to language and gender, to conduct a comprehensive search of articles on PTSD and breast cancer: (“neoplasms” or “cancer” or “tumor” or “carcinoma”) and (“Stress Disorders, Post-Traumatic” or “PTSD”). Search for articles in the seven databases was restricted to articles published within the period from the initial state of the databases to Jan 22, 2015. Efforts were made to retrieve the references of all relevant publications in order to obtain all eligible studies as possible.
Literature screening
Studies were considered eligible to following requirements: 1) they used cross-sectional, case-control or longitudinal/prospective methods; 2) subjects under investigation were breast cancer patients who had no history of other cancer before her/his breast cancer diagnosis; 3) diagnosis instrument for PTSD in details; 4) availability of raw data on PTSD prevalence or calculated that; 5) the language limited to English or Chinese. The exclusion criteria included: 1) only reported the lifetime PTSD prevalence; 2) duplicated publication, or repeated information from similar studies; 3) conference abstract, review, and editorial or commentary.
Data extraction
Two authors independently extracted data. Information retrieved from eligible articles included the first author, publication year, research country, study design, sample size, Caucasian (%), diagnosis assessment, patient’s mean age, PTSD prevalence, measurement point, tumor staging and quality score of literature. Some studies reported the mean time since post diagnosing and/or post treatment by varied units (such as d/wk rather than months). In such cases, d/wk was changed into months with an understanding that 30 d equal to one month.
Quality assessment
The assessment criteria for prevalence studies (18), were used for evaluating the methodology and quality of the available studies. Two study investigators completed the assessments independently. This type of quality literature evaluative instrument grades studies into eight methodological criteria. The total quality score ranges from zero to 8, with a higher score indicating higher study quality.
Statistical analysis
Freeman-Tukey Double Arcsine Transformation was used to estimate a pooled prevalence of PTSD among all patients diagnosed with breast cancer at 95% CI. Heterogeneity between study-specific estimates was assessed using inconsistency index (I2 statistic; low: I2<25%; moderate: 25%–50%; high: I2 >50%) (19). Fixed-effects model was considered for I2 ≤50%, while for I2 >50% existing substantial heterogeneity was considered and the random-effects model was then used instead of the fixed-effects model. Once heterogeneity was established, sub-group and meta-regression analyses were performed to investigate further between-study sources of heterogeneity. Sensitivity analysis was carried out to check the effect of every study on the summary prevalence estimate by removing low quality score studies and then pooling the remaining studies. Begg test and Egger funnel plot were used to examine the publication bias (20, 21). All analyses were performed using R 3.1.2 software metaprop and metafor packages and SPSS 21.0 (Chicago, IL, USA). For all tests, P< 0.1 was considered statistically significant.
Results
Brief description of study selection
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), 2396 studies were identified by seven electronic databases search and 1 through other source. Totally, 1732 articles were left for screening after 665 articles, which duplicated records, were removed. After screening, 1676 articles were then excluded because they were irrelevant to our study. The remaining 56 articles were thoroughly read to determine their eligibility for the study. Twenty-two articles were not eligible and thus, were excluded from the study. These articles were not eligible because two of them had the same information as the eligible articles, and the study population for the other two articles overlapped with some already eligible articles. Moreover, 10 of the articles did not measure the point prevalence of PTSD, and 2 just measured the lifetime prevalence of PTSD. In addition, 4 articles included some subjects, who were not breast cancer patients, in their study population. Finally, 1 article did not study the PTSD while the other article did not provide detailed information about the PTSD diagnosis instrument. After this screening process, 34 observational studies were eligible for this quantitative analysis (6–14, 22–46).
Characteristics and quality of included studies
The baseline characteristics of patients in the studies are shown in Table 1. All the subjects were female patients with mean age ranging from 43.3 to 65 yr. The average measure time of PTSD in breast cancer patients ranged from 0.1 to 216 months for post treatment and 0.5 to 60 months for post diagnosis. Noteworthy, is that the eligible studies included all cancer staging patients (0-IV and recurrence).
Table 1:
Characteristics and information of included studies on the prevalence of PTSD among breast cancer patients
| 1st author, year | Location | Caucasian (%) | Design | N | Mean age (yr) | Measurement point (months) | Diagnosis assessment | Staging | PTSD prevalence (%) | Score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-D | Post-T | Questionnaire | Clinical interview | cut-off | Cluster | Current | ||||||||
| Cordova, 1995 | USA | 82.3 | cross-sectional | 55 | 55.5 | 30.5 | PCL-C | NO | I-IIIA | 5.5 | 10.9 | 5 | ||
| Jacobsen, 1998 | USA | 98 | cross-sectional | 43 | 44.4 | 19.4 | PCL-C | NO | II-IV | 12 | 19 | 5 | ||
| Andrykowski, 1998 | USA | 95 | cross-sectional | 82 | 56.6 | 37 | PCL-C | SCID | I-IIIA | 5 | 6 | 6 | 6 | |
| Green, 1998 | USA | 66 | cross-sectional | 160 | 53.4 | 6.5 | IES | SCID | I-II | 2.5 | 6 | |||
| Tjemsland, 1998 | Norway | * | longitudinal | 106 | 50 | T1=1.4 | IES +GHQ | NO | I-II | 12 | 4 | |||
| T2=13.3 | IES +GHQ | NO | 14 | |||||||||||
| Cordova, 2000 | USA | 95.1 | cross-sectional | 142 | 56.4 | 35.6 | PCL-C | NO | 0-IV | 8.5 | 12.7 | 5 | ||
| Andrykowski, 2000 | USA | 95.7 | longitudinal | 46 | 56.4 | T1=29.8 | PCL-C | NO | 0-IIIA | 4.3 | 5 | |||
| T2=41.8 | 6.5 | |||||||||||||
| Naidich, 2000 | USA | * | cross-sectional | 31 | 54.77 | 15.96 | IES | CAPS-1 | I-III +unknown | 32.3 | 6 | |||
| Mundy, 2000 | USA | 100 | cross-sectional | 37 | G1=43.3 | 37.5 | 10.4 | NO | SCID | II-IV | 0 | 6 | ||
| G2=50.2 | 54.7 | 43.3 | NO | SCID | I-IV | 0 | ||||||||
| Pitman, 2001 | USA | * | cross-sectional | 50 | * | 20.5 | NO | CAPS | I-III | 14 | 6 | |||
| 37 | * | 20.5 | PCL-C | NO | I-III | 2.7 | ||||||||
| Amir, 2002 | Israel | * | cross-sectional | 39 | 50.4 | 78 | PTSD scale | NO | I-III | 18 | 4 | |||
| Boyer, 2002 | USA | 73.7 | cross-sectional | 133 | 65 | 37.2 | PTSD-RI | NO | 0-IV | 21.1 | 4 | |||
| Koopman, 2002 | USA | 88.8 | longitudinal | 117 | 50.2 | T1=1-12 | IES | NO | I-III | 5.1 | 4 | |||
| T2=13-24 | 8.5 | |||||||||||||
| Kornblith, 2003 | USA | 91 | cross-sectional | 153 | 65 | 216 | PCL-C | NO | I-II | 4.6 | 5 | |||
| Luecken, 2004 | USA | 98 | cross-sectional | 71 | 53 | 1–6 | NO | SCID | 0-III | 3 | 6 | |||
| Palmer, 2004 | USA | 80 | cross-sectional | 115 | 55.6 | 12–60 | IES | SCID | I-IV | 4 | 6 | |||
| Okamura 2005 | Japan | 0 | cross-sectional | 50 | 53 | 1–6 | SCID | recurence | 2 | 5 | ||||
| Levine, 2005 | USA | 77.9 | cross-sectional | 181 | 50.7 | 18 | PCL-C | NO | I-IV+unknown | 17.1 | 26 | 5 | ||
| 14.4 | ||||||||||||||
| Shelby, 2005 | USA | 93 | cross-sectional | 148 | 50.5 | 18 | 6 | PCL-C | NO | II-III | 2 | 6.8 | 5 | |
| Matsuoka, 2005 | Japan | 0 | cross-sectional | 155 | 46.8 | 9.16 | NO | SCID | I-III+unknown | 3.9 | 6 | |||
| Hegel, 2006 | USA | 96 | cross-sectional | 236 | 57.4 | ≥1 | PC-PTSD | NO | I-III | 10 | 4 | |||
| Mehnert, 2007 | Germany | * | longitudinal | 108 | 54.9 | 0.5 | 0.1 | IES-R | SCID | 0-IV | 18.5 | 2.4 | 6 | |
| 98 | * | 6.5 | 6.1 | IES-R | NO | 0-IV | 16.3 | |||||||
| 98 | * | 6.5 | 6.1 | PCL-C | NO | 11.2 | 16.3 | |||||||
| Cordova, 2007 | USA | 86 | cross-sectional | 65 | 52.3 | 9.4 | PCL-C | NO | I-III | 11 | 17 | 5 | ||
| Morrill, 2008 | USA | 85 | cross-sectional | 161 | 59 | 48 | PCL-C | NO | I-II | 1.9 | 5 | |||
| Shelby, 2008 | USA | 95 | prospective | 74 | 51 | ≥6 | PCL-C | SCID | II-III | 16.2 | 6 | |||
| Mehnert, 2008 | Germany | * | cross-sectional | 1083 | 61.8 | 46.5 | PCL-C | NO | I-IV+unknown | 12.1 | 5 | |||
| Gandubert, 2009 | France | * | Case-control | 144 | 53 | 21 | NO | Watson’s PTSD Inventory | I-III | 4.9 | 6 | |||
| Elklit, 2011 | Denmark | 100 | longitudinal | 81 | * | T1=1.4 | HTQ | NO | * | 7 | 4 | |||
| 64 | 56.3 | T2=13 | HTQ | NO | * | 13 | ||||||||
| O’Connor, 2011 | Denmark | * | longitudinal | 3318 | * | T1=3 | IES | NO | I-III | 20.1 | 5 | |||
| 2912 | * | T1=15 | IES | NO | I-III | 14.3 | ||||||||
| Liu bing, 2011 | China | 0 | cross-sectional | 21 | * | ≥1 | NO | CAPS | * | 9.5 | 5 | |||
| Yan lifu, 2011 | China | 0 | cross-sectional | 21 | * | ≥1 | NO | CAPS | * | 9.5 | 5 | |||
| Pan wen, 2013 | China | 0 | cross-sectional | 44 | * | ≥1 | NO | CAPS | * | 27.3 | 5 | |||
| Vin-Raviv, 2013 | USA | 68.8 | longitudinal | 1139 | * | 2-3 | IES | NO | I-III | 23 | ||||
| 69.3 | 1109 | * | 4 | IES | NO | I-III | 16.5 | 5 | ||||||
| 70.3 | 1076 | * | 6 | IES | NO | I-III | 12.6 | |||||||
| Kwakkenbos, 2014 | Canada | 72.3 | longitudinal | 437 | 54.2 | T1=2.18 | NO | SCID | * | 3 | 7 | |||
| 363 | 54.2 | T2=5.18 | NO | SCID | 2.5 | |||||||||
Note: post-D=post diagnosis; post-T=post treatment; T1,T2=time point 1,2; G1,G2=group1,2; GHQ=General Health Questionnaire; PTSD-RI =PTSD Reaction Index; HTQ = Harvard Trauma Questionnaire.
With respect to Loney’s (18) criteria for quality assessment of observational studies, 1 study was graded 7 points; 11 studies, 6 points each; 16 studies, 5 points each; and 6 studies, 4 points each. The main limitations were, generally, limited sample sizes, and absence of the 95%CI for the prevalence of PTSD. Therefore, biases in the outcome measurement would exist to some degree.
Prevalence of PTSD
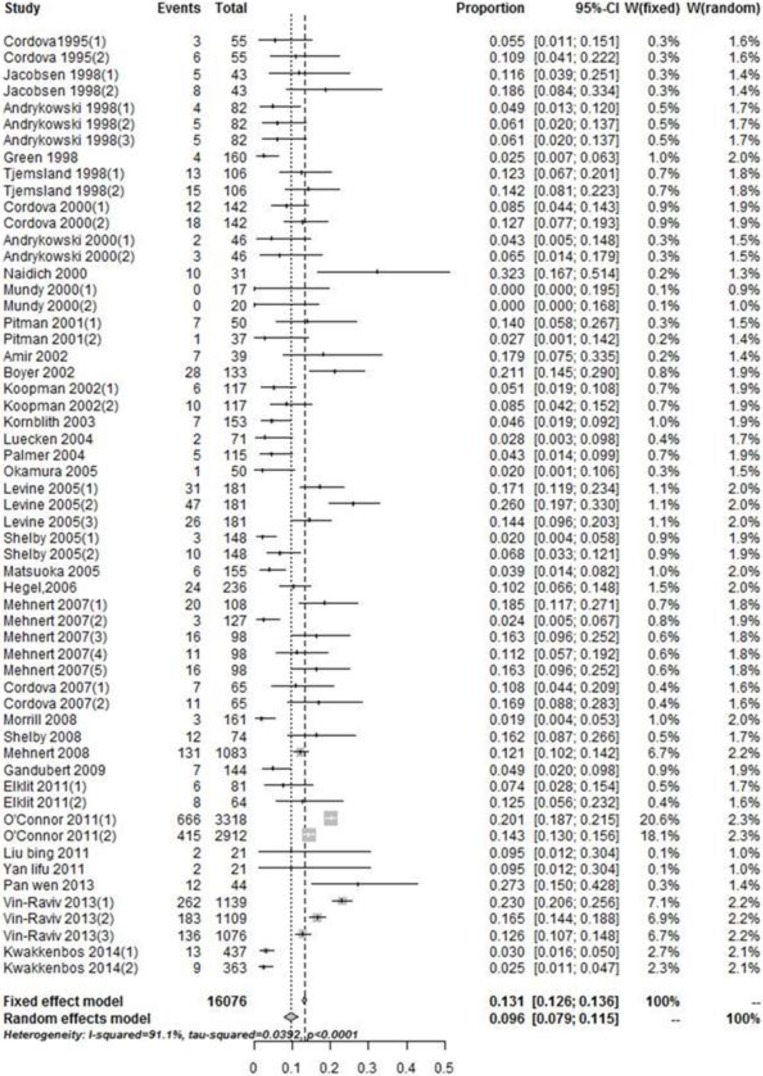
The prevalence of PTSD among breast cancer patients in the 34 eligible studies ranged from 0% to 32.3% (Fig. 1). The pooled analysis result gave an overall PTSD prevalence of 9.6% (95%CI=7.9–11.5%). The heterogeneous significance between all eligible studies was (I2=91.1%, P<0.001). Hence, the results reported by the random-effects model.
Fig. 1:
Forest plot showing the pooled prevalence of PTSD among breast cancer patients
Studies with clinical interview method yielded statistically significant lower estimate of prevalence (5.6%, 95%CI=3.3 - 8.4%, n=16) when compared with the questionnaire method (11.4%, 95%CI= 9.6 - 13.3%, n=18). These results were significant with Z=−2.702, P=0.007. The observed heterogeneity between these studies was 77.1%, and 88.3%, respectively. Moreover, the prevalence estimate of PTSD among studies with questionnaire cut-off method (10.9%, 95%CI= 8.8 - 13.2%, n=16) was marginally lower than the prevalence estimate of PTSD among studies with questionnaire cluster method (12.6%, 95%CI= 9.1 - 16.4%, n=13). However, this difference was not statistically significant (Z=−0.222, P=0.824). Furthermore, the observed heterogeneity between studies with questionnaire cut-off method was 90.2% while that between studies with questionnaire cluster method was 79.8%.
Studies with CAPS method alone yielded much higher prevalence (19.0%, 95%CI=13.1–25.5%, n=5) than SCID method alone (3.0%, 95%CI= 2.2–3.9%, n=11). These results were significant (Z= −2.912, P=0.004). The prevalence estimates of the studies that used PCL-C cut-off method, PCL-C cluster method and IES cut-off method were (7.0%, 95%CI= 3.9 - 10.8%, n=10), (11.5%, 95%CI= 8.6–15.6%, n=11) and (15.1%, 95%CI= 12.3 - 18.2%, n=4), respectively. These prevalence estimates were also statistically significant (H=7.319, P=0.026). The observed heterogeneity between-study ranged from I2=44.9% to I2= 92.3%.
Meta-regression analysis
Meta-regression analyses were conducted to investigate the heterogeneity among included studies. The variables, mean age, publication year, mean time post treatment, Caucasian (%) and mean time post diagnosis were entered into the meta-regression model to test their contribution to the heterogeneity between-study when using different PTSD assessment methods. The individually test, performed for each PTSD assessment method, indicated that, publication year was not significant for the pooled prevalence when using clinical interview method, SCID, or PCL-C, but was significant when using questionnaire method. Heterogeneity within studies was significantly reduced by considering more recent publications (R2=8.99%, P=0.064). Mean age was significantly associated with the pooled prevalence when using PCL-C. Heterogeneity between literature was greatly decreased by more measurements of older age (R2=16.21%, P=0.034). The contribution of Caucasian (%) to heterogeneity was meaningful when using the questionnaire method to estimate the prevalence of PTSD. However, heterogeneity between literature was greatly decreased by higher proportions of Caucasian individuals (R2=37.33%, P=0.004). In addition, the contribution to heterogeneity of mean time since post treatment was meaningful when using the questionnaire method to measure the prevalence of PTSD. Nevertheless, heterogeneity within studies was significantly reduced when mean time since post treatment increased (R2=12.63%, P=0.096).
A final composite model comprised four variables (publication year, Caucasian (%), mean age and mean time post treatment). A significance level of P=0.096 resulted in a 65.27% reduction in heterogeneity when using questionnaire method. However, when using PCL-C with those four variables, the reduction in heterogeneity was not statistically significant.
Sensitivity analysis
The excluded studies were graded 4 points each, with pooled breast cancer PTSD prevalence of 9.2% (95%CI=7.4 -11.3%, n=28), which is slightly lower than 9.6% pooled prevalence determined from the eligible studies. The small difference between the two-pooled prevalence indicates that the results of this study were credible.
Publication bias
The Egger’s funnel plot showed that the effect level of eligible studies was basically symmetrical and the Begg test obtained a P-value, P=0.504. Therefore, the publication bias was not obvious in this study.
Discussion
This is the first meta-analysis to observe pooled prevalence of breast cancer induced PTSD. The pooled prevalence of PTSD induced by breast cancer was 9.6%. Thus, the estimated overall magnitude of the occurrence of PTSD among breast cancer patients is not negligible. Although, there was significant heterogeneity among eligible studies, the outcomes of this study could reflect the significance of the overall and instrument-specific prevalence of PTSD among breast cancer patients.
As observed from the eligible studies, the variation of estimated prevalence of PTSD among breast cancer patients ranged from 0% (6) to 32.3% (7). This variation could be partly explained by the use of different PTSD assessment methods. For instance, self-report instrument presented a higher prevalence (11.4%) of PTSD than clinical interview method (5.6%). This was the case, probably, because the clinical interview is a kind of clinician administered evaluation method, often used to diagnose the disorder by trained and experienced clinicians, whereas self-report instrument mainly measures the severity and frequency of symptoms and may often be used by non-specialists. The accuracy of both instruments varies with respect to their sensitivity and specificity when matched with DSM diagnostic criteria. For example, the PCL-C (17) consists of 17 items in accordance with DSM-IV symptoms of PTSD, and it was the most common screening method used by the eligible studies. However, because this instrument has no single validated cut-off value, different studies could use different cut-off scores such as 50 (8) and 44 (31). Thus, the assessments used in the eligible studies perhaps overestimated the prevalence of PTSD. Moreover, the self-report measure method might be susceptible to reporting bias. For example, some patients perhaps showed more distress for seeking social support than others, and this could possibly distort the outcomes.
Interestingly, the pooled prevalence of PTSD among breast cancer patients was statistically different between the sub-group of SCID clinical assessment (3.0%, I2=44.9%) and the subgroup of CAPS (47) clinical assessment (19.0%, I2=48.1%). This was probably due to the different measurement instruments and the fact that SCID was more popularly used than CAPS. For example, 11 eligible articles had 1815 subjects assessed using the SCID-DSM-IV and only 5 had 167 subjects assessed using the CAPS. One eligible study did interviews using CAPS “in equipment laden medical facilities and offices, and this might have produced some elevations in PTSD scores” (7).
Results for self-reported questionnaire evaluation methods were also interesting. The prevalence estimate of PTSD for IES (48) cut-off method was 15.1%. This was much higher than the prevalence estimate of PTSD for PCL-C cut-off method, which was 7%, and slightly higher than PTSD for PCL-C cluster method that was 11.5%. This difference could, partially, be attributed to the varied clinical cut-off levels for IES, which ranged from 20 (24) to 35 (12). This wide scoring range perhaps resulted in more false positive for PTSD. Besides, this imparity could be attributed to the fact that IES does not cover the participant’s beliefs or feelings to the stressful events, and comparing to access the symptoms of PTSD, it could be more useful to measure adjustment problems and diffuse emotional distress (30, 36).
Ethnicity was a meaningful predictive factor of prevalence estimate on the questionnaire method. The prevalence estimate of PTSD reduced along with the proportions of Caucasian individuals in the sample increased. This observation might propose that non-Caucasian breast cancer patients were more likely to be diagnosed with PTSD than Caucasians. Asians and Blacks were more susceptible to breast cancer induced PTSD than Caucasians (13).
Being of younger age was related to the appearance and severity of PTSD among breast cancer patients (8, 22, 28, 30, 44, 49, 50). Results of our study suggest the similar outcomes when using PCL-C to assess the estimated prevalence. This was probably because the PTSD symptoms had a great impact on their lifestyle, careers and fertility issues. They could be more unwilling to accept the fact of breast cancer diagnosis than older. Publication year was another meaningful predictive factor of PTSD prevalence for the questionnaire method, that recent studies revealed higher estimates of PTSD. This was probably because more recent publications were done in the era where women are required to take on more social and domestic responsibilities with the increasing competition and role switching in the current society, and they needed to endure more pressure than before.
Prevalence decreased with the increase in mean time post treatment for the questionnaire method. Specifically, the prevalence of PTSD decreased with increase in mean time post treatment. However, it is always advisable to be cautious when interpreting the outcomes because there is a tendency of researchers to consider the two indices, post diagnosis or post treatment, as sources of trauma, which could induce PTSD, although no agreement, had been reached to consider these two indices as traumatic events that could induce PTSD. Researchers adopted at least one of the two indices, as the assessment time of PTSD among breast cancer patients. However, these moderators could not combine these two indices which resulted in low statistical power (15).
Compared to previous meta-analysis (15), this study obtained slightly lower estimated prevalence of PTSD among breast cancer patients when using clinical interview method (5.8% versus 5.6%), and lower with SCID (4.1% vs 3.0%). Also, a relatively higher estimated prevalence of PTSD among breast cancer patients was obtained when using questionnaire cut-off method (10.9% vs 6.4%) or questionnaire cluster method (12.6% vs 12.1%), and PCL-C cut-off method (7.0% vs 6.4%) or PCL-C cluster method (11.5% vs 11.2%). In contrast to the previous meta-analysis, the outcomes of this study provided the estimated prevalence specific to CAPS and IES, and it is suggested that publication year, mean age, the percentages of Caucasian and mean time post-treatment had effects on the heterogeneity between-study.
According to the global data, the total number of breast cancer cases in 2010 were 1643000 and the deaths resulting from breast cancer in 2010 were 425000 (3). Thus, there have been a considerable number of breast cancer patients at the risk of developing PTSD. Estimation of the prevalence of PTSD among breast cancer patients is the first step toward understanding the burden of this disease and the premise of clinical intervention and support. A higher prevalence of PTSD among breast cancer patients was related to the lower quality of life (38, 46), and would lead to lower compliance of breast cancer treatment (13). Increasing evidences have suggested that “individuals with PTSD have altered immune activity, including lower levels of natural killer cell activity and higher levels of circulating inflammatory markers” (29, 51). The compromised immune system related to PTSD may lead to cancer progression and shorten length of survival (13). Therefore, from the clinical standpoint, it is very important for clinicians to first evaluate the psychiatric status in women with breast cancer, and then provide psycho-physiologic support and clinical intervention (25, 29). Besides, accumulated evidence confirmed that psycho-educational support (such as health lecture) and intensive lifestyle change (taking some movement and mediation) could decrease the PTSD symptoms and increase quality of life and adjustment styles among breast cancer patients developed PTSD (31).
There were a number of limitations in this study emphasized and explained. Firstly, the assessment time since diagnosis or treatment of breast cancer had a substantial effect on the development of PTSD. However, the mean time for the measurement of PTSD observed in the reviewed articles for this study varied with wide margins, so it was difficult to combine these two indices. Future researchers are urged to report the specific time of the diagnosis or treatment of breast cancer when measuring the prevalence of PTSD and try to uniform these indices.
Secondly, advanced or recurrent cancer patients suffer more symptoms than early-stage cancer patients do (14, 23, 27, 37, 45). Although, most of our reviewed studies had covered almost all kinds of breast cancer stages, detailed information about staging was not available. Therefore, it was not possible to analyze the impact of cancer-stage on the prevalence of PTSD.
Finally, all the eligible studies for this meta-analysis used or accorded with DSM-III-R or DSM-IV criteria, but not with DSM-5 (52). The DSM-5 argues that a life-threatening illness is no longer described as a traumatic event. Given this reason, future researchers should be cautious in diagnosing cancer related PTSD (CR-PTSD). More investigations are necessary to find out whether the breast cancer could be seen as a single traumatic event rather than an ongoing distressing experience.
Conclusion
About 9.6% of the breast cancer patients would develop the PTSD symptoms. The estimated prevalence specific to diagnosis tools varied. Those who are younger, non-Caucasian and lately finished treatment would be at more risk of developing PTSD. In order to promote these individuals’ recovery from PTSD, the government should provide financial and mental health resources to support it.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgement
The Specialized Research Fund supported this article for the Doctoral Program of Higher Education (20130162110054). The authors declare that there is no conflict of interest.
References
- 1. Association AP (1994). Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. ed., American Psychiatric Association; Washington, DC. [Google Scholar]
- 2. Heim C, Nemeroff CB. (2009). Neurobiology of posttraumatic stress disorder. CNS Spectr, 14 (1 Suppl 1): 13–24. [PubMed] [Google Scholar]
- 3. Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJL, Naghavi M. (2011). Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet, 378: 1461–1484. [DOI] [PubMed] [Google Scholar]
- 4. Vahdaninia M, Omidvari S, Montazeri A. (2010). What do predict anxiety and depression in breast cancer patients? A follow-up study. Soc Psychiatry Psychiatr Epidemiol, 45: 355–61. [DOI] [PubMed] [Google Scholar]
- 5. Kangas M, Henry JL, Bryant RA. (2002). Posttraumatic stress disorder following cancer. A conceptual and empirical review. Clin Psychol Rev, 22: 499–524. [DOI] [PubMed] [Google Scholar]
- 6. Mundy EA, Blanchard EB, Cirenza E, Gargiulo J, Maloy B, Blanchard CG. (2000). Posttraumatic stress disorder in breast cancer patients following autologous bone marrow transplantation or conventional cancer treatments. Behav Res Ther, 38: 1015–27. [DOI] [PubMed] [Google Scholar]
- 7. Naidich JB, Motta RW. (2000). PTSD-Related Symptoms in Women with Breast Cancer. J Psychother Independent Practice, 1: 35–54. [Google Scholar]
- 8. Cordova MJ, Andrykowski MA, Kenady DE, McGrath PC, Sloan DA, Redd WH. (1995). Frequency and correlates of posttraumatic-stress-disorder-like symptoms after treatment for breast cancer. J Consult Clin Psychol, 63: 981–6. [DOI] [PubMed] [Google Scholar]
- 9. Andrykowski MA, Cordova MJ, McGrath PC, Sloan DA, Kenady DE. (2000). Stability and change in posttraumatic stress disorder symptoms following breast cancer treatment: a 1-year follow-up. Psychooncology, 9: 69–78. [DOI] [PubMed] [Google Scholar]
- 10. Cordova MJ, Studts JL, Hann DM, Jacobsen PB, Andrykowski MA. (2000). Symptom structure of PTSD following breast cancer. J Trauma Stress, 13: 301–19. [DOI] [PubMed] [Google Scholar]
- 11. Kornblith AB, Herndon JE, 2nd, Weiss RB, Zhang C, Zuckerman EL, Rosenberg S, Mertz M, Payne D, Jane Massie M, Holland JF, Wingate P, Norton L, Holland JC. (2003). Long-term adjustment of survivors of early-stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer, 98: 679–89. [DOI] [PubMed] [Google Scholar]
- 12. O’Connor M, Christensen S, Jensen AB, Moller S, Zachariae R. (2011). How traumatic is breast cancer? Post-traumatic stress symptoms (PTSS) and risk factors for severe PTSS at 3 and 15 months after surgery in a nationwide cohort of Danish women treated for primary breast cancer. Br J Cancer, 104: 419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vin-Raviv N, Hillyer GC, Hershman DL, Galea S, Leoce N, Bovbjerg DH, Kushi LH, Kroenke C, Lamerato L, Ambrosone CB, Valdimorsdottir H, Jandorf L, Mandelblatt JS, Tsai WY, Neugut AI. (2013). Racial disparities in posttraumatic stress after diagnosis of localized breast cancer: the BQUAL study. J Natl Cancer Inst, 105: 563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andrykowski MA, Cordova MJ, Studts JL, Miller TW. (1998). Posttraumatic stress disorder after treatment for breast cancer: prevalence of diagnosis and use of the PTSD Checklist-Civilian Version (PCL-C) as a screening instrument. J Consult Clin Psychol, 66: 586–90. [DOI] [PubMed] [Google Scholar]
- 15. Abbey G, Thompson SB, Hickish T, Heathcote D. (2015). A meta-analysis of prevalence rates and moderating factors for cancer-related post-traumatic stress disorder. Psychooncology, 24: 371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. First MB, Spitzer RL, Gibbon M, Williams JBW. (2002). Structured Clinical Interview for DSM-IVTR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). ed., New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- 17. Weathers FW LB, Herman DS, Huska JA, Keane TM. (1991). The PTSD Checklist—Civilian Version (PCL-C) . ed., Available from F.W. Weathers, National Center for PTSD, Boston Veterans Affairs Medical Center, 150 S. Huntington Avenue, Boston, MA, 02130. [Google Scholar]
- 18. Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. (1998). Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can, 19: 170–6. [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. (2003). Measuring inconsistency in meta-analyses. BMJ, 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Begg CB, Mazumdar M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50: 1088–101. [PubMed] [Google Scholar]
- 22. Green BL, Rowland JH, Krupnick JL, Epstein SA, Stockton P, Stern NM, Spertus IL, Steakley C. (1998). Prevalence of posttraumatic stress disorder in women with breast cancer. Psychosomatics, 39: 102–11. [DOI] [PubMed] [Google Scholar]
- 23. Jacobsen PBP, Widows MRBS, Hann DMP, Andrykowski MAP, Kronish LEBSN, Fields KKMD. (1998). Posttraumatic Stress Disorder Symptoms After Bone Marrow Transplantation for Breast Cancer. [Article]. Psychosom Med, 60: 366–371. [DOI] [PubMed] [Google Scholar]
- 24. Tjemsland L, Soreide JA, Malt UF. (1998). Posttraumatic distress symptoms in operable breast cancer III: status one year after surgery. Breast Cancer Res Treat, 47: 141–51. [DOI] [PubMed] [Google Scholar]
- 25. Pitman RK, Lanes DM, Williston SK, Guillaume JL, Metzger LJ, Gehr GM, Orr SP. (2001). Psychophysiologic assessment of posttraumatic stress disorder in breast cancer patients. Psychosomatics, 42: 133–40. [DOI] [PubMed] [Google Scholar]
- 26. Amir M, Ramati A. (2002). Post-traumatic symptoms, emotional distress and quality of life in long-term survivors of breast cancer: a preliminary research. J Anxiety Disord, 16: 195–206. [DOI] [PubMed] [Google Scholar]
- 27. Boyer BA, Bubel D, Jacobs SR, Knolls ML, Harwell VD, Goscicka M, Keegan A. (2002). Post-traumatic stress in women with breast cancer and their daughters. Am J Fam Ther, 30: 323– 338. [Google Scholar]
- 28. Koopman C, Butler LD, Classen C, Giese-Davis J, Morrow GR, Westendorf J, Banerjee T, Spiegel D. (2002). Traumatic stress symptoms among women with recently diagnosed primary breast cancer. J Trauma Stress, 15: 277–87. [DOI] [PubMed] [Google Scholar]
- 29. Luecken LJ, Dausch B, Gulla V, Hong R, Compas BE. (2004). Alterations in morning cortisol associated with PTSD in women with breast cancer. J Psychosom Res, 56: 13–5. [DOI] [PubMed] [Google Scholar]
- 30. Palmer SCP, Kagee AP, Coyne JCP, DeMichele AMDM. (2004). Experience of Trauma, Distress, and Posttraumatic Stress Disorder Among Breast Cancer Patients. [Article]. Psychosom Med, 66: 258–264. [DOI] [PubMed] [Google Scholar]
- 31. Levine EG, Eckhardt J, Targ E. (2005). Change in post-traumatic stress symptoms following psychosocial treatment for breast cancer. Psychooncology, 14: 618–35. [DOI] [PubMed] [Google Scholar]
- 32. Matsuoka Y, Inagaki M, Sugawara Y, Imoto S, Akechi T, Uchitomi Y. (2005). Biomedical and psychosocial determinants of intrusive recollections in breast cancer survivors. Psychosomatics, 46: 203–11. [DOI] [PubMed] [Google Scholar]
- 33. Shelby RA, Golden-Kreutz DM, Andersen BL. (2005). Mismatch of posttraumatic stress disorder (PTSD) symptoms and DSM-IV symptom clusters in a cancer sample: exploratory factor analysis of the PTSD Checklist-Civilian Version. J Trauma Stress, 18: 347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hegel MT, Moore CP, Collins ED, Kearing S, Gillock KL, Riggs RL, Clay KF, Ahles TA. (2006). Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer, 107: 2924–31. [DOI] [PubMed] [Google Scholar]
- 35. Cordova MJ, Giese-Davis J, Golant M, Kronenwetter C, Chang V, Spiegel D. (2007). Breast cancer as trauma: Posttraumatic stress and posttraumatic growth. J Clin Psychol Med Settings, 14: 308–319. [Google Scholar]
- 36. Mehnert A, Koch U. (2007). Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: a prospective study. Psychooncology, 16: 181–8. [DOI] [PubMed] [Google Scholar]
- 37. Mehnert A, Koch U. (2008). Psychological comorbidity and health-related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register-based sample of long-term breast cancer survivors. J Psychosom Res, 64: 383–91. [DOI] [PubMed] [Google Scholar]
- 38. Morrill EF, Brewer NT, O’Neill SC, Lillie SE, Dees EC, Carey LA, Rimer BK. (2008). The interaction of post-traumatic growth and post-traumatic stress symptoms in predicting depressive symptoms and quality of life. Psychooncology, 17: 948–53. [DOI] [PubMed] [Google Scholar]
- 39. Shelby RA, Golden-Kreutz DM, Andersen BL. (2008). PTSD diagnoses, subsyndromal symptoms, and comorbidities contribute to impairments for breast cancer survivors. J Trauma Stress, 21: 165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gandubert C, Carriere I, Escot C, Soulier M, Hermes A, Boulet P, Ritchie K, Chaudieu I. (2009). Onset and relapse of psychiatric disorders following early breast cancer: a case-control study. Psychooncology, 18: 1029–37. [DOI] [PubMed] [Google Scholar]
- 41. Elklit A, Blum A. (2011). Psychological adjustment one year after the diagnosis of breast cancer: a prototype study of delayed post-traumatic stress disorder. Br J Clin Psychol, 50: 350–63. [DOI] [PubMed] [Google Scholar]
- 42. Kwakkenbos L, Coyne JC, Thombs BD. (2014). Prevalence of posttraumatic stress disorder (PTSD) in women with breast cancer. J Psychosom Res, 76: 485–6. [DOI] [PubMed] [Google Scholar]
- 43. Yan LF. (2010) Prevalence and influencing factors for PTSD in patients with cancer in hospital. D. Tongji Medical College Huazhong University of Science and Technology, China.
- 44. Liu B, Qiu JX, Pan M, JX C. (2011). Post-traumatic Stress Disorders and the Risk factors in Cancer Patients. Shandong Medical, 51: 83–84. [Google Scholar]
- 45. Pan W. (2013). A correlative study of clinical psychological characteristics and heart rate variability in patients with cancer-related posttraumatic stress disorders . D. Affiliated First People’s Hospital, Soochow University, China.
- 46. Okamura M, Yamawaki S, Akechi T, Taniguchi K, Uchitomi Y. (2005). Psychiatric disorders following first breast cancer recurrence: prevalence, associated factors and relationship to quality of life. Jpn J Clin Oncol, 35: 302–9. [DOI] [PubMed] [Google Scholar]
- 47. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. (1995). The development of a Clinician-Administered PTSD Scale. J Trauma Stress, 8: 75–90. [DOI] [PubMed] [Google Scholar]
- 48. Horowitz M, Wilner N, Alvarez W. (1979). Impact of Event Scale: a measure of subjective stress. Psychosom Med, 41: 209–18. [DOI] [PubMed] [Google Scholar]
- 49. Alter CL, Pelcovitz D, Axelrod A, Goldenberg B, Harris H, Meyers B, Grobois B, Mandel F, Septimus A, Kaplan S. (1996). Identification of PTSD in cancer survivors. Psychosomatics, 37: 137–43. [DOI] [PubMed] [Google Scholar]
- 50. Andrykowski MA, Cordova MJ. (1998). Factors associated with PTSD symptoms following treatment for breast cancer: test of the Andersen model. J Trauma Stress, 11: 189–203. [DOI] [PubMed] [Google Scholar]
- 51. Pace TW, Heim CM. (2011). A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun, 25: 6–13. [DOI] [PubMed] [Google Scholar]
- 52. Association AP (2013). Diagnostic and Statistical Manual of Mental Disorders, (DSM-5). ed., American Psychiatric Association; Washington, DC. [Google Scholar]