Abstract
Background: Dupuytren disease is a common fibroproliferative disorder. Multiple procedural treatment options are available, with Collagenase Clostridium Histolyticum (CCH) injection being introduced in 2010. The purpose of this study was to investigate trends in the treatment of Dupuytren disease in the United States between 2007 and 2014. Methods: The PearlDiver Humana database was queried using International Classification of Diseases, Ninth Revision (ICD-9) and Current Procedural Terminology (CPT) codes for patients with Dupuytren disease that received percutaneous needle aponeurotomy (PNA), fasciotomy, fasciectomy, and CCH injection. Patients were filtered by age, number of comorbidities, and gender. Change in composition of treatments over time was analyzed for each demographic group between 2007 and 2014. Results: Patients presenting to clinic for Dupuytren disease increased from 1118 to 3280, with unchanging treatment percentage of 41. Percent fasciotomies and fasciectomies decreased from 5% to 3% and 33% to 21%, while CCH injection increased to 11% by 2012 to 2014. Percent fasciotomies decreased (P < .05) in younger healthier (age <65, 0-1 comorbidities) and older less healthy (age 65-74, 4+ comorbidities) populations. Percent fasciectomies decreased significantly in nearly all age and comorbidity groups, but by greater amounts in patients with 2+ comorbidities with increasing age. Percent CCH injections increased in all groups, at rates similar to the losses seen in open procedures. Conclusions: CCH injections have risen to substantial levels, with corresponding decreases in the percentage of patients receiving fasciotomies and fasciectomies. Patient age, comorbidities, and gender appear to have influence on the treatment chosen, likely due to their effects on surgical risk and the importance of timely return to activity.
Keywords: Dupuytren disease, aponeurotomy, fasciotomy, fasciectomy, collagenase clostridium histolyticum, fibroproliferative, disease
Introduction
Dupuytren disease is a fibroproliferative disorder affecting approximately 1% of the US population.9 The disease results in excessive proliferation of fibroblasts and production of collagen in palmar and digital fascia.13,24 Early signs include palmar nodules, with tenderness that typically resolves within several months.13 Nodules may progress over years to decades, forming fascial cords that extend distally into the fingers, most often the ring and small, causing stiffness and flexion contractures of metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints, and significant difficulty performing daily tasks.8,13 Most patients with Dupuytren disease present at ages greater than 50 years, and prevalence increases with age.8,24 Those presenting prior to age 50 often have more aggressive disease.21 It is most common in Caucasians of Northern European descent, with a male to female ratio of 1.7 for diagnosis and 3.0 for surgical treatment in the United States.1,13,21,22 Several risk factors have been identified, including diabetes, smoking, and alcohol abuse.7,11,17,22
Four main procedures are currently used in the treatment of Dupuytren disease, including open fasciectomy and fasciotomy, percutaneous needle aponeurotomy (PNA), and, most recently, Collagenase Clostridium Histolyticum (CCH) injection. Fasciectomies are the mainstay of modern treatment, involving the surgical excision of thickened palmar fascia, compared with simply transecting the fascia in fasciotomies.26 PNA are less invasive, performed by inserting a needle through the skin to weaken the fascia, followed by manipulation to rupture the cord.24 CCH injection was approved by the United States Food and Drug Administration (FDA) in 2010.10 Collagenase is injected into the fascial cord, which is usually ruptured 1 to 3 days later by manipulation but can be performed up to 1 week from injection.16 Auxilium Pharmaceuticals reports that more than 50,000 CCH injections have been administered since 2010; however, its effect on the landscape of Dupuytren disease treatments has yet to be examined.3 The purpose of this study was to investigate the trends in the treatment of Dupuytren disease in the United States between 2007 and 2014.
Materials and Methods
Patient data were obtained from the PearlDiver Patient Records Database (www.pearldiverinc.com; PearlDiver Inc, Fort Wayne, Indiana), a publicly available fee based Medicare database. Access to the database was provided for academic research via a protected server maintained by PearlDiver Inc. The de-identified patient records contain International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes, Current Procedural Terminology (CPT) codes, associated dates, and basic demographics including age and gender. The PearlDiver records examined in this study were from the Humana Claims Database, which contains information on all Humana Claims, including private/commercial insurance and Medicare programs, covering 16 million people between 2007 and the third quarter of 2014.
The database was queried for patients presenting with a primary diagnosis of Dupuytren disease (ICD-9 72.86). Patients receiving procedural treatment were identified using codes for PNA (CPT 26040), open fasciotomy (CPT 26045), open fasciectomy (CPT 26121, 26123, 26125), and CCH injection. Due to changes in the coding of CCH, patients were identified before 2012 according to insurance recommendations, by either injection of aponeurosis or unlisted procedure of hand (CPT 20550, 26989) with an unknown drug or biologic (CPT J3490, J3590, J9999, C9399), or injection of collagenase (CPT J0775). After 2012, CCH injections were identified specifically by enzyme injection of palmar fascial cord (CPT 20527). The number of each procedure was filtered by year. Fasciectomies were further analyzed by examining the number receiving release of 1 or more digits (CPT 26123, 26125) versus palmar-only surgery (CPT 26121).
Procedure groups were subdivided into 3 age groups, less than 65, 65 to 74, and greater than 74 years of age, then queried for comorbidities, including obesity (body mass index [BMI] > 30), smoking, diabetes mellitus, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic kidney disease, chronic liver disease, and chronic obstructive pulmonary disease, using appropriate ICD-9 diagnosis codes for each, grouping by patients with 0-1, 2-3, or 4+ comorbidities. Finally, the male to female ratio was calculated for each procedure. All groups were analyzed during 3 time frames: years 2007-2009 (before CCH), 2010-2011 (initial introduction of CCH), and 2012-2014 (after introduction of CCH).
Chi-square values were calculated for the change over time of each group examined, with P < .05 being considered statistically significant.
Results
Patients with a primary diagnosis of Dupuytren disease increased from 1118 to 3280 between 2007 and 2014. The total number of procedures increased correspondingly from 475 to 1334 (Figure 1). The percentage of patients receiving procedural treatment did not change significantly (P = .2866), remaining at approximately 41%. Between 2007 and 2014, the percentage of Dupuytren disease patients receiving fasciectomies decreased from 33% to 21% (P ≤ .0001), and fasciotomies decreased from 5% to 3% (P = .0232). PNA remained at 5% without significant change (P = .4546; Figure 2). Since the introduction of CCH in 2010, the percentage of patients receiving collagenase treatment increased to 11% by 2014. Examining fasciectomies further revealed no statistical change (P = .0748) in the percentage of procedures with release of 1 or more digits versus palmar-only procedures, with the ratio remaining at approximately 80:20, respectively (Figure 3).
Figure 1.
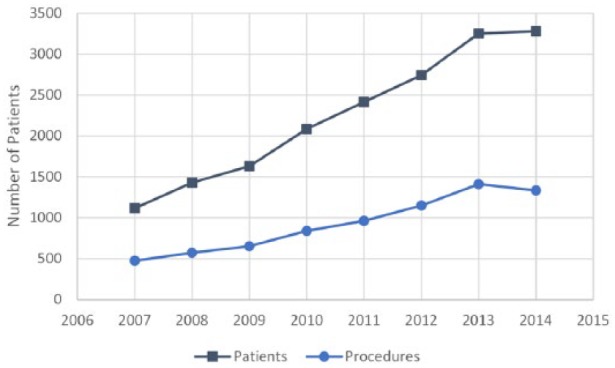
Number of Dupuytren disease patients receiving procedural treatment.
Figure 2.
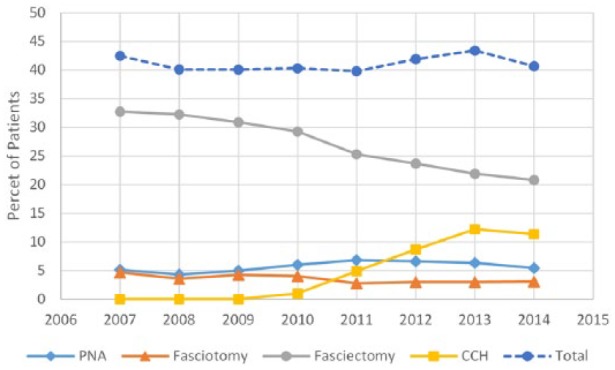
Percentage of Dupuytren disease patients receiving specific procedural treatments.
Note. PNA = percutaneous needle aponeurotomy; CCH = Collagenase Clostridium Histolyticum.
Figure 3.
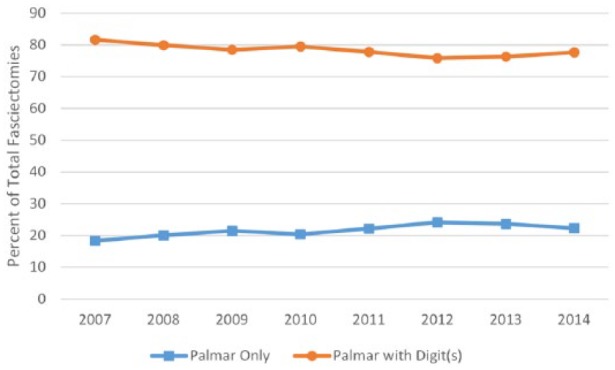
Percentage of fasciectomies performed with and without release of digit(s).
Dividing each procedure group into 3 subgroups by age demonstrated several differences. The percentage of treated patients receiving fasciectomies decreased significantly (P < .0001) across all age groups, although considerably more in those greater than 65 years old, decreasing by only 25% in those less than 65 years, compared with 35% and 32% in the 65 to 74 and >74 age groups (Table 1). Fasciotomies decreased significantly in patients less than 75 years of age, more so in those less than 65, which decreased by 44% (P = .0025) compared with 23% (P = .0488) in those 65 to 74 years of age. PNA changed significantly only in the 65 to 74 age group, increasing by 38% (P = .0488). The number of patients receiving CCH injections increased nearly equally across all age groups, increasing to 24%, 26%, and 25% of total procedures by the 2012 to 2014 time frame.
Table 1.
Dupuytren Disease Procedures Performed by Age.
| 2007-2009 | 2010-2011 | 2012-2014 | P (2007-2014) | |
|---|---|---|---|---|
| Age <65 | 456 | 360 | 649 | |
| PNA | 12% | 14% | 13% | .6382 |
| Fasciotomy | 12% | 8% | 7% | .0025 |
| Fasciectomy | 76% | 68% | 57% | <.0001 |
| CCH Injection | 0% | 9% | 24% | — |
| Age 65-74 | 809 | 958 | 2,056 | |
| PNA | 10% | 16% | 14% | .0060 |
| Fasciotomy | 10% | 9% | 8% | .0488 |
| Fasciectomy | 80% | 68% | 52% | <.0001 |
| CCH Injection | 0% | 7% | 26% | — |
| Age >74 | 396 | 451 | 1,060 | |
| PNA | 15% | 17% | 16% | .5659 |
| Fasciotomy | 9% | 8% | 7% | .4342 |
| Fasciectomy | 77% | 67% | 52% | <.0001 |
| CCH Injection | 0% | 8% | 25% | — |
Note. PNA = percutaneous needle aponeurotomy; CCH = Collagenase Clostridium Histolyticum.
Bold text signifies p < 0.05.
Each procedural age group was further analyzed by comorbidities. Fasciectomies as a percentage of total procedures decreased significantly across all age and comorbidity groups, with the exception of those less than 65 years of age with 2 to 3 comorbidities (P = .0614; Table 2). There was a greater decrease in fasciectomies in patients with 4+ comorbidities with increasing age, as well as a greater decrease in patients with 2 to 3 comorbidities at ages greater than 65 compared with those less than 65. Fasciotomies as a percentage of total procedures decreased in 2 separate populations, patients less than 65 with 0 or 1 comorbidity (P = .0052) and patients 65 to 74 with 4+ comorbidities (P = .0140). PNA increased significantly only in those age 65 to 74 with 4+ comorbidities (P = .0029). CCH injection as a percentage of total procedures increased fairly consistently across all age groups in patients with 0 or 1 comorbidity. There was a greater increase in patients with 4+ comorbidities with increasing age, as well as a greater increase in patients with 2 to 3 comorbidities at ages greater than 65 compared with less than 65.
Table 2.
Dupuytren Disease Procedures Performed by Age and Number of Comorbidities.
| 2007-2009 | 2010-2011 | 2012-2014 | P (2007-2014) | |
|---|---|---|---|---|
| Age <65 | 456 | 360 | 595 | |
| 0-1 comorbidities | 272 | 203 | 373 | |
| PNA | 14% | 18% | 14% | .8398 |
| Fasciotomy | 11% | 5% | 5% | .0052 |
| Fasciectomy | 75% | 69% | 53% | <.0001 |
| CCH Injection | 0% | 8% | 28% | — |
| 2-3 comorbidities | 117 | 105 | 150 | |
| PNA | 9% | 11% | 10% | .7746 |
| Fasciotomy | 15% | 14% | 8% | .0663 |
| Fasciectomy | 76% | 67% | 66% | .0614 |
| CCH Injection | 0% | 8% | 17% | — |
| 4+ comorbidities | 67 | 52 | 72 | |
| PNA | 10% | 6% | 12% | .7703 |
| Fasciotomy | 10% | 10% | 12% | .7703 |
| Fasciectomy | 79% | 69% | 63% | .0321 |
| CCH Injection | 0% | 15% | 13% | — |
| Age <65-74 | 809 | 958 | 1,876 | |
| 0-1 comorbidities | 362 | 446 | 989 | |
| PNA | 12% | 17% | 14% | .2726 |
| Fasciotomy | 8% | 9% | 7% | .5948 |
| Fasciectomy | 80% | 67% | 49% | <.0001 |
| CCH Injection | 0% | 7% | 29% | — |
| 2-3 comorbidities | 256 | 307 | 592 | |
| PNA | 9% | 17% | 12% | .2514 |
| Fasciotomy | 9% | 6% | 8% | .8436 |
| Fasciectomy | 82% | 70% | 54% | <.0001 |
| CCH Injection | 0% | 7% | 25% | — |
| 4+ comorbidities | 191 | 205 | 295 | |
| PNA | 8% | 15% | 17% | .0029 |
| Fasciotomy | 15% | 12% | 8% | .0140 |
| Fasciectomy | 77% | 67% | 57% | <.0001 |
| CCH Injection | 0% | 5% | 18% | — |
| Age >74 | 396 | 451 | 956 | |
| 0-1 comorbidities | 154 | 177 | 403 | |
| PNA | 16% | 19% | 17% | .7225 |
| Fasciotomy | 8% | 6% | 6% | .3491 |
| Fasciectomy | 76% | 64% | 50% | <.0001 |
| CCH Injection | 0% | 11% | 27% | — |
| 2-3 comorbidities | 122 | 166 | 354 | |
| PNA | 11% | 18% | 17% | .1401 |
| Fasciotomy | 11% | 8% | 7% | .1684 |
| Fasciectomy | 78% | 66% | 54% | <.0001 |
| CCH Injection | 0% | 8% | 22% | — |
| 4+ comorbidities | 120 | 108 | 199 | |
| PNA | 18% | 13% | 13% | .2662 |
| Fasciotomy | 7% | 8% | 11% | .2222 |
| Fasciectomy | 76% | 75% | 53% | <.0001 |
| CCH Injection | 0% | 4% | 23% | — |
Note. PNA = percutaneous needle aponeurotomy; CCH = Collagenase Clostridium Histolyticum.
Bold text signifies p < 0.05.
Finally, each procedure was filtered and analyzed by gender for the time frames of 2007-2009, 2010-2011, and 2012-2014. The male to female ratio of patients receiving fasciectomies decreased between 2007 and 2014 from 2.4 to 1.9 (P = .0077; Table 3). The ratio increased in PNA from 2.4 to 3.6 (P = .0343). The gender ratio of patients receiving fasciotomies did not change significantly, remaining at 2.1 (P = .8843). Since the introduction of CCH in 2010, the male to female ratio of patients receiving injection increased to 3.6 by the 2012-2014 time frame.
Table 3.
Male to Female Ratio of Patients Receiving Dupuytren Disease Procedures.
| 2007-2009 | 2010-2011 | 2012-2014 | P (2007-2014) | |
|---|---|---|---|---|
| PNA | 2.4 | 3.0 | 3.6 | .0343 |
| Fasciotomy | 2.1 | 1.9 | 2.1 | .8843 |
| Fasciectomy | 2.4 | 2.2 | 1.9 | .0077 |
| CCH Injection | 0.0 | 2.6 | 3.6 | — |
Note. PNA = percutaneous needle aponeurotomy; CCH = Collagenase Clostridium Histolyticum.
Bold text signifies p < 0.05.
Discussion
Dupuytren disease is a common acquired deformity of the hand.9 While there is no cure, there are multiple procedural treatments that can provide symptomatic relief. This study was designed to examine the direction that the US health care system is headed regarding the standard treatment of Dupuytren disease, and to identify possible medical and demographic factors that may be playing a role in that change.
The overall number of patients presenting to clinic with a primary diagnosis of Dupuytren disease increased nearly 3-fold between 2007 and 2014. As the PearlDiver database relies on ICD-9 coding, it is difficult to determine whether this represents an actual increase in the incidence of Dupuytren disease, or is simply an increase in patients presenting to clinic for treatment. The total number of procedures performed as treatment for Dupuytren disease increased as well; therefore, despite any changes in the number of patients seen or in the composition of procedures performed, the total percentage of Dupuytren disease receiving treatment remained the same at approximately 41. Since the introduction of CCH in 2010, major changes have occurred in the treatment of Dupuytren disease. Both fasciectomy and fasciotomy have decreased significantly, with the greatest decrease in fasciectomies. The percentage of PNA, however, did not change significantly. In contrast, CCH injections have increased substantially, at a rate similar to the decline in open surgeries.
Treatment recommendations are typically based on severity of disease. Open fasciectomy has been shown to be effective in the treatment of advanced Dupuytren disease, and is not normally indicated unless patients have an MCP contracture greater than 30 to 40 degrees, or a PIP contracture greater than 20 degrees.26,27 Both fasciotomies and PNA are less effective than fasciectomy at treating severe contracture, and are most effective for isolated MCP contractures.26,29,31 Recurrence rates are also higher, especially when involving a PIP contracture, with recurrence rates of 57% at the MCP and 70% at the PIP (85% overall) compared with 21% at both joints and overall for fasciectomies.30 The indications for CCH injections appear to overlap with both fasciectomies and fasciotomies. Initial trials demonstrated CCH to be most effective in patients with contractures less than 50 degrees, though it was still effective in some patients with more severe contractures, and a recent study found 3-year recurrence rates of 27% at MCP and 56% at PIP joints (35% overall).4,5,14,18 Our data show a major shift in the treatment of Dupuytren disease away from open procedures and toward CCH injection. The ability to use CCH to treat both mild and more advanced Dupuytren disease, with a recurrence rate falling somewhere between fasciectomies and fasciotomies, is likely 1 reason why CCH appears to be increasing in popularity in comparison with open procedures.18,30 Interestingly though, even with CCH being less effective at treating PIP contracture, fasciectomies decreased equally between palmar-only procedures and those involving release of 1 or more fingers.
Given the differences in invasiveness of each procedure, the associated risks were anticipated to play a role in the changing treatment of Dupuytren disease. Multiple studies have examined the risks of fasciectomy, with reported complication rates for digital nerve injury (3.4%), neuropraxia (0.4%-46%), arterial injury (2%), wound healing complications (22.9%), hematoma (2.1%), infection (2.4%), and flare reactions or complex regional pain syndrome (5.8%).8 This contrasts with CCH injection, which, during clinical trials, was frequently associated with a variety of local reactions, including peripheral edema, contusions, injection-site hemorrhage, and pain, but there were only 3 reports of more serious side effects, including tendon rupture and development of complex regional pain syndrome.14 CCH injection and subsequent manipulation can also be performed in clinic with only local anesthetic, compared with fasciectomy and fasciotomy which carry all of the additional risks of surgery under general anesthesia or targeted nerve block with sedation. Studies have shown that these risks of surgery, including both morbidity and mortality, increase with age as well as preoperative comorbidity status.23,28,32
Both age and comorbidities of the patients in our data set were analyzed to further elucidate the mechanics behind the shift from fasciectomies and fasciotomies to CCH injection. The percentage of patients receiving fasciectomies decreased significantly across all age groups, though by greater amounts in the older populations. Fasciectomies also decreased significantly in nearly all comorbidity groups; however, there was a greater decrease in patients with 2 to 3 and 4+ comorbidities with increasing age. The older, less healthy patients are likely less inclined to undergo the risks of invasive surgery, due to their increased rates of morbidity and mortality. While the risks of surgery may influence the treatment decisions for younger and healthier patients as well, the recovery process is probably also a major factor. Studies have shown that patients treated with CCH injection had shorter skin healing times and faster return to activities than patients receiving surgery.20 This is likely more important to younger and healthier patients, who may be more concerned with time out of work, loss of income, and interference with recreational activities. Open fasciotomies demonstrated similar trends, with significant decreases in both younger healthier populations (0-1 comorbidity, age less than 65) and older less healthy populations (4+ comorbidities, age 65-74), consistent with patients favoring shorter recovery times and less surgical risk. The percentage of patients receiving CCH injections nearly perfectly mirrored the changes seen in fasciectomies and fasciotomies, with increases across all age and comorbidity groups, and more dramatic changes in less healthy patients with increasing age.
Of note, in this study, is the apparent lack of effect of the introduction of CCH on PNA. Between 2007 and 2014, the percentage of patients receiving PNA remained unchanged. In addition, in patients aged 65 to 74 with 4+ comorbidities, PNA actually increased, despite rising numbers of CCH injection. This may also have contributed to the decreases seen in open surgeries within that population. The benefits of PNA include the ability to perform the procedure under local anesthesia in an outpatient setting, which minimizes risk to the patient, as well as a quicker recovery rate compared with open surgery.29,31 However, the procedure suffers from a high recurrence rate and is less effective than fasciectomy at treating severe contracture.29,31 In addition, despite the lesser risk, complications including damage to nerves and tendons and formation of pseudoaneurysms have still been reported.8,25 Despite the recurrence rate and possible complications, PNA continues to be a frequently used minimally invasive alternative to open surgery for the treatment of Dupuytren disease.
The final variable examined in this study was gender, which revealed several interesting trends regarding PNA, CCH injections, and fasciectomies. The initial male to female ratio of each procedure in the 2007 to 2009 time frame ranged from 2.1 to 2.4, similar to values obtained by Anthony et al. in 2008, which found the ratio to be 1.7 for diagnosis of Dupuytren disease and 3.0 for surgical intervention.1 Between 2007 and 2014, the male to female ratio of patients undergoing fasciectomy decreased significantly to 1.9, whereas PNA increased to 3.6. The gender profile of patients receiving CCH injections between 2012 and 2014 resembles that of PNA, with a male to female ratio of 3.6 as well. These trends seem contradictory to logic, given that males tend to present with more severe disease compared with females, and fasciectomies are more effective than both PNA and CCH injection at treating severe contracture.4,5,12,14,29,31 Further investigation into the role of gender in the treatment of Dupuytren disease is needed to fully understand the implications.
Although this study focused on the possible influences of age, comorbidities, and gender on the changes in the treatment of Dupuytren disease, cost of treatment is also likely to be playing a role. We were unable to analyze the cost of treatment for our populations using the PearlDiver database; however, other studies have examined the relative costs between procedures. Although CCH costs greater than $3000 per injection, studies have demonstrated fewer follow-up and therapy visits required post-procedure and significantly less overall costs for patients receiving CCH injection as compared to fasciectomy.2,6,15 Given the disparity in price between the 2 procedures, financial feasibility cannot be discounted as a possible factor involved in the decreasing percentage of patients receiving fasciectomy and the increasing rates of CCH injection. As CCH injections are more commercialized and marketed by the parent company toward individuals through various medias, the use can be expected to increase or at least discussed during clinic visits. Future studies could analyze the impact of cost and marketing on the decision making for treatment of Dupuytren disease. In addition, reimbursement rates for physicians could affect their decision on treatment modality though these likely vary between location, clinical setting, and experience. CCH injections are billed on 2 CPT codes: 20527 (injection) and 26341 (manipulation), which reimburse approximately $60 to $85 and $67 to $91, respectively. Fasciectomy (CPT: 20645) reimburses approximately $450 to $575 with additional reimbursement for additional digital fasciectomy. Given relative value units (RUV) variability, we can surmise that CCH injection reimbursement is less than surgery, even with multiple injections, as the overhead is often lower even though the cost of CCH is more than $3000. However, Peimer et al. found in a retrospective review that fewer injections were required to obtain similar outcomes as the clinical trials with CCH injections which could suggest a less expensive treatment modality as well.19 In terms of overall cost, CCH injections were found to be just below $700 less expensive than fasciectomy.2
This study had several limitations. The ability to query for procedures in the PearlDiver database is dependent on the availability of specific CPT codes. CCH injection for Dupuytren disease was not assigned a unique CPT code until 2012; therefore, for data prior to that time, we followed insurance provider recommendations for the coding of CCH injection, which included a diagnosis of Dupuytren disease and injection of aponeurosis or unlisted procedure of hand with an unknown drug or biologic. Although data returned for CCH injections during 2010 and 2011 appear consistent with the trends seen post 2012, there is still the possibility that some patients in the data set during that time period received a procedure or drug other than CCH injection. A second limitation is related to the format of the data returned by the PearlDiver database. Patients are returned in volumes and records cannot be accessed individually, hindering multivariate analysis of the variables examined in this study. Finally, given PearlDiver is a Medicare database, the generalizability may be more limited in scope.
In the years between 2007 and 2014, there have been major changes in the standard treatment of Dupuytren disease. Since its introduction in 2010, CCH injections have risen to substantial levels, with corresponding decreases in the percentage of patients receiving open surgeries, including both fasciotomies and fasciectomies. Patient age, comorbidities, and gender appear to have influence on the treatment chosen, likely due to their effects on surgical risk and the importance of timely return to activity. Despite its rapid adoption, CCH injection is still a young procedure, and the efficacy and safety have yet to be assessed in the long term. However, a better understanding of the direction that the treatment of Dupuytren disease is currently headed is the first step in assessing whether we are headed in the right direction.
Footnotes
Ethical Approval: The institutional review board approved the retrospective review of the medical charts.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all patients for being included in the study. All patient information was de-identified prior to accessing the database so no informed consent could be obtained personally by our group.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Anthony SG, Lozano-Calderon SA, Simmons BP, Jupiter JB. Gender ratio of Dupuytren’s disease in the modern U.S. population. Hand (N Y). 2008;3(2):87-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atroshi I, Strandberg E, Lauritzson A, Ahlgren E, Walden M. Costs for collagenase injections compared with fasciectomy in the treatment of Dupuytren’s contracture: a retrospective cohort study. BMJ Open. 2014;4(1):e004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Auxilium Pharmaceuticals I. Xiaflex patient brochure. https://www.xiaflex.com/_assets/pdf/Xiaflex-Patient-Brochure.pdf. Accessed July 20, 2015.
- 4. Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am. 2007;32(6):767-774. [DOI] [PubMed] [Google Scholar]
- 5. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636. [DOI] [PubMed] [Google Scholar]
- 6. Baltzer H, Binhammer PA. Cost-effectiveness in the management of Dupuytren’s contracture. a Canadian cost-utility analysis of current and future management strategies. Bone Joint J. 2013;95-B(8):1094-1100. [DOI] [PubMed] [Google Scholar]
- 7. Burge P, Hoy G, Regan P, Milne R. Smoking, alcohol and the risk of Dupuytren’s contracture. J Bone Joint Surg Br. 1997;79(2):206-210. [DOI] [PubMed] [Google Scholar]
- 8. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clin. 2015;31(2):345-354. [DOI] [PubMed] [Google Scholar]
- 9. DiBenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: Results from a population-based study. Hand. 2011;6(2):149-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. FDA approves xiaflex for debilitating hand condition. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm199736.htm. Accessed April 21, 2016.
- 11. Gamstedt A, Holm-Glad J, Ohlson CG, Sundstrom M. Hand abnormalities are strongly associated with the duration of diabetes mellitus. J Intern Med. 1993;234(2):189-193. [DOI] [PubMed] [Google Scholar]
- 12. Gudmundsson KG, Arngrimsson R, Sigfusson N, Bjornsson A, Jonsson T. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. the Reykjavik study.J Clin Epidemiol. 2000;53(3):291-296. [DOI] [PubMed] [Google Scholar]
- 13. Gudmundsson KG, Jonsson T, Arngrimsson R. Guillaume Dupuytren and finger contractures. Lancet. 2003;362(9378):165-168. [DOI] [PubMed] [Google Scholar]
- 14. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979. [DOI] [PubMed] [Google Scholar]
- 15. Mehta S, Belcher HJ. A single-centre cost comparison analysis of collagenase injection versus surgical fasciectomy for Dupuytren’s contracture of the hand. J Plast Reconstr Aesthet Surg. 2014;67(3):368-372. [DOI] [PubMed] [Google Scholar]
- 16. Mickelson DT, Noland SS, Watt AJ, Kollitz KM, Vedder NB, Huang JI. Prospective randomized controlled trial comparing 1- versus 7-day manipulation following collagenase injection for Dupuytren contracture. J Hand Surg Am. 2014;39(10):1933-1941. [DOI] [PubMed] [Google Scholar]
- 17. Noble J, Heathcote JG, Cohen H. Diabetes mellitus in the aetiology of Dupuytren’s disease. J Bone Joint Surg Br. 1984;66(3):322-325. [DOI] [PubMed] [Google Scholar]
- 18. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am. 2013;38(1):12-22. [DOI] [PubMed] [Google Scholar]
- 19. Peimer CA, Skodny P, Mackowiak JI. Collagenase clostridium histolyticum for Dupuytren contracture: patterns of use and effectiveness in clinical practice. J Hand Surg Am. 2013;38(12):2370-2376. [DOI] [PubMed] [Google Scholar]
- 20. Povlsen B, Shields AM, Bhabra GS. Resource utilisation associated with single digit Dupuytren’s contracture treated with either surgery or injection of collagenase clostridium histolyticum. Hand Surg. 2014;19(2):205-209. [DOI] [PubMed] [Google Scholar]
- 21. Reilly RM, Stern PJ, Goldfarb CA. A retrospective review of the management of Dupuytren’s nodules. J Hand Surg Am. 2005;30(5):1014-1018. [DOI] [PubMed] [Google Scholar]
- 22. Ross DC. Epidemiology of Dupuytren’s disease. Hand Clin. 1999;15(1):53-62. [PubMed] [Google Scholar]
- 23. Schmolders J, Friedrich MJ, Michel R, et al. Validation of the Charlson comorbidity index in patients undergoing revision total hip arthroplasty. Int Orthop. 2015;39(9):1771-1777. [DOI] [PubMed] [Google Scholar]
- 24. Swartz WM, Lalonde DH. MOC-PS(SM) CME article: Dupuytren’s disease. Plast Reconstr Surg. 2008;121(4 Suppl):1-10. [DOI] [PubMed] [Google Scholar]
- 25. Symes T, Stothard J. Two significant complications following percutaneous needle fasciotomy in a patient on anticoagulants. J Hand Surg Br. 2006;31(6):606-607. [DOI] [PubMed] [Google Scholar]
- 26. Townley WA. Dupuytren’s contracture unfolded. BMJ. 2006;332(7538):397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trojian TH, Chu SM. Dupuytren’s disease: diagnosis and treatment. Am Fam Physician. 2007;76(1):86-89. [PubMed] [Google Scholar]
- 28. Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203(6):865-877. [DOI] [PubMed] [Google Scholar]
- 29. van Rijssen AL, Gerbrandy FS, Ter Linden H, Klip H, Werker PM. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren’s disease: A 6-week follow-up study. J Hand Surg Am. 2006;31(5):717-725. [DOI] [PubMed] [Google Scholar]
- 30. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129(2):469-477. [DOI] [PubMed] [Google Scholar]
- 31. van Rijssen AL, Werker PM. Percutaneous needle fasciotomy in Dupuytren’s disease. J Hand Surg Br. 2006;31(5):498-501. [DOI] [PubMed] [Google Scholar]
- 32. Wolters U, Wolf T, Stutzer H, Schroder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217-222. [DOI] [PubMed] [Google Scholar]


