Abstract
More than 30 years ago, two unexpected findings were discovered that challenged conventional thinking in biology. The first was the identification of a misfolded protein with transmissible properties associated with a group of neurodegenerative diseases known as transmissible spongiform encephalopathies. The second was the discovery of a new pathway used for the extracellular release of biomolecules, including extracellular vesicles called exosomes. Two decades later, the convergence of these pathways was shown when exosomes were found to play a significant role in both the transmission and propagation of protein aggregates in disease. Recent research has now revealed that the majority of proteins involved in neurodegenerative diseases are transported in exosomes, and that external stresses due to age-related impairment of protein quality control mechanisms can promote the transcellular flux of these proteins in exosomes. Significantly, exosomes provide an environment that can induce the conformational conversion of native proteins into aggregates that can be transmitted to otherwise aggregate-free cells in the brain. Here we review the current roles of exosomes in the pathology of neurodegenerative diseases.
Keywords: Alzheimer disease, extracellular vesicles, Parkinson disease, prion, prion disease, exosomes
Introduction
Exosomes are released into the extracellular environment by the majority of cell types in the body. Originally identified to be involved in the non-degradative removal of the transferrin receptor during the maturation process of reticulocytes (1, 2), exosomes have now also been recognized as an important communication and signaling pathway in the body in both normal and disease settings. Exosomes differ from other extracellular vesicles (EVs)3 based on the secretion pathway used and the size of the vesicle released. Unlike other EVs that can bud from the plasma membrane, such as microvesicles (3), exosomes are created from intraluminal vesicles that form within multivesicular bodies (MVBs, or multivesicular endosomes). The subsequent fusion of the MVB at the plasma membrane releases these vesicles into the extracellular milieu where they are known as exosomes (Fig. 1). This secretion process results in a large number of exosomes being released in the body, with estimates of 3 × 106 exosomes per microliter of blood serum. Recent evidence has highlighted the importance of exosomes both for cellular communication and in the delivery of biomolecules.
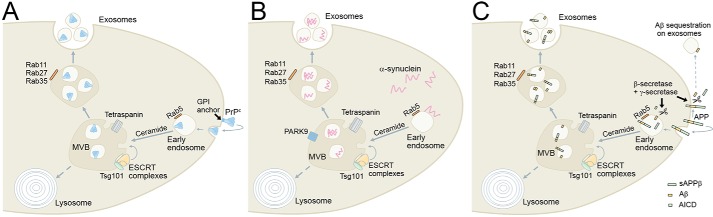
FIGURE 1.
Schematic depicting the pathways involved in the biogenesis of exosomes containing proteins associated with neurodegenerative diseases. A, the prion protein (PrPc) is found on the cell surface anchored by a glycosylphosphatidylinositol (GPI) linker to lipid rafts. PrPc can be internalized via early endosomes, and through a ceramide-dependent process (99), it can enter MVBs. MVB fusion with the cell surface results in the release of PrPc in exosomes. B, α-synuclein is a cytoplasmic protein that can be found on early endosomes. Through a process involving ceramide, tetraspanins, or endosomal sorting complexes required for transport (ESCRT) components, α-synuclein is directed into MVBs. Both PARK9 (51, 52) and Rab11 (48) have been shown to promote the exosomal release of α-synuclein. C, APP is a cell surface transmembrane protein that can be processed by a number of proteases. APP is found on early endosomes, where it can be cleaved by secretases (71) to form secreted APPβ (sAPPβ), Aβ, and APP intracellular domain (AICD). The AICD fragment can traffic to the nucleus, whereas Aβ can be trafficked into MVBs through a process involving ceramide, tetraspanins, or ESCRT components. Fusion of the MVB with the plasma membrane results in the subsequent release of exosomes containing Aβ from the cell. Exosome-independent pathways for Aβ release can result in extracellular Aβ that can be sequestered by exosomes, which can then be degraded by microglia (73).
The function of exosomes differs depending on the cell type from which they originate. Initial in vivo studies identified that exosomes derived from dendritic cells could express MHC class II molecules to promote an immune response (4). Since then, exosomes have been found to function in angiogenesis, inflammation, morphogen transportation, and programmed cell death (5). The richest area of exosome research, however, has come from disease studies, in particular the cancer field. Recent evidence has highlighted a role for exosomes to promote metastasis and regulate tumor immune response (6). Of particular interest is the ability of tumors to release exosomes that regulate distant cellular environments to initiate pre-metastatic niche formation (7–9). These findings highlight the potential for exosomes to spread disease pathways within the body (10).
The ability of exosomes to promote the spread of disease is also thought to play a role in neurodegenerative disorders. A common feature of these disorders is the deposition of misfolded, aggregated forms of specific proteins in defined neuroanatomical locations. As the diseases progress, these misfolded proteins spread along distinct pathways, suggesting that the pathological process may involve the movement of misfolded proteins from one site to another (11). Exosomes containing aggregation-prone proteins involved in Parkinson's disease (PD), Alzheimer's disease (AD), Creutzfeldt-Jakob disease (CJD), and amyotrophic lateral sclerosis (ALS) have all been found in the cerebral spinal fluid and blood of patients affected by these disorders. These and other findings have led to suggestions that neurodegenerative disorders may in fact be transmissible in the brain (12). Until recently, the prion protein (PrPSc) protein involved in CJD was the only known transmissible protein accountable for the spread of disease (13). However, recent evidence using both animal and cellular models has now shown that other neurodegenerative proteins may also be transmissible (14, 15). It should be made clear, however, that epidemiological data clearly show an extended time course for the generation of different disease states when compared with PrPSc related diseases. Given these new findings, it is important to understand the terminology used in describing protein aggregation and propagation involved in neurodegeneration.
Types of Aggregated Proteins in Neurodegenerative Diseases
A similar model of disease progression is thought to occur for the majority of neurodegenerative diseases. An abnormally folded disease-related protein self-associates into a β-sheet structure to form an ordered aggregate with the ability to propagate in a cell. However, the terminology used to define these structures and the ability to propagate not only within cells but also from cell to cell or between individuals is somewhat contentious. The term amyloid has been used widely for over 100 years to describe extracellular protein aggregates found in organs and tissue that are insoluble and resistant to degradation. The β-amyloid (Aβ) peptide that forms amyloids in Alzheimer's disease is perhaps the best known of these aggregates (16). A separate type of aggregate in neurodegeneration forms intracellularly, composed mainly of α-synuclein; they are termed Lewy bodies (17) and are known to be involved in a number of synucleinopathies such as PD and dementia with Lewy bodies (DLB). The term prion (proteinaceous infectious particle) was coined to describe an infectious protein aggregate capable of transmission between individuals (13). The PrPSc protein that is involved in CJD in humans, scrapie in sheep, and bovine spongiform encephalopathy in cows was identified to be the infectious agent in prion pathology. Based on this definition and recent discoveries in different neurodegenerative diseases about the transmission of pathology not involving PrP, further terms such as prionoid or prion-like (describing proteins capable of cell-to-cell propagation within individuals, but unable to infect other individuals) have been described (18). Although infectivity was thought to be the defining property of prions, a broader definition of prions has now also been suggested, describing prions as proteins that acquire alternative conformations and become self-propagating (19). This has caused considerable debate in the field (20); however, the semantics for the terminology used to describe aggregation-prone proteins and transmission in neurodegeneration may be unified in the future given recent findings describing the potential for iatrogenic transmission of Aβ pathology between humans (21). Here we review a number of neurodegenerative diseases with a focus on the role of exosomes in the transmission of protein aggregates.
Prion Diseases
Prions are the prototypical form of transmissible neurodegenerative disorders, and are thought to be composed principally of misfolded conformers of specific proteins that can induce further misfolding of these proteins in a catalytic mechanism. Prion diseases in humans include CJD, Gerstmann-Sträussler-Scheinker disease, and kuru; in animals, they manifest as scrapie in sheep and bovine spongiform encephalopathy in cattle. The “protein-only” hypothesis for the transmission of the disease was first speculated by Griffith (22) and was subsequently shown by Prusiner (13). It is now widely accepted that misfolding of the host-encoded prion protein, PrPC, into a disease-associated transmissible form, PrPSc, results in the transmission of pathology not only between cells but also from one organism to another. In humans, prion diseases manifest as rapidly progressing dementias with clinical signs of vision loss and cerebellar ataxia, and are currently untreatable.
Although the exact mechanism by which prion disease is transmitted intercellularly is yet to be identified, studies have shown that this may occur by cell-cell contact (23) or via tunneling nanotubes (24). The observation that prions can be detected in the lymphoreticular system of animals and humans with these diseases suggests that a mechanism exists whereby prion transmission can occur extracellularly without the need for cellular contact (25, 26). Over the last decade, a number of studies have highlighted a role for exosomes in the transmission of prions. PrPC and PrPSc isoforms of the prion protein were the first neurodegenerative proteins found to be secreted from the cell in association with exosomes (27). Exosomal PrPSc was found to transmit protein aggregation in rabbit kidney epithelial cells, whereas exosomes containing PrPC did not transmit aggregation. Subsequent in vivo experiments were able to show that exosomes derived from prion-infected mice were able to transmit aggregation to naive mice as well as cultured cells (28, 29). Importantly transmission between heterologous cell types has also been observed, indicating that exosomes may be involved in the transmission of disease from the periphery to the central nervous system, and this may explain the presence of PrPSc in the lymphoreticular system during the disease. A recent study has indicated that exosomal release is the main pathway for prion secretion from the cell (30); this is in contrast to other neurodegenerative proteins where exosomes are found to export only a small proportion of the total extracellular protein (31).
One aspect of prion biology that has become relevant for other proteins that misfold and have prion-like activity is the existence of strains. Prion strains are classically defined as distinct isolates that, when transmitted to a susceptible host, produce a unique clinical presentation, often measured in the incubation period, pattern of neuronal damage, and disease phenotype. In the absence of a nucleic acid genome, which could account for strain-specific differences, prion strains have been explained by distinct conformers of the PrPSc structure itself, which encodes these (32, 33). These have been identified using structural techniques and biochemical methods to identify unique patterns of proteolysis of PrPSc on Western blots. Recently, it has been demonstrated that both Aβ and α-synuclein can also exhibit distinct strain properties, which are maintained when oligomeric conformers of these proteins are transmitted to susceptible animals (34, 35). Whether the loading of oligomeric, neurotoxic forms of these proteins into exosomes is influenced by strain-specific conformers remains to be established.
Parkinson's Disease
Parkinson's disease is a neurodegenerative disorder with motor symptoms that affects ∼1% of the population over 65 years of age. It is characterized by histopathological lesions known as Lewy bodies (LBs) that predominantly contain the intrinsically disordered protein α-synuclein. Mutations, multiplications, and polymorphisms in the SNCA gene encoding α-synuclein are associated with familial forms of PD and susceptibility to idiopathic PD. Obvious motor symptoms are associated with the clinical phase of the disease combined with the loss of dopaminergic neurons of the substantia nigra; however, preceding this, there is a prodromal phase where patients can show a number of symptoms including hyposmia (loss of smell), disturbed sleep, and gastrointestinal dysfunction. These early symptoms, along with findings on the distribution pattern of α-synuclein aggregates, have led to the hypothesis that PD may start in either the enteric nervous system or olfactory bulbs before spreading to other regions of the brain during disease progression (11). How this transmission of PD occurs is currently not fully understood, but it has been suggested to occur through tunneling nanotubes, cell-cell contact, or exosomal transfer.
Extracellular Release of α-Synuclein
Although α-synuclein does not contain a sorting signal for extracellular release, soluble and aggregated α-synuclein has been detected in many body fluids, including brain interstitial fluid, plasma, and cerebral spinal fluid (CSF) (36, 37). In vitro experiments have identified extracellular α-synuclein secretion either through direct translocation across the plasma membrane or alternatively via a more specific mechanism in exosomes via the endosome pathway (38, 39). The first detection of exosomal α-synuclein indicated that the protein could be released from the cell in a calcium-dependent manner and could result in cell death in recipient cells (39). Subsequent studies have identified exosomal α-synuclein from multiple different cell types and also from patient-derived CSF and blood. The amount of exosomal α-synuclein found in PD patients when compared with controls has been shown to be variable, with some studies indicating an increase of exosomal α-synuclein in PD patients (40), whereas others have shown a decrease of exosomal α-synuclein in PD patients (31). Independent of this, the amount of exosomal α-synuclein has been found to vary within different synucleinopathies, with increased exosomal α-synuclein found in PD when compared with DLB (31). Together these findings suggest that exosomes may play a role in the transmission of α-synuclein in disease. However, it should be noted that the majority of extracellular α-synuclein is not contained in exosomes, with most α-synuclein found in CSF or conditioned medium being free and only a small fraction contained in exosomes (31, 40).
Exosomes Provide an Environment to Promote the Aggregation of α-Synuclein
If exosomes contain low levels of α-synuclein, are they really of any relevance to PD pathology? Recent evidence suggests that exosomes may provide a critical environment that promotes the aggregation of α-synuclein, potentially providing a platform for the propagation of PD pathology. Danzer et al. (41) showed the first evidence for oligomerized α-synuclein in exosomes using bioluminescent protein-fragment complementation assays. Importantly, this study identified that exosomal α-synuclein was delivered more efficiently to cells when compared with free α-synuclein, highlighting the importance of exosomal α-synuclein for transmission of oligomers between cells. Supporting these findings, exosomes from the CSF of PD patients was able to induce the oligomerization of α-synuclein when compared with control CSF (31). Further analysis of exosomal α-synuclein in CSF of both PD and DLB patients showed a linear correlation in the ability to induce the oligomerization of α-synuclein when compared with control CSF exosomes. Although the authors did not detect aggregates of α-synuclein in the exosomes from the patient CSF, the ability to promote the oligomerization of α-synuclein suggests that these species are present in exosomes from both PD and DLB patients.
To further understand the role of exosomes in the aggregation of α-synuclein, Gray et al. (42) investigated the aggregation kinetics of exosomal α-synuclein. Significantly, they found that exosomes could catalyze the aggregation of α-synuclein in a similar manner, as has been shown for preformed α-synuclein fibrils. Interestingly, phospholipids were found to inhibit α-synuclein aggregation; however, vesicles containing ganglioside lipids GM1 or GM3 were found to accelerate α-synuclein aggregation. These findings suggest that exosomes can provide an environment for the initial nucleation event giving rise to pathological states of α-synuclein. Assuming that exosome membranes may also provide a local concentration gradient of α-synuclein (as opposed to free α-synuclein in CSF, plasma, or interstitial fluid), as well as protection from degradation (38), indicates the potential of exosomes as a key environment for the transmission of PD pathology.
Mechanisms and Physiological Conditions for the Release of α-Synuclein in Exosomes
A number of pathways have been investigated for the release of α-synuclein in exosomes. A hallmark of PD is the failure of protein regulation by the ubiquitin proteasome system and the autophagy-lysosome pathway (ALP); both, when compromised, can result in intracellular protein aggregates. A failure of ALP has been found to be important for the release of extracellular α-synuclein. Lysosomal dysfunction by either pharmacological or genetic manipulation increases exosomal release of α-synuclein (43–45). In contrast, the induction of autophagy has been shown to inhibit the secretion of exosomes through the fusion of MVBs with autophagosomes (46). Interestingly, inhibition of ALP reduced intracellular α-synuclein aggregation but increased secretion of α-synuclein oligomers that can affect the surrounding environment, providing a mechanism for pathogenic transmission of α-synuclein (47). The GTPase Rab11, which is required for late endosomal vesicle formation, has been found to be important in this process. Overexpression of Rab11 has been shown to reduce aggregate formation in cells (48) while also increasing the release of exosomal α-synuclein (47).
Also in the lysosomal pathway is the gene PARK9, mutations in which cause Kufor-Rakeb syndrome, a juvenile-onset Parkinsonism (49). PARK9 has also been found to regulate extracellular α-synuclein (50). PARK9 deficiency causes lysosomal dysfunction and α-synuclein accumulation, whereas PARK9 overexpression suppresses cellular toxicity of α-synuclein (51, 52). Importantly, PARK9 is also involved in the biogenesis of exosomes and has been found to increase exosomal α-synuclein (51, 52). As such, PARK9 appears to function in a similar manner as Rab11 in regulating α-synuclein trafficking. However, both of these pathways appear to increase exosomal α-synuclein through an increase in the release of exosomes rather than specifically loading α-synuclein into exosomes. It is interesting to note that the increase in exosomal α-synuclein occurs concomitantly with a loss of protein aggregation in the cell, suggesting a survival mechanism that protects the cell from aggregates but can also result in the potential to propagate α-synuclein to the surrounding environment.
Are there specific pathways involved in the loading of α-synuclein into exosomes? Currently, no direct mechanism has been identified, although pathways involving sumoylation and environmental factors such as pesticides have been shown to increase exosomal α-synuclein (53, 54). It is expected that future studies will identify whether specific mechanisms are activated to promote exosomal α-synuclein release, greatly increasing our understanding of α-synuclein propagation with the potential for early intervention strategies in the disease process.
Exosomes in the Transmission of α-Synuclein Pathology
The concept of interneuronal transmission of α-synuclein originated from observations that α-synuclein pathology in the brain propagates from both the olfactory bulbs and also the brain stem in a caudal-rostral pattern toward the midbrain and neocortex (11, 55). Further support for the α-synuclein transmission hypothesis was provided in 2008 when two groups detected α-synuclein aggregates in transplanted embryonic neurons in Parkinson's disease patients' brains (56, 57). Although these studies did not equivocally prove that α-synuclein transmission had occurred between neurons, they provided substantial evidence. Subsequently, animal model studies have shown neuron-to-neuron transfer of α-synuclein in the mouse and primate brain (58, 59), internalization of exogenous α-synuclein fibrils, and induction of neuronal α-synuclein aggregation in vitro and in vivo (60–63). These experimental systems provided valuable proof of concept for the transmission of pathology, but the pathophysiological relevance of these data should be viewed with caution given the concentrations of aggregates used and the animal systems being investigated (predominately transgenic animals overexpressing α-synuclein).
Evidence for transmission of exosomal α-synuclein has mainly been limited to in vitro studies, although it is not clear whether studies using brain extracts that resulted in the propagation of α-synuclein pathology also contained exosomal α-synuclein (64). Recently, exosomes derived from the CSF of PD patients have been shown to transmit α-synuclein aggregation using a reporter cell line (31), but as yet, no direct proof for in vivo exosomal transmission of α-synuclein has been observed.
Alzheimer's Disease
AD is a late-onset neurological disorder causing progressive loss of memory and cognitive abilities as a result of excessive neurodegeneration. The exact etiology of Alzheimer's disease still remains a topic of debate; however, it is clear that the accumulation of Aβ peptides in plaques combined with neurofibrillary tangles of tau are important for the progression of the disease (65). Aβ peptides are derived from the proteolytic processing of the amyloid precursor protein (APP). This processing event can occur in multiple locations in the cell, with the importance of APP trafficking and processing highlighted by loci for late-onset AD mapping to processes regulating endosomal vesicle recycling (66–69). Significantly, the endosomal pathway is also critical for the formation of exosomes.
Extracellular Release of APP and Its Metabolites
Over 25 years ago, extracellular APP was identified and thought important for the generation of plaques in the brains of AD patients (70). This result was somewhat surprising given that APP is an intracellularly generated protein containing a transmembrane domain. The first identification of AD-linked proteins and peptides in exosomes was discovered while investigating the location of APP cleavage events. Rajendran et al. (71) determined that β-secretase cleaved APP on early endosomes, which subsequently resulted in the trafficking of Aβ to MVBs. A small fraction of the Aβ peptide was found to be sorted into intraluminal vesicles in MVBs, resulting in the export of Aβ in exosomes. The authors went on to show that exosomal proteins could be found to accumulate in the plaques of AD patient brains, suggesting a role for exosomes in the spread of pathogenesis in AD.
Since this discovery, the full-length APP protein and many of its metabolites have also been found in exosomes, both from in vitro cell culture studies and also from the CSF and blood of PD patients. APP and the C-terminal fragments of APP (CTFs-APP) have been consistently identified in exosomes, and importantly, many of the proteases involved in APP processing have also been found in exosomes (72). This suggests that proteolytic processing of APP may occur in situ in the exosome microenvironment; however, the function of these products in exosomes remains open to debate. A number of laboratories have determined that exosomal release of APP and its metabolites provides a protective role in neurodegeneration. Of note, it was found that neuron-derived exosomes can facilitate rapid conformational change of Aβ into nontoxic amyloid fibrils, which can subsequently be internalized by microglia for degradation (73). Exosomes from N2a neuroblastoma and BV-2 microglial cells have also been reported to promote degradation of Aβ through proteolysis via exosome-associated insulin-degrading enzyme (74, 75).
Although exosomes present a potential mechanism for the clearance of Aβ from the cell, it is clear that this mechanism can also pose a risk to surrounding cells, with the potential for exosomes to increase the aggregation potential of Aβ peptides. Similar to findings in PD with α-synuclein, studies have highlighted the role of ganglioside lipids (GMs) found in exosomes in the formation of Aβ aggregates, resulting in the trafficking of potentially pathogenic forms of the peptide. In particular, blocking GM1 formation can abrogate the aggregation of Aβ in exosomes (73, 76).
Mechanisms for the Release of APP and Its Metabolites
Currently, there is no specific mechanism known to be involved in the extracellular release of APP or its metabolites. Indeed, it is still unclear where exosomes containing Aβ are derived, with evidence for exosomal release of Aβ from both neurons as well as reactive microglia (73, 75). A consensus toward dysfunction in autophagy pathways has recently been established as a critical pathway in Aβ pathology. Autophagosomes have been found to contain proteases required for APP cleavage and more recently to be involved in the release of extracellular Aβ (77, 78). However, this is not a direct mechanism to promote the release of Aβ in exosomes, but rather a result of pathway failures in the cell. It remains to be determined whether there are specific mechanisms for the release of APP or any of the proteolytically cleaved products of APP.
Exosomes and the Transmission of AD Pathology
The role of exosomes in the transmission of AD pathology remains controversial; however, an association between exosomes and amyloid plaque formation in vivo has been reported. Exosomes were found to stimulate the aggregation of Aβ(1–42) by isolating exosomes from brain tissue of the 5×FAD mouse model of AD (79). Inhibition of exosome formation in this model using GW4869, an inhibitor of neutral sphingomyelinase, resulted in the reduction of amyloid plaques in the brain. Although these data provide evidence for exosomal transmission of Aβ in vivo, it should be noted that the 5×FAD mouse model better represents familial AD with the highly rapid production and accumulation of Aβ(1–42) (due to the additive effects of mutations in both APP and presenilin 1 (PS1)) in contrast to that observed in sporadic AD.
Tau
Separate from amyloid formation in AD, neurofibrillary tangles (NFTs), which are composed of abnormally phosphorylated tau protein, are correlated with progressive cognitive dysfunction and neuronal loss in AD. Importantly, tau and Aβ have been shown to function synergistically in AD pathology, with Aβ having been shown to promote NFTs (80), and tau having been found to promote Aβ toxicity at the synapse (81). A growing body of evidence now suggests that pathological tau protein can spread between cells, resulting in the recruitment of native tau into aggregates important in the development of AD.
Extracellular Release of Tau
Tau is a cytoplasmic protein known to function in the stabilization of microtubules. Extracellular tau was first discovered in CSF using an ELISA-based approach, revealing an increased level of the protein in AD patients when compared with controls (82). Subsequent inoculation of brain extracts containing aggregated tau or injection of preformed fibrillary tau protein into tau transgenic mice can cause AD-like NFT pathology in the brain (83–86). Unlike Aβ models of disease, tau pathology can be seeded by exogenous tau aggregates in non-transgenic (wild-type) mice (87). It should also be noted that tau pathology in animal models is also observed over a shorter time scale when compared with models for the transmission of Aβ or α-synuclein, suggesting that tau aggregation in vivo is kinetically favorable.
Nearly 20 years after the discovery of extracellular tau, the protein was found to be trafficked in exosomes (88). However, this finding was not supported by others who argued that extracellular tau was not found in exosomes (89). Other mechanisms involving tunneling nanotubes have also been suggested to be involved in the transmission of tau between cells (90). Recent discoveries, however, have now clearly implicated exosomes in tau pathology. Significantly, exosomal tau from microglia has been identified in the propagation of tau pathology in the brain (91). Both the depletion of microglia and the inhibition of exosome biogenesis using the small molecule GW4869 were shown to limit the propagation of tau in the brain. The authors suggest that microglia can phagocytose tau-containing cytopathic neurons and subsequently release tau in exosomes to transmit pathology; however, it is also possible that exosomal tau released from neurons could also be internalized by microglia before further exosomal transmission. This surprising method for the transmission of disease pathology through sequential cell processing of exosomes has also been described in cancer cell transmission (92).
Further evidence for a function of exosomes in tau propagation was recently discovered in a study in which exosomes from tau transgenic mice were found to propagate tau aggregation in a threshold-dependent manner (93). Interestingly, although tau was found to be phosphorylated in exosomes, it was not thought important for pathology at the known sites (AT8, AT100, and AT180). Taken together, there is now substantial evidence for the extracellular transport of tau in exosomes that can propagate tau aggregation in the brain.
ALS and Huntington's Disease
ALS is a neurodegenerative disorder of the motor neurons in the brain, brainstem, and spinal cord. Although the majority of ALS cases arise sporadically, mutations in a number of genes are associated with familial forms of disease. Superoxide dismutase 1 (SOD1) and TDP-43 are two such proteins mutated in these inherited forms of ALS, and both of these have exhibited template-directed induction of pathological misfolding of these proteins. Furthermore, both of these proteins have been found associated with exosomes, suggesting a potential role of EVs in the intercellular transfer of misfolded SOD1 and TDP43 (94, 95). It has also been demonstrated that huntingtin, the pathogenic protein that misfolds in Huntington's disease, can also exhibit prion-like mechanisms of misfolding. In a Drosophila model, it was shown that glia can phagocytose huntingtin aggregates, which can then induce the misfolding of monomeric forms of this protein (96).
Conclusion
After 30 years of research, it is now clear that exosomes provide a physiological platform for the transmission of information between cells. In the brain, exosomes can propagate the proliferation of misfolded proteins, a concept that evolved in the prion field, which now can potentially explain the pathogenesis of many neurodegenerative diseases. Given the extended time course of many neurodegenerative diseases, some of which take decades to develop, it is not surprising that prion diseases, which show a rapid onset of pathology, have provided researchers with a model to study the aggregation of transmissible proteins. It remains to be seen, however, whether current research, which uses transgenic animal models and large amounts of inoculated protein aggregates to speed up disease processes, accurately replicates the mechanisms observed in human disease. To this end, the role of exosomes outside of prion disease transmission has still not been proven conclusively in vivo for either PD or AD.
Although there appear to be a number of pathways that increase the transmission of protein pathology in exosomes, none provide a direct mechanism for the loading of aggregation-prone proteins into exosomes. Future research should identify whether there is an active transport pathway for these proteins, or whether they are simply a byproduct of disrupted cellular pathways in the aging brain. It is conceivable that there is a tipping point in the MVB pathway in which, under normal homeostasis, intraluminal vesicles are directed down the lysosomal pathway for the destruction of unwanted proteins; however, in disease states, this pathway is impaired, leading to a change in balance with a resulting increase in the flux of intraluminal vesicles through the exosomal pathway. This switch would maintain the removal of unwanted protein products from the cell, but could also result in the propagation of aggregation and the transmission of disease through exosome sequestration in recipient cells.
It should also be noted that exosome-independent pathways for the release of neurodegenerative proteins have also been reported. These include the non-vesicular release of Aβ and α-synuclein. Tunneling nanotubes, thin membranous channels formed between cells, have been shown to transfer tau, mutant huntingtin, PrPSc, and α-synuclein between cells (24, 90, 97, 98). These forms of protein transfer may also play a role in the neurodegenerative disease process.
An important aspect of the role of exosomes in neurodegenerative disease is the environment they can provide for the aggregation of proteins; in particular, it appears that the ganglioside content in exosomes potentiates the propensity for proteins to form β-sheet structures that can self-associate to form an initial nucleation site for aggregation. Several outstanding questions arise from the role that exosomes play in the pathogenesis of neurodegenerative diseases (Table 1). Identification of how neurodegenerative proteins are packaged into exosomes, the role of lipids in misfolding, and how exosomes are taken up by recipient cells will provide insights into how these crippling diseases may be therapeutically targeted in the future.
TABLE 1.
Outstanding questions in the field
|
The authors declare that they have no conflicts of interest with the contents of this article.
- EV
- extracellular vesicle
- MVB
- multivesicular body
- PD
- Parkinson's disease
- AD
- Alzheimer's disease
- FAD
- familial AD
- CJD
- Creutzfeldt-Jakob disease
- ALS
- amyotrophic lateral sclerosis
- DLB
- dementia with Lewy bodies
- PrP
- prion protein
- Aβ
- β-amyloid
- APP
- amyloid precursor protein
- ALP
- autophagy-lysosome pathway
- CSF
- cerebral spinal fluid
- NFT
- neurofibrillary tangle.
References
- 1. Pan B. T., and Johnstone R. M. (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978 [DOI] [PubMed] [Google Scholar]
- 2. Harding C., Heuser J., and Stahl P. (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raposo G., and Stoorvogel W. (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., and Amigorena S. (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4, 594–600 [DOI] [PubMed] [Google Scholar]
- 5. Colombo M., Raposo G., and Théry C. (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 [DOI] [PubMed] [Google Scholar]
- 6. Lo Cicero A., Stahl P. D., and Raposo G. (2015) Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr. Opin. Cell Biol. 35, 69–77 [DOI] [PubMed] [Google Scholar]
- 7. Hood J. L., San R. S., and Wickline S. A. (2011) Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801 [DOI] [PubMed] [Google Scholar]
- 8. Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C., Nitadori-Hoshino A., Hoffman C., Badal K., Garcia B. A., Callahan M. K., et al. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costa-Silva B., Aiello N. M., Ocean A. J., Singh S., Zhang H., Thakur B. K., Becker A., Hoshino A., Mark M. T., Molina H., Xiang J., Zhang T., Theilen T. M., García-Santos G., Williams C., et al. (2015) Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoshino A., Costa-Silva B., Shen T. L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., Singh S., Williams C., Soplop N., Uryu K., et al. (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braak H., Rüb U., Gai W. P., and Del Tredici K. (2003) Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. (Vienna) 110, 517–536 [DOI] [PubMed] [Google Scholar]
- 12. Brettschneider J., Del Tredici K., Lee V. M., and Trojanowski J. Q. (2015) Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat. Rev. Neurosci. 16, 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prusiner S. B. (1982) Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 [DOI] [PubMed] [Google Scholar]
- 14. Prusiner S. B., Woerman A. L., Mordes D. A., Watts J. C., Rampersaud R., Berry D. B., Patel S., Oehler A., Lowe J. K., Kravitz S. N., Geschwind D. H., Glidden D. V., Halliday G. M., Middleton L. T., Gentleman S. M., Grinberg L. T., and Giles K. (2015) Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. U.S.A. 112, E5308–E5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aguzzi A., and Rajendran L. (2009) The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 64, 783–790 [DOI] [PubMed] [Google Scholar]
- 16. Divry P. (1927) Histochemical study of senile plaques. J. Belge Neurol. Psychiatr. 27, 643–657 [Google Scholar]
- 17. Lewy F. H. (1912) Paralysis Agitans. I. Pathological Anatomy. In Lewandowsky's Manual of Neurology, III (Lewandowsky M., ed), pp. 920–933, Springer, Berlin [Google Scholar]
- 18. Aguzzi A. (2009) Cell biology: Beyond the prion principle. Nature 459, 924–925 [DOI] [PubMed] [Google Scholar]
- 19. Prusiner S. B. (2013) Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 47, 601–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Angot E., Steiner J. A., Hansen C., Li J. Y., and Brundin P. (2010) Are synucleinopathies prion-like disorders? Lancet Neurol. 9, 1128–1138 [DOI] [PubMed] [Google Scholar]
- 21. Jaunmuktane Z., Mead S., Ellis M., Wadsworth J. D., Nicoll A. J., Kenny J., Launchbury F., Linehan J., Richard-Loendt A., Walker A. S., Rudge P., Collinge J., and Brandner S. (2015) Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 525, 247–250 [DOI] [PubMed] [Google Scholar]
- 22. Griffith J. S. (1967) Self-replication and scrapie. Nature 215, 1043–1044 [DOI] [PubMed] [Google Scholar]
- 23. Kanu N., Imokawa Y., Drechsel D. N., Williamson R. A., Birkett C. R., Bostock C. J., and Brockes J. P. (2002) Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 12, 523–530 [DOI] [PubMed] [Google Scholar]
- 24. Gousset K., Schiff E., Langevin C., Marijanovic Z., Caputo A., Browman D. T., Chenouard N., de Chaumont F., Martino A., Enninga J., Olivo-Marin J. C., Männel D., and Zurzolo C. (2009) Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 11, 328–336 [DOI] [PubMed] [Google Scholar]
- 25. Kitamoto T., Mohri S., and Tateishi J. (1989) Organ distribution of proteinase-resistant prion protein in humans and mice with Creutzfeldt-Jakob disease. J. Gen. Virol. 70, 3371–3379 [DOI] [PubMed] [Google Scholar]
- 26. Hill A. F., Butterworth R. J., Joiner S., Jackson G., Rossor M. N., Thomas D. J., Frosh A., Tolley N., Bell J. E., Spencer M., King A., Al-Sarraj S., Ironside J. W., Lantos P. L., and Collinge J. (1999) Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 353, 183–189 [DOI] [PubMed] [Google Scholar]
- 27. Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., and Raposo G. (2004) Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 101, 9683–9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vella L. J., Sharples R. A., Lawson V. A., Masters C. L., Cappai R., and Hill A. F. (2007) Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J. Pathol. 211, 582–590 [DOI] [PubMed] [Google Scholar]
- 29. Coleman B. M., Hanssen E., Lawson V. A., and Hill A. F. (2012) Prion-infected cells regulate the release of exosomes with distinct ultrastructural features. FASEB J. 26, 4160–4173 [DOI] [PubMed] [Google Scholar]
- 30. Arellano-Anaya Z. E., Huor A., Leblanc P., Lehmann S., Provansal M., Raposo G., Andréoletti O., and Vilette D. (2015) Prion strains are differentially released through the exosomal pathway. Cell. Mol. Life Sci. 72, 1185–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stuendl A., Kunadt M., Kruse N., Bartels C., Moebius W., Danzer K. M., Mollenhauer B., and Schneider A. (2016) Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson's disease and dementia with Lewy bodies. Brain 139, 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weissmann C. (1991) A 'unified theory' of prion propagation. Nature 352, 679–683 [DOI] [PubMed] [Google Scholar]
- 33. Collinge J., Sidle K. C., Meads J., Ironside J., and Hill A. F. (1996) Molecular analysis of prion strain variation and the aetiology of 'new variant' CJD. Nature 383, 685–690 [DOI] [PubMed] [Google Scholar]
- 34. Petkova A. T., Leapman R. D., Guo Z., Yau W. M., Mattson M. P., and Tycko R. (2005) Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science 307, 262–265 [DOI] [PubMed] [Google Scholar]
- 35. Guo J. L., Covell D. J., Daniels J. P., Iba M., Stieber A., Zhang B., Riddle D. M., Kwong L. K., Xu Y., Trojanowski J. Q., and Lee V. M. (2013) Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154, 103–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El-Agnaf O. M., Salem S. A., Paleologou K. E., Cooper L. J., Fullwood N. J., Gibson M. J., Curran M. D., Court J. A., Mann D. M., Ikeda S., Cookson M. R., Hardy J., and Allsop D. (2003) α-Synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 17, 1945–1947 [DOI] [PubMed] [Google Scholar]
- 37. Emmanouilidou E., Elenis D., Papasilekas T., Stranjalis G., Gerozissis K., Ioannou P. C., and Vekrellis K. (2011) Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS One 6, e22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee H. J., Patel S., and Lee S. J. (2005) Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci. 25, 6016–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Emmanouilidou E., Melachroinou K., Roumeliotis T., Garbis S. D., Ntzouni M., Margaritis L. H., Stefanis L., and Vekrellis K. (2010) Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi M., Liu C., Cook T. J., Bullock K. M., Zhao Y., Ginghina C., Li Y., Aro P., Dator R., He C., Hipp M. J., Zabetian C. P., Peskind E. R., Hu S. C., Quinn J. F., Galasko D. R., Banks W. A., and Zhang J. (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol. 128, 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Danzer K. M., Kranich L. R., Ruf W. P., Cagsal-Getkin O., Winslow A. R., Zhu L., Vanderburg C. R., and McLean P. J. (2012) Exosomal cell-to-cell transmission of α synuclein oligomers. Mol. Neurodegener. 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grey M., Dunning C. J., Gaspar R., Grey C., Brundin P., Sparr E., and Linse S. (2015) Acceleration of α-synuclein aggregation by exosomes. J. Biol. Chem. 290, 2969–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee H. J., Cho E. D., Lee K. W., Kim J. H., Cho S. G., and Lee S. J. (2013) Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 45, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hasegawa T., Konno M., Baba T., Sugeno N., Kikuchi A., Kobayashi M., Miura E., Tanaka N., Tamai K., Furukawa K., Arai H., Mori F., Wakabayashi K., Aoki M., Itoyama Y., and Takeda A. (2011) The AAA-ATPase VPS4 regulates extracellular secretion and lysosomal targeting of α-synuclein. PLoS One 6, e29460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alvarez-Erviti L., Seow Y., Schapira A. H., Gardiner C., Sargent I. L., Wood M. J., and Cooper J. M. (2011) Lysosomal dysfunction increases exosome-mediated α-synuclein release and transmission. Neurobiol. Dis. 42, 360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fader C. M., Sánchez D., Furlán M., and Colombo M. I. (2008) Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in K562 cells. Traffic 9, 230–250 [DOI] [PubMed] [Google Scholar]
- 47. Poehler A. M., Xiang W., Spitzer P., May V. E., Meixner H., Rockenstein E., Chutna O., Outeiro T. F., Winkler J., Masliah E., and Klucken J. (2014) Autophagy modulates SNCA/α-synuclein release, thereby generating a hostile microenvironment. Autophagy 10, 2171–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Breda C., Nugent M. L., Estranero J. G., Kyriacou C. P., Outeiro T. F., Steinert J. R., and Giorgini F. (2015) Rab11 modulates α-synuclein-mediated defects in synaptic transmission and behaviour. Hum. Mol. Genet. 24, 1077–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramirez A., Heimbach A., Gründemann J., Stiller B., Hampshire D., Cid L. P., Goebel I., Mubaidin A. F., Wriekat A. L., Roeper J., Al-Din A., Hillmer A. M., Karsak M., Liss B., Woods C. G., Behrens M. I., and Kubisch C. (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 38, 1184–1191 [DOI] [PubMed] [Google Scholar]
- 50. Gitler A. D., Chesi A., Geddie M. L., Strathearn K. E., Hamamichi S., Hill K. J., Caldwell K. A., Caldwell G. A., Cooper A. A., Rochet J. C., and Lindquist S. (2009) α-Synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet. 41, 308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsunemi T., Hamada K., and Krainc D. (2014) ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. J. Neurosci. 34, 15281–15287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kong S. M., Chan B. K., Park J. S., Hill K. J., Aitken J. B., Cottle L., Farghaian H., Cole A. R., Lay P. A., Sue C. M., and Cooper A. A. (2014) Parkinson's disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum. Mol. Genet. 23, 2816–2833 [DOI] [PubMed] [Google Scholar]
- 53. Kunadt M., Eckermann K., Stuendl A., Gong J., Russo B., Strauss K., Rai S., Kügler S., Falomir Lockhart L., Schwalbe M., Krumova P., Oliveira L. M., Bähr M., Möbius W., Levin J., et al. (2015) Extracellular vesicle sorting of α-Synuclein is regulated by sumoylation. Acta Neuropathol. 129, 695–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pan-Montojo F., Schwarz M., Winkler C., Arnhold M., O'Sullivan G. A., Pal A., Said J., Marsico G., Verbavatz J. M., Rodrigo-Angulo M., Gille G., Funk R. H., and Reichmann H. (2012) Environmental toxins trigger PD-like progression via increased α-synuclein release from enteric neurons in mice. Sci. Rep. 2, 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Del Tredici K., and Braak H. (2016) Review: Sporadic Parkinson's disease: development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 42, 33–50 [DOI] [PubMed] [Google Scholar]
- 56. Li J. Y., Englund E., Holton J. L., Soulet D., Hagell P., Lees A. J., Lashley T., Quinn N. P., Rehncrona S., Björklund A., Widner H., Revesz T., Lindvall O., and Brundin P. (2008) Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 [DOI] [PubMed] [Google Scholar]
- 57. Kordower J. H., Chu Y., Hauser R. A., Freeman T. B., and Olanow C. W. (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 14, 504–506 [DOI] [PubMed] [Google Scholar]
- 58. Desplats P., Lee H. J., Bae E. J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., and Lee S. J. (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl. Acad. Sci. U.S.A. 106, 13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Recasens A., Dehay B., Bové J., Carballo-Carbajal I., Dovero S., Pérez-Villalba A., Fernagut P. O., Blesa J., Parent A., Perier C., Fariñas I., Obeso J. A., Bezard E., and Vila M. (2014) Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 75, 351–362 [DOI] [PubMed] [Google Scholar]
- 60. Luk K. C., Song C., O'Brien P., Stieber A., Branch J. R., Brunden K. R., Trojanowski J. Q., and Lee V. M. (2009) Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 106, 20051–20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Luk K. C., Kehm V., Carroll J., Zhang B., O'Brien P., Trojanowski J. Q., and Lee V. M. (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sacino A. N., Brooks M., McKinney A. B., Thomas M. A., Shaw G., Golde T. E., and Giasson B. I. (2014) Brain injection of α-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J. Neurosci. 34, 12368–12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sacino A. N., Brooks M., McGarvey N. H., McKinney A. B., Thomas M. A., Levites Y., Ran Y., Golde T. E., and Giasson B. I. (2013) Induction of CNS α-synuclein pathology by fibrillar and non-amyloidogenic recombinant α-synuclein. Acta Neuropathol. Commun. 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Luk K. C., Kehm V. M., Zhang B., O'Brien P., Trojanowski J. Q., and Lee V. M. (2012) Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209, 975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Selkoe D. J., and Hardy J. (2016) The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 8, 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jones L., Holmans P. A., Hamshere M. L., Harold D., Moskvina V., Ivanov D., Pocklington A., Abraham R., Hollingworth P., Sims R., Gerrish A., Pahwa J. S., Jones N., Stretton A., Morgan A. R., et al. (2010) Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer's disease. PLoS One 5, e13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rogaeva E., Meng Y., Lee J. H., Gu Y., Kawarai T., Zou F., Katayama T., Baldwin C. T., Cheng R., Hasegawa H., Chen F., Shibata N., Lunetta K. L., Pardossi-Piquard R., Bohm C., et al. (2007) The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 39, 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lambert J. C., Ibrahim-Verbaas C. A., Harold D., Naj A. C., Sims R., Bellenguez C., DeStafano A. L., Bis J. C., Beecham G. W., Grenier-Boley B., Russo G., Thorton-Wells T. A., Jones N., Smith A. V., Chouraki V., et al. (2013) Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat. Genet. 45, 1452–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhao Z., Sagare A. P., Ma Q., Halliday M. R., Kong P., Kisler K., Winkler E. A., Ramanathan A., Kanekiyo T., Bu G., Owens N. C., Rege S. V., Si G., Ahuja A., Zhu D., et al. (2015) Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 18, 978–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schubert D., LaCorbiere M., Saitoh T., and Cole G. (1989) Characterization of an amyloid β precursor protein that binds heparin and contains tyrosine sulfate. Proc. Natl. Acad. Sci. U.S.A. 86, 2066–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rajendran L., Honsho M., Zahn T. R., Keller P., Geiger K. D., Verkade P., and Simons K. (2006) Alzheimer's disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 103, 11172–11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sharples R. A., Vella L. J., Nisbet R. M., Naylor R., Perez K., Barnham K. J., Masters C. L., and Hill A. F. (2008) Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 22, 1469–1478 [DOI] [PubMed] [Google Scholar]
- 73. Yuyama K., Sun H., Mitsutake S., and Igarashi Y. (2012) Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 287, 10977–10989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bulloj A., Leal M. C., Xu H., Castaño E. M., and Morelli L. (2010) Insulin-degrading enzyme sorting in exosomes: a secretory pathway for a key brain amyloid-β degrading protease. J. Alzheimers Dis. 19, 79–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tamboli I. Y., Barth E., Christian L., Siepmann M., Kumar S., Singh S., Tolksdorf K., Heneka M. T., Lütjohann D., Wunderlich P., and Walter J. (2010) Statins promote the degradation of extracellular amyloid β-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J. Biol. Chem. 285, 37405–37414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yuyama K., Yamamoto N., and Yanagisawa K. (2008) Accelerated release of exosome-associated GM1 ganglioside (GM1) by endocytic pathway abnormality: another putative pathway for GM1-induced amyloid fibril formation. J. Neurochem. 105, 217–224 [DOI] [PubMed] [Google Scholar]
- 77. Agholme L., Hallbeck M., Benedikz E., Marcusson J., and Kågedal K. (2012) Amyloid-β secretion, generation, and lysosomal sequestration in response to proteasome inhibition: involvement of autophagy. J. Alzheimers Dis. 31, 343–358 [DOI] [PubMed] [Google Scholar]
- 78. Son S. M., Song H., Byun J., Park K. S., Jang H. C., Park Y. J., and Mook-Jung I. (2012) Altered APP processing in insulin-resistant conditions is mediated by autophagosome accumulation via the inhibition of mammalian target of rapamycin pathway. Diabetes 61, 3126–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dinkins M. B., Dasgupta S., Wang G., Zhu G., and Bieberich E. (2014) Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer's disease. Neurobiol. Aging 35, 1792–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Götz J., Chen F., van Dorpe J., and Nitsch R. M. (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Aβ42 fibrils. Science 293, 1491–1495 [DOI] [PubMed] [Google Scholar]
- 81. Ittner L. M., Ke Y. D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B. C., Christie M. J., Napier I. A., Eckert A., Staufenbiel M., Hardeman E., and Götz J. (2010) Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 [DOI] [PubMed] [Google Scholar]
- 82. Vandermeeren M., Mercken M., Vanmechelen E., Six J., van de Voorde A., Martin J. J., and Cras P. (1993) Detection of tau proteins in normal and Alzheimer's disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J. Neurochem. 61, 1828–1834 [DOI] [PubMed] [Google Scholar]
- 83. Clavaguera F., Bolmont T., Crowther R. A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A. K., Beibel M., Staufenbiel M., Jucker M., Goedert M., and Tolnay M. (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Iba M., Guo J. L., McBride J. D., Zhang B., Trojanowski J. Q., and Lee V. M. (2013) Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's-like tauopathy. J. Neurosci. 33, 1024–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ahmed Z., Cooper J., Murray T. K., Garn K., McNaughton E., Clarke H., Parhizkar S., Ward M. A., Cavallini A., Jackson S., Bose S., Clavaguera F., Tolnay M., Lavenir I., Goedert M., Hutton M. L., and O'Neill M. J. (2014) A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 127, 667–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boluda S., Iba M., Zhang B., Raible K. M., Lee V. M., and Trojanowski J. Q. (2015) Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer's disease or corticobasal degeneration brains. Acta Neuropathol. 129, 221–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lasagna-Reeves C. A., Castillo-Carranza D. L., Sengupta U., Guerrero-Munoz M. J., Kiritoshi T., Neugebauer V., Jackson G. R., and Kayed R. (2012) Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2, 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S., Jackson B., McKee A. C., Alvarez V. E., Lee N. C., and Hall G. F. (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287, 3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chai X., Dage J. L., and Citron M. (2012) Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol. Dis. 48, 356–366 [DOI] [PubMed] [Google Scholar]
- 90. Tardivel M., Bégard S., Bousset L., Dujardin S., Coens A., Melki R., Buée L., and Colin M. (2016) Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 4, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Asai H., Ikezu S., Tsunoda S., Medalla M., Luebke J., Haydar T., Wolozin B., Butovsky O., Kügler S., and Ikezu T. (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18, 1584–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zomer A., Maynard C., Verweij F. J., Kamermans A., Schäfer R., Beerling E., Schiffelers R. M., de Wit E., Berenguer J., Ellenbroek S. I., Wurdinger T., Pegtel D. M., and van Rheenen J. (2015) In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Polanco J. C., Scicluna B. J., Hill A. F., and Götz J. (2016) Extracellular vesicles isolated from the brains of rTg4510 mice seed Tau protein aggregation in a threshold-dependent manner. J. Biol. Chem. 291, 12445–12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Grad L. I., Pokrishevsky E., Silverman J. M., and Cashman N. R. (2014) Exosome-dependent and independent mechanisms are involved in prion-like transmission of propagated Cu/Zn superoxide dismutase misfolding. Prion 8, 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nonaka T., Masuda-Suzukake M., Arai T., Hasegawa Y., Akatsu H., Obi T., Yoshida M., Murayama S., Mann D. M., Akiyama H., and Hasegawa M. (2013) Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 4, 124–134 [DOI] [PubMed] [Google Scholar]
- 96. Pearce M. M., Spartz E. J., Hong W., Luo L., and Kopito R. R. (2015) Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat. Commun. 6, 6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Costanzo M., Abounit S., Marzo L., Danckaert A., Chamoun Z., Roux P., and Zurzolo C. (2013) Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J. Cell Sci. 126, 3678–3685 [DOI] [PubMed] [Google Scholar]
- 98. Abounit S., Bousset L., Loria F., Zhu S., de Chaumont F., Pieri L., Olivo-Marin J. C., Melki R., and Zurzolo C. (2016) Tunneling nanotubes spread fibrillar α-synuclein by intercellular trafficking of lysosomes. EMBO J. 35, 2120–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Guo B. B., Bellingham S. A., and Hill A. F. (2015) The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J. Biol. Chem. 290, 3455–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]