Abstract
The genus Klebsormidium (Klebsormidiales, Streptophyta) has a worldwide distribution in terrestrial habitats. In the present study, we focused on two strains of Klebsormidium flaccidum, the type species of the genus. The isolates used in this study were isolated from a soil and freshwater habitat. Photosynthetic activity was evaluated under different controlled gradients of light, temperature and desiccation. The data clearly indicate that both isolates of K. flaccidum exhibit conspicuously different photosynthetic response patterns to photon fluence rate, temperature and desiccation, and thus can be related to their different habitats. Although both strains represent the same species, their physiological response patterns to abiotic gradients, as well as their morphology differed to some extent, indicating high phenotypic plasticity of K. flaccidum, which was maintained even after long-term culture and thus can be explained by the formation of physiologically distinct ecotypes.
Keywords: biological soil crust, desiccation, ecological tolerance, ecotypic differentiation, photosynthesis, respiration
Introduction
The green algal genus Klebsormidium (Klebsormidiophyceae, Streptophyta) received much attention in recent years because of its worldwide distribution in terrestrial habitats such as soil, urban walls and rock surfaces as well as being an abundant member of so-called biological soil crusts in drylands (Rindi et al. 2011; Škaloud & Rindi 2013; Škaloud et al. 2014; Mikhailyuk et al. 2015; Ryšánek et al. 2015). In addition, some Klebsormidium species occur in aquatic habitats such as rivers, lakes and bogs (Rindi et al. 2011; Škaloud & Rindi 2013, and references therein). Klebsormidium has a low genetic diversity based on the conserved 18S rDNA gene, resulting in a relatively low number of species (Fabio Rindi, Pavel Škaloud, Tatiana Mikhailyuk pers. comm.). However, when using other molecular markers, such as the much more variable ITS1/2 or rbcL, the picture changes as reflected in extensive genetic diversity (Škaloud & Rindi 2013; Škaloud et al. 2014). Whether this genetic diversity reflects infraspecific differentiation or cryptic species is still an open question. Škaloud & Rindi (2013) investigated 70 strains of the Klebsormidium clade E lineage (K. flaccidum - K. nitens complex according to Rindi et al. 2011), that were collected from natural aeroterrestrial substrata (e.g. soil), anthropogenic aeroterrestrial substrata (e.g. building surfaces) and aquatic habitats. These authors demonstrated various sub-clades (phylogenetic lineages) within clade E that correlated with the three mentioned habitat preferences. Although these data are interesting and point to ecotypic differentiation, or even the presence of cryptic species, in morphologically similar strains, experimental evidence for physiological differences is still missing. Therefore, in the present study we focused on Klebsormidium flaccidum (Kützing) P.C.Silva, Mattox & Blackwell (1972), the type species of Klebsormidium; however, the poor characterization of the species still affects the circumscription of the whole genus. In the phylogenies of Rindi et al. (2011), strains morphologically assigned to K. flaccidum are polyphyletic and occur in three different clades (B, C, E). It has been impossible to assign the type specimen to any of these clades, as the original description by Kützing (1849) did not provide sufficient morphological information. Mikhailyuk et al. (2015) proposed a strain of K. flaccidum isolated by Lokhorst (1996), and now preserved in the SAG algal collection (Sammlung für Algenkulturen, Göttingen, Germany; strain SAG 2307), as epitype. Strain SAG 2307 corresponded well with the morphological description of K. flaccidum (Lokhorst 1996), although some minor differences such as the width of filaments and rare presence of H-like fragments of the cell wall were noted (Mikhailyuk et al. 2015).
Klebsormidium flaccidum strain SAG 2307 was originally isolated from a biofilm on a temperate clay soil in a field of beets in Lower Saxonia, Germany. This strain was assigned to clade C in the phylograms of Rindi et al. (2011) and Škaloud et al. (2014). For physiological comparison, another isolate of K. flaccidum (SAG 7.91) from a freshwater habitat in the former USSR was selected. This strain was assigned to clade B in the phylograms of Rindi et al. (2011) and Škaloud et al. (2014). It should be mentioned, however, that clade B and C show the smallest genetic distance compared to all other clades. Consequently, Mikhailyuk et al. (2015) combined clade B and C to a joint B/C K. flaccidum clade, in which SAG 2307 and SAG 7.91 are genetically very similar based even on the variable ITS-1 and ITS-2 rDNA sequences. Therefore, we consider both strains as genotypes of K. flaccidum in the present study. While strain SAG 7.91 was deposited 1989 to the SAG, strain SAG 2307 was deposited 20 years later. Both isolates have been kept under axenic and identical artificial laboratory conditions for at least six years, and hence they represent ideal model systems to comparatively study the physiological traits of two closely related genotypes.
In recent years, various ecophysiological and biochemical acclimation processes in Klebsormidium strains from all clades under gradients of irradiation (PAR and UV), temperature, pH and water potential have been reported (Holzinger et al. 2011, 2014; Kaplan et al. 2012; Karsten et al. 2010, 2016; Kitzing et al. 2014; Škaloud et al. 2014), and the generally wide physiological response patterns can be quite different depending on morphological and structural features such as strong or easily disintegrating filaments (Holzinger et al. 2011; Mikhailyuk et al. 2014). Some of the underlying molecular mechanisms have been discussed in the recently published genome of K. flaccidum strain NIES-2285 (Hori et al. 2014), which was isolated from a freshwater habitat. These authors showed, for example, the occurrence of a simple protective system against radiation stress in the sense of non-photochemical quenching (NPQ) that is considered as fundamental mechanism for streptophyte adaptation to terrestrial habitats (Hori et al. 2014). It comprises a cyclic electron flow activity at photosystem I, which is activated under high-light conditions and desiccation, and increases the proton gradient across the thylakoid membrane (Hori et al. 2014). As a consequence, non-photochemical quenching and ATP biosynthesis are induced, followed by the dissipation of excess radiation energy (Hori et al. 2014). This genomic approach is well supported by a transcriptomic study on K. crenulatum (Kützing) Lokhorst under desiccation (Holzinger et al. 2014), which showed, for example, the up-regulation of transcripts for photosynthesis, energy production, reactive oxygen species metabolism, as well as of plant proteins involved in early response to desiccation (ERD). These authors concluded that the streptophyte K. crenulatum exhibits similar molecular events during desiccation compared to land plants pointing to ancestral mechanisms for the successful colonization of terrestrial habitats (Holzinger et al. 2014).
As comprehensive ecophysiological studies on genetically closely related Klebsormidium strains from divergent habitats under controlled conditions are missing, the major goal was to characterize for the first time two K. flaccidum strains from a terrestrial and freshwater habitat. Photosynthetic activity as a function of temperature and light gradients, as well as under controlled dehydration and recovery conditions were investigated. The main question of this study was to evaluate whether strain-specific physiological traits are present that influence desiccation, temperature and light tolerance in both Klebsormidium genotypes, which would experimentally support ecotypic differentiation as suggested by Škaloud & Rindi (2013). However, by comparing only two strains, we will not be able to draw further conclusions on speciation concepts as suggested by these authors.
Material and Methods
Both Klebsormidium flaccidum (Kützing) P.C.Silva, Mattox & Blackwell (1972) strains SAG 2307 and SAG 7.91 were obtained from the SAG (The Culture Collection of Algae at the University of Göttingen, Germany – http://www.uni-goettingen.de/en/184982.html). SAG 2307 was collected before 1995 by Dr. G.M. Lokhorst (formerly University of Leiden, The Netherlands) from a temperate clay soil in a field of beets near Niederkruechten, Lower Saxonia, Germany, and originally termed this isolate KL1. In April 2009, Dr. H. Sluiman (Royal Botanic Garden Edinburgh, UK) deposited this strain at SAG (#2307) as well as sequence information on 18S rRNA gene, ITS1, 5.8S rRNA gene, ITS2 and partial 26S rRNA gene at GenBank (accession no. AM 490838). SAG 7.91 was collected in 1960 by Prof. B.V. Gromov (formerly St. Petersburg University, Russia) from a freshwater habitat in the former USSR and he originally named this isolate as Hormidium nitens Galn 44. In August 1989, Prof. Gromov deposited this strain at SAG (#7.91). In 2008 Dr. T. Mikhailyuk deposited sequence data at GenBank, namely partial 18S rRNA gene, ITS1, 5.8S rRNA gene, ITS2 and partial 26S rRNA gene (accession no. EU 434019). Both strains of K. flaccidum are kept axenic at SAG.
At the University of Innsbruck both Klebsormidium cultures were maintained in 250 mL Erlenmeyer flasks with modified Bold’s Basal Medium (3NBBM; Starr & Zeikus 1993). Algal cells were maintained at 20°C and 35–40 μmol photons m-2 s-1 at a light-dark cycle of 16:8 h L:D (Osram Daylight Lumilux Cool White lamps L36W/840, Osram, Munich, Germany). Photon fluence rates were measured with a Solar Light PMA 2132 cosine corrected PAR sensor connected to a Solar Light PMA 2100 radiometer (Solar Light Co., Inc., Philadelphia, USA). For all experiments, log-phase cultures exhibiting comparable cell densities were used.
Light microscopic examination of SAG 7.91 and SAG 2307 was performed using a Zeiss Axiovert 200 M microscope, equipped with a 63×1.4 NA objective and an Axiocam MRc5 camera controlled by Zeiss Axiovision software. Differential interference contrast (DIC) was applied to enhance the contrast. Images were further processed by Adobe Photoshop (CS5; Adobe Systems, San José, CA, USA).
Light-induced relative electron transport rates (rETR) were measured in both strains using a PAM 2500 (Heinz Walz GmbH, Effeltrich, Germany). Vital cells of each Klebsormidium strain were transferred on 4 replicate Whatman GF/F glass fibre filters (Whatman, Dassel, Germany) until a light green spot was visible. These moist filters were positioned on perforated metal grids on top of four glass columns inside a transparent 200 mL polystyrol box, which was filled with 100 mL tap water and sealed with a transparent top lid to guarantee a relative air humidity >95% (according to Karsten et al. 2014). The chambers were maintained at 22 ±1°C and 40 µmol photons m-2 s-1 PAR (culture conditions) (Osram light sources as above). The PAM fibre optics was positioned outside the cover lid of the boxes (always 2 mm distance) to guarantee undisturbed conditions inside, and thus all fluorescence measurements were performed through the polystyrol lids. The distance from the PAM light probe to the algal sample onto the glass fibre filters was a constant 10 mm. The Klebsormidium cells were exposed to 13 photon fluence densities (PFDs) for 30 s each (rapid light curves) ranging from 1 up to 1432 μmol photons m-2 s-1. The actinic light was provided by a red power LED (630 nm) of the PAM 2500. After each light exposure, a saturating pulse was given to detect Fm and ΔF/Fm’. The calculated rETR curve (Kromkamp & Forster 2003) was fitted according to Webb et al. (1974), as no indication of photoinhibition occurred. Therefore, the three photosynthetic parameters: α (positive slope at limiting photon fluence rates), Ik (initial value of light-saturated photosynthesis), and rETRmax (maximum electron transport rate) could be derived.
The effect of rising temperatures on respiratory and photosynthetic response patterns (referenced to chlorophyll a) in both Klebsormidium strains was determined using a Thermo Haake K20 refrigerated circulator (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) connected to a 3 mL thermostatic acrylic chamber (type DW1, Hansatech Instruments, Norfolk, UK) combined with a magnetic stirrer according to Remias et al. (2010). Both algal isolates were exposed to nine temperature steps ranging from 5 to 45°C in 5°C increments according to Karsten & Holzinger (2012). After treatment with the highest temperature (45°C), photosynthesis and respiration were measured again after a temperature reduction to 30°C to test for recovery effects.
A Presens Fibox 3 oxygen optode (Presens, Regensburg, Germany) was applied to record respiratory oxygen consumption rates in darkness for 10 min at each temperature step followed by photosynthetic oxygen production rates at saturating 200 μmol photons m−2 s−1 PAR for another 10 min. The oxygen sensor was attached to the DW1 chamber mentioned above (Remias et al. 2010). 2.8 mL log-phase Klebsormidium suspensions were added to the chamber supplemented by 0.2 mL of a NaHCO3 stock solution (resulting in 2 mM NaHCO3 final concentration) to guarantee sufficient carbon supply during measurements. All respiratory and photosynthetic O2 rates were normalised to the biomass proxy chlorophyll a (c. 4 to 5 µg 3 mL-1), which were determined according to Karsten et al. (2014). From the final photosynthetic and respiratory rates (µmol O2 mg-1 Chl. a h-1) the gross photosynthesis:respiration (P:R) ratios for each temperature were calculated.
The effect of desiccation and rehydration on the effective quantum yield of PSII (ΔF/F’m measured according to Genty et al. 1989) of both Klebsormidium strains was monitored by using a specially designed desiccation chamber (Karsten et al. 2014). Filament suspensions of SAG 2307 and SAG 7.91 were concentrated on four replicate Whatman GF/F glass fibre filters. Exactly 200 µL of the respective homogeneous algal suspension (ca. 1-2 mg Chl. a L-1, giving small, light green spots) were transferred onto each filter. Applying defined starting volumes of log-phase cultures guaranteed reproducibility, since it is impossible to obtain reliable water contents in small quantities of algal filaments. The moist filters were placed in the desiccation chambers filled with 100 g of activated silica gel (Silica Gel Orange, Carl Roth, Karlsruhe, Germany), which resulted in a relative air humidity of ~10% inside the sealed chamber (Karsten et al. 2014). The chambers were kept under constant PFDs (40 μmol photons m−2 s−1) at 22 ± 0.5°C, and the ΔF/F’m of the desiccating algae was determined by the saturation pulse approach.
The effective quantum yield was regularly determined during the dehydration period up to 22 h until no or only a minimal signal was detected in the samples of Klebsormidium. This was immediately followed by opening the chambers, transferring the filters to a polystyrol box filled with 100 mL tap water (relative air humidity >95%), and rehydrating the algae on the filters by adding 200 µL of the standard growth medium to each greenish spot to measure the recovery of the ΔF/F’m for ~2 days.
All measurements of the effective quantum yield (performed by PAM 2500) were performed in four independent replicates (n = 4), while all oxygen measurements (optode) were carried out with three independent replicates (n = 3). All data represent the respective mean values ± standard deviation.
Statistical significance of the means of all optode data, as well as the effective quantum yield of dehydrated and rehydrated samples were tested with one-way ANOVA followed by a Tukey’s multiple comparison test (P < 0.01) to find subgroups of means with significant differences. Comparison of cell dimensions (length, width, length:width ratio; n = 25) was performed by a standard two-sample t test (P < 0.05). Analyses were performed with InStat (GraphPad Software Inc., La Jolla, CA, USA).
Results
Phylogenetic position
As indicated in the introduction, Mikhailyuk et al. (2015) suggested a joint B/C K. flaccidum clade, based on morphological features and genetic markers, in which SAG 2307 and SAG 7.91 are integrated. Sequence comparison of the partial 18S rDNA gene of both strains indicates > 99% similarity, while the variable ITS-1 and ITS-2 rDNA sequences exhibited > 97% sequence similarity (data not shown). Therefore, SAG 2307 and SAG 7.91 were considered as genotypes of K. flaccidum in the present study.
Light microscopy
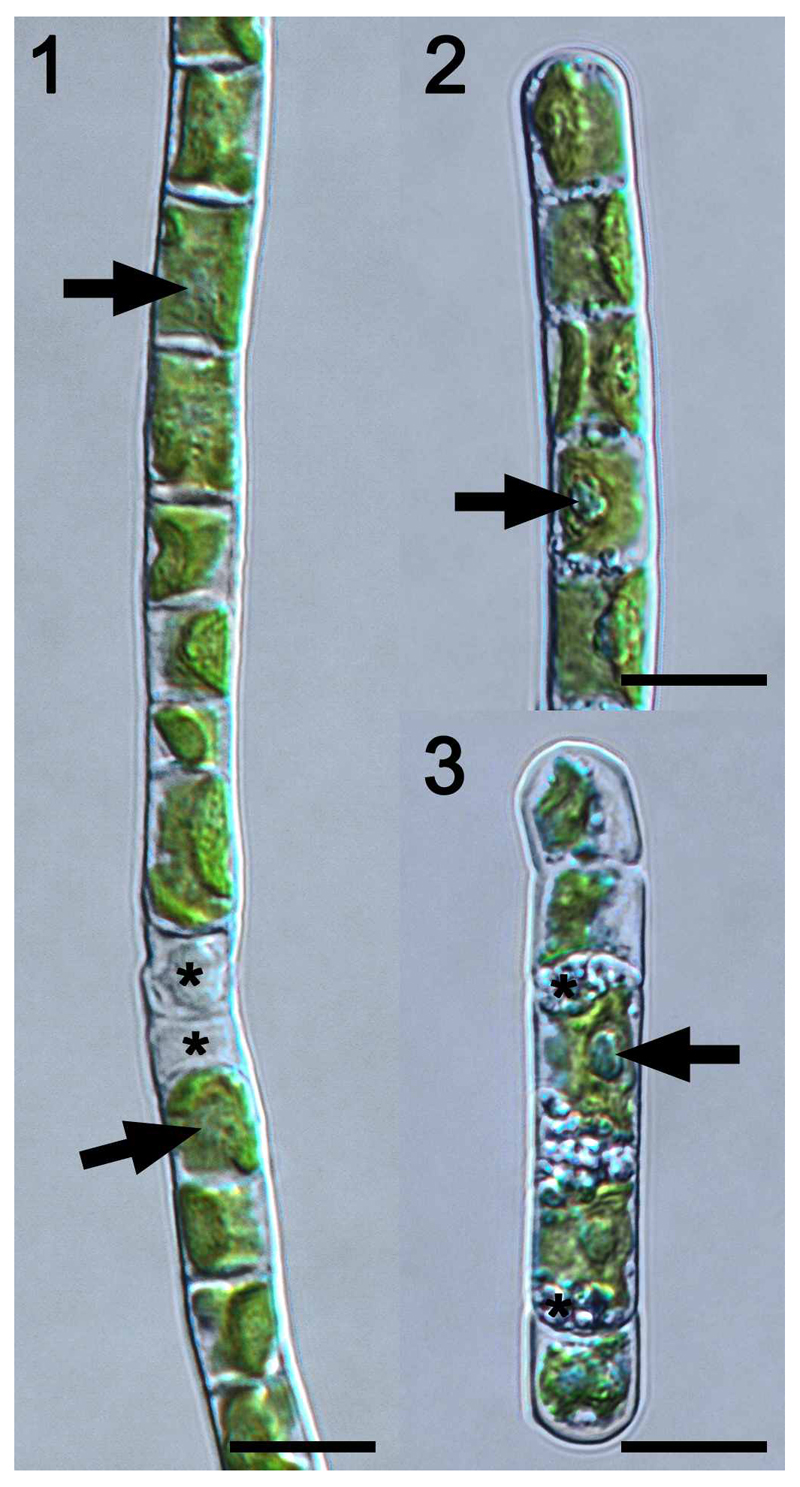
Cells of strictly uniseriate filaments of Klebsormidium flaccidum SAG 7.91 contained one parietal chloroplast with smooth (sometimes crenulated) margins, which covered 1/3 to 2/3 of the cell circumference and expanded to the cross cell walls (Fig. 1). One pyrenoid surrounded by starch grains was embedded in each chloroplast (Fig. 1). Occasionally, the filaments were covered by a thin mucilage layer. Fragmentation into short filaments was scarce, and filaments usually were not even disrupted along cross cell walls of dead cells (Fig. 1). Cell dimensions of SAG 7.91 are listed in Table 1. The morphology of SAG 2307, the epitype of K. flaccidum, was described in detail by Mikhailyuk et al. (2015), and these findings were confirmed in the present study. Cells formed strictly uniserate filaments and contained one parietal chloroplast with smooth margins, which covered the entire length of the cells and 1/3 – 2/3 of the cell circumference (Fig. 2). Each chloroplast contained one pyrenoid surrounded by starch grains (Fig. 2). Filaments were covered by a thin mucilage layer and fragmentation into shorter filaments (5 – 12 cells) was observed frequently (Fig. 3). Some cells contained numerous granular storage compounds close to the cross cell walls (Fig. 3). The cell width of SAG 2307 was significant higher (P < 0.05) compared with SAG 7.91, while the cell length and length:width ratio are similar (Table 1).
Figs 1-3.
Light microscopy of Klebsormidium flaccidum strain SAG 7.91. Scale bars = 10 µm.
Fig. 1. Filament with cells containing one parietal chloroplast covering the entire length of the cell and 1/3 to 2/3 of the cell circumference. One pyrenoid per chloroplast (arrows). Filaments do not break along cross cell walls between dead cells (asterisk).
Fig. 2. Similar to Fig 1 but the pyrenoids are more prominent (arrow).
Fig. 3. Short filament (5 cells) with one prominent pyrenoid per cell (arrow); 2 cells filled with storage compounds (asterisk) close to the cross cell walls.
Table 1.
Cell dimensions (length and width in µm and length:width ratio) Klebsormidium flaccidum strains SAG 7.91 and SAG 2307 (n = 25 ± s).
| length | width | length:width | |
|---|---|---|---|
| SAG 7.91 | 10.6 ± 2.3 | 7.5 ± 0.4 | 1.4 ± 0.3 |
| SAG 2307 | 11.1 ± 2.1 | 8.2 ± 0.4* | 1.4 ± 0.2 |
Cell length, width and the length:width ratio of both strains were compared and significant differences are indicated by an asterisk. They were determined by a standard two-sample t test (P < 0.05).
Light requirements for photosynthethic activity
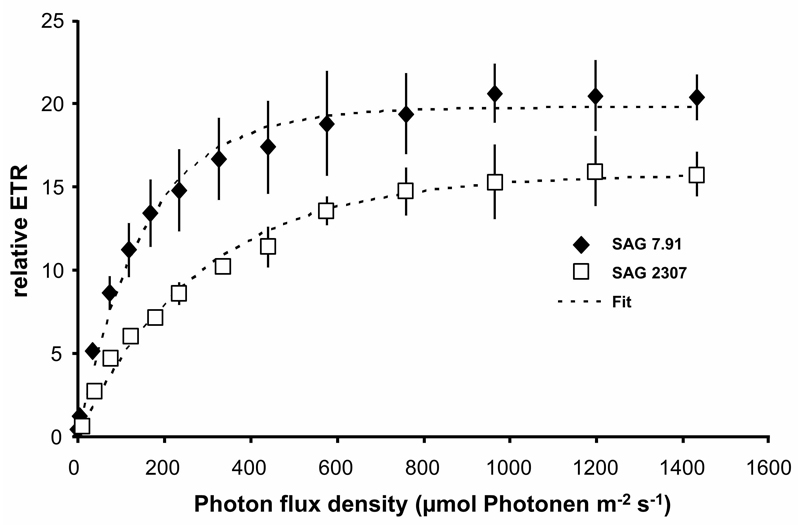
Rising photon fluence densities up to 1432 µmol photons m-2 s-1 stimulated the photosynthetic relative electron transport rates (rETR) in both strains of K. flaccidum without any photoinhibitory effects (Fig. 4). While SAG 2307 showed a rETRmax of 15.7, that of SAG 7.91 was slightly higher (19.6). In addition, SAG 2307 exhibited with 0.052 a lower α (positive slope at limiting photon fluence rates) and with 316 µmol photons m-2 s-1 a much higher Ik (initial value of light-saturated photosynthesis), compared to SAG 7.91 (α: 0.129, Ik: 152) (Fig. 4).
Fig. 4.
The effect of increasing photon fluence densities up to 1432 µmol photons m-2 s-1 on the relative electron transport rate (rETR) in the two studied strains of Klebsormidium flaccidum (n = 4, mean ± s). SAG 2307: terrestrial isolate; SAG 7.91: freshwater isolate. All PAM measurements were undertaken at 22 ± 1°C.
Photosynthetic activity under desiccation and rehydration
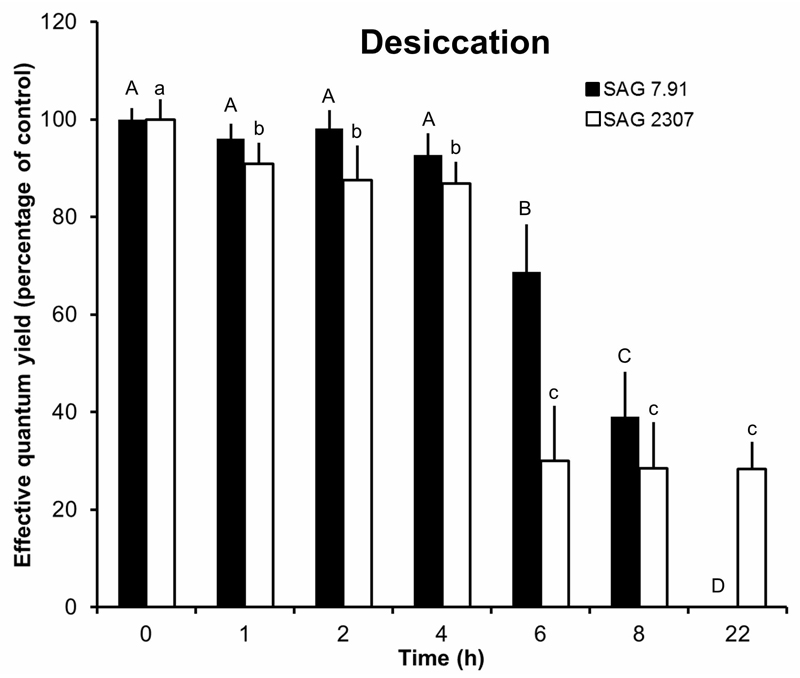
Both strains of K. flaccidum were desiccated under controlled conditions over silica gel. Within the first 4 h, the effective quantum yield of PSII (ΔF/F’m) was unchanged (SAG 7.91) or only slightly affected (SAG 2307) (Fig. 5). However, further increase of the desiccation period caused a strong decline of the photosynthetic activity. In SAG 7.91, the ΔF/F’m decreased to 70% of the control after 6 h treatment, to 40% after 8 h and no signal was recorded after 22 h (Fig. 5). SAG 2307 exhibited a much stronger loss of the photosynthetic activity between 4 and 6 h of incubation, leading to only 30% of the control value. Further desiccation, however, did not affect this minimum signal (Fig. 5).
Fig. 5.
The effect of controlled desiccation on the effective quantum yield (ΔF/F’m) of PSII as regularly measured with a PAM 2500 during the experiment (22 h) in the two studied strains of Klebsormidium flaccidum (n = 4, mean ± s). SAG 2307: terrestrial isolate; SAG 7.91: freshwater isolate. Effective quantum yield values of control algae under 40 µmol photons m-2 s-1 PAR was determined as 0.55-0.62 and standardized to 100% for better comparison. All PAM measurements were undertaken at 22 ± 1°C. Significances among the treatments were calculated by one-way ANOVA (P < 0.01). Different letters represent significant differences among the time points as revealed by Tukey’s post hoc test.
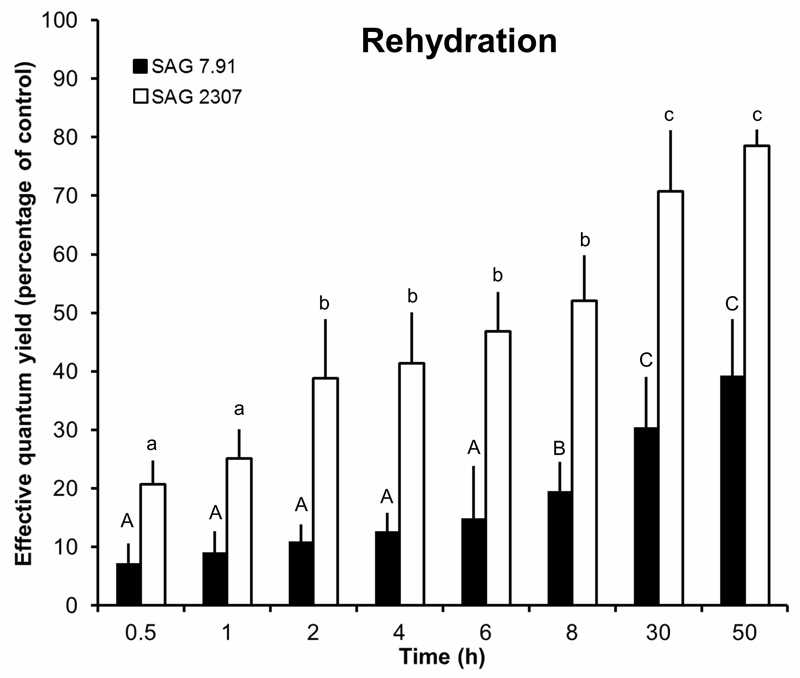
After rehydration of the dried K. flaccidum samples, SAG 2307 showed a continuous recovery of the effective quantum yield resulting in a value of 80% of the control after 50 h (Fig. 6). In sharp contrast, SAG 7.91 exhibited a much slower recovery rate reaching only 40% of the optimum effective quantum yield after 50 h (Fig. 6).
Fig. 6.
The effect of controlled recovery on the effective quantum yield (ΔF/F’m) of photosystem II as regularly measured with a PAM 2500 during the experiment (50 h) in the two studied strains of Klebsormidium flaccidum (n = 4, mean ± s). SAG 2307: terrestrial isolate; SAG 7.91: freshwater isolate. Effective quantum yield values of control algae (see Fig. 3) under 40 µmol photons m-2 s-1 PAR was determined as 0.55-0.62 and standardized to 100% for better comparison. All PAM measurements were undertaken at 22 ± 1°C. Significances among the treatments were calculated by one-way ANOVA (P < 0.01). Different letters represent significant differences among the time points as revealed by Tukey’s post hoc test.
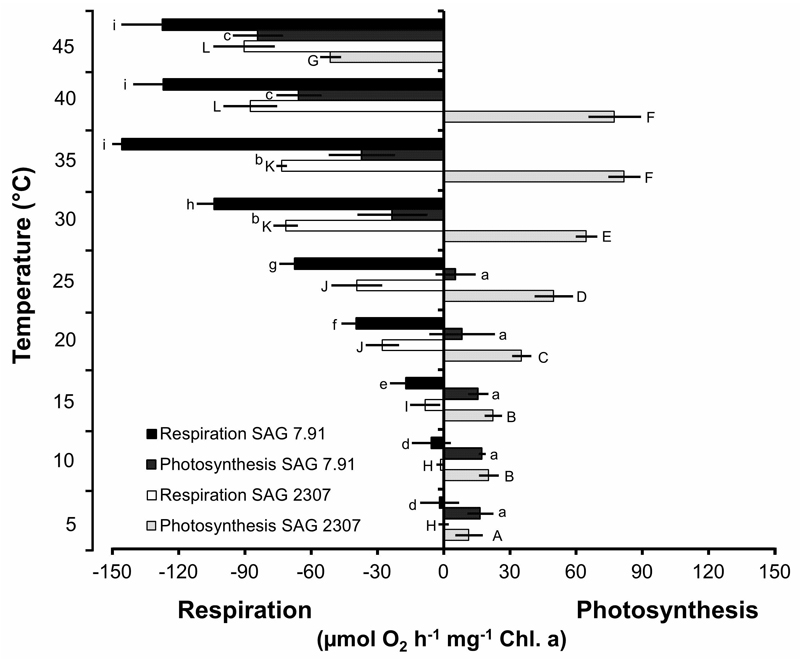
Photosynthesis and respiration as function of temperature
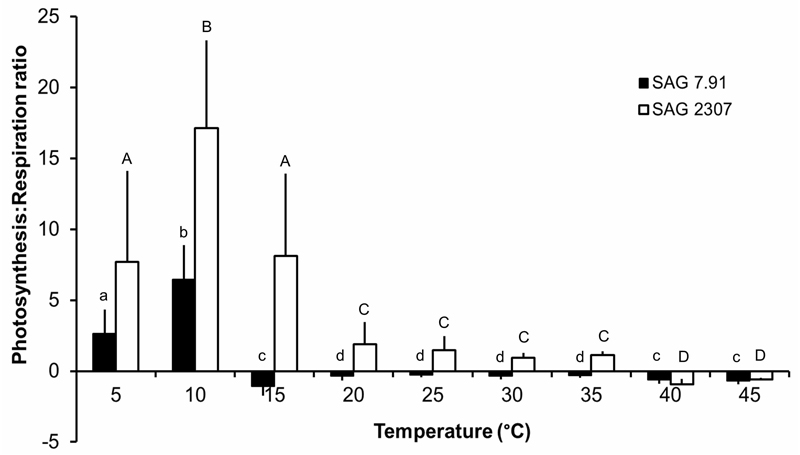
Both K. flaccidum strains exhibited a strongly temperature-dependent photosynthetic oxygen production and respiratory oxygen consumption, but with different temperature requirements for these physiological processes (Fig. 7). In SAG 2307, oxygen production continuously increased from 5 to 35 - 40°C, followed by a sharp decrease at 45°C, leading even to negative values (Fig. 7). In contrast, SAG 7.91 showed an almost identical photosynthetic rate between 5 and 25°C, which however, was lower than those of SAG 2307. In addition, further increase in temperature (> 25°C) led to strong reduction of oxygen production to negative values (Fig. 7). Respiration was very low in both K. flaccidum strains at low temperatures, and increased strongly > 10°C, before reaching a maximum at 35°C in SAG 7.91 and 40°C in SAG 2307 (Fig. 7). Based on the photosynthesis and respiration rates, the ratio of both physiological processes was calculated, and striking differences between both strains were observed (Fig. 8). SAG 2307 showed highest photosynthesis:respiration ratios between 5 and 15°C followed by much lower, but still positive values between 20 and 35°C, while at 40 and 45°C the ratios were negative (Fig. 8). In contrast, in SAG 7.91 only at 5 and 10°C positive photosynthesis:respiration ratios could be determined, while from 15 up to 45°C all ratios were negative (Fig. 8).
Fig. 7.
Net photosynthetic oxygen production and respiratory oxygen consumption in µmol O2 h-1 mg-1 Chl a measured at 200 µmol photons m-2 s-1 PAR as function of increasing temperatures in the two studied strains of Klebsormidium flaccidum (n = 4, mean value ± SD). SAG 2307: terrestrial isolate; SAG 7.91: freshwater isolate. Significances among the treatments were calculated by one-way ANOVA (P < 0.01). Different letters represent significant differences among the temperatures as revealed by Tukey’s post hoc test.
Fig. 8.
Net photosynthesis:respiration (P:R) ratios as function of increasing temperatures in the two studied strains of Klebsormidium flaccidum (n = 4, mean ± s). SAG 2307: terrestrial isolate; SAG 7.91: freshwater isolate. Significances among the treatments were calculated by one-way ANOVA (P < 0.05). Different letters represent significant differences among the temperatures as revealed by Tukey’s post hoc test.
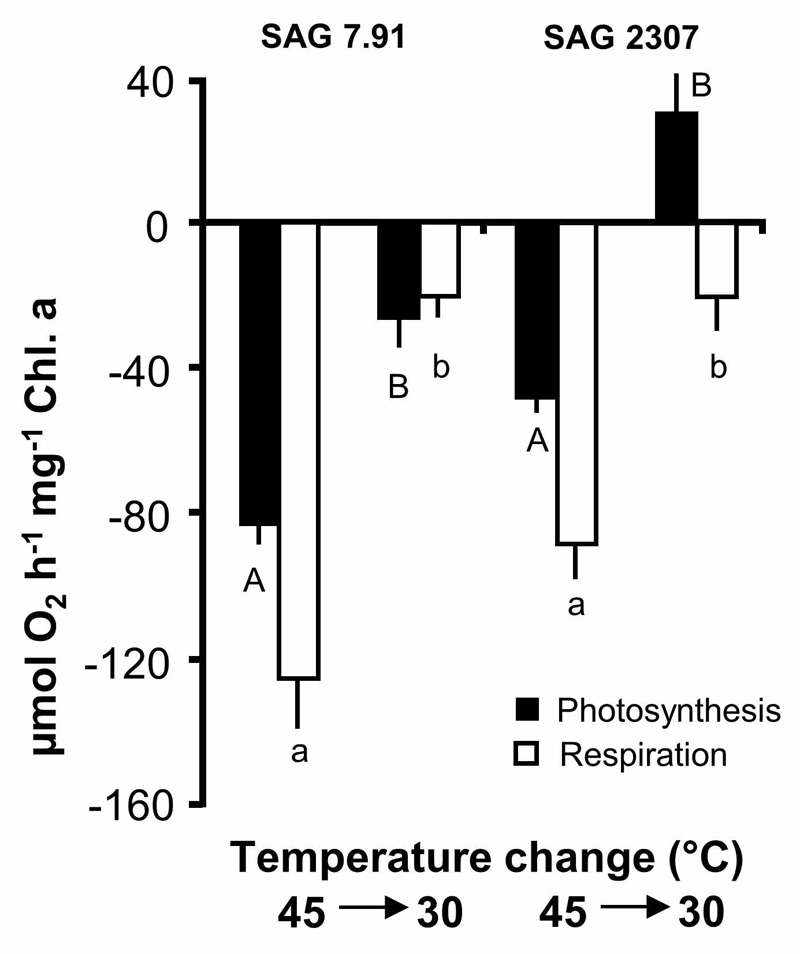
After exposing both strains to 45°C, temperature was decreased to 30°C and the short-term recovery potential of photosynthesis and respiration evaluated (Fig. 9). The K. flaccidum samples showed different response patterns, i.e. in SAG 7.91 with declining temperatures the negative photosynthetic and respiratory rates only partially recovered, but still with negative photosynthesis, while in SAG 2307 photosynthetic oxygen production reached 50% of the rate at 30°C (Fig. 9). The respiratory activity of SAG 2307 returned also to low rates.
Fig. 9.
Photosynthesis and respiration in µmol O2 h-1 mg-1 Chl. a measured at 200 µmol photons m-2 s-1 PAR at 45°C (see Fig. 5) and after transfer back to 30°C in the two studied strains of Klebsormidium flaccidum (n = 4, mean ± s). SAG 2307: terrestrial isolate; SAG 7.91: freshwater isolate. Significances among the treatments were calculated by one-way ANOVA (P < 0.01). Different letters represent significant differences among the temperatures as revealed by Tukey’s post hoc test.
Discussion
Both Klebsormidium flaccidum isolates investigated in the present study exhibited striking differences in the ecophysiological response patterns under light, temperature and desiccation gradients when considering photosynthesis as a key physiological process. As mentioned in the introduction, Škaloud & Rindi (2013) were the first to map habitat preferences on the phylogenetic tree of 70 closely related Klebsormidium clade E lineage strains. Although these authors reported reasonable correlations pointing to ecotypic differentiation, experimental data were missing at that time. The first experimental evidence for such infraspecific differentiation was provided on 5 isolates of Klebsormidium fluitans (F.Gay) Lokhorst, which were collected along an altitudinal gradient of the Alps and exposed to various ultraviolet conditions to follow physiological and biochemical acclimation mechanisms (Kitzing et al. 2014). More recently, Ryšánek et al. (2016) examined 12 Klebsormidium strains representing four different genotypes with respect to photosynthesis and growth, which were collected from sandstone and limestone rocks that were characterized by different surface pH values. These authors determined distinct physiological response patterns among distantly but also closely related lineages, pointing to infraspecific differentiation.
The data in the present study clearly indicate that both isolates of Klebsormidium flaccidum (SAG 2307, SAG 7.91) exhibit conspicuously different photosynthetic response patterns as a function of photon fluence rate, temperature and desiccation (Figs. 2-7), and hence are in agreement with the earlier investigations on ecotype formation in Klebsormidium. Various studies addressed physiological differences between geographically distinct or coexisting populations of algae (e.g. Rietema 1991, 1993; Nitschke et al. 2014, and references therein). Regardless, the designation of isolates of a species as ecotypes that are locally adapted to specific environmental conditions or habitats (sensu Turesson 1922) remains difficult. On the one hand, problems concerning species definition considerably hamper the interpretation of different ecotypes of one species vs. interspecific differences in the traits of interest. On the other hand, the presence of ecotypes as ecologically distinct units is accepted as a means of characterizing physiological diversity (Eggert et al. 2006; Ferris et al. 2003). Adaptation to specific environments and the formation of ecotypes can be caused by directional selection and/or environmental stress along gradients (Doebeli & Dieckmann 2003; Lexer & Fay 2005). Both K. flaccidum strains originated from distinct habitats (temperate clay soil vs. freshwater), and hence the ecotype concept is well supported by the presented data. However, we are aware that from investigating only two strains, no general conclusions on ecotypic differentiation in other K. flaccidum isolates can be drawn.
The measurement of relative electron transport rates with increasing photon fluence rates (PI curves) enables the light requirements of both K. flaccidum strains for photosynthesis to be characterized. While the terrestrial strain SAG 2307 showed a lower α together with a higher Ik value, the freshwater isolate SAG 7.91 exhibited the opposite, i.e. a higher α along with a lower Ik value. A high α/low Ik value (as in SAG 7.91) is typical for low-light adapted algae, and a low α/high Ik value (as in SAG 2307) is common for rather high-light adapted algae (Henley 1993). The diverging habitats of both K. flaccidum strains corroborate the contrasting PI curves, since terrestrial habitats such as open fields usually experience higher and more direct insolation compared to freshwater systems. In addition, the PAM measurements up to at least 1432 µmol photons m-2 s-1 PAR did not induce any photoinhibition. For SAG 7.91 this is a rather atypical photophysiological response, since in many other algae low-light adaptation of photosynthesis is generally reflected in photoinhibition under high photon fluence rates (e.g. Bischof et al. 1998). From the results available on other Klebsormidium species and closely related genera it seems that in these streptophytes photophysiological plasticity is common (Karsten et al. 2012, 2014; Herburger et al. 2016), which is consistent with the often terrestrial life style. In the field, various Klebsormidium species form multi-layered carpet-like structures interwoven with the upper millimetres of soil particles, contributing to a high degree of self-shading and hence photoprotection of individual filaments (Karsten et al. 2010).
The effect of increasing temperatures on respiration and photosynthetic oxygen production in both isolates of K. flaccidum showed striking differences in the temperature requirements of both physiological processes. While the terrestrial strain SAG 2307 exhibited optimum photosynthesis between 35°C and 40°C, the freshwater strain SAG 7.91 was already strongly inhibited > 25°C (Fig. 5). In addition, after treatment with 45°C both strains were exposed to a lower temperature (30°C) to evaluate any short-term recovery potential of photosynthesis and respiration (Fig. 7). While photosynthesis of SAG 7.91 did not recover, strain SAG 2307 exhibited photosynthetic oxygen production equivalent to 50% of control. These data also point to ecotypic differentiation and to a much higher temperature tolerance of the terrestrial isolate of K. flaccidum.
In contrast, respiration of both isolates was not detectable at 5°C, but showed highest rates between 35°C and 45°C. These data clearly indicate that respiration under higher temperatures is generally more efficient than photosynthesis, while the opposite is true at lower temperatures, where photosynthetic rates typically exhibit enhanced activity compared to respiration. The different temperature requirements for both physiological processes and between both K. flaccidum isolates can be again explained by the natural habitats. SAG 2307 will surely experience higher temperatures on the clay soil compared to SAG 7.91 from a Russian freshwater system. These striking differences in the temperature optima of photosynthesis and respiration have already been reported for closely related Klebsormidium, Interfilum, Hormidiella and Entransia species (Karsten et al. 2010, 2014; Karsten & Holzinger 2012; Herburger et al. 2016), and hence seem to represent a common trait for this group. An explanation for the reported different temperature demands for photosynthesis and respiration is related to the high light-dependency of the primary processes of photosynthesis, which are rather insensitive to temperature, while the opposite is true for respiration (Atkin & Tjoelker 2003). The respiration of algae consists of various catabolic reactions, localized in different cellular compartments and controlled by a number of specific enzymes with specific temperature optima. One partly inhibited respiratory enzyme under cool conditions would be sufficient to act as a bottleneck affecting the subsequent process (Atkin & Tjoelker 2003).
Due to the soil habitat, K. flaccidum SAG 2307 is more frequently exposed to desiccation stress than the freshwater strain SAG 7.91. This is reflected in the decreasing ΔF/F’m values during controlled desiccation and recovery (Figs. 3-4). SAG 7.91 failed to show measurable photosynthetic activity after 8 h desiccation, while the ΔF/F’m of SAG 2307 still amounted to 30% of the initial value, even after 22 h exposure. In addition, while SAG 2307 almost fully recovered within 50 h, SAG 7.91 showed only 40% of the control, which indicates some damage in the photosynthetic apparatus. Therefore, SAG 2307 might possess desiccation-tolerance mechanisms that are similar to those of Interfilum (Karsten et al. 2014) and other terrestrial green algae, which exhibited similar physiological responses under conditions of low water availability (Häubner et al. 2006).
In summary, the photosynthetic responses of both K. flaccidum strains to light and temperature gradients as well as to desiccation stress strongly differed, and hence can be related to the ecologically contrasting habitats where these algae were collected. Although SAG 2307 and SAG 7.91 represent the same species, their physiological response patterns to abiotic gradients, as well as their morphology differed to some extent, indicating high phenotypic plasticity of K. flaccidum, which was maintained even after long-term culture, and hence can be explained by ecotypic differentiation.
Acknowledgements
The present study was undertaken during the first author’s sabbatical at the University of Innsbruck, and it was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) (KA899/16-1/2/3/4) (U.K.), as well as a FWF grant P24242-B16 and FWF grant I 1951-B16 (A.H.). Our sincere thanks are extended to Dr. Tatiana Mikhailyuk (M.H. Kholodny Institute of Botany, National Academy of Sciences of Ukraine) for information concerning the phylogenetic positions of both isolates used.
References
- Atkin OK, Tjoelker MG. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science. 2003;8:343–351. doi: 10.1016/S1360-1385(03)00136-5. [DOI] [PubMed] [Google Scholar]
- Bischof K, Hanelt D, Tüg H, Karsten U, Brouwer PEM, Wiencke C. Acclimation of brown algal photosynthesis to ultraviolet radiation in Arctic coastal waters (Spitsbergen, Norway) Polar Biology. 1998;20:388–395. [Google Scholar]
- Doebeli M, Dieckmann U. Speciation along environmental gradients. Nature. 2003;421:259–264. doi: 10.1038/nature01274. [DOI] [PubMed] [Google Scholar]
- Eggert A, Visser RJW, Van Hasselt PR, Breeman AM. Differences in acclimation potential of photosynthesis in seven isolates of the tropical to warm temperate macrophyte Valonia utricularis (Chlorophyta) Phycologia. 2006;45:546–556. [Google Scholar]
- Ferris MJ, Kuhl M, Wieland A, Ward DM. Cyanobacterial ecotypes in different optical microenvironments of a 68 degrees C hot spring mat community revealed by 16S–23S rRNA internal transcribed spacer region variation. Applied and Environmental Microbiology. 2003;69:2893–2898. doi: 10.1128/AEM.69.5.2893-2898.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Häubner N, Schumann R, Karsten U. Aeroterrestrial algae growing on facades – response to temperature and water stress. Microbial Ecology. 2006;51:285–293. doi: 10.1007/s00248-006-9016-1. [DOI] [PubMed] [Google Scholar]
- Henley WJ. Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. Journal of Phycology. 1993;29:729–739. [Google Scholar]
- Herburger K, Karsten U, Holzinger A. Entransia and Hormidiella, sister lineages of Klebsormidium (Streptophyta), respond differently to light, temperature and desiccation stress. Protoplasma. 2016 doi: 10.1007/s00709-015-0889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A, Lütz C, Karsten U. Desiccation stress causes structural and ultra-structural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. Journal of Phycology. 2011;47:591–602. doi: 10.1111/j.1529-8817.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- Holzinger A, Kaplan F, Blaas K, Zechmann B, Komsic-buchmann K, Becker B. Transcriptomics of desiccation tolerance in the streptophyte green alga Klebsormidium reveal a land plant-like defense reaction. PLoS ONE. 2014;9:e110630. doi: 10.1371/journal.pone.0110630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Maruyama F, Fujisawa T, Togashi T, Yamamoto N, Seo M, et al. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nature Communications. 2014;5:3978. doi: 10.1038/ncomms4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Lewis LA, Wastian J, Holzinger A. Plasmolysis effects and osmotic potential of two phylogenetically distinct alpine strains of Klebsormidium (Streptophyta) Protoplasma. 2012;249:789–804. doi: 10.1007/s00709-011-0324-z. [DOI] [PubMed] [Google Scholar]
- Karsten U, Lütz C, Holzinger A. Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. Journal of Phycology. 2010;46:1187–1197. doi: 10.1111/j.1529-8817.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- Karsten U, Holzinger A. Light, temperature and desiccation effects on photosynthetic activity, and drought-induced ultrastructural changes in the green alga Klebsormidium disectum (Streptophyta) from a high alpine soil crust. Microbial Ecology. 2012;63:51–63. doi: 10.1007/s00248-011-9924-6. [DOI] [PubMed] [Google Scholar]
- Karsten U, Herburger K, Holzinger A. Dehydration, temperature and light tolerance in members of the aeroterrestrial green algal genus Interfilum (Streptophyta) from biogeographically different temperate soils. Journal of Phycology. 2014;50:804–816. doi: 10.1111/jpy.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten U, Herburger K, Holzinger A. Living in biological soil crust communities of African deserts – physiological traits of green algal Klebsormidium species (Streptophyta) to cope with desiccation, light and temperature gradients. Journal of Plant Physiology. 2016;194:2–12. doi: 10.1016/j.jplph.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzing C, Pröschold T, Karsten U. UV-induced effects on growth, photosynthetic performance and sunscreen contents in different populations of the green alga Klebsormidium fluitans (Streptophyta) from alpine soil crusts. Microbial Ecology. 2014;67:327–340. doi: 10.1007/s00248-013-0317-x. [DOI] [PubMed] [Google Scholar]
- Kromkamp JC, Forster RM. The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. European Journal of Phycology. 2003;38:103–112. [Google Scholar]
- Kützing FT. Species Algarum. Brockhaus; Leipzig, Germany: 1849. p. 902. [Google Scholar]
- Lexer C, Fay MF. Adaptation to environmental stress: a rare or frequent driver of speciation? Journal of Evolutionary Biology. 2005;18:893–900. doi: 10.1111/j.1420-9101.2005.00901.x. [DOI] [PubMed] [Google Scholar]
- Lokhorst GM. Comparative taxonomic studies on the genus Klebsormidium (Charophyceae) in Europe. Cryptogamic Studies. 1996;5:1–55. [Google Scholar]
- Mikhailyuk T, Holzinger A, Massalski A, Karsten U. Morphological and ultrastructural aspects of Interfilum and Klebsormidium (Klebsormidiales, Streptophyta) with special reference to cell division and thallus formation. European Journal of Phycology. 2014;49:395–412. doi: 10.1080/09670262.2014.949308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailyuk T, Glaser K, Holzinger A, Karsten U. Biodiversity of Klebsormidium (Streptophyta) from alpine biological soil crusts (Alps, Tyrol, Austria and Italy) Journal of Phycology. 2015;51:750–767. doi: 10.1111/jpy.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke U, Karsten U, Eggert A. Physiological performance of the red alga Stylonema alsidii (Stylonematophyceae) under varying salinities. Journal of Experimental Marine Biology and Ecology. 2014;460:170–176. [Google Scholar]
- Remias D, Albert A, Lütz C. Effects of simulated, but realistic, elevated UV irradiation on photosynthesis and pigment composition of the alpine snow alga Chlamydomonas nivalis and the Arctic soil alga Tetracystis sp. (Chlorophyceae) Photosynthetica. 2010;48:269–277. [Google Scholar]
- Rietema H. Evidence for ecotypic divergence between Phycodrys rubens populations from the Baltic Sea and North Sea. Botanica Marina. 1991;34:375–381. [Google Scholar]
- Rietema H. Ecotypic differences between Baltic and North-Sea populations of Delesseria sanguinea and Membranoptera alata. Botanica Marina. 1993;36:15–21. [Google Scholar]
- Rindi F, Mikhailyuk T, Sluiman HJ, Friedl T, Lopez-bautista JM. Phylogenetic relationships in Interfilum and Klebsormidium (Klebsormidiophyceae, Streptophyta) Molecular Phylogenetics and Evolution. 2011;58:218–231. doi: 10.1016/j.ympev.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Ryšánek D, Hrčková K, Škaloud P. Global ubiquity and local endemism of free-living terrestrial protists: phylogeographic assessment of the streptophyte alga Klebsormidium . Environmental Microbiology. 2015;17:689–698. doi: 10.1111/1462-2920.12501. [DOI] [PubMed] [Google Scholar]
- Ryšánek D, Holzinger A, Škaloud P. Influence of substrate and pH on the diversity of the aeroterrestrial alga Klebsormidium (Klebsormidiales, Streptophyta): a potentially important factor for sympatric speciation. Phycologia. 2016;55:347–358. doi: 10.2216/15-110.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škaloud P, Rindi F. Ecological differentiation of cryptic species within an asexual protest morphospecies: a case study of filamentous green alga Klebsormidium (Streptophyta) Journal of Eukaryotic Microbiology. 2013;60:350–362. doi: 10.1111/jeu.12040. [DOI] [PubMed] [Google Scholar]
- Škaloud P, Lukešová A, Malavasi V, Ryšánek D, Hrčková K, Rindi F. Molecular evidence for the polyphyletic origin of low pH adaptation in the genus Klebsormidium (Klebsormidiophyceae, Streptophyta) Plant Ecology and Evolution. 2014;147:333–345. [Google Scholar]
- Starr RC, Zeikus JA. UTEX—the culture collection of algae at the University of Texas at Austin 1993 list of cultures. Journal of Phycology. 1993;29:1–106. [Google Scholar]
- Turesson G. The genotypical response of the plant species to the habitat. Hereditas. 1922;3:211–350. [Google Scholar]
- Webb WL, Newton M, Starr D. Carbon dioxide exchange of Alnus rubra: a mathematical model. Oecologia. 1974;17:281–291. doi: 10.1007/BF00345747. [DOI] [PubMed] [Google Scholar]