Abstract
Background:
Prostate cancer (PCa) is the second most common malignancy in men worldwide. The purpose of this study was to provide a brief synthesis the current knowledge for the effects of physical activity (PA) and nutrition on PCa risk.
Methods:
A systematic review of English languages reviews, meta-analysis, and original articles published from 2009 to 2015 extracted from the following websites: MEDLINE, Web of Science, Health Source, Science Direct, and their references.
Results:
The review of the literature led to the selection of 12 review or meta-analysis studies and 15 lately published observational studies. Most of studies reported relationship of recreational and occupational PA and vegetables, fruits, vitamins, red/processed meats, and fats consumption with risk of PCa. Decreased risk for PCa associated with exercise was reported in seven of the ten articles on this topic. The inverse association of vegetables and/or fruit intake with PCa risk was reported in eight of 13 papers. The effect of meat/fat intake on PCa was estimated in four articles finding increased risk. There was heterogeneity between studies, and findings are inconsistent.
Conclusion:
Physical activity does not significantly reduce the risk of PCa; however, vigorous exercise may reduce the risk of aggressive tumor. Besides, there is a lack of definitive evidence supporting the preventive role of diet against PCa. Due to many other benefits of regular moderate-vigorous PA and a diet high in vegetables and fruits and low in red/processed meats and fats, these lifestyle patterns may be recommended.
Keywords: Prostate cancer, Physical activity, Nutrition, Prevention
Introduction
“Prostate cancer (PCa) is the second most common malignancy in men worldwide and the third leading cause of cancer death” (1). “According to global statistics, 1,111,689 the PCa incidence and 307,471 total deaths due to this cancer were estimated in 2012” (2). Epidemiological and laboratory findings suggest that PCa is a multifactorial disease undergoing to several non-modifiable risk factors (e.g. older age, race, family history of PCa) (3, 4) and possible modifiable risk factors such as lifestyle (5). Age is the major risk factor; the incidence of the disease increases with age and is more common in men above 50 yr of age being over 80% linked with men aged above 65 yr (3). Environmental factors (smoking, radiation, infections agents, industrial chemicals and air/water pollution, medications, obesity, physical inactivity, unhealthy nutrition) play an important role in the PCa pathology. Only 5%–10% of all cancers are linked with “genetic abnormalities” (6).
During the past two decades, a great interest has been placed in the modifiable risk factors, like physical inactivity (7–9) and diet poor in natural antioxidants (10–12) as the risk factors for cancer, including PCa. Moreover, lifestyle has been reported as an important aspect of quality of life for the PCa survivors and a factor slowing the cancer progression and reducing mortality (13). Biological benefits of regular moderate physical activity (PA) are consistently documented for primary cancer prevention, due to wide spectrum of its interactions (8, 14–18). In this respect, several previous reviews of epidemiological studies worldwide addressed the associations between PA and cancer risk, however, in the case of PCa the research is in the early stage comparing to breast or colorectal cancers. Some degree of a link between nutrition and PCa risk was demonstrated (6, 19–22).
This study provides a brief synthesis of the most important findings and conclusions available from the recent reviews and meta-analyses, concerning PCa incidence, the role of PA and diet in the cancer risk, and presents lately published epidemiologic findings on this topic not included in the previous review reports.
Methods
Four databases (Health Source, Science Direct, Web of Science, and MEDLINE) were searched from 2009 to Dec 2015. The search terms included prostate cancer, physical activity, exercise, diet, vegetables/fruits, nutrition, and supplements. In order to limit the size of the article and the number of references, meta-analyses and reviews of data not included in the analysis of the Second Expert Report (6) and lately published cohort and case-control studies on the interesting topic are cited. The reference lists from the relevant articles to check the retrieved data and to obtain additional information were also read. The search was limited to publications in English. Due to high level of heterogeneity in the study design, we have not carried out estimation of the overall quantitative synthesis of data across selected studies but display them in tables.
Results
Prostate cancer incidence
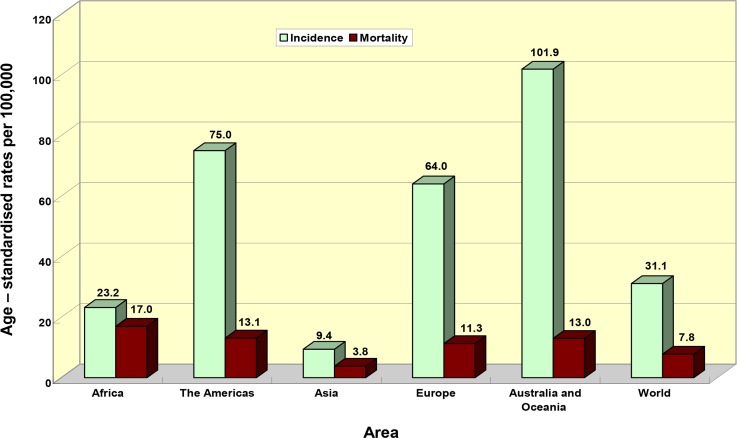
Epidemiologic data have shown large geographical variations in the PCa incidence (Fig. 1).
Fig. 1:
Age – standardized prostate cancer incidence and mortality rates in the area, 2012 (23)
The highest cancer incidence rates are observed in Australia and Oceania, and Northern America, while South-Central Asia and Northern Africa have the lowest rates (23). In general, PCa rates are 4.83 times higher in the more developed regions in comparison with less developed regions (69.5 vs 14.5 cases per 100000) (23). These geographic differences may be partially due to a fact that prostate-specific-antigen (PSA) screening in developed countries allows to diagnose cancers at earlier stage, as well as to difference in PCa treatment (24). Men who migrate from geographical regions with low PCa mortality to the developed countries with high mortality assume a higher prevalence rate (25). Besides, PCa is more often diagnosed among Afro-Americans (3). The disease prevalence and mortality rates increase strongly in countries with traditionally low the disease rates, like China or Japan. Increased rates for this disease are even observed in the more developed cities in the countries that are similar with respect of culture and geography, like countries of the South Asia (25).
Physical activity and prostate cancer
Physical activity (PA) is defined as any bodily movement using skeletal muscles resulting in energy expenditure (26). Four domains of PA are distinguished: household, transport, recreational, and occupational (26). For the association of PA with PCa risk, we selected four recently published reviews and meta-analyses (27–30) and six epidemiological studies (31–36) (Table 1). All retrieved reviews demonstrated the risk reduction with high moderate levels of occupational or recreational activity.
Table 1:
The association between physical activity and prostate cancer
|
Reviews and meta-analyses Study setting (reference) |
Main result RR or (95%CI) | Summary |
|---|---|---|
| 21 studies, 2004–2013 (27) |
Decreased risk Leisure time PA (moderate/vigorous) OR:0.84 (0.73–0.97)-0.35 (0.17–0.75) for advanced cancer Occupational PA (high activity levels) OR:0.55 (0.32–0.95)-0.90 (0.66–1.22) Increased risk Recreational + household activity ≥49.7MET-h per day OR:1.44 (1.08–1.92) Recreational OR=1.56 (1.16–2.10) Household ≥203 MET-h per week yearly OR:1.36 (1.05–1.76) |
Evidence that PA of moderate/vigorous intensity may reduce PCa risk directly or indirectly. Several studies have demonstrated decrease of PCa cells or their inhibition, increase apoptosis of cancer cells, suppression of metastasis and delay of tumor formation due to PA. |
| Six case-control studies and one cohort study 1999–2009 (28) |
Increased risk among workers with low levels of PA OR:1.33 (1.62–1.74)–2.13 (1.29–3.52) Decreased ORs among men with high levels vs low levels of occupational PA OR:0.44 (0.26–0.76)–0.78 (0.59–1.04) |
High levels of occupational PA were preventive against PCa, and sedentary job is a significant risk for PCa (case-control study). PCa was decreased by a 16% for the highest vs the lowest quartile of total PA cohort study. |
| Four cohort studies, 2005–2009 (29) |
Decreased risk for fatal PCa OR:0.59 (0.35–1.01) for ≥30 MET-hours/week vigorous recreational PA Decreased risk for aggressive cancer OR:0.69 (0.52–0.92) for >35 MET-hours/week recreational PA. Two cohort studies found no significant association. |
Underline an importance of several behavioral risk factors in the prevention of lethal PCa. |
| 19 cohort studies and 24 case-control studies 1998–2009 (30) |
Decreased risk Total PA: OR:0.90 (0.84–0.95). The reduction was found only for men between 20 and 45 yr of age and between 45 and 65 yr of age. Occupational PA: OR=0.81 (0.73–0.91) Recreational PA: OR:0.95(0.89–1.00) |
Occupational and long-lasting recreational PA may decrease the risk of PCa. |
| Epidemiological studies | ||
| Study setting | Physical activity measurement | Main result RR or OR (95% CI), adjustment factors |
| A prospective study (8,221 subjects), follow-up of 24.8 yr, 1052 cases among them 349 with advanced disease (31) | Regular recreational physical activity since the age 20 yr and current occupational. | Non-significant lower risk of advanced cancer OR:0.67 (0.42–1.07). |
| Physically active vs physically inactive. | A lack association between PA and overall or localized PCa; multivariable adjusted | |
| 286 patients. PA was assessed using Physical Activity Scale for Elderly (PASE) questionnaire (32) | Questions on the frequency and duration of household, occupational and recreational activity |
Decreased risks Total OR:0.146 (0.037–0.577) and OR:0.07 (0.006–0.764) for high-grade cancer among patients with increased PA; multivariable adjusted |
| Hospital-based case-control study (140 cases, 280 controls). Interview-administered questionnaire (33) | Active lifestyle (>40 min daily for exercise and sports) vs sedentary lifestyle | Decreased risk, OR:0.28 (0.13–0.58), P<0.05; age, marital status, residence, smoking, family history of PCa, red meat, fats and fruits intake |
| Population-based case-control study (1,436 cases and 1,349 controls, aged 39–70 yr) (34) | Occupational activity Manual handling of burdens (lifting and carrying of heavy burdens every day) |
No effect The top tertile vs the bottom tertile OR:1.01 (0.84–1.22) |
| Sedentary work | OR:1.00 (referent)/ OR:0.96 (0.78–1.25) OR:0.78 (0.62–0.99)/ OR:1.02 (0.81–1.29) Ptrend=0.94;/ unjusted models |
|
| Population-based case-control study (449 cases, 533 controls). Interview-administered questionnaire (35) | Lifetime occupational activity, job title and work description in MET score. Recreational activity during adult life |
Decreased risk, For at least 75% of work year spent in very active jobs OR:0.54 (0.31–0.95) <75% of work year spent in sedentary jobs OR=0.64 (0.41–0.98) Non-significant decrease for category often: 0.90 (0.67–1.20); smoking, alcohol consumption, farming |
| A case-control study (35 cases, 70 controls). Interview -the International Physical Activity Questionnaire (36) | Vigorous/moderate activity no vs yes |
Increased risk, non-significant Lifetime activity OR:1.4 (0.37–5.6) Ages: 18–24, OR:1.8(0.7–4.8),/ 25–34, OR:1.1(0.4–3.0),/35–44, OR:3.0(0.8–11.1),/ 45–54, OR:2.9(0.8–10.8), ≥55 years, OR:1.0(0.4–2.7); Age, family history, education level, BMI, total energy intake per day |
Abbreviations: OR – odds ratios, RR – relative risk, CI – confidence interval, PA – physical activity; PCa – prostate cancer.
The magnitude of risk reduction found in the reviews ranged from 10% to 56% for occupational PA and from 5% to 65% for recreational activity. Risk reduction was reported in three of six included case-control studies ranging from 72% to 93%. Similarly, as the authors of two reviews (28, 30), we noticed stronger risk reduction in case-control studies compared to cohort studies.
Nutrition and prostate cancer
For this topic, we selected seven recently published reviews and meta-analyses (37–43) and nine epidemiological studies (44–52).
An inverse association between high intake of vegetables and/or fruits was reported by three (37, 42, 43) of four reviews which considered these components of a diet and in five (44–46, 49, 52) of nine epidemiological studies. A statistically significant protective effect of vegetables or fruits on PCa found in case-control studies was strong (38%–91%), although non-significant decrease in the risk for vegetable intake or a lack of association for fruit consumption was also reported (49, 52). Much lower risk reductions for vegetable/fruit intake were estimated in meta-analyses (37, 42). One review (43) and three case-control studies (45, 50, 52) reported the positive association between red meat and/or grilled and processed meats and PCa risk. A high consumption of meats was significantly linked with increased risk at least by a 56%, especially for in-take of red or processed meats. One review (38) which analyzed the relationship for high intake of saturated fats and omega-6 fatty acids found the increased risk. One case-control study (48) found increased risks by 55% and 45% for oils use and fish consumption, respectively. Besides, one case-control study (51) reported an increase in the risk in the individuals consuming more than 3.5 eggs/week. This result was not confirmed in a meta-analysis (40). Supplementation of vitamin A, E was another reported risk factor for PCa (38, 48). Further, one review reported a high probability of the risk reduction in consumers of “Mediterranean diet” and its increase in consumers of western diet (37). Similarly, a cohort study (44) found a strong risk reduction (46%) in HPFS group for diet high in tomatoes and fatty fish and poor in processed meat. In addition, two reviews and one case-control study reported decrease of PCa due to tomatoes intake (37, 43, 52).
Discussion
Physical activity and prostate cancer
The reported evidence confirms that there is a direct association between PA and PCa risk what agrees with a summary evidence of 18 human and animal studies showing etiologic role of exercise and PA in prostate carcinogenesis (35). Our findings commonly indicated for strong conflicting epidemiological findings owing to large geographical difference in incidence rates of PCa and in genetic susceptibility, stage of cancer or a group of all PCa commonly considered (aggressive, benign prostatic and hyperplasia, BPH), heterogeneity in the PA intervention and methodology, and lifestyles. There exist several hypothesized molecular mechanisms by which PA can directly or indirectly (through protection against overweight/obesity and weight gain) affects all stages of PCa carcinogenesis (8, 14, 15, 18, 53, 54). The authors have underlined that PCa is a hormonally mediated cancer and inflammation plays a key role in the disease development.
Biological direct effects of PA include alteration of the sex hormones and metabolic hormones concentrations; decrease of insulin-like growth factor, IGF-1; increase of concentration of sex hormone binding globulin (SHBG) and insulin-like growth factor–binding protein-3 (IGFBP-3); reduction of proinflammatory factors levels; modulation of adipokines; reduction of systemic inflammation; improvement of immune function through influence on a number of components of immune function; reinforcement of DNA repair; improvement of antioxidant enzyme system ability; increase of p53 protein concentration; decrease of the IGF-1/IGFBP-1 ratio; suppression of 5-α reductase activity, and activation of protein kinases activities (8, 54, 55). Thus, PA may participate in the cell control and apoptosis, e.g. through effect on the reactive oxygen species (ROS) production, known as important messengers in intracellular signaling cascades. An important role of regular moderate PA is adaptation to oxidative stress (56, 57) that is commonly considered as initiator of cancer by DNA damage (58–59), however, acute PA may increase the tumor development (56, 60).
It is worth mentioning an experimental study evaluating the effect of serum from the men bicycling at increasing intensity by 60 min on PCa cell culture to grow. Exercise serum exhibited a 31% inhibition of cancer cell line (LNCaP) growth, due to increased levels of IGFBP-1 and a reduced concentration of epidermal growth factor (EGF) (60). Other studies also reported that serum from men engaged in physical exercise had the ability to decrease the PCa cells growth (54, 61). A systematic review of clinical trials on the effect of exercise on biomarkers showed that exercise modulates various cancer pathways, especially has a large effect on reinforcement of immune system function (55). As inflammation plays a key role in PCa development, this property of exercise may be clinically important.
Research on etiologic role of PA in PCa is very difficult due to the complex nature of PA interactions as well as complexity of the carcinogenesis process. The effect of PA on PCa may depend on the cancer subtype and stage, frequency, duration, intensity, and dose of PA and also other modifiable lifestyle factors. Moreover, the main problem of observational retrospective studies is the recall bias owing to reliability of data linked with the distant past, different methodology of studies, and often-small sample size, and inadequate control for confounders. These factors may, in part, explain the observed lack of consistency between findings and to cause that comparison of the findings across the examined studies is difficult.
The Second Expert Panel (6) recommends for adults engage in moderate-intensity PA at least 150 min per week or engage in at least 75 min per week vigorous intensity activity or combination of moderate and vigorous-intensity activities to sustain the equivalent of energy expenditure needed to protect against cancer adding that all types of PA are probable protective.
Nutrition and prostate cancer
Our study provides updated evidence supporting the previous findings that high intake of meats and animal fats increases prostate carcinogenesis, and the preventive role of vegetables and fruits in this respect.
The possible biological mechanisms for the association between processed meat, red meat, saturated fats and carcinogenesis include mainly generation of chemical carcinogens (6). These carcinogens were found to be extremely mutagenic and may affect all stages of carcinogenesis. They can damage DNA, stimulate formation of IGF-1, increase levels of endogenous hormones. DNA hypomethylation was reported as possible linked with PCa development and progression (62). Further, red meat – a rich source of heme iron is involved in generation of hydroxyl radicals, thus may indirectly affect cytoplasmic and nuclear signal transduction pathways (6, 12, 62). According to the Second Expert Panel judgments (6), the level of scientific evidence that processed meat increases PCa risk is limited-suggestive.
Vegetables and fruits as rich in microelements, carotenoids, vitamins, flavonoids, and fibers may play a key role in prevention of malignancy acting as effective antioxidants, exhibiting abilities to bind and to dilute carcinogens, and to alter hormone metabolism due to having sterols (12, 20, 63). Previous epidemiological, experimental and clinical research has consistently demonstrated strong evidence that vitamins A, D, and E, selenium may have high cancer preventive capacities (22, 63–65). Metabolites of vitamin A may reverse hyperplasia, regulate the growth and apoptosis of normal and malignant cells, increase levels of other antioxidants, and regulate DNA transcription (66). Although, no significant protective effects of provitamin A, such as lycopene, lutein, zeaxanthin were reported (20). Further, vitamins A and E were found to increase PCa risk at high doses (38). The Second Expert Panel (6) concluded that there is limited-suggestive evidence that vitamin E protects against PCa. Further, lycopene is considered as the most efficient antioxidant present in cell. The preventive effect of tomato consumption on PCa risk was found for a diet, which was rich in tomato products and lycopene (67). Unfortunately, there are conflicting findings on the lycopene-PCa risk relationship and the preventive role of tomato products (29). Lately evidence that selenium lowers PCa risk has been downgraded from strong to limited-suggestive, and the associations between PCa and foods containing lycopene and selenium supplements were determined as “no conclusion possible” (11). It is worth noting that a recent evidence from a review of 44 controlled trials of dietary, nutrition and PA intervention supports the role of diet low in fat and high in broccoli, soy, selenium, lycopene, green tea in reduction of PCa progression and mortality (68).
Conclusion
There is no definitive finding supporting the preventive role of PA against all PCa, however, there is some evidence that regular vigorous PA may reduce the risk of aggressive PCa. Similarly, it was impossible to identify the specific dietary patterns having the potential to prevent against the tumor. The methodological quality of epidemiological studies was different, studies varied in design and some of them have a high risk of bias. Variations in the mode, intensity, duration, and frequency of activity, composition of diet, way of meals preparation, caloric intake, different ranges during categorization of variables and a lack separate analysis for tumor grade and stage, might have effects on evidence.
Owing to the hypothesized biological mechanisms for association between PA and PCa, high intensity of activity was found to decrease testosterone levels and to alter the sex hormone receptors. In turn, regular moderate PA may enhance antioxidant systems, thereby prevent against oxidative stress. A diet poor in fat, red and processed meat, refined carbohydrates and reach in vegetables and fruits may decrease of insulin and IGF-1 levels.
The previous and current studies have underlined an important attribute of lifestyle patterns, like their modification. Obviously, many questions remain open and much research is still needed in this area. Future research should focus on methodological quality of epidemiological studies, like specific types of PCa, more precision in assessment of PA components, quantitatively assessment of nutrient intake, large samples size, and on effect of modification by confounding factors including ethnicity. There is a need for clinical trials, which will estimate intermediate biomarkers as predictors of outcome to give more inside on mechanisms by which lifestyle components may exert effects on the PCa development.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The authors declare that there is no conflict of interests.
References
- 1. Jain S, Saxena S, Kumar A. (2014). Epidemiology of prostate cancer in India. Meta Gene, 2: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ikromov O, Alkamal I, Magheli A, Ratert N, Sendeski M, Miller K, et al. Functional Epigenetic Analysis of Prostate Carcinoma: A Role for Seryl-tRNA Synthetase? J Biomark, 2014 . : 362164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daniyal M, Siddiqui ZA, Akram M, Asif HM, Sultana S, Khan A. (2014). Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac J Cancer Prev, 15: 9575–9578. [DOI] [PubMed] [Google Scholar]
- 4. Brandt A, Sundquist J, Hemminki K. (2011). Risk for incident and fatal prostate cancer in men with a family history of any incident and fatal cancer. Ann Oncol, 23: 251. [DOI] [PubMed] [Google Scholar]
- 5. Darlington GA, Kreiger N, Lightfood N, Purdham J, Sass-Kortsak A. (2007). Prostate cancer risk and diet, recreational physical activity and cigarette smoking. Chronic Dis Can, 27: 145–153. [PubMed] [Google Scholar]
- 6. World Cancer Research Fund/American Institute for Cancer Research Food, Nutrition, Physical Activity, and Prevention of Cancer: a Global Perspective. Washington YC: AICR, 2007. [Google Scholar]
- 7. Schmid D, Steindorf K, Leitzmann MF. (2014). Epidemiologic studies of physical activity and primary prevention of cancer. Dtsch Z Sportmed, 65: 5–10. [Google Scholar]
- 8. McTiernan A. (2008). Mechanisms linking physical activity with cancer. Nat Rev Cancer, 8: 205–211. [DOI] [PubMed] [Google Scholar]
- 9. Khan N, Afag F, Mukhtar H. (2010). Lifestyle as risk factor for cancer: evidence from human studies. Cancer Lett, 293: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prostate Cancer UK (2012). Diet and physical activity for men with prostate cancer. http://www.nhs.uk/ipgmedia/National/Prostate%20Cancer%20UK/assets/Dietandprostatecancer(PCC).pdf .
- 11. World Cancer Research Fund International (2014). Diet, Nutrition, Physical Activity, and Prostate Cancer. http://www.wcrf.org/sites/default/files/Prostate-Cancer-2014-Report.pdf .
- 12. Di Sebastiano K, Maturazakis M. (2014). The role of dietary fat throughout the prostate cancer trajectory. Nutrients, 6: 6095 –6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mina DS, Connor MK, Alibhai SM, Toren P, Guglietti C, Matthew AG, et al. (2013). Exercise effects on adipokines and the IGF axis in men with prostate cancer treated with androgen deprivation: a randomized study. Can Urol Assoc J, 7: E692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kruijsen-Jaarsma M, Revesz D, Bierlings MB, Buffart LM, Takken T. (2013). Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev, 19: 120–143. [PubMed] [Google Scholar]
- 15. Laszlo R, Hartveg P, Laszlo S, Otto S, Procopchuk D, Steinacker JM. (2014). Physical activity and cancer. OA Sports Med, 2: 1. [Google Scholar]
- 16. Kruk J, Czerniak U. (2013). Physical activity and its relation to cancer risk: updating the evidence. Asian Pac J Cancer Prev, 14: 3993–4003. [DOI] [PubMed] [Google Scholar]
- 17. Zheng X, Cui XX, Huang MT, Liu Y, Shih WJ, Lin Y, et al. (2009). Physical activity reduces prostate carcinogenesis in a transgenic model. Prostate, 69: 1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. (2002). Effect of diet and exercise on serum insulin IGF-1 and IGFBP-1 levels and growth LNCaP in vitro (United States). Cancer Causes Control, 13: 929–935. [DOI] [PubMed] [Google Scholar]
- 19. Wolk A. (2005). Diet, lifestyle and risk of prostate cancer. Acta Oncol, 44: 277–281. [DOI] [PubMed] [Google Scholar]
- 20. Garg MI, Dalela D, Goel A, Kumar M, Sankhwar SW. (2014). Prevention of prostate cancer with vitamins - current perspectives. Asian Pac J Cancer Prev, 15: 1897–1904. [DOI] [PubMed] [Google Scholar]
- 21. Zheng X, Cui XX, Khor TO, Huang Y, DiPaola R, Goodin S, et al. (2011). Inhibitory effect of a γ-tocopherol-rich mixture of tocopherols on the formation and growth of LNCaP prostate tumors in immunodeficient mice. Cancers (Basel), 3: 3762–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kovalenko PL, Zhang Z, Yu JG, Li Y, Clinton SK, Fleet JC. (2011). Dietary vitamin D and vitamin D receptor level modulate epithelial cell proliferation and apoptosis in the prostate. Cancer Prev Res (Phila), 4: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer, 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 24. Center MM, Jemal A, Lortel-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. (2012). International variation in prostate cancer incidence and mortality rates. Eur Urol, 61: 1079–1092. [DOI] [PubMed] [Google Scholar]
- 25. Moore MA, Ariyaratne Y, Badar F, Bhurgri Y, Datta K, Mathew A, et al. (2010). Cancer epidemiology in South Asia - past, present and future. Asian Pac J Cancer Prev, 11 (Suppl 2): 49– 66. [PubMed] [Google Scholar]
- 26. Caspersen CJ, Powell KE, Christenson GM. (1985). Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep, 100: 126–131. [PMC free article] [PubMed] [Google Scholar]
- 27. Wekesa A, Harrison M, Watson RW. (2015). Physical activity and its mechanistic effects on prostate cancer. Prostate Cancer Prostatic Dis, 18: 197–207. [DOI] [PubMed] [Google Scholar]
- 28. Doolan GW, Benke G, Giles GG, Severi G, Kauppinen T. (2014). A case control study investigating the effects of levels of physical activity at work as a risk factor for prostate cancer. Environ Health, 13: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilson KM, Giovannucci EI, Mucci LA. (2012). Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J Androl, 14: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Hu F, Li D, Wang F, Zhu L, Chen W, et al. (2011). Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol, 60: 1029–1044. [DOI] [PubMed] [Google Scholar]
- 31. Hrafnkelsdóttir SM, Torfadóttir JE, Aspelund T, Magnusson KT, Tryggvadóttir L, Gudnason V, et al. (2015). Physical activity from early adulthood and risk of prostate cancer: a 24-year follow-up study among Icelandic men. Cancer Prev Res (Phila), 8: 905–911. [DOI] [PubMed] [Google Scholar]
- 32. De Nunzio C, Presicce F, Lombardo R, Cancrini F, Petta S, Trucchi A, et al. (2015). Physical activity as a risk factor for prostate cancer diagnosis: a prospective biopsy cohort analysis. BJU Int, 117( 6B): E29–35. [DOI] [PubMed] [Google Scholar]
- 33. Bashir MN, Ahmad MR, Malik A. (2014). Risk factors of prostate cancer: a case-control study in Faisalabad, Pakistan. Asian Pac J Cancer Prev, 15: 10237–10240. [DOI] [PubMed] [Google Scholar]
- 34. Doolan G, Benke G, Giles G. (2014). An update on occupation and prostate cancer. Asian Pac J Cancer Prev, 15: 501–516. [DOI] [PubMed] [Google Scholar]
- 35. Parent ME, Rousseau MC, El-Zein M, Latreille B, Désy M, Siemiatycki J. (2011). Occupational and recreational physical activity during adult life and the risk of cancer among men. Cancer Epidemiol, 35: 151–159. [DOI] [PubMed] [Google Scholar]
- 36. Shahar S, Shafurah S, Shaari NSAH, Rajikan R, Rajab NF, Golkhalkhali B, Zainuddin ZM. (2011). Roles of diet, lifetime physical activity and oxidative DNA damage in the occurrence of prostate cancer among men in Klang Valley, Malaysia. Asian Pac J Cancer Prev, 12: 605–611. [PubMed] [Google Scholar]
- 37. Lin P-H, Aronson W, Freedland SJ. (2015). Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC Med, 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Masko EM, Allott EH, Freedland SJ. (2013). The relationship between nutrition and prostate cancer, is more always better? Eur Urol, 63: 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meng H, Hu W, Chen Z, Shen Y. (2014). Fruit and vegetable intake and prostate cancer risk: a meta-analysis. Asia Pac J Clin Oncol, 10: 133–140. [DOI] [PubMed] [Google Scholar]
- 40. Xie B, He H. (2012). No association between egg intake and prostate cancer risk: a meta-analysis. Asian Pac J Cancer Prev, 13: 4677–4681. [DOI] [PubMed] [Google Scholar]
- 41. Chua ME, Sio MC, Sorongon MC, Dy JS. (2012). Relationship of dietary intake of omega-3 and omega-6 fatty acids with risk of prostate cancer development: a meta-analysis of prospective studies and review of literature. Prostate Cancer, 2012: 826254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu B, Mao Q, Cao M, Xie L. (2012). Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol, 19: 134–141. [DOI] [PubMed] [Google Scholar]
- 43. Ma RW-L, Chapman K. (2009). A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet, 22: 187–199. [DOI] [PubMed] [Google Scholar]
- 44. Kenfield SA, Batista JL, Jahn JL, Downer MK, Van Blarigan EL, et al. (2015). Development and application of a lifestyle score for prevention of lethal prostate cancer. J Natl Cancer Inst, 108 (3): djv329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bashir MN, Malik MA. (2015). Case-control study of diet and prostate cancer in a rural population of Faisalabad, Pakistan. Asian Pac J Cancer Prev, 16: 2375–2378. [DOI] [PubMed] [Google Scholar]
- 46. Askari F, Parizi MK, Jessri M, Rashidkhani B. (2014). Fruit and vegetable intake in relation to prostate cancer in Iranian men: a case-control study. Asian Pac J Cancer Prev, 15: 5223–5227. [DOI] [PubMed] [Google Scholar]
- 47. De Stefani E, Moore M, Aune D, Deneo-Pellegrini HD, Ronco AL, Boffetta P, et al. (2011). Maté consumption and risk of cancer: a multi-site case-control study in Uruguay. Asian Pac J Cancer Prev, 12: 1089–1093. [PubMed] [Google Scholar]
- 48. Tyagi B, Manoharan N, Raina V. (2010). A case-control study on prostate cancer in Delhi. Asian Pac J Cancer Prev, 11: 397–401. [PubMed] [Google Scholar]
- 49. Aune D, De Stefani E, Ronco AL, Boffetta P, Deneo-Pellegrini D, Acosta G, Mendilaharasu M. (2009). Fruits, vegetables, and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev, 10: 419–428. [PubMed] [Google Scholar]
- 50. Aune D, De Stefani E, Ronco AL, Boffetta P, Deneo-Pellegrini D, Acosta G, Mendilaharasu M. (2009). Meat consumption and cancer risk: a case-control study in Uruguay. Asian Pac J Cancer Prev, 10: 429–436. [PubMed] [Google Scholar]
- 51. Aune D, De Stefani E, Ronco AL, Boffetta P, Deneo-Pellegrini D, Acosta G, Mendilaharasu M. (2009). Egg consumption and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev, 10: 869–876. [PubMed] [Google Scholar]
- 52. Subahir MN, Shah SA, Zainuddin ZM. (2009). Risk factors for prostate cancer in Universiti Kebangsaan Malaysia Medical Centre: a case-control study. Asian Pac J Cancer Prev, 10: 1015–1020. [PubMed] [Google Scholar]
- 53. Neuhouser ML, Schenk J, Song YJ, Tangen CM, Goodman PJ, Pollak M, et al. (2008). Insulin-like growth factor-1, insulin-like growth factor binding protein-3 and risk of benign prostate hyperplasia in the prostate cancer prevention trial. Prostate, 68: 1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leung PS, Aronson WJ, Ngo TH, Golding LA, Barnard RJ. (2004). Exercise alters the IGF axis in vivo and increases p53 protein in prostate tumor cells in vitro. J Appl Physiol (1985), 96: 450–454. [DOI] [PubMed] [Google Scholar]
- 55. Winzer BM, Whiteman DC, Reeves M, Paratz JD. (2011). Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control, 22: 811–826. [DOI] [PubMed] [Google Scholar]
- 56. Kruk J. (2011). Physical exercise and oxidative stress. Med Sport, 15: 30–40. [Google Scholar]
- 57. Friedenreich CM, Neilson HK, Lynch BM. (2010). State of epidemiological evidence on physical activity and cancer prevention. Eur J Cancer, 46: 2593–2604. [DOI] [PubMed] [Google Scholar]
- 58. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. (2010). Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med, 49: 1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Venza M, Wisalli M, Beninati C, De Gaetano GV, Teti D, Venza I. (2015). Cellular mechanisms of oxidative stress and action in melanoma. Oxid Med Cell Longev, 2015: 481782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rundqvist H, Augsten M, Strömberg A, Rullman E, Mijwel S, Kharaziha, et al. (2013). Effect of acute exercise on prostate cancer cell growth. PLoS One, 8 (7): e67579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Soliman S, Aronson WJ, Barnard RJ. (2011). Analyzing serum-stimulated prostate cancer cell lines after low-fat, high-fiber diet and exercise intervention. Evid Based Complement Alternat Med, 2011: 529053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zelic R, Fiano V, Grasso C, Zugna D, Pettersson A, Gillio-Tos A, et al. (2015). Global DNA hypomethylation in prostate cancer development and progression: a systematic review. Prostate Cancer Prostatic Dis, 18: 1–12. [DOI] [PubMed] [Google Scholar]
- 63. Kein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. (2011). Vitamin E and the risk of prostate cancer, the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA, 306: 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Travis RC, Crowe FL, Allen NE, Appleby PN, Roddam AW, Tjønneland A, et al. (2009). Serum vitamin D and risk of prostate cancer in a case-control analysis nested within the European prospective investigation into cancer and nutrition (EPIC). Am J Epidemiol, 169: 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hurst R, Hooper L, Norat T, Lau R, Aune D, Greenwood DC, et al. (2012). Selenium and prostate cancer: systematic review and meta-analysis. Am J Clin Nutr, 96: 111–122. [DOI] [PubMed] [Google Scholar]
- 66. Mondul AM, Watters JL, Mannisto S, Weinstein SJ, Snyder K, Virtamo J, et al. (2011). Serum retinol and risk of prostate cancer. Am J Epidemiol, 173: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Etminan M, Takkouche B, Caamano-Isorna F. (2004). The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev, 13: 340–345. [PubMed] [Google Scholar]
- 68. Hackshaw-McGeagh LE, Perry RE, Leach VA, Quandil S, Jeffreys M, Martin RM, Lane JA. (2015). A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control, 26: 1521–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]