Abstract
Background:
Migraine is a common debilitating primary headache disorder with current head pain attacks, which contributes to physical activity dysfunctions in chronic pain phase. PGE2 and PGI2 are two important prostaglandins synthesised by COX-2 enzymes, involved in migraine pain signals. COX-2 modulation is essential in treatment and pathogenesis of migraine. This study aimed to investigating the association between COX-2 gene polymorphisms with the risk of migraine susceptibility in migraine patients with related and unrelated parents.
Methods:
This case- control study was based on 100 migraine patients and 100 non-migraine subjects in Bushehr province, Iran in 2013. Genomic DNA of blood samples was extracted and genotyping of COX-2-765G>C (rs20417) and COX-2-1195A>G (rs689466) gene variants was investigated by PCR-RFLP method. Statistical analyses were accomplished using the SPSS software package.
Results:
There was a significant differences in the frequencies of the COX-2-765G>C and COX-2-1195A>G genotypes between migraine patients and controls (P≤0.05).
Conclusion:
COX-2-765CC, COX-2-765CG, COX-2-1195GG and COX-2-1195AG genotypes can increase the risk of migraine significantly. As the first study in Iran, we are hopeful to achieve greater results about the relevancy of COX-2 gene, migraine and pain signals pathway by repeating these experiments on more samples.
Keywords: Migraine, COX-2 gene, Polymorphism, RFLP, Iran
Introduction
Migraine originated from a Greece term meaning “hemicraine” or “half of the head” (1), usually associated with episodes of strong unilateral pulsating headache (2). Migraine disorder shows a high-related relevancy; approximately 50% of migraine cases have a first-degree related as a migraine sufferer (3). Familial clustering migraine points to importance of genetic factors in this illness, but its inheritance pattern is argumentative and supposed it is likely multifactorial, although autosomal dominant inheritance cannot account for it (3).
The prevalence of migraine impressed about 10%–12% of the white population (4), 24% of US populations and 12% of adults (5) contains both sexes (4), affected women more higher than men (17.1% in women and 5.6% in men) (5) and often involves the middle-aged people (6). Migraine is observed as an inherited brain disturbance, specified by neurotransmitter imbalances, especially, serotonin 5-hydroxytryptamine (7) that contribute to neuronal dysfunctions (8). This disorder is generally characterized by strong and recurrent head pains which typically lasting about 4–72 h and attended by some symptoms like vomiting, neurological disturbance, photophobia and phonophobia. According to the classification of International Headache Society (IHS), two main classes of migraine consist: migraine without aura (MO), which included 70% of all migraineurs and migraine with aura (MA), which affected the rest of migraine population (about 25%) (9), some sources have defined them as common and classical migraine respectively (10). Both sub-groups have an intense and valid genetic background, but according to recent epidemiological information, the genetic factors in increasing development of MA (25%–30%) are stronger than MO (11). The differences between MA and MO refer to the clinical symptoms of MA, it can include signals such as food craving, mood changes, neck stiffness, fatigue, reversible visual system symptoms, sensory and aphasic aura signs, each symptom might last from 5 min to 1 h (9).
Since migraine is a complicated and multifactorial disease, no distinct marker is in available to diagnosis the patient’s status, yet. Although, studies discovered prostaglandins synthesized by cyclooxygenase (COX) pathways, play a significant role in pathogenesis of this disorder, COX enzymes involved in pain mechanisms and migraine attacks, are the most important mediators of inflammation and pain (2). COX plays a noticeable role in prostanoids synthesis from arachidonic acid and arises this chemical reaction in both constitutive (COX-1) and inducible (COX-2) isoforms (12) which leads to sensibility and chronic pain in neuronal cells (13). Prostaglandin E2 (PGE2) and prostaglandin I2 (PGI2) are two important prostanoids that effect on pain signal considerably. Some pharmaceutics combination like nonsteroidal anti-inflammatory drugs (NSAIDs) can inhibit COX-1 and COX-2 pathway and degrade pain in migraineur by reducing the production of prostanoids consisting PGE2. Thereby, one of the important factor in migraine therapy is COX-2 modulation, hence, applying non-selective COX suppressors such as acetylsalicylic acid was been high usage. Moreover, a selective COX-2 inhibitor as rofecoxib is so effective in patient suffering from migraine with or without aura to tolerate the pain (2).
As for the substantial role of COX-2 in synthesize PGE2 and pathogenesis of migraine, we hypothesized that COX-2 gene basic polymorphisms may increase susceptibility to migraine. Therefore, we did this research project for the first time in Iran (Bushehr Province) and second time in the world.
The aim of study was to determine the association of COX-2-765G>C (rs20417) and COX-2-1195A>G (rs689466) promoter polymorphisms to migraine susceptibility in Iranian migraine patients for the first time in this region using RFLP method.
Materials and Methods
Samples
In this case-control study a total of 100 migraine patients (79 females, 21 males) and 100 controls (77 females, 23 males) were collected from July to Nov 2013 from Bushehr Province, eastern Iran and examined for promoter polymorphisms of COX-2 gene variant. Patient subjects were people conferred to Bushehr Abolfazl Therapeutic Clinic. Controls were chosen among the people volunteered to donate their blood in the Bushehr Blood Transforming Center.
The Ethics Board of Hospital for Migraine approved this study and all individuals providing samples signed informed consents covering aspects of the experiments conducted.
The numbers of 20 patient samples were the individuals suffered from MA and 80 samples from MO. Inclusion criteria were as follows: 1) patients with migraine referred to the mentioned clinic; 2) signing informed consents covering by patients itself; 3) diagnosis migraine by consultant. Exclusion criteria were as follows: 1) discontentment of migraine patients or control group; 2) unverified of migraine patients or control group by consultant. Both control and patients were interviewed and examined by a specialist neurologist, all of them answered to a complete and perfect questionnaire and expressed their specifications such as their sex, age of onset, inbreeding or outbreeding marriage, related migraine history and pain severity. After obtaining awarded consent, blood samples were taken from donors and assembled in EDTA containing tubes. The blood samples were transported to the laboratory and stored at −20 °C until needed for analysis. PCR products for COX-2 gene on Agarose gel with COX-2-765G>C (rs20417) and COX21195A>G (rs689466) primers showed fragments about 309 and 273 bp respectively (Fig. 1 and 2). The anticipated results after restriction for each gene are also mentioned in Table 1. The Agarose gel electrophoresis of PCR products after digested with AciI and PvuII enzymes are showed in Fig. 3 and 4, respectively.
Fig. 1:

Agarose gel stained with ethidium bromide, PCR products of COX-2 gene by using the COX-2-765G>C (rs20417) primer. Line 1–6 is 309 bp fragments, Line M is 100 bp DNA ladder
Fig. 2:
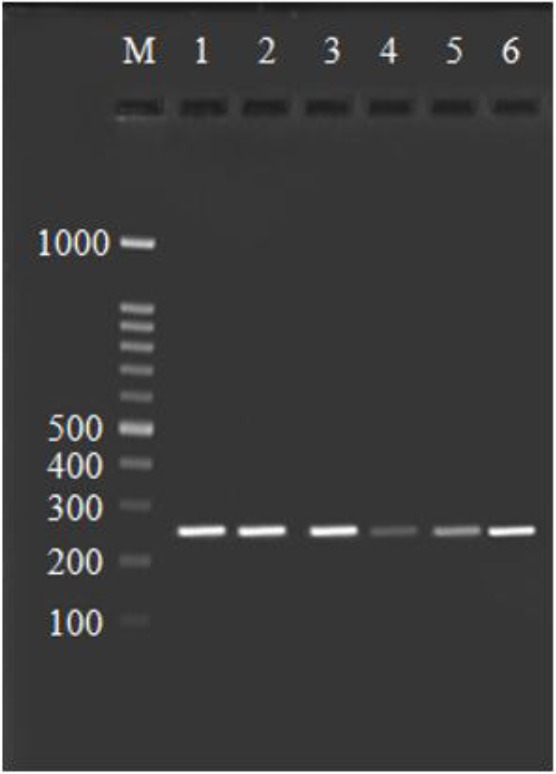
Agarose gel stained with ethidium bromide, PCR products of COX-2 gene by using the COX21195A→G (rs689466) primer. Line 1–6 is 273 bp fragments, Line M is 100 bp DNA ladder
Table 1:
PCR and RFLP procedures and products of COX-2-765G>C and COX-2-1195A>G genes
| Primers | Primer Sequences | Restriction Enzymes | PCR Products |
|---|---|---|---|
| COX-2-765G>C | 5′-AGGCAGGAAACTTTATATTGG-3′ | AciI | GG: 309 bp |
| (rs20417) | 5′-ATGTTTTAGTGACGACGCTTA-3′ | CC: 209 bp, 100 bp | |
| GC: 309 bp209 bp, 100 bp | |||
| COX-2-1195A>G | 5′-CCCTGAGCACTACCCATGAT-3′ | AA: 273 bp | |
| (rs689466) | PvuII | GG: 220 pb, 53 bp | |
| 5′-GCCTTCATAGGAGATACTGG-3′ | AG: 273 bp, 220 pb, 53 bp |
Fig. 3:
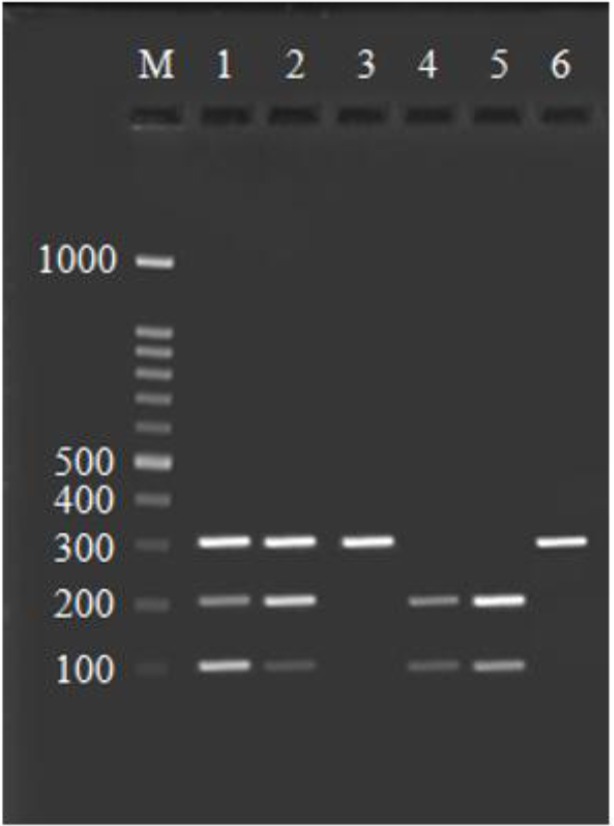
Result of enzyme digestion by AciI. Lane M is 100 bp ladder, lane 1 and 2: heterozygote polymorphic DNAs (GC) contained 309, 209 and 100 bp fragments, Lane 3 and 6: 309 bp normal DNAs (GG), Lane 4 and 5: homozygote polymorphic DNAs (CC) contained 209 and 100 bp fragments
Fig. 4:

Result of enzyme digestion by PvuII. Lane M is 100 bp ladder, Lane 1, 3 and 4: 273 bp normal DNAs (AA), Lane 2: homozygote polymorphic DNAs (GG) contained 220 and 53 bp fragments, Lane 5 and 6: heterozygote polymorphic DNAs (AG) contained 273, 220 and 53 bp fragments
DNA preparation
Total DNA was extracted from blood samples using DNA purification kit (DNP™, CinnaGen, Iran) according to the manufacturer’s recommendation. Purified DNA was immediately used or kept frozen at −20 °C until needed. The total extracted DNA was measured at 260 nm optical density (14).
PCR assay
In order to amplify the individual’s respective gene, PCR test was accomplished using specific primers (Table 1). Therefore, the final volume of 25 μl PCR reactions in 0.2 ml tubes containing 500 ng/μl of DNA template, 2 mM of MgCl2 concentration, 2 mM dNTPs, 10 pmol/μl of each primer, 5 μl of 10X PCR buffer and 1 U of Taq DNA polymerase (Fermentas, Germany) was performed. Thermal PCR conditions consisted of denaturation phase for 5 min at 95 °C, followed by 30 cycles of 94 °C for 1 min, temperature of 59 °C for COX-2-1195A>G (rs689466) primer and 56 °C for COX-2-765G>C (rs20417) primer for 1 min, and 72 °C for 1 min, with a final extension for 5 min at 72 °C. PCR-amplified products were examined by 2% Agarose gel and analysis of PCR products for presence of COX-2-1195A>G gene revealed 273 bp fragments (Fig. 2). After electrophoresis, DNA was observed and photographed in ultraviolet imager (UVI) doc gel documentation systems (UK).
Polymorphism analysis
Restriction Fragment Length Polymorphisms (RFLPs) were used for analysis sequence polymorphisms of proliferated DNA. The PCR product from second step digested for 4 h at 37 °C by AciI and PvuII restriction enzymes for COX-2-765G>C (rs20417) and COX-2-1195A>G (rs689466) SNPs, respectively, according to the manufacturer’s instructions. The results of enzymatic digestion were identified in a 2% agarose gel by electrophoresis and ethidium bromide staining. Molecular weight 100 bp plus marker (Fermentas, Co.) and undigested PCR products was included in each analysis. The genotypes were deduced from the fragmentation patterns of the amplified DNA, observed in UVIdoc gel documentation systems.
Statistical analysis
Statistical analyses were performed by the chi-square test using the SPSS 20 (SPSS Inc. Chicago, IL, USA) software. Related risk at 95% confidence intervals (CI) was calculated as the odds ratio (OR). Linkage disequilibrium between COX-2-765G>C and COX-2-1195A>G polymorphisms was performed using SHEsis software (http://analysis.bio-x.cn/myAnalysis.php) (15). The probability level for significance was P≤0.05.
Results
The mean age was 33.16±10.38 for patients (18–69 age), and 34.20±10.16 yr (18–60 age) for controls. There were symmetry between patient and control subjects in terms of sex prevalence and mean age and most of patients experienced intensive pain, which disorganized their functions and activities.
Table 2 shows the frequencies of COX-2-765G>C and COX-2-1195A>G genotype distributions in controls, MA and MO patients separately. There were statistically considerable differences in COX-2-765G>C and COX-2-1195A>G genotypes between the controls and patients. Carriers of COX-2-765 CC and GC genotypes (polymorph types) in patients were further than in the controls and frequencies of COX-2-765 GG genotype (the wild type) in controls were higher than in all the patients (P<0.001, χ2:34.03). In MA and MO patients, -765 CC and -765 GC genotypes were significantly higher than in controls (P<0.018, χ2:8.00 and P<0.001, χ2:36.83 respectively), and not marked diversities in -765 CC and -765 GC genotypes between MA and MO patients (P<0.084, χ2:4.95).
Table 2:
Frequencies of COX-2-765 G>C and COX-2-1195A>G genotype distributions in patients and controls
| Genotypes/alleles | Controls | MA | MO | All patients | P1 | P2 | P3 | P4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N: 100 | N: 20 | N: 80 | N: 100 | |||||||||
| N | % | N | % | N | % | N | % | |||||
| COX-2 765GC | ||||||||||||
| GG | 52 | 52.0 | 7 | 35.0 | 11 | 13.8 | 18 | 18.0 | ||||
| CC | 8 | 8.0 | 6 | 30.0 | 29 | 36.2 | 35 | 35.0 | ||||
| CG | 40 | 40.0 | 7 | 35.0 | 40 | 50.0 | 47 | 47.0 | 0. 018 | 0.001 | 0.084 | 0.001 |
| C allele | 56 | 28.0 | 19 | 47.5 | 98 | 61.2 | 117 | 58.5 | ||||
| G allele | 144 | 72.0 | 21 | 52.5 | 62 | 38.7 | 83 | 41.5 | 0.015 | 0.001 | 0.114 | 0.001 |
| C+(CC+CG) | 48 | 48.0 | 13 | 65.0 | 69 | 86.2 | 82 | 82.0 | 0.165 | 0.001 | 0.6 | 0.001 |
| G+(GG+CG) | 92 | 92.0 | 14 | 70.0 | 51 | 63.7 | 65 | 65.0 | 0.005 | 0.001 | 0. 6 | 0.001 |
| COX-2 1195 AG | ||||||||||||
| AA | 71 | 71.0 | 10 | 50.0 | 40 | 50.0 | 50 | 50.0 | ||||
| GG | 5 | 5.0 | 4 | 20.0 | 5 | 6.2 | 9 | 9.0 | ||||
| AG | 24 | 24.0 | 6 | 30.0 | 35 | 43.8 | 41 | 41.0 | 0.042 | 0.014 | 0.129 | 0.010 |
| A allele | 166 | 83.0 | 26 | 65.0 | 115 | 71.0 | 141 | 70.5 | ||||
| G allele | 34 | 17.0 | 14 | 35. 0 | 45 | 28.1 | 59 | 29.5 | 0.009 | 0.011 | 0.394 | 0.003 |
| A+ (AA+AG) | 95 | 95.0 | 16 | 16.0 | 75 | 75.0 | 91 | 91.0 | 0.02 | 0.72 | 0.055 | 0.268 |
| G+ (GG+AG) | 29 | 29.0 | 10 | 10.0 | 40 | 40.0 | 50 | 50.0 | 0.067 | 0.004 | 1.0 | 0.002 |
P1: P value of migraine with aura vs control.
P2: P value of migraine without aura vs control.
P3: P value of migraine with aura vs migraine without aura.
P4: P value of all patients vs control.
In COX-2-1195 polymorphism, only AA genotype (the wild type) was statistically higher in the controls than in the all patients (P<0.010, χ2:9.23) and determined that COX-2-1195 AA genotype had decreased risk for migraine. Conversely, COX-2-1195 GG and AG genotypes in patients were higher than in controls considerably (P<0.010, χ2: 9.23). We also looked into the prevalence of COX-2-765 C+ and COX-2-1195 G+ genotype dispensation in MA and MO patients and control subjects, separately (Table 2 and 3).
Table 3:
OR and 95% CI distributions in MA, MO patients and controls
| Genotypes/alleles | OR 1 | 95%CI 1 | OR 2 | 95%CI 2 | OR 3 | 95%CI 3 | OR 4 | 95%CI 4 |
|---|---|---|---|---|---|---|---|---|
| COX-2 765GC | ||||||||
| C allele | ||||||||
| G allele | 2.33 | 1.16 – 4.65 | 4.06 | 2.61 – 6.33 | 0.572 | 0.285 – 1.15 | 3.62 | 2.39 – 5.5 |
| C+(CC+CG) | 2.01 | 0.74 – 5.46 | 6.79 | 3.22 – 14.35 | 0.296 | 0.097–0.905 | 4.93 | 2.59 – 9.39 |
| G+(GG+CG) | 4.93 | 1.49 – 16.34 | 6.54 | 2.78 – 15.36 | 0.754 | 0.261 – 2.17 | 6.19 | 2.7 – 14.22 |
| COX-2 1195 AG | ||||||||
| A allele | ||||||||
| G allele | 0.38 | 0.18 – 0.803 | 0.523 | 0.316 – 0.867 | 0.727 | 0.348 – 1.52 | 0.489 | 0.303–0.789 |
| A+ (AA+AG) | 0.211 | 0.051 – 0.87 | 0.79 | 0.22 – 2.83 | 0.267 | 0.064 – 1.1 | 0.532 | 0.172 – 1.65 |
| G+ (GG+AG) | 0.41 | 0.154 – 1.08 | 0.41 | 0.221 – 0.756 | 1.0 | 0.375 – 2.664 | 0.408 | 0.228 – 0.732 |
1: Migraine with aura vs control.
2: Migraine without aura vs control.
P3: Migraine with aura vs migraine without aura. P4: Patients vs control.
OR: odds ratio
CI: confidence intervals
In despite of being considerable differences in frequencies of COX-2-765 C+ and COX-2-1195 G+ genotypes between migraine cases and healthy people (P<0.001, χ2:25.41, OR:4.93 CI= 2.59- 9.39 and P<0.002, χ2:9.23, OR:0.408 CI= 0.228- 0.732 respectively), but there were no significant diversity between MO and MA patients in COX-2-765 C+ and COX-2-1195 G+ genotypes (P<0.6, χ2:4.89 OR:0.296 CI= 0.097- 0.905 and P<1, χ2:0, OR:1 CI= 0.375- 2.664 respectively). On the other hand, carriers of COX-2-1195 G+ genotype (the mutant type) in patient subjects were higher than in all the migraine- free cases (P<0.002, χ2:9.23, OR: 0.408 95% CI= 0.228–0.732). COX-2-765 GG and COX-2-1195 AA genotypes decrease risk for migraine and COX-2-765 C+ and COX-2-1195 G+ genotypes contribute to increase risk of migraine susceptibility.
Table 2 and 3 also show the details of significant differences in frequencies of C allele and G allele from -765G>C polymorphism (P<0.001, χ2:37.9, OR: 3.62 CI= 2.39- 5.5) and an allele and G allele from 1195A>G polymorphism (P<0.003, χ2:8.76, OR: 0.489 CI= 0.303- 0.789) between control and migraine cases.
The association of migraine disorder with COX-2 gene polymorphisms was confirmed by haplotype analysis and specified that the frequencies of COX-2-765G: 1195A haplotype were significantly lower in patients as compared with those of controls (P<0.001), also revealed that frequencies of COX-2-765C: 1195A and COX-2-765C: 1195G haplotype frequencies were significantly higher in patients as compared with those of controls (P<0.002 and P<0.001) (Table 4). There was high linkage disequilibrium between COX-2-765G>C and COX-2-1195A>G polymorphisms. In this analysis, no significant difference was observed in frequencies of COX-2-765G: 1195G haplotype in patients as compared with controls (P<0.886).
Table 4:
The frequencies of haplotypes of COX-2 gene in patients and controls
| Number of Haplotype | Haplotype association | Frequency | Chi-square | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (N) | % | All patients (N) | % | Control(N) | % | ||||
| 1 | COX-2 765G: 1195A | 178 | 44.5 | 59 | 33.14 | 119 | 66.85 | 20.225 | 0.001 |
| 2 | COX-2 765C: 1195A | 130 | 32.5 | 83 | 63.84 | 47 | 36.15 | 9.969 | 0.002 |
| 3 | COX-2 765G: 1195G | 49 | 12.3 | 24 | 48.97 | 25 | 51.02 | 0.020 | 0.886 |
| 4 | COX-2 765C: 1195G | 43 | 10.7 | 34 | 79.06 | 9 | 20.93 | 14.53 | 0.001 |
We also looked into the frequencies of COX-2-765G>C and COX-2-1195A>G genotype distributions in patients with related or unrelated parents separately (Table 5).
Table 5:
Frequencies of COX-2-765G>C and COX-2-1195A>G genotype distributions in patients with related and unrelated marriage parents
| Genotypes/alleles | Controls | Related parent | Unrelated parent | All patients | P1 | P2 | P3 | P4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N: 100 | N: 55 | N: 45 | N: 100 | |||||||||
| N | % | N | % | N | % | N | % | |||||
| COX-2 765GC | ||||||||||||
| GG | 52 | 52.0 | 2 | 3.6 | 16 | 35.6 | 18 | 18.0 | ||||
| CC | 8 | 8.0 | 22 | 40.0 | 13 | 28.9 | 35 | 35.0 | ||||
| CG | 40 | 40.0 | 31 | 56.4 | 16 | 35.6 | 47 | 47.0 | 0. 001 | 0.004 | 0.001 | 0.001 |
| C allele | 56 | 28.0 | 75 | 68.2 | 42 | 46.6 | 117 | 58.5 | ||||
| G allele | 144 | 72.0 | 35 | 31.8 | 48 | 53.3 | 83 | 41.5 | 0.001 | 0.002 | 0.002 | 0.001 |
| C+(CC+CG) | 48 | 48.0 | 53 | 96.4 | 29 | 64.4 | 82 | 82.0 | 0.001 | 0.066 | 0.001 | 0.001 |
| G+(GG+CG) | 92 | 92.0 | 33 | 60.0 | 32 | 71.1 | 65 | 65.0 | 0.001 | 0.001 | 0.246 | 0.001 |
| COX-2 1195 AG | ||||||||||||
| AA | 71 | 71.0 | 10 | 18.2 | 40 | 88.9 | 50 | 50.0 | ||||
| GG | 5 | 5.0 | 9 | 16.4 | 0 | 0 | 9 | 9.0 | ||||
| AG | 24 | 24.0 | 36 | 65.5 | 5 | 11.1 | 41 | 41.0 | 0.001 | 0.047 | 0.001 | 0.010 |
| A allele | 166 | 83.0 | 56 | 50.9 | 85 | 94.4 | 141 | 70.5 | ||||
| G allele | 34 | 17.0 | 54 | 49.1 | 5 | 5.5 | 59 | 29.5 | 0.001 | 0.008 | 0.001 | 0.003 |
| A+ (AA+AG) | 95 | 95.0 | 46 | 83.6 | 45 | 100 | 91 | 91.0 | 0.018 | 0.127 | 0.004 | 0.268 |
| G+ (GG+AG) | 29 | 29.0 | 45 | 81.8 | 5 | 11.1 | 50 | 50.0 | 0.001 | 0.019 | 0.001 | 0.002 |
P1: P value of patients with related parent vs control.
P2: P value of patients with unrelated parent vs control.
P3: P value of patients with related parent vs patients with unrelated parent.
P4: P value of all patients vs control.
In COX-2-765G>C, quantity of COX-2-765 GG genotype (the wild type) in controls were higher as compared with the patients with related parents and unrelated parents, however, quantity of COX-2-765 CC and COX-2-765 GC genotypes in patients with related and unrelated parents were more than in migraine- free subjects (P<0.001, χ2: 44.67 and P<0.004, χ2: 11.30 respectively). Likewise, it can be perceived from the date in Table 5 that there are significant discrepancies between patients with related parents and patients with unrelated parents in COX-2-765 CC and COX-2-765 GC genotypes (P<0.001, χ2: 17.16).
Moreover, COX-2-1195 AA genotype (the wild type) in controls were higher than in patients with related parents and unrelated parents and besides, frequencies of COX-2-1195 GG and COX-2-1195 AG genotypes in control cases were lower than patients with related parents and unrelated parents (P<0.001, χ2: 39.76 and P<0.047, χ2: 6.12 respectively). Moreover, considerable differences were achieved in frequencies of COX-2-1195 GG and COX-2-1195 AG genotypes between patients with related parents and patients with unrelated parents (P<0.001, χ2: 49.94), consanguineous marriage would heighten the risk of susceptibility to migraine illness.
We also looked into the quantity of G allele, C allele, A allele and G allele distributions in patients with related parents, patients with unrelated parents and controls. On one hand Table 5 and 6 show significant differences in frequencies of G allele and C allele between patients with related parent and control subjects (P<0.001, χ2: 46.96, OR: 5.51 CI= 3.32–9.14), besides, frequencies of C allele in patients with unrelated parent were higher than in controls significantly (P<0.002, χ2: 9.67, OR: 2.25 CI= 1.34 – 3.77) and considerable diversity was observed between patients with related parents and patients with unrelated parents in frequencies of G allele and C allele (P<0.002, χ2: 9.44, OR: 2.45 CI= 1.38 – 4.36). Table 5 and 6 indicate diversities in amount of A allele and G allele in patients with related parents, patients with unrelated parents and controls on the other hand. Information clarify marked discrepancy in frequencies of A allele and G allele between patients with related parents and migraine free cases (P<0.001, χ2: 35.95, OR: 0.21 CI= 0.13–0.36), although significant difference between patients with unrelated parents and healthy cases was noted in quantity of A allele and G allele (P<0.008, χ2: 6.98, OR: 3.48 CI= 1.31–9.22). There are considerable diversity in frequencies of these two alleles between patients with related parents and patients with unrelated parents (P<0.001, χ2: 45.11, OR: 0.61 CI= 0.02–0.16). Moreover, consanguineous marriage plays a key role in boosting prevalence of migraine.
Table 6:
OR and 95% CI distributions in patients with related parents, unrelated parents and control
| Genotypes/alleles | OR 1 | 95%CI 1 | OR 2 | 95%CI 2 | OR 3 | 95%CI 3 | OR 4 | 95%CI 4 |
|---|---|---|---|---|---|---|---|---|
| COX-2 765GC | ||||||||
| C allele | ||||||||
| G allele | 5.51 | 3.32 – 9.14 | 2.25 | 1.34 – 3.77 | 2.44 | 1.38 – 4.36 | 3.62 | 2.39 – 5.5 |
| C+(CC+CG) | 28.70 | 6.63 – 124.27 | 1.96 | 0.95 – 4.05 | 14.62 | 3.14 – 68.07 | 4.93 | 2.59 – 9.39 |
| G+(GG+CG) | 7.66 | 3.11 – 18.89 | 4.67 | 1.77 – 12.30 | 1.64 | 0.708 – 3.80 | 6.19 | 2.7 – 14.22 |
| COX-2 1195 AG | ||||||||
| A allele | ||||||||
| G allele | 0.21 | 0.13 – 0.36 | 3.48 | 1.31 – 9.22 | 0.061 | 0.023 – 0.16 | 0.489 | 0.303–0.789 |
| A+ (AA+AG) | 0.27 | 0.085 – 0.848 | - | - | - | - | 0.532 | 0.172 – 1.65 |
| G+ (GG+AG) | 0.091 | 0.040 – 0.204 | 3.27 | 1.172 – 9.108 | 0.028 | 0.009 – 0.088 | 0.408 | 0.228 – 0.732 |
1: Migraineurs with related parents vs control.
2: Migraineurs with unrelated parents vs control.
P3: Migraineurs with related parents vs migraineurs with unrelated parents. P4: Patients vs control.
OR: odds ratio
CI: confidence intervals
We also achieved that carries of COX-2 765 C+ and COX-2 765 G+ genotypes in patients with related parents were higher and lower than in control cases respectively (P<0.001, χ2: 36.56, OR: 28.70 CI= 6.63–124.27 and P<0.001, χ2: 27.28, OR: 7.66 CI= 3.11–18.89 respectively).
Meanwhile, considerable difference in COX-2 765 C+ genotype between patients with related parents and patients with unrelated parents has mentioned in Table 5 and 6 (P<0.001, χ2: 17.08, OR: 14.62 CI= 3.14–68.07). Table 5 and 6 illustrate that there is considerable difference in COX-2-1195 G+ genotype between control and patient with related parents (P<0.001, χ2: 39.68, OR: 0.091 CI= 0.040–0.204) although in COX-2-1195 G+ genotype distribution, significant difference was observed between subjects with related parents and patients with unrelated parents (P<0.001, χ2=49.50, OR= 0.028 95%CI= 0.009–0.088). Generally, there were statistically considerable differences in COX-2-765G>C and COX-2-1195A>G SNPs between the MA patients, MO patients, patients with related parents, patients with unrelated parents as compared with control individuals, so these variants and consanguineous type of marriage can increase risk of migraine susceptibility.
Discussion
We have studied the impression of genetic polymorphisms of COX-2 gene and the risk of migraine susceptibility. In this study, there is a positive relation between the -765 GC, -765 CC, -1195 AG and -1195 GG and being adventured to migraine attacks. In other words C allele of COX-2-765G>C and G allele of COX-2-1195A>G play key role in increasing migraine risk and besides -765 GG and -1195 AA have protective effect against migraine. This genetic reality has been demonstrated in Turkey for the first time. We showed this association on Iranian population as the first study in Iran and second in the world. Recent studies are available now and have recognized four new genetic variants related to migraine. A new variant known as rs1835740 moderates glutamate homeostasis is associated with concepts of neurotransmitter disorders. This new variant may be more specific for acute sorts of migraine such as MA than MO (8). Another variant exists as rs11172113, involves the lipoprotein receptor LRP1 which may interact with neuronal glutamate receptors and pathway however the specify function of third variant rs2651889 (PRDM16) in migraine is unknown yet (8). The fourth variant that for the first time connected gene impressions on migraine pain pathways, was rs10166942 is in close proximity to TRPM8, which encodes for pain and cold receptors (8).
COX-2 derived prostanoids enumerated as crucial clinical mediators of pain and other inflammatory symptoms of knee osteoarthritis (2) suggested that COX-2 polymorphisms could contribute susceptibility to sarcoidosis (16). Promoter polymorphisms COX-2-765G>C and COX-2-1195A>G would alter the mRNA levels and transcriptional pattern (17). These polymorphisms would have a great impact on PGE2 production (18). There is a major relevance between COX-2 expression and the risk of vitiligo (19, 20). Correlation of COX-2 functional polymorphisms (COX-2-1195A>G, -765G>C, -8473T>C) and the risk of vitiligo has been reported (21). COX-2 overexpression stimulates tumor growth and prostate cancer progression (22). Furthermore, expression of the two main isoforms of cyclooxygenase (COX-1 and COX-2) in human cancerous prostatic tissues has been studied and overexpression of both of them is reported (23). Moreover, an increased propensity for lung cancer development was observed in individuals who caring the C allele of a polymorphism in the 3′-UTR of COX-2 (24). In a study on Iranian population, there was no significant relation between PTGS2 (COX-2)-765G>C gene polymorphism and the risk of sporadic colorectal cancer (25). Moreover, 75% of patient with poorly differentiated tumors and 84.6% of patient with moderately differentiated tumors had high scale of COX-2 in their primary tumor cells (26).
Induction of COX-2 in dementia of Alzheimer type and Down’s syndrome may contribute to the integration of free radicals and then may be related to neuronal degeneration (27). Selective COX-2 inhibitors can increase the risk of vascular events (28) myocardial infarction (29) and cardiovascular occurrences (30). Conversely, selective COX-2 inhibitors made a significantly reduction in the risk of breast cancer (31), this reality can be related to the finding that elevated COX-2 expression in breast cancers was seen as a common and ordinary point (32). COX-2 may be a target for the prohibition, treatment and abatement the risk of squamous cell carcinoma of the head and neck (HNSCC) (33). The COX-2 pathway can lead to the growth and apoptosis of pancreatic cancer (34) and interesting results are in available, pointed to that overexpression of COX-2 in multiple myeloma (MM) is correlated with reduced survival (35–36).
Conclusion
As the first study in Iran, demonstrates that COX-2 gene polymorphisms would increase the risk of affliction to migraine disorder. Further studies on other races in the other regions with different climate are necessary to compare various results. Regarding effective role of genetic factors in MA and related clustering migraine, more genetically based studies can be helpful in diagnosing the reasons, symptoms and inheritance prognosis of illness.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
The authors thank assistance of all members of Bushehr Transferring Blood Center and Abolfazl Therapeutic Clinic staff in collecting blood samples. We also express our gratitude to personal of the Biotechnology Research Center of Islamic Azad University of Shahrekord Branch in Iran for their synergetic cooperation. The authors declare that they have no conflicts of interest.
References
- 1. Evans RW. (2009). Migraine: a question and answer review. Med Clin North Am, 93( 2): 245–62. [DOI] [PubMed] [Google Scholar]
- 2. Dasdemir S, Cetinkaya Y, Gencer M, Ozkok E, Aydin M, Cakmakoglu B. (2013). Cox-2 gene variants in migraine. Gene, 518( 2): 292–5. [DOI] [PubMed] [Google Scholar]
- 3. Griffiths LR, Nyholt DR, Curtain RP, Goadsby PJ, Brimage PJ. (1997). Migraine association and linkage studies of an endothelial nitric oxide synthase (NOS3) gene polymorphism. Neurology, 49( 2): 614–7. [DOI] [PubMed] [Google Scholar]
- 4. Wessman M, Kallela M, Kaunisto MA, et al. (2002). A susceptibility locus for migraine with aura, on chromosome 4q24. Am J Hum Genet, 70( 3): 652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. (2007). Migraine prevalence, disease burden, and the need for preventive therapy. Neurology, 68( 5): 343–9. [DOI] [PubMed] [Google Scholar]
- 6. Lipton RB, Silberstein SD, Stewart WF. (1994). An update on the epidemiology of migraine. Headache, 34( 6): 319–28. [DOI] [PubMed] [Google Scholar]
- 7. Colson NJ, Lea RA, Quinlan S, Griffiths LR. (2006). No role for estrogen receptor 1 gene intron 1 Pvu II and exon 4 C325G polymorphisms in migraine susceptibility. BMC Med Genet, 7( 1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schürks M. (2012). Genetics of migraine in the age of genome-wide association studies. J Headache Pain, 13( 1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menon S. (2011). Migraine Molecular Genetic and Pharmacogenetic Studies. Griffith Australia : . [Google Scholar]
- 10. Wilkinson M, Blau JN. (1985). Are Classical and Common Migraine Different Entities? Headache, 25( 4): 211–213. [DOI] [PubMed] [Google Scholar]
- 11. Todt U, Dichgans M, Jurkat-Rott K, Heinze A, Zifarelli G, Koenderink JB, Goebel I, Zumbroich V, Stiller A, Ramirez A, Friedrich T, Göbel H, Kubisch C. (2005). Rare missense variants in ATP1A2 in families with clustering of common forms of migraine. Hum Mutat, 26( 4): 315–21. [DOI] [PubMed] [Google Scholar]
- 12. Lopez-Campos JL, Rodriguez-Rodriguez D, Rodriguez-Becerra E, et al. (2009). Cyclooxygenase-2 polymorphisms confer susceptibility to sarcoidosis but are not related to prognosis. Respir Med, 103( 3): 427–33. [DOI] [PubMed] [Google Scholar]
- 13. Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. (2001). Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature, 410( 6827): 471–5. [DOI] [PubMed] [Google Scholar]
- 14. Sambrook J, Russell DW. (2001). Molecular Cloning: A Laboratory Manual [Internet]. 3rd ed New York : : Cold Spring Harbour Laboratory Press, Cold Spring Harbour, New York ; ; 2001 . . 1 – 2100 p. [Google Scholar]
- 15. Shi YY, He L. (2005). SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 15( 2): 97–8. [DOI] [PubMed] [Google Scholar]
- 16. Ulrich CM, Bigler J, Sibert J, et al. (2002). Cyclooxygenase 1 (COX1) polymorphisms in African-American and Caucasian populations. Hum Mutat, 20( 5): 409–410. [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Miao X, Tan W, Ning B, Liu Z, Hong Y, Song W, Guo Y, Zhang X, Shen Y, Qiang B, Kadlubar FF, Lin D. (2005). Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology, 129( 2): 565–76. [DOI] [PubMed] [Google Scholar]
- 18. Sanak M, Szczeklik W, Szczeklik A. (2005). Association of COX-2 gene haplotypes with prostaglandins production in bronchial asthma. J Allergy Clin Immunol, 116( 1): 221–3. [DOI] [PubMed] [Google Scholar]
- 19. Azzam OA, Kadry DM, Rashed LA, El-Refaie AEA, Doss RW. (2012). Cyclooxygenase-2 and prostaglandin E2 in vitiligo patients: plasma and tissue levels. Journal of the Egyptian Women’s Dermatologic Society, 9 ( 2 ), 92 –97. [Google Scholar]
- 20. Esmaeili B, Rezaee SA, Layegh P, Tavakkol Afshari J, Dye P, Ghayoor Karimiani E, Kalalinia F, Rafatpanah H. (2011). Expression of IL-17 and COX2 gene in peripheral blood leukocytes of vitiligo patients. Iran J Allergy Asthma Immunol, 10 ( 2), 81–89. [PubMed] [Google Scholar]
- 21. Li M, Gao Y, Li C, Liu L, Li K, Gao L, Wang G, Zhang Z, Gao T. (2009). Association of COX2 functional polymorphisms and the risk of vitiligo in Chinese populations. J Dermatol Sci, 53( 3): 176–81. [DOI] [PubMed] [Google Scholar]
- 22. Fujita H, Koshida K, Keller ET, Takahashi Y, Yoshimito T, Namiki M, Mizokami A. (2002). Cyclooxygenase-2 promotes prostate cancer progression. Prostate, 53( 3): 232–40. [DOI] [PubMed] [Google Scholar]
- 23. Kirschenbaum A, Klausner AP, Lee R, Unger P, Yao S, Liu XH, Levine AC. (2000). Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology, 56( 4): 671–6. [DOI] [PubMed] [Google Scholar]
- 24. Campa D, Zienolddiny S, Maggini V, Skaug V, Haugen A, Canzian F. (2004). Association of a common polymorphism in the cyclooxygenase 2 gene with risk of non-small cell lung cancer. Carcinogenesis, 25( 2): 229–35. [DOI] [PubMed] [Google Scholar]
- 25. Daraei A, Salehi R, Mohamadhashem F. (2012). PTGS2 (COX2) -765G>C gene polymorphism and risk of sporadic colorectal cancer in Iranian population. Mol Biol Rep, 39( 5): 5219–24. [DOI] [PubMed] [Google Scholar]
- 26. Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK. (2001). Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer, 91( 6): 894–9. [DOI] [PubMed] [Google Scholar]
- 27. Oka A, Takashima S. (1997). Induction of cyclooxygenase 2 in brains of patients with Down’s syndrome and dementia of Alzheimer type: specific localization in affected neurones and axons. Neuroreport, 8( 5): 1161–4. [DOI] [PubMed] [Google Scholar]
- 28. Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. (2006). Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomized trials. BMJ, 332( 7553): 1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hippisley-Cox J, Coupland C. (2005). Risk of myocardial infarction in patients taking cyclooxygenase-2 inhibitors or conventional nonsteroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ, 330( 7504): 1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mukherjee D, Nissen SE, Topol EJ. (2001). Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA, 286( 8): 954–9. [DOI] [PubMed] [Google Scholar]
- 31. Harris RE, Beebe-Donk J, Alshafie GA. (2006). Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer, 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. (2002). Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res, 62( 3): 632–5. [PubMed] [Google Scholar]
- 33. Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ. (1999). Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res, 59( 5): 991–4. [PubMed] [Google Scholar]
- 34. Ding XZ, Tong WG, Adrian TE. (2000). Blockade of cyclooxygenase-2 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Anticancer Res, 20( 4): 2625–31. [PubMed] [Google Scholar]
- 35. Cetin M, Buyukberber S, Demir M, Sari I, Sari I, Deniz K, Eser B, Altuntas F, Camci C, Oztürk A, Turgut B, Vural O, Unal A. (2005). Overexpression of cyclooxygenase-2 in multiple myeloma: association with reduced survival. Am J Hematol, 80( 3): 169–73. [DOI] [PubMed] [Google Scholar]
- 36. Trojan A, Tinguely M, Vallet S, Seifert B, Jenni B, Zippelius A, Witzens-Harig M, Mechtersheimer G, Ho A, Goldschmidt H, Jäger D, Boccadoro M, Ladetto M. (2006). Clinical significance of cyclooxygenase-2 (COX-2) in multiple myeloma. Swiss Med Wkly, 136( 25–26): 400–3. [DOI] [PubMed] [Google Scholar]


