The presence of KRAS mutation in lung adenocarcinoma tumors predicts a shorter time to local recurrence after ablation that is independent of tumor size and ablation margin.
Abstract
Purpose
To establish the relationship between KRAS mutation status and local recurrence after image-guided ablation of lung adenocarcinoma.
Materials and Methods
This study consisted of a HIPAA-compliant institutional review board–approved retrospective review of 56 primary lung adenocarcinomas in 54 patients (24 men, 30 women; median age, 72 years; range, 54–87 years) treated with percutaneous image-guided ablation and with available genetic mutational analysis. KRAS mutation status and additional clinical and technical variables—Eastern Cooperative Oncology Group (ECOG) status, smoking history, stage at diagnosis, status (new primary or not), history of radiation, history of surgery, prior systemic treatment, modality of ablation, size of nodule, ablation margin, and presence of ground-glass appearance—were recorded and evaluated in relation to time to local recurrence, which was calculated from the time of ablation to the first radiographic evidence of recurrence. Predictors of outcome were identified by using a proportional hazards model for both univariate and multivariate analysis, with death as a competing risk.
Results
Technical success was 100%. Of the 56 ablated tumors, 37 (66%) were wild type for KRAS and 19 (34%) were KRAS mutants. The 1-year and 3-year cumulative incidences of recurrence were 20% and 35% for wild-type KRAS compared with 40% and 63% for KRAS mutant tumors. KRAS mutation status was a significant predictor of local recurrence at both univariate (P = .05; subdistribution hazard ratio [sHR], 2.32) and multivariate (P = .006; sHR, 3.75) analysis. At multivariate analysis, size (P = .026; sHR, 2.54) and ECOG status (P = .012; sHR, 2.23) were also independent significant predictors, whereas minimum margin (P = .066) was not.
Conclusion
The results of this study show that there is a relationship between KRAS mutation status and local recurrence after image-guided ablation of lung adenocarcinoma. Specifically, KRAS mutation status of the ablated lesion is a significant predictor of time to local recurrence, independent of size and margin.
© RSNA, 2016
Introduction
Lung cancer is the leading cause of cancer death in male and female patients in the United States (1). Non–small cell lung cancer (NSCLC) accounts for a majority of these cases, and the standard treatment for early-stage NSCLC is surgical resection. However, 25% of these patients are not surgical candidates because of medical comorbidities (2,3). In such patients, local-directed therapies such as stereotactic body radiation therapy (SBRT) and percutaneous image-guided ablation (PIA) may be offered (4). PIA includes radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation (CRA), and others.
Indications for PIA for NSCLC include patients with high-risk or unresectable disease and patients with recurrent or persistent disease despite treatment with surgery, radiation, or chemotherapy. Overall 2-year survival rates of approximately 70% for PIA appear to be similar to those for SBRT (5,6) and surgery after propensity score matching. Moreover, a multicenter trial (7) demonstrated no significant worsening of pulmonary function after PIA. Ongoing prospective clinical trials comparing the efficacy of SBRT, PIA, and surgery in patients with high risk aim to improve patient selection for each modality (8).
Various predictors of PIA success have been demonstrated in the literature. These include tumor-associated features such as tumor size (9,10) and technique-associated features such as ablation margin (9). Kodama et al (11) recently demonstrated excellent local tumor control for patients with ground-glass opacity–dominant lung adenocarcinoma. Ridge et al (12) showed improved local tumor control for metachronous and synchronous tumors compared with first primary lung cancers. To our knowledge, the role of biomarkers in predicting local recurrence in PIA has not been studied.
Lung adenocarcinomas are associated with several clinically important oncogenic driver mutations, although their prognostic role remains controversial. In a large meta-analysis, KRAS mutation status was associated with worse survival in patients with NSCLC (hazard ratio [HR], 1.35; 95% confidence interval [CI]: 1.16, 1.56) (13). However, D’Angelo et al (14) demonstrated similar overall survival rates between patients with KRAS mutations and patients with wild-type KRAS or wild-type EGFR. The impact of KRAS mutations on recurrence and survival has also been studied in relation to surgery (15) and SBRT (16). Given the high local recurrence rates with PIA (6), the role of these driver mutations may help better define cohorts appropriate for PIA or high-risk cohorts that may require more extensive ablation.
The purpose of our study was to establish the relationship between KRAS mutation status and local recurrence after image-guided ablation of lung adenocarcinoma. Our hypothesis was that KRAS mutation status is a predictor of local recurrence, independent of established clinical and technical variables such as size and margin.
Materials and Methods
Study Design
This was a retrospective study that included consecutive patients who underwent PIA of a lung adenocarcinoma and mutational analysis of the ablated tumor. The study was approved by the institutional review board, with a waiver of informed consent, and was compliant with the Health Insurance Portability and Accountability Act.
Patient Selection
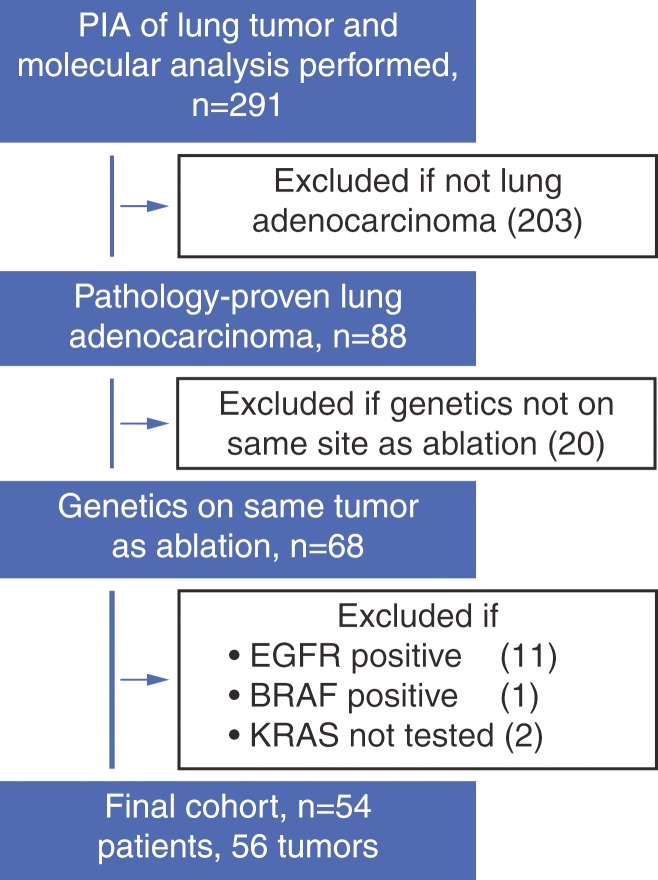
We performed an institutional database search that included consecutive patients from January 1, 2009, through July 1, 2014. Inclusion criteria were any patients who had undergone PIA for a lung tumor and had also undergone genetic testing (n = 291). We excluded patients who did not have pathologically proven lung adenocarcinoma (203 patients excluded; 88 patients remaining). We excluded patients if genetic testing was performed on a tumor other than the one that was ablated (20 patients excluded, 68 remaining). Metachronous tumors were defined on the basis of published criteria (17). From among the remaining patients, we excluded those who harbored a known oncogenic driver mutation other than KRAS (11 patients with EGFR mutation excluded, one patient with BRAF mutation excluded) and those in whom the KRAS mutation was not tested (two patients excluded). The final cohort included 56 tumors in 54 patients (two patients each had two separate tumors that were ablated and analyzed for mutations). A flowchart summarizing initial patient population and each exclusion step is included in Figure 1.
Figure 1:
Flow diagram of patient selection and exclusion criteria.
Lung Ablation
The decision to perform PIA was made by the interventional radiologist in conjunction with the medical oncologist, surgeon, and radiation oncologist, who form the thoracic tumor disease management team at our institution. All lung ablations were performed with general anesthesia by using computed tomographic (CT) guidance to monitor device placement. Ablations were performed by a fellowship-trained interventional radiologist with at least 6 years of experience at the beginning of the study (including S.B.S., C.T.S., J.P.E., and H.Y.). Ablation technique, including modality (RFA, MWA, or CRA), device type, needle, and number and length of treatments, was determined by operator preference, taking into account tumor location, size, and adjacent structures. Complications were categorized by using the Society of Interventional Radiology guidelines (18). Major complications were those that increased the level of care or required that the patient be hospitalized. All other complications were considered minor. Immediate postprocedure CT and chest radiography were performed to assess technical success and postprocedure complications, respectively.
Tissue Acquisition and Mutational Analysis
Patients with lung adenocarcinomas have their tumor(s) tested for multiple oncogenic mutations at our institution as part of the standard of care. The spectrum of genes tested has increased over time but has consistently included KRAS. Tumor specimens were obtained either through biopsy or from a surgical specimen. After microscopic examination results confirmed the diagnosis of adenocarcinoma, tissue was sent to a molecular diagnostic laboratory in the Department of Pathology for extraction of genomic DNA. All samples were determined to have adequate DNA quality prior to testing. KRAS mutations of codons 12 and 13 were detected by means of direct sequencing or mass spectrometry–based genotyping (Sequenom) (19–21).
Assessment of Tumor Recurrence
Postprocedural CT was performed according to standard guidelines (22), with baseline imaging typically performed at 1 month and follow-up imaging performed at regular intervals after that (typically 3, 6, and 12 months, and yearly after that). Positron emission tomography (PET)/CT surveillance was performed for tumors that were fluorine 18 fluorodeoxyglucose avid before the ablation, according to the operator and referring clinician preference, and in the setting of suspected local recurrence on the basis of CT findings. One of the investigators (E.Z.) reviewed the imaging studies in all patients to determine tumor size at time of ablation, margin size after ablation, and local recurrence. Local recurrence was defined by imaging criteria as the appearance of tumor foci within or at the edge of the ablation zone, according to previously published standard reporting guidelines (22). Distant metastases were not included in our definition.
Covariates
Patient clinical characteristics were collected (E.Z., E.N.P., and S.G., in consensus) and included age, smoking status (measured in pack-years), sex, Eastern Cooperative Oncology Group (ECOG) performance status (0 or > 0), clinical stage at the time of original diagnosis, new primary tumor versus recurrence, prior treatment with radiation at ablated site, prior surgery at ablated site, treatment with systemic chemotherapy, treatment modality (RFA, MWA, or CRA), tumor size (using a 2-cm threshold), margin ablation (using a 5-mm threshold), and presence of a ground-glass appearance in the tumor prior to treatment. Clinical stage was defined by the tumor, node, metastasis classification system, according to the 7th edition of the American Joint Committee on Cancer staging manual (23). We used the Martini and Melamed criteria (17) to distinguish between de novo primary tumors and recurrent or metastatic tumors (12). We also enhanced these criteria by using mutation data (presence or absence of driver mutation), when available, to help either confirm or refute similar histologic types. A ground-glass appearance of the tumor at CT imaging prior to ablation was defined as either completely ground glass or as mixed solid and ground glass). None of the ablated lesions were purely ground glass in appearance.
Statistical Analyses
For the 54 patients, clinical characteristics were compared among wild-type and KRAS mutant tumors by using the Fisher exact test for categoric variables and the Mann-Whitney U test for continuous variables. Analysis of data in the two patients who had undergone PIA of two metachronous lesions was based on their first tumor. Overall survival was measured from the time of PIA to patient death or most recent follow-up and was determined on the basis of a review of patient medical records. Overall survival rates were estimated by using the Kaplan-Meier method. For the 56 tumors, time to local recurrence was calculated from the time of PIA to the first radiologic evidence of progression at the ablation site. Our primary interest was to evaluate the association between KRAS and local recurrence. However, to determine which variables to include in the multivariate analysis, we needed to test multiple potential confounders. We used a standard competing-risks proportional hazards model (24) to analyze time to local recurrence with death as a competing risk and to obtain a predicted cumulative incidence function. Clustering was used to account for within-patient correlations. Univariable analysis was performed by using this model, and covariates with P < .25 were included in the multivariable analysis. Backward selection with a cutoff of P = .05 was performed to select significant predictors of outcome in multivariable analysis. Competing risk analysis was performed by using Stata12 software, while all the other analyses were performed by using SAS9.3 software. Tumor size (20 mm) and ablation margin (5 mm) were analyzed as categoric variables by using clinically well-established thresholds. Additional continuous variables, including age and smoking status, were analyzed as continuous variables to avoid imposing arbitrary thresholds.
Results
Technical success was 100%. Patient and tumor characteristics are summarized in Table 1. There were 54 patients, with a median age of 72 years (range, 54–87 years) and a median number of pack-years of smoking of 40 (range, 0–120 pack-years). There were 24 men and 30 women; 40 patients had an ECOG status of 0, and 13 had an ECOG status of 1 or 2. There were 56 ablated tumors: Forty-four (79%) were stage I, and 12 (21%) were stage II–IV; 32 (57%) were new primary cancers and 24 (43%) were recurrences. Nine (16%) tumors had previously been treated with radiation, 18 (32%) had previously been treated with surgical resection, and 27 (48%) had been treated with systemic chemotherapy; 43 (77%) tumors were treated with RFA, 10 (18%) were treated with MWA, and three (5%) were treated with CRA. The average tumor size was 17 mm (range, 8–39 mm), and 42 tumors were smaller than or equal to 20 mm, while 14 were larger than 20 mm. The average minimum margin was 4 mm (range, 0–11 mm), and 37 tumors had less than a 5-mm minimum margin, while 19 had a 5-mm or greater minimum margin. There were 15 tumors that demonstrated some ground-glass appearance. There were 19 (34%) tumors with KRAS mutations. Table 1 also summarizes associations between KRAS status and the rest of the covariates. There were no statistically significant associations, although there was a trend for KRAS mutant tumors to be less likely to have a ground-glass component (P = .061). There was no difference between the groups in terms of size or margin achieved. There was a trend for KRAS mutant tumors to be smaller (P = .106).
Table 1.
Patient, Tumor, and Treatment Characteristics
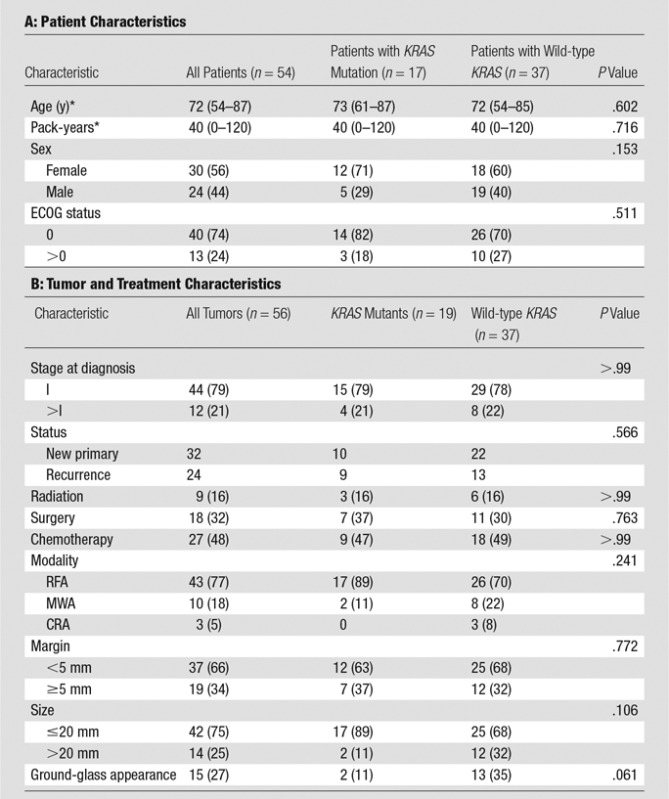
Note.—Unless otherwise specified, data are numbers of patients, with percentages in parentheses.
*Data are medians, with ranges in parentheses.
Complications were recorded as follows: There were 14 (25%) pneumothoraces that required chest tubes. There was one (2%) bronchopleural fistula, which was managed with a chest tube and antibiotics, and one (2%) abscess, which was managed with surgical débridement at video-assisted thorascopic surgery. No procedure-related deaths were recorded. There were no deaths recorded within 30 days.
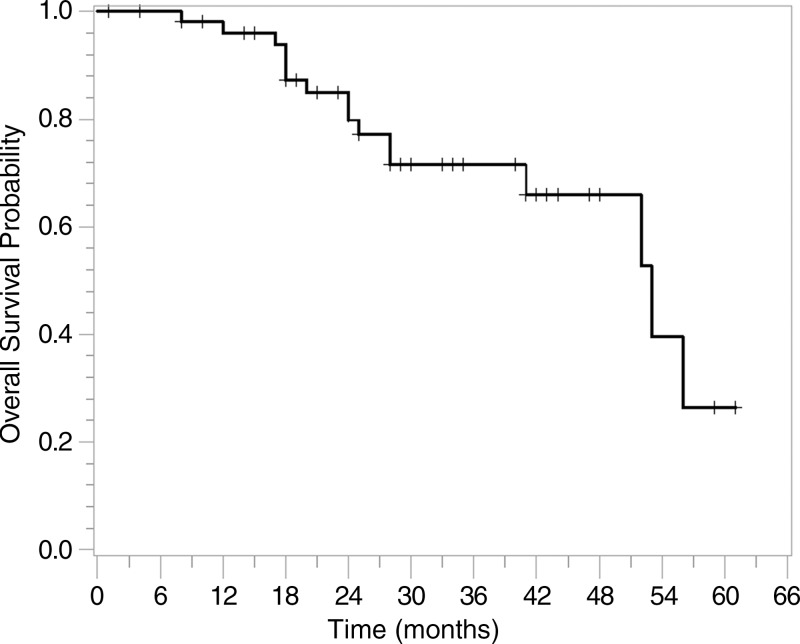
Figure 2 displays the Kaplan-Meier curve for overall survival for the 54 patients. The median overall survival probability was 53 months. The overall survival rate was 96% (95% CI: 85%, 99%) at 1 year, 80% (95% CI: 65%, 89%) at 2 years, and 72% (95% CI: 55%, 83%) at 3 years.
Figure 2:
Graph shows overall survival for all patients. The overall survival rate was 96% at 1 year, 80% at 2 years, and 72% at 3 years.
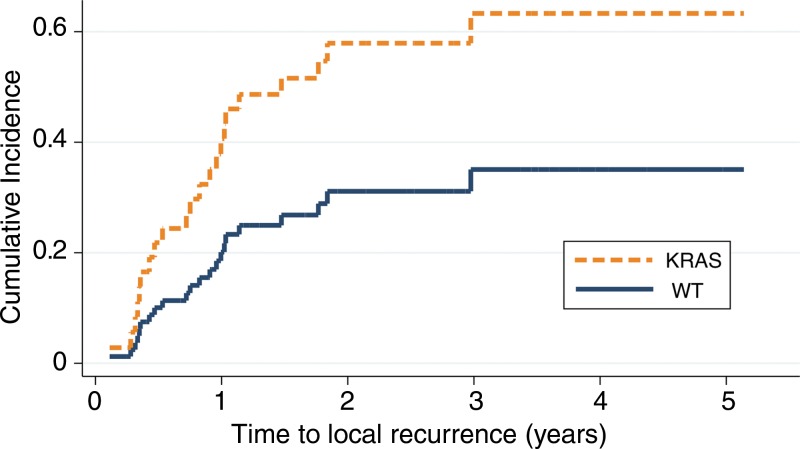
Results of univariate analysis of time to local recurrence are summarized in Table 2. Presence of KRAS mutation was associated with shorter time to local recurrence (P = .05; subdistribution HR, 2.32; 95% CI: 1.00, 5.39). The cumulative incidence function generated from the competing risk univariate analysis is presented in Figure 3. The 1-, 2-, and 3-year cumulative incidences of recurrences were 20%, 31%, and 35% for wild-type tumors, compared with 40%, 58%, and 63% for KRAS mutants, respectively. The presence of a ground-glass appearance in the nodule was associated with longer time to local recurrence (P = .04; subdistribution HR, 0.31; 95% CI: 0.01, 0.95). ECOG status, minimum margin, and tumor size were marginally associated with time to local recurrence (P < .25) and were thus included in the multivariate analysis.
Table 2.
Univariate Analysis of Time to Local Recurrence with Death as Competing Risk
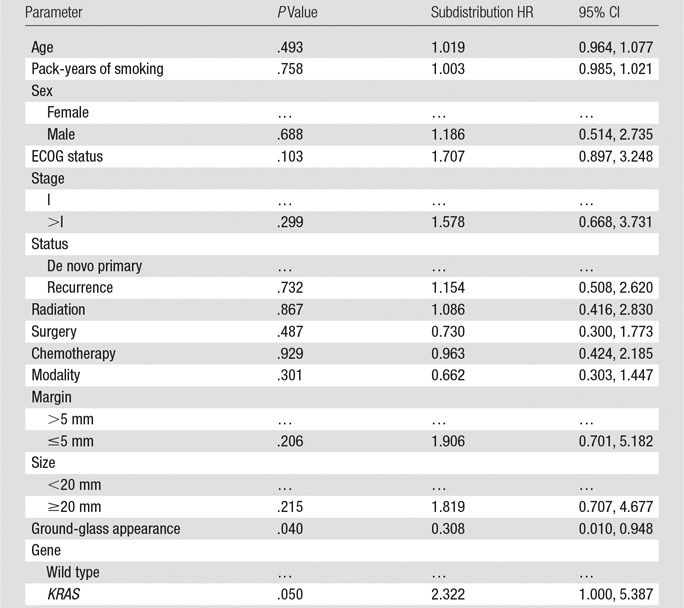
Figure 3:
Graph shows cumulative incidence function of time to local recurrence, with death as competing risk (P = .05), for wild-type (WT) KRAS versus KRAS mutation.
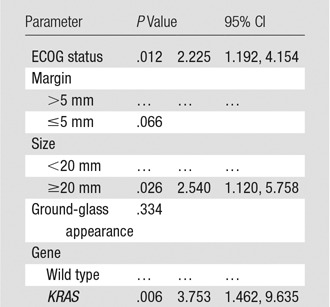
Table 3 lists results from the multivariate competing-risks proportional hazards model of time to local recurrence using the five covariates identified as either significant or marginally significant in the univariate analysis. KRAS mutation remained statistically significant (P = .006; subdistribution HR, 3.75; 95% CI: 1.46, 9.64). ECOG status and tumor size were also statistically significant in the multivariate analysis. Presence of ground-glass appearance and minimum margin were not significant in the multivariate analysis.
Table 3.
Multivariate Analysis of Time to Local Recurrence with Death as Competing Risk by Using Backward Selection with P < .05 as Cutoff
Discussion
Local recurrence after PIA in patients with lung adenocarcinoma remains an unresolved issue. In our series of patients, we found that KRAS mutation status was an independent predictor of time to local recurrence, with a significantly decreased cumulative incidence of recurrence (20% for wild-type KRAS tumors vs 40% for KRAS mutants at 1 year). Of note, the KRAS mutants were not more likely to be larger or to have smaller margins, suggesting an inherent aggressiveness of these tumors.
There has been much attention focused on the prognostic impact of KRAS mutation status for lung adenocarcinoma, largely focusing on overall survival. D’Angelo et al (14) reported similar overall survival in patients with and those without KRAS mutation with stage I–III lung adenocarcinoma, although KRAS mutations did predict shorter survival in a different study (25) in patients with advanced (stage IV) lung adenocarcinoma. Similar to our results, one report in the radiation oncology literature identified an association between KRAS mutation and lower freedom from recurrence (16), although these results are difficult to interpret because genotyping was performed in only 10 of 75 patients and likely included a biased subpopulation.
The predictive and prognostic role of other oncogenic driver mutations, including EGFR, has been well studied. Patients with EGFR mutation have a better prognosis than patients without EGFR mutation and are more likely to benefit from treatment with tyrosine kinase inhibitors (14,26,27). In the surgical literature, EGFR mutation did confer a benefit in overall survival over KRAS mutation that was independent of pathologic stage (15). One may make the conjecture that in our population, EGFR mutants may have better results because they may be candidates for tyrosine kinase inhibitor therapy. On the other hand, many of these tumors may have developed acquired resistance and failed tyrosine kinase inhibitor therapy. Because the natural history and treatment algorithms of these other mutants are so different, we chose to exclude patients with these mutations from our analysis. Subsequent analyses of other driver mutations, including EGFR, may yield further insight into these questions.
Many predictors of tumor response after lung ablation have been proposed. Traditional predictors of local recurrence in PIA include tumor size and ablation margin (28–30). We saw a significant association between tumor size and local recurrence. There was no significant association with minimum margin (9), although our study was small. Other markers of tumor biology may also be useful to assess. Sofocleous et al (31) have previously reported Ki-67 as a predictive biomarker after RFA. In their cohort of 47 lung tumors, 13 were known to represent primary lung cancers. Additionally, multiple reports establish the prognostic role of histologic subtype in lung adenocarcinoma (32). Because histologic subtyping is now performed in routine practice, future analysis should include this as an additional covariate.
Kodama et al (11) reported excellent local recurrence rates and overall survival in patients with ground-glass opacities who underwent RFA. Purely ground-glass opacities may actually represent adenocarcinoma in situ or minimally invasive adenocarcinoma (33,34). However, all of our nodules with a ground-glass appearance had at least partially solid components. Interestingly, we also saw a trend toward an association between ground-glass appearance and mutation status—KRAS mutant tumors were less likely to have a ground-glass component (P = .06). This finding is supported by recent work correlating CT imaging features with mutation status (35,36). This may also explain why the presence of a ground-glass appearance in the nodule was significant in the univariate analysis but not in the multivariate analysis.
Our overall survival rates at 1 year (96%) and 3 years (72%) are comparable with rates in the SBRT and surgery literature (37) and are in keeping with rates in a recently published report (6) that demonstrated comparable overall survival rates for RFA, SBRT, and surgery. These results support the need for prospective comparative trials between the three modalities. Interestingly, the presence of local recurrence after ablation does not appear to affect overall survival in primary lung cancer (6) but does affect overall survival in lung metastases (28).
There were several important limitations to our study. First, this was a retrospective study with a relatively small number of patients. We note that our multivariate model was at risk for overfitting and emphasize that this result is exploratory and should be validated in a separate cohort. The population was, moreover, heterogeneous and included tumors originally diagnosed as stage I–IV disease, although most were stage I (76%). Treatment was also heterogeneous and included RFA, MWA, and CRA. Additionally, there was an inherent bias in our population because patients were identified on the basis of molecular testing at a single institution. Although molecular analysis has become the standard of care for lung cancer, not all nodules are sampled for biopsy and not all biopsy samples are genetically evaluated, so our patients may not be representative of a general population. Additionally, genetic assays have evolved, so some driver mutations may have not been identifiable with earlier versions of the assay. Despite these limitations, the findings of our study support further prospective studies validating the role of KRAS as a prognostic biomarker of local recurrence and further explorative studies to identify additional potential biomarkers.
In conclusion, the presence of KRAS mutation in lung adenocarcinoma tumors predicts a shorter time to local recurrence after ablation that is independent of tumor size and ablation margin. Our study supports the utility of KRAS status as a prognostic indicator for local recurrence and, more broadly, underscores the importance of biomarkers in assessing outcomes and stratifying risk. Such information can be useful in identifying appropriate patients for PIA and establishing well-matched cohorts for future prospective comparison trials between PIA and SBRT or surgery. The importance of oncogene driver mutation status has not been well established in the PIA literature. Further prospective studies will enable better understanding of these potential prognostic markers in the setting of PIA.
Advances in Knowledge
■ KRAS mutation status was significantly predictive of local recurrence at univariate (P = .05) and multivariate (P = .006) analysis.
■ KRAS mutant cumulative incidence of recurrence at 1 year was 40% (65% at 3 years), compared with 20% (35% at 3 years) for wild-type KRAS.
■ Size (P = .026) and Eastern Cooperative Oncology Group status (P = .012) were also independently predictive of local recurrence, whereas ablation margin (P = .066) was not.
Implications for Patient Care
■ KRAS mutation status should be checked in lung adenocarcinomas undergoing ablation, as these tumors are more likely to recur even if they are small.
■ KRAS mutant tumors may require wider ablation margins or more careful monitoring after ablation.
Received January 22, 2016; revision requested March 22; revision received April 18; accepted May 2; final version accepted May 10.
This research was supported in part by the National Institutes of Health (grant P30 CA008748).
Disclosures of Conflicts of Interest: E.Z. disclosed no relevant relationships. J.P.E. disclosed no relevant relationships. H.Y. disclosed no relevant relationships. F.E.B. disclosed no relevant relationships. E.N.P. disclosed no relevant relationships. S.G. disclosed no relevant relationships. W.S. disclosed no relevant relationships. C.T.S. Activities related to the present article: none to disclose. Activities not related to the present article: is a consultant to Neuwave Medical (JNJ) and SIRTEX Medical; receives research support from Neuwave Medical (JNJ) and Perseon; is on the speakers bureaus of Medtronics and Perseon; owns stock in SIRTEX Medical. Other relationships: none to disclose. D.R.J. disclosed no relevant relationships. C.M.R. disclosed no relevant relationships. S.B.S. Activities related to the present article: none to disclose. Activities not related to the present article: has received personal fees from Medtronic and GE Healthcare; has received grants from GE Healthcare and Angiodynamics. Other relationships: none to disclose.
Abbreviations:
- CI
- confidence interval
- CRA
- cryoablation
- ECOG
- Eastern Cooperative Oncology Group
- HR
- hazard ratio
- MWA
- microwave ablation
- NSCLC
- non–small cell lung cancer
- PIA
- percutaneous image-guided ablation
- RFA
- radiofrequency ablation
- SBRT
- stereotactic body radiation therapy
References
- 1.Siegel RL, Sahar L, Portier KM, Ward EM, Jemal A. Cancer death rates in US congressional districts. CA Cancer J Clin 2015;65(5):339–344. [DOI] [PubMed] [Google Scholar]
- 2.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128(1):237–245. [DOI] [PubMed] [Google Scholar]
- 3.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341(16):1198–1205. [DOI] [PubMed] [Google Scholar]
- 4.Jones GC, Kehrer JD, Kahn J, et al. Primary treatment options for high-risk/medically inoperable early stage NSCLC patients. Clin Lung Cancer 2015;16(6):413–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petre EN, Solomon SB, Sofocleous CT. The role of percutaneous image-guided ablation for lung tumors. Radiol Med (Torino) 2014;119(7):541–548. [DOI] [PubMed] [Google Scholar]
- 6.Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121(19):3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9(7):621–628. [DOI] [PubMed] [Google Scholar]
- 8.Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013;145(3):692–699. [DOI] [PubMed] [Google Scholar]
- 9.de Baère T, Palussière J, Aupérin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology 2006;240(2):587–596. [DOI] [PubMed] [Google Scholar]
- 10.Okuma T, Matsuoka T, Yamamoto A, et al. Determinants of local progression after computed tomography-guided percutaneous radiofrequency ablation for unresectable lung tumors: 9-year experience in a single institution. Cardiovasc Intervent Radiol 2010;33(4):787–793. [DOI] [PubMed] [Google Scholar]
- 11.Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J Vasc Interv Radiol 2014;25(3):333–339. [DOI] [PubMed] [Google Scholar]
- 12.Ridge CA, Silk M, Petre EN, et al. Radiofrequency ablation of T1 lung carcinoma: comparison of outcomes for first primary, metachronous, and synchronous lung tumors. J Vasc Interv Radiol 2014;25(7):989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2005;92(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Angelo SP, Janjigian YY, Ahye N, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012;7(12):1815–1822. [DOI] [PubMed] [Google Scholar]
- 15.Sonobe M, Kobayashi M, Ishikawa M, et al. Impact of KRAS and EGFR gene mutations on recurrence and survival in patients with surgically resected lung adenocarcinomas. Ann Surg Oncol 2012;19(Suppl 3):S347–S354. [DOI] [PubMed] [Google Scholar]
- 16.Mak RH, Hermann G, Lewis JH, et al. Outcomes by tumor histology and KRAS mutation status after lung stereotactic body radiation therapy for early-stage non-small-cell lung cancer. Clin Lung Cancer 2015;16(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70(4):606–612. [PubMed] [Google Scholar]
- 18.Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol 2003;14(9 Pt 2):S293–S295. [DOI] [PubMed] [Google Scholar]
- 19.Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the Lung Cancer Mutation Consortium experience. J Thorac Oncol 2015;10(5):768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitale D, Gong Y, Taylor BS, et al. An integrated genomic analysis of lung cancer reveals loss of DUSP4 in EGFR-mutant tumors. Oncogene 2009;28(31):2773–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brevet M, Johnson ML, Azzoli CG, Ladanyi M. Detection of EGFR mutations in plasma DNA from lung cancer patients by mass spectrometry genotyping is predictive of tumor EGFR status and response to EGFR inhibitors. Lung Cancer 2011;73(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. J Vasc Interv Radiol 2014;25(11):1691–1705.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 24.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 25.Johnson ML, Sima CS, Chaft J, et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer 2013;119(2):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362(25):2380–2388. [DOI] [PubMed] [Google Scholar]
- 27.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 28.de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26(5):987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson EM, Lees WR, Gillams AR. Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol 2009;32(3):478–483. [DOI] [PubMed] [Google Scholar]
- 30.Gillams A, Khan Z, Osborn P, Lees W. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol 2013;36(3):724–730. [DOI] [PubMed] [Google Scholar]
- 31.Sofocleous CT, Garg SK, Cohen P, et al. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann Surg Oncol 2013;20(Suppl 3):S676–S683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6(2):244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardiner N, Jogai S, Wallis A. The revised lung adenocarcinoma classification: an imaging guide. J Thorac Dis 2014;6(Suppl 5):S537–S546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin X, Zhao SH, Gao J, et al. CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol 2015;25(9):2532–2540. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Zhang T, Han X, Liu XI, Zhou N, Liu Y. Impact of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of stage IA adenocarcinoma of the lung: correlation between computed tomography images and EGFR and KRAS gene mutations. Exp Ther Med 2015;9(6):2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Yang Y, Zhou X, et al. EGFR L858R mutation is associated with lung adenocarcinoma patients with dominant ground-glass opacity. Lung Cancer 2015;87(3):272–277. [DOI] [PubMed] [Google Scholar]
- 37.Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90(3):603–611. [DOI] [PubMed] [Google Scholar]