In patients undergoing hepatic radioembolization for metastatic colorectal adenocarcinoma, increased lung shunt fraction is predictive of significantly decreased survival but demonstrates only a weak correlation with serum carcinoembryonic antigen levels and tumor-to-liver volume ratio and no correlation to the presence or absence of extrahepatic metastatic disease.
Abstract
Purpose
To determine if high lung shunt fraction (LSF) is an independent prognostic indicator of poor survival in patients who undergo yttrium 90 radioembolization for unresectable liver-dominant metastatic colorectal cancer.
Materials and Methods
Retrospective data were analyzed from 606 patients (62% men; mean age, 62 years) who underwent radioembolization to treat liver metastases from colorectal adenocarcinoma between July 2002 and December 2011 at 11 U.S. centers. Institutional review board exemptions were granted prior to the collection of data at each site. Overall survival was estimated by using Kaplan-Meier survival and univariate Cox proportional hazards models to examine the effect of LSF on survival and to compare this to other potential prognostic indicators. Multivariate analysis was also performed to determine whether LSF is an independent risk factor for poor survival.
Results
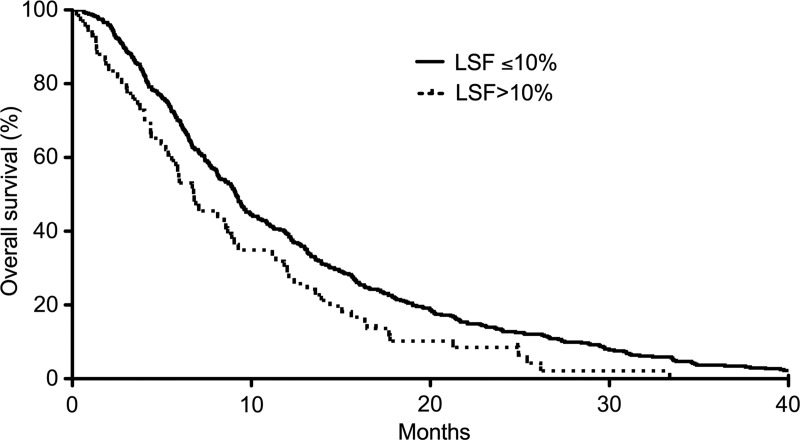
LSF higher than 10% was predictive of significantly decreased survival (median, 6.9 months vs 10.0 months; hazard ratio, 1.60; P < .001) and demonstrated a mild but significant correlation to serum carcinoembryonic antigen levels and tumor-to-liver volume ratio (Pearson correlation coefficients, 0.105 and 0.113, respectively; P < .05). A progressive decrease in survival was observed as LSF increased from less than 5% to more than 20% (P < .05). LSF did not correlate with the presence of extrahepatic metastases or prior administration of bevacizumab.
Conclusion
Increased LSF is an independent prognostic indicator of worse survival in patients undergoing radioembolization for liver-dominant metastatic colorectal adenocarcinoma. High LSF correlates poorly to other potential markers of tumor size, such as tumor-to-liver volume ratio or serum carcinoembryonic antigen level, and does not correlate to the presence of extrahepatic metastases.
© RSNA, 2016
Introduction
Radioembolization with yttrium 90 (90Y) microspheres has become a mainstay in the treatment of unresectable primary and metastatic liver cancer (1–3). 90Y radioembolization typically occurs after appropriate clinical and imaging evaluation to determine the extent of malignant disease, vascular supply of the hepatic malignancy, presence of hepatopulmonary shunting, and, if cirrhotic, Child-Pugh stage and Model for End-Stage Liver Disease score. Imaging studies used for these purposes typically include contrast material–enhanced abdominal computed tomography (CT) or magnetic resonance (MR) imaging, hepatic and mesenteric angiography, and technetium 99m (99mTc) microaggregated albumin (MAA) scintigraphy.
90Y particles infused directly into the feeding artery of a tumor may travel through arteriovenous shunts within the tumor and enter the systemic venous circulation via the hepatic vein, a phenomenon termed hepatopulmonary shunting. Successful radioembolization relies on an intact capillary bed within the tumor for 90Y microspheres to lodge and treat local tissue. Resin 90Y microspheres have a median diameter of 32.5 μm (range, 20–60 μm) and therefore cannot typically pass through capillaries (diameter, 8–10 μm). However, in the disorganized angiogenesis of tumor growth, these particles may potentially pass through intratumoral hepatic shunts and lodge in the capillaries of the lung. Shunting has been linked with tumor vascularity in hepatocellular carcinoma (4,5). If sufficient 90Y particle activity passes through such shunts and lodges in the lungs, radiation pneumonitis may lead to respiratory collapse and even intubation for mechanical ventilation (6). To minimize the risk of radiation pneumonitis, during planning of mesenteric angiography, patients undergo 99mTc-MAA infusion from the same hepatic arterial location as anticipated for subsequent 90Y microsphere delivery to calculate the lung shunt fraction (LSF) by using a planar or single photon emission computed tomography (SPECT) gamma camera. Ninety percent of the administered 99mTc-MAA particles measure 10–40 μm in size, similar to the size of 90Y microspheres (median diameter, 32.5 μm; range, 20–60 microns) and are used to estimate clinically relevant shunting (7). The calculated LSF is used to adjust the dose in patients who have a shunt fraction of less than 20%. Treatment with radioembolization is relatively contraindicated in patients with an LSF higher than 20% or estimated lung exposure of more than 30 Gy (8).
High LSF has been postulated as a prognostic indicator of poor survival and a correlate to the presence of circulating tumor cells (9). The hypothesis of whether LSF can serve as a prognostic indicator of survival was tested by means of retrospective review of a large cohort of 606 patients with metastatic colorectal carcinoma (mCRC) treated with 90Y resin microspheres (10).
Materials and Methods
Selection of Institutions and Patient Cases
A retrospective study was conducted of 606 consecutive patients who were referred to 11 U.S. tertiary care centers for 90Y resin microsphere (SIR-Spheres; Sirtex Medical, Sydney, Australia) treatment between July 2002 and December 2011 (clinicaltrials.gov identifier no. NCT01815879). Institutional review board exemptions were granted prior to the collection of data at each site. 90Y resin microsphere treatment was considered for patients with advanced liver-dominant mCRC (a) who were not suitable (or refused consent) to undergo surgery, ablation, or systemic therapy or (b) whose disease had progressed after systemic therapy or (c) who had become intolerant of at least one line of systemic therapy. All patients with a diagnosis of mCRC who underwent at least one radioembolization treatment and had at least one follow-up visit were then included in the analysis. Candidates for 90Y resin microsphere treatment had liver-only or liver-dominant mCRC, Eastern Cooperative Oncology Group performance status score of up to 2, and untreated life expectancy of at least 12 weeks. Patients with limited hepatic reserve, ascites, or other signs of liver failure (such as total bilirubin level > 2.0 mg/dL [34.2 μmol/L] or serum albumin level < 3.0 g/dL [30 g/L]) or with compromised bone marrow or pulmonary function were considered unsuitable for 90Y resin microsphere treatment. According to exceptional circumstances and with informed consent, some patients were treated outside of the outlined criteria on the basis of the clinical judgment of the treating physicians.
Data Collection and Analysis
Data were collated on the day of the first radioembolization treatment (day 0) and at all subsequent visits or until death. All results from hematologic, liver function, and blood biochemistry tests, physical examinations, and imaging studies were recorded and submitted to a centralized data collection center by using a unified case report form. Data collected included laboratory test results, volumes calculated from preprocedural computed tomography (CT)/magnetic resonance (MR) imaging (tumor and total liver volumes were used to calculate tumor-to-liver volume ratios), LSF, and prior treatment regimens. Survival was calculated from the day of the first radioembolization treatment to the day of death or last follow-up examination. Patients were censored at the time of their last follow-up examination if their status could not be established. All patient-identifying information was replaced with unique study numbers. Patient and disease characteristics are available in Table E1 (online). An analysis of the safety of the radioembolization procedure was recently published by using all 606 patients in the Metastatic colorectal cancer liver metastases Outcomes after Radioembolization, or MORE, cohort (10). The present analysis focuses on the usefulness of LSF as a prognostic indicator by using long-term follow-up data, while the prior work focused on the safety of the radioembolization procedure. This was an investigator-initiated study funded by Sirtex Medical Limited, Sydney, Australia. Authors who did not have consulting or proctoring relationships with Sirtex Medical had full control over the data and information submitted for publication and performed all important parts of the study, including data analysis and manuscript preparation (K.H.N., senior radiology resident physician with >5 years of experience; and M.V.B., biostatistician with >10 years of experience). Statistical analysis was conducted by using SAS XP Pro version 9.2 (SAS Institute, Cary, NC).
Radioembolization Procedure
The technique and rationale for radioembolization procedures to deliver radioactive 90Y resin microspheres into the hepatic artery were performed according to consensus guidelines that are well described elsewhere (8,11). Briefly, after multidisciplinary evaluation to establish candidacy for radioembolization therapy, visceral angiography was performed to map celiac, superior mesenteric, and hepatic arterial vessels; identify anatomic variants; and isolate the hepatic arterial circulation by performing prophylactic embolization of hepaticoenteric vessels. The gastroduodenal artery was embolized in 82.3% of patients (499 of 606) undergoing their first radioembolization treatment. After angiographic evaluation of the hepatic vasculature and prophylactic embolization, technetium 99m (99mTc)-MAA was injected into the appropriate hepatic artery to calculate LSF. It was the treating physician’s preference whether to treat lobar or bilobar disease in a single session. After review of the radionuclide scintigraphy images and calculation of the prescribed dose, the desired activity was extracted from the 3-GBq source vial and injected into the right, left, or proper hepatic artery according to established guidelines (11). The body surface area method was used for dosimetry calculations. Clinical judgment was used to assess the appropriateness of treatment in patients with relative contraindications, including compromised pulmonary function, presence of ascites, and inadequate liver reserve.
Pulmonary Scintigraphic Imaging and LSF Calculation
Centers were guided by the published consensus from the Radioembolization Brachytherapy Oncology Consortium and earlier reviews on pretreatment work-up and dosimetry (8). All patients underwent hepatic angiography and embolization of the gastroduodenal and other arterial branches to prevent nontarget embolization. A transcatheter injection of approximately 148 MBq of 99mTc-MAA particles (Macrotec; Bracco Diagnostics, Princeton, NJ) was introduced into the right, left, or proper hepatic artery, with flow rate and catheter position mimicking the anticipated 90Y microsphere infusion rate. Within 2 hours of 99mTc-MAA administration, planar scintigraphic images were acquired by using a gamma camera with a large field of view, with the patient in the supine position. The images consisted of anterior and posterior views of the lungs and liver, obtained over 5 minutes each. To calculate the LSF, regions of interest were drawn over the lungs and liver on the anterior and posterior images so that geometric means of the lung and liver activity could be calculated. LSF was calculated by dividing the geometric mean of the net counts from the lungs by the sum of the geometric mean of the net counts from both the lungs and the liver.
Statistical Methods
Overall survival was estimated by using the Kaplan-Meier method, and the log-rank test was used to assess statistical significance. Univariate Cox proportional hazards models were applied to identify univariate prognostic factors associated with survival. A multivariate proportional hazards model was applied to the statistically significant univariate variables by using either Kaplan-Meier or Cox proportional hazards models, and the analysis model was constructed on the basis of the maximum number of statistically significant variables (best subsets approach). Statistical significance was determined by using a two-sided α value of .05, and no adjustments were made for multiple comparisons. All statistical analyses were conducted by using statistical analysis software (SAS, Cary, NC).
Results
The study included 606 patients treated with 90Y radioembolization for liver-dominant colorectal cancer metastases (Table E1 [online]). There was extensive variability in the LSF measured at the time of radioembolization (Fig 1). Seventy patients (11.7%) had an LSF higher than 10%, and six patients (1.0%) had an LSF higher than 20%. LSF higher than 10% was predictive of significantly decreased survival compared with LSF of less than or equal to 10% (median, 6.9 months vs 10.0 months; hazard ratio, 1.60; P < .001; Fig 2). Increasing LSF was associated with a continuous decrease in survival across the entire cohort (Table 1, Fig E1 [online]). Other patient variables also correlated with decreased survival in this cohort, including Eastern Cooperative Oncology Group performance status, ascites, tumor-to-liver volume ratio, serum carcinoembryonic antigen (CEA) level, serum total bilirubin level, presence of extrahepatic metastases, and prior treatment with radioembolization, chemoembolization, or chemotherapy (P < .05). In a univariate proportional hazards model, LSF higher than 10% was associated with a hazard ratio of 1.60 (95% onfidence interval: 1.22, 2.08). A multivariate proportional hazards model was applied to the statistically significant univariate model variables, including LSF, presence of ascites, prior radioembolization treatments, presence of extrahepatic disease, number of prior chemotherapy treatments, tumor-to-liver volume ratio, and serum total bilirubin level (Table 2). LSF remained an independent prognostic indicator of survival in the multivariate model.
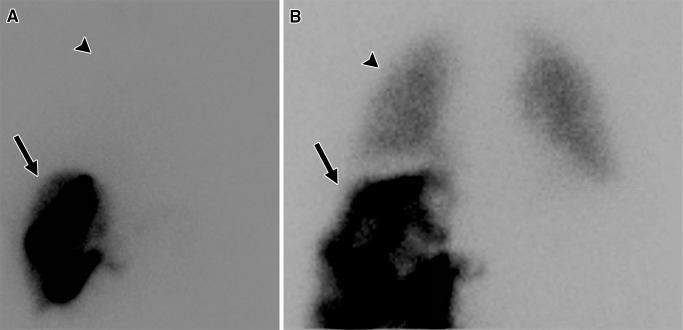
Figure 1:
Examples of 99mTc-MAA scintigraphic images obtained during evaluation prior to radioembolization. After 99mTc-MAA was injected into the hepatic artery to be treated, the patient underwent planar scintigraphy or SPECT to calculate LSF. A, In a patient with low LSF (4.4%), activity was primarily found in the liver (arrow), with little activity detected in the lungs (arrowhead). B, In a patient with high LSF (16%), significant activity was detected in the lungs (arrowhead), which was compared with the activity in the liver (arrow) to calculate LSF.
Figure 2:
Kaplan-Meier survival curve generated after the first 90Y radioembolization procedure. Patients with increased LSF of more than 10% demonstrate significantly poorer survival when compared with patients with low LSF.
Table 1.
Kaplan-Meier Statistics Indicating Significant Differences in Survival between Groups of Patients with Varying LSF
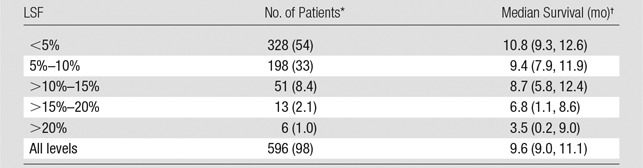
Note.—In the cohort of 606 patients, 10 patients (1.6%) had missing lung shunt data. A total of 503 of 606 patients (83%) died during the follow-up period. P values were less than .05 according to the proportional hazards model.
*Numbers in parentheses are percentages.
†Numbers in parentheses are 95% confidence intervals.
Table 2.
Multivariate Proportional Hazards Model Constructed by Using the Statistically Significant Univariate Variables with Either Kaplan-Meier or Cox Proportional Hazards Models
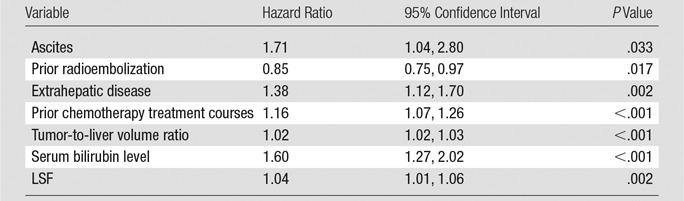
Note.—The analysis was constructed on the basis of the maximum number of statistically significant variables (best subsets approach).
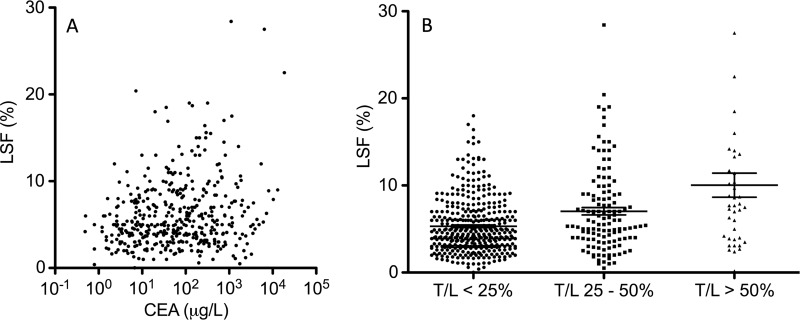
The relationship of LSF, a proposed scintigraphic marker of prognostic utility, to the existing preoperative prognostic marker of serum CEA levels was evaluated. There was a weak but statistically significant correlation between serum CEA level and LSF (Pearson correlation coefficient, 0.105; P = .043; Fig 3a). The tumor-to-liver volume ratios calculated (on the basis of three-dimensional volumetric measurements from preprocedural abdominal CT or MR images) demonstrated a weak but statistically significant correlation with LSF (Pearson correlation coefficient, 0.113; P = .013). Patients with tumor-to-liver volume ratios of 25%–50% or higher than 50% had higher LSFs compared with patients with tumor-to-liver volume ratios of less than 25% (Fig 3b).
Figure 3:
Scatterplots illustrate the relationship of LSF to other prognostic determinants. A, Scatterplot of serum CEA level against LSF demonstrates a weak correlation (Pearson correlation coefficient, 0.105; P = .043). B, Grouped column scatterplot of tumor-to-liver volume ratio (T/L) against LSF demonstrates a weak correlation between increased tumor-to-liver volume ratios of at least 25% and LSF (Pearson correlation coefficient, 0.113; P = .013).
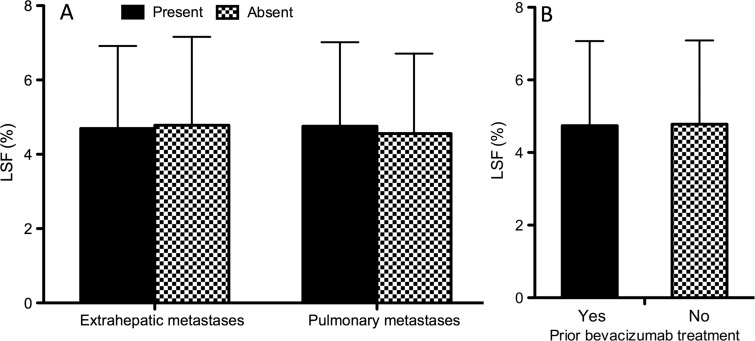
There was no statistical difference in LSF between patients with and those without extrahepatic or pulmonary metastases (Fig 4a), and there was no significant difference in LSF measurements between patients who had been previously treated with bevacizumab and those who had not (Fig 4b).
Figure 4:
Bar graphs show that high LSF does not indicate the presence of extrahepatic metastases or lack of prior bevacizumab treatment. A, In patients with extrahepatic metastases (left) or specifically pulmonary metastases (right), LSF was not significantly increased. B, In patients who had previously been treated with the antiangiogenic agent bevacizumab, LSF was not significantly decreased.
Discussion
Appropriate clinical and imaging evaluation prior to 90Y radioembolization includes measurement of the LSF during planning angiography. LSF is measured by means of planar scintigraphy after injection of 99mTc-MAA particles via a catheter positioned in the appropriate hepatic artery. The distribution of 99mTc-MAA particles (typical mean size, 20–40 μm) in the liver and lung is used to predict migration of radioembolic microspheres delivered during a subsequent treatment. An LSF of more than 20% or a calculated hypothetical single lung dose of more than 30 Gy is considered a contraindication to hepatic radioembolization because of the risk of radiation-induced lung toxicity (8). Excessive shunting of radioactive microspheres into the pulmonary circulation after hepatic radioembolization can rarely result in radiation pneumonitis, organizing pneumonia, or chronic pulmonary disease (12). Therefore, treatment guidelines have been developed to recommend a 20% decrease in administered radioactivity for patients with LSF of 10%–15% and a 40% decrease in administered radioactivity for patients with LSF of 15%–20% and to avoid radioembolization in patients with LSF of more than 20% (SIR-spheres 90Y microspheres package insert; Sirtex Medical). Although LSF and tumor-to-liver volume ratio are weakly correlated, LSF bears special significance because it can potentially prompt subtherapeutic radiation treatment dosing according to these guidelines.
Several prognostic scoring systems have been previously developed for patients with isolated metastatic disease in the liver who are considering undergoing resection or ablation, including the clinical risk score (13), modified clinical risk score (14), and Basingstoke predictive index (15). These scoring systems are used in an attempt to predict survival by incorporating available preprocedural data, such as number and size of liver tumors, serum CEA level, number of sites of extrahepatic disease, lymph node status, degree of differentiation of the primary cancer, and disease-free interval from discovery of the primary cancer to discovery of the liver metastases (synchronous vs metachronous). Such preprocedural risk stratification is thought to aid in appropriate patient selection for ablation and/or resection and to guide subsequent surveillance and follow-up regimens. Sofocleous et al recently validated many of these prognostic factors in a cohort of 53 heavily pretreated patients undergoing radioembolization for liver-dominant colorectal cancer metastases (16). In our cohort of patients with liver-dominant, unresectable metastatic colorectal cancer, indicators of poor survival included expected factors associated with tumor burden (tumor-to-liver volume ratio, serum CEA level, extrahepatic disease), hepatic function (serum bilirubin level, ascites), and prior treatment (prior radioembolization, prior chemotherapy). However, LSF was also found to be associated with poor survival, although it did not correlate well with variables that reflected tumor burden or prior treatment. Specifically, LSF correlated weakly with tumor-to-liver volume ratio (Pearson correlation coefficient, 0.113). Although LSF is available preprocedurally for patients with liver-dominant, unresectable metastatic colorectal cancer to determine their candidacy for radioembolization treatment, it is not widely used as an indicator of survival.
LSF has been proposed as a marker of prognostic utility in a small cohort of 62 patients with mCRC (9) and has been validated as a prognostic indicator of poor overall survival in a larger cohort of 366 patients with primary and secondary hepatic malignancies (17). We validated the prognostic utility of LSF retrospectively in a large cohort of 606 patients with mCRC and found that high LSF had a significant adverse effect on survival. Median survival among patients with low LSF (≤10%) was 10.0 months, while median survival among patients with high LSF (>10%) was only 6.9 months. High LSF only demonstrated weak correlation with other markers of tumor aggressiveness or size, such as serum CEA level or tumor-to-liver volume ratio, which further substantiates its role as an independent prognostic factor. Other groups have similarly found poor correlation between LSF and tumor size in smaller patient series (18,19).
Extrahepatic metastases are associated with poor survival in patients with stage 4 colorectal cancer (14,15) and may therefore be implicated in the poor survival of patients with increased LSF. If intratumoral shunts serve as conduits for metastasis of neoplastic cells, one might expect higher rates of extrahepatic metastatic disease among patients with high LSF, particularly in the lungs. However, we did not find a significant difference in LSF between patients with and those without extrahepatic colorectal adenocarcinoma metastases at the time of radioembolization. Therefore, LSF may represent a marker for more biologically aggressive tumors with upregulated angiogenesis rather than a conduit for extrahepatic metastases, although further histopathologic and molecular-genetic validation studies would be needed to corroborate this hypothesis.
Bevacizumab is an antiangiogenic agent that has recently been incorporated into many mCRC treatment regimens and may be expected to normalize the disorderly angiogenesis of tumor growth and thereby lower LSF. Accordingly, another potential explanation for the poor prognosis of patients with high LSF is that these patients have not been previously treated with bevacizumab, which may serve to decrease incidence of arteriovenous shunts and confer improved survival. However, prior treatment with bevacizumab did not bear any relationship to LSF or the presence of extrahepatic colorectal adenocarcinoma metastasis in our cohort. Although we did not find a significant difference in LSF between patients with and those without extrahepatic colorectal cancer metastases at the time of radioembolization, evaluation of a large prospective cohort of patients with no extrahepatic metastasis would help to confirm these results.
If high LSF serves as an indicator of tumor aggressiveness independent of any effect on the likelihood of metastasis, it is possible that patients with high LSF should be treated with aggressive liver-directed therapy rather than have their radiation dose decreased to avoid radiation pneumonitis, as is currently standard practice. Although the risk of radiation pneumonitis is important to consider, conservative treatment parameters designed to avoid this complication may be precluding patients with high LSF from receiving treatment doses needed for objective response or an extension in disease-free survival. This hypothesis is supported by a study in which 58 patients who received a cumulative lung dose of more than 30 Gy during hepatic radioembolization by using glass microspheres demonstrated no clinical or imaging evidence of radiation pneumonitis during follow-up, despite high LSF in the cohort studied (median LSF, 16.5%; range, 7.8%–26.6%) (20). To decrease rates of suboptimal response in patients with LSF of 10%–20%, we suggest that unnecessary reductions of therapeutic dose should be avoided when possible (21). First, calculation of absorbed lung dose should serve as a more precise method for adjusting therapeutic radiation dose than current guidelines, which recommend dose reduction on the basis of LSF alone. Then, above a threshold-absorbed lung dose, adjunctive measures should be used to reduce risk of radiation-induced lung injury aside from simply reducing treatment dose, such as temporary balloon occlusion of the hepatic veins (22) or transarterial embolization (23). In a retrospective study of 89 patients with LSF higher than 10%, one group has recently found that patients treated according to dose reduction guidelines had worse outcomes, although adjunctive techniques to reduce LSF (such as bland arterial embolization) may have contributed to better response rates in patients treated without dose reduction (24). Last, calculation of LSF on the basis of 99mTc-MAA distribution may not accurately model 90Y microsphere distribution. Real-time monitoring of tumor, liver, and lung dose could assist with these risk-reduction techniques, and intraprocedural positron emission tomography may provide this information in the future (25).
The primary limitation of our study is its retrospective nature. Future studies could be conducted to prospectively assess the relationship of high LSF with the development of distant metastases. Many patients in our cohort had already been treated with multiple chemotherapeutic regimens, which may confound evaluation of survival effects. Our study also focused on nonsurgical treatment of mCRC and therefore lacks surgically resected specimens, which could have been evaluated for histologic features, such as microvessel density or molecular tumor markers, such as 18q deletions. The relationship of LSF to histologic and molecular prognostic features could bear relevance in future studies. Also, our study is limited to the use of resin 90Y microspheres. Although we would expect similar results in a patient cohort treated with glass 90Y microspheres, this has not yet been validated.
In conclusion, our results indicate that LSF is a statistically significant covariate in a univariate proportional hazards model and contributes as an independent risk factor for poor survival in a multivariate model. In patients undergoing hepatic radioembolization for metastatic colorectal adenocarcinoma, increased LSF is predictive of significantly decreased survival but demonstrates only a weak correlation with serum CEA levels and tumor-to-liver volume ratio and no correlation to the presence or absence of extrahepatic metastatic disease. Increased LSF may serve as a useful preprocedural prognostic indicator of worse survival in this patient population.
Advances in Knowledge
■ High lung shunt fraction (LSF) (>10%) is predictive of poor survival in patients undergoing radioembolization for metastatic colorectal adenocarcinoma (hazard ratio = 1.60, P < .001).
■ Contrary to prior reports, high LSF was not related to the presence of extrahepatic metastases, including lung metastases (606 patients, P > .05).
■ LSF correlates poorly to other prognostic indicators, such as serum carcinoembryonic antigen level (Pearson correlation coefficient, 0.105; P = .043) and tumor-to-liver volume ratio (Pearson correlation coefficient, 0.113; P = .013) and is not related to prior treatment with the antiangiogenesis agent bevacizumab (P > .05).
Implication for Patient Care
■ Patients with high LSF have a poorer prognosis with limited overall survival, irrespective of other prognostic indicators.
SUPPLEMENTAL TABLE
SUPPLEMENTAL FIGURE
Acknowledgments
Acknowledgments
We express our gratitude to Angelica Robles, BA, and Norissa Gastelum, BA, for their assistance in the preparation of this article.
Received October 8, 2015; revision requested December 15 and received February 22, 2016; accepted April 12; final version accepted May 24.
K.H.N. supported by a National Institutes of Health T32 grant (T32EB005970) and an RSNA Research & Education Foundation Resident Grant (RR1452). A.S.K. supported by an investigator-initiated educational grant from Sirtex Medical, Sydney, Australia.
Disclosures of Conflicts of Interest: K.H.N. disclosed no relevant relationships. M.V.B. disclosed no relevant relationships. A.S.K. Activities related to the present article: author received a grant from Sirtex Medical. Activities not related to the present article: institution received a grant from Sirtex Medical. Other relationships: disclosed no relevant relationships. M.S. disclosed no relevant relationships. N.A. disclosed no relevant relationships. C.K.H. disclosed no relevant relationships. K.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from Sirtex Medical for being the vice president of global clinical affairs; author has stock and/or stock options in Sirtex Medical. Other relationships: disclosed no relevant relationships. J.M. disclosed no relevant relationships. D.B.S. disclosed no relevant relationships. S.J.C. disclosed no relevant relationships. M.C. disclosed no relevant relationships. D.M.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from Sirtex for consulting. Other relationships: disclosed no relevant relationships. A.D. Activities related to the present article: author received a grant from Sirtex Medical. Activities not related to the present article: author received payment from Sirtex Medical for speakers’ bureau fees. Other relationships: disclosed no relevant relationships. E.E. Activities related to the present article: author received money from Abbott Northwestern Hospital for data preparation. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. S.K. disclosed no relevant relationships. C.W.N. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from Sirtex Medical for proctoring. Other relationships: disclosed no relevant relationships. F.M.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from Sirtex Medical for consulting, service on speakers’ bureaus, and travel and/or accommodations. Other relationships: disclosed no relevant relationships. M.A.S. Activities related to the present article: author received a grant from Sirtex Medical. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. S.S. disclosed no relevant relationships. S.G.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from Sirtex Medical for consulting and service on speakers’ bureaus. Other relationships: disclosed no relevant relationships. N.K.S. Activities related to the present article: author received payment from Sirtex Medical for consulting and support for travel to meetings. Activities not related to the present article: author received payment from Sirtex Medical for consulting and for service on speakers’ bureaus. Other relationships: disclosed no relevant relationships. E.A.W. disclosed no relevant relationships. S.C.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from Sirtex Medical for proctoring and serving on a scientific advisory board; author is a stockholder in Sirtex Medical; author received payment from Embolx, Guerbet, and XLSciTech for consulting. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CEA
- carcinoembryonic antigen
- LSF
- lung shunt fraction
- MAA
- microaggregated albumin
- mCRC
- metastatic colorectal carcinoma
References
- 1.Nicolay NH, Berry DP, Sharma RA. Liver metastases from colorectal cancer: radioembolization with systemic therapy. Nat Rev Clin Oncol 2009;6(12):687–697. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy A. Radioembolization of hepatic tumors. J Gastrointest Oncol 2014;5(3):178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy A, Coldwell D, Sangro B, Wasan H, Salem R. Integrating radioembolization ((90)Y microspheres) into current treatment options for liver tumors: introduction to the international working group report. Am J Clin Oncol 2012;35(1):81–90. [DOI] [PubMed] [Google Scholar]
- 4.Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol 1997;52(1):36–40. [DOI] [PubMed] [Google Scholar]
- 5.Honda H, Tajima T, Kajiyama K, et al. Vascular changes in hepatocellular carcinoma: correlation of radiologic and pathologic findings. AJR Am J Roentgenol 1999;173(5):1213–1217. [DOI] [PubMed] [Google Scholar]
- 6.Leung TW, Lau WY, Ho SK, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys 1995;33(4):919–924. [DOI] [PubMed] [Google Scholar]
- 7.Leung WT, Lau WY, Ho SK, et al. Measuring lung shunting in hepatocellular carcinoma with intrahepatic-arterial technetium-99m macroaggregated albumin. J Nucl Med 1994;35(1):70–73. [PubMed] [Google Scholar]
- 8.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68(1):13–23. [DOI] [PubMed] [Google Scholar]
- 9.Deipolyi AR, Iafrate AJ, Zhu AX, Ergul EA, Ganguli S, Oklu R. High lung shunt fraction in colorectal liver tumors is associated with distant metastasis and decreased survival. J Vasc Interv Radiol 2014;25(10):1604–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol 2015;6(2):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salem R, Thurston KG. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol 2006;17(8):1251–1278. [DOI] [PubMed] [Google Scholar]
- 12.Wright CL, Werner JD, Tran JM, et al. Radiation pneumonitis following yttrium-90 radioembolization: case report and literature review. J Vasc Interv Radiol 2012;23(5):669–674. [DOI] [PubMed] [Google Scholar]
- 13.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230(3):309–318; discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology 2016;278(2):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees M, Tekkis PP, Welsh FKS, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247(1):125–135. [DOI] [PubMed] [Google Scholar]
- 16.Sofocleous CT, Violari EG, Sotirchos VS, et al. Radioembolization as a salvage therapy for heavily pretreated patients with colorectal cancer liver metastases: factors that affect outcomes. Clin Colorectal Cancer 2015;14(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing M, Lahti S, Kokabi N, Schuster DM, Camacho JC, Kim HS. 90Y radioembolization lung shunt fraction in primary and metastatic liver cancer as a biomarker for survival. Clin Nucl Med 2016;41(1):21–27. [DOI] [PubMed] [Google Scholar]
- 18.Ho S, Lau WY, Leung WT, et al. Arteriovenous shunts in patients with hepatic tumors. J Nucl Med 1997;38(8):1201–1205. [PubMed] [Google Scholar]
- 19.Powerski MJ, Erxleben C, Scheurig-Münkler C, et al. Hepatopulmonary shunting in patients with primary and secondary liver tumors scheduled for radioembolization. Eur J Radiol 2015;84(2):201–207. [DOI] [PubMed] [Google Scholar]
- 20.Salem R, Parikh P, Atassi B, et al. Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol 2008;31(5):431–438. [DOI] [PubMed] [Google Scholar]
- 21.Lam MGEH, Banerjee A, Goris ML, et al. Fusion dual-tracer SPECT-based hepatic dosimetry predicts outcome after radioembolization for a wide range of tumour cell types. Eur J Nucl Med Mol Imaging 2015;42(8):1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bester L, Salem R. Reduction of arteriohepatovenous shunting by temporary balloon occlusion in patients undergoing radioembolization. J Vasc Interv Radiol 2007;18(10):1310–1314. [DOI] [PubMed] [Google Scholar]
- 23.Rose SC, Hoh CK. Hepatopulmonary shunt reduction using chemoembolization to permit yttrium-90 radioembolization. J Vasc Interv Radiol 2009;20(6):849–851. [DOI] [PubMed] [Google Scholar]
- 24.Ward TJ, Tamrazi A, Lam MG, et al. Management of high hepatopulmonary shunting in patients undergoing hepatic radioembolization. J Vasc Interv Radiol 2015;26(12):1751–1760. [DOI] [PubMed] [Google Scholar]
- 25.Bourgeois AC, Chang TT, Bradley YC, Acuff SN, Pasciak AS. Intraprocedural yttrium-90 positron emission tomography/CT for treatment optimization of yttrium-90 radioembolization. J Vasc Interv Radiol 2014;25(2):271–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.