Despite not reporting isolated diminutive lesions at initial CT colonography screening, the positive rates at repeat CT colonography screening were lower, supporting a 5–10-year screening interval.
Abstract
Purpose
To determine the rate and types of polyps detected at repeat computed tomographic (CT) colonography screening after initial negative findings at CT colonography screening.
Materials and Methods
Among 5640 negative CT colonography screenings (no polyps ≥ 6 mm) performed before 2010 at one medical center, 1429 (25.3%; mean age, 61.4 years; 736 women, 693 men) patients have returned for repeat CT colonography screening (mean interval, 5.7 years ± 0.9; range, 4.5–10.7 years). Positive rates and histologic findings of initial and repeat screening were compared in this HIPAA-compliant, institutional review board-approved study. For all patients with positive findings at repeat CT colonography, the findings were directly compared against the initial CT colonography findings. Fisher exact, Pearson χ2, and Student t tests were applied as indicated.
Results
Repeat CT colonography screening was positive for lesions 6 mm or larger in 173 (12.1%) adults (compared with 14.3% at initial CT colonography screening, P = .29). In the 173 patients, 29.5% (61 of 207) of nondiminutive polyps could be identified as diminutive at the initial CT colonography and 12.6% (26 of 207) were missed. Large polyps, advanced neoplasia (advanced adenomas and cancer), and invasive cancer were seen in 3.8% (55 of 1429), 2.8% (40 of 1429), and 0.14% (two of 1429), respectively, at follow-up, compared with 5.2% (P = .02), 3.2% (P = .52), and 0.45% (P = .17), respectively, at initial screening. Of 42 advanced lesions in 40 follow-up screenings, 33 (78.6%) were right sided and 22 (52.4%) were flat, compared with 45.4% (P < .001) and 11.3% (P < .001), respectively, at initial screening. Large right-sided serrated lesions were confirmed in 20 individuals (1.4%), compared with 0.5% (P < .001) confirmed at initial screening.
Conclusion
Positive rates for large polyps at repeat CT colonography screening (3.7%) were lower compared with those at initial screening (5.2%). However, more advanced right-sided lesions were detected at follow-up CT colonography, many of which were flat, serrated lesions. The cumulative findings support both the nonreporting of diminutive lesions and a 5–10-year screening interval.
© RSNA, 2016
An earlier incorrect version of this article appeared online. This article was corrected on August 30, 2016.
Introduction
Computed tomographic (CT) colonography is a relatively new colorectal screening test that has been validated for initial screening in both the clinical trial (1–4) and clinical practice (5–9) settings. However, little or no data exist to date regarding its use in repeat screening after an initial negative study. Borrowing from the standard recommended screening intervals of 5 years and 10 years for double-contrast barium enema and optical colonoscopy (OC), respectively, the Working Group on Virtual Colonoscopy suggested 5–10 years following a negative CT colonography screening examination in its 2005 CT Colonography Reporting and Data System (C-RADS) publication (10). Other groups, including the American Cancer Society, have recommended a 5-year screening interval, but typically without explanation (11). Largely because isolated diminutive lesions (≤ 5 mm) are ignored at CT colonography, we chose to favor the 5-year interval following first-time CT colonography screening for our clinical program (12). Early results from our 1st year of clinical CT colonography screening showed a very low colorectal cancer rate at 5-year clinical follow-up that was lower than the published rates following OC screening (13), which prompted us to maintain this screening interval. We have now performed enough follow-up CT colonography screening examinations to formally assess the specific colorectal findings that were identified. Therefore, the purpose of this study was to determine the rate and types of polyps detected at repeat CT colonography screening after initial negative CT colonography screening.
Materials and Methods
This retrospective observational study was compliant with Health Insurance Portability and Accountability Act and approved by our institutional review board. The requirement for signed informed consent was waived.
Patient Population
Between 2004 and 2010, a total of 5640 asymptomatic adults underwent CT colonography screening at one medical center with a negative result, defined as no colorectal polyps 6 mm or larger detected. As of May 2015, a total of 1429 (25.3%) of these individuals (736 women [mean age, 61.3 years] and 693 men [mean age, 61.4 years]; mean age at follow-up CT colonography screening, 61.4 years) had returned for repeat CT colonography screening. Repeat colorectal screening, whether with CT colonography or another modality, was generally recommended at 5 years after the initial negative screening examination. The mean interval between screening CT colonography in this repeat CT colonography screening cohort of 1429 adults was 5.7 years ± 0.9 years (range, 4.5–10.7 years), with the majority occurring within the 5–10-year range suggested by C-RADS in 2005 (10). Specifically, 94.1% (1344 of 1429) of repeat CT colonography screening examinations occurred within the 5–10-year follow-up window, with 5.7% (82 of 1429) performed less than 5 years later (range, 4.5–4.9 years) and 0.2% (three of 1429) performed after 10 years (range, 10.2–10.7 years). As with initial CT screening, individuals returning for repeat CT colonography screening remained asymptomatic and at average risk for colorectal cancer. Most patients returned for screening without a formal reminder. In 2012, we began sending reminder letters to patients who were more than 3 years overdue for repeat screening.
CT Colonography Technique and Interpretation
The CT colonography technique used at our institution has been described in detail elsewhere (5,12). In summary, patients underwent a low-volume bowel preparation on the day prior to CT colonography by using a cathartic cleansing agent. Magnesium citrate was used at repeat CT colonography screening in 1379 (96.5%) of 1429 patients, with polyethylene glycol used in most of the remaining patients (14). Oral contrast material tagging of residual fecal material was achieved with 2.1% weight per volume barium sulfate, and fluid tagging was achieved with either diatrizoate or iohexol iodinated contrast material (15). During the CT colonography examination, colonic insufflation was maintained by using automated continuous low-pressure carbon dioxide delivered through a small flexible rectal catheter (16). Patients were routinely scanned in both supine and prone positions, with additional decubitus positioning as needed (17). Images were acquired with 16–64 section multi–detector row CT scanners by using 1.25-mm collimation, 1-mm reconstruction interval, 120 kVp, and tube-current modulation (range, 30–300 mA) with a noise index of 50.
All CT colonography examinations (initial and follow-up screening) were prospectively interpreted by one of 12 experienced board-certified radiologists practicing within our abdominal imaging section (mean of 14 years in practice; range, 2–33 years). Radiologist interpretation of CT colonography examinations was performed by using three-dimensional endoluminal fly-through for initial polyp detection and two-dimensional cross-sectional images for secondary detection and polyp confirmation (12,18). All studies were interpreted by using a dedicated CT colonography software system (Viatronix V3D Colon; Viatronix, Rocky Point, NY). For all nondiminutive lesions detected at CT colonography, lesion size, segmental location, and morphology (sessile, pedunculated, flat, mass) were prospectively recorded. Proximal “right-sided” lesions were defined as located within the cecum, ascending, or transverse colon. Patient-level C-RADS categorization for colorectal findings was also recorded (10). When indicated, OC examinations were performed by board-certified gastroenterologists, usually on the same day as CT colonography. A standard matching algorithm for comparing CT colonography and OC findings was applied, consisting of linear size match within 50% and location within the same or adjacent colonic segment (3). All resected polyps were sent for surgical pathologic examination, with histologic evaluation by a specialized gastrointestinal pathologist. Advanced neoplasia is defined by the presence of any of the following: adenoma or serrated lesion 10 mm or greater, prominent villous component (> 25% at histologic examination), high-grade dysplasia, or invasive cancer.
Review of Positive Cases
An additional retrospective review was primarily performed by one author (B.D.P., 6 years of experience with CT colonography) for all follow-up CT colonography screening studies positive for nondiminutive polyps (≥ 6 mm). Specifically, both the follow-up and initial CT colonography screening examinations were evaluated side by side to place each new nondiminutive lesion into one of the following categories: presumed de novo lesion (not seen at initial CT colonography), prior diminutive lesion (≤ 5 mm at initial CT colonography), and missed nondiminutive lesion (≥ 6 mm at initial CT colonography). For patients subsequently evaluated at OC, further categorization of lesions was possible, including CT colonography true-positive, false-positive (vs OC false-negative [19]), and false-negative lesions, as well as histologic correlation for resected confirmed lesions.
Positive results at repeat CT colonography screening were reported and analyzed according to both by-patient and by-polyp (≥ 6 mm) levels.
Statistical Analysis
Key categories of colorectal polyp findings at repeat CT colonography screening (eg, C-RADS categories C2-C4, advanced neoplasia, invasive cancer, lesion morphology, lesion location, etc) were compared against summary statistics from earlier programmatic results for initial CT colonography screening as reported in previous publications (5,20–22). Fisher exact test and Pearson χ2 test were used, where applicable, to test for differences in categorical variables. Student t test was used to test for differences in continuous variables. A two-tailed P value < .05 was used as the criterion for statistical significance.
Results
At the 6-mm threshold, the overall rate of positive findings at repeat CT colonography screening after initial negative screening was 12.1% (173 of 1429), compared with our previously established positive rate of 14.3% at first-time CT colonography screening (Table). The mean age of individuals at repeat screening was 61.4 years, compared with 56.7 years at initial screening. Slightly more than half of individuals returning for CT colonography screening were female, similar to initial screening (51.5% vs 54.1%). Patient-level results according to the C-RADS category are shown in the Table, including comparison with results at initial CT colonography screening. Of note, the frequency of patients with large polyps (C-RADS category 3) was decreased at repeat CT colonography screening (3.8% [55 of 1429] vs 5.2% [351 of 6769]; P = .037). The frequency of mass or masslike lesions (C-RADS category C4) at follow-up screening was half that seen at initial screening (0.3% [four of 1429] vs 0.6% [39 of 6769]; P = .224). Among the four patients with C4 categorization, findings in three proved to be benign, including advanced carpet lesions (tubulovillous adenoma, n = 2) and benign diverticular stricture (n = 1), and finding in one patient proved to be malignant (primary adenocarcinoma). In comparison, the overall frequency of small, category C2 polyps (6–9 mm) was similar at follow-up and initial screening (8.0% [114 of 1429] vs 8.6% [579 of 6769]; P = .497).
Comparison of Findings at Repeat versus Initial Routine CT Colonography Screening
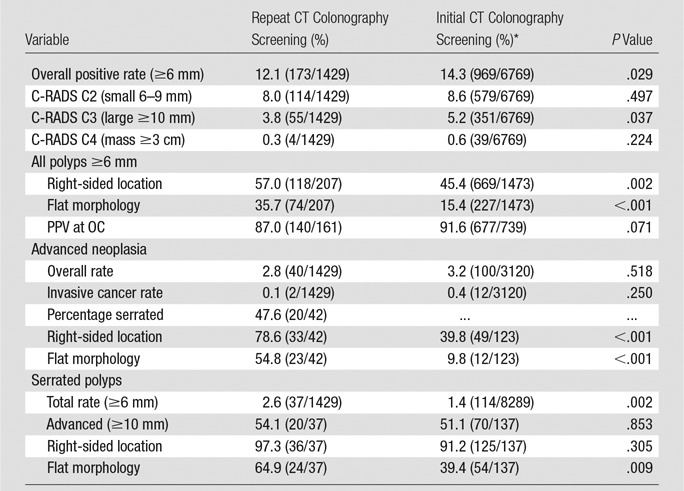
Note.—Data in parentheses are numerators and denominators. Data are reported both by-patient (n = 1429) and by-polyp (n = 207 ≥6 mm) level. PPV = positive predictive value.
Of the 173 patients with positive findings at prospective CT colonography follow-up screening, 122 (70.5%) underwent OC for confirmation and potential resection. The remaining patients with positive findings were enrolled in CT colonography surveillance, primarily for small polyps (C-RADS category C2). Of 161 total nondiminutive lesions identified at CT colonography in the 122 patients referred for OC, 140 (87.0%) were confirmed and resected (CT colonography true-positive), similar to our reported positive predictive value of 91.6% at initial CT colonography screening (P = .071) (21). The remaining 21 (13.0%) lesions were not seen at subsequent OC, representing either CT colonography false-positive or OC false-negative findings (19). In descending order of frequency, histologic results of confirmed resected lesions sent for pathologic evaluation included tubular adenoma (n = 62), sessile serrated polyps (n = 37), hyperplastic polyps (n = 16), tubulovillous adenoma (n = 13), mucosal/normal mucosa (n = 7), adenocarcinoma (n = 2), and inflammatory polyps (n = 2); one 6-mm polyp was resected but not retrieved. Of the 37 serrated lesions, 20 (54.1%) were 10 mm or larger, 24 (64.9%) were flat, and 36 (97.3%) were right sided (Table). Only one (0.7%) of the 137 nonmalignant lesions with histologic examination performed had a focus of high-grade dysplasia. An additional 14 nondiminutive lesions were identified at OC (CT colonography false-negative), all of which were benign and only four of which were 10 mm or larger (range, 6–15 mm).
After exclusion of the 21 presumed CT colonography false-positive lesions described earlier, there were 207 total nondiminutive lesions detected at repeat CT colonography screening in the positive cohort of 173 patients. Directed secondary review of the initial CT colonography screening examinations for these 173 patients revealed that 29.5% (61 of 207) of lesions could be identified as diminutive lesions (≤ 5 mm) that grew in the interval (Fig 1). An additional 12.6% (26 of 207) of lesions detected at repeat screening were retrospectively identified as already nondiminutive at the initial CT colonography screening, corresponding to lesions missed at the initial CT colonography (Figs 2, 3). Among the 17 lesions missed at initial CT colonography that were confirmed and resected at OC following repeat CT colonography, histologic results were all neoplastic, including sessile serrated polyp (n = 10), tubulovillous adenoma (n = 3), tubular adenoma (n = 3), and adenocarcinoma (n = 1). The remaining detected lesions could not be clearly identified at the initial screening CT colonography study, corresponding to either presumed de novo lesions (Fig 4), lesions not identifiable at initial CT colonography (eg, due to technical reasons and/or diminutive size), or unproven false-positive lesions.
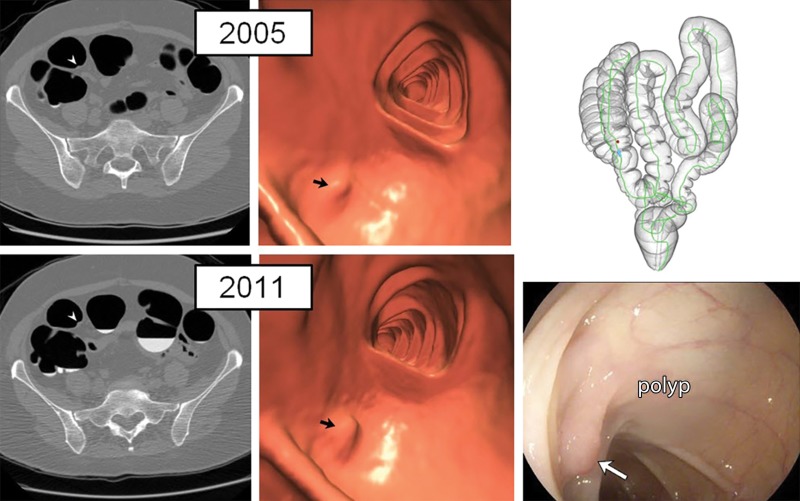
Figure 1:
Diminutive polyp at initial CT colonography screening that grew to small size at follow-up screening 6 years later in an asymptomatic woman (61 years old at initial screening; 67 years old at repeat screening). Top: two-dimensional (2D) (left) and three-dimensional (3D) (middle) images from the initial CT colonography screening in 2005 show a diminutive lesion (arrowhead for 2D, arrow for 3D) measuring less than 5 mm in the proximal transverse colon. The specific colonic location is indicated on the colon map (right) by the red dot. We do not report isolated diminutive lesions at CT colonography screening. Bottom: 2D (left) and 3D (middle) images from repeat CT colonography screening in 2011 show that the sessile polyp has grown in the intervening 6 years, now measuring 7 mm (arrowhead for 2D, arrow for 3D). The polyp was confirmed (arrow) and removed at same-day colonoscopy (right) and proved to be a tubular adenoma at pathologic evaluation.
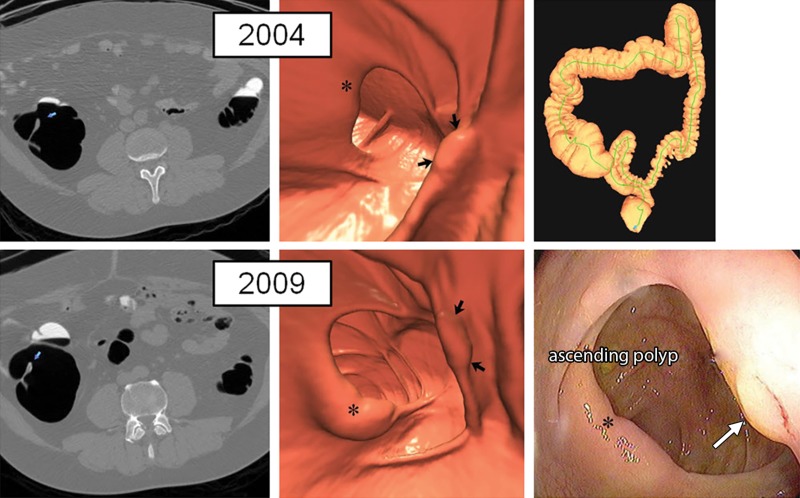
Figure 2:
Large right-sided flat serrated lesion missed at initial CT colonography screening that was detected at follow-up screening 5 years later in an asymptomatic man (50 years old at initial screening; 55 years old at repeat screening). Top: two-dimensional (2D) (left) and three-dimensional (3D) (middle) images from the initial CT colonography screening in 2004 show a subtle flat lesion (arrows) was missed in the ascending colon just distal to the ileocecal valve (*). The specific colonic location is indicated on the colon map (right) by the red dot. Little or no contrast material coating of the polyp surface is seen. Bottom: 2D (left) and 3D (middle) images from repeat CT colonography screening in 2009 show the same flat lesion (arrows), which measured 12 mm without significant change in size from 2004. The polyp was confirmed (arrow) and removed at same-day colonoscopy (right) and proved to be a sessile serrate polyp at pathologic evaluation. * indicates ileocecal valve.
Figure 3:
Missed flat lesion at initial CT colonography screening that progressed to localized cancer at follow-up screening 5 years later in an asymptomatic man (59 years old at initial screening; 64 years old at repeat screening). Top: two-dimensional (2D) (left) and three-dimensional (3D) (middle) images from the initial CT colonography screening in 2005 show a flat lesion (arrowhead for 2D, arrows for 3D) was missed in the proximal ascending colon, despite contrast material tagging. The specific colonic location is indicated on the colon map (right) by the red dot. Bottom: 2D (left) and 3D (middle) images from repeat CT colonography screening in 2010 now show an ulcerated 26-mm lesion (arrows). It was confirmed and biopsied at same-day colonoscopy and proved to be adenocarcinoma (arrow) at pathologic evaluation (right).
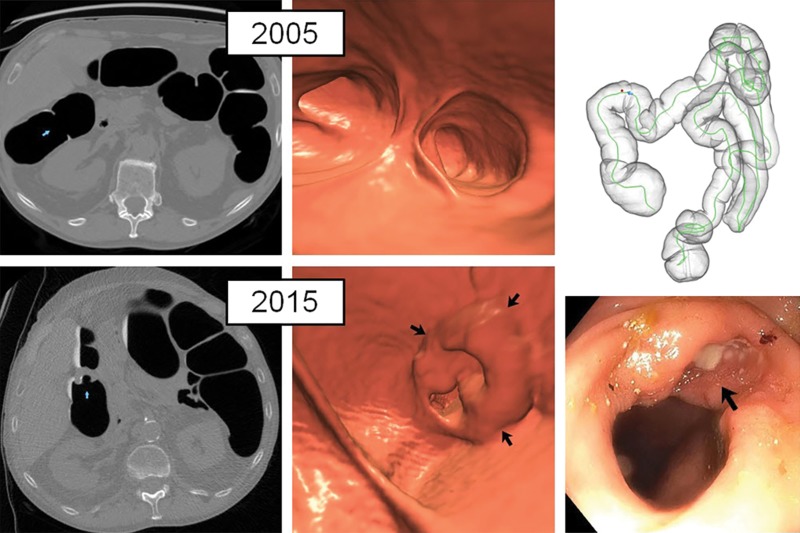
Figure 4:
Normal initial CT colonography screening (even in retrospect) in asymptomatic man (60 years old at initial screening; 70 years old at repeat screening) with obvious cancer detected 10 years later at follow-up screening. Top: on two-dimensional (2D) (left) and three-dimensional (3D) (middle) images from the initial CT colonography screening in 2005, no focal lesions were identified, even in retrospect, in the area of subsequent cancer in 2015. Bottom: 2D (left) and 3D (middle) images from repeat CT colonography screening in 2015 now show a semiannular mass near the hepatic flexure (arrows). Initial biopsy of mass (arrow) from same-day colonoscopy (right) revealed only tubular adenoma, but repeat colonoscopy with biopsy 4 months later revealed adenocarcinoma at pathologic evaluation.
A total of 42 confirmed advanced neoplasms in 40 patients were seen at follow-up CT colonography, corresponding to a rate of 2.8% (40 of 1429). Four (9.5%) of 42 advanced adenomas were 6–9 mm in size (all tubulovillous adenomas); the remainder were all large (range, 10–40 mm). Advanced lesion morphology was flat (n = 23), sessile (n = 12), pedunculated (n = 6), and annular mass (n = 1). Advanced lesions were more frequently right sided (78.6%, 33 of 42). Specific histology of advanced lesions included sessile serrated polyps (n = 20), tubulovillous adenoma (n = 13), tubular adenoma (n = 7), and adenocarcinoma (n = 2). High-grade dysplasia was reported in only one (2.4%) of 42 advanced adenomas. Compared with initial screening, advanced lesions at repeat CT colonography screening were more often right sided (78.6% vs 39.8%, P < .001) and flat (54.8% vs 9.8%, P < .001) (Table). The detection rate of large right-sided serrated lesions was also higher at repeat CT colonography screening (1.4% vs 0.5%).
Two interval cancers were identified at follow-up CT colonography screening (Figs 3, 4), corresponding to an incidence of 0.1% (two of 1429). In one case, a 64-year-old woman was found to have a 26-mm lesion in the ascending colon that proved to be malignant at biopsy from OC. At retrospective review of the initial CT colonography screening study from 5 years earlier, a flat 13-mm lesion was identified, which demonstrated surface tagging of oral contrast material (Fig 3). On the basis of size and appearance, this lesion was most likely an advanced adenoma at the time of initial CT colonography screening. This patient underwent laparoscopic right hemicolectomy, which revealed stage III low-grade mucinous adenocarcinoma (T3N1bMo). She subsequently underwent adjuvant chemotherapy (combination of infusional 5-fluorouracil, leucovorin, and oxaliplatin) and is without evidence of disease more than 5 years after surgery. The second patient, a 70-year-old man, was found to have an annular constricting mass near the hepatic flexure 10 years after an initial negative screening CT colonography. Even in retrospect, no precursor lesion could be identified in this location at the initial CT colonography (Fig 4). Initial biopsy at OC revealed only tubular adenoma, but repeat OC with biopsy 4 months later was positive for adenocarcinoma. The patient underwent laparoscopic right hemicolectomy for a stage I well-differentiated adenocarcinoma (T2N0M0) and is currently without evidence of disease. Among the 1429 patients who have returned for routine CT colonography screening, none had a clearly malignant lesion missed at initial CT colonography screening.
Discussion
The accuracy of colorectal evaluation at initial CT colonography screening has been well established in the clinical trial setting (1–4) and has been validated in clinical practice as well (5–9). However, because CT colonography is a relatively new technique and very few dedicated screening programs exist, the findings at repeat routine CT colonography screening after initial negative screening have not previously been reported. The maturity of our CT colonography screening program has now allowed for this assessment. Although the positive rate (defined as lesions ≥ 6 mm) was lower compared with that at initial CT colonography screening, as expected, we nonetheless found a number of clinically relevant lesions. Improved recognition of flat lesions, as discussed below, appears to be the most important difference between our initial and repeat CT colonography screening rounds. Not only does each flat lesion missed at initial CT colonography lower the first-round prevalence, but detecting them at repeat screening further increases the positive rate at the second round. The fact that the individuals screened were 5–10 years older at repeat screening likely contributes somewhat to the overall polyp prevalence as well.
The interval cancer rate at routine CT colonography surveillance was lower than the cancer rate seen at initial screening, as well as the reported interval rates at OC (23–25). We only identified two localized colon cancers, one that appeared to be a de novo growth 10 years after initial CT colonography screening and another that progressed over 5 years from a flat 13-mm lesion. The 0.1% incidence at repeat screening compares favorably with the reported colorectal cancer incidence rates ranging from 0.6% to more than 1% at surveillance OC (23–25). It is estimated that 70% of all interval cancers at surveillance OC represent missed or incompletely resected lesions, with the majority being missed at the initial OC examination, especially in the right colon (23,24). In one meta-analysis, the reported sensitivity of 96.1% for cancer at initial CT colonography evaluation was comparable to that of OC (94.7%) for trials where the endoscopists were blinded, but with fewer misses in the right colon for CT colonography (26). In addition, the high sensitivity of CT colonography for advanced adenomas at initial screening, also well above 90%, further contributes to the low interval colorectal cancer rate through cancer prevention. Our advanced neoplasia detection rate of 2.8% at repeat CT colonography was similar to the 2.4% rate reported at OC within 5.5 years of a negative baseline screening examination (25).
Nondiminutive lesions that were missed at initial CT colonography screening but detected at follow-up tended to be flat, right sided, and often serrated at histologic examination. The ability to detect these subtle lesions at CT colonography has greatly improved, with increased awareness of their existence and recognition of their propensity for surface coating with oral contrast material (22,27,28). We continue to believe that the dilute (2%) barium administered as part of our standard oral bowel preparation is what adheres to the surface of flat lesions (22,29). For CT colonography bowel preparations that utilize only an iodinated oral contrast material–or no contrast material at all–the detection rate for clinically relevant flat lesions is likely reduced, although we are not aware of specific data regarding serrated polyp detection in this setting. Regardless, flat lesions remain a challenge for CT colonography (and for OC) and are associated with lower diagnostic confidence and predictive values relative to polypoid lesions (21,30). Nonetheless, CT colonography and OC are likely complementary in detecting flat lesions (31–34). Furthermore, these flat lesions seem to follow a more indolent course in general, allowing for benign detection at repeat screening in most cases.
Of nondiminutive polyps (≥ 6 mm) detected at follow-up CT colonography screening, about 30% could be identified at the initial CT colonography screening as diminutive (≤ 5 mm). The previously established histology of diminutive (and small 6–9 mm) polyps is very benign, with invasive cancer being exceeding rare (35,36). We have previously investigated the natural history and in vivo growth rates of small (6–9 mm) polyps at surveillance CT colonography (37). Our study provides more insight into the natural history of subcentimeter colorectal lesions, especially for diminutive lesions. However, given that diminutive tubular adenomas are extremely common, with adenoma detection rates now exceeding 50% in some recent endoscopic screening series (38), it is clear from the reported rates of nondiminutive polyps from combined CT colonography-OC trials (2,3,5) that the majority do not grow beyond 5 mm. Therefore, our study only addresses the rare diminutive lesions that grow beyond 6 mm, and these results should not be extrapolated to all diminutive lesions in general. Nonetheless, our study clearly documents progression of some diminutive lesions to a nondiminutive size. In terms of detecting nondiminutive lesions, we previously showed that lesion detection at both the 6-mm and 10-mm thresholds for screening CT colonography is equivalent to that for screening OC. As such, our additional natural history observations of small 6–9 mm lesions at CT colonography are valid and likely generalizable.
Our results at repeat CT colonography screening provide further support for the practice of nonreporting of isolated diminutive lesions at CT colonography screening, adding to the limited clinical follow-up for cancer we reported previously (13). In addition, the suggested follow-up range of 5–10 years following a negative CT colonography screening examination originally proposed by C-RADS (10) appears to be reasonable based on the acceptably low rate of important findings seen at repeat CT colonography in our screening program. We continue to recommend that individuals return for the first follow-up CT colonography screening 5 years after the initial screening. However, if the second-round screening CT colonography remains negative, we often expand the recommended interval to 10 years in the absence of apparent diminutive lesions, technical inadequacies, or other notable risk factors.
We acknowledge limitations to the current study. Since patients with positive findings at CT colonography at initial screening were necessarily excluded from this routine CT colonography follow-up screening cohort, this study cohort would be expected to have a lower overall colorectal cancer risk profile from this selection bias. These results also reflect the experience of a single academic center and should not necessarily be extrapolated to practices where CT colonography technique or expertise may differ. Last, because we compared the current study results from repeat CT colonography screening against summary statistics of previously reported results from initial CT colonography screening, we were limited to Fisher exact, Pearson χ2, and Student t tests for this comparison.
In summary, we report our experience with repeat CT colonography screening after an initial negative examination 5 or more years earlier. The overall rate of positive colorectal findings at CT colonography follow-up screening was slightly lower than that at initial CT colonography screening, especially for more clinically relevant lesions, but was high enough to warrant ongoing surveillance. Approximately 30% of polyps 6 mm or larger could be identified as diminutive on the initial screening study, whereas missed nondiminutive lesions at initial CT colonography screening tended to be flat in morphology, right-sided in location, and serrated at histologic examination. Last, the interval cancer rate at routine CT colonography surveillance was very low. These collective findings support the practice of not reporting isolated diminutive lesions at CT colonography screening and of a 5–10-year screening interval after initial negative examination.
Advances in Knowledge
■ The overall rate of patients with positive colorectal findings at follow-up CT colonography screening (12.1% [173 of 1429]); mean interval, 5.7 years) was lower than at initial CT colonography screening (14.3%), especially for the more clinically relevant lesions.
■ Among nondiminutive polyps (≥6 mm) detected at follow-up CT colonography screening, the majority could not be found at dedicated retrospective review of initial CT colonography screening, whereas 29.5% (61 of 207) were previously diminutive and 12.6% (26 of 207) were nondiminutive at initial screening and were missed.
■ The interval cancer rate at routine follow-up CT colonography screening was low (two cancers in 1429 patients).
■ Nondiminutive lesions missed at initial CT colonography screening but detected at follow-up were more often flat and right sided and tended to be serrated at histologic examination.
Implications for Patient Care
■ The suggested follow-up range of 5–10 years following a negative CT colonography screening proposed with the CT Colonography Reporting and Data System appears to be reasonable based on the acceptably low rate of positive findings seen at repeat CT colonography in our screening program.
■ These findings provide further evidence that nonreporting of isolated diminutive lesions at CT colonography screening is a valid clinical approach.
■ The increased awareness of flat, right-sided lesions at CT colonography, many of which are serrated at histologic examination, should lead to increased prospective detection at initial CT colonography screening, which should result in even lower positive rates at repeat CT colonography screening in the future.
Received March 8, 2016; revision requested April 19; final revision received May 3; accepted June 2; final version accepted June 13.
Supported in part by the National Institutes of Health National Cancer Institute (1R01CA144835-01), the National Center for Advancing Translational Sciences (Ul1TR000427), the American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (grant MRSG-13-144-01-CPHPS), and the University of Wisconsin Institute for Clinical and Translational Research through the National Center for Advancing Translational Sciences (grant Ul1TR000427).
Abbreviations:
- C-RADS
- CT Colonography Reporting and Data System
- OC
- optical colonoscopy
Disclosures of Conflicts of Interest: P.J.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: cofounder of VirtuoCTC, shareholder in Cellectar Biosciences, Elucent, and SHINE, previous consultant for Bracco and Check-Cap, received research and travel support from Philips. Other relationships: disclosed no relevant relationships. B.D.P. disclosed no relevant relationships. I.M. disclosed no relevant relationships. J.M.W. disclosed no relevant relationships. D.H.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: cofounder of VirtuoCTC, consultant for Viatronix, and is on the medical advisory board of Digital Artforms. Other relationships: disclosed no relevant relationships.
References
- 1.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 2009;58(2):241–248. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008;359(12):1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003;349(23):2191–2200. [DOI] [PubMed] [Google Scholar]
- 4.Sali L, Mascalchi M, Falchini M, et al. Reduced and full-preparation CT colonography, fecal immunochemical test, and colonoscopy for population screening of colorectal cancer: a randomized trial. J Natl Cancer Inst 2015;108(2):djv319. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007;357(14):1403–1412. [DOI] [PubMed] [Google Scholar]
- 6.Pickhardt PJ, Taylor AJ, Kim DH, Reichelderfer M, Gopal DV, Pfau PR. Screening for colorectal neoplasia with CT colonography: initial experience from the 1st year of coverage by third-party payers. Radiology 2006;241(2):417–425. [DOI] [PubMed] [Google Scholar]
- 7.An S, Lee KH, Kim YH, et al. Screening CT colonography in an asymptomatic average-risk Asian population: a 2-year experience in a single institution. AJR Am J Roentgenol 2008;191(3):W100–W106. [DOI] [PubMed] [Google Scholar]
- 8.Nelson NJ. Virtual colonoscopy accepted as primary colon cancer screening test. J Natl Cancer Inst 2008;100(21):1492–1499. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PJ. CT colonography for population screening: ready for prime time? Dig Dis Sci 2015;60(3):647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: a consensus proposal. Radiology 2005;236(1):3–9. [DOI] [PubMed] [Google Scholar]
- 11.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58(3):130–160. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol 2007;189(2):290–298. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Pooler BD, Weiss JM, Pickhardt PJ. Five year colorectal cancer outcomes in a large negative CT colonography screening cohort. Eur Radiol 2012;22(7):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borden ZS, Pickhardt PJ, Kim DH, Lubner MG, Agriantonis DJ, Hinshaw JL. Bowel preparation for CT colonography: blinded comparison of magnesium citrate and sodium phosphate for catharsis. Radiology 2010;254(1):138–144. [DOI] [PubMed] [Google Scholar]
- 15.Johnson B, Hinshaw JL, Robbins JB, Pickhardt PJ. Objective and subjective intrapatient comparison of iohexol versus diatrizoate for bowel preparation quality at CT colonography. AJR Am J Roentgenol 2016;206(6):1202–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. AJR Am J Roentgenol 2006;186(6):1491–1496. [DOI] [PubMed] [Google Scholar]
- 17.Buchach CM, Kim DH, Pickhardt PJ. Performing an additional decubitus series at CT colonography. Abdom Imaging 2011;36(5):538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT colonography. AJR Am J Roentgenol 2007;189(6):1451–1456. [DOI] [PubMed] [Google Scholar]
- 19.Pooler BD, Kim DH, Weiss JM, Matkowskyj KA, Pickhardt PJ. Colorectal polyps missed with optical colonoscopy despite previous detection and localization with CT colonography. Radiology 2016;278(2):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pooler BD, Kim DH, Lam VP, Burnside ES, Pickhardt PJ. CT Colonography Reporting and Data System (C-RADS): benchmark values from a clinical screening program. AJR Am J Roentgenol 2014;202(6):1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickhardt PJ, Wise SM, Kim DH. Positive predictive value for polyps detected at screening CT colonography. Eur Radiol 2010;20(7):1651–1656. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, Matkowskyj KA, Lubner MG, et al. Serrated polyps at CT colonography: prevalence and characteristics of the serrated polyp spectrum. Radiology 2016 Feb 15. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut 2014;63(6):949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler J, Robertson DJ. Interval colorectal cancer after colonoscopy: exploring explanations and solutions. Am J Gastroenterol 2015;110(12):1657–1664; quiz 1665. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology 2007;133(4):1077–1085. [DOI] [PubMed] [Google Scholar]
- 26.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection—systematic review and meta-analysis. Radiology 2011;259(2):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DH, Hinshaw JL, Lubner MG, Munoz del Rio A, Pooler BD, Pickhardt PJ. Contrast coating for the surface of flat polyps at CT colonography: a marker for detection. Eur Radiol 2014;24(4):940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor SD, Summers RM, Choi JR, Pickhardt PJ. Oral contrast adherence to polyps on CT colonography. J Comput Assist Tomogr 2006;30(1):51–57. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor SD, Summers RM. Revisiting oral barium sulfate contrast agents. Acad Radiol 2007;14(1):72–80. [DOI] [PubMed] [Google Scholar]
- 30.Pickhardt PJ, Choi JR, Nugent PA, Schindler WR. The effect of diagnostic confidence on the probability of optical colonoscopic confirmation of potential polyps detected on CT colonography: prospective assessment in 1,339 asymptomatic adults. AJR Am J Roentgenol 2004;183(6):1661–1665. [DOI] [PubMed] [Google Scholar]
- 31.Pickhardt PJ, Kim DH. Performance of CT colonography for detecting small, diminutive, and flat polyps. Gastrointest Endosc Clin N Am 2010;20(2):209–226. [DOI] [PubMed] [Google Scholar]
- 32.Pickhardt PJ, Kim DH, Robbins JB. Flat (nonpolypoid) colorectal lesions identified at CT colonography in a U.S. screening population. Acad Radiol 2010;17(6):784–790. [DOI] [PubMed] [Google Scholar]
- 33.Pickhardt PJ, Lam VP, Weiss JM, Kennedy GD, Kim DH. Carpet lesions detected at CT colonography: clinical, imaging, and pathologic features. Radiology 2014;270(2):435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR Am J Roentgenol 2004;183(5):1343–1347. [DOI] [PubMed] [Google Scholar]
- 35.Pickhardt PJ, Hain KS, Kim DH, Hassan C. Low rates of cancer or high-grade dysplasia in colorectal polyps collected from computed tomography colonography screening. Clin Gastroenterol Hepatol 2010;8(7):610–615. [DOI] [PubMed] [Google Scholar]
- 36.Pickhardt PJ, Kim DH. Colorectal cancer screening with CT colonography: key concepts regarding polyp prevalence, size, histology, morphology, and natural history. AJR Am J Roentgenol 2009;193(1):40–46. [DOI] [PubMed] [Google Scholar]
- 37.Pickhardt PJ, Kim DH, Pooler BD, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol 2013;14(8):711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin GL, Fennimore B, Ahnen DJ. Can colonoscopy remain cost-effective for colorectal cancer screening? the impact of practice patterns and the Will Rogers phenomenon on costs. Am J Gastroenterol 2013;108(3):296–301. [DOI] [PubMed] [Google Scholar]