Abstract
Adipose tissue expansion occurs by increasing the size of existing adipocytes or by increasing the number of adipocytes via adipogenesis. Adipose tissue dysfunction in obesity is associated with adipocyte hypertrophy and impaired adipogenesis. We recently demonstrated that deletion of the ubiquitin ligase Siah2 is associated with enlarged adipocytes in lean or obese mice. In this study, we find that adipogenesis is impaired in 3T3-L1 preadipocytes stably transfected with Siah2 shRNA and that overexpression of Siah2 in non-precursor fibroblasts promotes adipogenesis. In the 3T3-L1 model, loss of Siah2 is associated with sustained β-catenin expression post-induction, but depletion of β-catenin only partially restores PPARγ expression and adipocyte formation. Using wild-type and Siah2−/− adipose tissue and adipose stromal vascular cells, we observe that Siah2 influences the expression of several factors that control adipogenesis, including Wnt pathway genes, β-catenin, Zfp432, and Bmp-4. Consistent with increased β-catenin levels in shSiah2 preadipocytes, Wnt10b is elevated in Siah2−/− adipose tissue and remains elevated in Siah2−/− primary stromal cells after addition of the induction mixture. However, addition of BMP-4 to Siah2−/− stromal cells reduces Wnt10b expression, reduces Zfp521 protein levels, and increases expression of Zfp423, a transcriptional regulator of peroxisome proliferator-activated receptor γ expression that controls commitment to adipogenesis and is repressed by Zfp521. These results indicate that Siah2 acts upstream of BMP-4 to regulate factors that control the commitment of adipocyte progenitors to an adipogenic pathway. Our findings reveal an essential role for Siah2 in the early events that signal undifferentiated progenitor cells to become mature adipocytes.
Keywords: adipogenesis, adipose tissue, β-catenin, ubiquitin, Wnt pathway, BMP-4, Siah2, ZFP423
Introduction
Adipose tissue expansion in response to excess caloric intake is central to storing the surplus energy as neutral lipids. However, excess adipose tissue is a risk factor for developing insulin resistance and type 2 diabetes as the lipid storage capacity of the adipose tissue is exceeded (1). The capacity to store lipids can be increased in two ways: by increasing the size of existing adipocytes (hypertrophy) and by recruiting stromal cells to form new adipocytes (hyperplasia). It has been consistently observed that adipocyte hypertrophy is associated with insulin resistance (2, 3) and strongly linked with adipose tissue inflammation as the limit of adipocyte expansion via hypertrophy is reached (4, 5). As macrophage-mediated removal of necrotic-like hypertrophied adipocytes progresses, new adipocytes are formed to maintain lipid storage capacity in the remodeled adipose tissue (5–7). The importance of adipocyte hyperplasia in maintaining adipose tissue function has prompted interest in understanding the factors controlling recruitment of resident adipose tissue stromal cells (8) or bone marrow-derived progenitor cells (9) to undergo adipogenesis.
The ubiquitin-proteasome system is well described as controlling the proteolysis and activity of key regulatory proteins that determine proliferation and differentiation of stem/progenitor cells of neural, hematopoietic, and mesenchymal origin (10–12). The ubiquitin-proteasome system functions as a set of highly ordered enzymes that activate and then transfer ubiquitin to a target protein, leading to proteasome-mediated degradation of the target protein or non-proteolytic regulation of the activity of the target protein (13). The proteasomal degradation of regulatory proteins required for commitment of mesenchymal progenitor cells to osteogenesis has been described for Wnt/β-catenin (14) and bone morphogenetic proteins (15). These pathways also regulate commitment to an adipogenic lineage.
Wnt10b, Wnt10a, and Wnt6 inhibit adipogenesis and promote osteogenesis via β-catenin mechanisms (16) that are terminated by proteasomal degradation of β-catenin (17). Conversely, Wnt5a promotes adipogenesis (18) and the degradation of β-catenin (19). Bone morphogenetic protein 4 (BMP-4)2 is a member of the TGFβ superfamily of growth factors that were initially identified as controlling bone formation (20). Subsequent data established a role for the BMPs in development of many tissues, including adipose tissue (21), where BMP-4 promotes commitment of mesenchymal stem cells to the adipocyte lineage in white adipose tissue (22–24). However, the role of the ubiquitin-proteasome system in the recruitment of mesenchymal stem cells to form adipocyte progenitor cells is not well defined.
Our studies in the 3T3-L1 model of adipogenesis indicate that the ubiquitin ligase Siah2 promotes adipogenesis (25). Moreover, adipocytes from Siah2−/− (Siah2KO) mice tend to be larger than wild-type adipocytes (26), suggesting that loss of Siah2 affects the ability to form new adipocytes in vivo. Thus, we investigated whether Siah2 affects adipogenesis via Wnt or BMP-4 pathways using primary adipose tissue stromal cells obtained from wild-type and Siah2KO mice. Here we report evidence that Siah2 functions upstream of BMP-4 to regulate commitment of adipose tissue stromal cells to the adipogenic pathway.
Results
Siah2 Expression Promotes Adipogenesis
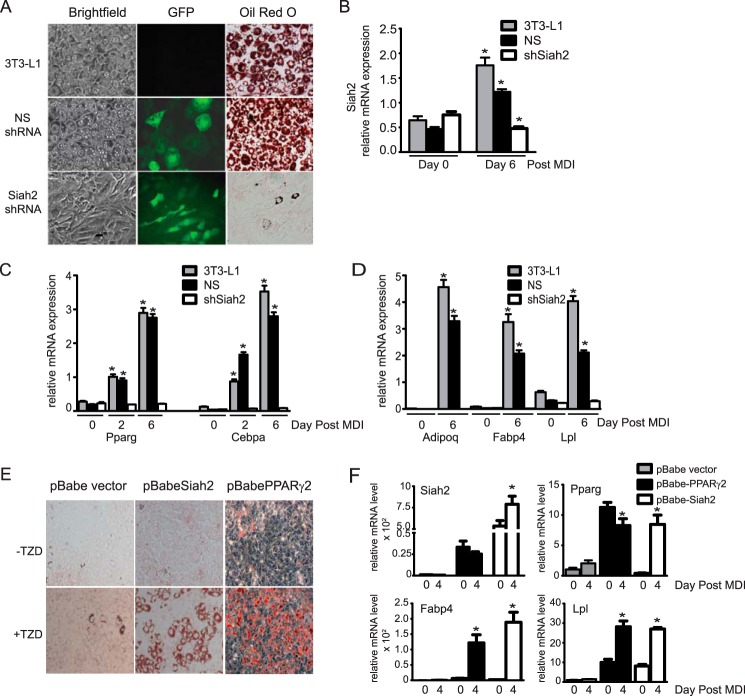
Our previous experiments using Siah2 siRNA in preadipocytes indicated that Siah2 is necessary for induction of adipogenesis (25). To confirm that result with more sustained reductions in Siah2 expression, we generated 3T3-L1 preadipocytes stably expressing non-silencing (NS) shRNA or Siah2 shRNA, each linked to GFP expression as a marker of transfection. Fig. 1A demonstrates that loss of Siah2 prior to induction of adipogenesis suppresses adipocyte formation, as assayed by neural lipid staining (Oil Red O) compared with untransfected 3T3-L1 preadipocytes (3T3-L1) or preadipocytes transfected with a non-silencing shRNA. Fig. 1B shows that Siah2 is depleted in shSiah2-transfected cells throughout the adipogenesis time course. In agreement with our previous finding that Siah2 is up-regulated during 3T3-L1 adipogenesis (25), Siah2 mRNA levels are increased in untransfected and non-silencing shRNA-transfected 3T3-L1 cells during adipogenesis (Fig. 1B). Consistent with a substantial reduction in lipid droplet formation in the absence of Siah2, mRNA levels of transcription factors required for adipogenesis (Pparg and Cebpa) (Fig. 1C) and markers of adipocyte formation (Adipoq, Fabp4, and Lpl) (Fig. 1D) are not up-regulated. To further evaluate whether Siah2 contributes to preadipocyte commitment, we ectopically expressed Siah2 in non-adipogenic NIH 3T3 fibroblasts. Ectopic expression of Siah2 stimulates adipogenesis, but only in the presence of a PPARγ ligand (Fig. 1E, +TZD). Gene expression of Pparg and the adipogenic markers Fabp4 and Lpl on day 4 post-induction with overexpression of Siah2 is comparable with the levels obtained with overexpression of PPARγ both in the presence of rosiglitazone (Fig. 1F). Although Siah2 stimulates PPARγ gene expression post-induction, ectopic overexpression of PPARγ induces Siah2 gene expression prior to adding the adipogenic mixture and rosiglitazone as a PPARγ ligand.
FIGURE 1.
Siah2 promotes adipogenesis in 3T3-L1 preadipocytes and non-precursor fibroblasts. A, 3T3-L1 preadipocytes did not undergo transfection (3T3-L1) or were stably transfected with non-silencing shRNA or Siah2 shRNA co-expressing GFP and induced to undergo adipogenesis. Accumulation of neutral lipids was detected using Oil Red O, and GFP was detected via fluorescence microscopy. B, Siah2 gene expression is up-regulated in untransfected and NS shRNA-transfected cells and specifically reduced in shSiah2-transfected cells during induction of adipogenesis. C and D, mRNA levels of transcription factors necessary for adipogenesis (C, Pparg and Cebpa) and late markers of adipogenesis (D; Adipoq, Fabp4, and Lpl) are not up-regulated in the absence of Siah2. E, neutral lipid accumulation is detected using Oil Red O in non-precursor NIH3T3 fibroblasts induced to undergo adipogenesis with or without rosiglitazone (2.5 μm, TZD) after ectopic expression of PPARγ or Siah2. F, mRNA levels of markers of adipogenesis (Pparg, Fabp4, and Lpl) are up-regulated in non-precursor fibroblasts with ectopic expression of Siah2. Gene expression was assayed using qRT-PCR. Statistical significance is compared with the corresponding day 0. *, p < 0.01.
Siah2 Regulates Expression of β-Catenin during Adipogenesis
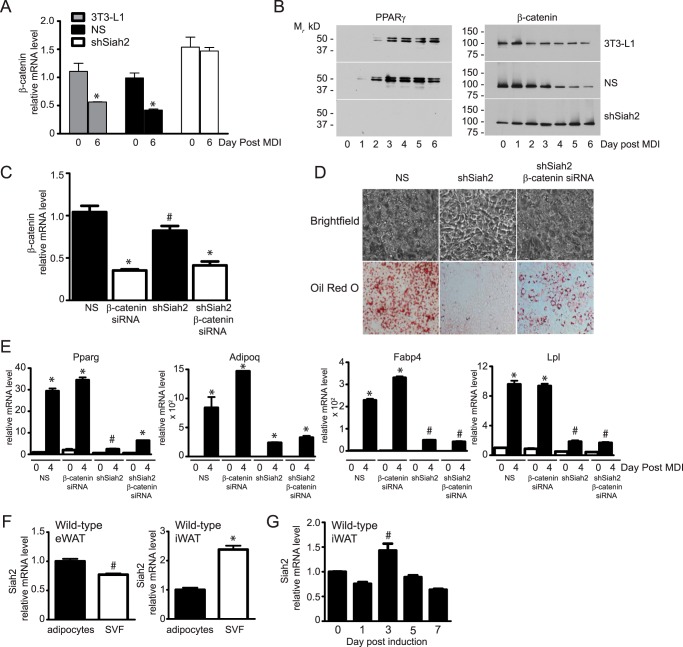
Stimulation of adipogenesis via up-regulation of PPARγ expression has the reciprocal effect of inhibiting osteogenesis via inhibition of β-catenin (27, 28), a transcriptional coactivator regulated by Wnt signaling that suppresses adipogenesis (16) and promotes osteoblast formation (29, 30). Activation of PPARγ during adipogenesis inhibits β-catenin activity by promoting proteasomal degradation of β-catenin (17). The absence of PPARγ protein expression in shSiah2 preadipocytes after treatment with the adipogenic mixture (Fig. 2B) prompted us to ask whether impaired adipogenesis correlated with increased β-catenin levels. This possibility was supported by experiments in HEK293 cells that found that non-canonical Wnt5a-mediated degradation of β-catenin involves Siah2 (19). In our experiments, loss of Siah2 in preadipocytes increases β-catenin gene and protein (Fig. 2, A and B) expression during adipogenesis. To determine whether elevated β-catenin expression accounts for the effect of Siah2 depletion on adipogenesis, we depleted β-catenin in the shSiah2 preadipocytes (Fig. 2C). Lipid accumulation (Oil Red O) is enhanced by reducing β-catenin expression in shSiah2 cells but does not return to the levels observed when both Siah2 and β-catenin are present (Fig. 2D). Expression of Pparg and other markers of adipocyte formation (Adipoq, Fabp4, and Lpl) were slightly increased in the Siah2, β-catenin double deletion but significantly less than in control cells (Fig. 2E). The failure of β-catenin depletion to fully restore adipocyte formation indicates that Siah2 functions “upstream” of β-catenin expression. Together with reduced expression of the transcriptional regulators of adipogenesis (Fig. 1C), the results suggest a role for Siah2 in determining commitment of preadipocytes to undergo adipogenesis. Consistent with that possibility, Siah2 mRNA is expressed in stromal vascular fraction (SVF) cells and mature adipocytes isolated from murine epididymal and inguinal adipose tissue (Fig. 2F). Moreover, the levels of Siah2 mRNA are 2-fold higher in the inguinal SVF compared with adipocytes (Fig. 2F). When primary cells obtained from the inguinal adipose tissue of wild-type mice are induced to undergo adipogenesis ex vivo, Siah2 expression increases slightly on day 3 post-induction but is below preinduction levels by day 7 post-induction (Fig. 2G).
FIGURE 2.
Siah2-mediated regulation of β-catenin expression does not directly account for the effect of Siah2 on adipogenesis. A, β-catenin gene expression was assayed prior to induction of adipogenesis (day 0) or 6 days post-induction in untransfected (3T3-L1), NS shRNA transfected and shSiah2-transfected 3T3-L1 preadipocytes. B, β-catenin and PPARγ protein expression was assayed by Western blotting analysis. C, depletion of β-catenin alone (β-catenin siRNA) or in combination with shSiah2 was confirmed using real-time qRT-PCR on day 6 post-induction. D, accumulation of neutral lipids was assayed via Oil Red O staining on day 4 post-induction of Siah2-depleted or Siah2/β-catenin-depleted 3T3-L1 preadipocytes. E, adipogenic markers were assayed on days 0 and 4 post-induction in control (NS), β-catenin, Siah2, or Siah2/β-catenin-depleted 3T3-L1 preadipocytes. F, Siah2 mRNA expression was determined in mature adipocytes and SVF cells isolated from visceral (epididymal white adipose tissue, eWAT) or subcutaneous (inguinal white adipose tissue, iWAT) fat depots. G, Siah2 mRNA levels were determined during induction of adipogenesis in adherent stromal cells isolated from wild-type iWAT. Gene expression was assayed using real-time qRT-PCR. Statistical significance was compared with corresponding day 0 (A, E, and G), non-silencing preadipocytes (C), or mature adipocytes (F). #, p < 0.05; *, p < 0.01.
Regulation of Wnt Pathway Genes by Siah2
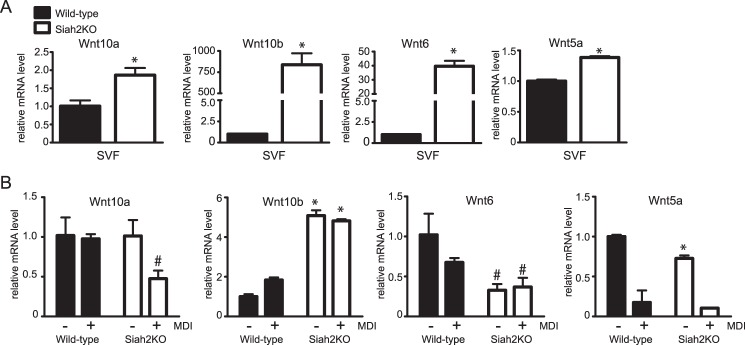
As an indicator of whether Siah2 influences commitment of adipose tissue mesenchymal precursors to undergo adipogenesis, we focused on the expression of Wnt10a, Wnt10b, Wnt6, and Wnt5a. Wnt pathway genes are well described as inhibiting commitment to the adipogenic pathway (16, 31) or, conversely, promoting adipogenesis in the case of WNT5a (18). Wnt10a, Wnt10b, Wnt6, and Wnt5a levels were determined in SVF cells isolated from the inguinal adipose tissue of wild-type and Siah2KO mice. As shown in Fig. 3A, Siah2 deletion is associated with substantially higher levels of Wnt10b and Wnt6 and significant but small increases for Wnt10a and Wnt5a. When the adherent population of stromal cells is induced to undergo adipogenesis, Wnt5a, Wnt6, and Wnt10a expression decreases, but Wnt10b mRNA levels are unchanged in Siah2-deficient cells (Fig. 3B). The correlation between loss of Siah2 and increased Wnt10b expression implies that Siah2 affects adipogenic potential via Wnt10b. To begin understanding whether regulation of Wnt10b accounts for Siah2KO-mediated inhibition of adipogenesis, we first assayed the levels of Wisp-2, Bmp-4, and Zfp423 as factors that interact with the Wnt pathway in adipose tissue to regulate adipogenesis.
FIGURE 3.
Loss of Siah2 regulates Wnt expression in adipose tissue and during adipogenesis. A and B, gene expression of Wnt5a, Wnt6, Wnt10a, and Wnt10b was assayed in inguinal adipose tissue SVF cells (A) or during induction of adipogenesis in adherent stromal cells isolated from wild-type and Siah2KO inguinal adipose tissue (B). Gene expression was assayed using real-time qRT-PCR. Statistical significance compared with the corresponding wild type. #, p < 0.05; *, p < 0.01.
Siah2 Acts Upstream of BMP-4 during Adipogenesis
Wnt1-inducible-signaling pathway protein 2 (WISP2) is a secreted protein that is highly expressed in adipose tissue SVF cells (32). WISP2 has been described as activating Wnt signaling (32–34) and inhibiting both adipocyte commitment and adipogenesis (32, 33). BMP-4 and Wisp2 activities intersect at regulation of Zfp423 (32, 34), a transcriptional coactivator of PPARγ that influences preadipocyte conversion to adipocytes at least in part by enhancing sensitivity to BMP-4 stimulation of adipogenesis (35). Zfp423 transcriptional coactivator function is restrained when bound by WISP2 in the cytoplasm. The Wisp2-Zfp423 complex is disrupted by BMP-4, allowing Zfp423 to enter the nucleus (32).
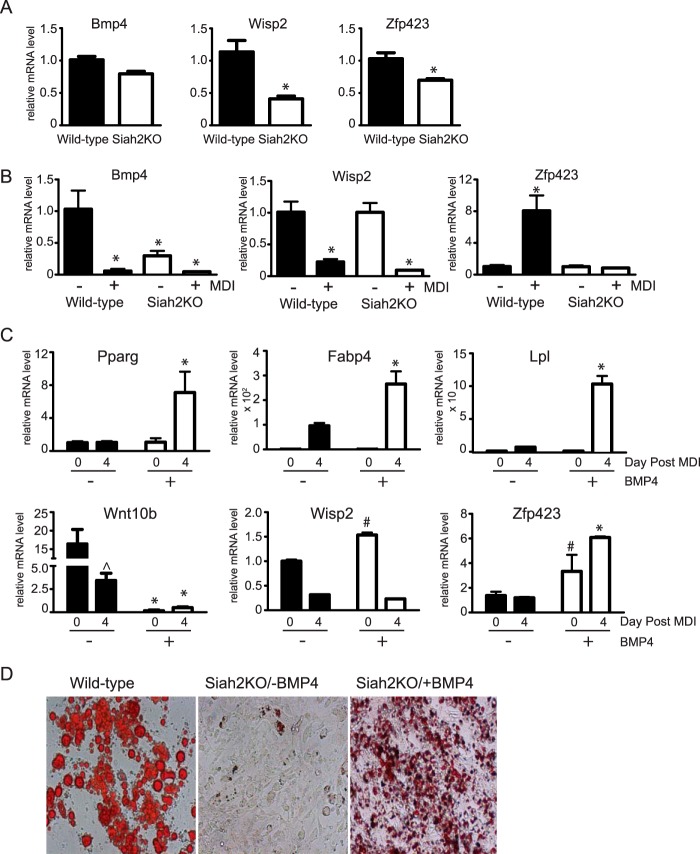
Contrary to our expectations, we found that wisp2 expression is reduced in the inguinal adipose tissue of Siah2KO mice, and expression of Bmp-4 and Zfp423 was also lower in the absence of Siah2 (Fig. 4A). Expression of wisp2 is not regulated by Siah2 during adipogenesis, whereas bmp-4 levels are significantly lower in the adherent stromal cell population of Siah2KO compared with the wild type and are further reduced when the cells are induced to undergo adipogenesis (Fig. 4B). In addition, Zfp423 gene expression is significantly up-regulated with adipogenesis in wild-type but unchanged in Siah2KO stromal cells (Fig. 4B). Because reduced Bmp-4, but not Wisp2, expression correlates with impaired adipogenic potential and reduced Zfp423 levels in the Siah2KO cells, we asked whether adding BMP-4 prior to induction could improve adipogenesis in Siah2KO cells. When BMP-4 (40 ng/ml) is present prior to induction of adipogenesis, the levels of Ppargamma, Fabp4, and Lpl are substantially increased, indicating that BMP-4 overrides inhibition of adipogenesis because of deletion of Siah2 (Fig. 4C). This change is associated with down-regulation of Wnt10b, up-regulation of Zfp423, and a small but significant increase in Wisp2 levels (Fig. 4C). To confirm that BMP-4 enhances adipogenesis in Siah2KO cells, lipid accumulation was visualized using Oil Red O staining in wild-type cells or Siah2KO cells induced to undergo adipogenesis in the absence or presence of BMP-4. As shown in Fig. 4D, lipid droplets accumulate only when Siah2KO cells are induced in the presence of BMP-4.
FIGURE 4.
Siah2 acts upstream of BMP-4 to promote adipogenesis. A and B, Bmp-4, Wisp2, and Zfp423 gene expression was assayed in wild-type and Siah2KO inguinal adipose tissue (A) or prior to induction of adipogenesis in wild-type and Siah2KO primary inguinal adherent stromal cells (−MDI) and on day 4 post induction (+MDI) (B). C, markers of adipogenesis (Pparg, Fabp4, and Lpl), Wnt10b, Wisp2, and Zfp423 gene expression were assayed during adipogenesis in the absence or presence of 40 ng/ml BMP-4. D, Oil Red O staining of neutral lipid accumulation on day 4 post-induction of adipogenesis in wild-type and Siah2KO stromal cells. Siah2KO stromal cells were incubated in the absence (Siah2KO/−BMP-4) or presence of 40 ng/ml BMP-4 (Siah2KO/+BMP-4). Statistical significance of Siah2KO was compared with the wild type (A), wild-type −MDI (B), or the corresponding day 0 (C). #, p < 0.05; *, p < 0.01; ∧, p < 0.01 compared with −MDI, day 0.
Siah2 Targets Zfp521 for Proteasomal Degradation
Siah2-mediated regulation of Zfp423 mRNA expression suggests that Siah2 functions to affect stability of a factor that controls transcription of Zfp423. Zfp521 is a transcriptional regulator that represses Zfp423 expression (36, 37) and acts to inhibit adipogenesis (37). Thus, we asked whether Zfp521 protein levels are altered during induction of adipogenesis in stromal cells from the inguinal adipose tissue of wild-type and Siah2KO mice. Fig. 5A shows that Zfp521 protein levels are increased prior to induction (day 0 post-induction) in the absence of Siah2 compared with the wild type and that Zfp521 levels increase during induction of adipogenesis in Siah2KO cells in the absence of BMP-4. When BMP-4 (40 ng/ml) is added 1 day prior to induction in Siah2KO cells, Zfp521 levels are substantially reduced by day 2 post-induction and are comparable with the wild type by day 4 post-induction. Experiments in vitro (Fig. 5B) demonstrate that HA-tagged ZFP521 levels are substantially reduced in the presence of FLAG-Siah2 but increased by proteasome inhibition (epoxomicin) when expressed alone or in the presence of FLAG-Siah2, indicating that Siah2 increases proteasomal degradation of Zfp521. MemCode staining (Fig. 5C) of the nitrocellulose membrane corresponding to the Western blot shown in Fig. 5B confirms equal loading of protein in each lane. Taken together, our results indicate that Siah2 regulates early steps in commitment to adipogenesis via regulation of Zfp521 protein levels in a BMP-4-dependent manner to promote Zfp423 mRNA expression (Fig. 5D).
FIGURE 5.
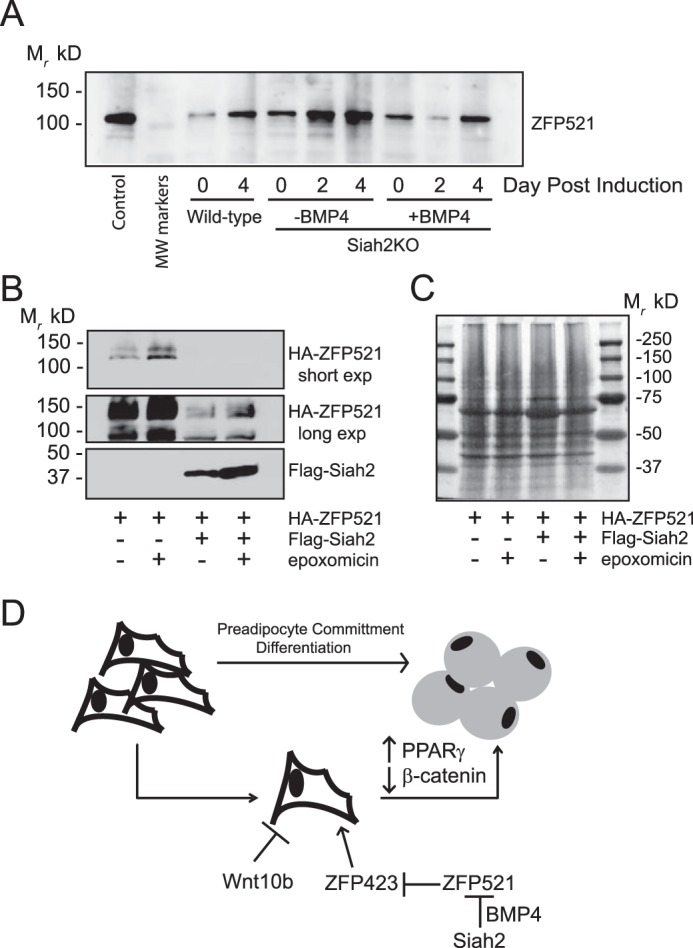
Siah2 regulates Zfp521 protein levels. A, Zfp521 protein levels were assayed during induction of adipogenesis in stromal cells isolated from wild-type and Siah2KO inguinal adipose tissue. Control Zfp521 is untagged mouse Zfp521 transiently expressed in HEK293 cells. Where indicated, BMP-4 (40 ng/ml) was added 2 days prior to induction of adipogenesis. B, HEK293 cells were transiently transfected with HA-Zfp521 and pcDNA3.1 or HA-Zfp521 and FLAG-Siah2 and treated with the proteasome inhibitor epoxomicin (1 μm) as indicated. The top panel is a short film exposure, and the bottom panel is a long film exposure to detect HA-Zfp521. Protein levels (A and B) were assayed by Western blotting analysis. C, MemCode staining of the total protein present in B. D, schematic depicting Siah2-mediated commitment to adipogenesis via regulation of a pathway that involves BMP-4, Zfp521, and Zfp423 expression in adipose tissue stromal vascular cells.
Discussion
Identifying the factors that participate in converting adipocyte stromal cells to mature adipocytes is fundamentally important to understanding the expansion of adipose tissue in obesity. Adipocytes in the visceral and subcutaneous adipose depots of lean or obese Siah2KO mice are hypertrophied, consistent with impaired adipogenesis in vivo that is related to loss of Siah2 (26). Although adipogenesis is impaired, both lean and obese Siah2KO mice were more insulin-sensitive compared with the wild type. Along with other studies showing that enlarged adipocytes can be associated with metabolic health (38), these results indicate that sufficient lipid storage capacity can occur in adipose tissue independent of a robust adipogenic response to energy challenges.
In this study, we investigated the role of Siah2 in adipogenesis. We found that the ubiquitin ligase Siah2 promotes adipogenesis by influencing early steps in commitment to the adipogenic pathway via regulation of Bmp-4 and Zfp423 expression and Zfp521 protein stability. Our previous experiments using the 3T3-L1 model of adipogenesis showed that Siah2 mRNA levels are low and that Siah2 protein is not detected in committed preadipocytes but increase during adipogenesis (25). This suggested that Siah2 expression is stimulated upon induction of adipogenesis and contributes to adipogenesis after progenitor cells are committed to the adipogenic pathway. However, induction of adipogenesis in adipose tissue stromal vascular cells argues for an earlier role for Siah2 in adipogenesis. Siah2 levels are higher in stromal vascular cells than in mature adipocytes, and Siah2 levels are only transiently up-regulated during adipogenesis of the adherent stromal cells. Impaired adipogenesis in the Siah2KO stromal cells correlates with reduced Bmp-4 levels and is overcome by ectopic expression of BMP-4, a bone morphogenetic protein that regulates commitment of progenitor cells to an adipogenic lineage (23). Thus, it is likely that Siah2 is found in stromal vascular cell types other than committed preadipocytes and that Siah2 affects processes upstream of BMP-4 to determine adipogenic potential.
Decreased expression of Bmp-4 in Siah2KO adipose tissue further supports the ex vivo data implicating Siah2 in promoting commitment of adipose stromal cells to undergo adipogenesis. BMP-4-mediated down-regulation of Wnt10b coupled with reduced expression of Wisp2 independent of Siah2 or BMP-4 upon induction of adipogenesis in Siah2KO stromal cells suggests that Wnt signaling does not directly account for the effect of Siah2 on adipogenic potential. However, Zfp423 expression is attenuated in the absence of Siah2 both in vivo and ex vivo, and restoration of adipogenesis by BMP-4 in Siah2KO stromal cells is associated with robust up-regulation of Zfp423. Zfp423 regulates commitment of stromal vascular cells to the adipogenic pathway in part via increasing sensitivity to BMP-4 signaling to promote adipogenesis (35). Zfp423 is also reported to link BMP-4 and Notch signaling (39), indicating a complex relationship between Zfp423 and BMP-4 in cell differentiation that may be regulated by Siah2. Our findings indicate that Zfp521, a paralog of Zfp423 that acts as a transcriptional repressor of Zfp423 (36, 37), is a likely target of Siah2 activity and that Siah2-mediated degradation of Zfp521 may function to derepress Zfp423 expression. Finally, down-regulation of Zfp521 protein by BMP-4 during induction of adipogenesis in Siah2KO stromal cells positions BMP-4 as mediating the effect of Siah2 on Zfp521 protein levels and, subsequently, Zfp423 expression.
Although enzymes of the ubiquitin-proteasome system are well described as regulating proliferation and differentiation of stem and progenitor cells, the focus has largely been on understanding the role of ubiquitin-proteasome system enzymes in regulating pluripotency and reprogramming in embryonic stem cells (10, 40). However, evidence is accumulating that ubiquitin system enzymes control the stability and activity of a range of proteins that regulate terminal adipogenesis (41–43). Given the reciprocal nature of adipocyte and osteoblast formation, evidence that the ubiquitin-proteasome system affects bone formation via regulation of bmp-2 expression (15) supports a role for ubiquitin system enzymes in determining whether mesenchymal progenitor cells are recruited to undergo adipogenesis. A more specific link to Siah2 is found in studies that show that c-Cbl, a cytoplasmic ubiquitin ligase that regulates osteoblast proliferation and differentiation (44), also partners with Siah2 to control signaling events in cell proliferation and differentiation (45, 46).
This study places Siah2 as an upstream regulation of zfp423 gene expression and indicates that Siah2 influences Zfp423 gene expression via regulation of Bmp-4 expression and Zfp521 protein stability. Generally, our results show that the mechanisms linking Zfp423 and BMP-4-mediated regulation of adipogenesis extend to control of Zfp423 and Bmp-4 gene expression. Our results clearly place the ubiquitin ligase Siah2 as a factor that mediates the relationship between Bmp-4 and Zfp423 expression in determining commitment of adipose stromal cells to an adipogenic lineage.
Experimental Procedures
Animal Experiments
Wild-type and global Siah2−/− (Siah2KO) C57BL/6J male mice were housed with a 12-h light-dark cycle at 24 °C. All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Pennington Biomedical Research Center Animal Care and Use Committee (protocol 876). At 4–8 weeks of age, the mice were euthanized between 8–11 a.m., and the inguinal and epididymal adipose tissue was harvested for whole tissue analysis or isolating SVF cells and mature adipocytes.
Cell Culture
Murine 3T3-L1 preadipocytes were plated and grown to 2 days post-confluence in DMEM high-glucose with 10% bovine serum, 100 units of penicillin/100 μg of streptomycin. The medium was changed every 48 h. Cells were induced to differentiate by changing the medium to DMEM high-glucose containing a standard induction mixture of 10% FBS, 0.5 mm isobutylmethylxanthine (IBMX), 1 μm dexamethasone, 1.7 μm insulin (MDI), and 100 units of penicillin/100 μg of streptomycin. After 48 h, this medium was replaced with DMEM high-glucose supplemented with 10% FBS, and cells were maintained in this medium. The NIH 3T3 fibroblast cell lines (ATCC) ectopically expressing Siah2 or PPARγ were induced to differentiate with standard induction mixture in the presence or absence of rosiglitazone (TZD, 2.5 μm). HEK293 cells were maintained in DMEM high-glucose with 10% FBS, 100 units of penicillin/100 μg of streptomycin.
Adipose tissue obtained from wild-type or Siah2KO mice was minced and suspended in PBS supplemented with 0.1% bovine serum and 0.1% collagenase type I (Worthington) prewarmed to 37 °C at 10 ml/2 g of tissue. The tissue was incubated in a shaking water bath at 37 °C for 1 h and centrifuged at 450 × g for 5 min at room temperature. Mature adipocytes from both inguinal and epididymal fat pads were collected from the supernatant and processed for RNA isolation. The pelleted SVF cells from both fat pads were also processed for RNA isolation. The SVF cells from the inguinal adipose tissue were resuspended in stromal medium (DMEM/Ham's F-12 medium, 15% FBS, 100 units of penicillin/100 μg of streptomycin), plated, and maintained as described previously (47). When the cells were 80–90% confluent, the stromal medium was exchanged for differentiation medium (DMEM/Ham's F-12 medium with 3% FBS, 0.5 mm IBMX, 33 μm biotin, 17 μm pantothenate, 1 μm insulin, 1 μm dexamethasone, 2.5 μm rosiglitazone, and 100 units of penicillin/100 μg of streptomycin). After 3 days, the medium was exchanged for maintenance medium that was identical to the differentiation medium except that IBMX and rosiglitazone were deleted. BMP-4 (Life Technologies, 40 ng/ml) was added to the medium 5 days prior to induction.
Generating Stable Cell Lines
Retrovirus-mediated stable expression of shRNA targeting Siah2 (SMARTvector lentiviral shRNA) or a non-silencing control hairpin sequence containing the TurboGFP marker in the pGIPZ vector (Dharmacon) was generated in 3T3-L1 preadipocytes according to the instructions of the manufacturer. Selection of preadipocytes containing the desired shRNA was carried out using puromycin (2.5 μg/ml) over 2 weeks. β-Catenin was depleted in the non-silencing pGIPZ and pGIPZ-shSiah 3T3-L1 preadipocytes via reverse transfection (48) using siRNA targeting β-catenin (Dharmacon SMARTpool On-Targetplus siRNA). The preadipocytes were transfected 1 day prior to inducing the cells to undergo adipogenesis. Depletion of target genes was confirmed using qRT-PCR. To overexpress Siah2 in NIH3T3 fibroblasts, Siah2 cDNA was amplified via PCR to contain a 5′ EcoR1 restriction site and a 3′ Sal1 restriction site. The PCR product was purified (Qiagen MiniElute PCR purification) and inserted into the pBabePuro vector (Addgene) using site-directed mutagenesis (Stratagene QuikChange). The sequence was confirmed by dideoxy sequencing prior to transfection of pBabePuro-Siah2 into NIH3T3 fibroblasts as described previously (49).
Transient Transfection of HA-Zfp521 and FLAG-Siah2
Mouse Zfp521 cDNA was obtained from OriGene (TrueClone cDNA), and the HA epitope tag was inserted after the start codon using site-directed mutagenesis (Stratagene QuikChange II) and confirmed by dideoxy sequencing as described previously (25). HEK293 cells were grown to 40–70% confluence, and transient transfections were carried out using a total of 2 μg of cDNA/well and Polyfect according to the instructions of the manufacturer (Qiagen). The cells were transfected with HA-Zfp521 and pcDNA3.1 or HA-Zfp521 and FLAG-Siah2. Forty-eight hours after transfection, the cells were treated with vehicle control (DMSO) or epoxomicin (1 μm), and whole cell extracts were harvested 4 h later.
Oil Red O Staining
Oil Red O staining was performed as described by Green and Kehinde (50).
Quantitative PCR
Total RNA was reverse-transcribed (200 ng of RNA) using Multiscribe reverse transcriptase (Applied Biosystems) with random primers at 37 °C for 2 h. Real-time PCR was performed with TaqMan chemistry using the 7900 real-time PCR system and universal cycling conditions (50 °C for 2 min; 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by 95 °C for 15 s, 60 °C for 15 s, and 95 °C for 15 s). The results were normalized to cyclophilin B or ubiquitin B mRNA levels and analyzed using the 2−ΔΔCT method.
Preparation of Whole Cell Extracts and Immunoblotting
Whole cell extracts were prepared by homogenizing in 50 mm Tris/Cl (pH 7.4) with 150 mm NaCl, 1 mm EDTA, 1% Igepal CA 630, 0.5% sodium deoxycholate, 0.1% SDS, 10 mm N-ethylmaleimide, protease inhibitors (1 mm PMSF, 10 μg/ml aprotinin, 1 μg/ml pepstatin, and 5 μg/ml leupeptin), and phosphatase inhibitor (2 mm sodium orthovanadate). The samples were centrifuged at 14,000 × g for 10 min at 4 °C, and protein concentrations were determined by BCA assay (Thermo Fisher Scientific).
Gel Electrophoresis and Immunoblotting
Proteins were separated in polyacrylamide (National Diagnostics) gels containing SDS and transferred to nitrocellulose (Bio-Rad). Following transfer, the membrane was blocked in 4% milk in 25 mm Tris/Cl (pH 8.0) with 150 mm NaCl, 0.1% Tween 20 (TBS-T) for 1 h at room temperature. The membranes were incubated with antibodies against PPARγ (Santa Cruz Biotechnology, sc-7273, 1:200; Abcam, 19481, 1:500), β-catenin (Bethyl Laboratories, A302-012A-M, 1:1000), Zfp521 (ProSci, 6859, 1:1000), HA epitope tag (BioLegend, 901513, 1:2000), and FLAG epitope tag (Sigma, F1804, 1:500) for 1–2 h at room temperature. The results were visualized with HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) and enhanced chemiluminescence (Thermo Fisher/Pierce). Equal loading was determined using MemCode (Thermo Fisher Scientific) staining of the nitrocellulose membrane.
Statistical Analysis
Statistical significance was determined using an unpaired two-tailed t test. GraphPad Prism 5 software was used for statistical analyses. Adipose tissue data (Fig. 4A) were obtained from 4 mice/group, and adipogenesis in primary SVF cells was carried out in pooled samples from three to four mice and repeated at least twice. Experiments in the 3T3-L1 adipocytes and HEK293 cells were repeated at least twice for a minimum of three replicates. All technical replicates were carried out in triplicate. Variability was expressed as the mean ± S.D.
Author Contributions
G. K. and Z. E. F. designed, performed, and analyzed the experiments shown in Figs. 1–4. D. H. B. provided technical assistance for the experiments shown in Fig. 1. Z. E. F. coordinated the study and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
This work used the Cell Biology and Bioimaging Core and the Genomics Core facilities at Pennington Biomedical Research Center, which are supported in part by COBRE (NIH 8P20-GM103528) and NORC (NIH 2P30-DK072476) center grants from the National Institutes of Health.
This work was supported by NIDDK, National Institutes of Health Grant 1R01DK099625 (to Z. E. F.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- BMP
- bone morphogenetic protein
- NS
- non-silencing
- PPAR
- peroxisome proliferator-activated receptor
- SVF
- stromal vascular fraction
- IBMX
- isobutylmethylxanthine
- qRT-PCR
- quantitative RT-PCR
- TZD
- thiazolidinedione.
References
- 1. Grundy S. M. (2000) Metabolic complications of obesity. Endocrine 13, 155–165 [DOI] [PubMed] [Google Scholar]
- 2. Stern J. S., Batchelor B. R., Hollander N., Cohn C. K., and Hirsch J. (1972) Adipose-cell size and immunoreactive insulin levels in obese and normal-weight adults. Lancet 2, 948–951 [DOI] [PubMed] [Google Scholar]
- 3. Lundgren M., Svensson M., Lindmark S., Renström F., Ruge T., and Eriksson J. W. (2007) Fat cell enlargement is an independent marker of insulin resistance and “hyperleptinaemia.” Diabetologia 50, 625–633 [DOI] [PubMed] [Google Scholar]
- 4. Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A. S., and Obin M. S. (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 [DOI] [PubMed] [Google Scholar]
- 5. Strissel K. J., Stancheva Z., Miyoshi H., Perfield J. W. 2nd, DeFuria J., Jick Z., Greenberg A. S., and Obin M. S. (2007) Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56, 2910–2918 [DOI] [PubMed] [Google Scholar]
- 6. Spalding K. L., Arner E., Westermark P. O., Bernard S., Buchholz B. A., Bergmann O., Blomqvist L., Hoffstedt J., Näslund E., Britton T., Concha H., Hassan M., Rydén M., Frisén J., and Arner P. (2008) Dynamics of fat cell turnover in humans. Nature 453, 783–787 [DOI] [PubMed] [Google Scholar]
- 7. Sun K., Kusminski C. M., and Scherer P. E. (2011) Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cawthorn W. P., Scheller E. L., and MacDougald O. A. (2012) Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J. Lipid Res. 53, 227–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crossno J. T. Jr, Majka S. M., Grazia T., Gill R. G., and Klemm D. J. (2006) Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J. Clin. Invest. 116, 3220–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naujokat C., and Sarić T. (2007) Concise review: role and function of the ubiquitin-proteasome system in mammalian stem and progenitor cells. Stem Cells 25, 2408–2418 [DOI] [PubMed] [Google Scholar]
- 11. Moran-Crusio K., Reavie L. B., and Aifantis I. (2012) Regulation of hematopoietic stem cell fate by the ubiquitin proteasome system. Trends Immunol. 33, 357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buckley S. M., Aranda-Orgilles B., Strikoudis A., Apostolou E., Loizou E., Moran-Crusio K., Farnsworth C. L., Koller A. A., Dasgupta R., Silva J. C., Stadtfeld M., Hochedlinger K., Chen E. I., and Aifantis I. (2012) Regulation of pluripotency and cellular reprogramming by the ubiquitin-proteasome system. Cell Stem Cell 11, 783–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welchman R. L., Gordon C., and Mayer R. J. (2005) Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 6, 599–609 [DOI] [PubMed] [Google Scholar]
- 14. Aberle H., Bauer A., Stappert J., Kispert A., and Kemler R. (1997) β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garrett I. R., Chen D., Gutierrez G., Zhao M., Escobedo A., Rossini G., Harris S. E., Gallwitz W., Kim K. B., Hu S., Crews C. M., and Mundy G. R. (2003) Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J. Clin. Invest. 111, 1771–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cawthorn W. P., Bree A. J., Yao Y., Du B., Hemati N., Martinez-Santibañez G., and MacDougald O. A. (2012) Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 50, 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moldes M., Zuo Y., Morrison R. F., Silva D., Park B. H., Liu J., and Farmer S. R. (2003) Peroxisome-proliferator-activated receptor γ suppresses Wnt/β-catenin signalling during adipogenesis. Biochem. J. 376, 607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishizuka M., Koyanagi A., Osada S., and Imagawa M. (2008) Wnt4 and Wnt5a promote adipocyte differentiation. FEBS Lett. 582, 3201–3205 [DOI] [PubMed] [Google Scholar]
- 19. Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P. J., and Yang Y. (2003) Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J. Cell Biol. 162, 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reddi A. H., and Cunningham N. S. (1993) Initiation and promotion of bone differentiation by bone morphogenetic proteins. J. Bone Miner. Res. 8, S499–502 [DOI] [PubMed] [Google Scholar]
- 21. Chen D., Zhao M., and Mundy G. R. (2004) Bone morphogenetic proteins. Growth Factors 22, 233–241 [DOI] [PubMed] [Google Scholar]
- 22. Dani C., Smith A. G., Dessolin S., Leroy P., Staccini L., Villageois P., Darimont C., and Ailhaud G. (1997) Differentiation of embryonic stem cells into adipocytes in vitro. J. Cell Sci. 110, 1279–1285 [DOI] [PubMed] [Google Scholar]
- 23. Bowers R. R., and Lane M. D. (2007) A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle 6, 385–389 [DOI] [PubMed] [Google Scholar]
- 24. Tang Q. Q., and Lane M. D. (2012) Adipogenesis: from stem cell to adipocyte. Annu. Rev. Biochem. 81, 715–736 [DOI] [PubMed] [Google Scholar]
- 25. Kilroy G., Kirk-Ballard H., Carter L. E., and Floyd Z. E. (2012) The ubiquitin ligase Siah2 regulates PPARγ activity in adipocytes. Endocrinology 153, 1206–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kilroy G., Carter L. E., Newman S., Burk D. H., Manuel J., Möller A., Bowtell D. D., Mynatt R. L., Ghosh S., and Floyd Z. E. (2015) The ubiquitin ligase Siah2 regulates obesity-induced adipose tissue inflammation. Obesity 23, 2223–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akune T., Ohba S., Kamekura S., Yamaguchi M., Chung U. I., Kubota N., Terauchi Y., Harada Y., Azuma Y., Nakamura K., Kadowaki T., and Kawaguchi H. (2004) PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 113, 846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rzonca S. O., Suva L. J., Gaddy D., Montague D. C., and Lecka-Czernik B. (2004) Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145, 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bain G., Müller T., Wang X., and Papkoff J. (2003) Activated β-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem. Biophys. Res. Commun. 301, 84–91 [DOI] [PubMed] [Google Scholar]
- 30. Glass D. A. 2nd, Bialek P., Ahn J. D., Starbuck M., Patel M. S., Clevers H., Taketo M. M., Long F., McMahon A. P., Lang R. A., and Karsenty G. (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 [DOI] [PubMed] [Google Scholar]
- 31. Ross S. E., Hemati N., Longo K. A., Bennett C. N., Lucas P. C., Erickson R. L., and MacDougald O. A. (2000) Inhibition of adipogenesis by Wnt signaling. Science 289, 950–953 [DOI] [PubMed] [Google Scholar]
- 32. Hammarstedt A., Hedjazifar S., Jenndahl L., Gogg S., Grünberg J., Gustafson B., Klimcakova E., Stich V., Langin D., Laakso M., and Smith U. (2013) WISP2 regulates preadipocyte commitment and PPARγ activation by BMP4. Proc. Natl. Acad. Sci. U.S.A. 110, 2563–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grünberg J. R., Hammarstedt A., Hedjazifar S., and Smith U. (2014) The novel secreted adipokine WNT1-inducible signaling pathway protein 2 (WISP2) is a mesenchymal cell activator of canonical WNT. J. Biol. Chem. 289, 6899–6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gustafson B., Hammarstedt A., Hedjazifar S., and Smith U. (2013) Restricted adipogenesis in hypertrophic obesity: the role of WISP2, WNT, and BMP4. Diabetes 62, 2997–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gupta R. K., Arany Z., Seale P., Mepani R. J., Ye L., Conroe H. M., Roby Y. A., Kulaga H., Reed R. R., and Spiegelman B. M. (2010) Transcriptional control of preadipocyte determination by Zfp423. Nature 464, 619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Addison W. N., Fu M. M., Yang H. X., Lin Z., Nagano K., Gori F., and Baron R. (2014) Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Mol. Cell. Biol. 34, 3076–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang S., Akerblad P., Kiviranta R., Gupta R. K., Kajimura S., Griffin M. J., Min J., Baron R., and Rosen E. D. (2012) Regulation of early adipose commitment by Zfp521. PLoS Biol. 10, e1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khan T., Muise E. S., Iyengar P., Wang Z. V., Chandalia M., Abate N., Zhang B. B., Bonaldo P., Chua S., and Scherer P. E. (2009) Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masserdotti G., Badaloni A., Green Y. S., Croci L., Barili V., Bergamini G., Vetter M. L., and Consalez G. G. (2010) ZFP423 coordinates Notch and bone morphogenetic protein signaling, selectively up-regulating Hes5 gene expression. J. Biol. Chem. 285, 30814–30824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atkinson S. P., Collin J., Irina N., Anyfantis G., Kyung B. K., Lako M., and Armstrong L. (2012) A putative role for the immunoproteasome in the maintenance of pluripotency in human embryonic stem cells. Stem Cells 30, 1373–1384 [DOI] [PubMed] [Google Scholar]
- 41. Pal P., Lochab S., Kanaujiya J. K., Kapoor I., Sanyal S., Behre G., and Trivedi A. K. (2013) E3 ubiquitin ligase E6AP negatively regulates adipogenesis by downregulating proadipogenic factor C/EBPα. PLoS ONE 8, e65330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gao Y., Koppen A., Rakhshandehroo M., Tasdelen I., van de Graaf S. F., van Loosdregt J., van Beekum O., Hamers N., van Leenen D., Berkers C. R., Berger R., Holstege F. C., Coffer P. J., Brenkman A. B., Ovaa H., and Kalkhoven E. (2013) Early adipogenesis is regulated through USP7-mediated deubiquitination of the histone acetyltransferase TIP60. Nat. Commun. 4, 2656. [DOI] [PubMed] [Google Scholar]
- 43. Watanabe M., Takahashi H., Saeki Y., Ozaki T., Itoh S., Suzuki M., Mizushima W., Tanaka K., and Hatakeyama S. (2015) The E3 ubiquitin ligase TRIM23 regulates adipocyte differentiation via stabilization of the adipogenic activator PPARγ. eLife 4, e05615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sévère N., Dieudonné F. X., and Marie P. J. (2013) E3 ubiquitin ligase-mediated regulation of bone formation and tumorigenesis. Cell Death Dis. 4, e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nadeau R. J., Toher J. L., Yang X., Kovalenko D., and Friesel R. (2007) Regulation of Sprouty2 stability by mammalian Seven-in-Absentia homolog 2. J. Cell. Biochem. 100, 151–160 [DOI] [PubMed] [Google Scholar]
- 46. Guy G. R., Jackson R. A., Yusoff P., and Chow S. Y. (2009) Sprouty proteins: modified modulators, matchmakers or missing links? J. Endocrinol. 203, 191–202 [DOI] [PubMed] [Google Scholar]
- 47. Yu G., Wu X., Kilroy G., Halvorsen Y. D., Gimble J. M., and Floyd Z. E. (2011) Isolation of murine adipose-derived stem cells. Methods Mol. Biol. 702, 29–36 [DOI] [PubMed] [Google Scholar]
- 48. Kilroy G., Burk D. H., and Floyd Z. E. (2009) High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS ONE 4, e6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Floyd Z. E., and Stephens J. M. (2003) STAT5A promotes adipogenesis in nonprecursor cells and associates with the glucocorticoid receptor during adipocyte differentiation. Diabetes 52, 308–314 [DOI] [PubMed] [Google Scholar]
- 50. Green H., and Kehinde O. (1975) An established preadipose cell line and its differentiation in culture: II: factors affecting the adipose conversion. Cell 5, 19–27 [DOI] [PubMed] [Google Scholar]