Abstract
Pulmonary X-ray computed tomographic (CT) and magnetic resonance imaging (MRI) research and development has been motivated, in part, by the quest to sub-phenotype common chronic lung diseases such as chronic obstructive pulmonary disease (COPD). For thoracic CT and MRI, the main COPD research tools, disease biomarkers are being validated that go beyond anatomy and structure to include pulmonary functional measurements such as regional ventilation, perfusion and inflammation. In addition, there has also been a drive to improve spatial and contrast resolution while at the same time reducing or eliminating radiation exposure. Therefore, this review focuses on our evolving understanding of patient-relevant and clinically-important COPD endpoints and how current and emerging MRI and CT tools and measurements may be exploited for their identification, quantification and utilization. Since reviews of the imaging physics of pulmonary CT and MRI and reviews of other COPD imaging methods were previously published and well-summarized, we focus on the current clinical challenges in COPD and the potential of newly emerging MR and CT imaging measurements to address them. Here we summarize MRI and CT imaging methods and their clinical translation for generating reproducible and sensitive measurements of COPD related to pulmonary ventilation and perfusion as well as parenchyma morphology. The key clinical problems in COPD provide an important framework in which pulmonary imaging needs to rapidly move in order to address the staggering burden, costs as well as the mortality and morbidity associated with COPD.
Keywords: phenotypes, pulmonary MRI, COPD, quantitative CT, dual energy CT, pulmonary ventilation and perfusion
INTRODUCTION -COPD MEASUREMENTS AND ENDPOINTS ARE URGENTLY NEEDED
Despite decades of research, therapies that modify chronic obstructive pulmonary disease (COPD) progression or mortality are lacking.1 Despite considerable efforts to discover and develop new COPD interventions, progress has been slow. This is in large part due to limited and suboptimal patient phenotyping that relies on spirometry measurements made at the mouth that cannot account for the regional and inter-subject variability of COPD. Moreover, while COPD is still diagnosed and classified on the basis of symptoms related to the presence of persistent airflow limitation,1 these measurements correlate weakly with important clinical outcomes.2
A wide variety of imaging methods may be used to study the pulmonary system, including those that rely on tissue absorption of x-ray radiation (chest x-ray and CT), radiofrequency stimulation (magnetic resonance imaging: MRI) or signals generated from injected or inhaled radioactive particles (simple gamma emission projection imaging, single photon emission tomography (SPECT) and Positron Emission Tomography (PET)) and inhaled or injected contrast agents. New insights into the basis for disease initiation and progression using these methods have the potential to break through the current conundrum, which is the fact that image acquisition and analysis tools are slow to be adopted because of a lack of meaningful interventions and yet the interventions are slow in coming because of a lack of understanding disease mechanisms and etiology. Therefore, as shown in Figure 1, we frame this review of CT and MRI measurements and phenotypes as potential solutions to the following major COPD problems: 1) COPD treatments are required that improve outcomes, not just symptoms, 2) a better understanding of COPD disease onset and mechanisms is urgently required in order to better design drugs targeted at underlying pathophysiology, 3) more sensitive measurements are required to better understand the links between ventilation, perfusion and inflammation, and, 4) better predictive measurements of COPD exacerbations and progression are critically needed. Here we summarize and compare MRI and CT tools and measurements of COPD because of their near-universal availability that makes their use in COPD multicenter clinical trials and COPD patient care, both practical and timely. Taken together, MRI and CT methods provide a way to identify the underlying pathologies associated with COPD and previous work has been summarized and previously reviewed3–5
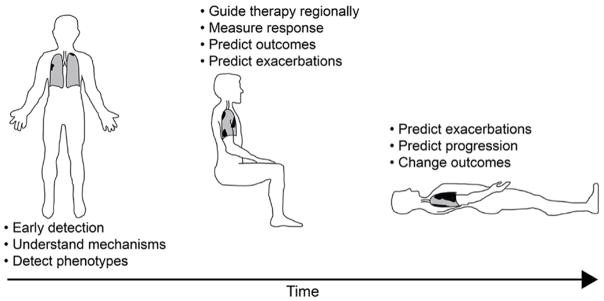
Figure 1. Current COPD challenges.
Three distinct COPD phases are outlined in schematic: 1) Early disease when patients are asymptomatic, clinical measurements typically do not reflect disease but imaging measurements provide evidence of mild emphysema, airways disease, perfusion heterogeneity, LV filling defects, etc. 2) Mild-moderate COPD as patients become symptomatic, clinical measurements are modestly abnormal while imaging measurements can be markedly abnormal revealing regional disease, LV filling defects can continue to worsen, co-morbidities can begin to appear including aortic aneurysms, coronary disease, lung nodules, osteoporosis, 3) Severe COPD with patients reporting severe symptoms and activity impairment, clinical measurements of airflow limitation, diffusing capacity of carbon monoxide and gas trapping are markedly abnormal and yet patients still can be differentiated into those with predominantly airway or predominantly parenchymal disease with marked differences in the distribution of parenchymal destruction.
PULMONARY CT MEASUREMENTS OF COPD
CT Structural Measurements and Phenotypes
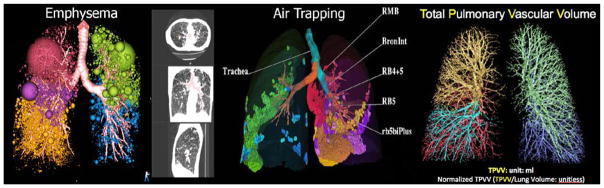
Computer-based methods6–9 for the objective quantitation of CT images are increasingly used in multi-center studies10–15 that aim to interrogate phenotype-genotype linkages and identify intermediary endpoints for the assessment of potential interventions. A cornerstone of this approach is the CT lung tissue attenuation or density mask that provides a way to estimate regional COPD-related emphysema. By empirically defining regional emphysematous lung using selected threshold tissue attenuation measurements (Hounsfield Unit: HU) at full inspiration (total lung capacity: TLC), one can count the number of voxels in the whole lung and express this measure. In this way, the percent of voxels reflecting emphysema can be expressed relative to the total volume of the lung or lung region.16–23 The CT density mask is particularly useful in classifying mild/moderate and severe emphysema24,25 and has been used in the National Emphysema Treatment Trial (NETT) to identify subgroups of patients who show benefit from lung volume reduction surgery.26 In the left panel of Figure 2, (using Apollo software provided by VIDA Diagnostics, Coralville, IA, USA), the spatially-heterogeneous distribution of emphysema, shown in spheres and regional clustering (sphere size) is shown by lung lobe. Similarly, a density mask may be used on the expiratory dataset (RV or FRC) to identify regions of air trapping as shown in the middle panel of Figure 2. In both the inspiratory and expiratory images, airways may also be segmented and labeled9,27,28 using commercially-available software such that relationships can be determined between the airway path, airway remodeling features, and parenchymal destruction or peripheral airway closure. CT-based density metrics have been used in numerous large-scale studies12–15, producing a wealth of literature over the past several years. These measures have identified correspondence of quantitative CT measures of emphysema and air trapping to, for example: genotypes,29,30 left ventricular (LV) filling,31 physiologic measures,32,33 environmental smoke exposure in childhood as a risk factor for emphysema,34 predictors of bronchoreversibility,35 association of cigarette smoking with sub-clinical disease,36 and trapped gas in severe asthma.37 It is also important to note that in addition to tissue attenuation or density masks, texture analyses may be employed to evaluate clusters of emphysema such38 as estimated using low attenuating clusters. Image analysis methods based on such local feature patterns provide a way to objectively differentiate between these subtypes and some of these methods have better classification rates than expert radiologists39. So-called low attenuation clusters are visually obvious, correlating with histopathology measurements40 and more closely reflecting scoring performed by a radiologist41
Figure 2.
CT Measurements: Threshold-based evaluation of the extemt and distribution of emphysema at full inspiration (far left panel), amount and air trapping at expiration to functional residual capacity (FRC) or residual volume (RV) (middle panels), airway geometry assessed in conjunction with distribution patterns of emphysema and air trapping (middle panels), vascular anatomy (total pulmonary vascular volume and total pulmonary arterial volume assessed from full inspiratory non-contrast enhanced CT scans (far right panel). Colour-coding differentiate between lung lobes.
However, CT quantification of the lung parenchyma is also challenging. Scanner mis-calibration, inconsistent use of reconstruction kernels, differences in reconstruction algorithms between manufacturers and poor coaching of the patient to the targeted lung volume can result in measurement variability.42–45 Accurate, quantitative CT requires image acquisition consistency46 and even then, subject age, sex, race/ethnicity and height influence the normal range of these measures (similar to lung function) as does weight.47 Current smoking status has a large and paradoxical effect on these measures,47 making precise measurements of smoking status (eg. Cotinine or other objective measure) important in longitudinal studies. Reference equations for both percent emphysema and total lung volume on CT are now available to account for most of these differences.47 The limitations due to concerns of radiation dose are being addressed with recent advances in CT technologies, including improvements in the x-ray tube, detector technology, adaptive exposure 48,49 and iterative image reconstruction,50–54 leading to clinically-adequate image quality with sub-mSv doses.48,55 Finally, despite considerable successes using quantitative CT to assess the presence and distribution of emphysema and airway wall remodeling56–61, critical underlying differences among disease sub-populations continue to emerge. For example, in an apparently discordant result, spatially-matched airway segmentation demonstrated that airway walls may become thinner rather than thicker in COPD, leaving open the possibility that airway wall remodeling may, itself have multiple phenotypes.9
Functional CT Measurements and Phenotypes
Pulmonary CT Vascular or Perfusion
It has long been thought that a better understanding of the pathologic response to inflammation in COPD and asthma will be important for the design of new therapies. What is well-understood is that in response to noxious particles and gases in cigarette smoke, the lung reacts by recruiting inflammatory cells. Pulmonary vascular changes, including thickening of the vessel walls, have been characterized early in the history of COPD.62,63 More recently it was observed that in the presence of inflammation, there is an enhanced delivery of progenitor cells to the lung.64,65 Remy-Jardin and colleagues recently observed an increased propensity for the lung to develop emphysema in regions of suspected inflammatory processes as defined by ill-defined ground glass opacities and micro nodules.66 In more than half of smokers, the inflammatory process was able to resolve itself with repair and maintenance of normal parenchymal anatomy and function. For the remainder (30–45% of smokers) there was parenchymal destruction. There is evidence in the literature suggesting that in humans and animals, hypoxic pulmonary vasoconstriction (HPV) is normally blocked in the presence of inflammation.25,67–69 For example, Alford et al.70 demonstrated that smokers with normal pulmonary function, but small visibly obvious signs of localized, apical centrilobular emphysema, have an increase in coefficient of variation (CV) of CT-based regional pulmonary blood flow (PBF) and mean transit time (MTT), supporting a hypothesis that one etiology of smoking-associated emphysema may be failure to maintain PBF to inflamed lung regions. Recent work published together with an associated editorial31,71 also demonstrated and discussed the strong relationship between impaired LV filling and percent emphysema in a group of non-smokers, ex-smokers and current smokers. The correlation between impaired LV filling with emphysema was greatest in current smokers compared with previous smokers, consistent with the notion that the inflammatory effects of smoking may determine this effect rather than emphysema alone.
With the observation that pulmonary perfusion heterogeneity may serve as a biomarker in smokers susceptible to centrilobular emphysema,70 it has been hypothesized that the lung normally inhibits hypoxic pulmonary vasoconstriction (HPV) in the presence of inflammation. Conversely, in patients unable to block HPV in inflamed lung regions, perfusion is reduced, thus prolonging inflammation and limiting repair mechanisms. With the use of single breath methods in conjunction with dual energy CT, efforts have been directed towards maintaining sensitivity to increased perfusion heterogeneity while simplifying imaging methodologies.
Dual energy CT (DECT) provides a way to double the temporal resolution of CT making it possible to image the entire lung field with a 0.62 mm slice thickness in 0.6 seconds. By having two x-ray guns together with two sets of detector rows, both capable of acquiring 128 slices of image data, there is now the possibility of dual energy CT which allows for sensitive discrimination between tissue types and contrast agents such as iodine for perfusion and xenon for ventilation. At two different peak kilo-voltages (kVp), the reconstructed CT densities for iodine or xenon are shifted significantly between the two resultant image data sets, but the body tissues are not. Thus, because the two image sets were acquired simultaneously, assuring alignment, with a modified form of image subtraction (material decomposition) it is possible to assess perfused blood volume or regional ventilation respectively. For regional assessment of perfused blood volume, when blood is equilibrated with a concentration of iodine (or gadolinium) through the slow infusion of contrast agent, an image of iodine (or gadolinium) can be directly related to regional blood volume.
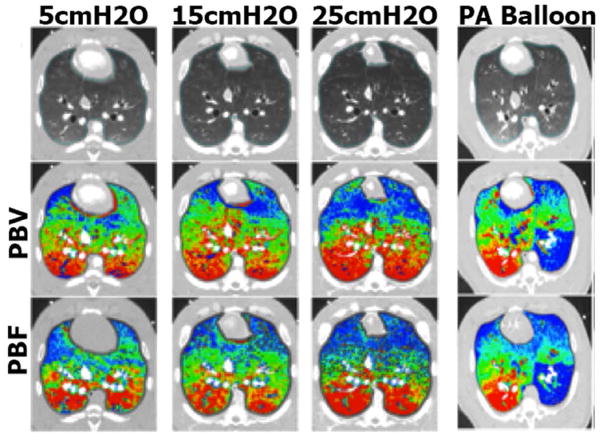
Other studies have demonstrated the equivalency of perfused blood volume (PBV), as assessed by dual energy CT, and true perfusion. Perfusion blood volume and perfused blood flow (PBF) likely reflect one another because peripheral vascular beds dilate and capillary beds are recruited with increased PBF. PBV may be evaluated using DECT during a slow infusion of x-ray contrast. As shown in Figure 3 modified72 from a report on interventions performed in pigs, regional perfusion (colour-coded from red to blue as percent of total perfused blood volume or total perfusion) was perturbed either by incrementally pulling back a balloon catheter placed in a pulmonary artery or by imaging the lung at various static inflation pressures which effectively reduces perfusion in the non-dependent lung regions.72 Under all conditions, PBF maps and PBV maps are strikingly similar and a strong relationship for PBF and PBV heterogeneity was also demonstrated.
Figure 3.
Gray-scale (top row), PBV (middle row), and PBF (lower row) MDCT scans. (Columns 1–3) Color map comparison of CT-derived PBF and PBV from pig imaged at 3 different lung volumes, used to achieve a range of pulmonary perfusion values. (Column 4) Color map comparison of CT-derived PBF (dynamic axial scanning) and PBV (dual energy spiral scanning) from pig studied with a balloon partially inflated in a left lower lobe pulmonary artery. Color coding is the same for each condition: percent of total PBV or PBV with low values in blue and high values in red. Modified from 72.
Pulmonary CT Ventilation
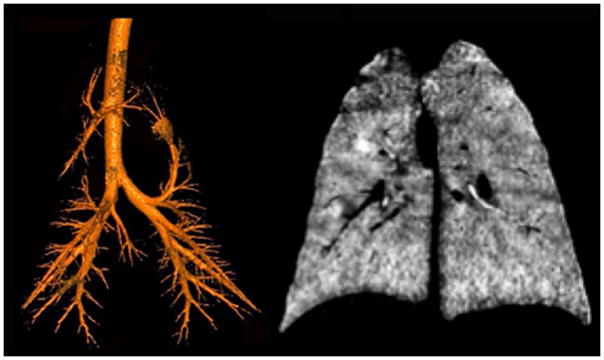
Xenon gas regional wash-in and wash-out kinetic studies were also explored using CT imaging73–75 and these methods have been translated clinical studies.76–82 Importantly, with the more recent development of dual-energy CT, it is possible to simplify the assessment of regional ventilation via use of single breath methods. As shown in Figure 4, by adjusting xenon inhalation of a Xe/O2 gas mixture, gas flow to the central airways may be monitored and compared with gas flow to the parenchyma. As shown in Figure 4 left panel, the central airway tree is identified by having the subject inhale to TLC, exhale the central dead space volume and re-inhale the same volume of a Xenon gas mixture. This leaves just the central airways filled with Xenon gas. In the right panel, results are shown from of a single inhalation of a Xenon gas mixture. It was also demonstrated that with a slow inhalation of Xenon gas mixtures, geometry and gas density influence or dominate gas distribution causing increased ventilation heterogeneity while with a rapid inspiration, ventilation is more homogeneous.83 Furthermore, the gravity-driven distribution of Xe gas can be eliminated by mixing Xe with helium (He), likely due to the change in the gas density mixture. Another contrast gas agent, Krypton (Kr) while less radio-dense than Xe84,85, has no anesthetic effects. As multi-spectral CT technologies, including Photon Counting CT86–88 evolve, it is expected that sensitivity to Kr gas contrast will improve, making it a potential translational/clinical method.
Figure 4.
Dual Energy CT scans of the airway tree (left) and lung parenchyma (right) of an anesthetized pig. For the scan in the left panel, the lung was inflated to 25 cmH2O airway pressure using room air. An amount of air approximately equal in volume to the central airway tree was removed and replaced with xenon gas. This provided a way to identify the central airway tree without the use of more conventional airway segmentation methods. In the right panel, the lungs were inflated from functional residual capacity to total lung capacity via a gas mixture of 80%xenon and 20%oxygen. Material decomposition image processing was used to generate an image representing the regional distribution of the inhaled xenon gas.
As an alternative to inhaled contrast agents, recent studies89,90 have shown that regional measures of lung ventilation can be assessed using CT images acquired at different volumes without the need for inhaled contrast agents. A two-lung volume (TLC or full inspiration and RV or full expiration) protocol has been standardized as part of “SPIROMICS”13 CT protocol that obtains isotropic sub-millimeter images of the entire lungs at TLC and RV. Using this protocol together with advanced image registration methods90–95, regional maps of lung ventilation can be obtained at spatial resolutions close to the size of a pulmonary acinus. Image matching of an inspiratory/expiratory lung image pair has also been employed7 to help differentiate air trapping from emphysema. Functional measurements may be directly generated from expiratory CT96,97 or a combination of inspiratory and expiratory CT, including those generated using parametric response maps.7 All of these important approaches have the potential to test the hypotheses generated using micro-CT98 about the pathological mechanisms that accompany the earliest airway and parenchyma changes in COPD.
There is well-developed software commercially available for evaluating the relationship of airway microstructural abnormalities with ventilation and emerging software tools are now being developed to probe the geometry of the pulmonary arterial and venous trees and vascular-structure-function relationships.99–101 The extracted pulmonary vascular tree from a non-contrast inspiratory MDCT volume of the lung is shown in the right panel of Figure 2, and the volume of this combined arterial and venous tree – the Total Pulmonary Vascular Volume or TPVV has been generated developed using methods that segment the arterial tree.101 The cross sectional area of the pulmonary trunk relative to the aorta has been shown102 to correlate with acute exacerbations in COPD patients and TPVV (normalized to total lung volume) is currently being used as an upstream marker of down-stream endothelial dysfunction.103
PULMONARY MRI MEASUREMENTS OF COPD
Conventional 1H MRI Structural and Functional Phenotypes
As pulmonary CT continues to advance with new capabilities and lower radiation doses, pulmonary MRI has also advanced to provide complimentary tools for the quantitative evaluation of lung structure and function. However, pulmonary MRI using conventional hardware platforms (1H methods) is very technically challenging and therefore currently, its clinical use, has been limited. These technical demands stem from the inherently low pulmonary 1H abundance and corresponding low 1H signal that can be measured using conventional MRI approaches. Furthermore, the multitude of lung air-tissue interfaces generate significant magnetic field distortions or susceptibility artifacts, further diminishing pulmonary 1H MRI signal. For these reasons, and until recently, the major applications of conventional pulmonary 1H MRI included intravenous contrast agents to evaluate pulmonary blood flow and vessel hemodynamics.104 Methods have also been devised that combine relaxation signals and intravenous contrast that provide a way to differentiate inflammation105, smooth muscle remodeling, edema and mucus deposition.104,105 Taken together, these methods provide a way to identify the underlying pathologies associated with COPD. However, because signal intensity is generally very low using 1H MRI, careful calibration with other organs in the same field of view is required. In addition, because of gravity-dependent changes occur, signal averaging over time may not provide physiologically-relevant information in the dependent lung regions, where atelectasis may occur in minutes while supine.
Another way to address these challenges is to reduce the time (echo time or TE) required to acquire the pulmonary MR signal. As shown in Figure 5, conventional, ultra-short TE (UTE) pulse sequences as suggested 2 decades ago106 significantly improve pulmonary MR signal so that emphysematous regions can be identified and quantified, based upon the difference between tissue-poor emphysematous bullae and more normal parenchyma. So-called ultrashort-echo or zero echo time MRI methods help address the inherent challenges of low tissue and 1H density107 by minimizing the effects of rapid MR signal decay. The relationship between MRI signal intensity and tissue density108, as previously shown for pulmonary CT, is clearly important for further development of the method. The first studies employing UTE methods reported that the tissue density was related to MR signal109 and T2*. UTE MRI was also used to measure signal intensity and T2* in emphysema110 and showed good correlations with histological measurements while T2* correlated with pulmonary function measurements111 and pulmonary signal intensity was related to tissue density, pulmonary function and CT density measurements.108 Very recently methods have been developed that exploit optimized112 and so-called zero echo time (ZTE)113 approaches and this has resulted in excellent, MR image quality and signal in pulmonary images that is very similar to CT. Another method for measuring regional lung function, involves using inhaled oxygen in combination with 1H MRI114 and this exploits the alteration of lung tissue 1H relaxation times by molecular O2. In this manner, wash-in or difference maps can be generated by using the inherent signal differences that stem from breathing room air and pure O2. While pulmonary ventilation is reflected by O2 wash-in maps, the alveolar-capillary transfer may be reflected by the O2-enhancement ratio.115 Like all 1H-based MRI, O2-enhanced MRI is limited by the weak proton signal and the fact that O2-enhanced measurements still require histopathological validation. In fact, for all MRI measurements of COPD, validation of the pathologies directly or indirectly measured is still pending.
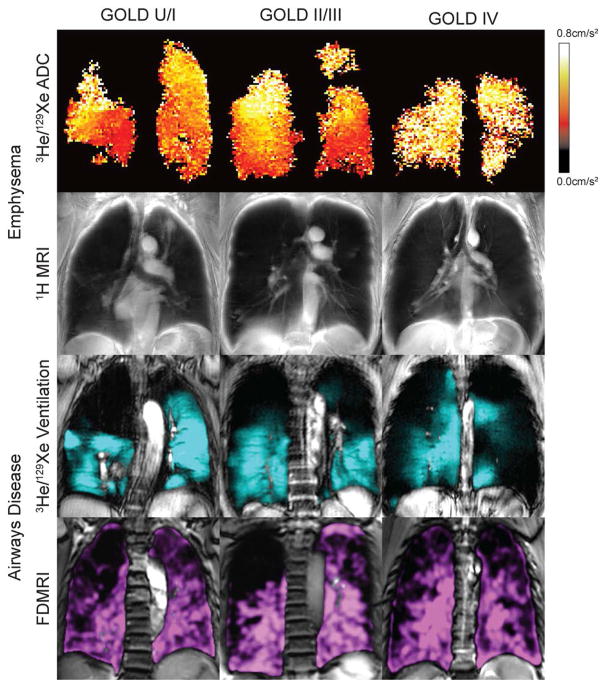
Figure 5.
MRI measurements of COPD for different GOLD stages including: emphysema (ADC, 1H MRI signal intensity), and ventilation (3He MRI and FDMRI).
To tackle the challenge of very low pulmonary 1H signal intensity, Bauman and colleagues116–118 proposed another ingenious approach that relies on MRI signal oscillations that occur with the differences in lung volume during normal tidal breathing. It was hypothesized that the 1H MRI signal oscillations during breathing could be employed to generate both ventilation and perfusion images. They developed a way119 using Fourier decomposition (FD) of oscillating 1H signal intensity120 related to the compression and expansion of the lung parenchyma and blood flow121 to generate pulmonary ventilation and/or perfusion measurements. Importantly, this method was recently shown in COPD subjects with emphysema 122 and contrast can be generated using static volumetric 1H MRI methods.123 Figure 5 shows very recent examples of Fourier-decomposition of pulmonary magnetic resonance imaging (FDMRI) in COPD subjects across GOLD grades and excellent comparisons were also shown in animal studies.124 FDMRI exploits free-breathing 1H MRI and non-rigid registration to generate ventilation images. Importantly, although image registration and analysis is complex, and again dependent on weak 1H MRI contrast, this approach does not depend on inhaled gas or injected contrast agents and therefore there is strong potential for clinical translation. Again, a more complete validation still needs to be undertaken.
Inhaled 19F and hyperpolarized 129Xe/3He MRI Structural and Functional Phenotypes
Similar to FD and O2-enhanced 1H MRI methods, MRI using inhaled 19F gas (after multiple breaths of perfluoropropane mixed with oxygen), or hyperpolarized noble gases such as 3He and 129Xe (after a single breath mixed with 4He or N2) provides a way to visualize pulmonary ventilation by taking advantage of high gas density in the lung. For inhaled 3He and 129Xe, increased nuclear polarization generates ventilation images of the airways and airspaces and to measure apparent diffusion coefficients that estimate gas displacement. For 129Xe, similar to Xe-CT methods, the fractional solubility of Xe gas in biological tissues125 has additional applications for measuring gas exchange, alveolar surface area and perfusion. For all three gases, the nuclear proton provides the MRI signal. In the case of 19F, MR signal is inherently strong because of the high gyromagnetic ratio of 19F such that extra polarization (and polarization equipment) is not required. For both 3He and 129Xe, however, nuclear polarization is achieved126,127 using laser polarization equipment. As shown in Figure 5, 3He and 129Xe MRI provide superior signal-to-noise ratio ventilation images in COPD subjects, and typically, image quality using 3He MRI is the greatest compared with polarized 129Xe and unpolarized 19F due to larger gyromagnetic ratio and high polarization rates for 3He. For these reasons, currently 3He MRI is most commonly used in research even though the global quantities of 3He are very limited and expensive. Although experience with 129Xe and 19F is still limited,128,129 these inhaled gas MRI methods provide the strongest translational potential because of the relative abundance and low cost of these gases. Notwithstanding these challenges, recent acute therapy studies in COPD post-salbutamol130 and post-COPD exacerbation therapy requiring hospitalization131 show the potential for inhaled gas MRI ventilation defect measurements to represent the subtle (post-exacerbation) and not so subtle (post-salbutamol) ventilation improvements after therapy. It is important to note that the visibly obvious and statistically-significant improvements in ventilation in these cases were not related to FEV1 improvements. In addition, in a number of these previous COPD studies132, and as shown in Fig. 6 ventilation measured using MRI was directly related to CT-derived measurements of abnormally-remodeled airways and emphysema. Importantly, previous work also showed the relationship of ventilation abnormalities with subclinical disease in smokers with normal lung function.133 Moreover, regional ventilation worsening in COPD has been measured over a follow-up period of two years134 even in patients with no change in FEV1. The Polarized Helium Imaging of the Lung (PHIL) study, was performed in three European centres in 122 COPD patients and is yet the largest COPD MRI-CT multicenter comparison study completed to date.132 This important study showed the potential for COPD biomarkers stemming from a comprehensive, prospectively planned, multi-centre and multimodality imaging approach. Another innovative example135 showed the potential of ventilation MRI to interrogate the role of regional collateral ventilation in COPD patients with bullous emphysema. Finally MRI measurements of ventilation in mild and moderate patients (GOLD I/II COPD)136 were also predictive of COPD exacerbation requiring hospital care, and in these patients, previous exacerbation and FEV1 were not.
Figure 6.
MR ventilation imaging reflecting the effects of both emphysema (slow filling units) and airways disease (airway obstruction) – a unique predictor of COPD exacerbations in mild disease.
For all inhaled gas MRI methods, diffusion due to random Brownian motion within the lung airways and airspaces can be measured using diffusion-weighted imaging similar to that used in conventional MRI.137 3He or 129Xe diffusion-weighted methods provide measurements of parenchyma microstructures, including the alveoli and acini that define the boundaries of the fundamental units for gas exchange.138 The ADC map provides quantitative regional airspace measurements information that is in agreement with the presence of emphysematous damage.139,140 Such measurements are consistent with alveolar changes related to differences in lung volumes141 gravitational dependence141–143 and ageing.144 Previous COPD studies have shown that ADC correlates with pulmonary function,142,145,146 histological measurements of lung surface area147 and is highly reproducible in COPD,141 while sensitive to subclinical disease148,149 and disease progression.150 Novel approaches have also been used to measure diffusion in longer time frames151, providing a way to generate information about acinar duct and airway connectivities, including communication and collateral ventilation.152,153 Long-range ADC appears to be more than twice as sensitive to parenchymal differences associated with COPD than short-range ADC154–156. Unfortunately this important information has not yet been exploited in clinical research studies.
Gadolinium-enhanced MRI Pulmonary Perfusion Phenotypes
Given the relative lack of 1H signal in the lung using conventional approaches, gadolinium-enhanced imaging of first-pass pulmonary perfusion provides sensitive and relatively reproducible measurements of signal increase and its change over time (slope). Fourier transformation of the signal change allows for the assessment of pulmonary blood flow, mean transit time and pulmonary blood volume. These measures can be attained over the whole lung or the lung periphery in order to evaluate microvascular perfusion.157–159 Pulmonary perfusion deficits were observed more frequently in COPD patients as compared to healthy volunteers; the largest study to date noted largely diminished pulmonary microvascular blood flow in mild to severe COPD with strong spatial correlations in severe disease to centrilobular and panlobular emphysema.160
CONCLUSIONS
As summarized in Table 1, pulmonary CT and MRI are on the threshold of providing regional, non-invasive measurements of ventilation, perfusion and parenchymal destruction as intermediate endpoints of COPD. However, whereas pulmonary CT measurements have been evaluated in COPDGene,12 ECLIPSE,14 MESA Lung,161 CanCOLD,15 and SPIROMICS,13 and cardiac MRI has been utilized in over 5000 participants in MESA, pulmonary MRI has not been exploited in large-scale studies. There are many reasons for this even though pulmonary MRI is rapid, well-tolerated and radiation-free, so it serves as an ideal platform for serial and longitudinal evaluations in patients. More widespread use of all imaging biomarkers has been limited for a number of key reasons including: 1) lack of support to harmonize image acquisition software, 2) universally available image analysis software, 3) regulatory boundaries for emerging approaches, and, 4) historically weak links between respiratory and radiology clinical programs. Not-withstanding these issues, CT and MRI measurements of COPD are being developed to provide a better understanding of disease onset, develop COPD treatments that improve outcomes, and to provide better predictive measurements of COPD exacerbations and progression.
Table 1.
MRI and CT COPD Phenotypes
| Airways Disease | Imaging Biomarkers of COPD | ||
|---|---|---|---|
| Emphysema | Perfusion abnormalities | ||
| CT | Lumen area Wall area % Pi10 PRM-gas trapping Xe gas ventilation |
Low attenuating clusters Low attenuating area RA950 RA856 PRM-emphysema |
Total pulmonary vascular volume Iodine perfusion |
| MRI | Ventilation defect percent Percent ventilated volume |
ADC | Gadolinium perfusion Xe perfusion/diffusion |
The unique and complementary ability of both MRI and CT to measure disease morphological and functional consequences and explore mechanisms of disease pathophysiology has not yet translated to improved COPD patient care. In COPD, there is an increasing recognition that different phenotypes exist162–164 and that these patient groups may have different responses to therapy. Moreover as therapies become more targeted and patient-specific, imaging will likely be one of the only ways to verify response and efficacy for an individual patient or group of patients. This will require imaging to become more quantitative, sensitive and accessible to justify its current cost and complexity. Although the risks related to tobacco smoking will decrease over time in the developed world, as the world becomes more industrialized and polluted, and smoking habits change in the developing world, respiratory illnesses will continue to increase in prevalence, morbidity and overall mortality. It is in this clinical context that development and appropriate utilization of pulmonary MRI and CT remain critically important.
Acknowledgments
We are grateful to our research teams and collaborators for their continued support in the development and application of new pulmonary CT and MRI tools for the measurement and monitoring of COPD. We also thank Khadija Sheikh, Dante Capaldi, Melissa Shirk, Jered Sieren and Ann Thompson for their assistance with figures and manuscript preparation. This work was the result of extensive discussions during the 6th International Workshop for Pulmonary Functional Imaging, Madison, WI.
References
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013 Feb 15;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Jones PW. Health status and the spiral of decline. Copd. 2009 Feb;6(1):59–63. doi: 10.1080/15412550802587943. [DOI] [PubMed] [Google Scholar]
- 3.Milne S, King GG. Advanced imaging in COPD: insights into pulmonary pathophysiology. Journal of thoracic disease. 2014 Nov;6(11):1570–1585. doi: 10.3978/j.issn.2072-1439.2014.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sverzellati N, Molinari F, Pirronti T, Bonomo L, Spagnolo P, Zompatori M. New insights on COPD imaging via CT and MRI. International journal of chronic obstructive pulmonary disease. 2007;2(3):301–312. [PMC free article] [PubMed] [Google Scholar]
- 5.van Beek EJ, Hoffman EA. Functional imaging: CT and MRI. Clinics in chest medicine. 2008 Mar;29(1):195–216. vii. doi: 10.1016/j.ccm.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006 Aug;3(6):519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galban CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012 Nov;18(11):1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro M, Fain SB, Hoffman EA, et al. Lung imaging in asthmatic patients: the picture is clearer. J Allergy Clin Immunol. 2011 Sep;128(3):467–478. doi: 10.1016/j.jaci.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith BM, Hoffman EA, Rennard S, Barr RG. Location, location, location: studying anatomically comparable airways is highly relevant to understanding COPD. Thorax. 2014 Nov;69(11):1049–1050. doi: 10.1136/thoraxjnl-2014-206188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Lung Screening Trial Research T. Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011 Jan;258(1):243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr RG, Ahmed FS, Carr JJ, et al. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. The European respiratory journal. 2012 Apr;39(4):846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010 Feb;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couper D, LaVange LM, Han MK, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2013 Sep 12;0:1–4. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faner R, Tal-Singer R, Riley JH, et al. Lessons from ECLIPSE: a review of COPD biomarkers. Thorax. 2014 Jul;69(7):666–672. doi: 10.1136/thoraxjnl-2013-204778. [DOI] [PubMed] [Google Scholar]
- 15.Bourbeau J, Tan WC, Benedetti A, et al. Canadian Cohort Obstructive Lung Disease (CanCOLD): Fulfilling the Need for Longitudinal Observational Studies in COPD. Copd-Journal of Chronic Obstructive Pulmonary Disease. 2014 Apr;11(2):125–132. doi: 10.3109/15412555.2012.665520. [DOI] [PubMed] [Google Scholar]
- 16.Adams H, Bernard M, McConnochie K. An appraisal of CT pulmonary density mapping in normal subjects. Clinical Radiology. 1991;43(4):238–242. doi: 10.1016/s0009-9260(05)80245-4. [DOI] [PubMed] [Google Scholar]
- 17.Kinsella M, Muller NL, Abboud RT, Morrison NJ, DyBuncio A. Quantitation of Emphysema by computed tomography using a “density mask” program and correlation with pulmonary function tests. Chest. 1990;97:315–321. doi: 10.1378/chest.97.2.315. [DOI] [PubMed] [Google Scholar]
- 18.Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94(4):782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 19.Gould GA, Macnee W, McLean A, et al. CT Measurements of Lung Density in Life can quantitate distal airspace enlargement-an essential defining feature of human emphysema. Am Rev Resp Dis. 1988;137:380–392. doi: 10.1164/ajrccm/137.2.380. [DOI] [PubMed] [Google Scholar]
- 20.Hartley PG, Galvin JR, Hunninghake GW, et al. High-resolution CT-derived measures of lung density are valid indexes of interstitial lung disease. J Applied Physiology. 1994;76(1):271–277. doi: 10.1152/jappl.1994.76.1.271. [DOI] [PubMed] [Google Scholar]
- 21.Coxson HO, Rogers RM. Quantitative computed tomography of chronic obstructive pulmonary disease. Acad Radiol. 2005 Nov;12(11):1457–1463. doi: 10.1016/j.acra.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Newell JD, Jr, Hogg JC, Snider GL. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. The European respiratory journal. 2004 May;23(5):769–775. doi: 10.1183/09031936.04.00026504. [DOI] [PubMed] [Google Scholar]
- 23.Stolk J, Ng WH, Bakker ME, et al. Correlation between annual change in health status and computer tomography derived lung density in subjects with alpha1-antitrypsin deficiency. Thorax. 2003 Dec;58(12):1027–1030. doi: 10.1136/thorax.58.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coxson HO. Quantitative chest tomography in COPD research: chairman’s summary. Proc Am Thorac Soc. 2008 Dec 15;5(9):874–877. doi: 10.1513/pats.200810-118QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006 Aug;3(6):519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003 May 22;348(21):2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 27.Tschirren J, Hoffman EA, McLennan G, Sonka M. Intrathoracic airway trees: segmentation and airway morphology analysis from low-dose CT scans. IEEE transactions on medical imaging. 2005 Dec;24(12):1529–1539. doi: 10.1109/TMI.2005.857654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tschirren J, McLennan G, Palagyi K, Hoffman EA, Sonka M. Matching and anatomical labeling of human airway tree. IEEE transactions on medical imaging. 2005 Dec;24(12):1540–1547. doi: 10.1109/TMI.2005.857653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho MH, Washko GR, Hoffmann TJ, et al. Cluster analysis in severe emphysema subjects using phenotype and genotype data: an exploratory investigation. Respiratory research. 2010;11:30. doi: 10.1186/1465-9921-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manichaikul A, Hoffman EA, Smolonska J, et al. Genome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014 Feb 15;189(4):408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010 Jan 21;362(3):217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Washko GR, Martinez FJ, Hoffman EA, et al. Physiological and computed tomographic predictors of outcome from lung volume reduction surgery. American journal of respiratory and critical care medicine. 2010 Mar 1;181(5):494–500. doi: 10.1164/rccm.200906-0911OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Washko GR, Criner GJ, Mohsenifar Z, et al. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. Copd. 2008 Jun;5(3):177–186. doi: 10.1080/15412550802093025. [DOI] [PubMed] [Google Scholar]
- 34.Lovasi GS, Diez Roux AV, Hoffman EA, Kawut SM, Jacobs DR, Jr, Barr RG. Association of environmental tobacco smoke exposure in childhood with early emphysema in adulthood among nonsmokers: the MESA-lung study. American journal of epidemiology. 2010 Jan 1;171(1):54–62. doi: 10.1093/aje/kwp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han MK, Wise R, Mumford J, et al. Prevalence and clinical correlates of bronchoreversibility in severe emphysema. The European respiratory journal. 2009 Nov 19; doi: 10.1183/09031936.00052509. [DOI] [PubMed] [Google Scholar]
- 36.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. American journal of respiratory and critical care medicine. 2009 Sep 1;180(5):407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busacker A, Newell JD, Jr, Keefe T, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009 Jan;135(1):48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishima M, Hirai T, Itoh H, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 1999 Aug 3;96(16):8829–8834. doi: 10.1073/pnas.96.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Sonka M, McLennan G, Guo J, Hoffman EA. MDCT-based 3-D texture classification of emphysema and early smoking related lung pathologies. IEEE Trans Med Imaging. 2006 Apr;25(4):464–475. doi: 10.1109/TMI.2006.870889. [DOI] [PubMed] [Google Scholar]
- 40.Madani A, Van Muylem A, de Maertelaer V, Zanen J, Gevenois PA. Pulmonary emphysema: size distribution of emphysematous spaces on multidetector CT images--comparison with macroscopic and microscopic morphometry. Radiology. 2008 Sep;248(3):1036–1041. doi: 10.1148/radiol.2483071434. [DOI] [PubMed] [Google Scholar]
- 41.Gietema HA, Muller NL, Fauerbach PV, et al. Quantifying the extent of emphysema: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol. 2011 Jun;18(6):661–671. doi: 10.1016/j.acra.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Stoel BC, Bakker ME, Stolk J, et al. Comparison of the sensitivities of 5 different computed tomography scanners for the assessment of the progression of pulmonary emphysema: a phantom study. Investigative Radiology. 2004 Jan;39(1):1–7. doi: 10.1097/01.rli.0000091842.82062.a3. [DOI] [PubMed] [Google Scholar]
- 43.Shaker SB, Dirksen A, Laursen LC, Skovgaard LT, Holstein-Rathlou NH. Volume adjustment of lung density by computed tomography scans in patients with emphysema. Acta radiologica. 2004 Jul;45(4):417–423. doi: 10.1080/02841850410005525. [DOI] [PubMed] [Google Scholar]
- 44.Boedeker KL, McNitt-Gray MF, Rogers SR, et al. Emphysema: effect of reconstruction algorithm on CT imaging measures. Radiology. 2004 Jul;232(1):295–301. doi: 10.1148/radiol.2321030383. [DOI] [PubMed] [Google Scholar]
- 45.Sieren JP, Newell JD, Judy PF, et al. Reference standard and statistical model for intersite and temporal comparisons of CT attenuation in a multicenter quantitative lung study. Medical physics. 2012 Sep;39(9):5757–5767. doi: 10.1118/1.4747342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman EA, Barr R. Thresholds to Lung Density Measures Hoffman and Barr Replies. Academic Radiology. 2010;17:339–401. [Google Scholar]
- 47.Hoffman EA, Ahmed FS, Baumhauer H, et al. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Annals of the American Thoracic Society. 2014 Jul;11(6):898–907. doi: 10.1513/AnnalsATS.201310-364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newell JD, Jr, Fuld MK, Allmendinger T, et al. Very low-dose (0.15 mGy) chest CT protocols using the COPDGene 2 test object and a third-generation dual-source CT scanner with corresponding third-generation iterative reconstruction software. Invest Radiol. 2015 Jan;50(1):40–45. doi: 10.1097/RLI.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sieren JP, Hoffman EA, Fuld MK, Chan KS, Guo J, Newell JD., Jr Sinogram Affirmed Iterative Reconstruction (SAFIRE) versus weighted filtered back projection (WFBP) effects on quantitative measure in the COPDGene 2 test object. Medical physics. 2014 Sep;41(9):091910. doi: 10.1118/1.4893498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pontana F, Pagniez J, Flohr T, et al. Chest computed tomography using iterative reconstruction vs filtered back projection (Part 1): Evaluation of image noise reduction in 32 patients. Eur Radiol. 2011 Mar;21(3):627–635. doi: 10.1007/s00330-010-1990-5. [DOI] [PubMed] [Google Scholar]
- 51.Yanagawa M, Honda O, Yoshida S, et al. Adaptive statistical iterative reconstruction technique for pulmonary CT: image quality of the cadaveric lung on standard- and reduced-dose CT. Academic radiology. 2010 Oct;17(10):1259–1266. doi: 10.1016/j.acra.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Honda O, Yanagawa M, Inoue A, et al. Image quality of multiplanar reconstruction of pulmonary CT scans using adaptive statistical iterative reconstruction. The British journal of radiology. 2011 Apr;84(1000):335–341. doi: 10.1259/bjr/57998586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heilbron BG, Leipsic J. Submillisievert coronary computed tomography angiography using adaptive statistical iterative reconstruction - a new reality. The Canadian journal of cardiology. 2010 Jan;26(1):35–36. doi: 10.1016/s0828-282x(10)70333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moscariello A, Takx RA, Schoepf UJ, et al. Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique-comparison with traditional filtered back projection. Eur Radiol. 2011 Oct;21(10):2130–2138. doi: 10.1007/s00330-011-2164-9. [DOI] [PubMed] [Google Scholar]
- 55.McCollough CH, Chen GH, Kalender W, et al. Achieving routine submillisievert CT scanning: report from the summit on management of radiation dose in CT. Radiology. 2012 Aug;264(2):567–580. doi: 10.1148/radiol.12112265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coxson HO, Leipsic J, Parraga G, Sin DD. Using pulmonary imaging to move chronic obstructive pulmonary disease beyond FEV1. American journal of respiratory and critical care medicine. 2014 Jul 15;190(2):135–144. doi: 10.1164/rccm.201402-0256PP. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman EA, Clough AV, Christensen GE, et al. The comprehensive imaging-based analysis of the lung: a forum for team science. Acad Radiol. 2004 Dec;11(12):1370–1380. doi: 10.1016/j.acra.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Jarjour NN, Erzurum SC, Bleecker ER, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. American journal of respiratory and critical care medicine. 2012 Feb 15;185(4):356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. The Lancet. Respiratory medicine. 2013 Apr;1(2):129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 60.Coxson HO. Using computed tomography to measure the site of airflow obstruction. Respirology. 2012 Jan;17(1):5–6. doi: 10.1111/j.1440-1843.2011.02084.x. [DOI] [PubMed] [Google Scholar]
- 61.Coxson HO. Quantitative chest tomography in COPD research: chairman’s summary. Proc Am Thorac Soc. 2008 Dec 15;5(9):874–877. doi: 10.1513/pats.200810-118QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American journal of respiratory and critical care medicine. 2001 Apr;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 63.Wright JL, Churg A. Advances in the pathology of COPD. Histopathology. 2006 Jul;49(1):1–9. doi: 10.1111/j.1365-2559.2006.02395.x. [DOI] [PubMed] [Google Scholar]
- 64.Peinado VI, Ramirez J, Roca J, Rodriguez-Roisin R, Barbera JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006 Mar;34(3):257–263. doi: 10.1165/rcmb.2005-0255OC. [DOI] [PubMed] [Google Scholar]
- 65.Ishizawa K, Kubo H, Yamada M, et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS letters. 2004 Jan 2;556(1–3):249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 66.Remy-Jardin M, Edme JL, Boulenguez C, Remy J, Mastora I, Sobaszek A. Longitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function tests. Radiology. 2002 Jan;222(1):261–270. doi: 10.1148/radiol.2221001154. [DOI] [PubMed] [Google Scholar]
- 67.Gust R, Kozlowski J, Stephenson AH, Schuster DP. Synergistic hemodynamic effects of low-dose endotoxin and acute lung injury. American journal of respiratory and critical care medicine. 1998 Jun;157(6 Pt 1):1919–1926. doi: 10.1164/ajrccm.157.6.9704110. [DOI] [PubMed] [Google Scholar]
- 68.Schuster DP, Marklin GF. The effect of regional lung injury or alveolar hypoxia on pulmonary blood flow and lung water measured by positron emission tomography. The American review of respiratory disease. 1986 Jun;133(6):1037–1042. doi: 10.1164/arrd.1986.133.6.1037. [DOI] [PubMed] [Google Scholar]
- 69.Easley RB, Fuld MK, Fernandez-Bustamante A, Hoffman EA, Simon BA. Mechanism of hypoxemia in acute lung injury evaluated by multidetector-row CT. Acad Radiol. 2006 Jul;13(7):916–921. doi: 10.1016/j.acra.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 70.Alford SK, van Beek EJ, McLennan G, Hoffman EA. Heterogeneity of pulmonary perfusion as a mechanistic image-based phenotype in emphysema susceptible smokers. Proceedings of the National Academy of Sciences of the United States of America. 2010 Apr 20;107(16):7485–7490. doi: 10.1073/pnas.0913880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vonk-Noordegraaf A. The shrinking heart in chronic obstructive pulmonary disease. N Engl J Med. 2010 Jan 21;362(3):267–268. doi: 10.1056/NEJMe0906251. [DOI] [PubMed] [Google Scholar]
- 72.Fuld MK, Halaweish AF, Haynes SE, Divekar AA, Guo J, Hoffman EA. Pulmonary perfused blood volume with dual-energy CT as surrogate for pulmonary perfusion assessed with dynamic multidetector CT. Radiology. 2013 Jun;267(3):747–756. doi: 10.1148/radiol.12112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chon D, Beck KC, Simon BA, Shikata H, Saba OI, Hoffman EA. Effect of low-xenon and krypton supplementation on signal/noise of regional CT-based ventilation measurements. J Applied Physiology. 2007 Apr;102(4):1535–1544. doi: 10.1152/japplphysiol.01235.2005. [DOI] [PubMed] [Google Scholar]
- 74.Chon D, Simon BA, Beck KC, et al. Differences in regional wash-in and wash-out time constants for xenon-CT ventilation studies. Respiratory physiology & neurobiology. 2005 Aug 25;148(1–2):65–83. doi: 10.1016/j.resp.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Hoffman EA, Chon D. Computed tomography studies of lung ventilation and perfusion. Proc Am Thorac Soc. 2005;2(6):492–498. 506. doi: 10.1513/pats.200509-099DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MK F, Simon BA, Van Beek EJ, Hudson M, Sieren J, Hoffman EA. Transitioning from the Laboratory to the Clinic: Adapting the Xe-CT Method for Human Scanning. American Thoracic Society Annual Meeting; 2008; Toronto, Canada. [Google Scholar]
- 77.Fuld M, van Beek E, Simon B, Morgan J, Hoffman EA. Establishing “Normal” Regional Ventilation Via Dynamic Xenon-CT in Humans. American Thoracic Annual Meeting; 2009; San Diego, CA. [Google Scholar]
- 78.Park HW, Jung JW, Kim KM, et al. Xenon ventilation computed tomography and the management of asthma in the elderly. Respirology. 2014 Apr;19(3):389–395. doi: 10.1111/resp.12242. [DOI] [PubMed] [Google Scholar]
- 79.Kong X, Sheng HX, Lu GM, et al. Xenon-enhanced dual-energy CT lung ventilation imaging: techniques and clinical applications. AJR Am J Roentgenol. 2014 Feb;202(2):309–317. doi: 10.2214/AJR.13.11191. [DOI] [PubMed] [Google Scholar]
- 80.Park EA, Goo JM, Park SJ, et al. Chronic obstructive pulmonary disease: quantitative and visual ventilation pattern analysis at xenon ventilation CT performed by using a dual-energy technique. Radiology. 2010 Sep;256(3):985–997. doi: 10.1148/radiol.10091502. [DOI] [PubMed] [Google Scholar]
- 81.Chae EJ, Seo JB, Goo HW, et al. Xenon ventilation CT with a dual-energy technique of dual-source CT: initial experience. Radiology. 2008 Aug;248(2):615–624. doi: 10.1148/radiol.2482071482. [DOI] [PubMed] [Google Scholar]
- 82.Jung JW, Kwon JW, Kim TW, et al. New insight into the assessment of asthma using xenon ventilation computed tomography. Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2013 Aug;111(2):90–95. e92. doi: 10.1016/j.anai.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 83.Fuld MK, Halaweish AF, Newell JD, Jr, Krauss B, Hoffman EA. Optimization of dual-energy xenon-computed tomography for quantitative assessment of regional pulmonary ventilation. Invest Radiol. 2013 Sep;48(9):629–637. doi: 10.1097/RLI.0b013e31828ad647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahnken AH, Jost G, Pietsch H. Krypton for Computed Tomography Lung Ventilation Imaging: Preliminary Animal Data. Invest Radiol. 2014 Dec 30; doi: 10.1097/RLI.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 85.Hachulla AL, Pontana F, Wemeau-Stervinou L, et al. Krypton ventilation imaging using dual-energy CT in chronic obstructive pulmonary disease patients: initial experience. Radiology. 2012 Apr;263(1):253–259. doi: 10.1148/radiol.12111211. [DOI] [PubMed] [Google Scholar]
- 86.Schmidt TG, Zimmerman KC, Sidky EY. The effects of extending the spectral information acquired by a photon-counting detector for spectral CT. Physics in medicine and biology. 2015 Jan 23;60(4):1583–1600. doi: 10.1088/0031-9155/60/4/1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Vries A, Roessl E, Kneepkens E, et al. Quantitative Spectral K-Edge Imaging in Preclinical Photon-Counting X-ray Computed Tomography. Invest Radiol. 2014 Dec 30; doi: 10.1097/RLI.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 88.Lee S, Choi YN, Kim HJ. Quantitative material decomposition using spectral computed tomography with an energy-resolved photon-counting detector. Physics in medicine and biology. 2014 Sep 21;59(18):5457–5482. doi: 10.1088/0031-9155/59/18/5457. [DOI] [PubMed] [Google Scholar]
- 89.Fuld MK, Easley RB, Saba OI, et al. CT-measured regional specific volume change reflects regional ventilation in supine sheep. J Appl Physiol (1985) 2008 Apr;104(4):1177–1184. doi: 10.1152/japplphysiol.00212.2007. [DOI] [PubMed] [Google Scholar]
- 90.Ding K, Cao K, Fuld MK, et al. Comparison of image registration based measures of regional lung ventilation from dynamic spiral CT with Xe-CT. Medical physics. 2012 Aug;39(8):5084–5098. doi: 10.1118/1.4736808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yin Y, Hoffman EA, Ding K, Reinhardt JM, Lin CL. A cubic B-spline-based hybrid registration of lung CT images for a dynamic airway geometric model with large deformation. Physics in medicine and biology. 2011 Jan 7;56(1):203–218. doi: 10.1088/0031-9155/56/1/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin Y, Choi J, Hoffman EA, Tawhai MH, Lin CL. A multiscale MDCT image-based breathing lung model with time-varying regional ventilation. Journal of computational physics. 2013 Jul 1;244(0):168–192. doi: 10.1016/j.jcp.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi S, Hoffman EA, Wenzel S, et al. Registration-based Assessment of Regional Lung Function via Volumetric CT Images of Normal vs. Severe Asthmatics. JAP. 2013 doi: 10.1152/japplphysiol.00113.2013. JAPPL-00113-02013R00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin Y, Hoffman EA, Lin CL. Lung lobar slippage assessed with the aid of image registration. Medical image computing and computer-assisted intervention: MICCAI … International Conference on Medical Image Computing and Computer-Assisted Intervention. 2010;13(Pt 2):578–585. doi: 10.1007/978-3-642-15745-5_71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jahani N, Yin Y, Hoffman EA, Lin CL. Assessment of regional non-linear tissue deformation and air volume change of human lungs via image registration. Journal of biomechanics. 2014 May 7;47(7):1626–1633. doi: 10.1016/j.jbiomech.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR American journal of roentgenology. 2013 Sep;201(3):W460–470. doi: 10.2214/AJR.12.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mets OM, Buckens CF, Zanen P, et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011 Oct 26;306(16):1775–1781. doi: 10.1001/jama.2011.1531. [DOI] [PubMed] [Google Scholar]
- 98.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. The New England journal of medicine. 2011 Oct 27;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Estepar RS, Kinney GL, Black-Shinn JL, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. American journal of respiratory and critical care medicine. 2013 Jul 15;188(2):231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao Z, Grout RW, Holtze C, Hoffman EA, Saha PK. A new paradigm of interactive artery/vein separation in noncontrast pulmonary CT imaging using multiscale topomorphologic opening. IEEE transactions on bio-medical engineering. 2012 Nov;59(11):3016–3027. doi: 10.1109/TBME.2012.2212894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saha PK, Gao Z, Alford SK, Sonka M, Hoffman EA. Topomorphologic separation of fused isointensity objects via multiscale opening: separating arteries and veins in 3-D pulmonary CT. IEEE transactions on medical imaging. 2010 Mar;29(3):840–851. doi: 10.1109/TMI.2009.2038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012 Sep 6;367(10):913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barr RG. The epidemiology of vascular dysfunction relating to chronic obstructive pulmonary disease and emphysema. Proc Am Thorac Soc. 2011 Nov;8(6):522–527. doi: 10.1513/pats.201101-008MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ley-Zaporozhan J, Ley S, Kauczor HU. Proton MRI in COPD. Copd. 2007 Mar;4(1):55–65. doi: 10.1080/15412550701198719. [DOI] [PubMed] [Google Scholar]
- 105.Vogel-Claussen J, Renne J, Hinrichs J, et al. Quantification of Pulmonary Inflammation after Segmental Allergen Challenge Using TIRM Magnetic Resonance Imaging. American journal of respiratory and critical care medicine. 2014 Jan 8; doi: 10.1164/rccm.201310-1825OC. [DOI] [PubMed] [Google Scholar]
- 106.Mayo JR, MacKay A, Muller NL. MR imaging of the lungs: value of short TE spin-echo pulse sequences. AJR Am J Roentgenol. 1992 Nov;159(5):951–956. doi: 10.2214/ajr.159.5.1414805. [DOI] [PubMed] [Google Scholar]
- 107.Mayo JR. Thoracic magnetic resonance imaging: physics and pulse sequences. Journal of thoracic imaging. 1993 Winter;8(1):1–11. [PubMed] [Google Scholar]
- 108.Ma W, Sheikh K, Svenningsen S, et al. Ultra-short echo-time pulmonary MRI: Evaluation and reproducibility in COPD subjects with and without bronchiectasis. J Magn Reson Imaging. 2014 Jun 26; doi: 10.1002/jmri.24680. [DOI] [PubMed] [Google Scholar]
- 109.Takahashi M, Togao O, Obara M, et al. Ultra-short echo time (UTE) MR imaging of the lung: comparison between normal and emphysematous lungs in mutant mice. Journal of magnetic resonance imaging: JMRI. 2010 Aug;32(2):326–333. doi: 10.1002/jmri.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zurek M, Boyer L, Caramelle P, Boczkowski J, Crémillieux Y. Longitudinal and noninvasive assessment of emphysema evolution in a murine model using proton MRI. Magnetic Resonance in Medicine. 2012;68(3):898–904. doi: 10.1002/mrm.23281. [DOI] [PubMed] [Google Scholar]
- 111.Ohno Y, Koyama H, Yoshikawa T, et al. T2* measurements of 3-T MRI with ultrashort TEs: capabilities of pulmonary function assessment and clinical stage classification in smokers. AJR American journal of roentgenology. 2011 Aug;197(2):W279–285. doi: 10.2214/AJR.10.5350. [DOI] [PubMed] [Google Scholar]
- 112.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med. 2013 Nov;70(5):1241–1250. doi: 10.1002/mrm.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weiger M, Wu M, Wurnig MC, et al. Rapid and robust pulmonary proton ZTE imaging in the mouse. NMR in biomedicine. 2014 Sep;27(9):1129–1134. doi: 10.1002/nbm.3161. [DOI] [PubMed] [Google Scholar]
- 114.Edelman RR, Hatabu H, Tadamura E, Li W, Prasad PV. Noninvasive assessment of regional ventilation in the human lung using oxygen-enhanced magnetic resonance imaging. Nat Med. 1996 Nov;2(11):1236–1239. doi: 10.1038/nm1196-1236. [DOI] [PubMed] [Google Scholar]
- 115.Ohno Y, Koyama H, Nogami M, et al. Dynamic oxygen-enhanced MRI versus quantitative CT: pulmonary functional loss assessment and clinical stage classification of smoking-related COPD. AJR Am J Roentgenol. 2008 Feb;190(2):W93–99. doi: 10.2214/AJR.07.2511. [DOI] [PubMed] [Google Scholar]
- 116.Bauman G, Puderbach M, Deimling M, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magn Reson Med. 2009;62(3):656–664. doi: 10.1002/mrm.22031. [DOI] [PubMed] [Google Scholar]
- 117.Bauman G, Lutzen U, Ullrich M, et al. Pulmonary functional imaging: qualitative comparison of Fourier decomposition MR imaging with SPECT/CT in porcine lung. Radiology. 2011 Aug;260(2):551–559. doi: 10.1148/radiol.11102313. [DOI] [PubMed] [Google Scholar]
- 118.Biederer J, Mirsadraee S, Beer M, et al. MRI of the lung (3/3)-current applications and future perspectives. Insights into imaging. 2012 Aug;3(4):373–386. doi: 10.1007/s13244-011-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chefd’hotel CHG, Faugeras O. A variational approach to multimodal image matching. Proceedings of the IEEE Workshop on Variational and Level Set Methods in Computer Vision (VLSM’2001); 2001; Vancouver, British Columbia, Canada. [Google Scholar]
- 120.Deimling MJV, Geiger B, Chefd’Hotel C. Time resolved lung ventilation imaging by Fourier decomposition. Paper presented at: Proceedings of the 16th Annual Meeting of ISMRM; Toronto, Ontario, Canada. 2008. [Google Scholar]
- 121.Bauman G, Puderbach M, Deimling M, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magn Reson Med. 2009 Sep;62(3):656–664. doi: 10.1002/mrm.22031. [DOI] [PubMed] [Google Scholar]
- 122.Capaldi DP, Sheikh K, Guo F, et al. Free-breathing Pulmonary H and Hyperpolarized He MRI: Comparison in COPD and Bronchiectasis. Acad Radiol. 2014 Dec 6; doi: 10.1016/j.acra.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 123.Pennati F, Quirk JD, Yablonskiy DA, Castro M, Aliverti A, Woods JC. Assessment of Regional Lung Function with Multivolume (1)H MR Imaging in Health and Obstructive Lung Disease: Comparison with (3)He MR Imaging. Radiology. 2014 Nov;273(2):580–590. doi: 10.1148/radiol.14132470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bauman G, Scholz A, Rivoire J, et al. Lung ventilation- and perfusion-weighted Fourier decomposition magnetic resonance imaging: in vivo validation with hyperpolarized 3He and dynamic contrast-enhanced MRI. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2013 Jan;69(1):229–237. doi: 10.1002/mrm.24236. [DOI] [PubMed] [Google Scholar]
- 125.MacDonald A, Wann K. Physiological Aspects of Anaesthetics and Inert Gases. London: Academic Press; 1978. [Google Scholar]
- 126.Goodson BM. Nuclear magnetic resonance of laser-polarized noble gases in molecules, materials, and organisms. J Magn Reson. 2002 Apr;155(2):157–216. doi: 10.1006/jmre.2001.2341. [DOI] [PubMed] [Google Scholar]
- 127.Saam BT. Magnetic resonance imaging with laser-polarized noble gases. Nat Med. 1996 Mar;2(3):358–359. doi: 10.1038/nm0396-358. [DOI] [PubMed] [Google Scholar]
- 128.Halaweish AF, Moon RE, Foster WM, et al. Perfluoropropane gas as a magnetic resonance lung imaging contrast agent in humans. Chest. 2013 Oct;144(4):1300–1310. doi: 10.1378/chest.12-2597. [DOI] [PubMed] [Google Scholar]
- 129.Couch MJ, Ball IK, Li T, et al. Pulmonary ultrashort echo time 19F MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: feasibility. Radiology. 2013 Dec;269(3):903–909. doi: 10.1148/radiol.13130609. [DOI] [PubMed] [Google Scholar]
- 130.Kirby M, Mathew L, Heydarian M, Etemad-Rezai R, McCormack DG, Parraga G. Chronic Obstructive Pulmonary Disease: Quantification of Bronchodilator Effects by Using Hyperpolarized He MR Imaging. Radiology. 2011 doi: 10.1148/radiol.11110403. [DOI] [PubMed] [Google Scholar]
- 131.Kirby M, Kanhere N, Etemad-Rezai R, McCormack DG, Parraga G. Hyperpolarized helium-3 magnetic resonance imaging of chronic obstructive pulmonary disease exacerbation. J Magn Reson Imaging. 2013 May;37(5):1223–1227. doi: 10.1002/jmri.23896. [DOI] [PubMed] [Google Scholar]
- 132.Van Beek EJ, Dahmen AM, Stavngaard T, et al. Hyperpolarised 3He MRI versus HRCT in COPD and normal volunteers: PHIL trial. Eur Respir J. 2009 Dec;34(6):1311–1321. doi: 10.1183/09031936.00138508. [DOI] [PubMed] [Google Scholar]
- 133.Woodhouse N, Wild JM, Paley MN, et al. Combined helium-3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imaging. 2005 Apr;21(4):365–369. doi: 10.1002/jmri.20290. [DOI] [PubMed] [Google Scholar]
- 134.Kirby M, Mathew L, Wheatley A, Santyr GE, McCormack DG, Parraga G. Chronic obstructive pulmonary disease: longitudinal hyperpolarized (3)He MR imaging. Radiology. 2010 Jul;256(1):280–289. doi: 10.1148/radiol.10091937. [DOI] [PubMed] [Google Scholar]
- 135.Marshall H, Deppe MH, Parra-Robles J, et al. Direct visualisation of collateral ventilation in COPD with hyperpolarised gas MRI. Thorax. 2012 Jan 27; doi: 10.1136/thoraxjnl-2011-200864. [DOI] [PubMed] [Google Scholar]
- 136.Kirby M, Pike D, Coxson HO, McCormack DG, Parraga G. Hyperpolarized (3)He ventilation defects used to predict pulmonary exacerbations in mild to moderate chronic obstructive pulmonary disease. Radiology. 2014 Dec;273(3):887–896. doi: 10.1148/radiol.14140161. [DOI] [PubMed] [Google Scholar]
- 137.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994 Mar;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 138.Saam B, Yablonskiy D, Kodibagkar V, et al. MR imaging of diffusion of 3He gas in healthy and diseased lungs. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2000;44:174–179. doi: 10.1002/1522-2594(200008)44:2<174::aid-mrm2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 139.Brookeman J, Muglerr JP, Knight-Scott J, Munger T, deLange E, Bogorad P. Studies of 3He diffusion coefficient in the human lung: Age-related distribution patterns. Eur J Radiol. 1999;9(B):21. [Google Scholar]
- 140.Kauczor HU, Ebert M, Kreitner KF, et al. Imaging of the lungs using 3He MRI: preliminary clinical experience in 18 patients with and without lung disease. J Magn Reson Imaging. 1997 May-Jun;7(3):538–543. doi: 10.1002/jmri.1880070314. [DOI] [PubMed] [Google Scholar]
- 141.Diaz S, Casselbrant I, Piitulainen E, et al. Hyperpolarized 3He apparent diffusion coefficient MRI of the lung: reproducibility and volume dependency in healthy volunteers and patients with emphysema. J Magn Reson Imaging. 2008 Apr;27(4):763–770. doi: 10.1002/jmri.21212. [DOI] [PubMed] [Google Scholar]
- 142.Morbach AE, Gast KK, Schmiedeskamp J, et al. Diffusion-weighted MRI of the lung with hyperpolarized helium-3: a study of reproducibility. J Magn Reson Imaging. 2005 Jun;21(6):765–774. doi: 10.1002/jmri.20300. [DOI] [PubMed] [Google Scholar]
- 143.Fichele S, Woodhouse N, Swift AJ, et al. MRI of helium-3 gas in healthy lungs: posture related variations of alveolar size. J Magn Reson Imaging. 2004 Aug;20(2):331–335. doi: 10.1002/jmri.20104. [DOI] [PubMed] [Google Scholar]
- 144.Fain SB, Altes TA, Panth SR, et al. Detection of age-dependent changes in healthy adult lungs with diffusion-weighted 3He MRI. Acad Radiol. 2005 Nov;12(11):1385–1393. doi: 10.1016/j.acra.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 145.Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP., 3rd Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes--initial experience. Radiology. 2002 Jan;222(1):252–260. doi: 10.1148/radiol.2221001834. [DOI] [PubMed] [Google Scholar]
- 146.Diaz S, Casselbrant I, Piitulainen E, et al. Validity of apparent diffusion coefficient hyperpolarized 3He-MRI using MSCT and pulmonary function tests as references. Eur J Radiol. 2009 Aug;71(2):257–263. doi: 10.1016/j.ejrad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 147.Woods JC, Choong CK, Yablonskiy DA, et al. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2006 Dec;56(6):1293–1300. doi: 10.1002/mrm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fain SB, Panth SR, Evans MD, et al. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology. 2006 Jun;239(3):875–883. doi: 10.1148/radiol.2393050111. [DOI] [PubMed] [Google Scholar]
- 149.Swift AJ, Wild JM, Fichele S, et al. Emphysematous changes and normal variation in smokers and COPD patients using diffusion 3He MRI. Eur J Radiol. 2005 Jun;54(3):352–358. doi: 10.1016/j.ejrad.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 150.Diaz S, Casselbrant I, Piitulainen E, et al. Progression of emphysema in a 12-month hyperpolarized 3He-MRI study: lacunarity analysis provided a more sensitive measure than standard ADC analysis. Acad Radiol. 2009 Jun;16(6):700–707. doi: 10.1016/j.acra.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 151.Wang C, Miller GW, Altes TA, de Lange EE, Cates GD, Jr, Mugler JP., 3rd Time dependence of 3He diffusion in the human lung: measurement in the long-time regime using stimulated echoes. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2006 Aug;56(2):296–309. doi: 10.1002/mrm.20944. [DOI] [PubMed] [Google Scholar]
- 152.Bartel SE, Haywood SE, Woods JC, et al. Role of collateral paths in long-range diffusion in lungs. J Appl Physiol. 2008 May;104(5):1495–1503. doi: 10.1152/japplphysiol.01005.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Conradi MS, Yablonskiy DA, Woods JC, et al. The role of collateral paths in long-range diffusion of 3He in lungs. Acad Radiol. 2008 Jun;15(6):675–682. doi: 10.1016/j.acra.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Woods JC, Yablonskiy DA, Choong CK, et al. Long-range diffusion of hyperpolarized 3He in explanted normal and emphysematous human lungs via magnetization tagging. J Appl Physiol. 2005 Nov;99(5):1992–1997. doi: 10.1152/japplphysiol.00185.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang C, Altes TA, Mugler JP, 3rd, et al. Assessment of the lung microstructure in patients with asthma using hyperpolarized 3He diffusion MRI at two time scales: comparison with healthy subjects and patients with COPD. J Magn Reson Imaging. 2008 Jul;28(1):80–88. doi: 10.1002/jmri.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang C, Miller GW, Altes TA, et al. Extending the range of diffusion times for regional measurement of the 3He ADC in human lungs. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2008 Mar;59(3):673–678. doi: 10.1002/mrm.21543. [DOI] [PubMed] [Google Scholar]
- 157.Fan L, Xia Y, Guan Y, et al. Capability of differentiating smokers with normal pulmonary function from COPD patients: a comparison of CT pulmonary volume analysis and MR perfusion imaging. European radiology. 2013 May;23(5):1234–1241. doi: 10.1007/s00330-012-2729-2. [DOI] [PubMed] [Google Scholar]
- 158.Fan L, Xia Y, Guan Y, Zhang TF, Liu SY. Characteristic features of pulmonary function test, CT volume analysis and MR perfusion imaging in COPD patients with different HRCT phenotypes. The clinical respiratory journal. 2014 Jan;8(1):45–54. doi: 10.1111/crj.12033. [DOI] [PubMed] [Google Scholar]
- 159.Xia Y, Guan Y, Fan L, et al. Dynamic contrast enhanced magnetic resonance perfusion imaging in high-risk smokers and smoking-related COPD: correlations with pulmonary function tests and quantitative computed tomography. COPD. 2014 Sep;11(5):510–520. doi: 10.3109/15412555.2014.948990. [DOI] [PubMed] [Google Scholar]
- 160.Sergiacomi G, Taglieri A, Chiaravalloti A, et al. Acute COPD exacerbation: 3 T MRI evaluation of pulmonary regional perfusion--preliminary experience. Respir Med. 2014 Jun;108(6):875–882. doi: 10.1016/j.rmed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 161.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. The New England journal of medicine. 2010 Jan 21;362(3):217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007 Feb;119(2):405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mathew L, Kirby M, Etemad-Rezai R, Wheatley A, McCormack DG, Parraga G. Hyperpolarized (3)He magnetic resonance imaging: Preliminary evaluation of phenotyping potential in chronic obstructive pulmonary disease. Eur J Radiol. 2009 Nov 20; doi: 10.1016/j.ejrad.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 164.Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, Sin DD. New and current clinical imaging techniques to study chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2009 Oct 1;180(7):588–597. doi: 10.1164/rccm.200901-0159PP. [DOI] [PubMed] [Google Scholar]