Abstract
Despite ongoing smoking cessation efforts and optimized perfusion, failed wound closure in the presence of peripheral arterial disease (PAD) and diabetes are common. A clinical effectiveness review was conducted in actively smoking diabetic patients diagnosed with PAD, treated with serial applications of a viable intact cryopreserved human placental membrane (vCPM) (Grafix, Osiris Therapeutics Inc, Columbia, MD) for recalcitrant lower extremity ulcerations (n = 6). More than half of the patients were not candidates for revascularization. Baseline vascular status in 5 of 6 lower-extremity wounds remained unchanged throughout the entire course of vCPM treatment. Daily cigarette consumption averaged 18 cigarettes per patient. Mean wound duration and mean surface area was 53 weeks and 4.6 cm2, respectively. Mean number of vCPM applications and time to closure was 7.0 grafts in 7.8 weeks. There were no wound-related infections or amputations and no vCPM-related adverse events. All 6 wounds remained closed at the 12-month follow-up visit. In conclusion, vCPM demonstrated clinically effective outcomes in 6 previously nonhealing ulcerations despite ongoing smoking habits in the presence of PAD and diabetes.
Keywords: peripheral arterial disease, cigarette smoking, treatment refractory, viable cryopreserved placental membrane
As the number of nonhealing lower extremity wounds continue to rise globally, significant societal costs are seen through lost productivity and an increased financial strain on the health care system.1-3 In the United States alone, chronic wounds affect an estimated 2% of the population with an associated cost of care rising over $50 billion annually.1 Even with a multidisciplinary approach that includes advanced therapies with consistent standard of care (SOC) measures such as infection control, debridement, offloading, revascularization, compression and the promotion of patient normoglycemia, a nonhealing or treatment refractory wound may occur in as many as one-third of cases.1-4
The management of a treatment refractory chronic wound will exceed $9000 per year as compared with the currently estimated cost of nonrefractory chronic wound care, which ranges from $3601 to $4282 yearly per ulcer.1,5 Common conditions such as peripheral arterial disease (PAD), chronic venous insufficiency (CVI), diabetes, and inhaled tobacco dependence, have been linked with the development of treatment refractory wounds.6-8 Together, these comorbidities lead to pathophysiologic abnormalities that further complicate ulcer management and pose a challenge to the wound care specialist worldwide.2,3
In particular, PAD, diabetes, and active smoking alongside the presence of a nonhealing lower extremity wound represent the greatest risk for ongoing tissue loss, infection, amputation, and potential mortality.6,9-11 Smoking, a well-known deterrent to cutaneous healing, is often a part of a patient’s medical history.12-18 The presence of PAD and diabetes together with smoking lead to vasoconstriction cumulative with each cigarette that is smoked, and to oxygen and nutrient deficiencies in tissues that independently contribute to the formation and exacerbation of nonhealing ulcerations.19-23
Revascularization is considered the gold-standard for promoting wound closure in patients with PAD.2 Despite ongoing smoking cessation efforts and optimized perfusion, failures of wound closure in the presence of PAD and diabetes are common. Rates of complete lower extremity wound closure may be as low as 45% following procedures such as open surgical bypass or endovascular intervention.20 Delayed healing and ongoing tissue loss in the affected limb may continue to occur, even with technically successful revascularization surgeries.4,20,24 Forty percent of initially healed patients will also experience ulcer recurrence within the first year.4 Nearly three-fourths of such wounds result in major amputation due to inadequate healing.4,20 Even with the available advanced wound care products, technologies and improved methods of accurate diagnosis, prolonged duration of treatment and an increased morbidity related to infection and amputation continue to occur.2,3,25,26
Materials and Methods
Study Design and Population
The purpose of this study is to present the clinical outcomes associated with the use of Grafix (Osiris Therapeutics, Inc, Columbia, MD), a viable intact cryopreserved human placental membrane (vCPM), for the outpatient management of refractory lower extremity wounds in actively smoking PAD patients. A single center retrospective chart review was conducted on all patients with chronic wounds managed with vCPM during a 1-year period (July 2014 to July 2015). Because of the retrospective nature of data collection, an internal review board approval was not required for this case series analysis.
Individual patient consents were obtained for the use of all de-identified materials. Selection for subject inclusion in this analysis was based on the following criteria: (1) presence of a nonhealing lower extremity wound with a known diagnosis of moderate to severe PAD, (2) a vascular status assessment to confirm the diagnosis of PAD and potential for revascularization, (3) an ongoing cigarette smoking habit, and (4) previous SOC treatment failure in addition to advanced therapies.
From the population of vCPM-treated patients (N = 86), a subset of 4 males and 1 female (n = 5) with 6 wounds met the prespecified inclusion criteria. In addition to ankle brachial index (ABI) values, the Fontaine classification system was used to classify the severity of PAD.27-30 Clinical manifestations of PAD ranged from moderate to severe intermittent claudication in conjunction with minor tissue loss and nonhealing ulcerations. The classification of a wound as recalcitrant depended on a minimum previous treatment duration of ≥120 days (4 months) characterized by a prior failure to respond SOC and ≥1 advanced therapy regimens.6
Patient Evaluation and Wound Management
Prior to the initiation of vCPM treatment, all patients were evaluated for the potential to improve their lower extremity perfusion through endovascular or open surgical intervention. All patients presented with claudication as a clinical symptom. Three subjects were categorized ineligible for revascularization, the remaining 2 patients were scheduled for invasive procedures: One patient received percutaneous stenting subsequent to treatment with vCPM; and the second patient underwent surgical revision of a previous femoral-arterial bypass graft, occurring day 30 after initiation of vCPM treatment.
All patients were managed with a multidisciplinary approach from podiatry, nursing, vascular surgery, nutrition, and endocrinology. Smoking cessation treatment was offered during every visit, including pharmacotherapy and nicotine replacement therapy.31 Appropriate dietary and blood glucose control was promoted and monitored in all diabetic patients.32 Wounds received weekly SOC treatment, including selective wound debridement, offloading, infection control, and exudate management.33 Modified compression dressings were applied for all lower extremity wounds of mixed arterial-venous etiology.34
Clinical Effectiveness Analysis
Evaluation of clinical effectiveness in vCPM-treated patients included (1) the incidence of complete wound closure, (2) the individual and mean percentage area reduction (PAR) in wound surface area at 4 weeks of ≥50%, (3) the mean time to closure, (4) the mean number of grafts required for closure, and (5) adverse events, defined as any wound-related amputation or infection occurring during the vCPM treatment phase. Patients were also assessed at a 12-month follow-up in order to evaluate all vCPM-related wound closures.
Results
The patient demographics and baseline wound characteristics are summarized in Table 1. Per Fontaine classification for PAD, 4 patients (80%) were categorized as stage IIa/b while the remaining patient was categorized as stage III. Daily cigarette smoking habits ranged from 0.5 to >1 pack per day (PPD) with a mean consumption of 18 cigarettes per day. Baseline surface area of the wounds (n = 6) ranged from 1.0 to 9.7 cm2 with a mean of 4.6 cm2 (SD 3.4). Average wound duration was 53 weeks (370.7 days; SD 346.2; median 220; range 121-1114 days). After initiation of treatment with vCPM, closure occurred in a mean time of 7.8 weeks (SD 4.4; range 2-15.4 weeks) with 7.0 applications (SD 3.8; range 2-14).
Table 1.
Baseline Patient Demographics, Wound Characteristics, and Mean Study Outcomes.
| Patient (Sex) | Age (Years) | BMI (kg/m2) | ABI | Fontaine Classification | Revascularization Status | Cigarettes (PPD) | Study Wound No. | Wound Size (cm2) | Wound Duration (Days) | Wound Etiology | Wound Location | 4-Week PAR (%) | vCPM Applications | Time to Closure (Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a (F) | 56 | 32.1 | 0.6 | Stage IIb | Right superior femoral artery stent after wound closure | 1 | 1 | 1.0 | 183 | Ischemic diabetic foot ulcer | Right fifth digit | N/A | 2 | 2 |
| 2a (M) | 63 | 39.5 | 0.5 | Stage III | Left femoral-popliteal bypass revision after 4 vCPM applications | 1 | 2 | 8.8 | 121 | Diabetic with mixed venous and arterial disease | Left anterolateral leg | 76.24 | 5 | 6 |
| 3 (M) | 43 | 28.5 | 1.0 | Stage IIa | Not a candidate for revascularization | 1 | 3 | 9.7 | 256 | Mixed venous and arterial disease | Right anterolateral leg | 24.5 | 9 | 11 |
| 0.8 | 4 | 3.2 | 412 | Mixed venous and arterial disease | Left dorsal midfoot | 98.75 | 5 | 5 | ||||||
| 4a (M) | 64 | 25.1 | 0.7 | Stage IIb | Not a candidate for revascularization | 1+ | 5 | 3.0 | 1114 | Neuroischemic diabetic foot ulcer | Right plantar forefoot | 63.7 | 14 | 15.4 |
| 5a (M) | 66 | 29.4 | 0.5 | Stage IIa | Not a candidate for revascularization | 0.5 | 6 | 1.9 | 138 | Ischemic diabetic foot ulcer | Right dorsal forefoot | 83.87 | 7 | 7.14 |
| Mean | 58.4 | 30.92 | 0.7 | 0.9 | 4.6 | 370.7 | 69.4 | 7 | 7.8 |
Abbreviations: ABI, ankle brachial index; BMI, body mass index; F, female; M, male; N/A, no applicable; PAR, percentage area reduction; PPD, packs per day (1 PPD = 20 cigarettes); vCPM, viable intact cryopreserved human placental membrane.
Diabetic.
Five of the ulcers met the minimum 28-day treatment time for calculation of the mean 4-week PAR. Four of the ulcers surpassed the minimum benchmark for clinically effective treatment progress (≥50%), resulting in mean PAR of 69.4% (SD 25.2%; range 24.5%-98.8%). Study wound 1 received only 2 serial graft applications (day 0 and day 7), and reached a 75% PAR at the first follow-up visit and complete wound closure by day 15. The patient with study wound 3 did not return for a period of 3 weeks after day 14 of initial treatment with vCPM (applications: day 0, day 7, and day 14). Study wound 3 had a 2-week PAR of 25.6%. This 20-day disruption of care resulted in an increased wound surface area. Wound size reduction was observed when vCPM applications were resumed, resulting in a 50.4 % PAR by day 49. Adjusting for the 20-day treatment gap, study wound 3 demonstrated a ≥50% in wound size with 4 active weeks of treatment with vCPM. This adjusted PAR was not included in the mean % PAR calculations.
Selected Case
Patient 3
A 43-year old male patient (body mass index 28.5 kg/m2) presented with 2 separate wounds on the left and right lower extremities, etiology included mixed venous and arterial disease (Patient 3, see Table 1). vCPM applications were initiated after both wounds had been present for 256 days. The patient had bilateral nonpalpable dorsalis pedis and posterior tibial pulses. ABI ranged from 0.8 on the right lower extremity to 1.0 on the left lower extremity, Fontaine classification IIa. The patient was not considered a candidate for revascularization procedures due to noncompliance, procedure refusal, and ongoing intravenous drug abuse. Past medical history included moderate PAD, hypertension, dyslipidemia, substance abuse, and deep vein thrombosis. Cigarette smoking habit equaled 1 PPD. Past treatment failures included acellular and cellular skin substitutes.
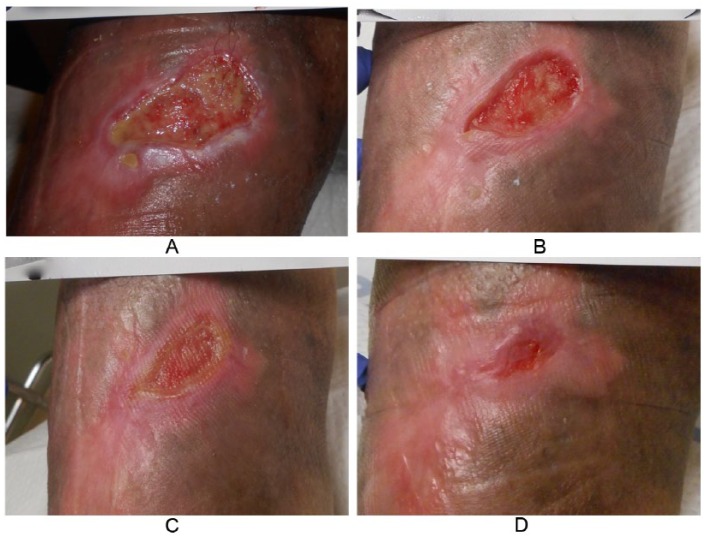
Study wound 3: 9.7 cm2 wound of the right anterolateral tibia with a previous duration of 256 days. Nine serial applications of vCPM were done with complete closure at day 77 (Figures 1A-D and 2).
Study wound 4: 3.2 cm2 wound of the left dorsal foot with a previous duration of 412 days. Complete closure at day 36 was achieved after 5 serial applications of vCPM.
Figure 1.
Study wound 3. (A) Baseline: 9.7 cm2 wound of the right anterolateral tibia with a duration of 256 days prior to application of vCPM. (B) Day 49: 50.5% PAR after 5 applications (C) Day 56: 71.5% PAR after 6 applications. (D) Day 77: Final wound closure following 9 vCPM applications. PAR, percentage area reduction; vCPM, viable intact cryopreserved human placental membrane.
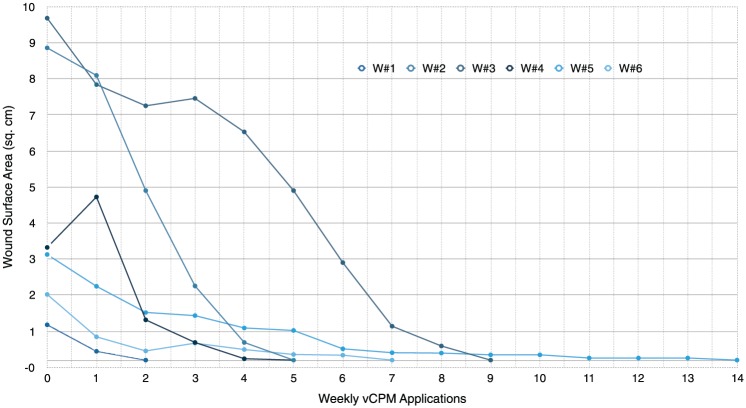
Figure 2.
Progressive reductions in wound surface area with serial viable intact cryopreserved human placental membrane (vCPM) applications.
Discussion
The clinical effectiveness of an advanced therapy can be assessed by the incidence and time to complete wound closure. The management-related clinical progress can also be monitored through reductions in wound size with treatment. The objective of this study was to evaluate the clinical effectiveness of vCPM in subjects with both pathophysiological and patient behavior–related challenges that prevent wound closure, even after reperfusion optimization.
Diabetes and an active smoking habit are associated with the most significant increases in treatment time and financial expenditures related to wound management.1,35 All patients included in this review were long-term heavy smokers with a mean consumption of nearly 1 PPD. Four patients with 5 wounds were also diabetic. These high-risk patients are typically excluded from randomized controlled clinical trials. Nonclosed wounds in such patients represent a prominent risk factor for infection leading to hospitalization and lower limb amputation.4,9
Although most individuals are initially considered for revascularization procedures, factors such as age, patient consent, comorbidities, and the extent and pattern of vessel occlusion may limit surgical reperfusion options.9,36 Many clinicians are left to pursue outpatient management instead of the more aggressive surgically based wound closure strategies.4,9,24 More than half of the patients included in this study were not candidates for revascularization procedures. As a result, baseline vascular status and ABI values for 5 of 6 lower extremity wounds remained unchanged throughout the entire course of vCPM treatment. Although the patient with study wound 2 underwent a surgical revision of his femoral-popliteal bypass on treatment day 30, a 76.2% reduction in wound surface area was recorded during the initial 4 weeks of vCPM treatment.
Even in wounds with a mixed etiology such as diabetic with arterial and venous insufficiency, literature indicates that surrogate markers with predictive value for clinical effectiveness, and thus for healing, can be identified.37 Lavery et al38 reported on the predictive value of early wound progression, where a ≥15% PAR at week 1 could be used to identify the likelihood of healing by 16 weeks versus the need for considering a change in treatment. In this study, an early shift from recalcitrance to a mean 15.8% PAR at week 1 of vCPM applications is consistent with predicting positive clinical outcomes. The continued mean reductions in surface area were 55.3%, 65.2%, and 79.3%, at weeks 2, 3, and 4, respectively. This progressive trajectory toward 100% closure is also considered indicative of long-term healing potential.32,38-41
A reevaluation of treatment regimen is also recommended for wounds with PAR <50% by week 4.39-42 Eighty-three percent (5/6) of the wounds were treated up to day 28 for calculations of surface area reduction with a mean 28-day PAR of 69.4%. Eighty percent of these wounds demonstrated a correlation between the ≥50% at the 4-week point and subsequent closure. Treated for a total of 11 weeks, study wound 3 may be categorized as an outlier since failure to meet the minimum benchmark for surface area reduction was due to a 20-day gap between vCPM applications 3 and 4, thus preventing documentation of wound size and/or surface area during this period of time.
Infections substantially increase the morbidity and mortality associated with open wounds, particularly diabetic foot ulcerations and pressure ulcers.30 PAD patients are almost 90 times more likely to receive lower extremity amputations once infection is present.4 PAD severity is independently correlated with reductions in primary healing while simultaneously increasing the rates of amputation and mortality.10,41 Despite a mean ABI of 0.7 with stage II/III PAD (per Fontaine classification), there were no treatment-related infections or wound-related amputations reported during this study. No vCPM-related adverse events were reported. In order to assess the quality of wound closure, patients were followed for 12 months. No subjects were lost to follow-up. All 6 wounds in 5 patients remained closed at the 1-year follow-up evaluation. Thus, we report durable wound closure versus the recurrence typically associated with transient wound coverage.32
In general, biological dressings or wound covers have not been shown to be vastly successful.2 However, vCPM represents an emerging tissue preservation technology that should be explored for its potential benefits in the management of nonhealing wounds.2 A prospective multi-center randomized clinical trial found vCPM to be beneficial for diabetic foot ulceration (62% complete wound closure versus 21% with SOC alone).33 While clear limitations such as the lack of smoking controls do exist in the design of this retrospective case series, the outcomes in this study suggest that vCPM should be considered in the wound management of PAD patients with otherwise limited options for reperfusion. vCPM may contribute to wound closure in high-risk smoking patients with a history of SOC and advanced treatment failures. vCPM and other innovative technologies aimed at improving patient outcomes should be continuously examined through multiple levels of evidence, supported by both clinician and researcher.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Jonathan Smedley is an employee of Precision Podiatry. He is a member of the speakers’ bureau at and consultant to Osiris Therapeutics, Inc. Georgina M. Michael and Yeabsera G. Tamire are employees of Osiris Therapeutics, Inc.
Funding: This publication was supported by Osiris Therapeutics, Inc.
References
- 1. Fife AC, Carter MJ, Walker D, Thomson B. Wound care outcomes and associated cost among patients treated in the US outpatient wound centers: data from the US Wound Registry. Wounds. 2012;24:10-17. [PubMed] [Google Scholar]
- 2. Mani R, Margolis DJ, Shukla V, et al. Optimizing technology use for chronic lower-extremity wound healing: a consensus document. Int J Low Extrem Wounds. 2016;15:102-119. [DOI] [PubMed] [Google Scholar]
- 3. Greer N, Foman NA, MacDonald R, et al. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med. 2013;159:532-542. [DOI] [PubMed] [Google Scholar]
- 4. Forsythe RO, Brownrigg J, Hinchliffe RJ. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes Metab. 2015;17:435-444. [DOI] [PubMed] [Google Scholar]
- 5. Rayner R. Effects of cigarette smoking on cutaneous wound healing. Prim Intention. 2006;14:100-102. [Google Scholar]
- 6. Harding KG. Nonhealing wounds: recalcitrant, chronic, or not understood? Ostomy Wound Manage. 2000;46(1A suppl):4S-7S. [PubMed] [Google Scholar]
- 7. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17:1-18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51:747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed but our methods have not. J Diabetes Sci Technol. 2011;5:1591-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jude EB, Oyibo SO, Chalmers N, Bouton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433-1437. [DOI] [PubMed] [Google Scholar]
- 11. Vartanian SM, Robinson KD, Ofili K, et al. Outcomes of neuroischemic wounds treated by a multidisciplinary amputation prevention service. Ann Vasc Surg. 2015;29:534-542. [DOI] [PubMed] [Google Scholar]
- 12. Knuutinen A, Kokkonen N, Risteli J, et al. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br J Dermatol. 2002;146:588-94. [DOI] [PubMed] [Google Scholar]
- 13. Freiman A, Bird G, Metelitsa A, Barankin B, Lauzon G. Cutaneous effects of smoking. J Cutan Med Surg. 2004;8:415-423. [DOI] [PubMed] [Google Scholar]
- 14. Gill JF, Yu SS, Neuhaus IM. Tobacco smoking and dermatologic surgery. J Am Acad Dermatol. 2013;68:167-172. [DOI] [PubMed] [Google Scholar]
- 15. Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized control trial. Ann Surg. 2003;238:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lind J, Kramhoft M, Bodtker S. The influence of smoking on complications after primary amputations of the lower extremity. Clin Orthop Relat Res. 1991;267:211-217. [PubMed] [Google Scholar]
- 17. Metelitsa AI, Lauzon GJ. Tobacco and the skin. Clin Dermatol. 2010;28:383-390. [DOI] [PubMed] [Google Scholar]
- 18. Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126:1131-1134. [DOI] [PubMed] [Google Scholar]
- 19. Muhs BE, Gagne P, Sheehan P. Peripheral arterial disease: clinical assessment and indications for revascularization in patients with diabetes. Curr Diab Rep. 2005;5:24-29. [DOI] [PubMed] [Google Scholar]
- 20. Henry JC, Peterson LA, Schlanger RE, Go MR, Sen CK, Higgins R. Wound healing in peripheral arterial disease: current and future therapy. J Vasc Med Surg. 2014;2:157. doi:10.4172/2329-6925 1000157. [Google Scholar]
- 21. Walker CM, Bunch FT, Cavros NG, Dippel EJ. Multidisciplinary approach to the diagnosis and management of patients with peripheral arterial disease. Clin Interv Aging. 2015;10:1147-1153. doi: 10.2147/CIA.S79355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siana JE, Rex S, Gottrup F. The effects of smoking on wound healing. Scand J Plast Reconstr Surg Hand Surg. 1989;23:207-209. [DOI] [PubMed] [Google Scholar]
- 23. Silverstein P. Smoking and wound healing. Am J Med. 1992;93:22S-24S. [DOI] [PubMed] [Google Scholar]
- 24. Marston WA, Davies SW, Armstrong B, et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg. 2006;44:108-114. [DOI] [PubMed] [Google Scholar]
- 25. Milne TE, Schoen DE, Bower VW, Burrows SA, Westphal C, Gurr JM. Healing time of diabetic foot ulcers: investigating the influence of infection and peripheral arterial disease. J Diab Foot Complications. 2013;5:29-38. [Google Scholar]
- 26. Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: is there any evidence for diabetic foot ulcer healing? Diabetes Metab. 2016;42:4-15. doi: 10.1016/j.diabet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 27. Gardner AW, Afaq A. Management of lower extremity peripheral arterial disease. J Cardiopulm Rehabil Prev. 2008;28:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890-2909. [DOI] [PubMed] [Google Scholar]
- 29. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 2007;45:S34. [DOI] [PubMed] [Google Scholar]
- 30. Kirsner RS, Vivas AC. Lower-extremity ulcers: diagnosis and management. Br J Dermatol. 2015;173:379-390. [DOI] [PubMed] [Google Scholar]
- 31. Coleman T. Smoking cessation: integrating recent advances into clinical practice. Thorax. 2001;56:579-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FDA Guidance Document: Chronic cutaneous ulcer and burn wounds—developing products for treatment. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformationGuidelines/ucm071324.pdf. Accessed 19 April 2016. [DOI] [PubMed]
- 33. Lavery LA, Fulmer J, Shebetka KA, et al. ; Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of Grafix® for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11:554-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marston W. Mixed arterial and venous ulcers. Wounds. 2011;23:351-356. [PubMed] [Google Scholar]
- 35. Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. Diabetic foot ulcers and amputations: estimates of health utility for use of cost-effectiveness analysis of new treatments. Diabetes Metab. 2004;30:549-556. [DOI] [PubMed] [Google Scholar]
- 36. Ward C, Gamberdella J, Mena-Hurtado C. Endovascular treatment of below the knee arteries. PanVascular Med. 2015;3195-3203. doi: 10.1007/978-3-642-37393-0. [DOI] [Google Scholar]
- 37. Cardinal M, Eisenbund DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 2008;16:19-22. [DOI] [PubMed] [Google Scholar]
- 38. Lavery LA, Barnes SA, Keith MS, Seaman JW, Jr, Armstrong DG. Prediction of postoperative diabetic wounds based on early wound area progression. Diabetes Care. 2008;31:26-29. [DOI] [PubMed] [Google Scholar]
- 39. Coerper S, Beckert S, Kuper MA, Konigsrainer A. Fifty percent area reduction after 4 weeks treatment is a reliable indicator for healing- analysis of a single center cohort of 704 diabetic patients. J Diabetes Complications. 2009;23:49-53. [DOI] [PubMed] [Google Scholar]
- 40. Warriner R, Snyder R, Cardinal M. Differentiating diabetic foot ulcers that are unlikely to heal by 12 weeks following achieving 50% percent area reduction at 4 weeks. Int Wound J. 2011;8:632-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bettin CC, Gower K, McCormick K, et al. Cigarette smoking increases complication rate in forefoot surgery. Foot Ankle Int. 2015;36:488-493. [DOI] [PubMed] [Google Scholar]
- 42. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879-1882. [DOI] [PubMed] [Google Scholar]