Intraesophageal hyperthermia can enhance direct intratumoral chemotherapy on rat orthotopic esophageal cancers, which may open new avenues for effective treatment of esophageal malignancies with simultaneous integration of radiofrequency technology, interventional oncology, and direct intratumoral chemotherapy.
Abstract
Purpose
To determine the feasibility of using intraesophageal radiofrequency (RF) hyperthermia to enhance local chemotherapy in a rat model with orthotopic esophageal squamous cancers.
Materials and Methods
The animal protocol was approved by the institutional animal care and use committee and the institutional review board. Human esophageal squamous cancer cells were transduced with luciferase lentiviral particles. Cancer cells, mice with subcutaneous cancer esophageal xenografts, and nude rats with orthotopic esophageal cancers in four study groups of six animals per group were treated with (a) combination therapy of magnetic resonance imaging heating guidewire–mediated RF hyperthermia (42°C) plus local chemotherapy (cisplatin and 5-fluorouracil), (b) chemotherapy alone, (c) RF hyperthermia alone, and (d) phosphate-buffered saline. Bioluminescent optical imaging and transcutaneous ultrasonographic imaging were used to observe bioluminescence signal and changes in tumor size among the groups over 2 weeks, which were correlated with subsequent histologic results. The nonparametric Mann-Whitney U test was used for comparisons of variables.
Results
Compared with chemotherapy alone, RF hyperthermia alone, and phosphate-buffered saline, combination therapy with RF hyperthermia and chemotherapy induced the lowest cell proliferation (relative absorbance of formazan: 23.4% ± 7, 44.6% ± 7.5, 95.8% ± 2, 100%, respectively; P < .0001), rendered the smallest relative tumor volume (0.65 mm3 ± 0.15, P < .0001) and relative bioluminescence optical imaging photon signal (0.57 × 107 photons per second per square millimeter ± 0.15, P < .001) of mice with esophageal cancer xenografts, as well as the smallest relative tumor volume (0.68 mm3 ± 0.13, P < .05) and relative photon signal (0.56 × 107 photons per second per square millimeter ± 0.11. P < .001) of rat orthotopic esophageal cancers.
Conclusion
Intraesophageal RF hyperthermia can enhance the effect of chemotherapy on esophageal squamous cell cancers.
© RSNA, 2016
Introduction
Esophageal cancer remains one of the leading causes of cancer-related mortality, with an estimated 455 800 new cases and 400 200 deaths per year worldwide (1,2). Surgery offers limited benefits for patients with advanced esophageal cancers, with extremely poor overall 5-year survival ranging from 15% to 25% (1,3,4). Radiation therapy as a palliative treatment is even less effective than surgical resection and is associated with a high likelihood of major complications such as tracheoesophageal fistula (1). Recent efforts have been focused on combination treatment with surgery, radiation therapy, and systemic chemotherapy to achieve better local control of disease and improve overall survival of patients with this deadly disease (1). However, randomized clinical trials have shown mixed results with respect to progression-free or overall survival benefit with such combination therapy (1,4).
It is well known that systemic chemotherapy is often unable to deliver sufficient chemotherapeutic drugs into target tumors and is often plagued by low chemotherapeutic sensitivity, resistance to chemotherapy, and high risk of systemic toxicity to other vital organs (5–7). On the other hand, imaging-guided minimally invasive interventional oncologic techniques enable delivery of high doses of chemotherapeutic agents into target tissue with improved tumoricidal activity but fewer systemic adverse effects (8,9).
Recent studies have confirmed that nonablative hyperthermia at approximately 42°C can significantly enhance the sensitivity of cancer cells to chemotherapeutic drugs and reverse chemotherapy resistance (10–12). In current clinical trials, nonablative hyperthermia for treatment of malignancies is generated by means of either systemic whole-body or external hyperthermia (13,14). However, because of the deep anatomic location of the esophagus, which is surrounded by vital organs including the heart, lungs, trachea, and spine, it is difficult to generate highly focused heat only at the site of esophageal tumors by using systemic or external hyperthermia. Generation of external hyperthermia in the esophagus poses the risk of injury to adjacent vital organs, such as the spinal cord (15,16). In the past decade, we have developed a U.S. Food and Drug Administration–approved magnetic resonance (MR) imaging heating guidewire that can be used not only for high-spatial-resolution luminal wall imaging and guiding interventions but also may be applied as an intraluminal heating source for enhancing local gene and chemotherapy treatment (8,17,18). The purpose of this study was to determine the feasibility of using intraesophageal radiofrequency (RF) hyperthermia to enhance the effect of local chemotherapy in rats with orthotopic esophageal squamous cancers.
Materials and Methods
Study Design
We divided the study into three stages: (a) in vitro evaluation with the use of human esophageal squamous cancer cells to establish “proof-of-principle” of the new concept of RF hyperthermia–enhanced chemotherapy, (b) in vivo confirmation of this new concept in a mouse model with subcutaneous esophageal squamous cancer xenografts, and (c) preclinical validation of the feasibility of the new technique in a rat model of molecular imaging–detectable orthotopic esophageal cancer.
In Vitro Evaluation
Cell culture and RF hyperthermia–enhanced killing effect of chemotherapeutic drugs.—Human esophageal squamous cancer cells (JCRB Cell Bank, Osaka, Japan) were transduced with luciferase–red fluorescence protein–lentivirus gene to create luciferase- and red fluorescence protein–positive esophageal squamous cancer cells according to the manufacturer’s protocol (GeneCopoeia, Rockville, Md). Luciferase- and red fluorescence protein–positive cells were sorted by using a fluorescence-activated cell sorting technique (Aria II; Becton Dickinson, Franklin Lakes, NJ). Cells were then seeded in four-chamber cell culture slides (NalgeNunc International, Rochester, NY) and maintained in Delbecco’s modified Eagle’s medium and F12 (1:1), supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY). RF hyperthermia was performed as described in the literature (18). RF hyperthermia was performed by placing a 0.022-inch MR imaging heating guidewire under the bottom of chamber four of the chamber slides. A 400-μm fiber optical temperature probe (PhotonControl, Burnaby, British Columbia, Canada) was placed in the chamber for temperature measurement. By adjusting RF output power at approximately 10 W, the temperature was kept at 41 °C ± 1. Cells in different groups were treated with (a) cisplatin (4.5μg/mL) and fluorouracil (15μg/mL) plus 30 minutes of RF hyperthermia at 41 °C ± 1; (b) cisplatin (4.5μg/mL) plus fluorouracil (15μg/mL) alone; (c) 30 minutes of RF hyperthermia alone; and (d) phosphate-buffered saline (PBS) to serve as a control group. We used the 30% inhibitory concentration doses of cisplatin and fluorouracil for cell treatment, which were determined by means of MTS assay (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, Wis). Cisplatin and fluorouracil are the first-line chemotherapeutic drugs for treating patients with esophageal cancers (19).
Cell proliferation assay.—Cell proliferation was evaluated by means of MTS assay 48 hours after the treatments. Relative cell proliferation of different cell groups was calculated by using the equation Atreated − Ablank/Acontrol − Ablank, where A is absorbance of formazan. Formazan is the bioreduced product of MTS by viable cells. Cells on cell culture slides were subsequently washed twice with PBS, fixed in 4% paraformaldehyde, counterstained with 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, Calif), and then imaged with a fluorescence microscope. All experiments for each of the cell groups were repeated six times.
Apoptosis assay.—The percentages of viable and apoptotic cells were determined by means of flow cytometry with Annexin V-fluorescein isothiocyanate and propidium iodide (BD Biosciences, San Diego, Calif). Cells were stained with the staining kit in a binding buffered solution along with the appropriate control solution. The total number of fluorescein and propidium–positive cells were counted by using a flow cytometer (FACScan; BD Biosciences). The data were analyzed by using software (FlowJo version 10; FloJo Data Analysis Software, Ashland, Ore).
In Vivo Confirmation
Creation of animal models.—The animal protocol was approved by our institutional animal care and use committee. The animals were anesthetized with 1%–3% isoflurane (Piramal Health care, Andhra Pradesh, India) in 100% oxygen. For creation of mouse models with subcutaneous esophageal squamous cancer xenografts, we used 24 nude mice aged 4–6 weeks (NU/NU mice; Charles River Laboratories, Wilmington, Mass). Each mouse was inoculated subcutaneously in the unilateral back with luciferase- and red fluorescence protein–positive embryonic stem cells (5 × 106 to 1 × 107) in 100 µL of an extracellular matrix (Matrigel; Corning Life Sciences, Corning, NY). Once the size of the tumor reached 5–10 mm in diameter, we began the experimental procedures.
For creation of rat models with orthotopic esophageal squamous cancers, we used 24 nude rats (weight, 180–220 g), and all procedures were performed by using a real-time ultrasonograpic (US) imaging–guided minimally invasive approach. The nude rats were positioned supine on the surgical table. A 0.035-inch guidewire was transorally introduced into the esophagus, and then a custom microcatheter was advanced into the cervical esophagus over the guidewire. After the guidewire was withdrawn, a custom microcoaxial needle that had a curved tip was positioned into the target esophagus through the microcatheter. Under real-time US imaging guidance, the curved needle tip was advanced into the cervical esophageal wall with a controlled penetration depth of 3 mm, at which point, 5 × 106 to 1 × 107 luciferase- and red fluorescence protein–positive esophageal squamous cancer cells in 100 µL of the gelatinous protein mixture were injected into the target esophageal site.
In vivo confirmation of the concept: RF hyperthermia–enhanced chemotherapy in a mouse model with subcutaneous esophageal squamous cancer xenografts.—The 24 mice with esophageal squamous cancer xenografts were randomly allocated into four groups. Six mice in each of the four groups were treated with (a) direct intratumoral injections of 2 mg/kg of cisplatin and 25 mg/kg of fluorouracil in 100 µL of PBS, immediately followed by local RF heating at 41 °C ± 1 for 30 minutes; (b) intratumoral injection of 2 mg/kg of cisplatin and 25 mg/kg of fluorouracil in 100 µL of PBS alone; (c) 30 minutes of RF hyperthermia alone; or (d) intratumoral injection of 100 µL of PBS to serve as the controls. A Hamilton microsyringe was used for injection of the chemotherapeutic drugs at a rate of 100 µL per 3 minutes. RF hyperthermia was performed as described in the literature (8). RF heating was performed by inserting a 0.022-inch MR imaging heating guidewire into the tumor, with its hyperthermia source at the center of the tumor. A 400-μm fiber optical temperature probe was placed subcutaneously parallel to the MR imaging heating guidewire at the margin of the tumor to measure the temperature.
In vivo validation of the new technique: intraesophageal RF hyperthermia–enhanced direct intratumoral chemotherapy in a rat model with orthotropic esophageal squamous cancers.—When the volume of tumors grew to approximately 50 mm3, chemotherapeutic drugs were directly injected into the esophageal tumor through the intraesophageal delivery needle under US imaging guidance at a rate of 100 uL per 3 minutes. We used the same concentration of cisplatin and fluorouracil as was used in mice, and chemotherapeutic drug solutions at 100 μL per 100 mm3 tumor were injected into tumors. A Hamilton microsyringe was used for injection of the chemotherapeutic drugs at a rate of 100 μL per 3 minutes. Immediately after drug delivery, intraesophageal RF hyperthermia was generated by inserting a 0.022-inch MR imaging heating guidewire into the esophagus with its heating element centered in the tumor. The MR imaging heating guidewire was made of a coaxial copper cable with a 3-cm extension of its inner conductor. When external RF thermal energy is delivered, the wire can create a very localized and controlled heat source, with the heating core at the junction of the inner conductor and outer conductor of the wire (19). The RF power distribution is homogeneous within a distance of 1 cm around the heating spot of the wire (20,21). A fiber optical temperature probe was placed in the esophagus next to the MR imaging heating guidewire to allow simultaneous monitoring of temperature. The intraesophageal RF heat was set at 41 °C ± 1 for 30 minutes.
Posttreatment follow-up with optical and US imaging.—We used optical imaging to follow up on tumor response at days 0, 7, and 14 after treatment. Optical imaging was conducted with an MR imaging system (Bruker In vivo Xtreme; Bruker, Billerica, Mass). Each animal was imaged at day 0 before treatment and days 7 and 14 after treatment. For mice, optical imaging of red fluorescence protein–positive tumors was acquired with an excitation wavelength of 570 nm and emission wavelength of 600 nm. For rats, optical images were achieved 20 minutes after an intraperitoneal injection of d-luciferin at 150 mg/kg (Pierce D-Luciferin; ThermoFisher Scientific, Rockford, Ill). Signal intensity was quantified by using the Bruker software. Relative signal intensity (RSI) was calculated by using the following equation: RSI = SIDn/SID0, where SI is signal intensity, Dn represents days after treatment, and D0 is the day before treatment.
US imaging was then performed to assess tumor growth (Sonosite; Bothel, Wash) at days 0, 7, and 14 after treatment. The axial (x) and longitudinal (y) diameters of tumors and tumor depths (z) were measured on the US images with maximal tumor sizes. The volume of each tumor was then calculated according to the following equation for volume: v = x · y · z · π/6. Data were expressed as relative tumor volume (RTV) by using the following equation: RTV = VDn/VD0, where V is tumor volume, Dn represents days after treatments, and D0 is the day before treatments.
Histologic correlation and confirmation.—Since this study was focused primarily on the new technical development, we did not continue following up until complete tumor disappearance. Tumors were harvested at day 14 after treatment. Tumor tissue was embedded in optimal cutting temperature compound, frozen in liquid nitrogen, kept frozen at −80°C, and then cryosectioned into 10-µm slices, which were placed on slides for apoptosis staining. The level of apoptosis was determined by means of terminal deoxynucleotidyltransferased deoxyuridine triphosphate nick-end labeling assay by using a kit (TACS XL Blue Label kit; Trivegen, Gaithersburg, Md) according to the manufacturer’s instructions. Cells with dark blue dots in the cytoplasm were recognized as apoptotic cells. On each slide, six high-powered fields were pictured randomly by using a digital camera (DP72; Olympus, Tokyo, Japan). Results were expressed as the apoptotic index (AI), which was calculated by using the following equation: AI = AC/TC · 100%, where AC is the number of apoptotic cells and TC is the total number of cells.
Statistical Analysis
Statistical software (SPSS 19.0; SPSS, Chicago, Ill) was used for all data analyses (8). A nonparametric Mann-Whitney U test was used to compare (a) relative proliferation rates among different cell groups; (b) relative optical signal intensity values; and (c) relative tumor volumes at different time points among the animal groups with various treatments. A P value of less than .05 was considered to indicate a significant difference.
Results
In Vitro Evaluation: RF Hyperthermia–enhanced Chemotherapeutic Effect on Esophageal Squamous Cancer Cells
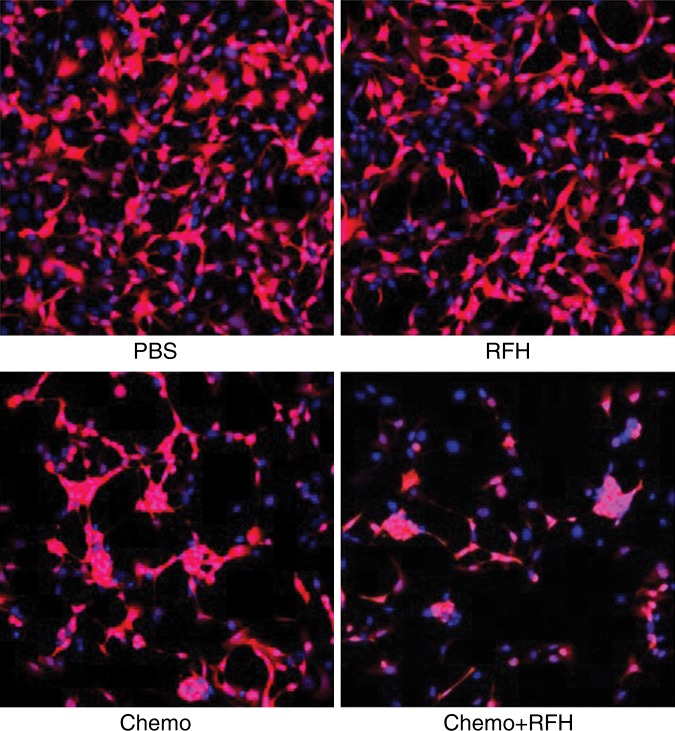
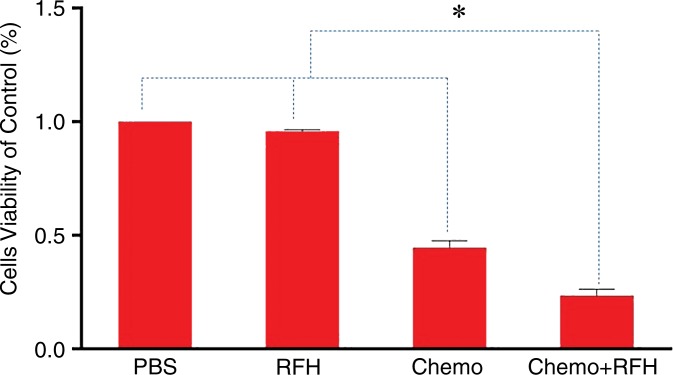
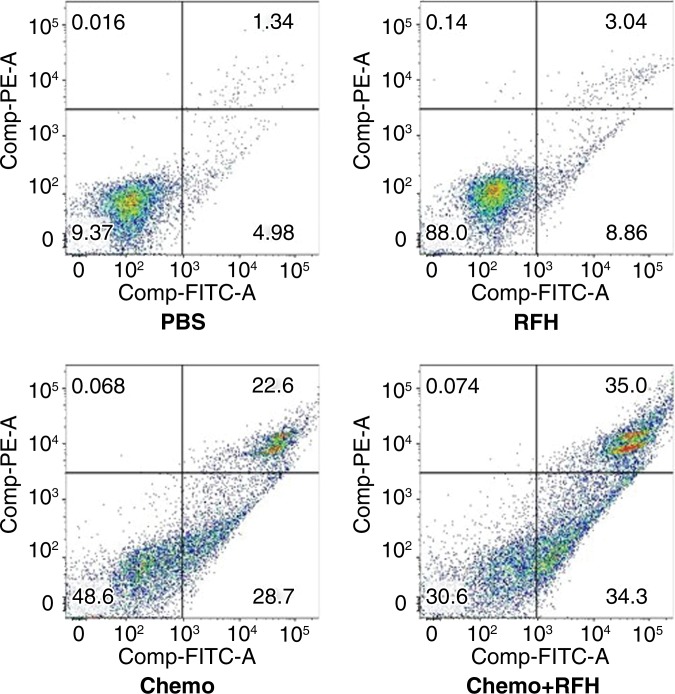
Confocal microscopy performed 48 hours after treatment demonstrated diminished cell survival with combination therapy compared with that in the other three treatment groups (Fig 1a). Quantitative MTS assay allowed further confirmation that cell proliferation with combination therapy (relative absorbance of formazan: 23.4% ± 7) was significantly lower than that seen in the groups treated with chemotherapy alone, RF hyperthermia alone, and PBS (44.6% ± 7.5, 95.8% ± 2, and 100%, respectively; P < .0001) (Fig 1b). After staining the cells with Annexin V-FITC/PI, we used flow cytometry to quantify apoptosis in the four study groups. Flow cytometry results confirmed the results of confocal microscopy and MTS assay, showing more apoptotic cells in the combination therapy group than in the other study groups (Fig 1c).
Figure 1a:
(a) Confocal photomicrograph shows the lowest cell survival in the group receiving combination therapy (chemotherapy and RF hyperthermia), compared with those in the other three treatment groups. (b) MTS assay further shows the lowest cell viability (* = P < .0001). (c) Apoptosis analysis confirms more apoptotic cells in the combination therapy group than in the other three groups. Comp-PE-A = compensation-phycoerythrin-adjustment, Comp-FITC-A = compensation-fluorescein-isothiocyanate-adjustment, RFH = radiofrequency heat.
Figure 1b:
(a) Confocal photomicrograph shows the lowest cell survival in the group receiving combination therapy (chemotherapy and RF hyperthermia), compared with those in the other three treatment groups. (b) MTS assay further shows the lowest cell viability (* = P < .0001). (c) Apoptosis analysis confirms more apoptotic cells in the combination therapy group than in the other three groups. Comp-PE-A = compensation-phycoerythrin-adjustment, Comp-FITC-A = compensation-fluorescein-isothiocyanate-adjustment, RFH = radiofrequency heat.
Figure 1c:
(a) Confocal photomicrograph shows the lowest cell survival in the group receiving combination therapy (chemotherapy and RF hyperthermia), compared with those in the other three treatment groups. (b) MTS assay further shows the lowest cell viability (* = P < .0001). (c) Apoptosis analysis confirms more apoptotic cells in the combination therapy group than in the other three groups. Comp-PE-A = compensation-phycoerythrin-adjustment, Comp-FITC-A = compensation-fluorescein-isothiocyanate-adjustment, RFH = radiofrequency heat.
In Vivo Confirmation: RF Hyperthermia–enhanced Chemotherapy in a Mouse Model with Esophageal Squamous Cancer Xenografts
All animals survived the experimental procedures without complications. Follow-up US imaging demonstrated the smallest relative tumor volume in the combination therapy group (0.65 mm3 ± 0.15) compared with those in the other three groups (1.28 mm3 ± 0.12, 2.67 mm3 ± 0.48, and 2.71 mm3 ± 0.3, respectively; P < .0001) (Fig 2a, 2c). Optical imaging was used to follow tumor response to the treatments, and it demonstrated significantly lower relative photon signal in the combination therapy group (0.57 × 107 photons per second per square millimeter ± 0.15) compared with that in the chemotherapy-only group (1.38 × 107 photons per second per square millimeter ± 0.3; P = .0003), RF hyperthermia–only group (2.4 × 107 photons per second per square millimeter ± 0.47, P < .0001), and PBS group (2.56 × 107 photons per second per square millimeter ± 0.63, P < .0001) (Fig 2b, 2d). Histologic analysis of tumors showed the smallest tumor (Fig 3, upper panel) and a higher apoptotic index in the group treated with combination therapy (46.5% ± 13.6) than in the groups treated with RF hyperthermia only (7.8% ± 2.2, P < .001) or chemotherapy only (24.2% ± 8.1, P = .0063) (Fig 3, bottom panel), which was correlated with subsequent imaging findings.
Figure 2a:
(a) US images and (b) optical and x-ray images show tumor growth and responses to treatments at days 0, 7, and 14. There was a statistically significant decrease in both relative tumor volume (arrows on a; graph c) and fluorescent signal intensity (yellow-red color on b, graph d) with combination therapy (chemotherapy and RF hyperthermia) compared with the other three treatment groups. RFH = radiofrequency heat.
Figure 2c:
(a) US images and (b) optical and x-ray images show tumor growth and responses to treatments at days 0, 7, and 14. There was a statistically significant decrease in both relative tumor volume (arrows on a; graph c) and fluorescent signal intensity (yellow-red color on b, graph d) with combination therapy (chemotherapy and RF hyperthermia) compared with the other three treatment groups. RFH = radiofrequency heat.
Figure 2b:
(a) US images and (b) optical and x-ray images show tumor growth and responses to treatments at days 0, 7, and 14. There was a statistically significant decrease in both relative tumor volume (arrows on a; graph c) and fluorescent signal intensity (yellow-red color on b, graph d) with combination therapy (chemotherapy and RF hyperthermia) compared with the other three treatment groups. RFH = radiofrequency heat.
Figure 2d:
(a) US images and (b) optical and x-ray images show tumor growth and responses to treatments at days 0, 7, and 14. There was a statistically significant decrease in both relative tumor volume (arrows on a; graph c) and fluorescent signal intensity (yellow-red color on b, graph d) with combination therapy (chemotherapy and RF hyperthermia) compared with the other three treatment groups. RFH = radiofrequency heat.
Figure 3:
Upper panel photographs of representative tumors harvested from four different groups of mice show smallest tumor size in combination therapy group (Chemo+RFH) compared with the others. Bottom panel photomicrographs (magnification, ×20) show that apoptosis analysis with deoxyuridine triphosphate nick-end staining further confirmed more apoptotic cells (blue dots) in combination therapy group than in other three groups. RFH = RF heat.
Intraesophageal RF Hyperthermia-enhanced Direct Intratumoral Chemotherapy of Orthotopic Esophageal Squamous Cancers
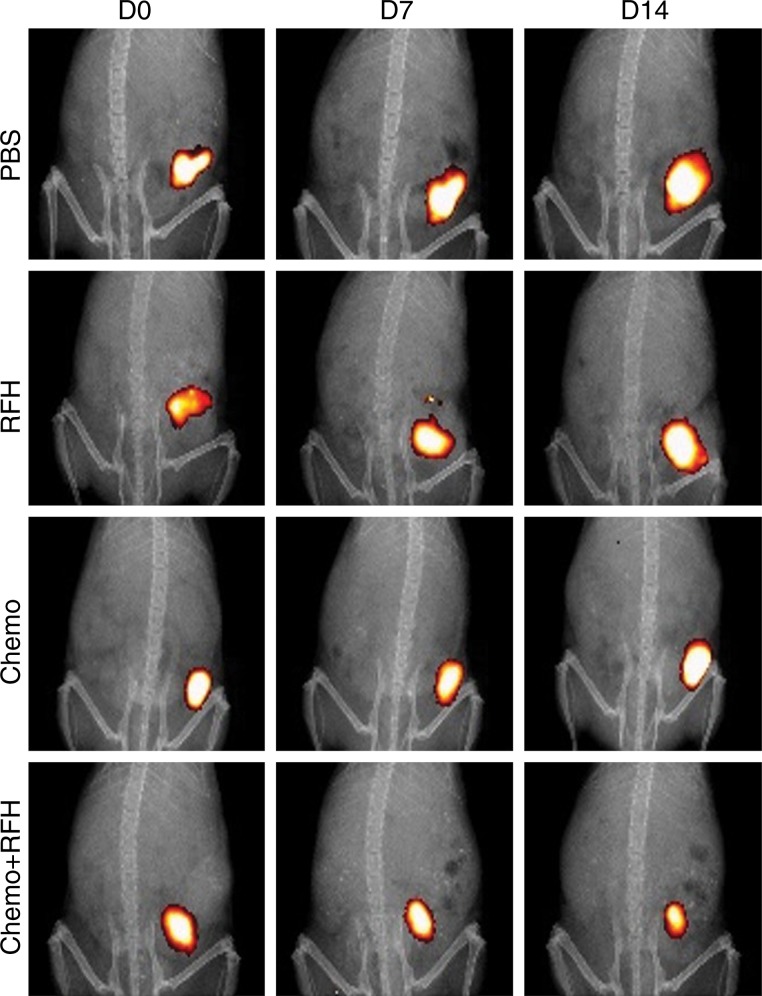
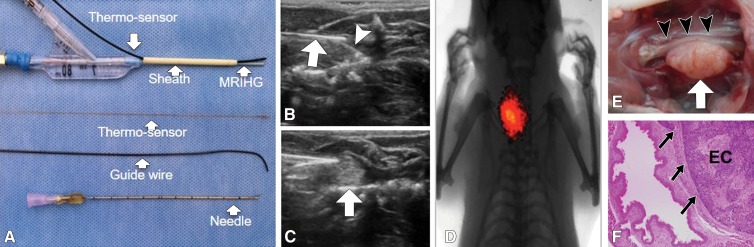
We designed and manufactured a microintraesophageal agent delivery and RF hyperthermia heating system (Fig 4, A), which enabled us to (a) locally implant esophageal squamous cancer cells into the target esophageal segment, (b) deliver chemotherapeutic agents directly into the developing esophageal tumors, and (c) transfer RF heat within the esophageal lumen to locally enhance chemotherapy of the target tumors. Under real-time US imaging guidance, we inoculated the wall of the cervical esophagus with a luciferase- and red fluorescence protein–positive esophageal squamous cancer cell suspension by means of an intraesophageal approach. US imaging allowed detection of the cell pellet in the tissue adjacent to the wall of the esophagus (Fig 4, B). Approximately 3 weeks later, both US and optical imaging demonstrated the presence of a tumor mass in the tissue (Fig 4, C, D).
Figure 4:
A, Photograph shows microintraesophageal agent–delivery and RF-heating system. B, US image shows guided positioning of microagent delivery system (arrow) and then intraesophageal inoculation of the target esophageal region with luciferase-labeled human esophageal carcinoma cells through the tip-curved needle (arrowhead). C, US image shows subsequent injection of cell pellet, which appears as an inhomogeneous hyperechoic signal intensity (arrow). D, Optical image shows bioluminescence signal from the generated esophageal tumor. E, Gross specimen displays tumor (arrow) adhering to esophageal wall (arrowheads). F, Photomicrograph (hematoxylin and eosin–stain; magnification, ×4) further confirms esophageal carcinoma (EC) with tumor invasion (arrows). RFH = RF heat.
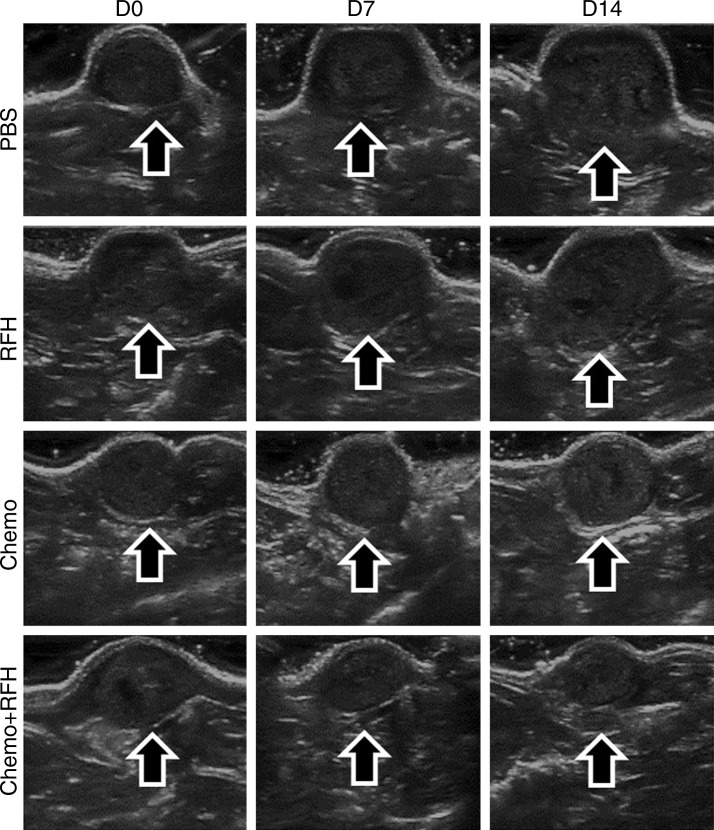
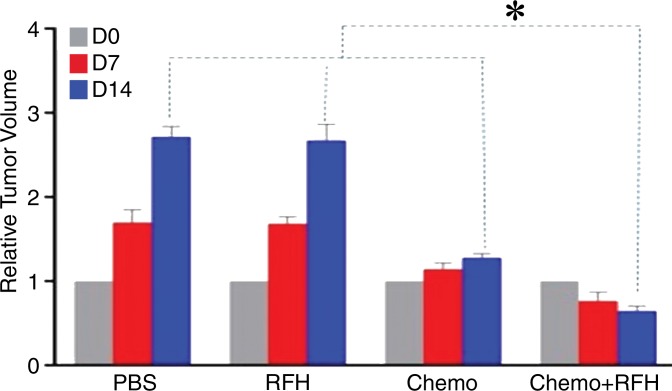
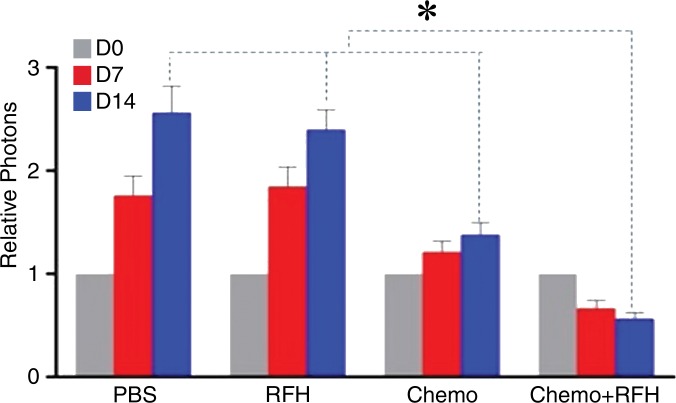
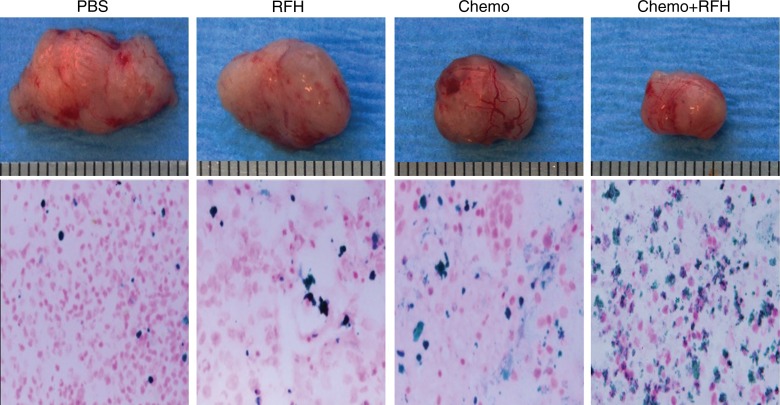
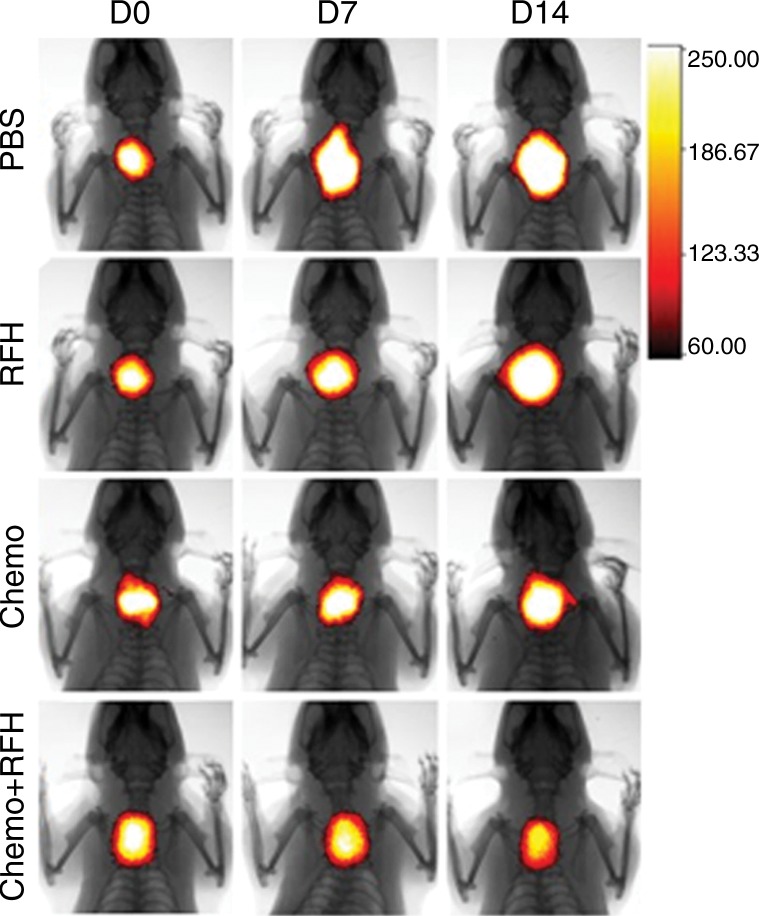
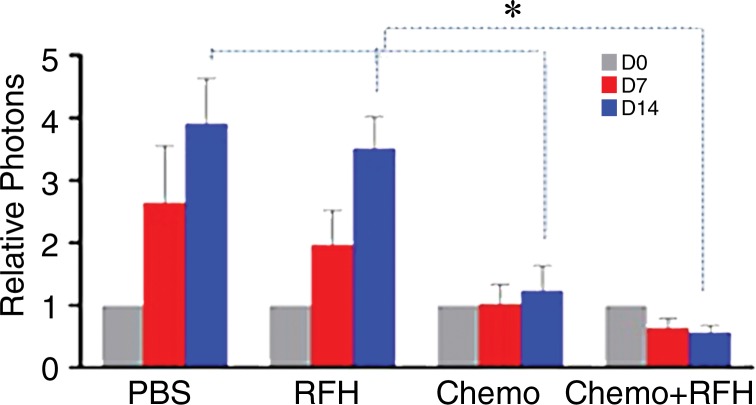
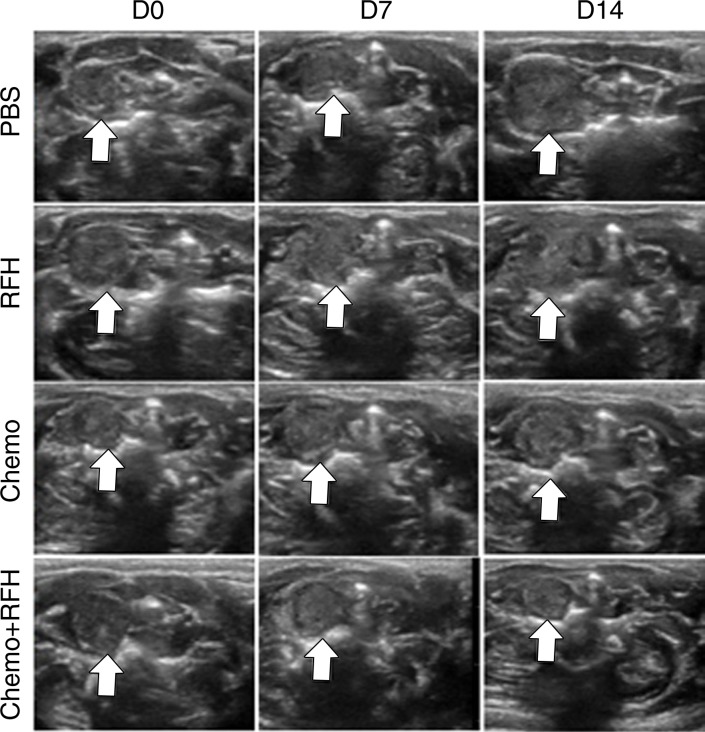
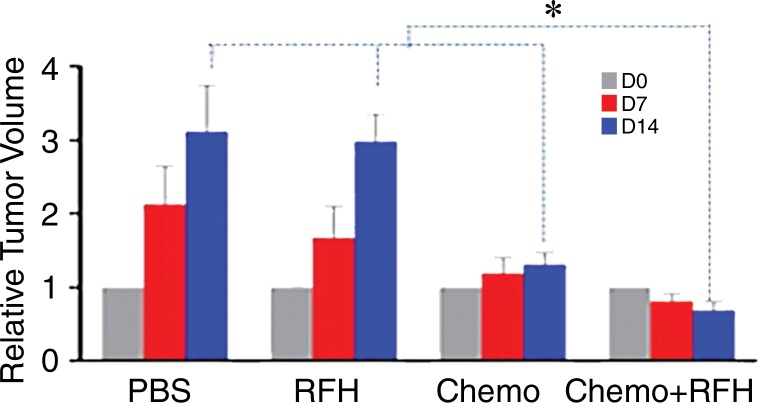
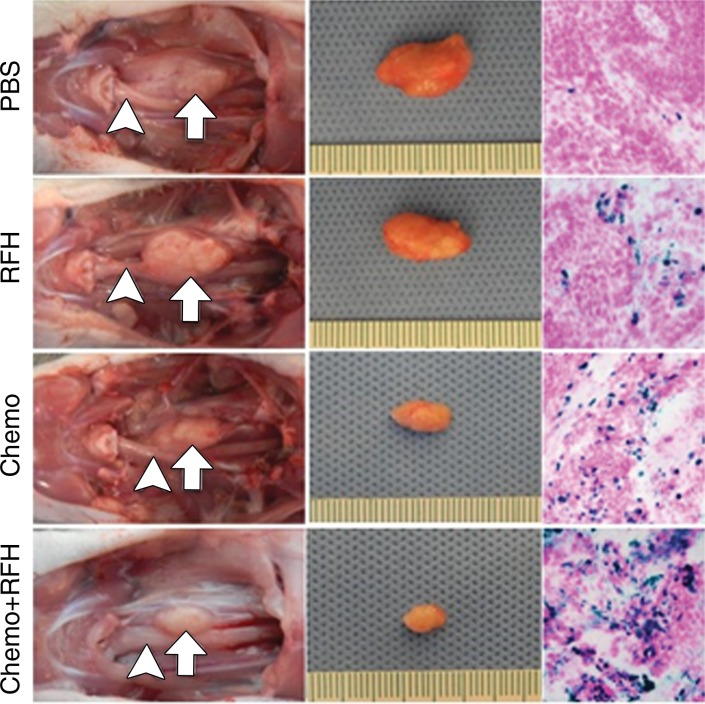
All animals survived all experiments without obvious complications. Examinations of gross specimens obtained at the end of the experiments revealed tumor adherent to the wall of esophagus, which was confirmed by means of subsequent pathologic correlation (Fig 4, E, F). Optical imaging demonstrated significantly lower relative photon signal in the combination therapy group (0.56 × 107 photons per second per square millimeter ± 0.11) compared with that in the chemotherapy-only group (1.23 × 107 photons per second per square millimeter ± 0.41, P < .05), RF hyperthermia–only group (3.51 × 107 photons per second per square millimeter ± 0.51; P < .0001), and PBS group (3.91 × 107 photons per second per square millimeter ± 0.73, P < .0001) (Fig 5a, 5c).We also performed US imaging to evaluate changes in tumor size before and after treatments (Fig 5b). We detected the smaller relative tumor volume in the combination therapy group (0.68 ± 0.13) compared with the chemotherapy-only group (1.31 ± 0.16, P = .0039), RF hyperthermia-only group (2.98 ± 0.37, P < .0001), and PBS group (3.11 ± 0.64, P < .0001) (Fig 5d). Gross specimens obtained at the end of the experiment revealed the smallest tumor size in the combination therapy group compared with those in the other three groups. Histologic analysis of apoptosis by means of deoxyuridine triphosphate nick-end labeling assay staining further confirmed more apoptotic cells in the combination therapy group (41.8% ± 13.8) than in the groups treated with RF hyperthermia only (5.7% ± 2.3, P < .001) or chemotherapy only (21.0% ± 7.7, P = .0092) (Fig 5e).
Figure 5a:
(a) Optical and x-ray images and (b) US images show tumor response as assessed according to signal intensity (yellow-red colors on a) and tumor size (arrows on b) in the four groups. There was a larger decrease in both signal intensity and tumor size in the combination therapy group compared with the other three groups. Graphs show quantitative analysis, which further confirms the significant decrease in both photon intensity according to (c) optical imaging and (d) relative tumor volume according to US imaging after combination therapy (chemotherapy and RF hyperthermia) compared with the other groups. (e) Photographs of representative tumors harvested from four different animal groups show smallest tumor (arrows) size in the combination therapy group compared with the others (left and middle columns, arrowheads indicate esophageal wall). Photomicrographs of apoptosis analysis further confirmed more apoptotic cells (blue dots) in the combination therapy group than in the other three groups (right column, magnification, ×20). RFH = RF heat.
Figure 5c:
(a) Optical and x-ray images and (b) US images show tumor response as assessed according to signal intensity (yellow-red colors on a) and tumor size (arrows on b) in the four groups. There was a larger decrease in both signal intensity and tumor size in the combination therapy group compared with the other three groups. Graphs show quantitative analysis, which further confirms the significant decrease in both photon intensity according to (c) optical imaging and (d) relative tumor volume according to US imaging after combination therapy (chemotherapy and RF hyperthermia) compared with the other groups. (e) Photographs of representative tumors harvested from four different animal groups show smallest tumor (arrows) size in the combination therapy group compared with the others (left and middle columns, arrowheads indicate esophageal wall). Photomicrographs of apoptosis analysis further confirmed more apoptotic cells (blue dots) in the combination therapy group than in the other three groups (right column, magnification, ×20). RFH = RF heat.
Figure 5b:
(a) Optical and x-ray images and (b) US images show tumor response as assessed according to signal intensity (yellow-red colors on a) and tumor size (arrows on b) in the four groups. There was a larger decrease in both signal intensity and tumor size in the combination therapy group compared with the other three groups. Graphs show quantitative analysis, which further confirms the significant decrease in both photon intensity according to (c) optical imaging and (d) relative tumor volume according to US imaging after combination therapy (chemotherapy and RF hyperthermia) compared with the other groups. (e) Photographs of representative tumors harvested from four different animal groups show smallest tumor (arrows) size in the combination therapy group compared with the others (left and middle columns, arrowheads indicate esophageal wall). Photomicrographs of apoptosis analysis further confirmed more apoptotic cells (blue dots) in the combination therapy group than in the other three groups (right column, magnification, ×20). RFH = RF heat.
Figure 5d:
(a) Optical and x-ray images and (b) US images show tumor response as assessed according to signal intensity (yellow-red colors on a) and tumor size (arrows on b) in the four groups. There was a larger decrease in both signal intensity and tumor size in the combination therapy group compared with the other three groups. Graphs show quantitative analysis, which further confirms the significant decrease in both photon intensity according to (c) optical imaging and (d) relative tumor volume according to US imaging after combination therapy (chemotherapy and RF hyperthermia) compared with the other groups. (e) Photographs of representative tumors harvested from four different animal groups show smallest tumor (arrows) size in the combination therapy group compared with the others (left and middle columns, arrowheads indicate esophageal wall). Photomicrographs of apoptosis analysis further confirmed more apoptotic cells (blue dots) in the combination therapy group than in the other three groups (right column, magnification, ×20). RFH = RF heat.
Figure 5e:
(a) Optical and x-ray images and (b) US images show tumor response as assessed according to signal intensity (yellow-red colors on a) and tumor size (arrows on b) in the four groups. There was a larger decrease in both signal intensity and tumor size in the combination therapy group compared with the other three groups. Graphs show quantitative analysis, which further confirms the significant decrease in both photon intensity according to (c) optical imaging and (d) relative tumor volume according to US imaging after combination therapy (chemotherapy and RF hyperthermia) compared with the other groups. (e) Photographs of representative tumors harvested from four different animal groups show smallest tumor (arrows) size in the combination therapy group compared with the others (left and middle columns, arrowheads indicate esophageal wall). Photomicrographs of apoptosis analysis further confirmed more apoptotic cells (blue dots) in the combination therapy group than in the other three groups (right column, magnification, ×20). RFH = RF heat.
Discussion
The findings of our study showed that intraesophageal RF hyperthermia can significantly enhance the direct intratumoral chemotherapy of human esophageal squamous cancers, as manifested in decreased survival of esophageal squamous cancer cells in in vitro experiments and shrunken tumor volumes and decreased optical signal intensity values in both in vivo confirmation and validation experiments. Our study results also provide evidence that (a) both chemotherapeutic agents and RF hyperthermia can be delivered locally to esophageal tumors by means of an intraesophageal approach, (b) intraesophageal RF hyperthermia can be used to enhance the direct intratumoral chemotherapy of esophageal cancers, and (c) both optical imaging and US imaging offer useful tools to assess the response of esophageal cancers to chemotherapeutic regimens. This new technique should provide useful diagnostic and therapeutic imaging-guided methods for further laboratory study of esophageal malignancies, and further study may allow its translationto clinical practice.
Over the past several decades, numerous devices and techniques have been developed for generation of external hyperthermia (22), including electromagnetic energy–based RF or microwave energy and high-intensity focused ultrasound. However, penetration depth of electromagnetic energy from a single applicator is limited to a few centimeters, particularly with microwave energy. In addition, adipose tissue at the fat-muscle interface can be overheated easily because of large reflections of electromagnetic energy (23). High-intensity focused ultrasound can result in high absorption by bone and can cause overheating, while reflection of high intensity focused ultrasound energy at bone and tissue interfaces can negatively affect energy focus at the target sites (24). Electromagnetic and high-intensity focused ultrasound-based approaches are well suited to treat tumors in large and superficially located organs such as the liver, lung, and kidney. These organs are readily accessible by using standard percutaneous interventional approaches (25,26). However, precise and effective delivery of local hyperthermia with electromagnetic energy and high-intensity focused ultrasound to deep-seated luminal malignancies such as esophageal cancers still remains a technical challenge in current clinical practice.
To overcome these clinical limitations of electromagnetic and high-intensity focused ultrasound-based techniques, in this study we specifically used the MR imaging heating guidewire to deliver thermal energy locally to esophageal cancers through an intraluminal approach. For the specific purpose of treating esophageal malignancies, the generation of intraesophageal RF hyperthermia with the MR imaging heating guidewire offers superior advantages over external hyperthermia with electromagnetic energy or high-intensity focused ultrasound. The thermal energy distribution pattern induced by the MR imaging heating guidewire is cylindrically symmetric and homogeneous, and thermal energy can be delivered within a distance of approximately 1–2 cm around the target site (21). This capability is well suited to deliver localized RF heat into the tumor alone, without causing thermal injury to adjacent vital organs (such as the spinal cord) and heat absorption by fat tissue and bones.
To assess the transferability of this methodology to humans, experiments in mid- to large-sized animal models would be most appropriate. However, to our knowledge, no such models for the study of esophageal cancer have been described. We created a rat model of molecular imaging–detectable orthotopic esophageal cancer that meets the requirement that a specific animal tumor model not only simulates the pathophysiologic properties of human esophageal cancer but also reveals the appropriate interactions between cancer cells and host organs (27,28). This rat model of orthotopic esophageal cancer will be a useful tool for further laboratory investigations into esophageal malignancy. Its advantages include (a) high reproducibility and cost effectiveness, allowing precise, targeted tumor inoculation with US imaging guidance; (b) relatively rapid tumor growth, enabling the evaluation of therapeutic efficacy in a short period of time; (c) suitability for use with intraesophageal interventional approaches; and (d) possibility of using both optical imaging and US imaging to assess the response of tumors to various treatments.
We designed and manufactured a microintraesophageal agent delivery and RF heating system that can be positioned precisely at the target site in the esophagus with real-time US imaging guidance. By using this microinterventional system, we could accomplish precisely targeted injection of esophageal squamous cancer cells into the esophageal wall to create a rat model of esophageal cancer. The system also allowed local delivery of chemotherapeutic agents and RF hyperthermia to enhance the chemotherapeutic effect in esophageal cancers. Because we prelabeled the esophageal squamous cancer cells with luciferase and red fluorescence protein genes, tumor response to RF hyperthermia–enhanced chemotherapy could be assessed by means of optical imaging, which is a sensitive molecular imaging tool for noninvasive evaluation of optical signal changes that reflect response of tumors during treatment.
Our study had limitations. We need additional experiments to optimize the temperature and heating duration to maximize the enhancing effect of RF hyperthermia on chemotherapy. In addition, we placed a fiber optical thermal sensor to monitor the real-time temperature change in the esophagus lumen, which lacks the capability to reflect the temperature in the relative deep tissue, such as the spinal cord. In future studies we will investigate the feasibility of using real-time MR thermometry to monitor the temperature changes in the RF heated areas to warrant the safe use of this technique. Moreover, since our study was focused on establishing the “proof-of-principle” of the technique, we did not include a control group with systemic chemotherapy to highlight the value of local RF hyperthermia-enhanced chemotherapy. In future studies, we will perform a quantitative analysis of chemotherapeutic drug deposit in esophageal cancers under different circumstances of drug delivery approaches, including local intratumoral injection, systemic administration, and RF hyperthermia-enhanced drug delivery. In addition, long-term tumor follow-up, rather than 14-day follow-up is necessary to investigate the effect of RF hyperthermia–enhanced chemotherapy on tumor mass–caused dysphagia.
In conclusion, intraesophageal hyperthermia can enhance direct intratumoral chemotherapy on rat orthotopic esophageal cancers, which may open new avenues for effective management of esophageal malignancies by means of simultaneous integration of RF technology, interventional oncology, and direct intratumoral chemotherapy.
Advance in Knowledge
■ Combination therapy with MR imaging and heating guidewire–mediated radiofrequency (RF) hyperthermia and chemotherapy induced the lowest cell proliferation, rendered the smallest relative tumor volume and relative bioluminescence optical imaging photon signal in mice with esophageal cancer xenografts, and resulted in the smallest relative tumor volume and relative photon signal in rat orthotopic esophageal cancers compared with groups treated with chemotherapy alone, RF hyperthermia alone, and phosphate-buffered saline.
Implication for Patient Care
■ RF hyperthermia can enhance the effect of local chemotherapy with cisplatin and fluorouracil in rat orthotopic esophageal cancers, which indicates that intraesophageal RF hyperthermia–enhanced local chemotherapy shows potential for development as an alternative treatment for esophageal cancers.
Received October 16, 2015; revision requested December 14; revision received March 29, 2016; accepted April 18; final version accepted April 26.
F.Z. and X.Y. supported by Society of Interventional Radiology (Pilot Research Grant). F.Z. and X.Y. supported by National Institutes of Health (RO1EBO12467).
Current address: Department of Tumor Interventional Treatment, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China.
Y.S. and F.Z. contributed equally to this work.
Disclosures of Conflicts of Interest: Y.S. disclosed no relevant relationships. F.Z. disclosed no relevant relationships. Z.B. disclosed no relevant relationships. J.W. disclosed no relevant relationships. L.Q. disclosed no relevant relationships. Y.L. disclosed no relevant relationships. Y.M. disclosed no relevant relationships. K.V. disclosed no relevant relationships. X.Y. disclosed no relevant relationships.
Abbreviations:
- PBS
- phosphate-buffered saline
- RF
- radiofrequency
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349(23):2241–2252. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg 2009;87(4):1048–1054; discussion 1054–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6(9):659–668. [DOI] [PubMed] [Google Scholar]
- 5.Medical Research Council Oesophageal Cancer Working Group . Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359(9319):1727–1733. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Wang X, Song S, et al. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol Oncol 2014;8(1):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilson DH. Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res 2008;2(2):85–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Le T, Wu X, et al. Intrabiliary RF heat-enhanced local chemotherapy of a cholangiocarcinoma cell line: monitoring with dual-modality imaging–preclinical study. Radiology 2014;270(2):400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F, Li J, Meng Y, et al. Development of an intrabiliary MR imaging-monitored local agent delivery technique: a feasibility study in pigs. Radiology 2012;262(3):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partanen A, Yarmolenko PS, Viitala A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia 2012;28(4):320–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyer HR, Delman KA. The role of hyperthermia in optimizing tumor response to regional therapy. Int J Hyperthermia 2008;24(3):251–261. [DOI] [PubMed] [Google Scholar]
- 12.Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001;17(1):1–18. [DOI] [PubMed] [Google Scholar]
- 13.Robins HI, Dennis WH, Neville AJ, et al. A nontoxic system for 41.8 degrees C whole-body hyperthermia: results of a Phase I study using a radiant heat device. Cancer Res 1985;45(8):3937–3944. [PubMed] [Google Scholar]
- 14.Ohguri T, Yahara K, Moon SD, et al. Deep regional hyperthermia for the whole thoracic region using 8 MHz radiofrequency-capacitive heating device: relationship between the radiofrequency-output power and the intra-oesophageal temperature and predictive factors for a good heating in 59 patients. Int J Hyperthermia 2011;27(1):20–26. [DOI] [PubMed] [Google Scholar]
- 15.Kok HP, van Haaren PM, van de Kamer JB, et al. Prospective treatment planning to improve locoregional hyperthermia for oesophageal cancer. Int J Hyperthermia 2006;22(5):375–389. [DOI] [PubMed] [Google Scholar]
- 16.van Haaren PM, Kok HP, Zum Vörde Sive Vörding PJ, et al. Reliability of temperature and SAR measurements at oesophageal tumour locations. Int J Hyperthermia 2006;22(7):545–561. [DOI] [PubMed] [Google Scholar]
- 17.Yang X. Imaging of vascular gene therapy. Radiology 2003;228(1):36–49. [DOI] [PubMed] [Google Scholar]
- 18.Du X, Qiu B, Zhan X, et al. Radiofrequency-enhanced vascular gene transduction and expression for intravascular MR imaging-guided therapy: feasibility study in pigs. Radiology 2005;236(3):939–944. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Zhang F, Meng Y, et al. Diffusion-weighted MRI monitoring of pancreatic cancer response to radiofrequency heat-enhanced intratumor chemotherapy. NMR Biomed 2013;26(12):1762–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu B, El-Sharkawy AM, Paliwal V, et al. Simultaneous radiofrequency (RF) heating and magnetic resonance (MR) thermal mapping using an intravascular MR imaging/f heating system. Magn Reson Med 2005;54(1):226–230. [DOI] [PubMed] [Google Scholar]
- 21.Qiu B, Yeung CJ, Du X, Atalar E, Yang X. Development of an intravascular heating source using an MR imaging guidewire. J Magn Reson Imaging 2002;16(6):716–720. [DOI] [PubMed] [Google Scholar]
- 22.Stauffer PR. Evolving technology for thermal therapy of cancer. Int J Hyperthermia 2005;21(8):731–744. [DOI] [PubMed] [Google Scholar]
- 23.van Haaren PM, Hulshof MC, Kok HP, et al. Relation between body size and temperatures during locoregional hyperthermia of oesophageal cancer patients. Int J Hyperthermia 2008;24(8):663–674. [DOI] [PubMed] [Google Scholar]
- 24.Cheung AY, Neyzari A. Deep local hyperthermia for cancer therapy: external electromagnetic and ultrasound techniques. Cancer Res 1984;44(10 Suppl):4736s–4744s. [PubMed] [Google Scholar]
- 25.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14(3):199–208. [DOI] [PubMed] [Google Scholar]
- 26.Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treat Rev 2012;38(5):346–353. [DOI] [PubMed] [Google Scholar]
- 27.Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Peritoneal metastatic model for human scirrhous gastric carcinoma in nude mice. Clin Exp Metastasis 1996;14(1):43–54. [DOI] [PubMed] [Google Scholar]
- 28.Fidler IJ. Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst 1995;87(21):1588–1592. [DOI] [PubMed] [Google Scholar]