Abstract
Background:
Current food safety issues are deleteriously reshaping the lifestyle of the population in the developing world. The globalization of food supply impacts patterns of foodborne disease outbreaks worldwide, and consumers are having increased concern about microbiological food safety.
Methods:
A total of 2305 samples including sauced meat, sausage, smoked meat, shrimp, sashimi and shellfish were collected from different farmer’s markets and supermarkets. The prevalence of selected foodborne pathogens was evaluated in cooked meat and seafood from 2010 to 2013 in Shandong Province, China.
Results:
The average contamination rate was 6.39% (93.1456) for the selected pathogens in cooked meat and 16.84% (143.849) for V. parahaemolyticus in seafood. For the selected pathogens, 0.55%, 1.03%, 1.17%, 3.64% and 16.84% samples were contaminated with E.coli O157: H7, Salmonella spp., L. monocytogenes, S. aureus and VP, respectively. There was a significant (P<0.05) difference in the contamination rate between the farmer’s markets and supermarkets.
Conclusion:
The contamination was decreasing in cooked meat and maintaining a relatively high level in seafood from 2010 to 2013. E. coli O157: H7, S. aureus, L. monocytogenes and Salmonella spp. existed at a relatively low rate in retail foods. For VP, the contamination rate has been maintained at a relatively high level in Shandong Province in China. Moreover, cooked meat and seafood obtained from farmer’s markets are more susceptible to be contaminated compared to those from supermarkets.
Keywords: Food safety, Foodborne pathogens, Cooked meat, Seafood
Introduction
Food production and consumption is now making an important contribution to development of countries, food safety associated with foodborne pathogens is undoubtedly facing great challenges (1). Annually, one-third of the world population is infected by foodborne pathogens (2). Even in the United States, 9.4 million foodborne episodes, 55961 hospitalizations and 1351 deaths were due to 31 major pathogens every year (3–5). Therefore, the importance of monitoring foodborne pathogens from various sources was necessary, including cooked meat (6, 7) and seafood (8, 9).
With the improvement of living standards, the consumption of cooked meat and seafood is increasing for the higher value and richer in micro-nutrients (10). Food safety has become a global challenge, and most countries are victims of foodborne illnesses (11). The incidence of food-borne illnesses do increase in the every summer and autumn, and mainly for two reasons. First, there are the natural causes, foodborne pathogen grow faster in the warm summer and autumn months. Second, outside activities do increase and the safety controls do implement difficulty (12, 13).
Being convenient and nutritious, the cooked meat and seafood are very popular worldwide. However, for inadequate hygienic measures during preparation, retailing and consumption, foods can be easily contaminated by foodborne pathogens such as Escherichia coli, Salmonella spp., Listeria, and Staphylococcus aureus (S. aureus) (14). Due to culture, climate and economic status, every country and region has its own unique food safety issues, but foodborne diseases caused by pathogens are similar. For cooked meat, the common foodborne pathogens include Escherichia coliO157: H7 (E. coli O157: H7), S. aureus, Listeria monocytogenes (L. monocytogenes) and Salmonella spp. (15, 16); for seafood, the most important pathogen is Vibrio parahaemolyticus (VP) (17, 18).
In the United States, each year more than 63000 illnesses, 2138 hospitalizations, and 20 deaths were caused by the food infected with E. coli O157: H7 (4). It associated with products ranging from ground beef to processed foods. L. monocytogenes is foodborne pathogen that can cause listeriosis in humans. The foodborne listeriosis outbreaks have been reported and recognized worldwide as a major public health concern (19). Salmonellosis and staphylococcal food poisoning are two of the main food-borne diseases (20). Salmonellosis is often caused by the contaminated foods such as meat products, eggs and dairy products (21, 22). Moreover, the existence of S. aureus in ready to eat (RTE) food has been reported by many countries, such as Korea, Brazil, and Greece (23–25). Moreover, the foodborne disease outbreak caused by VP mainly was associated with different kinds of seafood and occurs in summer and autumn (26).
In Shandong Province, a populous and major tourism province, food safety is particularly important. However, the contamination status of foodborne pathogens is not clear for cooked meat and seafood. In light of their importance, the aim of this research was to assess to what extent the selected foodborne pathogens are prevalent in cooked meat and seafood in Shandong Province. The results of this study may provide useful information for updating and assessing the risks for concerned departments, which oversee food safety.
Methods
Collection of food samples
There are two types of retail markets in China: supermarkets and farmer’s markets. Supermarkets are indoors, often air-conditioned, typically offer controlled-temperature environments. In contrast, farmer’s markets are traditional open-air markets where foodstuffs are sold without air-conditioned. Cooked meat products and seafood products were purchased randomly from geographically different farmer’s markets and supermarkets in Shandong Province from 2010 to 2013. Shandong Province consists of 17 prefectures. The sampling sites included 12 supermarkets and 6 farmer’s markets in 6 prefectures from 2010 to 2011.According to our real works, the sites were enlarged to 34 supermarkets and 17 farmer’s markets in 17 prefectures respectively in 2012 and 2013.
The sample size was determined by following Manual for Risk Surveillance on Foodborne Pathogens issued by National Health and Family Planning Commission of the People’s Republic of China. Three categories of cooked meat were collected including smoked meat, sausage and sauced meat. Three categories of seafood were collected including shrimp, sashimi and shellfish. All samples were transported to the laboratory in the insulated box with ice to maintain the temperature at 4 °C–6 °C. After removing the substandard samples, the actual number of tested samples was shown in Table 1 and Table 2.
Table 1:
The samples of cooked meat were collected in Shandong Province from 2010 to 2013
| Years | Seasons | Supermarkets | Farmer’smarkets | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoked meat | Sausage | Sauced meat | Smoked meat | Sausage | Sauced meat | ||||||||
| Packages | Sliced forms | Packages | Sliced forms | Packages | Sliced forms | Packages | Sliced forms | Packages | Sliced forms | Packages | Sliced forms | ||
| 2010 | Summer | 0 | 4 | 5 | 2 | 0 | 16 | 0 | 0 | 0 | 4 | 0 | 15 |
| Autumn | 6 | 10 | 14 | 7 | 3 | 73 | 0 | 4 | 0 | 0 | 0 | 3 | |
| Total | 6 | 14 | 19 | 9 | 3 | 89 | 0 | 4 | 0 | 4 | 0 | 18 | |
| 2011 | Summer | 0 | 11 | 14 | 11 | 12 | 27 | 0 | 0 | 0 | 0 | 0 | 9 |
| Autumn | 4 | 7 | 8 | 8 | 8 | 36 | 0 | 5 | 0 | 1 | 1 | 15 | |
| Total | 4 | 18 | 22 | 19 | 20 | 63 | 0 | 5 | 0 | 1 | 1 | 24 | |
| 2012 | Summer | 5 | 15 | 30 | 17 | 11 | 137 | 0 | 15 | 4 | 5 | 1 | 77 |
| Autumn | 4 | 3 | 43 | 20 | 23 | 121 | 2 | 16 | 7 | 13 | 2 | 51 | |
| Total | 9 | 18 | 73 | 37 | 34 | 258 | 2 | 31 | 11 | 18 | 3 | 128 | |
| 2013 | Summer | 20 | 14 | 48 | 5 | 65 | 74 | 4 | 6 | 10 | 5 | 7 | 61 |
| Autumn | 19 | 6 | 10 | 8 | 42 | 55 | 0 | 3 | 0 | 0 | 0 | 29 | |
| Total | 39 | 20 | 58 | 13 | 107 | 129 | 4 | 9 | 10 | 5 | 7 | 90 | |
Table 2:
The samples of seafood were collected in Shandong Province from 2010 to 2013
| Years | Seasons | Supermarkets | Farmer’s markets | ||||
|---|---|---|---|---|---|---|---|
| Shrimp | Sashimi | Shellfish | Shrimp | Sashimi | Shellfish | ||
| 2010 | Summer | 2 | 8 | 0 | 0 | 4 | 1 |
| Autumn | 16 | 28 | 42 | 9 | 1 | 13 | |
| Total | 18 | 36 | 42 | 9 | 5 | 14 | |
| 2011 | Summer | 18 | 33 | 25 | 8 | 6 | 7 |
| Autumn | 21 | 45 | 18 | 5 | 5 | 3 | |
| Total | 39 | 78 | 43 | 13 | 11 | 10 | |
| 2012 | Summer | 22 | 36 | 30 | 7 | 23 | 41 |
| Autumn | 18 | 46 | 30 | 18 | 20 | 32 | |
| Total | 40 | 82 | 60 | 25 | 43 | 73 | |
| 2013 | Summer | 13 | 40 | 27 | 5 | 6 | 8 |
| Autumn | 8 | 26 | 6 | 29 | 18 | 22 | |
| Total | 21 | 66 | 33 | 34 | 24 | 30 | |
E. coli O157: H7
25g sample was homogenized with 225 mL of sterile mEC+n enrichment broth (Luqiao, China) and incubated at 36 °C for 20 h. Following incubation, one loop of broth was streaked onto Chromogenic Agar O157 (Chromagar, France) and incubated at 36 °C for 20 h. Suspected colonies was picked and confirmed by GN cards (BioMérieux, France) (27).
S. aureus
Twenty-five g sample was homogenized with 225 mL of 7.5% NaCl broth (Huankai, China) and incubated at 36 °C for 20 h. One loop of broth was streaked onto S.aureus chromogenic agar (Chromagar) and incubated at 36°C for 20 h. Suspected colonies was picked and confirmed by GP cards (BioMérieux, France) (28).
L. monocytogenes
25g food sample was homogenized in 225 mL of listeria enrichment broth (Luqiao) with listeria selective enrichment supplement and incubated for 48 h at 30°C. After incubation, one loop of broth was streaked onto listeria chromogenic agar (Chromagar) and incubated at 36 °C for 24–48 h. Suspected colonies was picked and confirmed by GP cards (BioMérieux, France) (29).
Salmonella spp.
25g food sample was homogenized in 225 mL of buffered peptone water (BPW) (Huankai) and incubated at 36 °C for 20 h. Then, 0.1 mL of the broth was added to 10 mL of selenite cystine broth (Huankai) and was incubated at 42 °C overnight. Following incubation, one loop of broth was streaked onto Salmonella chromogenic agar (Chromagar) and incubated at 36 °C for 24 h. Suspected colonies was picked and confirmed by GN cards (BioMérieux, France) (30).
Vibrio parahaemolyticus
25g food sample was homogenized in 225 mL of alkaline peptone water (Huankai) with 3% NaCl and incubated at 36 °C for 20 h. One loop of mixture was streaked onto vibrio chromogenic agar (Chromagar) and incubated at 36 °C for 20 h. Typical suspected colonies was picked and confirmed by GN cards (31).
Statistical analysis
The relationship between the contaminated samples and the different kinds of samples was analyzed using chi-squared analysis. All statistical and chi-squared analyses were performed using SPSS 19.0. A P<0.05 was used for statistical significance.
Results
From 2010 to 2013, 2305 samples including sauced meat, sausage, smoked meat, shrimp, sashimi and shellfish were collected from different farmer’s markets and supermarkets in Shandong Province. 236 positive strains were identified by the method of chromogenic media and biochemical test among them, E. coli O157:H7 accounted for 8, S. aureus for 53, L. monocytogenes for 17, Salmonella spp. for 17 and VP for 143.
For cooked meat, the average contamination rate was 6.39% (93.1456) for the selected pathogens in the past four years. According to the annual statistics, the prevalence of the selected pathogens decreased (3.26%, 16.491) in 2013, have a significant differences when it compared to the year 2010 (9.04%, 15.166), 2011 (7.34%, 13.177) and 2012 (7.88%, 49.622).
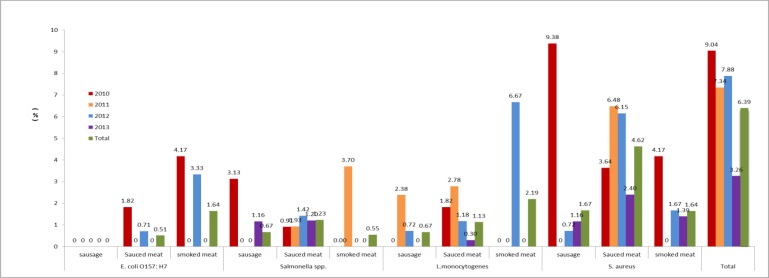
According to the statistics of tested food, the detection rate of tested pathogens in sausage (3.01%, 9.299) was significantly lower compared with in bacon (6.01%, 11.183) and in sauced meat (7.49%, 73.974) (Fig. 1).
Fig. 1:
The contamination rate of foodborne pathogens in cooked meat in Shandong Province from 2010 to 2013
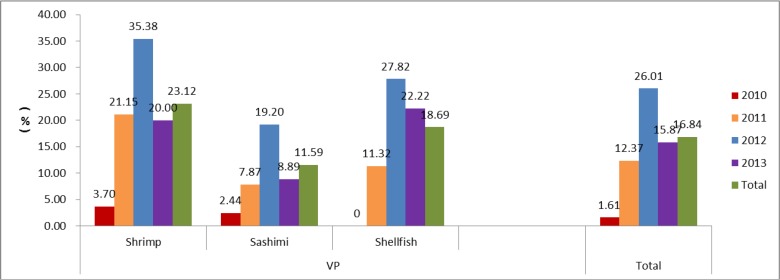
For seafood, the average contamination rate was 16.84% (143.849) for VP in the past four years. According to the annual statistics, the contamination rate was lower in 2010 (1.61%, 2.124), with significant differences compared to the year 2011 (12.37%, 24.194), 2012 (26.01%, 84.323) and 2013 (15.87%, 33.208). According to the statistics of tested foods, the positive rate of shrimp (23.12%, 46.199) was the highest compared to sashimi (11.59%, 40.345) and shellfish (18.69%, 57.305) (Fig. 2).
Fig. 2:
The contamination rate of foodborne pathogens in seafood in Shandong Province from 2010 to 2013
For the selected pathogens, 8 (0.55%) samples were contaminated with E. coli O157: H7, and among them, 5 (0.51%) and 3 (1.64%) sauced meat and smoked meat samples were contaminated with E. coli O157: H7, no E. coli O157: H7 was detected in 299 samples of sausage; 15 (1.03%) Salmonella spp. isolates were detected, and among them, 12 (1.23%), 2 (0.67%) and 1 (0.55%) were detected in sauced meat, sausage and smoked meat samples, respectively; 17 (1.17%) samples were contaminated with L. monocytogenes, and among them, 11(1.13%), 2 (0.67%) and 4 (2.18%) sauced meat, sausage and bacon samples were contaminated with L. monocytogenes, respectively; 53 (3.64%) S. aureus isolates were detected, and among them, 45 (4.62%), 5 (1.67%) and 3 (1.64%) were detected in sauced meat, sausage and bacon samples, respectively (Fig. 1).
Based on the cooked meat and seafood in the four years, generally, there was a significant (P<0.05) difference in the selected contamination rate of the pathogens between the farmer’s markets and supermarkets (Tables 3, 4). For cooked meat products, the contamination was decreasing from 2010 to 2013 in farmer’s markets and supermarkets (Table 3). For seafood, however, the contamination was maintaining a relatively high level in every year from 2010 to 2013 (Table 4).
Table 3:
The contamination rate of cooked meat in different markets in Shandong Province from 2010 to 2013
| Years | Super markets | Farmer’s markets | Total | Contaminated samples | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supermarkets | Farmer’s markets | Total | ||||||||
| number | (%) | number | (%) | number | (%) | |||||
| 2010 | 140 | 26 | 166 | 10 | 7.14 | 5 | 19.23 | 15 | 9.04 | 0.06 |
| 2011 | 146 | 31 | 177 | 6 | 4.11 | 7 | 22.58 | 13 | 7.34 | 0.00 |
| 2012 | 429 | 193 | 622 | 18 | 4.20 | 31 | 16.06 | 49 | 7.88 | 0.00 |
| 2013 | 366 | 125 | 491 | 6 | 1.64 | 10 | 8.00 | 16 | 3.26 | 0.00 |
| Total | 1081 | 375 | 1456 | 40 | 3.70 | 53 | 14.13 | 93 | 6.39 | 0.00 |
Table 4:
The contamination rate of seafood in different markets in Shandong Province from 2010 to 2013
| Years | Super markets | Farmer’s markets | Total | Contaminated samples | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supermarkets | Farmer’s markets | Total | ||||||||
| number | (%) | number | (%) | number | (%) | |||||
| 2010 | 96 | 28 | 124 | 1 | 1.04 | 1 | 3.57 | 2 | 1.61 | 0.40 |
| 2011 | 160 | 34 | 194 | 15 | 9.36 | 9 | 26.47 | 24 | 12.37 | 0.02 |
| 2012 | 182 | 141 | 323 | 47 | 25.82 | 37 | 26.24 | 84 | 26.01 | 1.00 |
| 2013 | 119 | 89 | 208 | 12 | 10.08 | 21 | 23.6 | 33 | 15.87 | 0.00 |
| Total | 557 | 292 | 849 | 75 | 13.91 | 68 | 21.94 | 143 | 16.84 | 0.00 |
Discussion
Cooked meat can provide a source of readily available and nutritious meals for the consumers, but as these foods do not receive any heat treatment before consumption, the first priority should be their safety and microbiological quality. For cooked meat, 3.64% of samples were positive for S. aureus, which was lower than the contamination rate of RTE foods (5.98%) reported in Korea (32) and post-cooked samples (16.7%) in Trinidad (33); 1.17% of samples were positive for L. monocytogenes, which was higher than the reported incidences (0.37%) in United Kingdom (34) and significantly lower than Brazil where the rates was 42.50% (35); 1.00% of samples were positive for Salmonella spp., which was consistent to the rate of cooked ham samples (1.89%) reported in Spain (36); the rate of E. coli O157: H7 (0.55%) was lower than other selected pathogens. The fact that the selected pathogens were isolated from samples of cooked meat products suggests that either the heat treatment had been inadequate, or post-processing contamination of the food products had occurred. Another possible explanation is bacterial contamination at retail process, where improper handling and cross-contamination during transportation and storage are possible. The results that the prevalence of pathogens significantly decreased in 2013 compared to other years suggest the supervision of the food at the factory and circulation is effectual and should be strengthened to reduce food contamination continuously in China.
Seafood is gaining popularity worldwide because it is considered healthy and nutritious. However, infections by VP from seafood are frequently reported in worldwide (37) including in China (38), this is caused by consumption of raw or partially cooked seafood contaminated with VP (39, 40). In this study, we noticed that the average contamination rate of seafood was 16.84% for VP in the past four years, especially for shrimp, the average contamination rate was 23.12%. Furthermore, the contamination rate has been maintained at a relatively high level except in 2010. These facts suggested that the high prevalence of VP in seafood presents maybe a great threat to human health and effective microbial inactivation methods should be employed in seafood postharvest processing to reduce seafood illness caused by VP in China. If possible, the effective intervention methods, for example, physical methods (41), chemical methods (42) and biological methods (43), for reducing VP used during seafood processing and consumption should be evaluated in the further studies. For physical methods, thermal processing, low-temperature freezing, high-pressure processing (HPP) and irradiation are reported to effectively inactivate or kill VP (41). Similar to the physical methods, chemical reagents and persistent broad-host-range Phages have been used for reducing the bacterial contamination in seafood (42–44). However, none of these absolutely dislodge VP from seafood, and further research is required that focuses on the screening and development of new methods.
There are two types of retail markets in China: supermarkets and farmer’s markets. In this study, the result that a significant (P<0.05) difference in the selected pathogens contamination rate between the supermarkets and farmer’s markets may suggest farmer’s markets are more susceptible to cross-contamination than supermarkets, which may be caused by constant exposure to environmental factors such as dust, rodents, and insects (45,46). Moreover, farmer’s markets are traditional open-air markets and not air-conditioned; these increase the risk of bacterial contamination and proliferation. According to Chinese standards of RTE food, foodborne pathogenic bacteria cannot be detected. Therefore, efforts must be made by vendors and supervisors to improve the levels of hygienic conditions to reach that of supermarkets at least. For example, vendors should strengthen disinfection and sterilization of RTE food and marketing conditions, and supervisors should strengthen supervision and monitoring.
Conclusion
E. coli O157: H7, S. aureus, L. monocytogenes and Salmonella spp. existed at a relatively low rate in retail foods. For VP, the contamination rate has been maintained at a relatively high level in Shandong Province in China. The potential of these foodborne pathogens in cooked meat and seafood should not be neglected. The supervision department should set rigorously national standard to ensure food safety. Meanwhile, special emphasis must be given to public education and mass awareness programs for strengthening food safety controls. The prevalence of microbial contamination in retail cooked meat and seafood can provide a foundation for future studies. Cooked meat and seafood obtained from farmer’s markets are more susceptible to be contaminated compared to those from supermarkets. If possible, further research is necessary to understand the origin and transmission route of these pathogens in cooked meat and seafood, which can help reduce foodborne diseases.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This study was supported by grants from the Health and Family Planning Commission of Shandong Province (2013WS0163, 2011HZ057) National Science and Technology Major Project of the Ministry of Science and Technology of China (2012ZX10004215) and National Science Foundation of China (NO. 81202260). The authors declare that there is no conflict of interest.
References
- 1. WHO (2008). Foodborne disease outbreaks: Guidelines for investigation and control. http://www.who.int/foodsafety/publications/foodborne_disease/outbreak_guidelines.pdf .
- 2. WHO (2004). Food safety at risk in Asia and the Pacific. http://www.fao.org/Newsroom/en/news/2004/43073/index.html . [PubMed]
- 3. Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. (2011). Foodborne illness acquired in the United States—unspecified agents. Emerg Infect Dis, 17( 1): 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. (2011). Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis, 17( 1): 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gould LH, Walsh KA, Vieira AR, Herman K, Williams IT, Hall AJ, Cole D. (2013). Surveillance for foodborne disease outbreaks - United States, 1998–2008. MMWR Surveill Summ, 62( 2): 1–34. [PubMed] [Google Scholar]
- 6. Nyenje ME, Odjadjare CE, Tanih NF, Green E, Ndip RN. (2012). Foodborne pathogens recovered from ready-to-eat foods from roadside cafeterias and retail outlets in Alice, Eastern Cape Province, South Africa: public health implications. Int J Environ Res Public Health, 9( 8): 2608–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ohk SH, Bhunia AK. (2013). Multiplex fiber optic biosensor for detection of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella enterica from ready-to-eat meat samples. Food Microbiol, 33( 2): 166–171. [DOI] [PubMed] [Google Scholar]
- 8. Coly I1, Sow AG, Seydi M, Martinez-Urtaza J. (2013). Vibrio cholerae and Vibrio parahaemolyticus detected in seafood products from Senegal. Foodborne Pathog Dis, 10( 12): 1050–1058. [DOI] [PubMed] [Google Scholar]
- 9. Hara-Kudo Y, Konuma H, Kamata Y, Miyahara M, Takatori K, Onoue Y, Sugita-Konishi Y, Ohnishi T. (2013). Prevalence of the main food-borne pathogens in retail food under the national food surveillance system in Japan. Food Addit Contam Part A Chem Anal Control Expo Risk Assess, 30( 8): 1450–1458. [DOI] [PubMed] [Google Scholar]
- 10. Broglia A, Kapel C. (2011). Changing dietary habits in a changing world: emerging drivers for the transmission of foodborne parasitic zoo-noses. Vet Parasitol, 182( 1): 2–13. [DOI] [PubMed] [Google Scholar]
- 11. Akhtar S, Sarker MR, Hossain A. (2014). Microbiological food safety: a dilemma of developing societies. Crit Rev Microbiol, 40( 4): 348–359. [DOI] [PubMed] [Google Scholar]
- 12. Wilson E. (2015). Foodborne illness and seasonality related to mobile food sources at festivals and group gatherings in the state of Georgia. J Environ Health, 77( 7): 8–11. [PubMed] [Google Scholar]
- 13. Ravel A, Smolina E, Sargeant JM, Cook A, Marshall B, Fleury MD, Pollari F. (2010). Seasonality in human salmonellosis: assessment of human activities and chicken contamination as driving factors. Foodborne Pathog Dis, 7( 7): 785–794. [DOI] [PubMed] [Google Scholar]
- 14. Kotzekidou P. (2013). Microbiological examination of ready-to-eat foods and ready-to-bake frozen pastries from university canteens. Food Microbiol, 34( 2): 337–43. [DOI] [PubMed] [Google Scholar]
- 15. Ananchaipattana C1, Hosotani Y, Kawasaki S, Pongsawat S, Latiful BM, Isobe S, Inatsu Y. (2012). Prevalence of foodborne pathogens in retailed foods in Thailand. Foodborne Pathog Dis, 9( 9): 835–40. [DOI] [PubMed] [Google Scholar]
- 16. Jamali H1, Radmehr B, Ismail S. (2014). Prevalence and antimicrobial resistance of Listeria, Salmonella, and Yersinia species isolates in ducks and geese. Poult Sci, 93( 4): 1023–1030. [DOI] [PubMed] [Google Scholar]
- 17. Suffredini E, Mioni R, Mazzette R, Bordin P, Serratore P, Fois F, Piano A, Cozzi L, Croci L. (2014). Detection and quantification of Vibrio parahaemolyticus in shellfish from Italian production areas. Int J Food Microbiol, 184: 14–20. [DOI] [PubMed] [Google Scholar]
- 18. Jones EH, Feldman KA, Palmer A, Butler E, Blythe D, Mitchell CS. (2013). Vibrio infections and surveillance in Maryland, 2002–2008. Public Health Rep, 128 ( 6 ): 537 –545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maertens de Noordhout C, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, Havelaar A, Speybroeck N. (2014). The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis, 14( 11): 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. (1999). Food-related illness and death in the United States. Emerg Infect Dis, 5( 5): 607–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention (2006). Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food--10 States, United States, 2005. MMWR Morb Mortal Wkly Rep, 55( 14): 392–395. [PubMed] [Google Scholar]
- 22. Knudsen GM, Sommer HM, Sørensen ND, Olsen JE, Aabo S. (2011). Survival of Salmonella on cuts of beef carcasses subjected to dry aging. J Appl Microbiol, 111( 4): 848–854. [DOI] [PubMed] [Google Scholar]
- 23. Sergelidis D, Abrahim A, Papadopoulos T, Soultos N, Martziou E, Koulourida V, Govaris A, Pexara A, Zdragas A, Papa A. (2014). Isolation of methicillin-resistant Staphylococcus spp. from ready-to-eat fish products. Lett Appl Microbiol, 59( 5): 500–506. [DOI] [PubMed] [Google Scholar]
- 24. Kim NH, Yun AR, Rhee MS. ( 2011 . ). Prevalence and classification of toxigenic Staphylococcus aureus isolated from refrigerated ready-to-eat foods (sushi, kimbab and California rolls) in Korea . J Appl Microbiol, 111 ( 6 ): 1456 –1464. [DOI] [PubMed] [Google Scholar]
- 25. Rizek CF, Matté MH, Dropa M, Mamizuka EM, de Almeida LM, Lincopan N, Matté GR, Germano PM. (2011). Identification of Staphylococcus aureus carrying the mecA gene in ready-to-eat food products sold in Brazil. Foodborne Pathog Dis, 8( 4): 561–563. [DOI] [PubMed] [Google Scholar]
- 26. Wang R, Zhong Y, Gu X, Yuan J, Saeed AF, Wang S. (2015). The pathogenesis, detection, and prevention of Vibrio parahaemolyticus. Front Microbiol, 6: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MFLP-80 Isolation and Identification of E. coli O157:H7 in Foods . https://www.bd.com/ds/technicalCenter/charts/ch-ecoli_workflow_fda_usda.pdf
- 28. Hu Kim, Hu, Goepfert Jm. Isolation and enumeration of the Bacillus cereus group in foods . pubmedcentralcanada.ca/pmcc/articles/PMC376367/pdf/applmicro00120-0119.pdf .
- 29. MFLP-74 Enumeration of Listeria monocyto-genes in foods ( 2011 . ). http://www.hc-sc.gc.ca/fn-an/res-rech/analy-meth/microbio/volume3-eng.php
- 30. MFHPB-20 , Isolation and Identification of Salmonella from Foods . http://doc.mbalib.com/view/d2b3d2bfed24ef386714adab0de8c57c.html . (In Chinese).
- 31. MFLP-37 Part 1: Detection of Halophilic Vibrio species in Seafood ( 2006 . ). http://www.hc-sc.gc.ca/fn-an/res-rech/analy-meth/microbio/volume3-eng.php
- 32. Oh SK, Lee N, Cho YS, Shin DB, Choi SY, Koo M. (2007). Occurrence of toxigenic Staphylococcus aureus in ready-to-eat food in Korea. J Food Prot, 70: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 33. Syne SM, Ramsubhag A, Adesiyun AA. ( 2013 . ). Microbiological hazard analysis of ready-to-eat meat processed at a food plant in Trinidad, West Indies . Infect Ecol Epidemiol. 3 : 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gormley FJ1, Little CL, Grant KA, de Pinna E, McLauchlin J. (2010). The microbiological safety of ready-to-eat specialty meat from markets and specialty food shops: a UK wide study with a focus on Salmonella and Listeria monocytogenes. Food Microbiol, 27( 2): 243–249. [DOI] [PubMed] [Google Scholar]
- 35. Fai AE, de Figueiredo EA, Verdin SE, Pinheiro NM, Braga AR, Stamford TL. (2011). Salmonella spp. and Listeria monocytogenes in fully cooked ham commercialized in supermarkets of Fortaleza (CE, Brazil): risk factor for public health. Cien Saude Colet, 16( 2): 657–662. [DOI] [PubMed] [Google Scholar]
- 36. Cabedo L, Picart i Barrot L, Teixidó i Canelles A. (2008). Prevalence of Listeria monocytogenes and Salmonella in ready-to-eat food in Catalonia, Spain. J Food Prot, 71( 4): 855–859. [DOI] [PubMed] [Google Scholar]
- 37. WHO (2011). Risk assessment of Vibrio parahaemolyticus in seafood. In: WHO (Ed.), MRA Series, Joint FAO/WHO expert meetings on microbiological risk assessment. [Google Scholar]
- 38. Ma C1, Deng X, Ke C, He D, Liang Z, Li W, Ke B, Li B, Zhang Y, Ng L, Cui Z. (2014). Epidemiology and etiology characteristics of foodborne outbreaks caused by Vibrio parahaemolyticus during 2008–2010 in Guangdong province, China. Foodborne Pathog Dis, 11( 1): 21–29. [DOI] [PubMed] [Google Scholar]
- 39. Yeung PS1, Boor KJ. (2004). Epidemiology, pathogenesis, and prevention of foodborne Vibrio parahaemolyticus infections. Foodborne Pathog Dis, 1( 2): 74–88. [DOI] [PubMed] [Google Scholar]
- 40. Nair GB, Ramamurthy T, Bhattacharya SK, Dutta B, Takeda Y, Sack DA. (2007). Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin Microbiol Rev, 20( 1): 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chae MJ1, Cheney D, Su YC. (2009). Temperature effects on the depuration of Vibrio parahaemolyticus and Vibrio vulnificus from the American oyster (Crassostrea virginica). J Food Sci, 74( 2): M62–66. [DOI] [PubMed] [Google Scholar]
- 42. Shams AM1, O’Connell H, Arduino MJ, Rose LJ. (2011). Chlorine dioxide inactivation of bacterial threat agents. Lett Appl Microbiol, 53( 2): 225–230. [DOI] [PubMed] [Google Scholar]
- 43. Teplitski M1, Wright AC, Lorca G. (2009). Biological approaches for controlling shellfish-associated pathogens. Curr Opin Biotechnol, 20( 2): 185–190. [DOI] [PubMed] [Google Scholar]
- 44. Ren T, Su YC. (2006). Effects of electrolyzed oxidizing water treatment on reducing Vibrio parahaemolyticus and Vibrio vulnificus in raw oysters. J Food Prot, 69( 8): 1829–1834. [DOI] [PubMed] [Google Scholar]
- 45. Duedu KO, Yarnie EA, Tetteh-Quarcoo PB, Attah SK, Donkor ES, Ayeh-Kumi PF. (2014). A comparative survey of the prevalence of human parasites found in fresh vegetables sold in supermarkets and open-aired markets in Accra, Ghana. BMC Res Notes, 7: 836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vital PG, Dimasuay KG, Widmer KW, Rivera WL. (2014). Microbiological quality of fresh produce from open air markets and supermarkets in the Philippines. ScientificWorldJournal, 2014: 219534. [DOI] [PMC free article] [PubMed] [Google Scholar]