Abstract
Small molecule inhibitors for bromodomain and extra-terminal (BET) proteins have recently emerged as potential therapeutic agents in clinical trials for various cancers. However, to date, it is unknown whether these inhibitors have side effects on bone structures. Here, we report that inhibition of BET bromodomain proteins may suppress chondrocyte differentiation and restrain bone growth. We generated a luciferase reporter system using the chondrogenic cell line ATDC5 in which the luciferase gene was driven by the promoter of Col2a1, an elementary collagen of the chondrocyte. The Col2a1-luciferase ATDC5 system was used for rapidly screening both activators and repressors of human collagen Col2a1 gene expression, and we found that BET bromodomain inhibitors reduce the Col2a1-luciferase. Consistent with the luciferase assay, BET inhibitors decrease the expression of Col2a1. Furthermore, we constructed a zebrafish line in which the enhanced green fluorescent protein (EGFP) expression was driven by col2a1 promoter. The transgenic (col2a1-EGFP) zebrafish line demonstrated that BET inhibitors I-BET151 and (+)-JQ1 may affect EGFP expression in zebrafish. Furthermore, we found that I-BET151 and (+)-JQ1 may affect chondrocyte differentiation in vitro and inhibit zebrafish growth in vivo. Mechanistic analysis revealed that BET inhibitors influenced the depletion of RNA polymerase II from the Col2a1 promoter. Collectively, these results suggest that BET bromodomain inhibition may have side effects on skeletal bone structures.
Keywords: anticancer drug, bone, cartilage, chondrocyte, epigenetics, BET inhibitors, Col2a1
Introduction
Bromodomain and extra-terminal (BET)2 proteins (containing four mammalian members, BRD2, BRD3, BRD4, and BRDT) function as key epigenetic readers by recognizing histone acetylation through binding to ϵ-N-lysine acetylation motifs of histone, such as Lys-5, Lys-12, and Lys-16 residues of H4 histone (1, 2) and Lys-27 residues of H3 histone (3). The BET proteins interact with the positive transcription elongation factor P-TEFβ and RNA polymerase II (Pol II) to facilitate gene transcription (4–6). A list of drugs, such as I-BET151, I-BET762, PFI-1, and (+)-JQ1, were discovered to target BET proteins and block the interaction between BET proteins and acetyl-lysine of histones (2, 7).
BET proteins and BET inhibitors have been reported to be involved in a variety of biological processes. Pharmacological inhibition of BET proteins lowers the expression of key transcription factors such as oncogene c-MYC (8), clamps the transductions of PI3K signaling (9), inhibits Gli1 transcription and Hedgehog pathway (10), blocks transcription in neurons (11), represses VEGF-induced angiogenesis and vascular permeability (12), reduces cell viability of osteosarcoma cells and inhibits osteoblastic differentiation (13), and restrains osteoclastogenesis (14). Meanwhile, potent BET inhibitors have been identified as showing antitumor efficacy in a number of preclinical cancer models in recent years, including leukemia, multiple myeloma, lymphoma, melanoma, and gastric cancer (15, 16). This led to clinical studies focusing mostly on the treatment of leukemia and lymphoma. As a result of these studies, the first encouraging signs of efficacy have already been reported (17). Furthermore, previous studies showed that BET inhibitors also control neuronal differentiation and cause an autism-like syndrome (18). However, BET inhibitors were not examined extensively in these studies to determine their side effects on skeletal bone structures.
The long bone is mainly formed through endochondral bone formation, which starts with the formation of a cartilage template from condensed mesenchymal cells. The chondrocytes of the cartilage template proliferate axially and subsequently undergo hypertrophy and expansion in cellular volume (19). In addition to cartilage development, reports show that the chondrocytes secrete a variety of collagen proteins (20). Type II and X collagens (Col2a1 and Col10a1) contribute to chondrocyte maintenance and proliferation (20, 21). Hypertrophic chondrocytes will then switch from the synthesis of the cartilage-characteristic collagens to the synthesis of type I collagen (Col1a1), an osteoblast-characteristic collagen that organizes a mineralizing bone matrix (20, 21).
To further examine the underlying mechanisms for chondrocyte proliferation and growth inhibition, we established a chondrogenic ATDC5 cell line with the Col2a1 promoter (Col2a1-luc ATDC5 system) and screened a list of epigenetic compounds. Strikingly, six different BET inhibitors were shown to reduce Col2a1-luc, consistent with the decrease of Col2a1 mRNA and protein. Furthermore, we found that BET inhibitors blocked the differentiation of chondrocyte culture. Consistent with this, BET inhibitors I-BET151 and (+)-JQ1 could induce retardation of the growth of zebrafish. Taken together, these data suggest that the Col2a1-luc ATDC5 system may be used to screen chondrogenic factors. Furthermore, these data suggest that anticarcinogenic BET inhibitors may potentially injure chondrocytes and the bones of patients.
Results
BET Inhibitors Suppressed the Expression of Col2a1 in Chondrogenic ATDC5 Cells
Type II collagen is the specific and major matrix protein of cartilage. The Col2a1 promoter has been used to identify the molecules related to chondrogenesis (22, 23). To explore novel activators or repressors of chondrogenic differentiation, we generated an ATDC5 cell line that includes the 3-kb human Col2a1 promoter ligated to the open reading frame of firefly luciferase (Fig. 1A). We checked the responses of Col2a1-luc to the heterocyclic molecule kartogenin (KGN) and transcriptional factor SOX9, which have been widely recognized to promote the expression of Col2a1 and chondrocyte differentiation (24, 25). In agreement with previous reports (24, 25), both KGN and SOX9 increased Col2a1 promoter activities in a dose-dependent manner (Fig. 1, B and C), suggesting that the Col2a1-luc ATDC5 system could be used to screen chondrogenic activators or repressors.
FIGURE 1.

Col2a1 promoter-luciferase reporter. A, schematic diagram of the Col2a1 promoter-luciferase reporter construct. B, relative luciferase activity in cells treated with gradient KGN in μm. The KGN values 0.1, 1, and 10 are reported as 0.1, 1, and 10 μm, respectively. C, top, relative luciferase activity in cells transfected with gradient Sox9. Bottom, Western blotting analysis. For B and C, values represent mean ± S.D. (n = 3). p values were obtained from t tests with paired or unpaired samples: *, p < 0.05; **, p < 0.01. Error bars represent S.D.
Epigenetic factors (i.e. chromatin modifiers) regulate normal and disease processes and are mediated in part by the methylation status of DNA as well as by chemical modifications of histones, including acetylation, methylation, phosphorylation, and ubiquitination (26). Chondrogenic processes are also influenced by epigenetic regulation, such as methylation of Lys-27 residues on histone H3 (27). To uncover more epigenetic regulators, we screened a collection of 38 chemicals involved in epigenetic regulations using the Col2a1-luc ATDC5 system (Table 1). After 48-h treatment in 10 μm final concentration of BET inhibitors, we found that a battery of epigenetic inhibitors decreased luciferase activities of Col2a1 promoter (Fig. 2A). Among them, all BET inhibitors, including bromosporine, I-BET-762, RVX208, I-BET151, PFI-1, and (+)-JQ1, could significantly suppress the luciferase activity of the Col2a1 promoter (Fig. 2A) compared with the non-response of the promoter of a soybean translation elongation factor EF1α (Fig. 2B).
TABLE 1.
List of 38 epigenetic compounds screened
CAS, Chemical Abstracts Service; N/A, not applicable; PCPA, phenylcyclopropylamine; CTPB, N-(4-chloro-3-trifluoromethyl-phenyl)-2-ethoxy-6-pentadecylbenzamide; HAT, histone acetyltransferase; HIF, hypoxia-inducible factor.
| Category | No. | Product name | CAS no. | Catalog no. | Targets |
|---|---|---|---|---|---|
| DNA methyltransferase | 1 | Azacitidine | 320-67-2 | S1782 | DNA methyltransferase |
| 2 | Lomeguatrib | 192441-08-0 | S8056 | DNA methyltransferase | |
| 3 | 5-Aza-2-deoxycytidine | GR-345 | 2353-33-5 | DNA Me-transferase inhibitor | |
| 4 | Decitabine | 2353-33-5 | S1200 | DNA Methyltransferase | |
| 5 | Zebularine | GR-344 | 3690-10-6 | DNA Me-transferase inhibitor | |
| 6 | Zebularine | 3690-10-6 | S7113 | DNA Methyltransferase | |
| 7 | RG108 | 48208-26-0 | S2821 | Transferase, DNA Methyltransferase | |
| Epigenetic reader domain | 8 | UNC1215 | 1415800-43-9 | S7088 | Epigenetic reader domain |
| 9 | SGC-CBP30 | N/A | S7256 | Epigenetic reader domain | |
| 10 | Bromosporine | N/A | S7233 | Epigenetic reader domain | |
| 11 | I-BET-762 | 1260907-17-2 | S7189 | Epigenetic reader domain | |
| 12 | RVX-208 | 1044870-39-4 | S7295 | Epigenetic reader domain | |
| 13 | I-BET151 | 1300031-49-5 | S2780 | Epigenetic reader domain | |
| 14 | PFI-1 | 1403764-72-6 | S1216 | Epigenetic reader domain | |
| 15 | (+)-JQ1 | 1268524-70-4 | S7110 | Epigenetic reader domain | |
| Histone methyltransferase | 16 | EPZ5676 | 1380288-87-8 | S7062 | Histone methyltransferase |
| 17 | SGC 0946 | N/A | S7079 | Histone methyltransferase | |
| 18 | EPZ-6438 | 1403254-99-8 | S7128 | Histone methyltransferase | |
| 19 | 3-Deazaneplanocin A (DZNeP) | 102052-95-9 | S7120 | Histone methyltransferase | |
| 20 | EPZ004777 HCl | 1380316-03-9 | S7032 | Histone methyltransferase | |
| 21 | MM-102 | 1417329-24-8 | S7265 | Histone methyltransferase | |
| 22 | Entacapone | 130929-57-6 | S3147 | Histone methyltransferase | |
| 23 | EPZ004777 | 1338466-77-5 | S7353 | Histone methyltransferase | |
| 24 | EPZ005687 | 1396772-26-1 | S7004 | Histone methyltransferase | |
| Histone demethylases | 25 | GSK J4 HCl | 1373423-53-0 | S7070 | Histone demethylases |
| 26 | Tranylcypromine (2-PCPA) HCl | 4548-34-9 | S4246 | Histone demethylases | |
| 27 | JIB-04 | 199596-05-9 | S7281 | Histone demethylases | |
| 28 | OG-L002 | 1357302-64-7 | S7237 | Histone demethylases | |
| 29 | Tranylcypromine | EI-217 | 13492-01-8 | Lysine demethylase inhibitor | |
| 30 | 2,4-Pyridinedicarboxylic acid | A-280 | 499-80-9 | Histone demethylase inhibitor | |
| Histone acetyltransferase | 31 | CTPB | 420–033 | 586976-24-1 | HAT inhibitor |
| 32 | Garcinol | GR-343 | 78824-30-3 | HAT inhibitor | |
| 33 | Butyrolactone 3 | 270-411 | 778649-18-6 | HAT inhibitor | |
| 34 | C646 | 328968-36-1 | S7152 | Histone acetyltransferase | |
| 35 | Anacardic acid | 270-381 | N/A | HAT inhibitor | |
| HIF | 36 | IOX2 | 931398-72-0 | S2919 | HIF |
| 37 | FG-4592 | 808118-40-3 | S1007 | HIF | |
| 38 | 2-Methoxyestradiol (2-MeOE2) | 362-07-2 | S1233 | HIF |
FIGURE 2.
Screen of epigenetic compounds that influence Col2a1 transcription by luciferase reporter assay. A, the effect of epigenetic compounds was assessed by luciferase activity analysis. All of the epigenetic compounds were added to achieve a final concentration of 10 μm. B, E, and H, luciferase activity analysis of EF1α promoter in ATDC5 cells (B), C3H10 cells (E), and primary chondrocytes (H). All of the epigenetic compounds were added to 10 μm final concentration. C, F, and I, analysis of Col2a1 transcriptional levels by quantitative RT-PCR using RNA isolated from ATDC cells (C), C3H10 cells (F), and primary chondrocytes (I) treated with 1 μm BET inhibitors, respectively. D and G, luciferase activity analysis of Col2a1 promoter in C3H10 cells (D) and primary chondrocytes (G).Values represent mean ± S.D. (n = 3). p values were obtained from t tests with paired or unpaired samples: *, p < 0.05; **, p < 0.01. Error bars represent S.D.
Consistent with the luciferase activity result, the RNA levels of Col2a1 were also significantly reduced in cells treated with BET inhibitors compared with vehicle alone (DMSO) (Fig. 2C). Consistently, we discovered that BET inhibitors decreased Col2a1 expression in mesenchymal progenitor cell line C3H/10T1/2 (C3H10) (Fig. 2, D–F) and primary chondrocytes (Fig. 2, G–I).
The half-maximal inhibitory concentration and cytotoxicity concentration were unequal for different BET inhibitors. To lower the impact of cell cytotoxicity of the various BET inhibitors, we further examined the effects of five BET inhibitors (I-BET-762, RVX208, I-BET151, PFI-1, and (+)-JQ1) on Col2a1 expression at various doses. We performed treatment with five BET inhibitors at gradient concentrations of 0.1, 1, and 5 μm. As shown in Fig. 3, A–E (ATDC5) and F–J (C3H10), all of the BET inhibitors were capable of dose-dependently decreasing the luciferase activity of the Col2a1 promoter. Consistent with this, the expression levels of Col2a1, as determined by quantitative RT-PCR, were dose-dependently reduced by various BET inhibitors (Fig. 3, K–O (ATDC5) and P–T (primary chondrocytes)).
FIGURE 3.
Effects of BET inhibitors on Col2a1 expression. A–E and F–J, the effect of BET inhibitors in gradient concentrations was assessed by luciferase activity analysis in ATDC5 cells (A–E) and C3H10 cells (F–J). K–O and P–T, the Col2a1 expression in ATDC5 cells (K–O) and primary chondrocytes (P–T) treated with gradient concentrations of BET inhibitors. The reported values of 0.1, 1, and 5 are indicated as 0.1, 1, and 5 μm, respectively. Values represent mean ± S.D. (n = 3). p values were obtained from t tests with paired or unpaired samples: *, p < 0.05; **, p < 0.01. Error bars represent S.D.
BET Inhibitors Dose-dependently Suppressed the Expression of Chondrogenic Marker Genes in Primary Chondrocytes
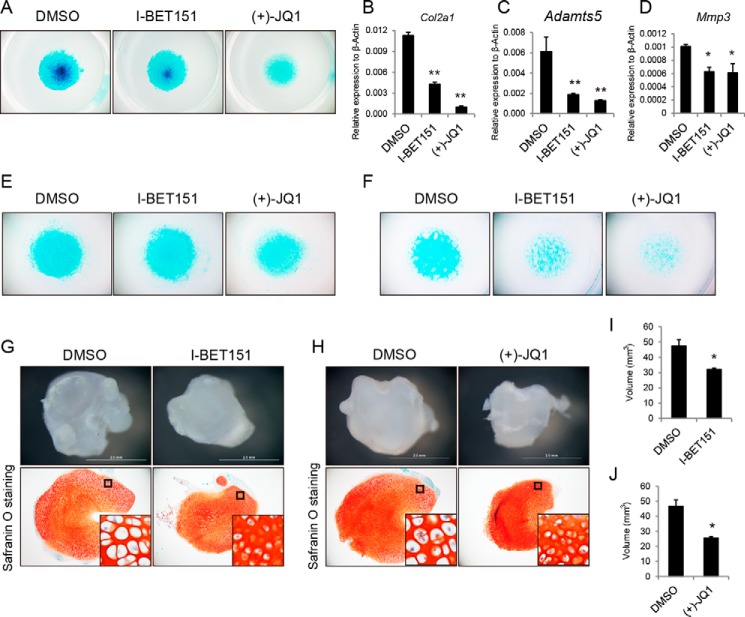
BET bromodomain inhibition results in the suppression of Col2a1, which is one of the most essential collagens expressed during cartilage maintenance and differentiation. We hypothesized that BET inhibitors may also play fundamental roles in chondrocyte proliferation and development. To test our hypothesis, we isolated chondrocytes from the articular cartilage of 3-day-old wild-type mice and examined the effects of BET bromodomain inhibition on the expression of chondrocyte marker genes. Total RNA and protein were collected from the cultured chondrocytes that were treated with I-BET151 and (+)-JQ1 at final concentrations of 0.2, 1, and 5 μm (I-BET151) and 0.2, 0.5, and 1 μm (+)-JQ1) for 72 h. Using quantitative RT-PCR, we observed a progressive transcription suppression of Col2a1, a disintegrin and metalloproteinase with thrombospondin motifs 5 (Adamts5), and matrix metalloproteinase-3 (Mmp3) (Fig. 4, A–F). However, the key transcriptional factor of chondrogenesis, Sox9, showed no obvious response to the BET bromodomain inhibition (data not shown). We also examined the protein levels of different chondrocyte marker genes after I-BET151 and (+)-JQ1 treatment. As shown in Fig. 4, G and H, immunoblotting analysis demonstrated that both BET151 and (+)-JQ1 may lead to decreased protein levels of COL2A1, ADAMTS5, and MMP3, correlating with RNA level inhibition. These data indicated that both I-BET151 and (+)-JQ1 treatments may have suppressive effects on chondrogenesis.
FIGURE 4.
I-BET151 and (+)-JQ1 affected the expression of Col2a1, Adamts5, and Mmp3 in chondrocytes. A–F, the expression level of chondrocyte-specific marker genes Col2a1, Adamts5, and Mmp3 as analyzed by quantitative RT-PCR using RNA isolated from cells treated as indicated. The values 0.2, 0.5, 1, and 5 are reported as 0.2, 0.5, 1, and 5 μm, respectively. Values represent mean ± S.D. (n = 3). p values were obtained from t tests with paired or unpaired samples: *, p < 0.05; **, p < 0.01. G and H, indicated protein level was assessed by Western blotting after treatment of chondrocytes with I-BET151 and (+)-JQ1. Error bars represent S.D.
BET Inhibition with I-BET151 and (+)-JQ1 Arrests Chondrogenesis and Differentiation
To further characterize the efficacy of inhibiting BET proteins in chondrogenic differentiation, we examined the effect of I-BET151 and (+)-JQ1 by using the chondrocyte micromass culture system. Inhibition of BET bromodomain proteins induced a remarkable decrease in matrix accumulation, which was monitored by Alcian blue staining after 7 days in micromass culture (Fig. 5A). Accordingly, both I-BET151 and (+)-JQ1 strongly suppressed the expression of chondrogenesis-related genes, such as Col2a1, Adamts5, and Mmp3 (Fig. 5, B–D). Consistently, the chondrogenic differentiation of C3H10 and ATDC5 cells was inhibited by I-BET151 and (+)-JQ1 as well (Fig. 5, E and F).
FIGURE 5.
BET inhibitors repressed chondrocyte differentiation. A, E, and F, micromass culture of chondrocytes derived from cartilage articularis of 4-day-old mice (A), C3H10 cells (E), and ATDC5 cells (F). The cultures were treated with I-BET151 and (+)-JQ1 at 5 and 1 μm, respectively for 7 days and stained using Alcian blue. B–D, quantitative RT-PCR analysis was performed for the indicated genes using RNA isolated from micromass cultures of primary chondrocytes (A). G, articular cartilage from distal femora of newborn mice was treated with I-BET151 at 5 μm and vehicle alone (DMSO) for 1 week. Top, comparison of cartilage in DMSO or I-BET151 treatment. Bottom, a cartilage-like particle was sectioned and stained with safranin O. H, articular cartilage from distal femora of newborn mice was treated with (+)-JQ1 at 0.5 μm and vehicle alone (DMSO) for 1 week. Top, comparison of cartilage in DMSO or (+)-JQ1 treatment. Bottom, a cartilage-like particle was sectioned and stained with safranin O. I and J, comparison of cartilage volume in DMSO and I-BET151 (I) or (+)-JQ1 treatment (J). Values represent mean ± S.D. (n = 3). p values were obtained from t tests with paired or unpaired samples: *, p < 0.05; **, p < 0.01. Error bars represent S.D. Scale bars, 2 mm in G and H.
Next, we examined the effects of BET inhibitors on ex vivo mouse articular cartilage cultures. Articular cartilage of distal femora was isolated from newborn mice and cultured in chondrogenic differential medium. I-BET151 at 5 μm or (+)-JQ1 at 0.5 μm was added to the culture medium with an equal amount of DMSO as the control. After 1 week of culture, we observed that both I-BET151 and JQ1 could suppress the growth of cartilage as the size of the I-BET151 and JQ1 treatment group was significantly smaller than the DMSO control group (Fig. 5, G–J). We examined the cartilage by positive safranin O staining and found that both I-BET151 and (+)-JQ1 can inhibit the hypertrophy of chondrocytes as determined by the size of the chondrocytes following treatment (Fig. 5, G and H).
I-BET151 and (+)-JQ1 Modulated the Accumulation of Pol II on Col2a1 Promoter
BET inhibitors are capable of blocking the interaction of acetylated lysine with BET proteins, which could regulate gene transcription driven by Pol II (28). First, we detected that the protein level of Pol II was not impacted by BET inhibitor treatment (Fig. 6A). We next examined whether BET inhibitors could affect the recruitment of Pol II to the Col2a1 promoter. For the 2.5-kb promoter upstream of the transcription start site of mouse Col2a1, we analyzed four loci (Fig. 6B, primers 1–4). DNA was purified after chromatin immunoprecipitation with Pol II antibody in primary chondrocytes treated with DMSO, I-BET151, or (+)-JQ1 for 72 h. The relative amount of promoter-associated Pol II was evaluated by quantitative RT-PCR followed by normalization with the IgG sample. We observed that Pol II associates with the Col2a1 promoter, especially DNA fragments close to the transcription start site (Fig. 6C). In addition, the association between Pol II and the Col2a1 promoter was arrested by BET bromodomain inhibition by I-BET151 and (+)-JQ1 (Fig. 6C). These data indicate that the recruitment of Pol II to the Col2a1 promoter was interrupted by BET inhibitors.
FIGURE 6.

BET inhibitors abolished the binding of Pol II to the promoter regions of the Col2a1. A, Pol II protein level was assessed by Western blotting after treatment of chondrocytes with I-BET151 and (+)-JQ1. B, schematic representation of the mouse Col2a1 gene locus. Positive ChIP-PCR amplification was obtained using primers 1–4. TSS, transcription start site. C, ChIP-quantitative PCR analysis of the distinct regions in Col2a1 promoter showing enrichment with Pol II antibody compared with IgG controls. Values represent mean ± S.D. (n = 3). p values were obtained from t tests with paired or unpaired samples: **, p < 0.01. Error bars represent S.D.
I-BET151 and (+)-JQ1 Retarded the Growth of Zebrafish
Zebrafish is a good, widely used model in developmental and functional studies of bone and cartilage (29). To determine whether BET inhibitors could influence Col2a1 as well as animal growth in vivo, we next examined the effects of BET inhibitors on zebrafish. We generated a transgenic zebrafish line harboring a col2a1 promoter-EGFP transgene (Fig. 7A). As shown in Fig. 7B, the EGFP fluorescence could be detected at 1 day postfertilization (dpf) and was further enhanced at 2 dpf. The EGFP fluorescence localized at the vertebrae, which contain collagen 2-positive chondrocytes. We incubated 12-h postfertilization embryos with different doses of I-BET151 (1, 5, and 10 μm) and (+)-JQ1 (0.1, 0.5, and 1 μm) for 36 h. As shown in Fig. 7, C and D, 10 μm I-BET151 and 1 μm (+)-JQ1 induced serious malformations in the zebrafish. In addition, both I-BET151 and (+)-JQ1 led to decreased EGFP fluorescence compared with the vehicle alone (DMSO), indicating that BET inhibitors could decrease col2a1 expression in vivo (Fig. 7, C and D).
FIGURE 7.

BET inhibitors regulated zebrafish skeletal development. A, schematic diagram of col2a1 promoter-EGFP construct. B, the remarkable EGFP fluorescence signal was detected at 2 dpf. C and D, EGFP fluorescence images of transgenic zebrafish embryos containing EGFP initiated by col2a1 promoter that were treated as indicated at 2 dpf. E and H, wild-type zebrafish were continuously treated as indicated for 2 weeks. F and I, body length of wild-type zebrafish continuously treated as indicated for 2 weeks. G and J, survival percentage of zebrafish undergoing the indicated treatment. K–N, alizarin red and Alcian blue staining of 3-week-old zebrafish. M and N are high magnification of K and L, respectively. O and P, statistics analysis of the length of centrum 15 (c15) under different treatment. Scale bars, 1 mm in E and H, 2 mm in K and L, and 500 μm in M and N. Values represent mean ± S.D. (n = 3). p values were obtained from t tests with paired or unpaired samples: *, p < 0.05.
Prior research studies have demonstrated that long bones are formed through endochondral ossification of chondrocytes in which col2a1 is one of the most requisite matrix collagens (30). Thus, we hypothesized that the inhibition of BET bromodomain proteins may also affect the growth of the organism. To test this hypothesis, we incubated 2-dpf embryos with I-BET151 (1 and 5 μm) and (+)-JQ1 (0.1 and 0.5 μm) for 2 weeks and measured the body length of the zebrafish following 2 weeks of cultivation. As shown in Fig. 7, E and F, zebrafish treated with I-BET151 at both 1 and 5 μm developed a specified amount of atrophy compared with vehicle alone (DMSO). However, I-BET151 at high concentrations (10 μm) impacted zebrafish viability (Fig. 7G). Consistently, we also observed that 0.5 μm (+)-JQ1 suppressed the length extension of the zebrafish, whereas 0.1 μm (+)-JQ1 showed no obvious functions (Fig. 7, H–J). Next, we characterized the whole mount skeleton of zebrafish by alizarin red and Alcian blue staining at 3 weeks old and observed similar shortened body length (Fig. 7, K and L). To analyze the bone growth restriction, we measured the length of centrum 15 of caudal vertebrae as a representative and observed the shorter vertebral bodies in I-BET151 (both 1 and 5 μm)- and (+)-JQ1 (0.5 μm)-treated zebrafish, whereas 0.1 μm (+)-JQ1 showed no obvious functions (Fig. 7, M–P).
To demonstrate that BET inhibitors also played fundamental roles in zebrafish chondrogenesis, we isolated total RNAs of tail fins from 3-week-old zebrafish under different treatments. Using quantitative RT-PCR, we observed a progressive transcription suppression of cartilage and bone marker genes, such as col2a1, adamts5, col1a1 (type I collagen), and runt-related transcription factor 2a and 2b (runx2a and runx2b) (Fig. 8, A–J). Consistent with this, the protein level of COL2A1 was reduced in paraffin sections of 3-week-old zebrafish treated with both I-BET151 and (+)-JQ1 (Fig. 8K). Taken together, our data suggest that BET inhibitors restrained chondrogenesis and differentiation both in vitro and in vivo.
FIGURE 8.
The expression of marker genes in zebrafish. A–J, analysis of the transcript levels of the indicated genes by quantitative PCR using RNA isolated from wild-type zebrafish that were continuously treated with I-BET151 (1 μm), (+)-JQ1 (0.5 μm), and DMSO as a control for 3 weeks. Values represent mean ± S.D. (n = 3). p values were obtained from t tests with paired or unpaired samples: *, p < 0.05; **, p < 0.01. K, immunohistochemistry analysis of COL2A1 in paraffin sections of DMSO-, I-BET151-, and (+)-JQ1-treated fish. Scale bars, 100 μm. Error bars represent S.D.
Discussion
In the present study, we generated a Col2a1-luc ATDC5 system to identify activators or repressors of chondrogenic differentiation. We found that six BET inhibitors restrain the expression of Col2a1 and impede chondrogenesis as determined by micromass culture and ex vivo cartilage culture. We also established a Col2-EGFP transgenic zebrafish line and demonstrated that BET inhibitors can decrease Col2-EGFP expression. Consistently, BET inhibitors retard the growth of zebrafish in vivo. These data demonstrate that BET inhibitors could inhibit chondrogenic differentiation and restrain bone growth in vitro and in vivo.
The BET subfamily of bromodomain-containing proteins is involved in a number of hematological and solid tumors (15, 17). Currently, several active clinical trials aiming to treat malignancies are using BET inhibitors, such as FT-1101, ZEN003694, BMS-986158, INCB054329, RVX-208, I-BET 762, OTX 015, CPI-0610, and TEN-010 (www.clinicaltrials.gov). In addition to this, multiple other BET inhibitors have been well studied and have revealed great potential for clinical application. For instance, (+)-JQ1 suppresses breast cancer cells (31), gastric cancer cells (32), acute myeloid leukemia cells (33), and osteosarcoma cells (13). Furthermore, I-BET151 has been shown to induce cell apoptosis in myeloma and leukemia (16, 34). However, because BET bromodomain proteins function as epigenetic regulators and play a key role in transcriptional regulation of essential genes in the cell cycle and apoptosis, such as c-Myc (8), nuclear factor κ-light chain enhancer of activated B cells (NF-κB) (35), and B-cell lymphoma 2 (BCL2) (8, 36), the inhibitory factors may affect other important biological processes.
Our current study demonstrated that BET inhibitors could interfere with chondrogenic differentiation and bone growth, highlighting the necessity of studying the role of bromodomain-containing proteins in chondrogenesis to monitor and avoid possible side effects of BET inhibitors on cartilage and bone. Besides bone formation, bone homeostasis is maintained by the balance between osteoblastic bone formation and osteoclastic bone resorption, whereas the growth of long bones is from endochondral ossification of chondrocytes derived from cartilage (15). Previous studies have shown that BET inhibitors may affect osteoclastogenesis (14) and the bone-associated tumor vicious cycle (13). In this study, we demonstrated BET inhibitors as repressors of chondrogenesis. Taken together, previous studies in addition to our studies demonstrate that preclinical pharmacological studies of BET inhibitors on human bone warrant further investigation.
Experimental Procedures
Cell Culture
Mouse embryonic cell line ATDC5 was maintained in DMEM (Corning) containing Ham's F-12 (1:1) (Corning), 2 mm glutamine, and 5% fetal bovine serum (FBS). The mouse mesenchymal stem cell line (C3H/10T1/2) was obtained from ATCC and maintained in low glucose α-MEM (Corning) containing 10% FBS. The cells were plated at 1.5 × 106/10-cm dish. To acquire primary chondrocytes, articular cartilage from 3-day-old wild-type mice was isolated and digested for 1 h in enzymatic hydrolysate containing 50 ml of low glucose α-MEM, 100 mg of Dispase II (D4693, Sigma-Aldrich), and 50 mg collagenase (C0130, Sigma). For chondrogenesis cultures and Alcian blue staining, chondrocytes were centrifuged at 300 × g for 5 min and cultured in low glucose α-MEM containing 10% FBS.
Culture and Differentiation of Chondrocyte Cells
Two-dimensional micromass culture was initiated by spotting 2.5 × 105 chondrocytes in 15 μl to a well of a 12-well plate and maintained in chondrogenic differentiation medium (DMEM supplemented with 1% insulin-transferrin-selenium, 500 nm/ml TGFβ3 (243-B3-200, R&D Systems), 10 nm dexamethasone, 10 mm β-glycerophosphate, and 0.1 mm l-ascorbic acid 2-phosphate. After a 7-day differentiation, the micromass culture was stained with 1% Alcian blue. For cartilage-like particle differentiation, articular cartilage was isolated from distal femora of newborn wild-type mice and cultured in chondrogenic differentiation medium and growth medium (low glucose α-MEM containing 10% FBS) for 7 days.
Phenotype Analyses
For histology analyses, cartilage-like particles were fixed in 4% paraformaldehyde overnight at 4 °C, dehydrated using graded ethanol overnight, and embedded in paraffin. The paraffin-embedded tissue samples were sectioned at 5 μm. For safranin O staining, sections were first dewaxed, immersed in 1% acetic acid, and stained with 0.05% fast green and 0.5% safranine. Immunohistochemistry staining was performed as described previously (37). Images were captured using a microscope (Olympus BX51). Alizarin red and Alcian blue staining was performed as described previously (38).
Zebrafish were maintained as described (39). For compound treatment, DMSO and BET inhibitors were added as indicated at 1 dpf. Images were captured using an Olympus BZX16 stereomicroscope.
Luciferase Reporter Assays
ATDC5 cells grown in 10-cm dishes were transiently transfected using polyethylenimine (765090, Sigma-Aldrich) with the luciferase reporter constructs. pRL-TK (Promega, Madison, WI) was co-transfected to provide a normalization control for transfection efficiency. Eight hours after transfection, ATDC5 cells with luciferase and control Renilla were digested and plated at 1.5 × 105/well in 12-well plate. Four hours later, cells were treated with different compounds. I-BET151 and (+)-JQ1 were first dissolved in DMSO at 5 mm and then diluted to final concentrations as described. The vehicle, DMSO, was added as a blank control. After 48-h cultivation, cells were lysed, and luciferase activity was measured using the Dual-Luciferase Assay kit (Promega).
Reverse Transcription and Real Time PCR
Total RNA was prepared from cells or tissues using TRIzol (T9424, Sigma) and reverse transcribed into cDNA using PrimeScriptTM RT Reagent kit (PR037A, TakaRa Bio Inc., Kusatsu, Siga, Japan). The cDNA quantity was analyzed using the Bio-Rad CFX96 system. The primer sets used were as follows: for mouse genes, β-actin, 5′-AGATGTGGATCAGCAAGCAG-3′ (sense) and 5′-GCGCAAGTTAGGTTTTGTCA-3′ (antisense); Col2a1, 5′-CGGTCCTACGGTGTCAGG-3′ (sense) and 5′-GCAGAGGACATTCCCAGTGT-3′ (antisense); Adamts5, 5′-GACACGAGTCTGGAGGTGAGCA-3′ (sense) and 5′-CGTCATGAGAAAGGCCAAGTAG-3′ (antisense); Mmp3, 5′-GACGATGATGAACGATGGACAG-3′ (sense) and 5′-TTGAGAGAGATGGAAACGGGAC-3′ (antisense); for zebrafish genes, actin β1, 5′-TCCTGGGTATGGAATCTTGCG-3′ (sense) and 5′-ACGGTCAGCAATGCCAGGGTA-3′ (antisense); col2a1, 5′-CCCTGAATGGAAGAGCGGTGAC-3′ (sense) and 5′-GCTCTTGCTTGTCCACCAGTTC-3′ (antisense); col1a1, 5′-AAGTCCCTGAGCCAGCAGATTG-3′ (sense) and 5′-TGACGCAAGTCTCGCCAGTTTC-3′ (antisense); acan, 5′-TCTGAAGAGGATTGCGTTGTGA-3′ (sense) and 5′-TGGCTTCTCCCACTGTCCGTCT-3′ (antisense); adamts5, 5′-CGCTCAACGGCTCTGTCCTCAAC-3′ (sense) and 5′-TCCTGGCTGGGAGTGGCGTTT-3′ (antisense); mmp13a, 5′-CGACGCAGTCCTCTACAAGGAAG-3′ (sense) and 5′-ATACTGGAAGGCTGCGGTGAC-3′ (antisense); mmp13b, 5′-GACGCTGCCTATGAGAACCCA-3′ (sense) and 5′-GATCGTGGGTATCCAGTGTCC-3′ (antisense); bsp, 5′-GAGATAGACAACATCGAGGCAAAT-3′ (sense) and 5′-TCCTCACTATCCGACTCTTCTTC-3′ (antisense); ocn, 5′-TCTTCTGCTGCCTGATGACTGTGT-3′ (sense) and 5′-AGGCGGTGATGATTCCAGACG-3′ (antisense); runx2a, 5′-CCAGCAACATTCACCTACACCC-3′ (sense) and 5′-TATGGTGTGCTGCTGGTTTGGA-3′ (antisense); runx2b, 5′-CGCCAGTTCCCTGCCTTCTCC-3′ (sense) and 5′-CGGAGGAAGCACCGTAATAGAG-3′ (antisense).
Western Blotting
Cell extracts were prepared with EBC buffer (50 mm Tris-HCl (pH 7.5), 120 mm NaCl, 0.5% Nonidet P-40, and protease inhibitor mixture (S8820, Sigma-Aldrich)). Protein lysates (20 μg) were separated on 10% SDS-polyacrylamide gels, transferred onto PVDF membranes, blocked with 5% blotting grade milk in Tris-buffered saline with Tween (20 mm Tris-HCl (pH 7.6), 137 mm NaCl, and 0.5% Tween 20), probed with the indicated primary antibodies, incubated with the corresponding secondary antibodies, and detected using a chemiluminescence assay (EMD Millipore, Billerica). Membranes were exposed to X-ray film (Amersham Biosciences) for visualization.
ChIP Assay
About 107 chondrocytes were cultured with 1 μm I-BET151, 0.1 μm (+)-JQ1, or DMSO added. After 72-h treatment, cells were resuspended in IP buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1 mm PMSF) and subjected to gentle sonication and centrifuged (12,000 × g for 10 min) to obtain cell extracts. ChIP was performed as described (40). IgG (I5006, Sigma-Aldrich) and RNA polymerase II antibody (05-623, EMD Millipore) were used at 1 μg/106 cells.
Plasmids and PCR Primers
A 3-kb Col2a1 region was obtained from total mouse genome using primers Col2-F (5′-TTAATTACTAGTTAGGTCGACTTTCATATGTGGTTTTCTGTAATGATCT-3′) and Col2-R (5′-TTTAATTGCGGCCGCTGGCAAACCGGCCCAAACCGCGGGCCGCCCCCT-3′) and inserted into pHAGE vector using restriction enzymes SpeI and NotI. A 255-bp poly(A) sequence was inserted at the 5′ terminus of Col2a1 promoter using restriction enzymes SalI and NdeI. The poly(A) sequence was as follows: GACTCTAGATCATAATCAGCCATACCACATTTGTAGAGGTTTTACTTGCTTTAAAAAACCTCCCACACCTCCCCCTGAACCTGAAACATAAAATGAATGCAATTGTTGTTGTTAACTTGTTTATTGCAGCTTATAATGGTTACAAATAAAGCAATAGCATCACAAATTTCACAAATAAAGCATTTTTTTCACTGCATTCTAGTTGTGGTTTGTCCAAACTCATCAATGTATCTTAAGGCGTAAATTGTAAGCGTT. To generate the HA-SOX9 construct, Sox9 was amplified from the total cDNA library of 10.5-day-old embryonic (E10.5) mice using primers SOX9-F (5′-GCGGCCGCATGAATCTCCTGGACCCCTTCATGA-3′) and SOX9-R (5′-GGATCCTCAGGGTCTGGTGAGCTGTGTGTAG-3′) and cloned into the modified pHAGE vector containing HA using restriction enzymes NotI and BamHI. To generate the col2a1-EGFP construct, a 1.9-kb col2a1 promoter region was obtained from total mouse genome using primers Col2a1-5-XhoI (5′-AATTAACTCGAGCGGCCCTCTGACACCTGATGCCAATT-3′) and Col2a1-3-SmaI (5′-AATTAACCCGGGGTCTAAAGATTAGACATGCAGGT-3′) and inserted into plasmid pEsce1.
Antibodies
Anti-HA antibody (SC-7392) was from Santa Cruz Biotechnology; anti-GFP antibody (66002-1) was from Proteintech; anti-COL2A1 antibody (ab34712), anti-MMP3 antibody (ab52915), monoclonal anti-histone H3 antibody (ab1791), and anti-tubulin antibody (SC-23948) were from Santa Cruz Biotechnology; and anti-ADAMTS5 antibody (PA5-14350) was from Thermo Scientific.
Statistical Analysis
For statistical analysis, in most graphs mean values with standard deviation (S.D.) are shown. Data were generated from several independently obtained data sets. p values were obtained from two-tailed student t tests. A p value less than 0.05 was considered a significant difference, and a p value less than 0.01 was considered an extremely significant difference. Error bars represent S.D.
Author Contributions
W. Z. and N. N. conceived and supervised the project. R. S. and N. N. generated the Col2a1-luc ATDC5 system and performed the screening of the epigenetic compounds. G. Y. generated transgenic zebrafish line Tg (col2a1-EGFP). N. N. performed other experiments and data analysis. N. N. and W. Z. prepared the manuscript.
Acknowledgment
We thank the members of the Zou laboratory for valuable discussions.
This work was supported in part by 973 Program from the Chinese Ministry of Science and Technology Grants 2015CB964503 and 2014CB964704 and National Natural Science Foundation of China Grants 31371463 and 31501170. The authors declare that they have no conflicts of interest with the contents of this article.
- BET
- bromodomain and extra-terminal
- KGN
- kartogenin
- dpf
- day(s) postfertilization
- EGFP
- enhanced green fluorescent protein
- Pol II
- RNA polymerase II
- luc
- luciferase
- C3H10
- C3H/10T1/2
- ADAMTS5
- a disintegrin and metalloproteinase with thrombospondin motifs 5
- MMP
- matrix metalloproteinase-3
- runx
- runt-related transcription factor
- MEM
- minimum Eagle's medium.
References
- 1. LeRoy G., Rickards B., and Flint S. J. (2008) The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell 30, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Filippakopoulos P., and Knapp S. (2014) Targeting bromodomains: epigenetic readers of lysine acetylation. Nat. Rev. Drug Discov. 13, 337–356 [DOI] [PubMed] [Google Scholar]
- 3. Dhalluin C., Carlson J. E., Zeng L., He C., Aggarwal A. K., and Zhou M. M. (1999) Structure and ligand of a histone acetyltransferase bromodomain. Nature 399, 491–496 [DOI] [PubMed] [Google Scholar]
- 4. Whyte W. A., Orlando D. A., Hnisz D., Abraham B. J., Lin C. Y., Kagey M. H., Rahl P. B., Lee T. I., and Young R. A. (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang Z., Yik J. H., Chen R., He N., Jang M. K., Ozato K., and Zhou Q. (2005) Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell 19, 535–545 [DOI] [PubMed] [Google Scholar]
- 6. Jang M. K., Mochizuki K., Zhou M., Jeong H. S., Brady J. N., and Ozato K. (2005) The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534 [DOI] [PubMed] [Google Scholar]
- 7. Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W. B., Fedorov O., Morse E. M., Keates T., Hickman T. T., Felletar I., Philpott M., Munro S., McKeown M. R., Wang Y., Christie A. L., et al. (2010) Selective inhibition of BET bromodomains. Nature 468, 1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delmore J. E., Issa G. C., Lemieux M. E., Rahl P. B., Shi J., Jacobs H. M., Kastritis E., Gilpatrick T., Paranal R. M., Qi J., Chesi M., Schinzel A. C., McKeown M. R., Heffernan T. P., Vakoc C. R., et al. (2011) BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stratikopoulos E. E., Dendy M., Szabolcs M., Khaykin A. J., Lefebvre C., Zhou M. M., and Parsons R. (2015) Kinase and BET inhibitors together clamp inhibition of PI3K signaling and overcome resistance to therapy. Cancer Cell 27, 837–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang Y., Gholamin S., Schubert S., Willardson M. I., Lee A., Bandopadhayay P., Bergthold G., Masoud S., Nguyen B., Vue N., Balansay B., Yu F., Oh S., Woo P., Chen S., et al. (2014) Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat. Med. 20, 732–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korb E., Herre M., Zucker-Scharff I., Darnell R. B., and Allis C. D. (2015) BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat. Neurosci. 18, 1464–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang M., Qiu Q., Xiao Y., Zeng S., Zhan M., Shi M., Zou Y., Ye Y., Liang L., Yang X., and Xu H. (2016) BET bromodomain suppression inhibits VEGF-induced angiogenesis and vascular permeability by blocking VEGFR2-mediated activation of PAK1 and eNOS. Sci. Rep. 6, 23770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamoureux F., Baud'huin M., Rodriguez Calleja L., Jacques C., Berreur M., Rédini F., Lecanda F., Bradner J. E., Heymann D., and Ory B. (2014) Selective inhibition of BET bromodomain epigenetic signalling interferes with the bone-associated tumour vicious cycle. Nat. Commun. 5, 3511. [DOI] [PubMed] [Google Scholar]
- 14. Park-Min K. H., Lim E., Lee M. J., Park S. H., Giannopoulou E., Yarilina A., van der Meulen M., Zhao B., Smithers N., Witherington J., Lee K., Tak P. P., Prinjha R. K., and Ivashkiv L. B. (2014) Inhibition of osteoclastogenesis and inflammatory bone resorption by targeting BET proteins and epigenetic regulation. Nat. Commun. 5, 5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu L. L., Tian M., Li X., Li J. J., Huang J., Ouyang L., Zhang Y., and Liu B. (2015) Inhibition of BET bromodomains as a therapeutic strategy for cancer drug discovery. Oncotarget 6, 5501–5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fong C. Y., Gilan O., Lam E. Y., Rubin A. F., Ftouni S., Tyler D., Stanley K., Sinha D., Yeh P., Morison J., Giotopoulos G., Lugo D., Jeffrey P., Lee S. C., Carpenter C., et al. (2015) BET inhibitor resistance emerges from leukaemia stem cells. Nature 525, 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jung M., Gelato K. A., Fernández-Montalván A., Siegel S., and Haendler B. (2015) Targeting BET bromodomains for cancer treatment. Epigenomics 7, 487–501 [DOI] [PubMed] [Google Scholar]
- 18. Sullivan J. M., Badimon A., Schaefer U., Ayata P., Gray J., Chung C. W., von Schimmelmann M., Zhang F., Garton N., Smithers N., Lewis H., Tarakhovsky A., Prinjha R. K., and Schaefer A. (2015) Autism-like syndrome is induced by pharmacological suppression of BET proteins in young mice. J. Exp. Med. 212, 1771–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mackie E. J., Tatarczuch L., and Mirams M. (2011) The skeleton: a multi-functional complex organ. The growth plate chondrocyte and endochondral ossification. J. Endocrinol. 211, 109–121 [DOI] [PubMed] [Google Scholar]
- 20. Seghatoleslami M. R., Lichtler A. C., Upholt W. B., Kosher R. A., Clark S. H., Mack K., and Rowe D. W. (1995) Differential regulation of COL2A1 expression in developing and mature chondrocytes. Matrix Biol. 14, 753–764 [DOI] [PubMed] [Google Scholar]
- 21. Bianco P., Cancedda F. D., Riminucci M., and Cancedda R. (1998) Bone formation via cartilage models: the “borderline” chondrocyte. Matrix Biol. 17, 185–192 [DOI] [PubMed] [Google Scholar]
- 22. Zhou G., Lefebvre V., Zhang Z., Eberspaecher H., and de Crombrugghe B. (1998) Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J. Biol. Chem. 273, 14989–14997 [DOI] [PubMed] [Google Scholar]
- 23. Kan A., Ikeda T., Saito T., Yano F., Fukai A., Hojo H., Ogasawara T., Ogata N., Nakamura K., Chung U. I., and Kawaguchi H. (2009) Screening of chondrogenic factors with a real-time fluorescence-monitoring cell line ATDC5-C2ER: identification of sorting nexin 19 as a novel factor. Arthritis Rheum. 60, 3314–3323 [DOI] [PubMed] [Google Scholar]
- 24. Johnson K., Zhu S., Tremblay M. S., Payette J. N., Wang J., Bouchez L. C., Meeusen S., Althage A., Cho C. Y., Wu X., and Schultz P. G. (2012) A stem cell-based approach to cartilage repair. Science 336, 717–721 [DOI] [PubMed] [Google Scholar]
- 25. Bell D. M., Leung K. K., Wheatley S. C., Ng L. J., Zhou S., Ling K. W., Sham M. H., Koopman P., Tam P. P., and Cheah K. S. (1997) SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16, 174–178 [DOI] [PubMed] [Google Scholar]
- 26. Kouzarides T. (2007) Chromatin modifications and their function. Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 27. Yapp C., Carr A. J., Price A., Oppermann U., and Snelling S. J. (2016) H3K27me3 demethylases regulate in vitro chondrogenesis and chondrocyte activity in osteoarthritis. Arthritis Res. Ther. 18, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang W., Prakash C., Sum C., Gong Y., Li Y., Kwok J. J., Thiessen N., Pettersson S., Jones S. J., Knapp S., Yang H., and Chin K. C. (2012) Bromodomain-containing protein 4 (BRD4) regulates RNA polymerase II serine 2 phosphorylation in human CD4+ T cells. J. Biol. Chem. 287, 43137–43155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fisher S., Jagadeeswaran P., and Halpern M. E. (2003) Radiographic analysis of zebrafish skeletal defects. Dev. Biol. 264, 64–76 [DOI] [PubMed] [Google Scholar]
- 30. Wei X., Hu M., Mishina Y., and Liu F. (2016) Developmental regulation of the growth plate and cranial synchondrosis. J. Dent. Res. 95, 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feng Q., Zhang Z., Shea M. J., Creighton C. J., Coarfa C., Hilsenbeck S. G., Lanz R., He B., Wang L., Fu X., Nardone A., Song Y., Bradner J., Mitsiades N., Mitsiades C. S., et al. (2014) An epigenomic approach to therapy for tamoxifen-resistant breast cancer. Cell Res. 24, 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montenegro R. C., Clark P. G., Howarth A., Wan X., Ceroni A., Siejka P., Nunez-Alonso G. A., Monteiro O., Rogers C., Gamble V., Burbano R., Brennan P. E., Tallant C., Ebner D., Fedorov O., et al. (2016) BET inhibition as a new strategy for the treatment of gastric cancer. Oncotarget 7, 43997–44012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fiskus W., Sharma S., Qi J., Shah B., Devaraj S. G., Leveque C., Portier B. P., Iyer S., Bradner J. E., and Bhalla K. N. (2014) BET protein antagonist JQ1 is synergistically lethal with FLT3 tyrosine kinase inhibitor (TKI) and overcomes resistance to FLT3-TKI in AML cells expressing FLT-ITD. Mol. Cancer Ther. 13, 2315–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaidos A., Caputo V., Gouvedenou K., Liu B., Marigo I., Chaudhry M. S., Rotolo A., Tough D. F., Smithers N. N., Bassil A. K., Chapman T. D., Harker N. R., Barbash O., Tummino P., Al-Mahdi N., et al. (2014) Potent antimyeloma activity of the novel bromodomain inhibitors I-BET151 and I-BET762. Blood 123, 697–705 [DOI] [PubMed] [Google Scholar]
- 35. Ceribelli M., Kelly P. N., Shaffer A. L., Wright G. W., Xiao W., Yang Y., Mathews Griner L. A., Guha R., Shinn P., Keller J. M., Liu D., Patel P. R., Ferrer M., Joshi S., Nerle S., et al. (2014) Blockade of oncogenic IκB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc. Natl. Acad. Sci. U.S.A. 111, 11365–11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asangani I. A., Dommeti V. L., Wang X., Malik R., Cieslik M., Yang R., Escara-Wilke J., Wilder-Romans K., Dhanireddy S., Engelke C., Iyer M. K., Jing X., Wu Y. M., Cao X., Qin Z. S., et al. (2014) Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 510, 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wein M. N., Jones D. C., Shim J. H., Aliprantis A. O., Sulyanto R., Lazarevic V., Poliachik S. L., Gross T. S., and Glimcher L. H. (2012) Control of bone resorption in mice by Schnurri-3. Proc. Natl. Acad. Sci. U.S.A. 109, 8173–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Z., Yao X., Yan G., Xu Y., Yan J., Zou W., and Wang G. (2016) Mediator MED23 cooperates with RUNX2 to drive osteoblast differentiation and bone development. Nat. Commun. 7, 11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fenaroli F., Westmoreland D., Benjaminsen J., Kolstad T., Skjeldal F. M., Meijer A. H., van der Vaart M., Ulanova L., Roos N., Nyström B., Hildahl J., and Griffiths G. (2014) Nanoparticles as drug delivery system against tuberculosis in zebrafish embryos: direct visualization and treatment. ACS Nano 8, 7014–7026 [DOI] [PubMed] [Google Scholar]
- 40. Adamo A., Sesé B., Boue S., Castaño J., Paramonov I., Barrero M. J., and Izpisua Belmonte J. C. (2011) LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat. Cell Biol. 13, 652–659 [DOI] [PubMed] [Google Scholar]