Abstract
Acute myeloid leukemia (AML) is characterized by the proliferation of immature myeloid lineage blasts. Due to its heterogeneity and to the high rate of acquired drug resistance and relapse, new treatment strategies are needed. Here, we demonstrate that IFNγ promotes AML blasts to act as effector cells within the context of antibody therapy. Treatment with IFNγ drove AML blasts toward a more differentiated state, wherein they showed increased expression of the M1-related markers HLA-DR and CD86, as well as of FcγRI, which mediates effector responses to therapeutic antibodies. Importantly, IFNγ was able to up-regulate CD38, the target of the therapeutic antibody daratumumab. Because the antigen (CD38) and effector receptor (FcγRI) were both simultaneously up-regulated on the AML blasts, we tested whether IFNγ treatment of the AML cell lines THP-1 and MV4-11 could stimulate them to target one another after the addition of daratumumab. Results showed that IFNγ significantly increased daratumumab-mediated cytotoxicity, as measured both by 51Cr release and lactate dehydrogenase release assays. We also found that the combination of IFNγ and activation of FcγR led to the release of granzyme B by AML cells. Finally, using a murine NSG model of subcutaneous AML, we found that treatment with IFNγ plus daratumumab significantly attenuated tumor growth. Taken together, these studies show a novel mechanism of daratumumab-mediated killing and a possible new therapeutic strategy for AML.
Keywords: antibody, Fc receptor, interferon, signal transduction, tumor immunology
Introduction
Acute myeloid leukemia (AML)3 is the most common type of acute leukemia in adults and affects over 20,830 people each year (1, 2). AML is a hematologic malignancy characterized by a proliferation of myeloid precursors (“blasts”), which infiltrate the bone marrow, blood, and other tissues (3, 4). Despite the existence of multiple biologically distinct subtypes of AML, the current methodology of treatment includes a regimen of chemotherapy and stem cell transplant (5, 6). Allogeneic hematopoietic stem cell transplantation can be curative for certain patients with AML; however, very few patients are candidates for this procedure (7, 8). Patients over 60 years of age have a worse prognosis due to both chemoresistance and intolerance to intensive chemotherapy, with a median survival of 5–10 months (3, 5, 9, 10). Hence, there is an urgent need for the development of safer and more effective therapeutics for AML.
Monoclonal antibodies are being utilized as a treatment for many different types of cancer and are being actively pursued as a treatment for AML (11, 12). Perhaps the most well known antibody in clinical use for AML was the toxin-conjugated anti-CD33 antibody, gemtuzumab ozagomicin (Mylotarg®). This took advantage of the rapid internalization of CD33 upon antibody binding, thereby delivering the toxin into CD33-expressing cells. However, it was withdrawn from the market due to toxicity issues (13, 14). Today, other CD33-targeting drug-antibody conjugates, such as SGN-CD33A and Fc-engineered anti-CD33 antibodies, are being studied in AML (1, 12, 15). The targeting of FcγRI has similarly been proposed, especially after the finding that IFNγ could increase the expression of the high affinity Fcγ receptor, FcγRI (16–19). Recently, a study was completed using a monoclonal antibody to CD123 that has been humanized, affinity-matured, and Fc-engineered for increased affinity toward CD16 (FcγRIIIa), which showed an effect against AML both in vitro and in vivo in an environment with adequate NK cell function (20).
CD38 is a transmembrane glycoprotein expressed in many different cells, including lymphocytes (21–24). The anti-CD38 monoclonal antibody daratumumab has shown a favorable safety profile and encouraging efficacy in patients with refractory multiple myeloma (25–27), and the anti-CD38 SAR650984 is being examined as a treatment for CD38+ hematological malignancies, including AML (clinicaltrials.gov registration NCT01084252). Here, we have found that treatment of AML cell lines and primary AML apheresis samples with IFNγ leads to the up-regulation of M1-related markers and of the daratumumab target CD38. IFNγ also induced AML cell fratricide in vitro and reduced tumor growth in vivo, an effect that was significantly enhanced by the addition of anti-CD38. Interestingly, IFNγ also led to FcγR-mediated granzyme B production in AML cell lines. These results suggest that IFNγ can cause the AML cells themselves to become immune effectors and that IFNγ plus anti-CD38 antibody may be an effective treatment for AML.
Results
IFNγ Promotes an M1-related Phenotype in AML Cells
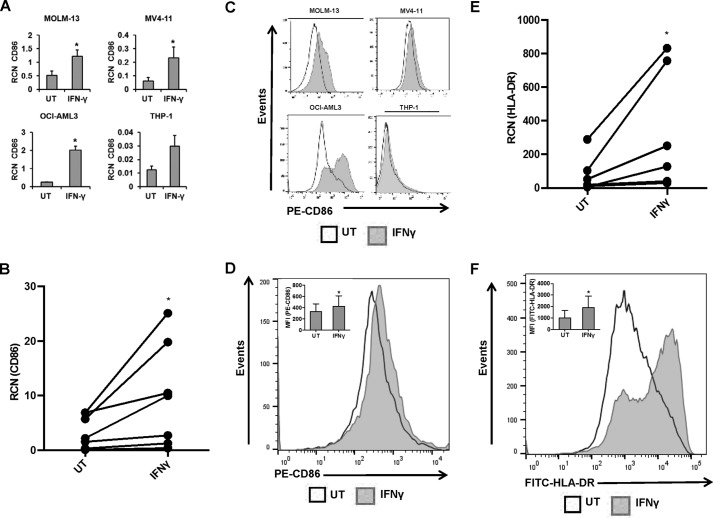
Myeloid cells within the context of tumors commonly display M2-like characteristics, which serve to promote tumor growth and survival (28, 29). Here, we tested whether treatment with IFNγ could lead to a shift toward an M1-like phenotype. M1 macrophages can be identified by many different phenotypic markers, including CD80/B7-1, CD86/B7-2, HLA-DR, and NOS2 (28, 30). To test this, we treated AML cell lines MV4-11, MOLM-13, OCI-AML3, and THP-1 for 24 or 48 h and primary AML samples for 18 h, with or without 10 ng/ml IFNγ. Levels of CD86/B7–2 (T-cell co-activator molecule), NOS2, and HLA-DR were measured using qPCR. CD80/B7-1, which works in tandem with CD86/B7-2 as a T-cell co-activator, is expressed at low levels on most M4/M5 AML cells (31). In agreement with this, we found little to no CD80 transcript in the four AML cell lines tested (data not shown).
Results showed that IFNγ significantly increased the transcript of CD86 in all cell lines except THP-1, which showed a strong trend toward significance (Fig. 1A). In primary AML apheresis samples, CD86 increased after 18-h IFNγ treatment (Fig. 1B). We then verified that these increases occurred at the cell surface using flow cytometry, with results showing that treatment with IFNγ for 24 h (MV4-11, OCI-AML3, and THP-1) or 48 h (MOLM-13) led to increases in CD86 expression (Fig. 1C). This was recapitulated in primary AML apheresis samples (Fig. 1D). Similarly, IFNγ significantly increased transcript and surface expression of HLA-DR in primary AML apheresis samples (Fig. 1, E and F, respectively), although NOS2 was variable (data not shown).
FIGURE 1.
IFNγ promotes an M1-related phenotype in AML cells. AML cell lines MOLM-13, MV4-11, OCI-AML3, and THP-1 (n = 3 or more separate experiments each) and primary AML apheresis samples were treated with or without 10 ng/ml IFNγ for 18 h (qPCR) or for 24 h (flow cytometry, except for MOLM-13 treated for 48 h). A and B, CD86 expression in AML cell lines (A) and primary AML apheresis samples (B, n = 7 donors) was measured by qPCR. C and D, CD86 expression in AML cell lines (C) and primary AML apheresis samples (D, n = 7 donors, representative histogram shown; inset bar graph depicts all donors) was measured by flow cytometry. E and F, HLA-DR expression in primary cells was measured by qPCR (E, n = 6 donors) and flow cytometry (F, n = 7, representative histogram shown; inset bar graph depicts all donors). *, p ≤ 0.05. Error bars, S.D.
IFNγ Increases FcγRI Expression and Phagocytic Ability in AML Cells
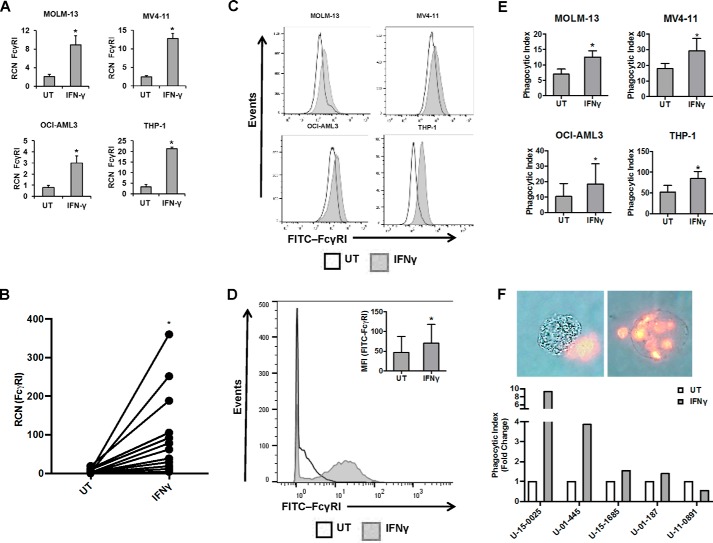
Because we observed that IFNγ is able to enhance the expression of M1-related markers, we hypothesized that IFNγ could also enhance Fcγ receptor functions, such as phagocytosis. It has previously been shown that IFNγ could increase the expression of FcγRI in AML cells, which led at one point to the exploration of IFNγ treatment combined with drug-conjugated anti-FcγRI antibody as a potential therapy for AML (17). However, FcγRI is not only a candidate therapeutic target; it is also a major effector of phagocytosis in myeloid cells (16, 18, 19). Hence, we tested the possibility that IFNγ treatment not only would increase FcγRI expression, but would also enhance the phagocytic ability of AML cells. We began by treating AML cell lines (MOLM-13, MV4-11, OCI-AML3, and THP-1) and primary AML apheresis samples with or without 10 ng/ml IFNγ and measured FcγRI transcript by qPCR (18-h treatment) and flow cytometry (24-h treatment). As expected, results showed that IFNγ increased the transcript (Fig. 2, A and B, for cell lines and primary AML apheresis samples, respectively) and surface expression (Fig. 2, C and D, for cell lines and primary AML apheresis samples, respectively) of FcγRI.
FIGURE 2.
IFNγ increases FcγRI expression and phagocytic ability in AML cells. AML cell lines MOLM-13, MV4-11, OCI-AML3, and THP-1 (n ≥ 3 separate experiments each) and primary AML apheresis samples were treated with or without 10 ng/ml IFNγ for 18 h (qPCR) or for 24 h (flow cytometry). A–D, FcγRI expression in AML cell lines (A) and primary AML apheresis samples (B, n = 12 donors) was measured by qPCR. FcγRI expression in AML cell lines (C) and primary AML apheresis samples (D, n = 3 donors, representative histogram shown; inset bar graph depicts all donors) was measured by flow cytometry. E, AML cell lines were treated with or without 10 ng/ml IFNγ for 24 h (MV4-11 cells for 48 h) and then incubated with opsonized sheep red blood cells. Phagocytosis was counted via microscopy in a blinded fashion. The phagocytic index represents the number of red blood cells ingested by 100 AML cells for each respective cell line. F, primary AML apheresis samples (n = 5 donors) were treated with or without 10 ng/ml IFNγ for 24 h and then incubated for 60 min with opsonized sheep red blood cells. Phagocytosis was counted via fluorescence microscopy in a blinded fashion. The phagocytic index represents the number of red blood cells ingested by 100 AML cells for each donor. *, p ≤ 0.05. Error bars, S.D.
To assess the effects of IFNγ on phagocytic ability, cell lines were treated with 10 ng/ml IFNγ for either 24 h (MOLM-13, OCI-AML3, and THP-1) or 48 h (MV4-11, which appeared to show a delayed response to IFNγ). Phagocytosis of fluorescently labeled opsonized sheep red blood cells was measured. As shown in Fig. 2E, IFNγ treatment significantly enhanced the phagocytic ability of all cell lines. We then treated primary AML cells for 24 h with IFNγ and tested them in a phagocytosis assay. Results showed that four of the five donors performed better with IFNγ (Fig. 2F). We also measured surface expression of FcγRI in these samples and compared changes in receptor expression with changes in phagocytic ability following IFNγ treatment. We found that the four donors with IFNγ-mediated enhancements of phagocytic ability also showed increases in FcγRI. Likewise, the nonresponding donor showed no change in FcγRI expression with IFNγ. Pearson correlation analysis showed a positive correlation between FcγRI surface expression and phagocytosis with regard to IFNγ response (p = 0.015, r = 0.945; Table 1). These results suggest that IFNγ can enhance the expression and function of FcγR in AML cells and that the degree of enhanced phagocytic ability is related at least in part to the degree of increased FcγRI expression.
TABLE 1.
Changes in phagocytic ability and FcγRI expression in primary AML cells following IFNγ treatment
AML apheresis samples (n = 5 donors) were treated without or with 10 ng/ml IFNγ for 24 h and then subjected to a phagocytosis assay as described under “Experimental Procedures.” Flow cytometry was also done to measure changes in FcγRI expression. The phagocytic index (mean number of opsonized sheep red blood cells ingested by 100 donor cells) and mean fluorescence intensity of FcγRI surface expression are shown. MFI, mean fluorescence intensity.
| Donor | Percentage change |
|
|---|---|---|
| Phagocytic index | MFI, FcγRI | |
| % | % | |
| U-15-0025 | 830.00 | 478.30 |
| U-01-445 | 286.11 | 25.00 |
| U-15-1685 | 54.24 | 25.25 |
| U-01-187 | 40.00 | 11.11 |
| U-11-0891 | −45.30 | 0.00 |
IFNγ Increases CD38 Expression in AML Cells
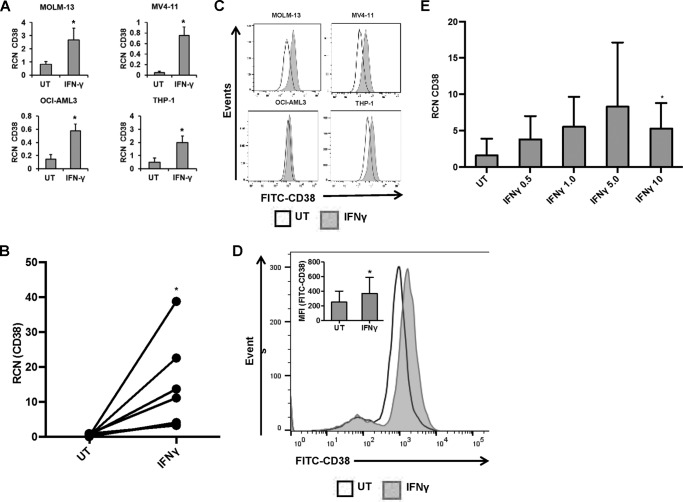
Because IFNγ is able to activate these immature myeloid cancer cells, we next tested whether IFNγ would have an effect on CD38 expression. We treated the AML cell lines MOLM-13, MV4-11, OCI-AML3, and THP-1, as well as primary AML apheresis samples, with or without 10 ng/ml IFNγ and measured the expression of CD38 using qPCR (18-h treatment) and flow cytometry (24-h treatment). Results showed that IFNγ treatment led to an increase in CD38 transcript in cell lines (Fig. 3A) and primary AML apheresis samples (Fig. 3B). Surface expression also increased for cell lines and primary AML apheresis samples (Fig. 3, C and D, respectively). No changes in CD33 were observed (data not shown). Hence, not only does IFNγ promote increased FcγR expression and function in AML cells; it also increases the expression of a candidate antibody target, CD38. Next, to test which concentration of IFNγ was required for this up-regulation of CD38, primary AML apheresis samples were treated for 18 h with 0–10 ng/ml IFNγ, and CD38 transcript was measured via qPCR. Results showed trends toward an increase at 0.5 ng/ml but a statistically significant increase at only 10 ng/ml (Fig. 3E).
FIGURE 3.
IFNγ increases CD38 expression in AML cells. AML cell lines MOLM-13, MV4-11, OCI-AML3, and THP-1 (n = 3 or more separate experiments each) and primary AML apheresis samples were incubated with or without 10 ng/ml IFNγ for 18 h (qPCR) or for 24 h (flow cytometry). A and B, CD38 expression in AML cell lines (A) and primary AML apheresis samples (B, n = 7 donors) was measured by qPCR. C and D, CD38 expression in AML cell lines (C) and primary AML apheresis samples (D, n = 8 donors, representative histogram shown; inset bar graph depicts all donors) was measured by flow cytometry. E, primary AML apheresis samples (n = 4 donors) were treated for 18 h with concentrations of IFNγ from 0 to 10 ng/ml. qPCR was done to measure transcript levels of CD38. *, p ≤ 0.05 compared with untreated (UT). Error bars, S.D.
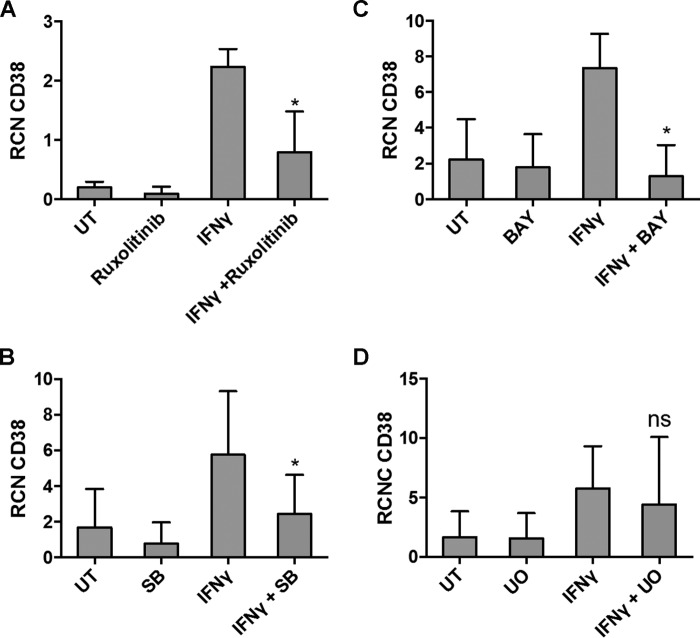
IFNγ-mediated CD38 Up-regulation Requires p38, NF-κB, and JAK/STAT
Previous studies have shown increased expression of CD38 by IFNγ in chronic lymphocytic leukemia to be JAK/STAT and T-bet-dependent (20). To determine which downstream signaling pathways were required for the up-regulation of CD38 seen with IFNγ treatment, primary AML apheresis samples were pretreated with inhibitors for ERK, PI3K, p38 MAPK, JAK/STAT, and NF-κB. Results showed that IFNγ-mediated up-regulation of CD38 was prevented by the inhibitors for JAK/STAT, p38, and NF-κB (Fig. 4, A–C, respectively). CD38 did not seem to be regulated by ERK (Fig. 4D) or PI3K (data not shown). Western blots with phospho-specific antibodies were performed to verify the efficacy of the inhibitors (data not shown).
FIGURE 4.
IFNγ-mediated CD38 up-regulation requires p38, NF-κB, and JAK/STAT. A–D, primary AML apheresis samples (n = 3) were pretreated for 30 min with a 5 nm concentration of the JAK1/2 inhibitor ruxolitinib (A), 5 μm p38 inhibitor SB203580 (B), 5 μm NF-κB inhibitor BAY 11-7085 (C), or 25 μm ERK inhibitor U0126 (D) and then treated for 18 h with or without 10 ng/ml IFNγ. qPCR was done to measure CD38 transcript. *, p ≤ 0.05. UT, untreated. Error bars, S.D.
IFNγ Enhances Antibody-mediated Fratricide in AML Cells
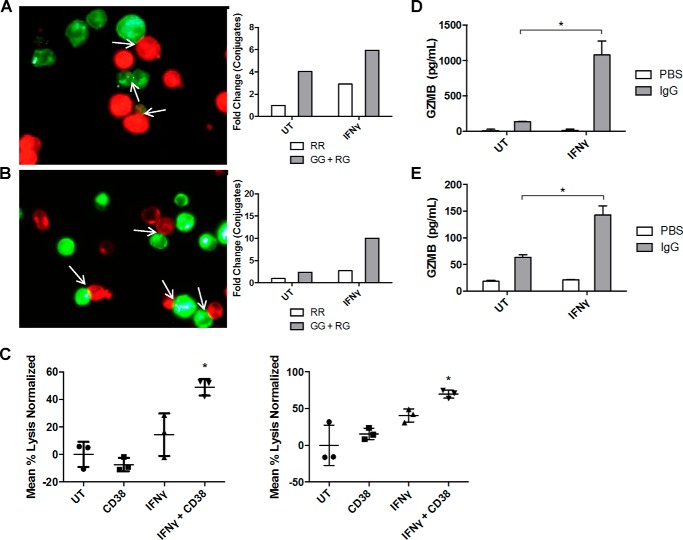
Results have shown that IFNγ treatment of AML cells promoted their shift toward an M1-related phenotype, enhanced the expression and function of FcγRI, and increased the expression of an antigen target for antibody therapy. Next, we tested the ability of IFNγ to promote antibody-mediated killing within pools of AML cells, a phenomenon termed fratricide (32, 33). We treated THP-1 and MV4-11 cells for 48 h with or without 10 ng/ml IFNγ and then split each group of samples in half to be labeled with either a red or green dye. Green-stained samples within each cell line were opsonized with anti-CD38 antibody on ice, washed with media, and then incubated with their corresponding non-opsonized red cells for 3 h. Conjugate formation was scored using fluorescence microscopy, with non-antibody-mediated conjugates represented as red-red (RR) interactions and antibody-mediated conjugates represented as red-green (RG) plus green-green (GG) (Fig. 5). Results showed that IFNγ led to increased antibody-mediated conjugates for both the THP-1 (Fig. 5A) and MV4-11 (Fig. 5B) cell lines.
FIGURE 5.
IFNγ enhances antibody-mediated fratricide in AML cells. A and B, THP-1 cells were treated for 48 h with or without IFNγ (10 ng/ml) and then split in half to be labeled with either a red or green dye. 10 μg/ml anti-CD38 antibody was added to the green stained samples, and samples were incubated at 4 °C for 1 h. Red and green stained untreated (UT) samples were mixed, incubated for 1 h, and fixed in 4% paraformaldehyde. 30 random images were taken by fluorescence microscopy, and conjugates were counted in a blinded fashion. The same was done for the IFNγ-treated samples. Red-red (RR) conjugates represent non-antibody-mediated conjugate formation, whereas green-green (GG) or red-green (RG) conjugates represent antibody-mediated conjugate formation. Data are represented as -fold change compared with untreated sample (n = 2 separate experiments, average shown) (A). The same procedure was repeated with MV4-11 cells (n = 2 separate experiments, average shown) (B). C, THP-1 cells (left) and MV4-11 cells (right) were treated with or without IFNγ (10 ng/ml) for 48 h, loaded with 51Cr, and labeled with anti-CD38 or IgG control antibodies. After 48 h of incubation, levels of 51Cr in supernatants were measured using a γ counter (n = 3). *, p ≤ 0.05 versus both untreated + CD38 or IFNγ + IgG. D and E, THP-1 cells were treated with or without IFNγ (10 ng/ml) for 18 h and then plated on IgG-coated 96-well plates. 24 h later, supernatants were collected, and granzyme B levels were detected via an ELISA (n = 4). *, p ≤ 0.05 versus IgG alone (D). The same procedure was repeated with MV4-11 cells (E). Error bars, S.D.
To further quantify the amount of antibody-mediated fratricide, we measured cytotoxicity using a 51Cr release assay. Cells were treated with or without 10 ng/ml IFNγ, loaded with 51Cr, opsonized with either anti-CD38 or control IgG, and then incubated for 48 h. Results showed that there was significantly greater antibody-mediated 51Cr release in samples treated with IFNγ within both the THP-1 and MV4-11 sets (Fig. 5C, left and right, respectively). In fact, the combination of anti-CD38 and IFNγ seemed to have a synergistic effect on cytotoxicity, suggesting that the effectiveness of anti-CD38 was dependent on IFNγ-mediated up-regulation of the CD38 antigen.
Granzymes are serine proteases that have been shown to be important effectors of natural killer cell and cytotoxic T cell responses (34). We have recently shown that monocytes treated with TLR8 agonists produced granzyme B and that this contributed to antibody-dependent cellular cytotoxicity (ADCC) (35), so we tested whether IFNγ treatment plus FcγR activation might elicit granzyme B from AML cells. We pretreated THP-1 and MV4-11 cells with or without IFNγ for 18 h and then incubated cells with or without immobilized IgG for 24 h. Results showed that this combination led to significant increases in granzyme B production in both THP-1 (Fig. 5D) and MV4-11 (Fig. 5E) cells.
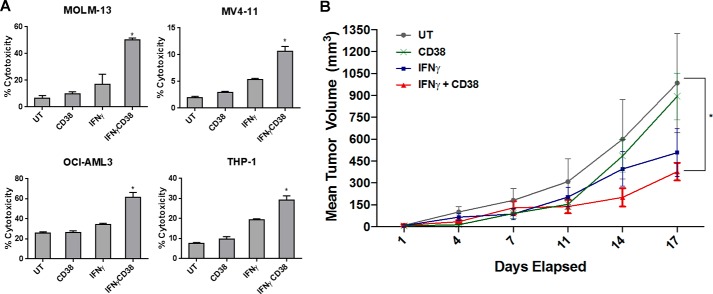
Next, to further confirm cytotoxicity and to extend the studies to all four AML cell lines, we treated MOLM-13, MV4-11, OCI-AML3, and THP-1 for 24 h with or without 10 ng/ml IFNγ and incubated them for an additional 3 h with anti-CD38 antibody. Cell death was measured via lactate dehydrogenase (LDH) release. As shown in Fig. 6A, IFNγ significantly enhanced antibody-mediated killing within each respective AML cell line. To test whether this was due to a toxic effect of anti-CD38, we incubated the AML cell lines on immobilized anti-CD38 for the same length of time and found that LDH release was not affected (data not shown). This again suggests that the anti-CD38 antibody led to cell-against-cell killing, which was triggered and enhanced by IFNγ pretreatment.
FIGURE 6.
IFNγ enhances anti-CD38 therapy in vivo. A, AML cell lines MOLM-13, MV4-11, OCI-AML3, and THP-1 (n ≥ 3 separate experiments each) were treated for 24 h with or without 10 ng/ml IFNγ and then incubated for 3 h with or without 10 μg/ml anti-CD38 antibody (CD38). IFNγ was added to the respective treatments for 18 h, and LDH assays were performed to measure cytotoxicity. *, p ≤ 0.05 versus untreated (UT). B, NSG mice were subcutaneously injected with 2.5 × 106 MV4-11 cells and then treated with either PBS, anti-CD38 antibody, IFNγ, or a combination of anti-CD38 and IFNγ (n = 6/group). Tumor growth rate as well as final tumor volumes were measured. *, p ≤ 0.05 versus PBS control at day 17 for rate of tumor growth. Error bars, S.D.
IFNγ Enhances Anti-CD38 Therapy in Vivo
We next tested whether IFNγ-induced antibody-mediated fratricide could occur in vivo. To do this, we injected MV4-11 cells subcutaneously into the flanks of NSG mice, which are deficient in B, T, NK, and dendritic cell activity (36), whereas the parent NOD/scid mice show defects in macrophage maturation (37). After 1 week (to permit tumor growth), mice were treated with PBS, anti-CD38 antibody, IFNγ, or a combination of anti-CD38 and IFNγ. Both the rate of tumor growth and the final tumor volumes were measured. Results showed that neither of the single-agent treatments had an effect on the rate of growth, but the combination of anti-CD38 and IFNγ led to a significantly reduced growth rate (Fig. 6B, p < 0.01). Final tumor sizes (day 17) were also compared, and results showed that both the IFNγ-treated and the combination-treated groups had significantly smaller tumors (p < 0.01 and p < 0.001, respectively; Fig. 6B). These results suggest that the combination treatment was superior, although single-agent IFNγ also showed an effect on final tumor size. Because NSG mice are severely immunodeficient, these results suggest that at least a large portion of the antitumor effects seen with IFNγ and daratumumab were mediated by the AML cells themselves.
Discussion
Herein, we have shown that treatment of AML blasts with IFNγ enables them to perform antibody-mediated fratricide. IFNγ treatment of AML cells was accompanied by increased expression of M1-related markers, resulting in an activated phenotype and increased expression of CD38, the target for the daratumumab. In addition, IFNγ significantly enhanced the IgG-mediated production of granzyme B, suggesting that the AML cells had taken on bona fide effector functions.
Previous work has shown that myeloid leukemia cells could be induced to differentiate by external signals. For example, all-trans-retinoic acid can cause acute promyelocytic leukemia cells to differentiate (37), and an agonistic antibody for the thrombopoietin receptor has been shown to cause AML cells to differentiate into active effector cells with characteristics of both dendritic and natural killer cells. These cells expressed substantial levels of granzyme B, perforin, and IFNγ and were capable of attacking one another via contact with needle-like filopodia (38).
The potential for IFNγ to stimulate effector functions on AML cells was of particular interest to us in light of its known role in up-regulating FcγRI expression (17). Attempts to exploit this have been made by linking anti-FcγRI to ricin, and this was tested in vitro and in vivo (17). However, although FcγRI is known to mediate cytotoxicity against tumor cells (39), IFNγ treatment for the purpose of stimulating antibody-mediated fratricide had not been fully explored. It is likely that the success of this strategy was not due solely to the up-regulation of FcγRI, however, because we also observed changes in M1-related markers. Such non-FcγR-mediated effects would be expected (and desired). Indeed, STAT1 and STAT3 were among the top responders to treatment with a pleiotropic thrombopoietin agonist antibody that elicited a shift toward DC/NK phenotypes in AML cells (38). Results from the present study suggest that, although IFNγ alone may not drive the full differentiation of AML cells as seen by Yea et al. (38), it is sufficient to promote antibody-mediated fratricide and enhance efficacy of antibody therapy. Traditionally, antibodies exert antitumor activity through antibody-dependent cellular cytotoxicity or complement-mediated cytotoxicity. Here we show that combination treatment with IFNγ resulted in a novel mechanism of action of daratumumab against AML blasts. As such, IFNγ may represent a broader treatment that could be tailored to a number of different antibody-based strategies.
One unexpected finding in the present study was that IFNγ led to the up-regulation of CD38 on AML cells. Although CD38 is known for its pro-survival signaling (40, 41), higher levels of CD38 could also make it more targetable by therapeutic antibodies, such as daratumumab. Indeed, certain agents are already being investigated for their ability to modulate CD38 expression for this purpose. For example, it was recently shown that all-trans-retinoic acid (ATRA) increased CD38 expression in multiple myeloma cell lines and in primary patient samples, significantly enhancing the effects of daratumumab in vitro and in vivo (42). Within the context of AML, ATRA is already being used as a therapy in the M3 subtype, acute promyelocytic leukemia, due to its ability to promote terminal differentiation of malignant cells. Although ATRA does not show single-agent efficacy in other AML subtypes (43, 44), its use with daratumumab is clearly a potential treatment strategy that should be explored.
IFNγ (IFNγ1b, Actimmune®, Horizon Pharma, Inc.) is an approved treatment for preventing infections in chronic granulomatous disease and for delaying severe malignant osteopetrosis (45–48). IFNγ is also being evaluated in combination with checkpoint inhibitors (nivolumab) in solid tumors (NCT02614456). The preclinical data presented here provide a strong rationale for combination of IFNγ and daratumumab in patients with acute myeloid leukemia. For example, Toll-like receptor agonists have been previously shown as effective enhancers of immune responses. In fact, the TLR7 agonist imiquimod is being used as a treatment for superficial basal cell carcinoma and HPV infection (49, 50). NK cells express both TLR7 and TLR8 and have been shown to produce IFNγ when stimulated by TLR7/8 ligands (51). TLR agonists combined with antibody therapy may stimulate a more localized production of IFNγ, which could be of benefit where there is binding of therapeutic antibody.
In summary, we have found that IFNγ can stimulate AML blasts to become effector cells and target one another in an antibody-dependent manner. Hence, strategies to enhance IFNγ production, including exogenous administration, may offer an effective way to improve the efficacy of antibody therapy for AML.
Experimental Procedures
Cell Culture
The AML cell lines used in this study (MOLM-13, MV4-11, OCI-AML3, and THP-1) were purchased from the ATCC and cultured according to ATCC recommendations. Cells were maintained below 1 × 106 cells/ml in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated FBS (Hyclone Laboratories, Grand Island, NY), 2 mm l-glutamine (Invitrogen), and penicillin/streptomycin (56 units/ml/56 μg/ml; Invitrogen) at 37 °C in an atmosphere of 5% CO2.
Primary Cells
White blood cells apheresed from AML patients were obtained after written informed consent in accordance with the Declaration of Helsinki under a protocol approved by the Ohio State University institutional review board. Cells were stored in liquid nitrogen in 20% FBS and 10% DMSO until needed for experiments. At the time of the experiment, cells were thawed at 37 °C and incubated in RPMI 1640 medium (Gibco) supplemented with 20% FBS, 2 mm l-glutamine (Invitrogen), and penicillin/streptomycin (56 units/ml/56 μg/ml; Invitrogen) at 37 °C in an atmosphere of 5% CO2 for 1 h. Cells were then centrifuged and incubated in RPMI 1640 medium (Gibco) supplemented with 20% FBS, 2 mm l-glutamine (Invitrogen), and penicillin/streptomycin (56 units/ml/56 μg/ml; Invitrogen) and were either left untreated or treated with 10 ng/ml recombinant human IFNγ (R&D Systems, Minneapolis, MN) and incubated for 18 h at 37 °C. The next day, cells were counted using trypan blue exclusion and used for assays.
Cytokines and Antibodies
Recombinant human IFNγ (R&D Systems) was added to cell cultures at a concentration of 10 ng/ml. For the LDH assays, anti-human CD38 (clone HIT2; BD Biosciences) was used to coat cells. Briefly, cells were incubated with 10 μg/ml antibody for 1 h on ice, washed once with PBS, and resuspended in medium. Daratumumab was used for ADCC (20 μg/ml), conjugate studies (10 μg/ml), and in vivo experiments (1 μg/g mouse weight) and was supplied by commercial sources (Ohio State University, Columbus, OH).
For flow cytometry, unconjugated mouse anti-human CD64 (clone 32.2) with an FITC goat anti-mouse secondary antibody (Invitrogen), anti-human CD38 conjugated to FITC (clone HIT2; BD Biosciences), anti-human CD86 conjugated to phycoerythrin (clone 2331 (FUN-1); BD Biosciences), and anti-human CD80 conjugated to APC-H7 (clone L307.4; BD Biosciences) were used. Briefly, cells were incubated with 1 μg of antibody at a concentration of 1 × 106 cells/ml for 30 min at 4 °C. Cells were washed twice with FACS buffer (PBS, 0.09% sodium azide, 10% FBS). Secondary only (anti-human-CD64) and isotype control (all others) labeling was done in parallel to control for nonspecific staining. Samples were analyzed using an LSRII flow cytometer (BD Bioscience) and FlowJo software (FLOWJO, LLC, Ashland, OR).
Inhibitors
The PI3K inhibitor LY294002 (used at 20 μm) and ERK inhibitor U0126 (used at 25 μm) were purchased from Calbiochem (Billerica, MA). The p38 inhibitor, SB203580 (used at 5 μm), was purchased from Sigma. The NF-κB inhibitor, BAY 11-7085 (used at 5 μm), was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). The JAK1/2 inhibitor, ruxolitinib (used at 50 nm), was purchased from Selleck Chemicals (Houston, TX).
Real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) and chloroform extraction followed by DNase treatment (Invitrogen). RNA was reverse transcribed and subjected to quantitative real-time PCR using Power SYBR Green Master Mix (Applied Biosystems, Grand Island, NY). The following primers were used: GAPDH (forward primer, 5′-ATT CCC TGG ATT GTG AAA TAG TC-3′; reverse primer, 5′-ATTAAAGTCACCGCCTTCTGTAG-3′), FcγRI (CD64) (forward primer, 5′-GGCAAGTGGACACCACAAAGGCA-3′; reverse primer, 5′-GCTGGGGGTCGAGGTCGAGGTCTGAGT-3′), CD38 (forward primer, 5′-GCTCAATGGATCCCGCAGT-3′; reverse primer, 5′-TCCTGGCARAAGTCTCTGG-3′), CD80 (forward primer, 5′-GGTCTGGCTGGTCTTTCT-3′; reverse primer, 5′-CACTCGTATGTGCCCTCGTC-3′), CD86 (forward primer, 5′-GGGCCGCACAAGTTTTGA-3′; reverse primer, 5′-GCCCTTGTCCTTGATCTGAA-3′). qPCR primers for HLA-DQ (Hs.PT.58.15134093), HLA-DR (Hs.PT.58.15096946), NOS-2 (Hs.PT.58.14740388), and SDF-1 (Hs.PT.58.27881121) were purchased from Integrated DNA Technology (San Diego, CA). GAPDH was used for normalization of the genes of interest. Data were presented as mean relative copy number for at least three separate experiments using relative copy number = 2−ΔCt × 100 (52), where ΔCt is the Cttarget − CtGAPDH.
Lactate Dehydrogenase Assay
Antibody-coated (anti-CD38) or uncoated cells (5 × 104/100 μl of medium) that were pretreated for 24 h with 10 ng/ml IFNγ or left untreated were plated in quadruplicate in a 96-well plate. IFNγ (10 ng/ml) was added to appropriate wells and then incubated for 18 h at 37 °C. The CyTox 96 non-radioactive cytoxicity assay (Promega, Madison, WI) was used to measure released LDH from cells. This assay was performed as per the manufacturer's instructions. After an 18-h incubation, plates were centrifuged at 250 × g for 4 min, supernatants were collected and transferred to an ELISA plate, and then the supernatants were incubated with LDH substrate for 30 min. Stop solution was added, and the plates were read on a plate reader at a wavelength of 490 nm. Percentage cytotoxicity was calculated as (experimental absorbance/LDH maximum release) × 100. The assay was repeated at least three times for each cell line.
Phagocytosis
Phagocytosis assays were performed as described previously with minor adaptations for the experimental requirements of this study (53). Briefly, sheep red blood cells (SRBCs; Colorado Serum Company, Denver, CO) were labeled with PKH26 fluorescent cell membrane dye (Sigma) and then opsonized with anti-SRBC antibody (Sigma). SRBCs were added to the respective AML cell lines (treated with IFNγ for 24 or 48 h) or primary AML apheresis samples (treated with IFNγ for 24 h), gently pelleted by slow centrifugation, and then incubated at 37 °C for 1 h. Non-phagocytosed SRBCs were lysed with red blood cell lysis buffer (eBioscience, San Diego, CA) at room temperature for 10 min and washed with PBS before fixation with 4% paraformaldehyde. The SRBCs ingested by the AML cells were counted in a blinded fashion using fluorescence microscopy, with three separate such counts per condition. For each set of counts, 100 phagocytes/condition were examined. The phagocytic index is defined as the total number of SRBCs ingested by 100 phagocytes.
Conjugate Formation Assay
Conjugate formation assays were performed using MV4-11 and THP-1 cell lines. Cells were treated with or without 10 ng/ml IFNγ and incubated at 37 °C for 48 h. Following incubation, samples were washed twice with PBS and split evenly into two tubes, one-half to be stained red and the other to be stained green. Staining was done using the CellVue® claret far red fluorescent cell linker kit and PKH67 green fluorescent cell linker kit, both from Sigma-Aldrich, according to the manufacturer's instructions. Following this, selected cell groups were opsonized by incubating with 10 μg/ml anti-CD38 antibody on ice for 1 h, followed by two washes with complete medium. For the assay, red and green cells were combined into 1 ml of medium and incubated at 37 °C for 1 h. Samples were washed in PBS three times and fixed in 4% paraformaldehyde. 30 random images were taken using a fluorescence microscope, and conjugates were counted in a blinded fashion.
Murine Model of Antibody Therapy
Female non-obese diabetic severe combined immunodeficient-γ (NSG) mice were purchased from Jackson ImmunoResearch Laboratories (Ban Harbor, ME). MV4-11 cells (0.25 × 106 cells resuspended in PBS) were subcutaneously injected into the right flank of 6-week-old NSG mice and allowed to grow for 7 days (36). Intraperitoneal injections with PBS vehicle, IFNγ (3,000 units/mouse), daratumumab (anti-CD38 antibody; 1 μg/g mouse weight), or IFNγ + daratumumab (anti-CD38 antibody) were administered twice per week for 2 weeks, with tumor measurements recorded on each treatment day in a blinded fashion. Tumor volumes were calculated as π/6(length × width × height) (54). All in vivo experiments were performed in strict accordance with guidelines set by the institutional animal care and use committee, under an approved protocol.
ADCC
ADCC assays were done as described previously (55). In brief, THP-1 and MV4-11 cells were treated with or without IFNγ (10 μg/ml), loaded with 51Cr, coated with anti-CD38 antibody or IgG antibody, and plated in V-bottom 96-well plates. After 48 h of incubation, levels of 51Cr in supernatants were measured using a γ counter. The percentage cytotoxicity was calculated as (sample − minimum)/(maximum − minimum) × 100, where minimum consisted of untreated cells not incubated with antibody and maximum was measured as cells that had been lysed with 10% SDS.
Statistical Analyses
The qPCR and phagocytosis data were analyzed using paired Student's t tests. To evaluate the association of IFNγ-mediated changes in phagocytosis and changes in FcγRI expression (Table 1), we first transformed the changes relative to baseline by using log transformation to fit the normality assumption, followed by performing Pearson correlation analysis. Cell death assays using released LDH were analyzed by mixed effect modeling, incorporating repeated measures for each sample. Tumor volumes from the in vivo study were first baseline-subtracted and then analyzed by mixed effect modeling, incorporating repeated measures for each tumor. Tumor growth rates and final tumor volumes were both compared. SAS 9.4 (SAS Inc., Cary, NC) was used for analyses.
Author Contributions
K. F., E. L. M., B. F. R., H. F., R. S., S. G., S. E., P. M., N. J. B., and G. M.-R. designed and performed experiments and collected and summarized data; X. M. performed statistical analyses and designed experiments; J. S. B. provided critical human samples and designed experiments; S. V., D. M. B., W. E. C., and J. C. B. designed experiments, provided reagents and helped write the paper; J. P. B. and S. T. designed experiments, analyzed results, and wrote the paper along with K. F., B. F. R., and S. V.; all authors reviewed and revised the manuscript and approved the final version.
Acknowledgments
We thank Kathleen McConnell and Adrienne Dorrance, Ph.D., for the NSG mice. We are also grateful to Jeshua Avila-Estrada for assistance with qPCR and phagocytosis experiments.
This work was supported by National Institutes of Health Grants P01-CA095426 (to S. T. and J. C. B.); R01 CA162411 (to S. T. and J. C. B.), T32 HL007946 (to B. F. R.), and P30 CA016058 (to the Ohio State University Comprehensive Cancer Center). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- AML
- acute myeloid leukemia
- qPCR
- quantitative PCR
- ADCC
- antibody-dependent cellular cytotoxicity
- LDH
- lactate dehydrogenase
- ATRA
- all-trans-retinoic acid.
References
- 1. Stein E. M., and Tallman M. S. (2016) Emerging therapeutic drugs for AML. Blood 127, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Estey E., Levine R. L., and Löwenberg B. (2015) Current challenges in clinical development of “targeted therapies”: the case of acute myeloid leukemia. Blood 125, 2461–2466 [DOI] [PubMed] [Google Scholar]
- 3. Döhner H., Weisdorf D. J., and Bloomfield C. D. (2015) Acute myeloid leukemia. N. Engl. J. Med. 373, 1136–1152 [DOI] [PubMed] [Google Scholar]
- 4. Masoumi-Dehshiri R., Hashemi A., Neamatzadeh H., and Zare-Zardeini H. (2014) A case report: acute myeloid leukemia (FAB M7). Iran. J. Ped. Hematol. Oncol. 4, 188–190 [PMC free article] [PubMed] [Google Scholar]
- 5. Löwenberg B., Downing J. R., and Burnett A. (1999) Acute myeloid leukemia. N. Engl. J. Med. 341, 1051–1062 [DOI] [PubMed] [Google Scholar]
- 6. Thomas E. D., Buckner C. D., Clift R. A., Fefer A., Johnson F. L., Neiman P. E., Sale G. E., Sanders J. E., Singer J. W., Shulman H., Storb R., and Weiden P. L. (1979) Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N. Engl. J. Med. 301, 597–599 [DOI] [PubMed] [Google Scholar]
- 7. Curran E., Corrales L., and Kline J. (2015) Targeting the innate immune system as immunotherapy for acute myeloid leukemia. Front. Oncol. 5, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta V., Tallman M. S., and Weisdorf D. J. (2011) Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood 117, 2307–2318 [DOI] [PubMed] [Google Scholar]
- 9. Dombret H., and Gardin C. (2016) An update of current treatments for adult acute myeloid leukemia. Blood 127, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Löwenberg B., and Rowe J. M. (2016) Introduction to the review series on advances in acute myeloid leukemia (AML). Blood 127, 1. [DOI] [PubMed] [Google Scholar]
- 11. Scott A. M., Wolchok J. D., and Old L. J. (2012) Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287 [DOI] [PubMed] [Google Scholar]
- 12. Kung Sutherland M. S., Walter R. B., Jeffrey S. C., Burke P. J., Yu C., Kostner H., Stone I., Ryan M. C., Sussman D., Lyon R. P., Zeng W., Harrington K. H., Klussman K., Westendorf L., Meyer D., et al. (2013) SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 122, 1455–1463 [DOI] [PubMed] [Google Scholar]
- 13. Cohen A. D., Luger S. M., Sickles C., Mangan P. A., Porter D. L., Schuster S. J., Tsai D. E., Nasta S., Gewirtz A. M., and Stadtmauer E. A. (2002) Gemtuzumab ozogamicin (Mylotarg) monotherapy for relapsed AML after hematopoietic stem cell transplant: efficacy and incidence of hepatic veno-occlusive disease. Bone Marrow Transplant. 30, 23–28 [DOI] [PubMed] [Google Scholar]
- 14. Kell J. (2016) The addition of gemtuzumab ozogamicin to chemotherapy in adult patients with acute myeloid leukemia. Expert Rev. Anticancer Ther. 16, 377–382 [DOI] [PubMed] [Google Scholar]
- 15. Vasu S., He S., Cheney C., Gopalakrishnan B., Mani R., Lozanski G., Mo X., Groh V., Whitman S. P., Konopitzky R., Kössl C., Bucci D., Lucas D. M., Yu J., Caligiuri M. A., Blum W., et al. (2016) Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood 127, 2879–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Poel C. E., Spaapen R. M., van de Winkel J. G., and Leusen J. H. (2011) Functional characteristics of the high affinity IgG receptor, FcγRI. J. Immunol. 186, 2699–2704 [DOI] [PubMed] [Google Scholar]
- 17. Zhong R. K., van de Winkel J. G., Thepen T., Schultz L. D., and Ball E. D. (2001) Cytotoxicity of anti-CD64-ricin a chain immunotoxin against human acute myeloid leukemia cells in vitro and in SCID mice. J. Hematother. Stem Cell Res. 10, 95–105 [DOI] [PubMed] [Google Scholar]
- 18. Ravetch J. V., and Clynes R. A. (1998) Divergent roles for Fc receptors and complement in vivo. Annu. Rev. Immunol. 16, 421–432 [DOI] [PubMed] [Google Scholar]
- 19. Bevaart L., Jansen M. J., van Vugt M. J., Verbeek J. S., van de Winkel J. G., and Leusen J. H. (2006) The high-affinity IgG receptor, FcγRI, plays a central role in antibody therapy of experimental melanoma. Cancer Res. 66, 1261–1264 [DOI] [PubMed] [Google Scholar]
- 20. Busfield S. J., Biondo M., Wong M., Ramshaw H. S., Lee E. M., Ghosh S., Braley H., Panousis C., Roberts A. W., He S. Z., Thomas D., Fabri L., Vairo G., Lock R. B., Lopez A. F., and Nash A. D. (2014) Targeting of acute myeloid leukemia in vitro and in vivo with an anti-CD123 mAb engineered for optimal ADCC. Leukemia 28, 2213–2221 [DOI] [PubMed] [Google Scholar]
- 21. Keyhani A., Huh Y. O., Jendiroba D., Pagliaro L., Cortez J., Pierce S., Pearlman M., Estey E., Kantarjian H., and Freireich E. J. (2000) Increased CD38 expression is associated with favorable prognosis in adult acute leukemia. Leuk. Res. 24, 153–159 [DOI] [PubMed] [Google Scholar]
- 22. Grimaldi J. C., Balasubramanian S., Kabra N. H., Shanafelt A., Bazan J. F., Zurawski G., and Howard M. C. (1995) CD38-mediated ribosylation of proteins. J. Immunol. 155, 811–817 [PubMed] [Google Scholar]
- 23. Howard M., Grimaldi J. C., Bazan J. F., Lund F. E., Santos-Argumedo L., Parkhouse R. M., Walseth T. F., and Lee H. C. (1993) Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 262, 1056–1059 [DOI] [PubMed] [Google Scholar]
- 24. Mehta K., Shahid U., and Malavasi F. (1996) Human CD38, a cell-surface protein with multiple functions. FASEB J. 10, 1408–1417 [DOI] [PubMed] [Google Scholar]
- 25. Lokhorst H. M., Plesner T., Laubach J. P., Nahi H., Gimsing P., Hansson M., Minnema M. C., Lassen U., Krejcik J., Palumbo A., van de Donk N. W., Ahmadi T., Khan I., Uhlar C. M., Wang J., et al. (2015) Targeting CD38 with daratumumab monotherapy in multiple myeloma. N. Engl. J. Med. 373, 1207–1219 [DOI] [PubMed] [Google Scholar]
- 26. van de Donk N. W., Janmaat M. L., Mutis T., Lammerts van Bueren J. J., Ahmadi T., Sasser A. K., Lokhorst H. M., and Parren P. W. (2016) Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol. Rev. 270, 95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Weers M., Tai Y. T., van der Veer M. S., Bakker J. M., Vink T., Jacobs D. C., Oomen L. A., Peipp M., Valerius T., Slootstra J. W., Mutis T., Bleeker W. K., Anderson K. C., Lokhorst H. M., van de Winkel J. G., and Parren P. W. (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 186, 1840–1848 [DOI] [PubMed] [Google Scholar]
- 28. Quatromoni J. G., and Eruslanov E. (2012) Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am. J. Transl. Res. 4, 376–389 [PMC free article] [PubMed] [Google Scholar]
- 29. Heusinkveld M., and van der Burg S. H. (2011) Identification and manipulation of tumor associated macrophages in human cancers. J. Transl. Med. 9, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mosser D. M. (2003) The many faces of macrophage activation. J. Leukoc. Biol. 73, 209–212 [DOI] [PubMed] [Google Scholar]
- 31. Costello R. T., Mallet F., Sainty D., Maraninchi D., Gastaut J. A., and Olive D. (1998) Regulation of CD80/B7-1 and CD86/B7-2 molecule expression in human primary acute myeloid leukemia and their role in allogenic immune recognition. Eur. J. Immunol. 28, 90–103 [DOI] [PubMed] [Google Scholar]
- 32. Madera S., Rapp M., Firth M. A., Beilke J. N., Lanier L. L., and Sun J. C. (2016) Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J. Exp. Med. 213, 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura K., Nakayama M., Kawano M., Ishii T., Harigae H., and Ogasawara K. (2013) NK-cell fratricide: dynamic crosstalk between NK and cancer cells. Oncoimmunology 2, e26529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoves S., Trapani J. A., and Voskoboinik I. (2010) The battlefield of perforin/granzyme cell death pathways. J. Leukoc. Biol. 87, 237–243 [DOI] [PubMed] [Google Scholar]
- 35. Elavazhagan S., Fatehchand K., Santhanam V., Fang H., Ren L., Gautam S., Reader B., Mo X., Cheney C., Briercheck E., Vasilakos J. P., Dietsch G. N., Hershberg R. M., Caligiuri M., Byrd J. C., et al. (2015) Granzyme B expression is enhanced in human monocytes by TLR8 agonists and contributes to antibody-dependent cellular cytotoxicity. J. Immunol. 194, 2786–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ranganathan P., Yu X., Na C., Santhanam R., Shacham S., Kauffman M., Walker A., Klisovic R., Blum W., Caligiuri M., Croce C. M., Marcucci G., and Garzon R. (2012) Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood 120, 1765–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shultz L. D., Schweitzer P. A., Christianson S. W., Gott B., Schweitzer I. B., Tennent B., McKenna S., Mobraaten L., Rajan T. V., and Greiner D. L. (1995) Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154, 180–191 [PubMed] [Google Scholar]
- 38. Yea K., Zhang H., Xie J., Jones T. M., Lin C. W., Francesconi W., Berton F., Fallahi M., Sauer K., and Lerner R. A. (2015) Agonist antibody that induces human malignant cells to kill one another. Proc. Natl. Acad. Sci. U.S.A. 112, E6158–E6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graziano R. F., and Fanger M. W. (1987) FcγRI and FcγRII on monocytes and granulocytes are cytotoxic trigger molecules for tumor cells. J. Immunol. 139, 3536–3541 [PubMed] [Google Scholar]
- 40. Funaro A., Morra M., Calosso L., Zini M. G., Ausiello C. M., and Malavasi F. (1997) Role of the human CD38 molecule in B cell activation and proliferation. Tissue Antigens 49, 7–15 [DOI] [PubMed] [Google Scholar]
- 41. Moreno-Garcia M. E., López-Bojórques L. N., Zentella A., Humphries L. A., Rawlings D. J., and Santos-Argumedo L. (2005) CD38 signaling regulates B lymphocyte activation via a phospholipase C (PLC)-γ2-independent, protein kinase C, phosphatidylcholine-PLC, and phospholipase D-dependent signaling cascade. J. Immunol. 174, 2687–2695 [DOI] [PubMed] [Google Scholar]
- 42. Nijhof I. S., Groen R. W., Lokhorst H. M., van Kessel B., Bloem A. C., van Velzen J., de Jong-Korlaar R., Yuan H., Noort W. A., Klein S. K., Martens A. C., Doshi P., Sasser K., Mutis T., and van de Donk N. W. (2015) Upregulation of CD38 expression on multiple myeloma cells by all-trans-retinoic acid improves the efficacy of daratumumab. Leukemia 29, 2039–2049 [DOI] [PubMed] [Google Scholar]
- 43. Breitman T. R., Collins S. J., and Keene B. R. (1981) Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood 57, 1000–1004 [PubMed] [Google Scholar]
- 44. Wang Z. Y., and Chen Z. (2000) Differentiation and apoptosis induction therapy in acute promyelocytic leukaemia. Lancet Oncol. 1, 101–106 [DOI] [PubMed] [Google Scholar]
- 45. Errante P. R., Frazao J. B., and Condino-Neto A. (2008) The use of interferon-γ therapy in chronic granulomatous disease. Recent Pat. Antiinfect. Drug Discov. 3, 225–230 [DOI] [PubMed] [Google Scholar]
- 46. Marciano B. E., Wesley R., De Carlo E. S., Anderson V. L., Barnhart L. A., Darnell D., Malech H. L., Gallin J. I., and Holland S. M. (2004) Long-term interferon-γ therapy for patients with chronic granulomatous disease. Clin. Infect. Dis. 39, 692–699 [DOI] [PubMed] [Google Scholar]
- 47. Nunoi H., Ishibashi F., Mizukami T., and Hidaka F. (2004) Clinical evaluation of interferon-γ treatment to chronic granulomatous disease patients with splice site mutations. Jpn. J. Infect. Dis. 57, S25–S26 [PubMed] [Google Scholar]
- 48. Wilson C. J., and Vellodi A. (2000) Autosomal recessive osteopetrosis: diagnosis, management, and outcome. Arch. Dis. Child. 83, 449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bong A. B., Bonnekoh B., Franke I., Schön M., Ulrich J., and Gollnick H. (2002) Imiquimod, a topical immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology 205, 135–138 [DOI] [PubMed] [Google Scholar]
- 50. So E. Y., and Ouchi T. (2010) The application of Toll like receptors for cancer therapy. Int. J. Biol. Sci. 6, 675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adib-Conquy M., Scott-Algara D., Cavaillon J. M., and Souza-Fonseca-Guimaraes F. (2014) TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol. Cell Biol. 92, 256–262 [DOI] [PubMed] [Google Scholar]
- 52. Gavrilin M. A., Bouakl I. J., Knatz N. L., Duncan M. D., Hall M. W., Gunn J. S., and Wewers M. D. (2006) Internalization and phagosome escape required for Francisella to induce human monocyte IL-1β processing and release. Proc. Natl. Acad. Sci. U.S.A. 103, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Joshi T., Ganesan L. P., Cao X., and Tridandapani S. (2006) Molecular analysis of expression and function of hFcγRIIbl and b2 isoforms in myeloid cells. Mol. Immunol. 43, 839–850 [DOI] [PubMed] [Google Scholar]
- 54. Tomayko M. M., and Reynolds C. P. (1989) Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 24, 148–154 [DOI] [PubMed] [Google Scholar]
- 55. Butchar J. P., Mehta P., Justiniano S. E., Guenterberg K. D., Kondadasula S. V., Mo X., Chemudupati M., Kanneganti T. D., Amer A., Muthusamy N., Jarjoura D., Marsh C. B., Carson W. E. 3rd, Byrd J. C., and Tridandapani S. (2010) Reciprocal regulation of activating and inhibitory Fcγ receptors by TLR7/8 activation: implications for tumor immunotherapy. Clin. Cancer Res. 16, 2065–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]