Abstract
Recent research into local circuit GABAergic inhibitory interneurons of the mammalian central nervous system has provided unprecedented insight into our understanding of the mechanics of neuronal circuitry and its dysfunction. The recognition that inhibitory interneurons represent a broad array of anatomically and neurochemically diverse cell types suggests that each occupy an equally diverse functional role. Although neurogliaform cells were observed by Cajal over a century ago, our understanding of their functional roles is in its infancy. However, it is rapidly becoming clear that this cell type operates under a distinct repertoire of rules to provide novel forms of inhibitory control of numerous afferent pathways.
Although GABAergic local circuit inhibitory interneurons represent only ~20% of the total cortical cell population their anatomical diversity is unparalleled in the mammalian central nervous system; for example there are currently upwards of 20 acknowledged distinct members within the CA1 hippocampal formation alone1. Their anatomical diversity is rich, with the morphologies of many cell types remaining local to a particular subfield, while other cell types extend wide arbor dendrites and axons that cross numerous cortical and hippocampal layers and subfields. Inhibitory interneurons often demonstrate exquisite targeting of their axons to differential postsynaptic structures. For example, axons can target selective subcellular domains (e.g. the perisomatic, axon initial segment or specific dendritic domains) to compartmentalize or time electrical activity in either a positive or negative manner. Alternatively, axons can make projections several millimeters in length, to innervate thousands of postsynaptic targets to co-ordinate the activity of both homogeneous and distributed neuronal ensembles2,3–5. A comparative newcomer to the interneuron scene is a small distinctive cell that resides primarily within the hippocampal stratum radiatum and lacunosum-moleculare (SLM), and both the superficial and deep layers of the neocortex; commonly referred to as the neurogliaform cell (NGF). The purpose of the present review is to integrate the current literature to highlight the unique properties and roles played by this cell type.
Distinctive morphology of NGF cells
In 1899 Santiago Ramón y Cajal6 wrote of a “short axon cell type” observed in 1 month old human motor cortex tissue.
“Cells with a Short Axon —
B) Dwarf or Neurogliaform Cells. These very small cells with a short axon, which we discovered in the human cerebral cortex are distinguished by a tiny perikaryon, as well as by the thinness and abundance of their radiating dendrites. They are found throughout the cortex, particularly in deeper layers. ………… their polygonal cell body issues a great many thin, varicose, very poorly branched, short dendrites from each of its crests. At first glance, these neurons could be mistaken for astrocytes with short processes if it were not for the absence of lateral outgrowths on the dendrites and the presence of an axon. The latter is very thin, to a point of only staining yellow with silver chromate. Shortly after arising it generates a very dense arborization of delicate, moniliform branches that can be examined only with an apochromatic objective. Sometimes only the arborization is impregnated, with the perikaryon and dendrites remaining invisible; this feature enhances its examination.”
This cell type, which he identified as existing across many cortical areas, he interchangeably referred to as dwarf, spiderweb, arachniform or neurogliaform. Surprisingly, in his subsequent documentation of cells with short axons within the hippocampal formation, Cajal did not describe the NGF cell and it would take several decades before this cell type would be definitively identified in the hippocampus. The seminal publication of Lacaille and Schwartzkroin7 (see figure 3a of that publication) may provide the first image of a neurogliaform-like cell within the hippocampal CA1 SLM. Subsequent work by the groups of Ben-Ari, Buhl, and Capogna clearly established this cell as a major hippocampal inhibitory interneuron subtype8–10.
Figure 3.
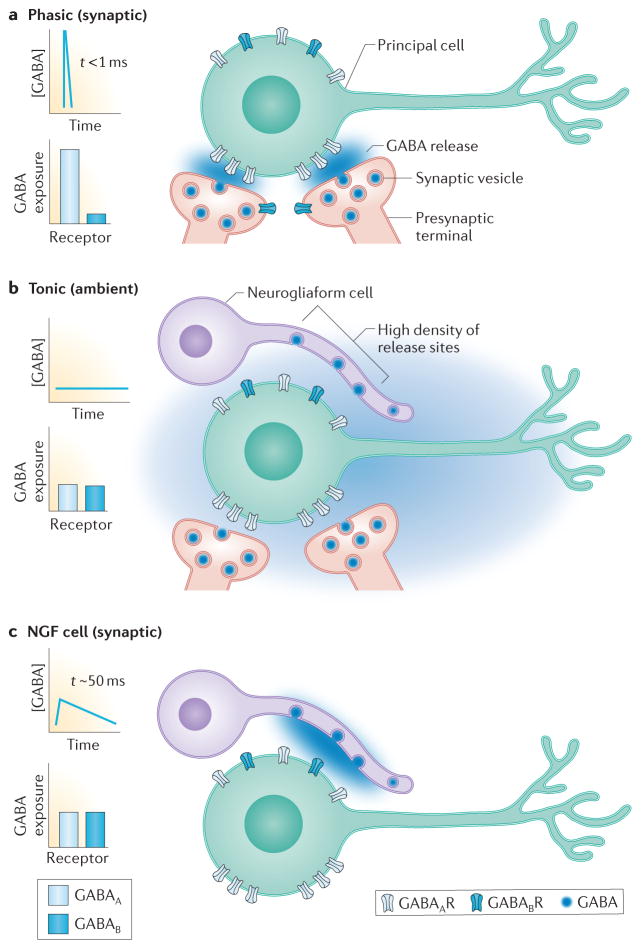
Distinct forms of signaling generate diverse GABA receptor-mediated responses. The concentration profile of GABA at receptors and the relative likelihood that GABAA versus GABAB receptors are exposed to GABA during each mode of transmission is illustrated in the left of each panel A. Phasic or synaptic transmission results from release of GABA-containing vesicles at presynaptic terminals directly opposed to postsynaptic clusters of GABAA receptors. The small volume of the synaptic cleft and the close proximity of receptors to the release site results in a high concentration of GABA (blue shading) that rapidly declines due to diffusion and GABA transport. GABAA and GABAB receptors located outside the synapse are exposed to GABA only during repetitive stimulation or when many closely spaced release sites are synchronously active to generate GABA spillover. B. Tonic activation of GABAA and GABAB receptors results from ambient GABA (illustrated by diffuse blue shading) in the extracellular space. The low level of extracellular GABA is set by activity of GABA transporters and can fluctuate based on surrounding synaptic activity (not shown). Presynaptic GABAB receptors on NGF cells are activated by ambient GABA. C. Synaptic transmission mediated by NGF cells results from release of GABA-containing vesicles from a high density of terminals on NGF cell axons that can release GABA into the extracellular space. Extrasynaptic GABAA and GABAB receptors, as well as presynaptic GABAB receptors, can be exposed to lower concentrations of GABA that persist for tens of milliseconds. This mode of transmission has characteristics of spillover-mediated signaling.
NGF cells represent approximately 10% of the total hippocampal inhibitory interneuron population3. NGF cells of both cortex and hippocampus are immunopositive for neuropeptide Y, reelin, α-actinin-2, COUPTFII and nNOS3, 11–13. However, with the exception of NPY no single marker labels the entire NGF cell population (almost all NGF cells are NPY-positive but not all NPY-positive cells are NGF cells). Unlike the cortex where all NGF cells are derived from the caudal ganglionic eminence (CGE) 11, NGF cells of the hippocampus have their origins within both the CGE and the medial ganglionic eminence (MGE) 13 (Box 1). MGE-derived NOS+ NGF cells share many features in common with another population of MGE-derived hippocampal NPY+ inhibitory interneurons, the so-called Ivy cells, which reside in all layers of the CA1 hippocampus with the exception of the SLM12, 14, 15. Ivy cells represent the largest single population of hippocampal inhibitory interneuron (23% of total inhibitory interneurons)3, and like many NGF cells are both NPY+, COUPTFII+ and nNOS+ but unlike NGF cells do not express reelin. Whether the neocortex harbors a similar Ivy cell population remains an open question. Finally, NPY-negative NGF-cells have been reported in both cortical layer I (termed the elongated NGF cell)16 and striatum17 raising the possibility that there exists a third NGF cell type that has not been previously included in any classification schemes or whose origins have been identified through genetic approaches.
Box 1. Developmental Origins of NGF cells.
Inhibitory interneurons of the neocortex and hippocampal formations are generated in the neurogenic medial and caudal ganglionic eminences (MGE and CGE respectively) of the ventral telencephalon86–88. Although the cortex and hippocampal structures share many of the same rules for interneuron embryogenesis a number of notable exceptions exist13, 14, 89, 90.
The vast majority of neocortical NGFs are reelin-, NPY- and COUPTFII-positive with only a small percentage positive for nNOS11. Neocortical NGF cells have their origins within the CGE91. The initiation and peak production of neocortical NGF cells occurs at E12.5 and E16.5 respectively11,91. In contrast hippocampal NGF cells arise from both the MGE and CGE. Like their neocortical counterparts, NOS-negative NGF cells arise exclusively from the CGE13, 14 between E12.5 and E16.5. In contrast, the vast majority of nNOS-positive NGF cells arise from the MGE, with only a small number of nNOS+ NGF cells originating from the CGE13, 91. MGE-derived hippocampal nNOS+ NGF cells are generated earlier than their CGE counterparts, between E9.5 and E13.5; with the majority of nNOS+ cells (>50%) being generated at ~E13.5.
Neurochemically heterogeneous NGF cells with distinct embryonic and temporal origins suggests a duplication of the NGF cell occurred during evolution, which has given rise to anatomically and functionally similar cell types that either contain or lack nNOS. The observation that cortical and hippocampal CGE-derived NGF cells are nNOS-negative and that both are born and migrate with similar time frames to superficial layers of each structure (the SLM is essentially the layer 1–2 of the hippocampus) suggest that these cells represent a single population of NGF cells. MGE-derived nNOS-positive NGF cells are generated earlier and provide a second distinct population, which tend to migrate to the deeper SLM and are more often found are the border between the SLM and St. radiatum 13 (Figure 1). The presence or absence of nNOS may endow each cell type with a distinct role in spatially coordinating hippocampal haemodynamics with changes in local network activity13,52, 92. Furthermore nNOS can act as a retrograde transmitter suggesting that nNOS containing NGF cells may have a select role in regulating activity in its pre- and postsynaptic partners53.
In the CA1 subfield the somata of NGF cells are confined largely to the SLM, with a smaller distinct population of cells located at the border of SLM that penetrate stratum radiatum (~15% of total NGF cells)10, 13. In CA1 NGF cells possess a small spherical soma of ~10–20mm in diameter. Their dendrites branch repeatedly to form a small dense stellate plexus around the somata, which are often contained within the axon cloud (Figure 1). The dense axonal arborization of NGF cells, which can originate from either the soma or dendritic compartment18, 19 typically remains close to the somato-dendritic profile (Figure 1, Figure 2a). Peter Somogyi and colleagues20 observed that the axonal expansion of a single CA1 SLM NGF was ~ 500 μm in the mediolateral axis and 1200 μm in the septotemporal axis. The axonal arborization has been calculated to approximate 140,000 μm in length3 compared to ~46,000 μm for a typical parvalbumin basket cell21. The axons of CA1 NGF cells can cross the fissure and penetrate the dentate gyrus20. Similarly, axons of NGF cells within the stratum moleculare of the dentate gyrus can penetrate the fissure and cross into the CA1 subfield18. This cross subfield axonal arborization may serve to functionally link the dentate gyrus and CA1. Recent evidence suggests that the ultimate spatial position of migrating cortical NGF cells as well as the elaboration of it axonal arborization is controlled both by the cells intrinsic neuronal activity as well as appropriate inputs from specific afferent projections 22,23.
Figure 1.
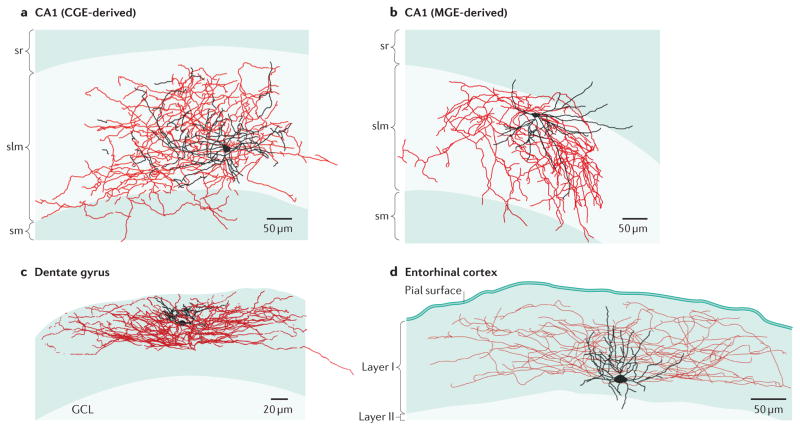
The diversity of neurogliaform (NGF) cell morphology is exemplified by Neurolucida reconstructions of biocytin recoveries from the hippocampal CA1 medial and caudal ganglionic eminence (MGE- and CGE)-derived NGF cells (parts a and b), dentate gyrus (part c) and entorhinal cortex (part d). Soma and dendrites are shown in black, axons are shown in red. Although the different types of neurogliaform cells illustrate a “stereotypic” anatomy, i.e. small cell body and compact dendrites with dense axonal plexus that stay close to the orbit of the somatodendritic compartment, it is clear that there is marked divergence in their overall anatomical profile that is likely dictated by the network in which they are embedded. CA1 NGFs are taken from Tricoire et al 201013. Dentate gyrus NGF cell used with permission of the authors and taken from Armstrong et al 201118. Entorhinal cortex NGF cell taken from Craig and McBain 2015 93
Figure 2.
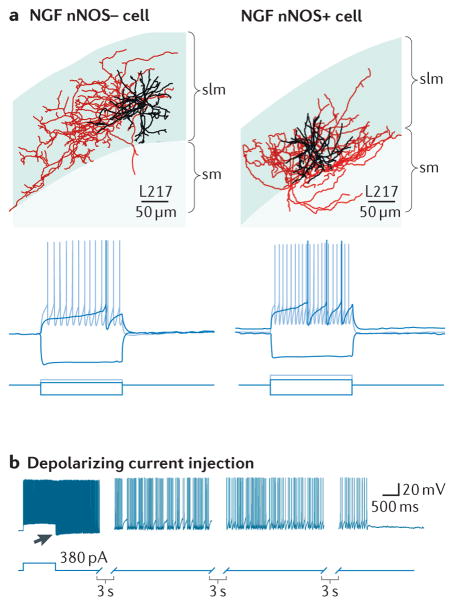
Anatomical and electrophysiological characterization of neuronal nitric oxide synthase (nNOS)-negative and nNOS-positive CA1 hippocampal NGF cells. A. ‘Neurolucida’ reconstructions of biocytin filled nNOS-negative and nNOS-positive NGFs cells (soma and dendrites are shown in black, axons are shown in red; scale bar 60μm). Bottom panels show voltage responses of cell types shown in A to three current step injections (hyperpolarizing, just suprathreshold, and twice the current for just suprathreshold). A prominent delay to first action potential is observed in both NGF cell types (black traces). B. NGF cells display persistent firing triggered by repetitive injections of depolarizing current (only the last current step is shown). Persistent firing consists of a period of high frequency spontaneous action potentials (indicated by arrow) that arise from a hyperpolarized membrane potential that gradually diminishes over the course of tens of seconds. Part A taken from Tricoire et al 201013. Part b used with permission of the authors and taken from Krook-Magnuson et al., 201136.
Despite occupying a relatively small volume the presynaptic bouton density of NGF cells is amongst the highest of all hippocampal interneurons with the average bouton density close to 42 per 100 μm of axon (interbouton separation 2.5 μm)3. For comparison, parvalbumin basket cells and somatostatin-positive OLM cells each have an average bouton density of ~23/100 μm3. Olah et al (2009)24 observed that the density of boutons on a single NGF matched the release site density of 5–6 overlapping basket cell axons. ~92% of hippocampal NGF cell synapses are apposed to pyramidal neurons with the remainder being made onto other inhibitory interneurons and Cajal Retzius cells of the SLM3, 25. One feature of NGF cells that separates them from all other inhibitory interneurons is the observation that the vast majority of synaptic boutons are spatially located at a larger than usual distance from their target dendrites. In somatosensory cortex the separation between NGF axons and the dendrite targets was calculated to be 2.7μm (range 1.1 – 5.0 μm)24. This observation coupled with their dense bouton arrangement has led to the widespread belief that NGF cells are not involved primarily with “point to point” synaptic transmission but release GABA in a target independent, volume- or “cloud-like”- manner to generate a non specific form of inhibitory control (see below).
Intrinsic Physiology of NGF Cells
The intrinsic firing properties of NGF cells differ in many respects from other inhibitory interneuron types and are likely tuned to reflect the unique roles played by these cells in the circuits in which they are embedded. CA1 hippocampal NGF cells possess resting membrane potentials close to −60mV, have relatively low input resistances and fast membrane time constants13. A hallmark feature of NGF cells is a delay to generate action potentials when challenged by a just suprathreshold current injection (Figure 2b)26. This “late spiking” phenotype27 is consistent between nNOS-containing and nNOS-lacking NGF cells. In fact a comparison of numerous intrinsic properties of CA1 nNOS-containing versus nNOS-lacking NGFs revealed no differences between the two cell types13 (Figure 2a,b). This late spiking activity may allow the NGF cell to act as a slow integrator of changes in either membrane potential or incoming activity across many 10s of milliseconds prior to its output of action potential activity.
Action potential amplitude is relatively small, duration is moderate and typically followed by a brief, but large after hyperpolarization (Figure 2b)13. Their firing patterns are largely non-accommodating and often accelerate as the depolarizing pulse proceeds. Of interest, NGF cells of the dentate gyrus molecular layer have more negative resting membrane potentials18. The physiological consequences of this are at present unclear, but negative resting potentials are also a hallmark feature of granule cells (GC), the principal neuron of the dentate gyrus, suggesting a homeostatic balance in regional excitability may exist.
Cells that possess a late spiking phenotype typically demonstrate a slow depolarizing ramping of their just subthreshold voltage trajectory (Figure 2b). This slow depolarization likely indicates an intrinsic voltage conductance with either time dependent activation or inactivation. Although no study to date has characterized voltage gated conductances in targeted NGF cells, interneurons of the SLM possess a complement of outward voltage-gated K+ conductances distinct from other hippocampal inhibitory interneurons28–31. These interneurons lack a prominent transient A-type current and possess a rapidly activating and slowly inactivating “delayed rectifier” current likely formed by Kv3.232, 33.
NGF cells of several cortical and hippocampal regions possess a novel action potential firing profile known as persistent or retroaxonal barrage firing34–37 (Figure 2c). Barrage firing represents a novel form of slow integration that is triggered in response to prolonged action potential activity. This persistent firing is generated within the axon compartment by an as yet unidentified mechanism and once generated can persist for several minutes after the trigger has elapsed. Once initiated NGF cells fire at frequencies ranging from 20 – 130Hz depending on the particular region under study37. Persistent firing is not blocked by antagonists of GABAA and GABAB, AMPA or NMDA receptors34 but its induction can be inhibited (at least in hippocampal NPY-positive Ivy cells) by activation of μ-opioid peptide receptors36, which act to either hyperpolarize the NGF or to inhibit the locally gap junction connected NGF network. Importantly, persistent firing, which likely represents a mechanism to provide a global “brake” on local excitability, occurs in NGF cells both in vitro and in vivo although it appears to occur with less frequency in the latter and to require a more prolonged barrage of action potential activity for its initiation in neocortical NGF cells in vivo37. As discussed below the inhibitory synaptic output of NGF cells rapidly declines during sustained trains of presynaptic activity, therefore its unclear exactly how much inhibition is being provided to postsynaptic targets during NGF cell barrage firing.
Afferent input onto NGF cells
A quantitative assessment of CA1 local circuitry has calculated that inhibitory interneurons within the CA1 subfield receive ~ 5–10% of all available glutamatergic synaptic inputs38. Based on these observations a “hypothetical” interneuron of the CA1 subfield would typically receive between ~8000–17000 Schaffer collateral inputs and an additional 1300 entorhinal inputs3 with the ratios of these inputs largely dictated by the anatomical location of the cell’s soma and dendrites.
The position of NGF cells within the CA1 hippocampal SLM largely overlaps with glutamatergic afferents arriving from the entorhinal cortex and thalamus, and suggests that they probably receive minimal Schaffer collateral input from the CA3 subfield. However, the small population of NGF cells with cell bodies close to the SLM-radiatum border and whose dendrites extend into stratum radiatum likely receive additional excitatory input via Schaffer collateral inputs. Although NGF cells receive glutamatergic synaptic input via both AMPA- and NMDA-preferring receptors8, 18, surprisingly little is known about the biophysical properties of these inputs. In early recordings NGF cells of the CA1 subfield were shown to receive excitatory input from both entorhinal- and Schaffer collateral inputs8 that demonstrated either short-term depression of synaptic transmission or initial facilitation followed by depression of transmission8. NGF cells of the dentate gyrus molecular layer receive AMPA receptor mediated excitatory input via the perforant path that shows paired pulse facilitation. Although evidence points to NGF cells expressing both AMPA and NMDA receptor synaptic receptors, no information exists about the relative contributions of these receptors. Schaffer collateral inputs onto the closely related MGE-derived NPY-positive, Ivy cell reveals an AMPA:NMDA ratio of ~5:139. NMDA receptor mediated synaptic currents are a minor conductance on these and other MGE-derived inhibitory interneurons compared to Schaffer collateral inputs onto CGE-derived interneurons. Whether this MGE-versus CGE-dependent segregation of AMPA:NMDA ratios is also mirrored in entorhinal inputs onto different NGF cells remains to be determined.
One intriguing aspect of excitatory input onto NGF cells was recently demonstrated by Quattrocolo and Maccaferri (2014)40 who revealed a monosynaptic glutamatergic input onto NGF cells originating from Cajal-Retzius cells located within the CA1 SLM. Optogenetically driven glutamate release from Cajal-Retzius cells activated both AMPA- and NMDA-receptor mediated synaptic currents in NGF cells. The functional role of this connection is at present unclear but given the essential role for Cajal-Retzius cells in cellular and dendritic development one could speculate that this connection has a role in structural and functional development and plasticity of NGF cells.
Early studies demonstrated that stimulation of almost all hippocampal CA1 subfields could trigger monosynaptic GABAA and GABAB receptor mediated inhibitory input onto NGFs9, 41. Paired pulse stimulation revealed these inhibitory inputs to possess relatively slow time constants of decay (~40msec) and to be largely depressing in nature. Paired recordings between hippocampal NGF cells revealed that the vast majority of cells were coupled through both electrical and chemical synapses8. Recordings in human and rodents also revealed that cortical NGF cells receive monosynaptic GABAA and GABAB receptor mediated inhibitory input from other NGF cells42. These monosynaptic events were longer lasting; rise times and half widths were ~6msec and 45msec respectively. Blockade of GABAB receptors reduced the response half width by ~20% underscoring a role for both GABAA and GABAB receptors. The observation that hippocampal NGF cells possess spontaneous inhibitory postsynaptic currents (IPSCs) with a variety of time courses suggested that other sources of inhibitory input onto NGF cells must also exist43.
Output of NGF cells
There are two well established modes of GABAAR-mediated inhibition with phasic (or synaptic) signaling referring to conventional point-to-point transmission whereas tonic signaling results from activation of receptors by ambient GABA in the extracellular space. The spatiotemporal concentration profiles of transmitter mediating these two signaling processes have distinct consequences for receptor-mediated currents, with the high and brief GABA transient in the synaptic cleft (> 1 mM for < 0.5 ms) driving rapid receptor activation and deactivation, while persistent low concentrations of GABA favors a gradual equilibration of receptors between desensitized and open states44 (Figure 3a and b) Interestingly, NGF cells mediate a third form of GABAergic transmission intermediate between phasic and tonic signaling, which in CA1 has been referred to as GABAA,slow26. In contrast to the rapid IPSCs produced at typical synapses made by perisomatic-projecting interneurons such as basket cells, NGF cells generate slower IPSCs that result from prolonged GABA transients with a low peak concentration43, 45, 46. The unusual GABA concentration transient likely results from the densely spaced NGF cell presynaptic terminals, some without postsynaptic specializations, that allow GABA to reach both synaptic and non-synaptic receptors located at a distance from release sites, in a form of volume transmission24 (Figure 3c). Thus synaptic currents evoked by NGF cells exhibit hallmarks of spillover signaling that reflect the action of GABA outside the synapse 43, 46, 47.
What are the functional consequences of volume transmission from NGF cells? First, the low and prolonged GABA transient favors postsynaptic receptor desensitization, resulting in use-dependent synaptic depression as receptors accumulate in desensitized states43. Second, in contrast to other interneuron subtypes that precisely target distinct subcellular domains, inhibition mediated by NGF cells generally lacks target cell and synaptic specificity. That is, the cloud of GABA released from NGF cells can act on GABAA receptors located on any nearby neuronal element, including the releasing cell itself, potentially producing suppression of neural activity in a widespread area dictated by their dense NGF cell axonal arbor24, 43 (Figure 3). Third, NGF cells provide a major source of slow GABAB receptor-mediated inhibition. Whereas other interneuron subtypes require high frequency stimulation to engage pre- and postsynaptic GABAB receptors that are typically located outside the synapse48, 49, single action potentials in NGF cells can activate postsynaptic GABAB receptor-mediated inhibition as well as presynaptic GABAB receptors that mediate homosynaptic and heterosynaptic depression8, 18, 24, 42, 50. These characteristics of synaptic signaling sharply contrast with transmission from other interneuron subtypes that exhibit strong temporal and spatial selectivity. The unexpected specificity of presynaptic GABAB receptor activation by NGF cells in the barrel cortex51 however highlights the need for additional understanding of synaptic transmission mediated by NGF cells and its role in network activity that appears more complex than expected.
An additional topic ripe for exploration is the role of other neuroactive peptides, such as NPY, reelin, nNOS and insulin, in NGF cell function. Notably, NGF cells and Ivy cells comprise a major fraction of nNOS-expressing interneurons and NOS is a well-known retrograde modulator of synaptic transmission as well as a mediator of neurovascular coupling19,52. Physiologically-relevant firing patterns induces release of NO from NGF cells that inhibits GABA release from impinging NGF cell terminals, in a form of retrograde signaling reminiscent of depolarization-induced suppression of inhibition (DSI) mediated by endocannabinoids53. NO release is induced by back propagating action potentials that trigger L-type Ca2+ transients in NGF cell dendrites and the resultant suppression of inhibition is sufficient to transiently enhance excitatory integration of EPSPs. NGF cells in the cortex are also a major source of insulin. Insulin released by NGF cells in response to locally applied glucose can mimic the effects on synaptic activity induced by bath applied insulin, suggesting that NGF cells might contribute to endogenous insulin signaling54. In contrast to NO release, however, insulin does not appear to be released by NGF cell spiking. A greater understanding of the signaling mechanisms mediated by NGF cells is needed to realize their specific roles in network activity.
NGF cells, network activity and oscillations
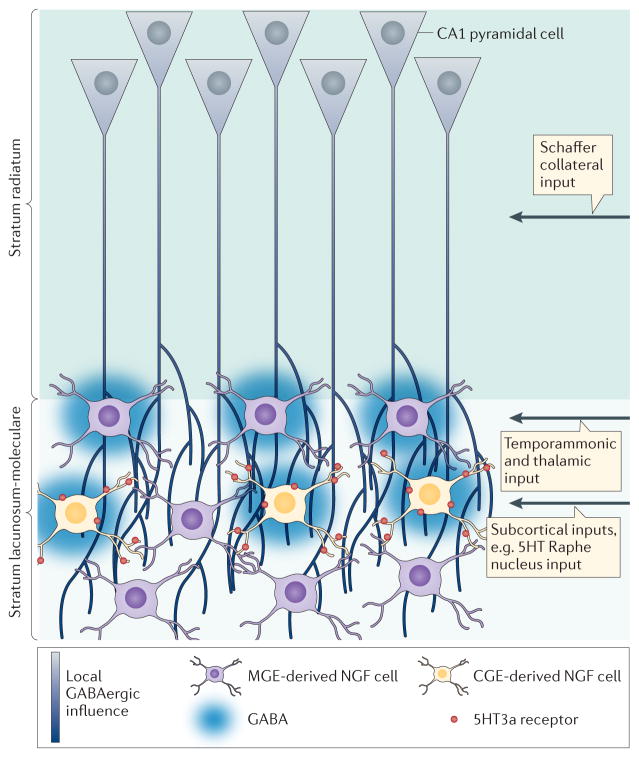
In CA1 SLM apical tufts of CA1 pyramidal cells are innervated by perforant path afferents that originate in layer III of the entorhinal cortex (also called the temporoammonic pathway; TA). The long electrotonic distance to the soma means that TA inputs are relatively weak but can generate dendritic spikes that drive CA1 spiking when facilitated by Schaffer collateral inputs55. Localization in the SLM predicts that NGF cells provide feed-forward inhibition that restricts the time window for integration of TA inputs as well as suppresses TA-induced dendritic spiking (Figure 4). Desensitization of postsynaptic GABAA receptors during repetitive stimuli, in concert with prominent presynaptic GABAB-mediated and NO-induced auto-inhibition of GABA release, results in an activity-dependent suppression of NGF feed-forward inhibition43, 50,53. Together these mechanisms for dynamic suppression of dendritic feedforward inhibition, which are prominent at theta frequency (4–10Hz), are likely to enhance integration of TA excitatory inputs. Interestingly, the lack of GABAB receptors at TA terminals will provide a measure of synapse specificity of spillover-mediated suppression of GABA (but not glutamate) release50, 56. Alternatively, CGE-derived NGF cells (but not MGE-derived NGF cells) express the serotonergic ionotropic 5HT3a receptor57, 58, and likely receive excitatory input from serotonin fibers originating in the Raphe Nucleus. Activation of 5HT3a receptors on nNOS-negative NGF cells may trigger a widespread inhibition of the distal dendrites of CA1 pyramidal cells to shunt activity arriving via the temporoammonic pathway of the entorhinal cortex. This localized inhibition may serve to allow preferential activation of CA1 pyramidal cells via their Schaffer collateral inputs from CA3 (Figure 4). However this scenario is likely an oversimplification as serotonergic raphe fibers also release glutamate and innervate other interneurons59, potentially leading to more complex outcomes that require further investigation.
Figure 4.
Two examples of how CA1 Stratum lucidum NGF cells may act as a global gate for afferent information flow. In hippocampus both medial ganglionic eminence (MGE)- and caudal ganglionic eminence (CGE)-derived NGF cells are mainly localized to the stratum lacunosum-moleculare (SLM) or at the SLM-radiatum border. Both cell types act to provide local feed-forward inhibition onto CA1 pyramidal cells (blue) that will modulate the influence of both temporoammonic and thalamic inputs. Alternatively, both MGE-derived NGF cells and CGE-derived NGF cells, which are also rich in ionotropic 5HT3a receptors, may be activated by glutamate and serotonin co-released from subcortical fibers originating in the Raphe Nucleus. Such activation may provide a widespread shunt of activity onto the distal dendrites of CA1 pyramidal cells by virtue of NGF cells inhibitory volume transmission. Shunting excitatory input of temporoammonic inputs will allow input from the CA3 Schaffer collaterals to dominate excitation of CA1 pyramids.
Another example of functional specificity of presynaptic inhibition mediated by NGF cells is evident in the somatosensory cortex, where temporal precision of sensory responses in layer IV is maintained by strong thalamic recruitment of feedforward inhibition from PV+ interneurons. This feed-forward inhibition is suppressed by layer IV NGF cells that generate spillover-mediated activation of GABAB receptors on PV+ terminals51, in a typical example of presynaptic silencing by NGF volume transmission24, 50. Despite expression of functional GABAB receptors on thalamic axon terminals, however, GABA release from NGF cells does not affect thalamic glutamate release51. This specificity of GABAB mediated presynaptic inhibition is surprising, since heterosynaptic depression of glutamate release from cortical terminals provided important evidence for the spatially nonselective nature of NGF volume transmission24. Results from the barrel cortex51 suggest that mechanisms to promote synaptic or spatial specificity of signaling by NGF cells could endow them with unexpected roles in fine-tuning circuit function. Specificity of glutamatergic and cholinergic input to NGF cells in the somatosensory cortex is also likely to refine their involvement in circuit functions 60,61.
NGF cells form prolific chemical and electrical synapses with other interneuron subtypes, suggesting that regulation of inhibitory circuits may be an important circuit function. Unlike most interneuron subtypes that are electrically coupled primarily with other cells of the same subtype, heterologous coupling with distinct interneuron subtypes suggests that NGF cells are poised to monitor network activity by non-synaptic communication 62,63. Interestingly, Maccaferri and colleagues showed that synaptic GABAergic potentials can also propagate via gap junctions between interneurons, including NGF cells, in SLM 64. Propagation of depolarizing GABA potentials via gap junctions contributed to oscillatory network activity generated by the potassium channel blocker 4-AP, suggesting a combination of synaptic and electrical signaling provides a mechanism for synchronizing interneuron networks containing NGF cells.
Work by Pearce and colleagues suggested that purely synaptic interactions between hippocampal interneurons that generate slow IPSCs (termed GABAA,slow) and interneurons that generate IPSCs with fast rise and decay phases (termed GABAA,fast) could contribute to oscillatory activity. Initial studies determined that these two kinetic classes of IPSCs were mediated by distinct interneuron subtypes, with GABAA,slow arising from NGF cells26. In slice preparations, most spontaneous IPSCs reflect activity of GABAA,fast interneurons, such as perisomatic projecting basket and axo-axonic cells. Banks et al., (2000)65 showed that activation of GABAA,slow suppresses the rate and amplitude of spontaneous IPSCs in CA1 pyramidal cells for hundreds of milliseconds. Thus NGF cells are poised to regulate the firing patterns of other interneurons. Interestingly, GABAA,slow-induced suppression of GABAA,fast recovered with a time constant in the theta frequency, leading these authors to postulate that GABAA,slow provides an intrinsic hippocampal mechanism for theta-frequency modulation of gamma oscillations66.
Networks of inhibitory interneurons generate oscillations that provide temporal and spatial organization of principal cell activity4. Local circuits of connected interneurons with strong and fast synaptic connections, like PV+ fast-spiking cells, are thought to generate the gamma-frequency oscillations exhibited in many brain regions67, however, the source and mechanisms underlying theta oscillations has been debated. In hippocampus, theta modulation of perforant path EPSCs in vivo supports the idea that theta activity is mainly relayed from the cortex via excitation from the entorhinal cortex68. Consistent with this idea, theta oscillations are strongest in the TA termination zone in the SLM. But, the ability to generate theta oscillations under various pharmacological conditions in isolated brain slices has also pointed to the existence of intrinsic mechanisms. White et al, (2000)66 used network modeling to suggest that interactions between networks of GABAA,slow and GABAA,fast interneurons were sufficient to generate mixed gamma and theta rhythms, an idea also supported by the striking dependence of theta on functional inhibition of PV+ interneurons69. Since NGF cells are a major source of IPSCs with slow kinetics, these results suggest that they could contribute to theta rhythms as well as theta frequency modulation of gamma oscillations via synaptic interactions with other interneuron subtypes26, 70. However, additional studies are needed to understand the specific contribution of NGF cells to oscillatory behavior in isolated and intact preparations.
Activity patterns recorded in vivo during exploratory behavior suggest how complex interactions between interneurons, cortical and septal inputs and modulators generate the temporal organization of hippocampal neuronal activity during theta oscillations70. In vivo, NGFs and the related Ivy cells are slow-spiking interneurons with broad action potentials. NGFs fire at low frequency during the peak of theta whereas Ivy cells fire at the trough of each oscillation, consistent with afferent input arising from the TA and Schaffer collaterals, respectively12, 20. Whereas PV+ interneurons dynamically modulate firing patterns during movement, sleep and transitions between behavioral states, Ivy/NGFs maintain similar low rates and patterns across behavioral states and oscillatory patterns, suggesting highly distinct roles in structuring network activity71.
Less is known about the role of interneuron subtypes in the activity patterns of the dentate gyrus, mainly due to the relative paucity of in vivo recordings from this region that exhibits unusually sparse population coding. Many in vitro and modeling studies have indicated, however, that the dentate gyrus exhibits particularly strong inhibitory circuitry that is important for maintaining activation of only small subsets of granule cells (GCs). Precisely how NGF cells contribute to sparse dentate GC activation is unclear, but their unique properties suggest NGF cells complement the role of other interneuron subtypes. For example, perforant path afferents preferentially recruit fast-spiking basket cells over GCs due to higher excitatory current densities, triggering strong feed-forward inhibition72 (Figure 5a). This scenario could be similar for NGFs that receive robust perforant path input across their small somatodendritic domains18. Basket cells, however, also receive feedback excitation that promotes frequency-dependent burst firing during repetitive perforant path stimulation72, 73 whereas NGF cells in the molecular layer are unlikely to participate in feedback inhibition. Furthermore, prolific chemical and electrical synapses with other interneuron subtypes, as well as the ability to generate GABAB-mediated inhibition, suggest NGF cells have complex interactions that contribute to network functions18. In fact, stimulation in the molecular layer generates slow inhibition of perisomatic-projecting interneurons and prolonged suppression of spontaneous IPSCs in mature GCs47 (Figure 5b), similar to the interactions between GABAA,slow and GABAA,fast circuits previously described in CA165. Thus NGF cells across hippocampal subregions may have similar network functions.
Figure 5.
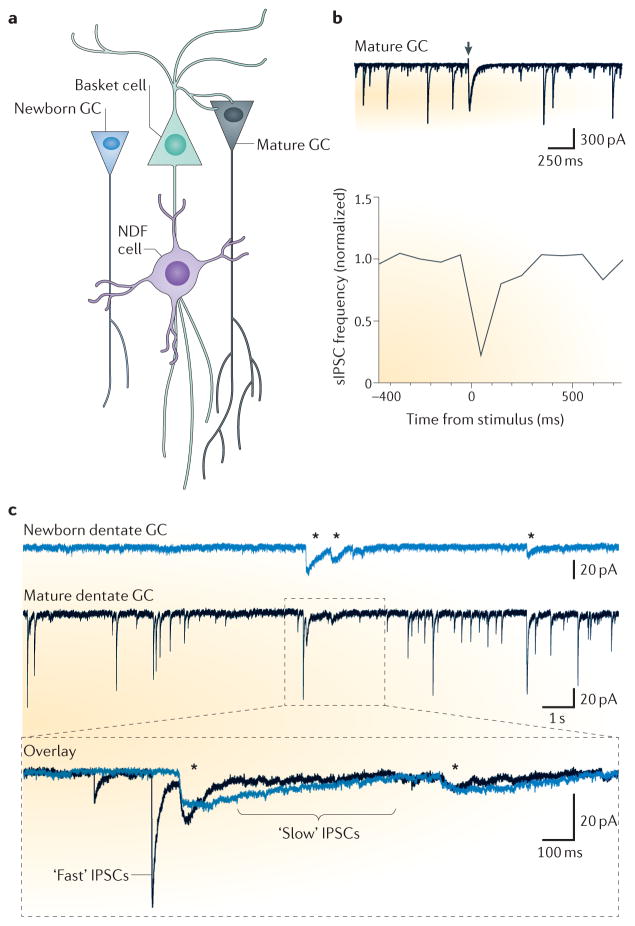
NGF cells coordinate activity in the neurogenic adult dentate gyrus. A. Schematic diagram showing the synaptic connectivity between NGF cells and dentate granule cells (GCs). GABA released from NGF cell terminals provides inhibition to other interneurons (such as basket cells) and mature GCs, and provides simultaneous depolarization of newborn GCs due to their level of intracellular Cl−. B, Stimulation in the molecular layer (top panel, arrowhead) generates a slow IPSC in mature GCs (and in basket cells, not shown) that is associated with a reduction in the frequency of fast spontaneous IPSCs recorded in mature GCs (lower panel). This suggests that NGF cells inhibit perisomatic-projecting interneurons like basket cells. C. Simultaneous recordings of spontaneous GABAergic synaptic activity in a newborn dentate GC and neighboring mature dentate GC. Mature GCs exhibit IPSCs with both fast and slow time courses that represent synaptic input from a variety of interneurons whereas newborn GCs exhibit IPSCs with only slow kinetics (asterisks). The expanded view illustrates that slow IPSCs in the newborn GC showed a high coincidence with slow, but not fast, sIPSCs in the neigbouring mature GC, suggesting that both arise from a common presynaptic interneuron with properties of NGF cells. (Modified with permission from ref 80).
A role for NGF cells in adult born neuron circuit integration
High levels of intracellular chloride in progenitor cells and immature neurons leads to GABAA receptor-mediated depolarization, enabling GABAergic activity to trigger Ca2+-dependent signaling cascades essential for proliferation, survival and growth. GABAergic signaling therefore has trophic functions in the embryonic brain as well as in adult neurogenic regions74, 75. The early appearance of NGF cells in developing cortical circuits and their target-independent mechanism of signaling makes NGF cells attractive candidates for providing trophic GABA signaling to developing principal neurons. Other signaling factors expressed by NGF cells including reelin, NOS and NPY are also known to participate in circuit formation.
The role of GABA mediated depolarization in neural maturation has been studied extensively in the context of hippocampal adult neurogenesis, where resident stem cells continually produce new dentate GCs that integrate into the preexisting circuit and acquire mature physiological characteristics over the course of many weeks. Pivotal early studies showed that impairing GABA mediated depolarization by knocking down the sodium-potassium-chloride exchanger NKCC1 that maintains a high intracellular [Cl−] in immature neurons dramatically impairs subsequent maturation and survival76, 77. The robust consequences of manipulating GABAergic signaling on adult neurogenesis raised the question of whether specific interneuron subtypes have preferential control over neural development 75. This idea was suggested by infrequent GABAergic postsynaptic currents with slow rise and decay phases in newborn neurons, whereas mature neurons display numerous IPSCs with heterogeneous fast and slow kinetics78, 79. Markwardt et al, (2009)80 showed that GABAergic postsynaptic currents in new adult born neurons exhibited characteristics of signaling from NGF cells, including high sensitivity to blockade of GABA transporters and to low affinity GABAA receptor antagonists. Innervation by NGF cells is also consistent with slow spontaneous synaptic currents in newborn GCs that are correlated with slow, but not fast, sIPSCs in simultaneous recordings of neighboring mature GCs (Figure 5c)80. Yet evoking synaptic currents using focal stimulation made it difficult to unambiguously differentiate signaling from NGF cells from non-specific GABA spillover from nearby basket cell terminals, hence, subsequent paired recordings confirmed that stimulation of single NGF is sufficient to generate slow GABA postsynaptic currents in newborn GCs47. These results suggest a local circuit in which newborn GCs are initially responsive primarily to GABA release from NGF cells with subsequent innervation from other interneurons developing during the course of maturation75.
GABAergic signaling by NGF cells constitutes a form of volume transmission that potentially activates perisynaptic or extrasynaptic GABAA and GABAB receptors, both of which have been implicated in regulation of progenitor proliferation prior to synapse formation75, 81. This form of transmission may be optimized for trophic signaling to cells in a dynamic period of dendrite motility and outgrowth prior to synapse stabilization, in a mechanism of signaling that could also be accomplished by spillover from densely packed conventional synapses for other interneuron subtypes82, 83. In addition to promoting depolarization-induced Ca2+ influx needed for regulation of proliferation and growth, Chancey et al (2013)84 demonstrated that GABAergic depolarization directly contributes to excitatory synaptogenesis of adult born neurons. Newborn neurons at an early stage of maturation have glutamatergic transmission mediated solely by NMDAR-only containing (silent) synapses that incorporate AMPA receptors in response to neural activity. Both NMDAR activation and GABA-mediated depolarization that allows relief from voltage-dependent block of NMDARs is required for this initial synapse unsilencing84. Hence there is a need for coordinated GABA and glutamate-mediated transmission. Initial silent synapses on adult born neurons arise from glutamatergic hilar mossy cells that comprise the associational/commissural pathway. Selective optogenetic activation of hilar mossy cell axons generates not only NMDAR EPSCs, but also di-synaptic depolarizing GABA currents85. Although further studies are required to determine whether di-synaptic GABA release arises from NGF cells, the slow time course comparable to NMDAR EPSCs supports that possibility. Together these results suggest that NGF cells contribute to activity-dependent regulation of neuronal maturation as well as early synaptogenesis during circuit assembly.
Conclusions
Once a cell type largely unexplored, NGF cells are emerging as an interneuron class capable of governing many diverse processes from circuit development to sculpting the activity of large neuronal ensembles. Their unique anatomy coupled to a hybrid phasic/tonic mode of GABAergic signaling mechanisms endows NGF cells with mechanisms for widespread local inhibition as well as a surprising precision in some cases. The presence of a variety of neuromodulators such as nNOS and reelin also suggest they they can exert a broad influence over their circuits independently from simple traditional GABAergic synaptic mechanisms.
In a recent review of fast spiking parvalbumin (PV) interneurons Peter Jonas and colleagues5, pointed out that the full court press of 20 years of research into PV cells has shown us that we can “close the gaps between the molecular, cellular, network and behavioral levels…and that these results may form the basis for PV interneurons as therapeutic targets”. That such an armament of information has been amassed regarding the PV basket cell is both a satisfying and rewarding accomplishment for the field. That a similar amount of insight could be obtained for all other inhibitory interneuron subtypes is a truly head spinning but realistic proposition and the advent of modern molecular, genetic, physiological and optogenetic approaches makes this tenable within the next decade. Although we consider it a worthwhile pursuit to apply similar research pressure to all inhibitory interneurons, we particularly feel this to be the case for neurogliaform cells. It is time for the field to turn its attention to this deserving cell type and be drawn into its spiderweb.
References
- 1.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–7. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 3.Bezaire MJ, Soltesz I. Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus. 2013;23:751–85. doi: 10.1002/hipo.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roux L, Buzsaki G. Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology. 2014;88:10–23. doi: 10.1016/j.neuropharm.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345:1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- 6.Ramon y Cajal S. Histology of the Nervous System. Oxford University Press; 1995. [Google Scholar]
- 7.Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988;8:1411–24. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price CJ, et al. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25:6775–86. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khazipov R, Congar P, Ben-Ari Y. Hippocampal CA1 lacunosum-moleculare interneurons: modulation of monosynaptic GABAergic IPSCs by presynaptic GABAB receptors. J Neurophysiol. 1995;74:2126–37. doi: 10.1152/jn.1995.74.5.2126. [DOI] [PubMed] [Google Scholar]
- 10.Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol. 1998;506( Pt 3):755–73. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyoshi G, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–94. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuentealba P, et al. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–29. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricoire L, et al. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30:2165–76. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricoire L, et al. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31:10948–70. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somogyi J, Szabo A, Somogyi P, Lamsa K. Molecular analysis of ivy cells of the hippocampal CA1 stratum radiatum using spectral identification of immunofluorophores. Front Neural Circuits. 2012;6:35. doi: 10.3389/fncir.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–8. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz-Manchado AB, et al. Novel Striatal GABAergic Interneuron Populations Labeled in the 5HT3aEGFP Mouse. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong C, Szabadics J, Tamas G, Soltesz I. Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward gamma-aminobutyric acidergic modulators of entorhinal-hippocampal interplay. J Comp Neurol. 2011;519:1476–91. doi: 10.1002/cne.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong C, Krook-Magnuson E, Soltesz I. Neurogliaform and Ivy Cells: A Major Family of nNOS Expressing GABAergic Neurons. Front Neural Circuits. 2012;6:23. doi: 10.3389/fncir.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuentealba P, et al. Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci. 2010;30:1595–609. doi: 10.1523/JNEUROSCI.4199-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–65. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Marco Garcia NV, Karayannis T, Fishell G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011;472:351–5. doi: 10.1038/nature09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Marco Garcia NV, Priya R, Tuncdemir SN, Fishell G, Karayannis T. Sensory inputs control the integration of neurogliaform interneurons into cortical circuits. Nat Neurosci. 2015;18:393–401. doi: 10.1038/nn.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olah S, et al. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–81. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quattrocolo G, Maccaferri G. Novel GABAergic circuits mediating excitation/inhibition of Cajal-Retzius cells in the developing hippocampus. J Neurosci. 2013;33:5486–98. doi: 10.1523/JNEUROSCI.5680-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capogna M, Pearce RA. GABA A, slow: causes and consequences. Trends Neurosci. 2011;34:101–12. doi: 10.1016/j.tins.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995;15:2638–55. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chikwendu A, McBain CJ. Two temporally overlapping “delayed-rectifiers” determine the voltage-dependent potassium current phenotype in cultured hippocampal interneurons. J Neurophysiol. 1996;76:1477–90. doi: 10.1152/jn.1996.76.3.1477. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, McBain CJ. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J Physiol. 1995;488( Pt 3):661–72. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, McBain CJ. Voltage-gated potassium currents in stratum oriensalveus inhibitory neurones of the rat CA1 hippocampus. J Physiol. 1995;488( Pt 3):647–60. doi: 10.1113/jphysiol.1995.sp020997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morin F, Haufler D, Skinner FK, Lacaille JC. Characterization of voltage-gated K+ currents contributing to subthreshold membrane potential oscillations in hippocampal CA1 interneurons. J Neurophysiol. 2010;103:3472–89. doi: 10.1152/jn.00848.2009. [DOI] [PubMed] [Google Scholar]
- 32.Atzori M, et al. H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking. Nat Neurosci. 2000;3:791–8. doi: 10.1038/77693. [DOI] [PubMed] [Google Scholar]
- 33.Weiser M, et al. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949–72. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheffield ME, Best TK, Mensh BD, Kath WL, Spruston N. Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. Nat Neurosci. 2011;14:200–7. doi: 10.1038/nn.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheffield ME, et al. Mechanisms of retroaxonal barrage firing in hippocampal interneurons. J Physiol. 2013;591:4793–805. doi: 10.1113/jphysiol.2013.258418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krook-Magnuson E, Luu L, Lee SH, Varga C, Soltesz I. Ivy and neurogliaform interneurons are a major target of mu-opioid receptor modulation. J Neurosci. 2011;31:14861–70. doi: 10.1523/JNEUROSCI.2269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki N, Tang CS, Bekkers JM. Persistent barrage firing in cortical interneurons can be induced in vivo and may be important for the suppression of epileptiform activity. Front Cell Neurosci. 2014;8:76. doi: 10.3389/fncel.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takacs VT, Klausberger T, Somogyi P, Freund TF, Gulyas AI. Extrinsic and local glutamatergic inputs of the rat hippocampal CA1 area differentially innervate pyramidal cells and interneurons. Hippocampus. 2012;22:1379–91. doi: 10.1002/hipo.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matta JA, et al. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci. 2013;16:1032–41. doi: 10.1038/nn.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quattrocolo G, Maccaferri G. Optogenetic activation of cajal-retzius cells reveals their glutamatergic output and a novel feedforward circuit in the developing mouse hippocampus. J Neurosci. 2014;34:13018–32. doi: 10.1523/JNEUROSCI.1407-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams S, Samulack DD, Beaulieu C, LaCaille JC. Membrane properties and synaptic responses of interneurons located near the stratum lacunosum-moleculare/ radiatum border of area CA1 in whole-cell recordings from rat hippocampal slices. J Neurophysiol. 1994;71:2217–35. doi: 10.1152/jn.1994.71.6.2217. [DOI] [PubMed] [Google Scholar]
- 42.Olah S, et al. Output of neurogliaform cells to various neuron types in the human and rat cerebral cortex. Front Neural Circuits. 2007;1:4. doi: 10.3389/neuro.04.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karayannis T, et al. Slow GABA transient and receptor desensitization shape synaptic responses evoked by hippocampal neurogliaform cells. J Neurosci. 2010;30:9898–909. doi: 10.1523/JNEUROSCI.5883-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overstreet LS, Jones MV, Westbrook GL. Slow desensitization regulates the availability of synaptic GABA(A) receptors. J Neurosci. 2000;20:7914–21. doi: 10.1523/JNEUROSCI.20-21-07914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–5. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 46.Szabadics J, Tamas G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci U S A. 2007;104:14831–6. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markwardt SJ, Dieni CV, Wadiche JI, Overstreet-Wadiche L. Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat Neurosci. 2011;14:1407–9. doi: 10.1038/nn.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–75. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 49.Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–81. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 50.Price CJ, Scott R, Rusakov DA, Capogna M. GABA(B) receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J Neurosci. 2008;28:6974–82. doi: 10.1523/JNEUROSCI.4673-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chittajallu R, Pelkey KA, McBain CJ. Neurogliaform cells dynamically regulate somatosensory integration via synapse-specific modulation. Nat Neurosci. 2013;16:13–5. doi: 10.1038/nn.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cauli B, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–9. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li G, Stewart R, Canepari M, Capogna M. Firing of hippocampal neurogliaform cells induces suppression of synaptic inhibition. J Neurosci. 2014;34:1280–92. doi: 10.1523/JNEUROSCI.3046-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molnar G, et al. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci. 2014;34:1133–7. doi: 10.1523/JNEUROSCI.4082-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci. 2005;8:1667–76. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- 56.Colbert CM, Levy WB. Electrophysiological and pharmacological characterization of perforant path synapses in CA1: mediation by glutamate receptors. J Neurophysiol. 1992;68:1–8. doi: 10.1152/jn.1992.68.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varga V, et al. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–53. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- 60.Wozny C, Williams SR. Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb Cortex. 2011;21:1818–26. doi: 10.1093/cercor/bhq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brombas A, Fletcher LN, Williams SR. Activity-dependent modulation of layer 1 inhibitory neocortical circuits by acetylcholine. J Neurosci. 2014;34:1932–41. doi: 10.1523/JNEUROSCI.4470-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon A, Olah S, Molnar G, Szabadics J, Tamas G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci. 2005;25:6278–85. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zsiros V, Maccaferri G. Electrical coupling between interneurons with different excitable properties in the stratum lacunosum-moleculare of the juvenile CA1 rat hippocampus. J Neurosci. 2005;25:8686–95. doi: 10.1523/JNEUROSCI.2810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zsiros V, Aradi I, Maccaferri G. Propagation of postsynaptic currents and potentials via gap junctions in GABAergic networks of the rat hippocampus. J Physiol. 2007;578:527–44. doi: 10.1113/jphysiol.2006.123463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banks MI, White JA, Pearce RA. Interactions between distinct GABA(A) circuits in hippocampus. Neuron. 2000;25:449–57. doi: 10.1016/s0896-6273(00)80907-1. [DOI] [PubMed] [Google Scholar]
- 66.White JA, Banks MI, Pearce RA, Kopell NJ. Networks of interneurons with fast and slow gamma-aminobutyric acid type A (GABAA) kinetics provide substrate for mixed gamma-theta rhythm. Proc Natl Acad Sci U S A. 2000;97:8128–33. doi: 10.1073/pnas.100124097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 68.Pernia-Andrade AJ, Jonas P. Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron. 2014;81:140–52. doi: 10.1016/j.neuron.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wulff P, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–6. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cutsuridis V, Hasselmo M. GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations. Hippocampus. 2012;22:1597–621. doi: 10.1002/hipo.21002. [DOI] [PubMed] [Google Scholar]
- 71.Lapray D, et al. Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci. 2012;15:1265–71. doi: 10.1038/nn.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ewell LA, Jones MV. Frequency-tuned distribution of inhibition in the dentate gyrus. J Neurosci. 2010;30:12597–607. doi: 10.1523/JNEUROSCI.1854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sambandan S, Sauer JF, Vida I, Bartos M. Associative plasticity at excitatory synapses facilitates recruitment of fast-spiking interneurons in the dentate gyrus. J Neurosci. 2010;30:11826–37. doi: 10.1523/JNEUROSCI.2012-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–84. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 75.Dieni CV, Chancey JH, Overstreet-Wadiche LS. Dynamic functions of GABA signaling during granule cell maturation. Front Neural Circuits. 2012;6:113. doi: 10.3389/fncir.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jagasia R, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–77. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esposito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–86. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–32. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 80.Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci. 2009;29:15063–72. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaiarsa JL, Porcher C. Emerging neurotrophic role of GABAB receptors in neuronal circuit development. Front Cell Neurosci. 2013;7:206. doi: 10.3389/fncel.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23:2618–26. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song J, et al. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci. 2013;16:1728–30. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chancey JH, et al. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci. 2013;33:6614–22. doi: 10.1523/JNEUROSCI.0781-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chancey JH, Poulsen DJ, Wadiche JI, Overstreet-Wadiche L. Hilar mossy cells provide the first glutamatergic synapses to adult-born dentate granule cells. J Neurosci. 2014;34:2349–54. doi: 10.1523/JNEUROSCI.3620-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 87.Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gelman DM, Marin O. Generation of interneuron diversity in the mouse cerebral cortex. Eur J Neurosci. 2010;31:2136–41. doi: 10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- 89.Chittajallu R, et al. Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT(3A)R expression. Nat Neurosci. 2013;16:1598–607. doi: 10.1038/nn.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–26. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tricoire L, Vitalis T. Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Frontiers in Neural Circuits. 2012;6 doi: 10.3389/fncir.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rancillac A, et al. Glutamatergic Control of Microvascular Tone by Distinct GABA Neurons in the Cerebellum. J Neurosci. 2006;26:6997–7006. doi: 10.1523/JNEUROSCI.5515-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Craig MT, McBain CJ. Navigating the circuitry of the brain's GPS system: Future challenges for neurophysiologists. Hippocampus. 2015 doi: 10.1002/hipo.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]