Abstract
Hyperactive Wnt/β-catenin signaling is linked to cancer progression and developmental abnormalities, making identification of mechanisms controlling Wnt/β-catenin signaling vital. Transforming growth factor β type III receptor (TβRIII/betaglycan) is a transmembrane proteoglycan co-receptor that exists with or without heparan and/or chondroitin sulfate glycosaminoglycan (GAG) modifications in cells and has established roles in development and cancer. Our studies here demonstrate that TβRIII, independent of its TGFβ co-receptor function, regulates canonical Wnt3a signaling by controlling Wnt3a availability through its sulfated GAG chains. Our findings revealed, for the first time, opposing functions for the different GAG modifications on TβRIII suggesting that Wnt interactions with the TβRIII heparan sulfate chains result in inhibition of Wnt signaling, likely via Wnt sequestration, whereas the chondroitin sulfate GAG chains on TβRIII promote Wnt3a signaling. These studies identify a novel, dual role for TβRIII/betaglycan and define a key requirement for the balance between chondroitin sulfate and heparan sulfate chains in dictating ligand responses with implications for both development and cancer.
Keywords: cancer biology, cell signaling, glycosaminoglycan, transforming growth factor β (TGF-β), Wnt signaling, TBRIII, betaglycan
Introduction
Wnt glycoproteins regulate three distinct Wnt signaling pathways to mediate cell fate, proliferation, and apoptosis as well as cancer initiation and progression in multiple cancers, including ovarian (1–9). Activation of the canonical Wnt/β-catenin pathway begins with the binding of Wnt to its cell surface receptors, Frizzled and LDL receptor-related proteins 5/6 (LRP5/6),3 followed by phosphorylation of LRP5/6, recruitment of Dishevelled to the plasma membrane to interact with Frizzled, and stabilization of cytosolic β-catenin (10). Axin interaction with phosphorylated LRP5/6 and Dishevelled leads to inactivation of the β-catenin destruction complex, accumulation of β-catenin, and translocation to the nucleus to regulate Wnt target genes by binding to TCF/LEF transcription factors (10, 11). The Wnt signaling cascade is controlled in part by transmembrane proteoglycans, which interact with Wnt signaling components and can either stimulate or inhibit signaling activity. For instance, the HSPG glypican-3 and syndecan-1 stimulate canonical Wnt signaling (12, 13), whereas others, including glypican-1 and glypican-6, suppress Wnt signaling (13, 14).
Type III TGF-β receptor (TβRIII)/betaglycan is a transmembrane proteoglycan with loss resulting in embryonic lethality in mice (15). Beyond its roles in regulating TGF-β signaling, TβRIII also controls several other pathways to inhibit cell migration, invasion, cell growth, and angiogenesis in both in vitro and in vivo cancer models (16–22) and regulating differentiation through FGF2 signaling (23). Mechanistically, TβRIII regulates these pathways either by altering the actin cytoskeleton, via TβRIII/β-arrestin2 cytoplasmic interactions (24), or by GAG chain interactions with FGF2 (23). Overall, TβRIII also acts as a tumor suppressor in prostate (19), lung (25), pancreatic (18), and breast cancer (16, 21, 26, 27) but has been shown to promote metastasis in specific mesenchymal stem-like breast cancers (28), indicating its complex roles for TβRIII in cancer.
Although the TβRIII core can bind TGF-β superfamily members with high affinity (22, 29, 30) the extracellular domain also contains two sites of heparan and chondroitin sulfate GAG chain modifications, resulting in TβRIII existing in multiple forms in vivo (30–32). Given that Wnt glycoproteins have a high affinity for both heparan and chondroitin GAG chains on proteoglycans (13, 33), we initiated studies to determine the possible role of TβRIII in canonical Wnt3a signaling.
We found, using both cancer and normal epithelial cells and a combination of loss and gain of function approaches, that TβRIII suppresses Wnt3a signaling both at the signal reception level and through inhibition of β-catenin transcriptional activity by binding Wnt3a via its sulfated GAG chains. In contrast, TβRIII chondroitin sulfate chains promote Wnt3a signaling, suggesting that the composition of the GAG chains may significantly alter the cellular response to TβRIII and thereby Wnt signaling. Consistent with a lack of a role for TβRIII GAG chains in TβRIII functions as a TGF-β co-receptor (30), TβRIII suppression of canonical Wnt3a signaling is independent of TGF-β signaling and independent of the TβRIII cytoplasmic domain interactions described previously (24, 34, 35). These results demonstrate an intricate mode of Wnt3a signaling regulation by TβRIII mediated largely by its heparan and chondroitin chains, laying the foundation to advance the current understanding of the various roles that proteoglycans, with different GAG chains, have in maintaining cellular homeostasis, specifically through control of Wnt availability and signaling.
Results
TβRIII Suppresses Wnt/β-Catenin Activity at the Level of Signal Reception
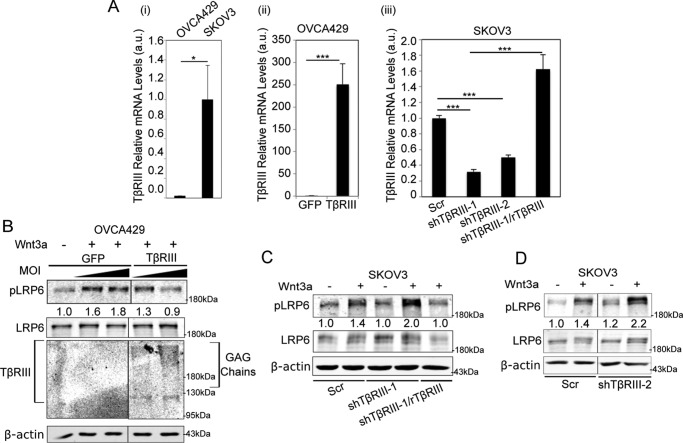
To investigate the role of TβRIII in signaling by Wnt glycoproteins, which have high affinities for both HSPG and CSPG (33, 36, 37), we expressed TβRIII in the ovarian cancer cell line OVCA429 that we and others have established as expressing low levels of TβRIII (Fig. 1A and Refs. 17 and 24). Conversely, we reduced the expression of TβRIII by shRNA-mediated knockdown in the ovarian cancer cell line SKOV3, which expresses higher levels of TβRIII (Fig. 1A, iii and Ref. 38). We examined whether TβRIII can affect canonical Wnt signaling as determined by phosphorylation of co-receptor LRP6, one of the first steps initiated by the binding of Wnt to their signaling co-receptors (39). We found that although Wnt3a robustly phosphorylated LRP6 at serine 1490 (40) in OVCA429 cells (low TβRIII levels), transiently increasing TβRIII expression in OVCA429 cells suppressed Wnt-induced LRP6 phosphorylation in a TβRIII dose-dependent manner (Fig. 1B). Total LRP6 levels remained stable in TβRIII-expressing OVCA429 cells when compared with OVCA429 cells with low levels of TβRIII (Fig. 1B). In SKOV3 cells, which express high levels of TβRIII (Fig. 1A, i), reducing TβRIII expression using shRNA resulted in increased LRP6 phosphorylation when compared with Wnt3a-stimulated SKOV3 control cells expressing high endogenous TβRIII (Fig. 1, C and D). To confirm that the effect of shTβRIII was specific to TβRIII, we utilized shRNA-resistant rat TβRIII (21, 23) to rescue TβRIII expression and examined Wnt-induced LRP6 phosphorylation. We found that rescue of TβRIII expression in shTβRIII cells (Fig. 1A, iii) suppressed Wnt-induced LRP6 phosphorylation compared with cells containing endogenous TβRIII (Fig. 1C). Total LRP6 levels were not significantly altered by shRNA to TβRIII or transient expression of rat TβRIII in SKOV3 cells when compared with control cells (Fig. 1C). Consistently, a second shRNA to TβRIII (shTβRIII-2) also resulted in increased LRP6 phosphorylation when compared with Wnt3a-stimulated control cells (Fig. 1D). These results indicate that TβRIII may regulate Wnt signaling at the signal reception level by suppressing canonical Wnt signaling.
FIGURE 1.
TβRIII suppresses Wnt/β-catenin activity at the level of signal reception. A, TβRIII mRNA expression by qRT-PCR analysis to detect endogenous TβRIII levels in OVCA429 and SKOV3 cells (i), overexpression of TβRIII in OVCA429 cells as indicated (ii), and expression in SKOV3 cells of the indicated shRNA and rescue conditions generated as described under “Experimental Procedures” (iii). Ct values are normalized in graph i to endogenous TβRIII levels in SKOV3 cells (lane 2), in graph ii to GFP (lane 1), and in graph iii to Scrambled (Scr) TβRIII levels (lane 1). Quantitations represent the average of two independent biological trials, each conducted in triplicate. B, OVCA429 cells transiently expressing increasing doses of TβRIII (5 and 10 multiplicities of infection (MOI) of TβRIII-expressing adenoviral constructs) or control (GFP) were stimulated with 50 ng ml−1 Wnt3a for 1 h followed by immunoblotting of lysates for phospho-LRP6 (Ser-1490) (pLRP6), LRP6, TβRIII, and β-actin. C, SKOV3 cells transiently expressing shRNA to TβRIII (shTβRIII-1) or Scrambled control (“Experimental Procedures”) and transiently transfected with rat TβRIII (rTβRIII) or control vector (pcDNA 3.1) for 24 h for rescue of TβRIII expression (as seen in A, right panel) and then stimulated with 50 ng ml−1 Wnt3a for 1 h followed by immunoblotting of lysates for phospho-LRP6 (Ser-1490) (pLRP6), LRP6, and β-actin. D, SKOV3 cells transiently expressing a second independent shRNA to TβRIII (shTβRIII) or Scrambled control (“Experimental Procedures”) were stimulated with 50 ng ml−1 Wnt3a for 1 h followed by immunoblotting of lysates for phospho-LRP6 (Ser-1490) (pLRP6), LRP6, and β-actin. B–D, quantitations represent pLRP6:LRP6 ratios and are normalized to the untreated sample. All panels (A–D) represent at least two independent biological trials.
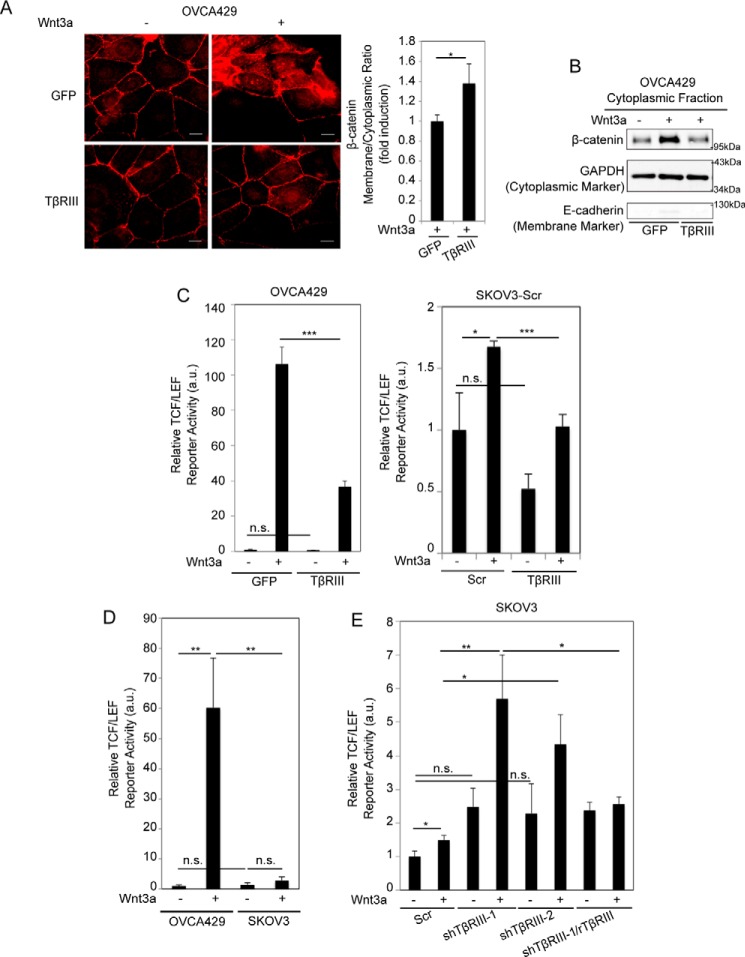
Activation of the canonical Wnt pathway leads to stabilization and accumulation of cytosolic β-catenin, which then enters the nucleus and regulates Wnt target genes (10). Consistent with reduced LRP6 phosphorylation, Wnt-induced β-catenin cytosolic accumulation was significantly reduced in the presence of TβRIII (Fig. 2, A and B).
FIGURE 2.
TβRIII suppresses Wnt-induced β-catenin cytoplasmic accumulation and transcriptional activity. A, left, OVCA429 cells transiently expressing control (GFP) or TβRIII were stimulated with 50 ng ml−1 Wnt3a for 1 h and immunostained for β-catenin (red). Scale bars: 20 μm. Right, the graph represents quantitation of β-catenin fluorescence at the membrane versus cytoplasm (“Experimental Procedures”). n ≥ 30 cells/condition, representative of at least two independent biological trials. Values are normalized to control GFP. B, cytoplasmic fractions obtained after subcellular fractionation (“Experimental Procedures”) of OVCA429 cells transiently expressing TβRIII or GFP stimulated with 50 ng ml−1 Wnt3a for 1 h followed by immunoblotting of lysates for β-catenin, GAPDH (positive cytoplasmic marker), and E-cadherin (negative cytoplasmic marker), representative at least two independent biological trials. C–E, the indicated cells expressing TβRIII, shTβRIII, or shTβRIII with rTβRIII, as described in Fig. 1, B and C, were transfected with a Wnt-responsive luciferase reporter and SV40 control vector and left untreated or treated with 50 ng ml−1 Wnt3a for 24 h. Luciferase activity was then measured as described under “Experimental Procedures.” All values are normalized to the untreated sample and represent the average of two independent biological trials, each conducted in duplicate. Data were analyzed using two-tailed Student's t test and represent the mean ± S.E. Scr, Scrambled; n.s., not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Upon β-catenin accumulation and stabilization, activation of TCF/LEF-sensitive transcription by β-catenin provides a robust readout of the Wnt-stimulated canonical pathway (41). To test whether TβRIII-mediated changes on LRP6 phosphorylation and β-catenin accumulation would translate to downstream effects on TCF/LEF activity, we analyzed the activity of a TCF/LEF-sensitive reporter, which contains multiple β-catenin binding sites (42). We found that Wnt3a significantly increased TCF/LEF reporter activity in OVCA429 cells (Fig. 2C). Increasing TβRIII expression in these cell lines resulted in a significant suppression of Wnt3a-induced activation of the TCF/LEF reporter compared with control Wnt-treated cells (Fig. 2C). Similar to trends seen in OVCA429 cells, overexpressing TβRIII in SKOV3 cells (high TβRIII) resulted in suppression of Wnt3a-induced TCF/LEF activity compared with control Wnt-treated cells (Fig. 2C). Side-by-side analysis of Wnt3a-stimulated TCF/LEF activity in SKOV3 (high TβRIII) and OVCA429 (low TβRIII) cells in the same experiment revealed lower Wnt3a-induced TCF/LEF activity in SKOV3 cells when compared with Wnt3a-treated ovarian cancer OVCA429 cells (Fig. 2D), which we hypothesized was in part due to higher endogenous TβRIII expression in SKOV3 cells (Fig. 1A, left graph). This hypothesis was confirmed in SKOV3 cells using shRNA to TβRIII (Fig. 1A, right graph), which resulted in enhanced Wnt-induced TCF/LEF reporter activity compared with control cells (Fig. 2E). This increased Wnt signaling in shTβRIII cells was suppressed upon restoring TβRIII expression using shRNA-resistant rat TβRIII (Fig. 2E), consistent with increased LRP6 activation observed in SKOV3 cells upon knockdown of TβRIII (Fig. 1C). Regulation of TCF/LEF reporter activity by TβRIII was not restricted to ovarian cancer cells, as TβRIII expression also repressed Wnt-induced TCF/LEF reporter activity in 4T1 (breast cancer) cells (Fig. 4D), indicating a broad-based impact of TβRIII on Wnt signaling regulation.
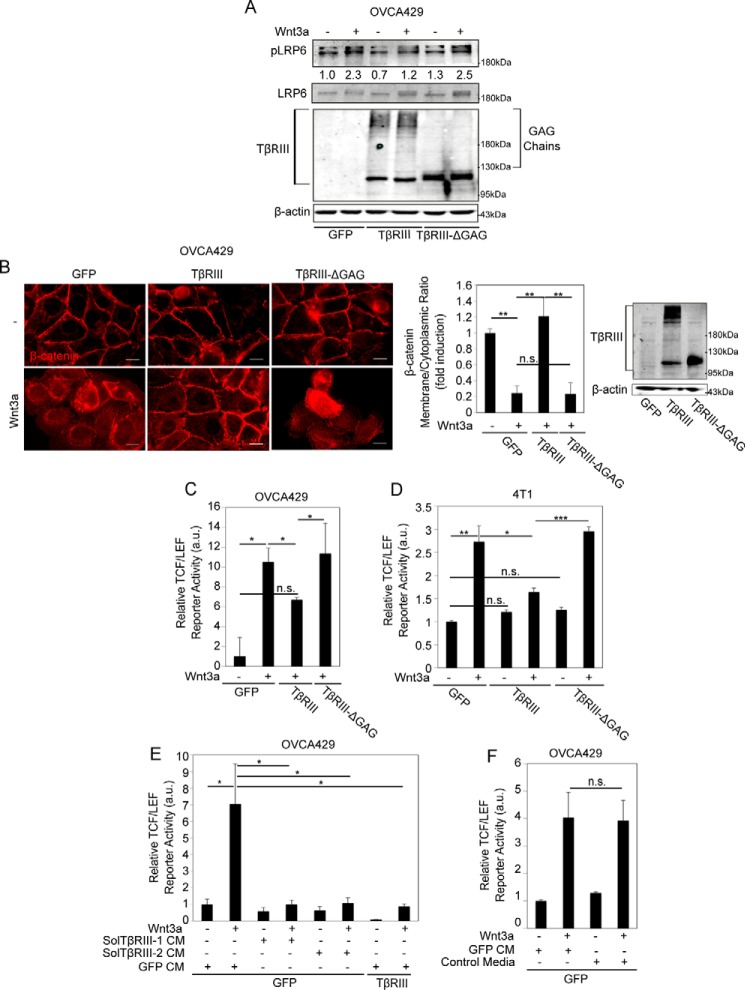
FIGURE 4.
GAG chains of TβRIII suppress Wnt signaling. A, OVCA429 cells transiently expressing full-length TβRIII, TβRIII-ΔGAG, or GFP (control) were stimulated with 50 ng ml−1 Wnt3a. Cells were then lysed after 1 h and phospho-LRP6 (Ser1490) (pLRP6), LRP6, and β-actin levels assessed by immunoblotting. Quantitations represent pLRP6:LRP6 ratios and are normalized to the untreated sample. B, the indicated OVCA429 cells were stimulated with 50 ng ml−1 Wnt3a for 1 h and immunostained for β-catenin (red). Scale bars: 20 μm. The graph represents quantitation of β-catenin fluorescence at the membrane versus cytoplasm (“Experimental Procedures”). n ≥ 30 cells/condition. Western analysis shows TβRIII and β-actin levels. Data were analyzed using analysis of variance followed by a post hoc Shapiro-Wilk test and represent the mean ± S.E. All values were normalized to the untreated sample. C–F, the indicated cells were stimulated with 50 ng ml−1 Wnt3a alone (C and D); 50 ng ml−1 Wnt3a in the presence of CM from cells expressing only TβRIII-ECD (Sol-TβRIII-1) (E), CM from cells expressing full-length TβRIII (Sol-TβRIII-2), or CM from control GFP-expressing cells for 24 h (E); or 50 ng ml−1 Wnt3a in the presence of control media from untransfected cells or from cells transiently expressing GFP (F). Cells were lysed after 24 h, and luciferase activity was measured in 1% serum as described under “Experimental Procedures.” All values were normalized to the untreated sample. All luciferase data (C–F) are representative of at least two independent biological trials, each conducted in duplicate. Data were analyzed using two-tailed Student's t test and represent the mean ± S.E. of a biological replicate. All values are normalized to the untreated sample, and all figures represent at least two independent biological trials. n.s., not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
TGF-β Signaling Does Not Limit TβRIII the Ability to Suppress Wnt/β-Catenin Signaling
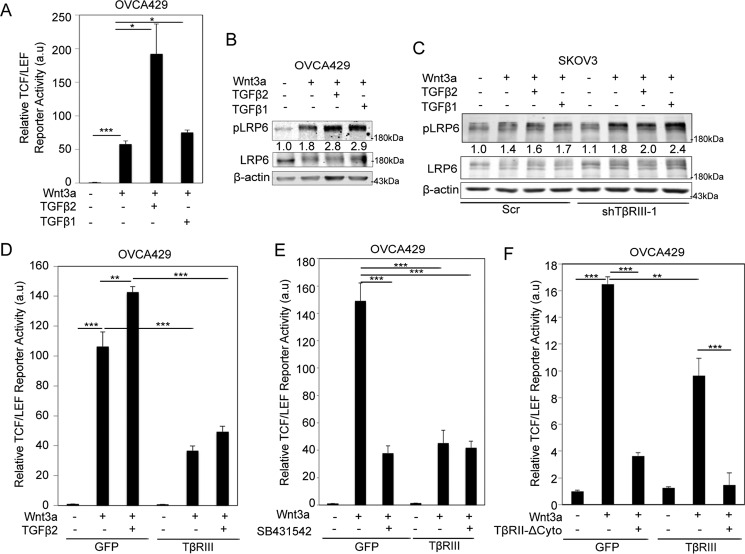
To begin elucidating the mechanisms by which TβRIII regulates Wnt signaling, we examined whether the presence of TGF-β, a high affinity ligand for the TβRIII core domain (43–45), impacts the ability of TβRIII to suppress Wnt signaling. We found that both TGF-β1 and TGF-β2 enhanced Wnt-induced LRP6 phosphorylation and TCF/LEF activity (Fig. 3, A and B) in OVCA429 cells and, to a lesser extent, in SKOV3 cells (high TβRIII) (Fig. 3C, lanes 1–4), indicating a cooperative role for TGF-β ligands in Wnt signaling that may be repressed by TβRIII. Treating TβRIII knockdown SKOV3 cells (shTβRIII) with TGF-β resulted in an enhancement of Wnt3a-TGF-β cooperativity compared with control TβRIII-expressing SKOV3 cells treated with Wnt3a and TGF-β (Fig. 3C, lanes 5–8). Because TGF-β2 binds the core domain of TβRIII with higher affinity than TGF-β1 (46), and it showed the most robust enhancement of Wnt3a-induced TCF/LEF activity (Fig. 3A), this ligand was chosen to determine TGF-β signaling-mediated changes on the suppression of Wnt3a-induced TCF/LEF activity by TβRIII. We found that Wnt-induced TCF/LEF activity, both in the absence and presence of TGF-β2, was dampened by TβRIII expression in OVCA429 cells (Fig. 3D).
FIGURE 3.
TGF-β signaling does not limit ability of TβRIII to suppress Wnt/β-catenin signaling. A, OVCA429 cells transfected with a Wnt-responsive luciferase reporter (500 ng) and a SV40 control vector were either untreated or stimulated with 50 ng ml−1 Wnt3a and 400 pm TGF-β1 or TGF-β2 for 24 h. Luciferase activity was then measured as described under “Experimental Procedures.” B, OVCA429 cells were either untreated or stimulated with 50 ng ml−1 Wnt3a and 400 pm TGF-β1 and TGF-β2 as indicated for 1 h. Cells were lysed, and the levels of phospho-LRP6 (Ser-1490) (pLRP6) and β-actin were assessed by immunoblotting. Quantitations represent pLRP6:LRP6 ratios and are normalized to the untreated sample. C, SKOV3 cells stably expressing control or TβRIII shRNA-1 were either untreated or stimulated with 50 ng ml−1 Wnt3a and 400 pm TGF-β1 or TGF-β2 for 1 h. Cells were assessed as described in the legend for Fig. 1B. Quantitations represent pLRP6:LRP6 ratios and are normalized to the untreated sample. D–F, OVCA429 cells transiently expressing either control (GFP) or TβRIII were transfected with a Wnt-responsive luciferase reporter and a SV40 control vector and incubated with 50 ng ml−1 Wnt3a in the presence or absence of 400 pm TGF-β2 (D), 50 ng ml−1 Wnt3a and 5 μm SB431542 (E), or 50 ng ml−1 Wnt3a and a dominant negative form of the type II TGF-β receptor (TβRII-ΔCyto) or pcDNA 3.1 control vector (F) for 24 h. Luciferase activity was measured as described under “Experimental Procedures.” Luciferase data are representative of at least two independent biological trials each conducted in duplicate. Data were analyzed using two-tailed Student's t test and represent the mean ± S.E.; *, p < 0.05; **, p < 0.01; ***, p < 0.001. Western blotting analysis data represent at least two independent biological trials. All values were normalized to the untreated sample.
To confirm that TβRIII does not require TGF-β signaling receptors to suppress Wnt signaling, we first utilized SB431542 (inhibitor of TβRI kinase activity) and analyzed Wnt-induced TCF/LEF activity in OVCA429 cells. We found that inhibition of TβRI suppressed Wnt signaling independent of TβRIII expression in control cells (Fig. 3E). However, inhibition of TβRI did not affect the ability of TβRIII to suppress Wnt-induced TCF/LEF activity in OVCA429 cells when compared with control cells (Fig. 3E), indicating that repression of Wnt signaling by TβRIII is independent of TβRI kinase activity. Several TGF-β-independent roles for TβRIII have been reported through its interactions with the type II TGF-β receptor, TβRII (43). However, transient expression of TβRII lacking its cytoplasmic domain (TβRII-ΔCyto), and therefore unable to interact with TβRIII (43, 47), did not affect the ability of TβRIII to suppress Wnt-induced TCF/LEF activity when compared with control cells (Fig. 3F). Similar to what is shown in Fig. 3E, the removal of the TβRII cytoplasmic domain (TβRII-ΔCyto) in GFP-expressing cells led to a suppression of Wnt-induced TCF/LEF activity when compared with control cells (Fig. 3F). These TβRIII-independent observations of the effects of TβRII-ΔCyto and SB431542 on TCF/LEF activity may point to autocrine TGF-β-Wnt signaling mechanisms unrelated to the ability of TβRIII to suppress Wnt-dependent Wnt signaling. Collectively, these data suggest that even in the presence of the high affinity ligand TGF-β2, the absence of TGF-β signaling, and TβRIII-TβRII interaction, TβRIII is still able to suppress Wnt signaling.
GAG Chains of TβRIII Regulate Wnt Signaling
Wnt glycoproteins have been shown to have a high affinity for GAG chains on transmembrane proteoglycans (33), and the extracellular TβRIII domain contains two sites of heparan and chondroitin sulfate GAG chains (23, 48). To determine whether the chains on TβRIII are involved in suppressive effects on Wnt signaling, we expressed full-length TβRIII (TβRIII), TβRIII lacking GAG chain modifications (TβRIII-ΔGAG) (30), or control vectors in OVCA429 cells and assessed the levels of phosphorylation of LRP6, cytosolic β-catenin accumulation, and TCF/LEF activity induced by exogenous Wnt3a. We found that, unlike full-length TβRIII, TβRIII-ΔGAG failed to suppress LRP6 phosphorylation in OVCA429 cells (Fig. 4A). Consistently, TβRIII-ΔGAG did not suppress Wnt3a-dependent β-catenin cytoplasmic accumulation compared with full-length TβRIII; instead, β-catenin cytoplasmic accumulation in the presence of TβRIII-ΔGAG resembled the cytoplasmic β-catenin levels observed in Wnt3a-treated control cells (Fig. 4B). TβRIII-ΔGAG cells also failed to suppress TCF/LEF activity when compared with full-length TβRIII (Fig. 4C). The effect of the TβRIII GAG chains on Wnt signaling was not restricted to ovarian cells, as TβRIII-ΔGAG also failed to suppress Wnt signaling when compared with full-length TβRIII in the murine mammary 4T1 cells (Fig. 4D).
To test whether the extracellular domain (ECD) of TβRIII was sufficient to suppress Wnt-induced signaling, we used two parallel approaches. We treated OVCA429 cells, in the absence and presence of Wnt3a, with either conditioned media (CM) from cells expressing only the TβRIII ECD (Sol-TβRIII-1) (16, 18, 44) or CM from cells expressing full-length TβRIII containing soluble TβRIII in the media due to shedding (Sol-TβRIII-2) (30, 44) (Fig. 4E). CM from control vector (GFP) expressing cells was used as control (GFP-CM, Fig. 4E). These conditions were compared with OVCA429 cells expressing full-length TβRIII in the same experiment (Fig. 4E). We found that both the shed and soluble forms of TβRIII were able to significantly suppress Wnt-induced TCF/LEF activity to the same extent as they expressed full-length TβRIII (Fig. 4E). To control for possible artifacts associated with infection of vectors, we also tested media from uninfected cells (Fig. 4F) and found that infection with GFP did not impact TCF/LEF activity (Fig. 4F). Taken together, these data confirm that TβRIII ECD, with its GAG chains, is sufficient to suppress Wnt-induced signaling.
TβRIII Interacts with Wnt, and the Balance between Sulfated Heparan and Chondroitin Chains Determines TβRIII Ability to Regulate Wnt/β-Catenin Signaling
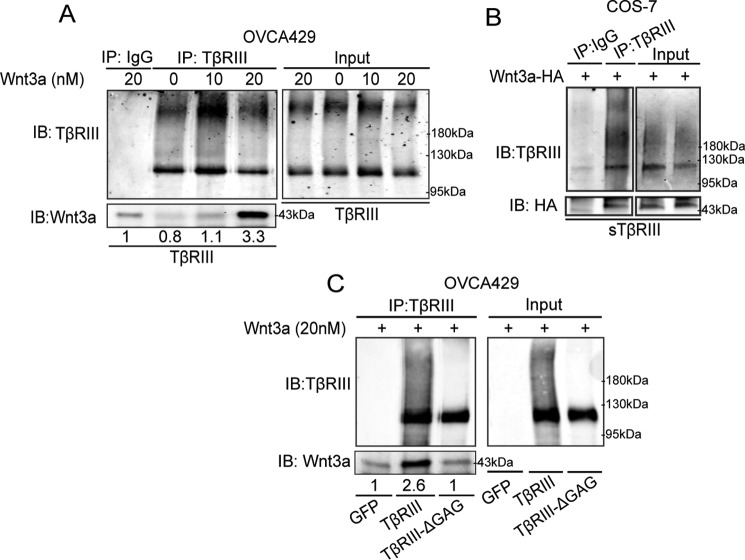
To determine whether TβRIII binds Wnt3a, we used co-immunoprecipitation of recombinant Wnt3a and TβRIII, a methodology commonly used to study Wnt interactions with its receptors (13, 49). We found a Wnt dose-dependent interaction between TβRIII and Wnt3a in OVCA429 cells (Fig. 5A). Consistent with the extracellular domain of TβRIII as sufficient to suppress Wnt signaling (Fig. 4E), we found that soluble TβRIII was also able to interact with Wnt3a, as determined by using CM from COS-7 cells expressing full-length TβRIII and HA-tagged Wnt3a (Fig. 5B). To determine whether the TβRIII-Wnt3a interaction is mediated through the TβRIII GAG chains as suggested by our Wnt signaling assays (Fig. 4), we incubated OVCA429 cell lysates with recombinant Wnt3a and performed co-immunoprecipitation in cells expressing TβRIII, TβRIII-ΔGAG, or control (see “Experimental Procedures”). We observed immunoprecipitation of Wnt3a and TβRIII reduced to background levels in cells expressing TβRIII-ΔGAG (Fig. 5C). These data indicate that the interaction/binding capacity of TβRIII-ΔGAG is significantly less than full-length TβRIII. These findings are consistent with TβRIII-ΔGAG being unable to inhibit Wnt3a signaling (Fig. 4, A–C).
FIGURE 5.
TβRIII interacts with Wnt through its GAG chains. A, OVCA429 cells transiently expressing full-length TβRIII were immunoprecipitated (IP) and analyzed for Wnt3a-TβRIII interactions using the Wnt pulldown assay described under “Experimental Procedures” and previously (13). All values were normalized to the IgG sample. B, conditioned medium from COS-7 cells transiently expressing full-length TβRIII and Wnt3a-HA was immunoprecipitated using anti-TβRIII and immunoblotted using anti-HA and anti-TβRIII to analyze Wnt3a-TβRIII interactions as described under “Experimental Procedures” and previously (21, 27, 77). C, OVCA429 cells transiently expressing GFP (control), full-length TβRIII, or TβRIII-ΔGAG were immunoprecipitated using anti-TβRIII and immunoblotted (IB) using anti-Wnt3a and anti-TβRIII to analyze Wnt3a-TβRIII interactions using the Wnt pulldown assay described under “Experimental Procedures” and previously (13) All values were normalized to the GFP sample, and all figures represent at least two independent biological trials.
Because TβRIII represses Wnt signaling and appears to interact with Wnt3a through its GAG chains, we aimed to test whether the regulation of Wnt signaling by TβRIII GAG chains was dependent on the sulfation state of the TβRIII GAG chains. We treated TβRIII-expressing OVCA429 cells with sodium chlorate, a competitive inhibitor of ATP-sulfurylase, which resulted in proteoglycans arriving at the cell surface bearing nonsulfated heparan sulfate or chondroitin sulfate chains (50). We found that non-sulfated GAG chains on TβRIII significantly stimulated Wnt-induced TCF/LEF activity (Fig. 6A). Treatment with sodium sulfate, which overcomes the effects of sodium chlorate and restores sulfation of proteoglycans (50), decreased Wnt-induced TCF/LEF activity compared with TβRIII-expressing OVCA429 cells treated only with sodium chlorate (Fig. 6A). These results demonstrate that the sulfation of the TβRIII GAG chains is required for TβRIII-mediated suppression of Wnt signaling and that loss of sulfation results in increased Wnt-induced signaling.
FIGURE 6.
The balance between sulfated heparan and chondroitin chains on TβRIII determines the ability of TβRIII to regulate Wnt/β-catenin signaling. A, OVCA429 cells transiently expressing full-length TβRIII, transfected with a Wnt-responsive luciferase reporter and a SV40 control vector were pretreated with 50 mm NaClO3 with or without 10 mm Na2SO4 as indicated for 2 h. Cells were then stimulated with 50 ng ml−1 Wnt3a, and luciferase activity was measured as described under “Experimental Procedures.” All values were normalized to the Wnt-treated sample. B–F, CHO K1, pgsD-677, or OVCA429 cells expressing TβRIII or GFP were transfected with a Wnt-responsive luciferase reporter and a SV40 control vector and pretreated with 100 mU ml−1 chondroitinase (Ch) (D and E) or 20 mU ml−1 heparanase (Hp) (F) for 2 h before overnight incubation with 50 ng ml−1 Wnt3a. Luciferase activity was measured as described under “Experimental Procedures.” All values were normalized to the untreated sample. Western blotting analysis (D) shows TβRIII expression in pgsD-677 cells after CS chain removal using 100 mU ml−1 chondroitinase. All data represent at least two independent biological trials. Data were analyzed using two-tailed Student's t test and represent the mean ± S.E. G, model of canonical Wnt/β-catenin signaling regulation by TβRIII. n.s., not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Because the GAG chains on TβRIII comprise both heparan sulfate (HS) and chondroitin sulfate (CS) chains (32), we aimed to isolate the individual effects of the different GAG chains of TβRIII on Wnt signaling. To do this, we first determined whether the suppressive role of TβRIII in Wnt signaling was conserved in parental CHO K1 cells, where TβRIII expresses both HS and CS chains (51). Although CHO cells have a modest response to Wnt stimulation as observed previously (33, 52) and by us here (Fig. 6, B and C), we observed a significant decrease in Wnt signaling upon TβRIII-expression in CHO K1 cells compared with control cells (Fig. 6B), consistent with our observations in ovarian and breast cancer cells (Figs. 2 and 4). To determine the role of TβRIII CS chains in Wnt signaling, we utilized the CHO cell line derivative pgsD-677; these cells lack both N-acetylglucosaminyltransferase and glucuronyltransferase activities and are unable to synthesize heparan sulfate but can produce high amounts of chondroitin sulfate (51). We increased TβRIII expression in pgsD-677 (ΔHS) cells (as described under “Experimental Procedures”) and examined Wnt-induced TCF/LEF activity. Strikingly, we observed a significant increase in Wnt signaling in TβRIII-expressing pgsD-677 cells compared with control cells (Fig. 6C). Furthermore, the removal of the TβRIII CS chains with chondroitinase (Fig. 6D, right panel, Ch) reduced Wnt-induced TCF/LEF activity in TβRIII-expressing pgsD-677 cells (Fig. 6D). Because pgsD-677 cells express only CS GAG chains (51), we tested whether CS chains promote Wnt signaling in cells that make both HS and CS GAG chains. Similar to our results in pgsD-677 cells (Fig. 6D), we found that TβRIII was able to further repress Wnt signaling in OVCA429 cells treated with chondroitinase as compared with control cells (2× repressed, Fig. 6E). In contrast, heparanase treatment of TβRIII-expressing OVCA429 cells resulted in increased TCF/LEF activity compared with heparanase (Hp)-untreated cells (5× increased, Fig. 6F). These data suggest that HS and CS chains on TβRIII contribute, in an opposing fashion, to the availability of Wnt for signaling. Therefore, we propose that the HS chains of TβRIII are responsible for Wnt3a sequestration and subsequent TβRIII-mediated suppression of Wnt3a signaling. In contrast, TβRIII CS chains increase Wnt availability and signaling (Fig. 6G).
Discussion
We provide novel evidence for the TβRIII/betaglycan-mediated regulation of canonical Wnt signaling through distinct functions of its heparan- and chondroitin-sulfated GAG chains. Our studies demonstrate that the HS chains of TβRIII are responsible for the suppression of Wnt3a signaling, most likely via sequestering Wnt, in contrast with the CS chains of TβRIII, which promote Wnt signaling. Based on our findings, we propose that Wnt interactions with the HS chains on TβRIII result in the sequestration of Wnt away from LRP6 and Frizzled, which decreases the levels of signaling-productive complexes between the ligand and its receptors. This hypothesis was confirmed upon examining the inability of TβRIII to suppress Wnt signaling upon removal of its GAG chains (Fig. 4). Mechanistically, our pulldown assays in TβRIII-expressing cells (Fig. 5) indicate an interaction between TβRIII and Wnt glycoproteins, which have a high affinity for polyanionic compounds such as heparin (53), and reveal that the GAG chains significantly increase Wnt-TβRIII interaction to suppress Wnt signaling. Strikingly, the TβRIII CS chains promote Wnt3a signaling in the absence of its HS chains (Fig. 6, B and D). To support this conclusion, chondroitinase treatment in pgsD-677 and OVCA429 cells resulted in a loss of Wnt signaling, thus indicating an exciting new role for the chondroitin chains of TβRIII in stimulating Wnt signaling.
The role of GAG chains in Wnt signal transduction may also depend on the core protein and specific biochemical cues, as our data indicate opposing functions for TβRIII HS and CS chains in Wnt signaling. In support of our hypothesis, it has been shown that exogenous chondroitin sulfate, heparin, and GAG are unable to stimulate Wnt3a signaling, whereas endogenous CSPG promote Wnt signaling in mouse L-cell fibroblasts, suggesting that the core proteins of CSPG may be involved in regulating Wnt3a activity (36). We speculate that the localization, sulfation, and/or chain length of GAG chains attached to core proteins could contribute to differences in ligand availability and signaling.
Studies have shown also that cell context can determine the role that proteoglycans and GAG chains play in cancer progression. The enzymatic elimination of chondroitin sulfate molecules in primary breast tumors, for example, increases lung metastases in mice (54), whereas the digestion of cell surface CS on lung cancer cells injected into tail veins leads to a reduction in the number of tumor cells able to populate and metastasize (55). These results suggest that CS molecules may have opposing roles during cancer progression: an anti-metastatic function in primary tumor tissue and a pro-metastatic role during extravasation (circulating cancer cell interaction with endothelial cells) (56). Other proteoglycans have also been shown to function as either tumor promoters or suppressors depending on the protein core, GAG chains attached, associated molecules, proteoglycan localization, and tumor type (57). Perlecan, for example, can both promote tumor invasiveness (58) and inhibit angiogenesis (59), whereas glypicans and syndecans may promote local cancer cell growth and metastatic potential in some cancer tissues (37, 60) but inhibit tissue growth, invasion, and metastasis in others (61, 62). Together, these data show a requirement for the proteoglycan core domain and cellular environment in deciding GAG chain function.
In addition to the contributions made by the proteoglycan core domain and environment, the sulfation state of the proteoglycan also plays a major role in its ability to regulate signaling pathways. Upon treatment of our TβRIII-expressing OVCA429 cells with sodium chlorate, an ATP-sulfurylase-competitive inhibitor that causes proteoglycans to arrive at the cell surface bearing nonsulfated heparan sulfate or chondroitin sulfate chains (50), we found TβRIII unable to repress Wnt signaling, indicating that the sulfation of TβRIII GAG chains is required for proper Wnt signal regulation by TβRIII (Fig. 6A), consistent with previous reports for glypican-1 (33). Studies in Drosophila have also shown that, upon treatment of Drosophila cells with sodium chlorate or in the absence of an HS N-deacetylase/N-sulfotransferase, cells are completely deficient in HS chain sulfation and Wingless (Wg) signaling is disrupted (63–66). HS chain sulfation plays a vital role in regulating FGF signaling as well. Consistently, the HS chains of TβRIII can also regulate FGF signaling and play a critical role in tumor progression (23).
Previous reports indicate that FGF signal transduction is dependent on sulfation of the 2-O and 6-O positions on HS chains, which control FGF1 binding to heparin and FGF1-dependent dimerization and activation of the FGFR1 receptor, respectively (67–69). In articular cartilage, studies reveal a Wnt signaling promoter role for CS chains that is dependent on the sulfation of the CS chain (70). Taken together, these studies, combined with our data, suggest that sulfation plays a significant role in growth factor signaling regulation by GAG chains on proteoglycans.
It is possible that different expression levels of β1,4-N-acetylgalactosaminyltransferase-I (β4GalNAcT-I) and/or α1,4-N- acetylglucosaminyltransferase-I (α4GlcNAcT-I), which initiate the synthesis of CS or HS chains, respectively, may also contribute to the TβRIII proteoglycan state and subsequent effects on Wnt signaling. Moreover, within a single core protein, Ser-Gly residues in a hydrophobic pocket might signal heparan sulfate attachment, whereas Ser-Gly residues in an exposed hydrophilic environment might signal chondroitin sulfate attachment. These different local environments could achieve selectivity by modulating the activity of β4GalNAcT-I and α4GlcNAcT-I (71). Other biochemical cues may include the location of N-linked glycosylation sites (Asn-Phe-Ser) as described for syndecan-1 (72). Attachment of an N-linked sugar at a GAG chain attachment site would likely prevent subsequent recognition by the xylosyltransferase and GAG chain attachment to the TβRIII core protein.
The precise mechanism by which CS chains of TβRIII increase Wnt availability remains to be determined. Future studies into the biochemical cues involved in determining the proteoglycan state of HSPG such as TβRIII, as well as the role of TβRIII in regulating Wnt signaling, will help shed light on Wnt signaling regulation and increase our understanding of the diverse roles that proteoglycans like TβRIII play in signaling and disease.
Experimental Procedures
Cell Lines and Reagents
Ovarian epithelial carcinoma cell lines SKOV3, and OVCA429 were obtained from the Duke Gynecology/Oncology Bank (Durham, NC). Authentication of cell lines was carried out at the University of Colorado (Denver) sequencing facility. Monkey kidney COS-7 (ATCC® CRL-1651TM) cells, mouse mammary tumor cell line 4T1 (ATCC® CRL2539TM), normal CHO epithelial cell lines pgsA-745 (ATCC® CRL-2242TM), and pgsD-677 (ATCC® CRL-2244TM) were obtained from ATCC (Manassas, VA). Epithelial carcinoma cell lines SKOV3, 4T1, and OVCA429 were cultured in RPMI 1640 (ATCC® 30-2001ATCCTM) containing l-glutamine, 10% FBS, and 100 units of penicillin-streptomycin. COS-7 cells were maintained in DMEM (ATCC® 30–2002TM) containing 10% FBS and 100 units of penicillin-streptomycin. CHO cell lines pgsA-745 and pgsD-677 were cultured in Kaighn's modification of Ham's F-12 medium (ATCC® 30-2004TM) containing l-glutamine, 10% FBS, and 100 units of penicillin-streptomycin. All cells lines were maintained at 37 °C in a humidified incubator at 5% CO2. The antibodies used were as follows. Phospho-LRP6 (Ser-1490) (catalog No. 2568), LRP6 (catalog No. 2560), β-catenin (D10A8) XP® rabbit mAb (catalog No. 8480), GAPDH rabbit mAb (catalog No. 14C10), HA rabbit mAb (catalog No. 3724), and Wnt3a (C64F2) rabbit mAb (catalog No. 2721) were from Cell Signaling Technology (Danvers, MA). Mouse E-cadherin mAb was purchased from BD Biosciences (catalog No. 610181). Human TβRIII antibody (catalog No. AF-242-PB) was purchased from R&D Biosystems (Minneapolis, MN) and actin (catalog No. A2228) from Sigma-Aldrich. Mouse HA antibody (catalog No. 32-6700) from Invitrogen. Inhibitor SB431542 hydrate (catalog No. S4317) was purchased from Sigma-Aldrich. Sodium chlorate (NaClO3) was obtained from Thomas Scientific (Swedesboro, NJ) and sodium sulfate anhydrous (Na2SO4) (catalog No. S421-500) from ThermoFisher Scientific. Heparinase III (catalog No. H8891) and chondroitinase ABC (catalog No. C3667) were obtained from Sigma-Aldrich, and recombinant TGF-β1, TGF-β2, and Wnt3a were purchased from R&D Systems.
Plasmid Constructs and Stable Cell Lines
TβRIII constructs used in this study have been described previously (16, 19, 23, 73, 74). Full-length TβRIII consists of TβRIII-HA in pcDNA 3.1 as described previously (29, 73). The TβRIII-ΔGAG construct consists of human TβRIII-HA, with serine-to-alanine point mutations at amino acids 534 and 545 to prevent GAG attachment (29, 48, 75, 76). rTβRIII is a HA-tagged rat TβRIII in the pcDNA 3.1 vector (19). Adenoviral constructs were used at multiplicities of infection between 5 and 100 particles/cell, and infections were performed as described previously (21, 23, 24). shRNA sequences for TβRIII were obtained from Sigma-Aldrich with the following sequences: shRNA33430 (shTβRIII-1),CCGGCCAAGCATGAAGGAACCAAATCTCGAGATTTGGTTCCTTCATGCTTGGTTTTTG; and shRNA33432 (shTβRIII-2), CCGGCGTGCTTTATCTCTCCATATTCTCGAGAATATGGAGAGATAAAGCACGTTTTTG in a pLKO.1-puro backbone (TβRIII shRNA construct and non-targeted control). Lentiviral particles were generated at the Center for Targeted Therapeutics Core Facility and the University of South Carolina (Columbia). For TβRIII knockdown, SKOV3 cells were infected with 1× TβRIII shRNA lentivirus. Cells were then selected in the presence of 1 μg ml−1 puromycin. Stable cell lines were maintained in 0.5 μg ml−1 puromycin.
Wnt3a-HA (catalog No. 18030) and TβRII-ΔCyto (catalog No. 14051) plasmids were purchased from Addgene (Cambridge, MA) (47). The soluble human TβRIII construct was a kind gift from G. Blobe (Duke University, Durham, NC). Conditioned media containing soluble TβRIII were generated by transfecting cells with the indicated expression vectors and collected 48 h after transfection under serum-free conditions. Transient DNA transfections were performed using Lipofectamine 2000 (catalog No. 11668019) from Life Technologies or FuGENE® 6 (catalog No. E2691) from Promega (Madison, WI) according to the manufacturer's instructions. The cell fractionation kit to analyze β-catenin localization came from Cell Signaling Technology (catalog No. 9038). The luciferase assay kit (catalog No. E1500) came from Promega, and M50 Super 8× TOPFlash (42) used to measure luciferase activity was a gift from Randall Moon (Addgene plasmid 12456).
Quantitative RT-PCR
For qRT-PCR, total RNA was isolated from ∼200,000 cells using TRIzol reagent (Invitrogen). RNA was retrotranscribed using iScriptTM Reverse Transcription Supermix (catalog No. 1708841) and SsoAdvanced Universal SYBR Green Supermix (#1725271) from Bio-Rad. The qRT-PCR primer sequences used were: RPL13A-forward, AGATGGCGGAGGTGCAG; RPL13A-reverse, GGCCCAGCAGTACCTGTTTA; TβRIII-forward, CGTCAGGAGGCACACACTTA; and TβRIII-reverse, CACATTTGACAGACAGGGCAAT.
Immunoprecipitation and Western Blotting
Immunoprecipitation and Western blotting were performed using standard techniques as described previously (21, 27, 77). For co-immunoprecipitation in COS-7 cells, TβRIII-expressing cells were transfected with the indicated Wnt3a-HA construct, and the culture medium was collected 48 h after transfection under serum-free conditions. TβRIII was then immunoprecipitated by incubating the cell lysates overnight with anti-human TβRIII antibody. The next day, protein G-Sepharose beads were added to the lysates for 2 h at 4 °C. The beads were then washed three times with cold PBS and resuspended in sample buffer. The amount of TβRIII or Wnt3a bound to the beads was detected by Western blotting with anti-human TβRIII or Wnt3a antibodies.
Wnt3a-TβRIII Pulldown Assay
This assay was performed as described previously (13, 49). Briefly, OVCA429 cells were lysed in non-denaturing COIP lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm of NaCl, 1% Nonidet P-40, 10% glycerol, 1 mm DTT, 25 mm NaF, 1 mm Na3VO4 and 1× protease inhibitor mixture (catalog No. P8340, Sigma-Aldrich)). TβRIII-HA was then immunoprecipitated by incubating the cell lysates overnight with an anti-human TβRIII antibody. The next day, protein G-Sepharose beads were added to the lysates for 2 h at 4 °C. Beads were then washed three times with PBS and incubated with 20 nm Wnt3a-conditioned medium for 2 h at 4 °C. After two more washes with PBS, the beads were resuspended in sample buffer, and the amount of Wnt3a bound to TβRIII was detected by Western blotting using anti-Wnt3a and anti-TβRIII antibodies.
Luciferase Assay
The indicated cells were seeded in 24-well plates and co-transfected with a Luciferase reporter vector containing a β-catenin-responsive promoter (to drive luciferase expression (TOPFlash, catalog No. 12456, Addgene)) and SV40 (Renilla internal control vector). One day after transfection and infection, cells were incubated overnight with 50 ng ml−1 Wnt3a and then lysed. Luciferase activity (Luciferase assay system, Promega) was measured by calculating the ratio between luciferase and Renilla activities (to normalize for transfection efficiency) and then normalizing the values to the untreated sample.
Immunofluorescence and Intensity Analysis
The indicated cells were seeded onto coverslips in 12-well plates at a density of 5 × 104 cells/well. After infections and treatment with 50 ng ml−1 Wnt3a, cells were washed with ice-cold PBS and fixed with 100% methanol for 10 min followed by PBS washes. Cells were permeabilized with 0.1% Triton X-100 in PBS and then blocked with 3% BSA or 0.2% gelatin in PBS for 30 min at room temperature followed by an overnight incubation at 4 °C with a rabbit anti-β-catenin antibody. After extensive washing with PBS, the cells were incubated with an Alexa-conjugated secondary antibody (Molecular Probes, Eugene, OR). Cells were mounted in mounting medium and analyzed under an Olympus IX81 motorized inverted microscope (Shinjuku, Tokyo, Japan). Fluorescence intensity for the β-catenin was analyzed using ImageJ 1.50d software (National Institutes of Health) by drawing a fixed line of interest over the membrane and cytoplasm followed by averaging the maximum intensities obtained from the plot profile plugin. To estimate the change in β-catenin localization after Wnt treatment in the presence and absence of TβRIII, the ratio between the membrane and cytoplasmic fractions of β-catenin fluorescence was calculated. The statistical significance of the data was analyzed in SigmaPlot version 11 software. p values < 0.05 were considered to be statistically significant.
Subcellular Fractionation
The indicated cells were seeded in 12-well plates and infected to express TβRIII. 48 h post-infection, the cells were treated with 50 ng ml−1 Wnt3a for 1 h and then lysed. Subcellular fractionation of β-catenin, the cytoplasmic marker GAPDH, and the plasma membrane marker E-cadherin was carried out using the cell fractionation kit (Cell Signaling Technology) according to the manufacturer's instructions.
Author Contributions
L. M. J., P. S., A. V., K. O. C., S. S., and H. V. F. performed all of the experiments. N. Y .L. helped analyze the data. L. M. J. and K. M. designed all of the experiments, analyzed the data, and wrote the manuscript.
Acknowledgments
We thank Drs. John Lavigne and Fabienne Poulain (University of South Carolina) for helpful discussions.
This work was supported in part by Grant 258785 from the Ovarian Cancer Research Fund (to K. M.), National Institutes of Health Grant P20 GM109091 (to K. M.), and a Minority Graduate Research Fellowship from the University of South Carolina (to L. M. J.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- LRP
- LDL receptor-related protein
- TβRI
- -II, and -III, transforming growth factor β receptor type I, II, and III
- ECD
- extracellular domain
- GAG
- glycosaminoglycan
- HS
- heparan sulfate
- HSPG
- heparan sulfate proteoglycan
- CS
- chondroitin sulfate
- CSPG
- chondroitin sulfate proteoglycan
- TCF/LEF
- T-cell factor/lymphoid enhancer factor
- CM
- conditioned medium
- qRT-PCR
- quantitative RT-PCR
- mU
- milliunits.
References
- 1. Rask K., Nilsson A., Brännström M., Carlsson P., Hellberg P., Janson P. O., Hedin L., and Sundfeldt K. (2003) Wnt signalling pathway in ovarian epithelial tumours: increased expression of β-catenin and GSK3β. Br. J. Cancer 89, 1298–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qi L., Sun B., Liu Z., Cheng R., Li Y., and Zhao X. (2014) Wnt3a expression is associated with epithelial-mesenchymal transition and promotes colon cancer progression. J. Exp. Clin. Cancer Res. 33, 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badiglian Filho L., Oshima C. T., De Oliveira Lima F., De Oliveira Costa H., De Sousa Damião R., Gomes T. S., and Gonçalves W. J. (2009) Canonical and noncanonical Wnt pathway: a comparison among normal ovary, benign ovarian tumor and ovarian cancer. Oncol. Rep. 21, 313–320 [PubMed] [Google Scholar]
- 4. Tung E. K., Wong B. Y., Yau T. O., and Ng I. O. (2012) Upregulation of the Wnt co-receptor LRP6 promotes hepatocarcinogenesis and enhances cell invasion. PloS One 7, e36565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verras M., Brown J., Li X., Nusse R., and Sun Z. (2004) Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 64, 8860–8866 [DOI] [PubMed] [Google Scholar]
- 6. Vinyoles M., Del Valle-Pérez B., Curto J., Viñas-Castells R., Alba-Castellón L., García de Herreros A., and Duñach M. (2014) Multivesicular GSK3 sequestration upon Wnt signaling is controlled by p120-catenin/cadherin interaction with LRP5/6. Mol. Cell 53, 444–457 [DOI] [PubMed] [Google Scholar]
- 7. Usongo M., Li X., and Farookhi R. (2013) Activation of the canonical WNT signaling pathway promotes ovarian surface epithelial proliferation without inducing β-catenin/Tcf-mediated reporter expression. Dev. Dyn. 242, 291–300 [DOI] [PubMed] [Google Scholar]
- 8. King M. L., Lindberg M. E., Stodden G. R., Okuda H., Ebers S. D., Johnson A., Montag A., Lengyel E., MacLean Ii J. A., and Hayashi K. (2015) WNT7A/β-catenin signaling induces FGF1 and influences sensitivity to niclosamide in ovarian cancer. Oncogene 34, 3452–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asad M., Wong M. K., Tan T. Z., Choolani M., Low J., Mori S., Virshup D., Thiery J. P., and Huang R. Y. (2014) FZD7 drives in vitro aggressiveness in Stem-A subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Cell Death Dis. 5, e1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arend R. C., Londoño-Joshi A. I., Straughn J. M. Jr., and Buchsbaum D. J. (2013) The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol. Oncol. 131, 772–779 [DOI] [PubMed] [Google Scholar]
- 11. Gatcliffe T. A., Monk B. J., Planutis K., and Holcombe R. F. (2008) Wnt signaling in ovarian tumorigenesis. Int. J. Gynecol. Cancer 18, 954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alexander C. M., Reichsman F., Hinkes M. T., Lincecum J., Becker K. A., Cumberledge S., and Bernfield M. (2000) Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat. Genet. 25, 329–332 [DOI] [PubMed] [Google Scholar]
- 13. Capurro M., Martin T., Shi W., and Filmus J. (2014) Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J. Cell Sci. 127, 1565–1575 [DOI] [PubMed] [Google Scholar]
- 14. Shiau C. E., Hu N., and Bronner-Fraser M. (2010) Altering Glypican-1 levels modulates canonical Wnt signaling during trigeminal placode development. Dev. Biol. 348, 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stenvers K. L., Tursky M. L., Harder K. W., Kountouri N., Amatayakul-Chantler S., Grail D., Small C., Weinberg R. A., Sizeland A. M., and Zhu H. J. (2003) Heart and liver defects and reduced transforming growth factor β2 sensitivity in transforming growth factor β type III receptor-deficient embryos. Mol. Cell. Biol. 23, 4371–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong M., How T., Kirkbride K. C., Gordon K. J., Lee J. D., Hempel N., Kelly P., Moeller B. J., Marks J. R., and Blobe G. C. (2007) The type III TGF-β receptor suppresses breast cancer progression. J. Clin. Invest. 117, 206–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hempel N., How T., Dong M., Murphy S. K., Fields T. A., and Blobe G. C. (2007) Loss of betaglycan expression in ovarian cancer: role in motility and invasion. Cancer Res. 67, 5231–5238 [DOI] [PubMed] [Google Scholar]
- 18. Gordon K. J., Dong M., Chislock E. M., Fields T. A., and Blobe G. C. (2008) Loss of type III transforming growth factor β receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis 29, 252–262 [DOI] [PubMed] [Google Scholar]
- 19. Turley R. S., Finger E. C., Hempel N., How T., Fields T. A., and Blobe G. C. (2007) The type III transforming growth factor-β receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 67, 1090–1098 [DOI] [PubMed] [Google Scholar]
- 20. Mythreye K., and Blobe G. C. (2009) Proteoglycan signaling co-receptors: roles in cell adhesion, migration and invasion. Cell. Signal. 21, 1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mythreye K., Knelson E. H., Gatza C. E., Gatza M. L., and Blobe G. C. (2013) TβRIII/β-arrestin2 regulates integrin α5β1 trafficking, function, and localization in epithelial cells. Oncogene 32, 1416–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gatza C. E., Oh S. Y., and Blobe G. C. (2010) Roles for the type III TGF-β receptor in human cancer. Cell. Signal. 22, 1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knelson E. H., Gaviglio A. L., Tewari A. K., Armstrong M. B., Mythreye K., and Blobe G. C. (2013) Type III TGF-β receptor promotes FGF2-mediated neuronal differentiation in neuroblastoma. J. Clin. Invest. 123, 4786–4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mythreye K., and Blobe G. C. (2009) The type III TGF-beta receptor regulates epithelial and cancer cell migration through β-arrestin2-mediated activation of Cdc42. Proc. Natl. Acad. Sci. U.S.A. 106, 8221–8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finger E. C., Turley R. S., Dong M., How T., Fields T. A., and Blobe G. C. (2008) TβRIII suppresses non-small cell lung cancer invasiveness and tumorigenicity. Carcinogenesis 29, 528–535 [DOI] [PubMed] [Google Scholar]
- 26. Meyer A. E., Gatza C. E., How T., Starr M., Nixon A. B., and Blobe G. C. (2014) Role of TGF-β receptor III localization in polarity and breast cancer progression. Mol. Biol. Cell 25, 2291–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oh S. Y., Knelson E. H., Blobe G. C., and Mythreye K. (2013) The type III TGFβ receptor regulates filopodia formation via a Cdc42-mediated IRSp53-N-WASP interaction in epithelial cells. Biochem. J. 454, 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jovanović B., Beeler J. S., Pickup M. W., Chytil A., Gorska A. E., Ashby W. J., Lehmann B. D., Zijlstra A., Pietenpol J. A., and Moses H. L. (2014) Transforming growth factor β receptor type III is a tumor promoter in mesenchymal-stem-like triple negative breast cancer. Breast Cancer Res. 16, R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen W., Kirkbride K. C., How T., Nelson C. D., Mo J., Frederick J. P., Wang X. F., Lefkowitz R. J., and Blobe G. C. (2003) β-Arrestin 2 mediates endocytosis of type III TGF-β receptor and down-regulation of its signaling. Science 301, 1394–1397 [DOI] [PubMed] [Google Scholar]
- 30. López-Casillas F., Payne H. M., Andres J. L., and Massagué J. (1994) Betaglycan can act as a dual modulator of TGF-β access to signaling receptors: mapping of ligand binding and GAG attachment sites. J. Cell Biol. 124, 557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Segarini P. R., and Seyedin S. M. (1988) The high molecular weight receptor to transforming growth factor-β contains glycosaminoglycan chains. J. Biol. Chem. 263, 8366–8370 [PubMed] [Google Scholar]
- 32. Cheifetz S., Andres J. L., and Massagué J. (1988) The transforming growth factor-β receptor type III is a membrane proteoglycan: domain structure of the receptor. J. Biol. Chem. 263, 16984–16991 [PubMed] [Google Scholar]
- 33. Ai X., Do A. T., Lozynska O., Kusche-Gullberg M., Lindahl U., and Emerson C. P. Jr. (2003) QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J. Cell Biol. 162, 341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blobe G. C., Liu X., Fang S. J., How T., and Lodish H. F. (2001) A novel mechanism for regulating transforming growth factor β (TGF-β) signaling: functional modulation of type III TGF-β receptor expression through interaction with the PDZ domain protein, GIPC. J. Biol. Chem. 276, 39608–39617 [DOI] [PubMed] [Google Scholar]
- 35. Finger E. C., Lee N. Y., You H. J., and Blobe G. C. (2008) Endocytosis of the type III transforming growth factor-β (TGF-β) receptor through the clathrin-independent/lipid raft pathway regulates TGF-β signaling and receptor down-regulation. J. Biol. Chem. 283, 34808–34818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nadanaka S., Ishida M., Ikegami M., and Kitagawa H. (2008) Chondroitin 4-O-sulfotransferase-1 modulates Wnt-3a signaling through control of E disaccharide expression of chondroitin sulfate. J. Biol. Chem. 283, 27333–27343 [DOI] [PubMed] [Google Scholar]
- 37. O'Connell M. P., Fiori J. L., Kershner E. K., Frank B. P., Indig F. E., Taub D. D., Hoek K. S., and Weeraratna A. T. (2009) Heparan sulfate proteoglycan modulation of Wnt5A signal transduction in metastatic melanoma cells. J. Biol. Chem. 284, 28704–28712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steller M. D., Shaw T. J., Vanderhyden B. C., and Ethier J. F. (2005) Inhibin resistance is associated with aggressive tumorigenicity of ovarian cancer cells. Mol. Cancer Res. 3, 50–61 [PubMed] [Google Scholar]
- 39. Bilic J., Huang Y. L., Davidson G., Zimmermann T., Cruciat C. M., Bienz M., and Niehrs C. (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619–1622 [DOI] [PubMed] [Google Scholar]
- 40. Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., and He X. (2004) A mechanism for Wnt coreceptor activation. Mol. Cell 13, 149–156 [DOI] [PubMed] [Google Scholar]
- 41. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 42. Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H., and Moon R. T. (2003) Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680–685 [DOI] [PubMed] [Google Scholar]
- 43. Blobe G. C., Schiemann W. P., Pepin M. C., Beauchemin M., Moustakas A., Lodish H. F., and O'Connor-McCourt M. D. (2001) Functional roles for the cytoplasmic domain of the type III transforming growth factor β receptor in regulating transforming growth factor β signaling. J. Biol. Chem. 276, 24627–24637 [DOI] [PubMed] [Google Scholar]
- 44. Mendoza V., Vilchis-Landeros M. M., Mendoza-Hernández G., Huang T., Villarreal M. M., Hinck A. P., López-Casillas F., and Montiel J. L. (2009) Betaglycan has two independent domains required for high affinity TGF-β binding: proteolytic cleavage separates the domains and inactivates the neutralizing activity of the soluble receptor. Biochemistry 48, 11755–11765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheifetz S., and Massagué J. (1989) Transforming growth factor-β (TGF-β) receptor proteoglycan: cell surface expression and ligand binding in the absence of glycosaminoglycan chains. J. Biol. Chem. 264, 12025–12028 [PubMed] [Google Scholar]
- 46. Kubiczkova L., Sedlarikova L., Hajek R., and Sevcikova S. (2012) TGF-β: an excellent servant but a bad master. J. Transl. Med. 10, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Siegel P. M., Shu W., Cardiff R. D., Muller W. J., and Massagué J. (2003) Transforming growth factor β signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc. Natl. Acad. Sci. U.S.A. 100, 8430–8435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andres J. L., DeFalcis D., Noda M., and Massagué J. (1992) Binding of two growth factor families to separate domains of the proteoglycan betaglycan. J. Biol. Chem. 267, 5927–5930 [PubMed] [Google Scholar]
- 49. Sakane H., Yamamoto H., and Kikuchi A. (2010) LRP6 is internalized by Dkk1 to suppress its phosphorylation in the lipid raft and is recycled for reuse. J. Cell Sci. 123, 360–368 [DOI] [PubMed] [Google Scholar]
- 50. Rapraeger A. C., Guimond S., Krufka A., and Olwin B. B. (1994) Regulation by heparan sulfate in fibroblast growth factor signaling. Methods Enzymol. 245, 219–240 [DOI] [PubMed] [Google Scholar]
- 51. Lidholt K., Weinke J. L., Kiser C. S., Lugemwa F. N., Bame K. J., Cheifetz S., Massagué J., Lindahl U., and Esko J. D. (1992) A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 89, 2267–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Cat B., Muyldermans S. Y., Coomans C., Degeest G., Vanderschueren B., Creemers J., Biemar F., Peers B., and David G. (2003) Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J. Cell Biol. 163, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Willert K., and Nusse R. (2012) Wnt proteins. Cold Spring Harbor Perspect. Biol. 4, a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prinz R. D., Willis C. M., van Kuppevelt T. H., and Klüppel M. (2014) Biphasic role of chondroitin sulfate in cardiac differentiation of embryonic stem cells through inhibition of Wnt/β-catenin signaling. PloS One 9, e92381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li F., Ten Dam G. B., Murugan S., Yamada S., Hashiguchi T., Mizumoto S., Oguri K., Okayama M., van Kuppevelt T. H., and Sugahara K. (2008) Involvement of highly sulfated chondroitin sulfate in the metastasis of the Lewis lung carcinoma cells. J. Biol. Chem. 283, 34294–34304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Willis C. M., and Klüppel M. (2014) Chondroitin sulfate-E is a negative regulator of a pro-tumorigenic Wnt/β-catenin-collagen 1 axis in breast cancer cells. PloS One 9, e103966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fjeldstad K., and Kolset S. O. (2005) Decreasing the metastatic potential in cancers: targeting the heparan sulfate proteoglycans. Curr. Drug Targets 6, 665–682 [DOI] [PubMed] [Google Scholar]
- 58. Cohen I. R., Murdoch A. D., Naso M. F., Marchetti D., Berd D., and Iozzo R. V. (1994) Abnormal expression of perlecan proteoglycan in metastatic melanomas. Cancer Res. 54, 5771–5774 [PubMed] [Google Scholar]
- 59. Mongiat M., Sweeney S. M., San Antonio J. D., Fu J., and Iozzo R. V. (2003) Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 278, 4238–4249 [DOI] [PubMed] [Google Scholar]
- 60. Capurro M. I., Xiang Y. Y., Lobe C., and Filmus J. (2005) Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 65, 6245–6254 [DOI] [PubMed] [Google Scholar]
- 61. Peters M. G., Farías E., Colombo L., Filmus J., Puricelli L., and Bal de Kier Joffé E. (2003) Inhibition of invasion and metastasis by glypican-3 in a syngeneic breast cancer model. Breast Cancer Res. Treat. 80, 221–232 [DOI] [PubMed] [Google Scholar]
- 62. Dhodapkar M. V., Abe E., Theus A., Lacy M., Langford J. K., Barlogie B., and Sanderson R. D. (1998) Syndecan-1 is a multifunctional regulator of myeloma pathobiology: control of tumor cell survival, growth, and bone cell differentiation. Blood 91, 2679–2688 [PubMed] [Google Scholar]
- 63. Lin X., and Perrimon N. (1999) Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature 400, 281–284 [DOI] [PubMed] [Google Scholar]
- 64. Toyoda H., Kinoshita-Toyoda A., Fox B., and Selleck S. B. (2000) Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu: Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J. Biol. Chem. 275, 21856–21861 [DOI] [PubMed] [Google Scholar]
- 65. Reichsman F., Smith L., and Cumberledge S. (1996) Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J. Cell Biol. 135, 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dhoot G. K., Gustafsson M. K., Ai X., Sun W., Standiford D. M., and Emerson C. P. Jr. (2001) Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science 293, 1663–1666 [DOI] [PubMed] [Google Scholar]
- 67. Pye D. A., Vivès R. R., Hyde P., and Gallagher J. T. (2000) Regulation of FGF-1 mitogenic activity by heparan sulfate oligosaccharides is dependent on specific structural features: differential requirements for the modulation of FGF-1 and FGF-2. Glycobiology 10, 1183–1192 [DOI] [PubMed] [Google Scholar]
- 68. Schlessinger J., Plotnikov A. N., Ibrahimi O. A., Eliseenkova A. V., Yeh B. K., Yayon A., Linhardt R. J., and Mohammadi M. (2000) Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 6, 743–750 [DOI] [PubMed] [Google Scholar]
- 69. Jemth P., Kreuger J., Kusche-Gullberg M., Sturiale L., Giménez-Gallego G., and Lindahl U. (2002) Biosynthetic oligosaccharide libraries for identification of protein-binding heparan sulfate motifs: exploring the structural diversity by screening for fibroblast growth factor (FGF)1 and FGF2 binding. J. Biol. Chem. 277, 30567–30573 [DOI] [PubMed] [Google Scholar]
- 70. Shortkroff S., and Yates K. E. (2007) Alteration of matrix glycosaminoglycans diminishes articular chondrocytes' response to a canonical Wnt signal. Osteoarthritis Cartilage 15, 147–154 [DOI] [PubMed] [Google Scholar]
- 71. Kokenyesi R., and Bernfield M. (1994) Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J. Biol. Chem. 269, 12304–12309 [PubMed] [Google Scholar]
- 72. Saunders S., Jalkanen M., O'Farrell S., and Bernfield M. (1989) Molecular cloning of syndecan, an integral membrane proteoglycan. J. Cell Biol. 108, 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Eickelberg O., Centrella M., Reiss M., Kashgarian M., and Wells R. G. (2002) Betaglycan inhibits TGF-β signaling by preventing type I-type II receptor complex formation: glycosaminoglycan modifications alter betaglycan function. J. Biol. Chem. 277, 823–829 [DOI] [PubMed] [Google Scholar]
- 74. Henis Y. I., Moustakas A., Lin H. Y., and Lodish H. F. (1994) The types II and III transforming growth factor-β receptors form homo-oligomers. J. Cell Biol. 126, 139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mythreye K., and Blobe G. C. (2009) The type III TGFβ receptor regulates directional migration: new tricks for an old dog. Cell Cycle 8, 3069–3070 [DOI] [PubMed] [Google Scholar]
- 76. Lambert K. E., Huang H., Mythreye K., and Blobe G. C. (2011) The type III transforming growth factor-β receptor inhibits proliferation, migration, and adhesion in human myeloma cells. Mol. Biol. Cell 22, 1463–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J. P., and He X. (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530–535 [DOI] [PubMed] [Google Scholar]