Abstract
Background:
Breast cancer is the most frequent cancer in women. Cancer/Testis antigens are immunogenic proteins ectopically expressed in human neoplasms. Synaptonemal complex protein 3 (SYCP3) belongs to cancer/testis genes family involved in meiotic events and spermatogenesis. The aim of this study was to express analysis of SYCP3 in breast cancer and validate it as a breast cancer biomarker.
Methods:
Expression of SYCP3 transcripts in 47 breast tumors, 6 breast cancer cell lines (MCF7, SKBR3, T47D, BT474, MDA-MB-231 and MDA-MB 468), 5 normal breast and 2 testis tissues was studied by Real Time RT-PCR reaction. The reference genes phosphoglucomutase 1 and hypoxanthine guanine phosphoribosyl transferase were used as reactions normalizers. The software tool REST 2009 was applied for statistical analysis of the data. The research was conducted from Apr 2014 to August 2015 in Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Results:
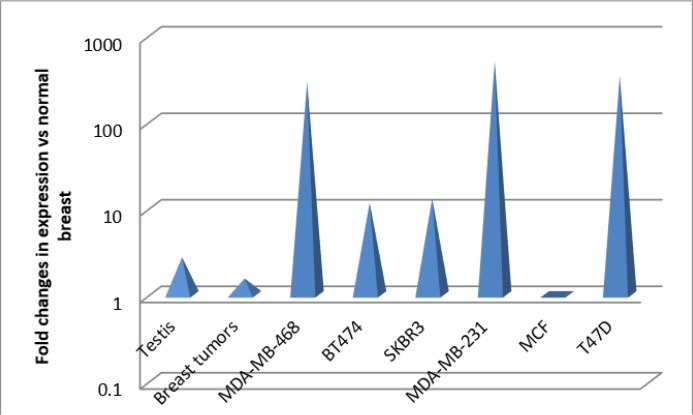
All of the studied breast cancer cell lines showed very high levels of SYCP3 overexpression in comparison to normal breast (P=0.001) and even to normal testis (P=0.001), except for MCF7 cell line. Breast tumors showed moderately increasing in transcript changes in comparison to normal breast.
Conclusion:
SYCP3 is a known testis-specific gene, but interestingly five out of six studied breast cancer of cell lines showed higher expression levels of SYCP3 in comparison to normal testis and normal breast tissues. SYCP3 has critical role in cell division with known interaction with the tumor suppressor genes, BRCA1 and BRCA2, which are critical genes in breast cancer.
Keywords: Breast cancer biomarkers, Targeted therapy, Cancer/testis genes, SYCP3, Breast cancer cell lines
Introduction
Breast cancer is the most commonly diagnosed type of cancer among women in 2013, accounting for 29% of all new cancer cases and the second most common cause of cancer death in women (1). Carcinoembryonic antigen and CA 15-3 used as weak detection markers in breast cancer as well as the Mucin 1, which expresses in both affected and unaffected individuals (2, 3). Because, there is no gold standard treatment for breast cancer as a highly heterogeneous disease, nowadays there is special attention focused on targeted therapies and specific predictive and prognostic targets for individualized therapeutics and screening approaches (4). In the last decades development of skills in morphological features and clinical staging in breast cancer has caused the early detection of the disease as well as novel adjutant therapies, reduced the mortality rate in patients (4).
A group of tumor-associated antigens, the Cancer/Testis antigens (CTAs), has recently attracted the attention of the researchers, to develop cancer vaccines and specific targeted therapies (5).
Aberrant expression of CTAs has been shown in different cancers while testis shows the highest normal expression levels and no significant expression have been reported in the other normal tissues (6, 7, 8). The tight junctions between the Sertoli cells in testis, generates the testis blood barrier which prevents communication between germ cells and the immune cells circulating within blood vessels and prevents them from eliciting an immune response which causes to the unique feature of testis as an immune privileged organ. Resistance against cancer will induce, if someone is exposed to male germ cell proteins (9).
The important similarities between the processes of germ-cell and cancer cell development strengthen the possibility that cancer commandeers parts of the gametogenic programs in the process of cancer cell development (6). Malignant characteristics may be induced by the same pathways of gametogenic programs that have been silent in fetal development in somatic tissues (6). There is a growing list of CT antigens in different cancers; some of them are in clinical trials such as lung cancer (10).
The protein product made by Synaptonemal Complex Protein 3 (SYCP3) is the key structural ingredient presents in chromosomal synapsis. It is essential for appropriate chromosomal segregation and possible recombination of the chromosomes passing meiosis.
Synaptonemal complex protein 3 known as a meiotic gene, but dysfunction of the mitotic: meiotic switch has been reported as a possible reason for neoplastic changes of primordial germ cells (11). The protein encoded by SYCP3 is mainly located at the cells nucleoplasm, interacts with the tumor suppressor genes, BRCA1 (12) and BRCA2 (13) which are the main disease causing genes in breast cancer.
According to the critical role of SYCP3 in cell division, we conducted this study to analyze its transcripts in breast tumors and breast cancer of cell lines. The cell lines were selected based on their specific criteria has to cover all pathological characteristics and derivatives of breast cancer (14).
Materials and Methods
Patients and normal samples
Forty-seven breast tumors, five normal epithelial cells, and two normal testes were subjected to transcript analysis. Confirmed normal breast epithelial cells were collected from the surgical removals of the mamoplastic surgeries from the individuals referred to the hospitals related to Tehran University of Medical Sciences during April 2014 to August 2015. Normal testes were obtained from the orchiectomy surgeries and fresh frozen breast tumors were obtained from the National Tumor Bank of Cancer Institute of Tehran University of Medical Sciences. All tissues were stored in −70 °C until experiments.
The Ethics Committee of Tehran University of Medical Sciences has approved the research. Informed constants were obtained from all participants.
Cell Lines
The selected breast cancer cell lines including, BT-474, MDA-MB-468, MDA-MB-231, T47D, SKBR3 and MCF7 were purchased from Pasteur institute, Tehran. Cells were cultured according to ATCC instructions in RPMI-1640 or DMEM media by adding 10% fetal calf serum (Invitrogen) and 1% penicillin/streptomycin (Invitrogen). Cells were incubated at 37 °C in a humidified incubator with 5% CO2. The flasks with about 2×106 cell counts were separated for RNA extraction.
RNA isolation and cDNA synthesis
Isolation of total RNA from the cell lines and fifty micrograms of tumors and normal tissues were done using Tripure reagent (Roche, Mannheim, Germany) following the manufacturer’s protocol. To determine the quality and quantity of extracted RNA, NanoDrop spectrophotometer (Nano-Drop® Technologies) and gel electrophoresis were done. One microgram of total RNA was converted to cDNA using Takara cDNA synthesis kit (Takara, Japan), according to the manufacturer protocol with minor modifications. As the reaction primer, a mix of random hexamers and oliogo dT was used. RT-negative tubes without adding the RT enzyme were used as the negative control of the experiments. Quality of cDNAs was confirmed by the amplification of the housekeeping gene Phosphoglucomutase 1(PGM1) (Table 1).
Table 1:
Primers sequences
| Gene | Primer sequences | Amplicon (bp) |
|---|---|---|
| SYCP3 | F: GACTGGCTTTTCTCCTGTGC | 220 |
| R: GATCTTCCACAGACGGCTTC | ||
| PGM1 | F: AGCATTCCGTATTTCCAGCAG | 120 |
| R: GCCAGTTGGGGTCTCATACAAA | ||
| HPRT | F: CCTGGCGTCGTGATTAGTGAT | 131 |
| R: AGACGTTCAGTCCTGTCCATAA |
F: Forward primer, R: Reverse primer, bp: base pairs
Real-time quantitative polymerase chain reaction
Quantitative Real-Time PCR was performed to determine the transcript levels of the target genes, using SYBR GreenI Premix (Takara, Japan) and 200 ng/μl of each of the cDNAs, following the manufacturer’s protocol with minor modifications.
Amplifications were done in 40 cycles using Rotor-GeneTM 6000 machine (Corbette Life ScienceTM, Germany). To determine the maximum efficiency of each primer pairs, serial dilution of 10 pmol/μl of mixed primers was performed and 0.5μl for each primer was considered as the best efficiency in a final volume of 20 μl of reactions. PGM 1 and Hypoxanthine phosphoribosyltransferase (HPRT) were used as internal controls to normalize the target genes. The sequences of the related primers are listed in Table 1.
Statistical analysis
To analyze the relative gene expression data, REST 2009 software was used (15) using the amplification efficiencies and cycle thresholds from comparative quantification analysis in Rotor gene software. The final relative expression obtained relative to the normalizer genes and normal breast tissues. The pair-wise fixed reallocation randomization test with 2000 iterations in the REST 2009 software was used to determine the significances.
Results
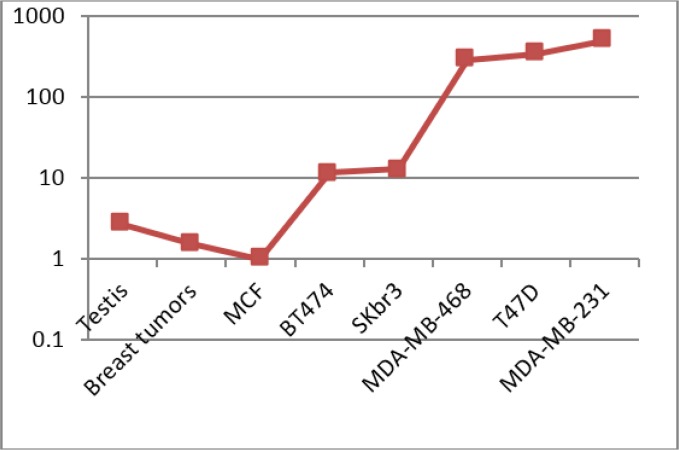
The testis-specific gene, SYCP3 was subjected to transcript analysis by employing the quantitative Real Time RT-PCR in six breast cancer cell lines, 5 normal breast tissues, 2 normal testis tissues and 47 breast tumors. PGM1 and HPRT were used to normalize the gene expression as the internal controls. After normalizing the expressions levels, normal breast epithelial tissues showed low levels of SYCP3 transcripts in comparison to the expression of reference genes, whereas the SYCP3 transcript levels in testis were higher than normal breast but not at a statistically significant level (P= 0.321). Breast tumors showed moderately increasing in transcript fold changes in comparison to normal breast (P=0.745), whereas all of the studied breast cancer cell lines showed very high levels of overexpression (P= 0.001) in comparison to normal breast, except for MCF7 cell line (P= 0.834) (Fig. 1). However SYCP3 is known as a testis-specific gene, interestingly five out of six studied breast cancer cell lines showed very significantly up-regulation of SYCP3 in comparison to normal testis (P=0.001) (Fig. 1, 2).
Fig. 1:
Fold changes of SYCP3 expressions in comparison to normal breast tissues in breast tumors and cell lines
Fig. 2:
Comparative fold expressions changes of SYCP3 in comparison to normal breast tissues in breast tumors and cell lines
Discussion
In our experiment the breast cancer cell lines except for MCF7, showed higher expression levels than those in testis, however SYCP3 has normally enriched expression in testis. Breast tumors showed overexpressed SYCP3 in comparison with normal breast as well, but the differences were not statistically significant.
The cells in breast tumors are usually heterogeneous; otherwise, contaminated tumor cells with stromal cells may cause this difference as well (16).
MDA-MB-231, the Claudin-low subtype of breast cancer, T47D, the Luminal A subtype and MDA-MB-468, the Basal subtype of breast cancer showed the highest levels of transcripts. SKBR3, the HER2-enriched subtype and BT474, the Luminal B subtypes showed the next rank in elevated transcript in comparison to normal breast (Fig. 2). MCF7 as the other Luminal A subtype has no SYCP3 overexpression. SYCP3 could be a potential diagnostic marker for breast cancer, however, further investigations are needed to extend and more validate the results.
Most of breast cancer knowledge has been obtained from in vivo and in-vitro studies on breast cancer cell lines. They are unlimited and easily exploited sources of self-replicating material, without impurity with stromal cells. Cell lines are indicative models of the tumors which are derived from and potent tools to test the efficiency of therapeutic drugs (16). Working on cell lines has some disadvantages as well, such as being the representative of the original stromal environment, which they come from. Their sustained growth in culture processes might modify the phenotype and lead to an accumulation of genome alterations (16).
We suggest SYCP3 as a new Cancer/Testis antigen in breast cancer. Testis is an immune privileged organ without expressing HLA molecules. CTAs are proposed as possible biomarkers for targeted treatments. Unlike chemotherapy, which kills healthy dividing cells in addition to tumor cells with important side effects, CTAs can be used for specific targeting of cancer cells by using monoclonal antibodies or dendritic cell-based immunotherapy (2). Functional roles of CTAs including modulating the gene expression, contribution to tumor signaling pathways, tumor cell division and apoptosis have been indicated (17). A growing inventory of various CTAs in breast cancer is available (18).
We previously reported overexpression of cancer/testis gene TSGA10 (19), OIP5 and TAF7L (20), and AURKC (7) in breast tumors as well.
In our previous research SYCP3 was the subject of expression analysis in different cancers using conventional RT-PCR. Elevated expression showed in the moderately differentiated gemistocytic astrocytoma, pituitary adenoma, ovarian tumor and glioma (21). SYCP3 has been proposed as a prognostic marker in cervical cancer (22), non-small cell lung cancer (23) and overexpressed in cutaneous T-cell lymphoma (24) and acute lymphoblastic leukemia as well (25). It has an essential meiotic function in spermatogenesis and mutations in this gene are associated with spermatogenesis arrest in males and susceptibility to pregnancy loss in females as well (26, 27).
The science on CTAs is not deep enough to explain the nature, reasons, outcomes, factors and how working the activation system of CTAs in various cancers. The genetic attributes (28) and environmental effects (29) on the expression of CTAs in cancers remain unclear. Because of the absence of accredited biomarkers in breast cancer and importance of early detection of the disease to warranty the diagnosis and effective treatments in early stages, the possible candidates such as SYCP3 must be the subject of more studies to dig their efficiency as an appropriate biomarker and immunotherapeutic objective in various cancers including breast cancer.
Conclusion
The RNA transcript levels of the testis specific gene; Synaptonemal Complex Protein 3 was detected in breast cancer tumors and five breast cancer cell lines, where it should not be normally expressed. The SYCP3 aberrant expression in breast cancer has the potential role to use as a detection marker for early detection of the disease as well as a suitable candidate for targeted therapy, which can be subject of further studies.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This study was supported by a financial grant from Cancer Research Center of Tehran University of Medical Sciences. The authors thank Dr Mohammad Ali Mohagheghi and Dr. Kazem Zendehdel for their kind supports. The authors have no conflict of interests to declare.
References
- 1. Siegel R, Naishadham D, Jemal A. (2012). Cancer statistics. Ca Cancer J Clin, 62( 1): 10–2. [DOI] [PubMed] [Google Scholar]
- 2. Saini S, Jagadish N, Gupta A, Bhatnagar A, Suri A. (2013). A novel cancer testis antigen, AKinase anchor protein 4 (AKAP4) is a potential biomarker for breast cancer. PLoS One, 8( 2): e57095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, et al. (2007). American Society of Clinical Oncology 2007 Update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol, 25( 33): 5287–312. [DOI] [PubMed] [Google Scholar]
- 4. Pandey A, Kurup A, Shrivastava A, Radhi S, Nguyen DD, Arentz C, et al. (2012). Cancer testes antigens in breast cancer: biological role, regulation, and therapeutic applicability. Int Rev Immunol, 31( 5): 302–20. [DOI] [PubMed] [Google Scholar]
- 5. Grizzi F, Mirandola L, Qehajaj D, Cobos E, Figueroa JA, Chiriva-Internati M. (2015). Cancer-testis antigens and immunotherapy in the light of cancer complexity. Int Rev Immunol, 34( 2): 143–53. [DOI] [PubMed] [Google Scholar]
- 6. Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. (2005). Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer, 5( 8): 615–25. [DOI] [PubMed] [Google Scholar]
- 7. Mobasheri MB, Shirkoohi R, Zendehdel K, Jahanzad I, Talebi S, Afsharpad M, Modarressi MH. (2015). Transcriptome analysis of the cancer/testis genes, DAZ1, AURKC, and TEX101, in breast tumors and six breast cancer cell lines. Tumour Biol, 36( 10): 8201–6. [DOI] [PubMed] [Google Scholar]
- 8. Yazarloo F, Shirkoohi R, Mobasheri MB, Emami A, Modarressi MH. (2013). Expression analysis of four testis-specific genes AURKC, OIP5, PIWIL2 and TAF7L in acute myeloid leukemia: a gender-dependent expression pattern. Med Oncol, 30( 1): 368. [DOI] [PubMed] [Google Scholar]
- 9. Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz L. (2013). Increased risk of cancer among azoospermic men. Fertil Steril, 100( 3): 681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly RJ, Giaccone G. (2011). Lung cancer vaccines. Cancer J, 17( 5): 302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adamah DJ, Gokhale PJ, Eastwood DJ, Rajpert De-Meyts E, Goepel J, Walsh JR, et al. (2006). Dysfunction of the mitotic:meiotic switch as a potential cause of neoplastic conversion of primordial germ cells. Int J Androl, 29( 1): 219–27. [DOI] [PubMed] [Google Scholar]
- 12. Kouznetsova A, Wang H, Bellani M, Camerini-Otero RD, Jessberger R, Höög C. (2009). BRCA1-mediated chromatin silencing is limited to oocytes with a small number of asynapsed chromosomes. J Cell Sci, 122 ( Pt 14 ): 2446 –2452. [DOI] [PubMed] [Google Scholar]
- 13. Hosoya N1, Okajima M, Kinomura A, Fujii Y, Hiyama T, Sun J, Tashiro S, Miyagawa K. (2011). Synaptonemal complex protein SYCP3 impairs mitotic recombination by interfering with BRCA2. EMBO Rep, 23; 13 ( 1 ): 44 –51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holliday Deborah L, Speirs Valerie. (2011). Choosing the right cell line for breast cancer-research. Breast Cancer Res, 13( 4): 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfaffl MW, Horgan GW, Dempfle L. (2002). Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res, 30 ( 9 ): e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charafe-Jauffret, Ginestier C, Monville1 F, Finetti P, Adélaïde J, et al. (2006). Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene, 25( 15): 2273–84. [DOI] [PubMed] [Google Scholar]
- 17. Whitehurst AW. (2014). Cause and consequence of cancer/testis antigen activation in cancer. Annu Rev Pharmacol Toxicol, 54: 251–72. [DOI] [PubMed] [Google Scholar]
- 18. Ghafouri-Fard S, Shamsi R, Seifi-Alan M, Javaheri M, Tabarestani S. (2014). Cancer-testis genes as candidates for immunotherapy in breast cancer. Immunotherapy, 6( 2): 165–79. [DOI] [PubMed] [Google Scholar]
- 19. Dianatpour M1, Mehdipour P, Nayernia K, Mobasheri MB, Ghafouri-Fard S, Savad S, et al. (2012). Expression of Testis-Specific Genes TSGA10, TEX101 and ODF3 in Breast Cancer. Iran Red Crescent Med J, 14( 11): 722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mobasheri MB, Shirkoohi R, Modarressi MH. (2015). Cancer/Testis OIP5 and TAF7L Genes are Up-Regulated in Breast Cancer. Asian Pac J Cancer Prev, 16( 11): 4623–8 [DOI] [PubMed] [Google Scholar]
- 21. Mobasheri MB, Jahanzad, Mohagheghi MA, Aarabi M, Farzan S, Modarressi MH. (2007). Expression of two testis-specific genes, TSGA10 and SYCP3, in different cancers regarding to their pathological features. Cancer Detect Prev, 31( 4): 296–302. [DOI] [PubMed] [Google Scholar]
- 22. Cho H, Noh KH, Chung JY, Takikita M, Chung EJ, Kim BW, et al. (2014). Synaptonemal complex protein 3 is a prognostic marker in cervical cancer. PLoS One, 6; 9 ( 6 ): e98712 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung JY, Kitano H, Takikita M, Cho H, Noh KH, Kim TW. (2013). Synaptonemal complex protein 3 as a novel prognostic marker in early stage non-small cell lung cancer. Hum Pathol, 44( 4): 472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Litvinov IV, Cordeiro B, Huang Y, Zargham H, Pehr K, Doré MA, et al. (2014). Ectopic expression of cancer-testis antigens in cutaneous T-cell lymphoma patients. Clin Cancer Res, 15; 20 (14): 3799– 808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niemeyer P1, Türeci O, Eberle T, Graf N, Pfreundschuh M, Sahin U. (2003). Expression of serologically identified tumor antigens in acute leukemias. Leuk Res, 27( 7): 655–60. [DOI] [PubMed] [Google Scholar]
- 26. Bolor H1, Mori T, Nishiyama S, Ito Y, Hosoba E, Inagaki H, et al. (2009). Mutations of the SYCP3 gene in women with recurrent pregnancy loss. Am J Hum Genet, 84( 1): 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aarabi M1, Modarressi MH, Soltanghoraee H, Behjati R, Amirjannati N, Akhondi MM. (2006). Testicular expression of synaptonemal complex protein 3 (SYCP3) messenger ribonucleic acid in 110 patients with nonobstructive azoospermia. Fertil Steril, 86( 2): 325–31. [DOI] [PubMed] [Google Scholar]
- 28. Miryounesi M, Nayernia K, Mobasheri MB, Dianatpour M, Oko R, Savad S, et al. (2014). Evaluation of in vitro spermatogenesis system effectiveness to study genes behavior: monitoring the expression of the testis specific 10 (Tsga10) gene as a model. Arch Iran Med, 17( 10): 692–7. [PubMed] [Google Scholar]
- 29. Azam R, Ghafouri-Fard S, Tabrizi M, Modarressi MH, Ebrahimzadeh-Vesal R, et al. (2014). Lactobacillus acidophilus and Lactobacillus crispatus culture supernatants downregulate expression of cancer-testis genes in the MDA-MB-231 cell line. Asian Pac J Cancer Prev, 15( 10): 4255–9. [DOI] [PubMed] [Google Scholar]