Abstract
The balance between proliferation and differentiation is a fundamental aspect of multicellular life. Perhaps nowhere is this delicate balance more palpable than in the multiciliated cells (MCCs) that line the respiratory tract, the ependyma, and the oviduct. These cells contain dozens to hundreds of motile cilia that beat in a concerted fashion to generate directed fluid flow over the tissue surface. Although MCCs have exited the cell cycle, remarkably, they retain the ability to duplicate their centrioles and to mature those centrioles into ciliary basal bodies—two features, which are known to be normally under strict cell cycle control (Firat‐Karalar & Stearns, 2014). How post‐mitotic MCCs retain this ability, remains unclear. In the past several months, four research articles, including one from Terré et al in this issue of The EMBO Journal, have described a vital role for the geminin coiled‐coil domain‐containing protein (Gemc1) in the MCC gene expression program in multiple tissues and organisms, that bring us closer to understanding this question (Kyrousi et al, 2015; Zhou et al, 2015; Arbi et al, 2016; Terré et al, 2016).
Subject Categories: Cell Adhesion, Polarity & Cytoskeleton; Development & Differentiation
Over the past 10 years, multiple MCC differentiation factors have been identified (Brooks & Wallingford, 2014), most of which are transcriptional regulators that act together to launch a massive, MCC‐specific gene expression program that turns on hundreds of regulatory and structural ciliary genes. Interestingly, some of these transcription factors, including c‐Myb, and E2F4 and E2F5 (E2F4/5), also control cell cycle events in other cells. Gemc1 (also known as Gmnc) is a member of the geminin family of nuclear proteins and has a previously defined role in cell cycle‐regulated DNA replication (Balestrini et al, 2010). Data show that Gemc1 is essential for MCC differentiation, and acts together with the other two geminin family members, Geminin and Mcidas, at the top of the transcriptional cascade that drives motile ciliogenesis. These studies clarify our understanding of early MCC gene expression events and support the idea that centriole assembly in quiescent MCCs may still somehow fall within the purview of cell cycle regulators.
As cells adopt the MCC fate, they activate the MCC gene expression program and assemble centrioles in the cytoplasm, which then traffic to the cell surface and elongate the motile axoneme (Fig 1). Our understanding of the MCC gene expression program has grown increasingly complex (reviewed in Brooks & Wallingford, 2014). It is generally accepted that an initial Notch signaling event among neighboring epithelial cells is required for MCC fate acquisition, such that cells with low Notch activity become MCCs. Downstream of Notch, it was found that Mcidas is both necessary and sufficient to drive motile ciliogenesis as part of a transcriptional regulatory complex along with the transcription factors E2F4/5 and Dp1. This complex activates numerous motile ciliogenesis genes in addition to other downstream transcription factors such as c‐Myb, Foxj1, and Rfx family members (Fig 1), which then, too, turn on or sustain the expression of MCC genes. There are likely complicated relationships between regulatory events and factors through which MCCs launch, sustain, and ultimately cease transcriptional activity during the course of motile ciliogenesis, and we are still very far from complete understanding of this process. We can now add two new players to this regulatory pathway in the form of Gemc1 and Geminin. The four recent studies in sum demonstrate that Gemc1 acts downstream of Notch signaling together with E2F4/5 and DP1 to turn on Mcidas expression, making it the earliest acting transcriptional activator in the MCC gene expression program. The role of Geminin is less well explored (Arbi et al, 2016; Terré et al, 2016), but it appears to act as a repressor of Gemc1 and Mcidas function before MCC differentiation. These discoveries not only identify new regulators of motile ciliogenesis, but also open the field to the complex relationship between Gemc1, Mcidas, and Geminin.
Figure 1. Geminin family members within the MCC gene expression program.
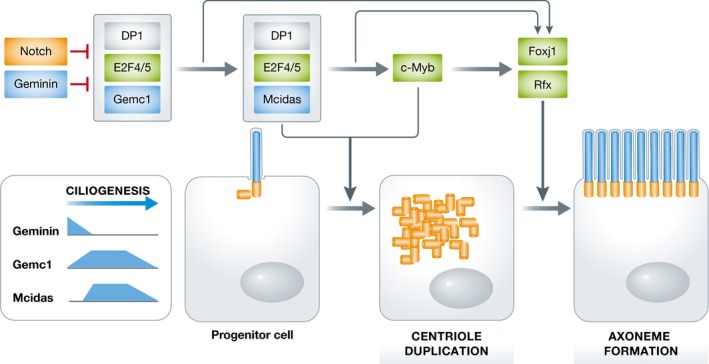
MCC differentiation depends on the transcription of hundreds of structural and regulatory factors. Upstream Notch signaling negatively regulates the acquisition of the MCC fate. When progenitor cells exit the cell cycle, they initially have high Geminin expression, which inhibits the transcription of MCC genes. Geminin levels begin to decline, and Gemc1 and Mcidas levels increase. Gemc1 acts together with DP1 and the E2F4 or E2F5 transcription factor to turn on Mcidas and other ciliary genes.
Gemc1 and Mcidas both act as transcriptional activators as part of a complex with the transcription factors E2F4/5 and DP1 (termed the EDG and EDM complexes). These complexes can be detected by co‐immunoprecipitation and chromatin immunoprecipitation, and it was shown that E2F4/5 have no or only weak transcriptional activity on MCC targets in the absence of Gemc1 or Mcidas (Ma et al, 2014; Arbi et al, 2016; Terré et al, 2016). Gemc1 and Mcidas appear to function similarly within the respective EDG/M complexes, as mutation of identical residues in either protein abolishes E2F4/5 binding and transcriptional activity (Ma et al, 2014; Terré et al, 2016). However, the two complexes are far from identical in behavior. Gemc1 appears to be expressed first during differentiation, and it turns on MCC target genes, including Mcidas. Mcidas does not appear to be able to activate Gemc1 expression (Arbi et al, 2016), but it can turn on itself and MCC targets. This is consistent with a mechanism where the EDG complex initiates MCC gene expression and activates the EDM complex, which then takes over MCC transcriptional activity. Thus, the two complexes provide both differential regulation and redundancy in the expression of MCC targets to promote differentiation. However, the relative contribution of Gemc1 and Mcidas to the long‐term maintenance of the MCC gene expression remains to be explored.
The E2F family regulates multiple events during cell cycle progression (Thurlings & de Bruin, 2016), notably, a complex of E2F1, DP1, and the retinoblastoma transcriptional regulator is essential for entry into S‐phase. E2F4/5 are known to act as transcriptional repressors in cycling cells, although in MCCs they appear to have been repurposed as transcriptional activators within the EDG/M complexes. These, however, are not the only cell cycle regulators that operate in the MCC gene expression program, as the c‐Myb transcription factor and cyclin O are both also known to drive cell cycle events in dividing cells. Clearly, MCCs possess a permissive environment for centriole assembly, which normally occurs only during S‐phase of the cell cycle, and it appears that a gene expression program driven by cell cycle regulators is essential for this process. It will be vital to understand how these factors are able to turn on centriole assembly targets, but not those required for DNA replication or other events during cell cycle progression. Likely, transcriptional activators like Gemc1 and Mcidas and repressors will be a key aspect of the regulatory mechanism. Thus, while the discovery of GemC1 as the earliest transcriptional regulator of the MCC lineage is certainly an important advance, it is clear that the pathway is more complex than a simple linear hierarchy of transcriptional regulators.
We have ample evidence that the three members of the geminin family all play vital roles in MCC differentiation, yet they do so in quite distinct ways. During the course of ciliogenesis, Geminin is down‐regulated just as GemC1 and Mcidas expression begin to increase. While Gemc1 and Mcidas are the major activators of MCC gene expression, Geminin suppresses this activity and thus inhibits MCC gene expression. This yin and yang of the Geminin‐Gemc1/Mcidas function appears to be a conserved feature, as Geminin is known as a negative regulator of DNA replication during cell cycle progression, whereas GemC1 and Mcidas are positive regulators of the process (Caillat et al, 2015).
How does Geminin repress Gemc1 and Mcidas function? While there is no evidence that Geminin interacts with E2F complexes by itself, biochemical experiments indicate that all three geminin family members can form homo‐ and heterodimers and regulate each other's activity through such interactions (Caillat et al, 2015). Thus, it is possible that Geminin can sequester Gemc1 or Mcidas away from the EDG/M complex. Another possibility is that the EDG/M complexes themselves rely on Gemc1 or Mcidas homo‐ or heterodimers, and a Geminin‐Gemc1/Mcidas heterodimer may block transcriptional activity. However, these proposed mechanisms will require lengthy experimental validation.
There are several apparent advantages to placing MCC differentiation under the control of the geminin family. Gemc1 and Mcidas provide differential regulation and redundancy, but converge on the same transcriptional targets. The Geminin block to gene expression may function as a MCC checkpoint by controlling the timing and kinetics of motile ciliogenesis. Generating numerous motile cilia is energetically expensive and to our knowledge an irreversible process. Premature motile ciliogenesis due to ectopic Gemc1 expression during development also has detrimental effects on ependymal differentiation in the adult neurogenic niche (Kyrousi et al, 2015). Thus, Geminin could be safeguarding precursor cells from the hazards of prematurely embarking on MCC differentiation.
Multiciliogenesis is an ancient cellular feature, present in animals from many invertebrate and all vertebrate lineages and in unicellular ciliates such as Paramecium and Tetrahymena (Brooks & Wallingford, 2014). However, it is unclear how well conserved is the role of geminin family regulators. Zhou et al point out that GemC1 and Mcidas are absent in planaria, a flatworm that uses motile cilia for locomotion. However, while the MCCs of planaria look similar to other MCCs, deuterosomes (ciliogenic structures unique to MCCs) have not been reported in these cells, suggesting that the overall mode of multiciliogenesis may be quite distinct. Perhaps more interesting are the potential differences in vertebrate Gemc1 function. While studies in mouse and Xenopus MCCs (Kyrousi et al, 2015; Arbi et al, 2016; Terré et al, 2016) clearly demonstrate that Gemc1 is both necessary and sufficient for motile ciliogenesis, this does not appear to be conserved in zebrafish kidney tubule MCCs (Zhou et al, 2015), where it was found to be necessary, but not sufficient for the process. Ectopic expression of Gemc1 together with E2F4/5 was also insufficient to drive MCCs formation, suggesting that perhaps even this relationship is different in fish. Consistent with this, Zhou et al were unable to detect an interaction between Gemc1 and E2F4/5. We may, therefore, conclude that MCC gene expression in zebrafish may somehow be under a variant regulatory pathway.
The addition of Gemc1 and Geminin to the transcriptional hierarchy of the MCC gene expression program is an important contribution to the field, and as with all significant discoveries, we are left with many outstanding questions. How is Gemc1 expression regulated? What are the events that span Notch signaling and Gemc1 activation? How does Geminin suppress ciliogenesis during this window, and how is it lost from MCCs? These are among the most critical gaps in our knowledge of MCC differentiation, but it will also be essential to understand how Gemc1 and Mcidas each contribute to the expression of ciliary genes. Comparison of direct transcriptional targets in a single system will be necessary to delineate the differential targets of Gemc1 and Mcidas. Additionally, while the identification of cell cycle proteins, including the geminin family members, as regulators of the MCC gene expression program draws a compelling parallel between motile ciliogenesis and cell cycle regulation, many mechanistic details need to be uncovered to define the cell cycle state of MCCs.
Lastly, most of the focus to date has been on the activation of the MCC gene expression program as a whole. However, it is desirable to eventually understand how the transcription of specific targets is initiated and eventually terminated. This will help clarify many confounding aspects of ciliary biology, for example, key differences in the generation and maintenance of the centriolar and axonemal structures of motile cilia. Centriole biogenesis is an apparently irreversible process. MCCs make a remarkably consistent number of centrioles for a given apical surface area, then cease centriole assembly. In contrast, axoneme formation seems to be more malleable. Axonemes rapidly regrow after deciliation, indicating that mature MCCs maintain a stockpile of axonemal proteins. Hopefully, future experiments on the MCC gene expression program will clarify how centriolar and axonemal transcriptional targets are differentially regulated in MCCs. Clearly, this is an exciting time to be working on these unique and enigmatic cells.
See also: B Terré et al (May 2016) and M Arbi et al (March 2016)
References
- Arbi M, Pefani DE, Kyrousi C, Lalioti ME, Kalogeropoulou A, Papanastasiou AD, Taraviras S, Lygerou Z (2016) GemC1 controls multiciliogenesis in the airway epithelium. EMBO Rep 17: 400–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini A, Cosentino C, Errico A, Garner E, Costanzo V (2010) GEMC1 is a TopBP1‐interacting protein required for chromosomal DNA replication. Nat Cell Biol 12: 484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks ER, Wallingford JB (2014) Multiciliated cells. Curr Biol 24: R973–R982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillat C, Fish A, Pefani DE, Taraviras S, Lygerou Z, Perrakis A (2015) The structure of the GemC1 coiled coil and its interaction with the Geminin family of coiled‐coil proteins. Acta Crystallogr D Biol Crystallogr 71: 2278–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat‐Karalar EN, Stearns T (2014) The centriole duplication cycle. Philos Trans R Soc Lond B Biol Sci 369: 20130460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrousi C, Arbi M, Pilz GA, Pefani DE, Lalioti ME, Ninkovic J, Gotz M, Lygerou Z, Taraviras S (2015) Mcidas and GemC1 are key regulators for the generation of multiciliated ependymal cells in the adult neurogenic niche. Development 142: 3661–3674 [DOI] [PubMed] [Google Scholar]
- Ma L, Quigley I, Omran H, Kintner C (2014) Multicilin drives centriole biogenesis via E2f proteins. Genes Dev 28: 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terré B, Piergiovanni G, Segura‐Bayona S, Gil‐Gómez G, Youssef SA, Attolini CS, Wilsch‐Bräuninger M, Jung C, Rojas AM, Marjanović M, Knobel PA, Palenzuela L, López‐Rovira T, Forrow S, Huttner WB, Valverde MA, de Bruin A, Costanzo V, Stracker TH (2016) GEMC1 is a critical regulator of multiciliated cell differentiation. EMBO J 35: 942–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlings I, de Bruin A (2016) E2F transcription factors control the roller coaster ride of cell cycle gene expression. Methods Mol Biol 1342: 71–88 [DOI] [PubMed] [Google Scholar]
- Zhou F, Narasimhan V, Shboul M, Chong YL, Reversade B, Roy S (2015) Gmnc is a master regulator of the multiciliated cell differentiation program. Curr Biol 25: 3267–3273 [DOI] [PubMed] [Google Scholar]


