Abstract
Social behavior is regulated by conserved neural networks across vertebrates. Variation in the organization of neuropeptide systems across these networks is thought to contribute to individual and species diversity in network function during social contexts. For example, oxytocin (OT) is an ancient neuropeptide that binds to OT receptors (OTRs) in the brain and modulates social and reproductive behavior across vertebrate species, including humans. Central OTRs exhibit extraordinarily diverse expression patterns that are associated with individual and species differences in social behavior. In voles, OTR density in the nucleus accumbens (NAc)—a region important for social and reward learning—is associated with individual and species variation in social attachment behavior. Here we test whether OTRs in the NAc modulate a social salience network (SSN)—a network of interconnected brain nuclei thought to encode valence and incentive salience of sociosensory cues—during a social context in the socially monogamous male prairie vole. Using a selective OTR antagonist, we test whether activation of OTRs in the NAc during sociosexual interaction and mating modulates expression of the immediate early gene product Fos across nuclei of the SSN. We show that blockade of endogenous OTR signaling in the NAc during sociosexual interaction and mating does not strongly modulate levels of Fos expression in individual nodes of the network, but strongly modulates patterns of correlated Fos expression between the NAc and other SSN nuclei.
Keywords: Immediate early gene, Fos, Social behavior, Sexual behavior, Nucleus accumbens, Striatum, Functional connectivity, Functional coupling, Social decision making network, Social behavior network, Pair bonding network
1. Introduction
Selective pressures favoring adaptive behavioral interaction with conspecifics have led to the evolution of diverse forms of social behavior within and across species (Hofmann et al., 2014). In vertebrates, social behavior is thought to be modulated by deeply conserved and neuro-peptide-sensitive neural networks. For example, the social decision-making network (SDMN) represents a synthesis of previously described social behavior and mesolimbic reward networks, and is thought to modulate social communication, parenting, sexual, and aggressive behaviors across vertebrates (Goodson, 2005; O'Connell and Hofmann, 2011, 2012).
Research efforts have aimed to identify organizing principles that describe both the anatomy and function of the SDMN. These efforts have led to models that emphasize distributed, interregional information processing among reciprocally interconnected nodes as a critical level of network function (Goodson, 2005; Goodson and Kabelik, 2009). It is explicitly hypothesized that distributed patterns of activity across the network more strongly reflect social context and behavior than activity within individual brain regions. Within this framework, individual nodes are hypothesized to serve dynamic functional roles depending on their functional “connectivity” or “coupling” with other nodes (Goodson and Kabelik, 2009; McIntosh, 2004; Teles et al., 2015). It is further hypothesized that organizational plasticity of steroid hormone and neuropeptide systems across the SDMN is an important mechanism contributing to diversity in distributed network function and social behavior within and across species (Barrett et al., 2013; Cardoso et al., 2015; Goodson, 2005; Goodson and Kabelik, 2009; Johnson and Young, 2015; Newman, 1999).
Although hypothesized links between neuropeptide signaling and distributed network function are consistent with a large body of literature, few studies have directly tested them. The oxytocin (OT) system is well poised for investigating these links. OT is a highly conserved neuro-peptide that modulates reproductive physiology and behavior across bilaterian animals, including nematode worms, rodents, and humans (Garrison et al., 2012; Grinevich et al., 2016; Lee et al., 2009). In vertebrates, the OT system innervates forebrain networks that modulate social behavior. Central distribution patterns and region-specific densities of OT receptors (OTRs) have undergone rapid evolutionary divergence between closely related species of birds and mammals that exhibit divergent social behavior (Anacker and Beery, 2013; Goodson, 2008; Johnson and Young, 2015). The critical functional roles of central and region-specific OTR populations in modulating social behavior have been demonstrated using selective OTR antagonists (OTAs), viral vector-mediated gene transfer, and/or RNAi knockdown in fishes (Goodson and Bass, 2000); birds (Goodson et al., 2009; Pedersen and Tomaszycki, 2012); and mammals (Bosch and Neumann, 2012; Burkett et al., 2016; D'Cunha et al., 2011; Donaldson and Young, 2008; Dumais et al., 2016; Johnson et al., 2016; Keebaugh et al., 2015; Nakajima et al., 2014; Ross et al., 2009b; Song et al., 2016; Young et al., 2001; Yu et al., 2016). Together these data suggest that evolutionary plasticity in region-specific OTR expression is a deeply conserved mechanism contributing to social behavioral diversity within and across species.
Previous experiments in non-mammalian species have used correlated expression of cytochrome oxidase (a metabolic marker) and immediate early genes (IEGs) to demonstrate that distributed patterns of transcriptional and metabolic activity across SDMN nuclei are strongly predictive of social context and behavior (Hoke et al., 2005; Sakata et al., 2000; Teles et al., 2015; Yang and Wilczynski, 2007). We recently used a similar approach to investigate how central OTR activation during a social context modulates IEG expression across a hypothesized social salience network (SSN) in male prairie voles. Briefly, the SSN is a neural network that is hypothesized to modulate selective social attachment and other forms of associative social learning by attaching valence and incentive salience to sociosensory cues. The SSN includes multiple SDMN nodes as well as multiple non-SDMN nodes, including the anterior olfactory nucleus (AON) and prefrontal cortex (PFC)—where OTR activation is critical for social learning and behavior in rodents (Nakajima et al., 2014; Oettl et al., 2016; Young et al., 2001)—and the paraventricular nucleus of the hypothalamus (PVN), a primary site of OT synthesis. We previously found that endogenous central OTR signaling during sociosexual interaction does not strongly modulate levels of Fos-immunoreactivity (Fos-ir) within individual SSN nuclei, but strongly modulates patterns of correlated Fos-ir across SSN nuclei, consistent with hypotheses that central OTRs modulate distributed neural network activity during social contexts (Johnson et al., 2016). These experiments, however, did not address the functional roles of region-specific OTR populations. To our knowledge, no experiments to date have tested whether endogenous activation of region-specific OTR populations during social contexts modulates activity-dependent markers (e.g. IEGs) across distributed neural networks. Here we aim to address this question by site-specifically manipulating endogenous OTR signaling in the NAc of male prairie voles during naturalistic sociosexual interaction with a female.
Briefly, the NAc is a SSN (and SDMN) nucleus in which OTRs (or homologous OT-like receptors) are variably expressed across distant vertebrate lineages (O'Connell and Hofmann, 2012). The NAc is anatomically positioned as an integration hub within the SSN, receiving direct projections from every other SSN nucleus—with the exception of the AON—including direct OTergic axonal projections from the PVN (Ross et al., 2009a). OTR densities in the NAc correlate with socially monogamous mating strategies and other forms of social behavioral variation in prairie voles and other mammals (Insel and Shapiro, 1992; Mooney et al., 2015; Olazabal, 2014; Olazabal and Young, 2006b; Ophir et al., 2012; Ross et al., 2009b); and blockade of endogenous OTR (and other neuromodulatory receptor) signaling in the NAc inhibits pair bond formation and other forms of social behavior in prairie voles and other rodents (Bosch et al., 2016; Keebaugh et al., 2015; Olazabal and Young, 2006a; Ross and Young, 2009; Young et al., 2001; Yu et al., 2016). In humans, intranasal OT administration to romantically attached males modulates BOLD responses in the NAc during viewing of a partner's face, raising the possibility of conserved neural systems modulating selective social attachment in rodents and humans (Scheele et al., 2013). For these reasons, here we administer a selective OTA to test whether endogenous OTR activation in the NAc during sociosexual interaction and mating modulates Fos-ir across the SSN in male prairie voles.
2. Methods
2.1. Subjects
Sexually naïve male prairie voles were group housed until stereotaxic surgery during adulthood (60–180 days), after which they remained singly housed for 5–6 days until behavioral testing. Subjects were housed in ventilated 26 × 18 × 19 cm Plexiglass cages filled with Bedo'cobbs Laboratory Animal Bedding (The Andersons; Maumee, Ohio) kept at 22 °C under a 14:10 h light/dark cycle with ad libitum access to food (Lab Rabbit Diet HF #5326, LabDiet) and water. Subjects were drawn from our laboratory breeding colony originally derived from field captured voles in Champaign, Illinois. Stimulus animals were sexually experienced, ovariectomized, estrogen-primed (see below under “Cohabitation”), adult female prairie voles. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Emory University Institutional Animal Care and Use Committee.
2.2. Cannulation
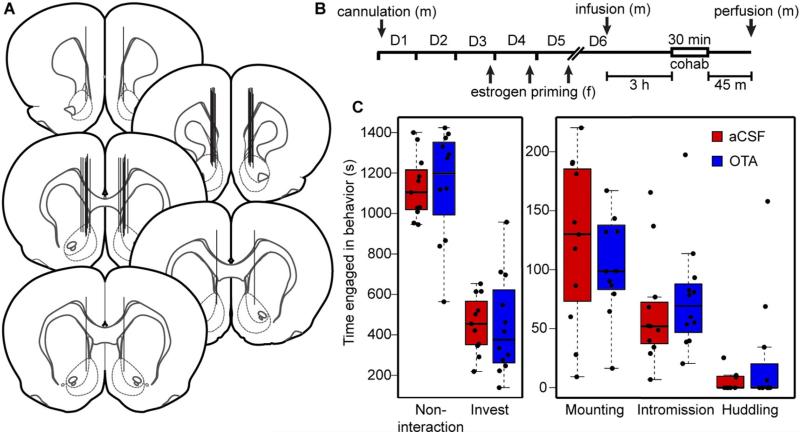
Adult male prairie voles were anesthetized by continuous administration of 1.5–2.5% isofluorane during stereotaxic implantation of a bilateral 26-gauge guide cannula (catalog no. C235G-2.0/SPC; Plastics One; Roanoke, VA) targeting the dorsal NAc shell with stereotaxic coordinates (from Bregma: A/P + 1.7 mm; M/L ± 1.0 mm; D/V – 4.5 mm; Fig. 1A). The guide cannula was fixed to the skull with a combination of Jet Acrylic Liquid and Jet Denture Repair Powder (Lang Dental Manufacturing Co., Inc.; Wheeling, IL). All subjects recovered for 5–6 days following surgery (Fig. 1B).
Fig. 1.
Site-specific administration of OTA into the NAc has no effect on male sociosexual behaviors. Fig. 1A depicts cannula placement across all subjects, with cannula tracks represented by solid black vertical lines and the NAc (shell and core) represented by dotted boundary lines. Fig. 1B shows a schematic of the experimental timeline and design (D = day; m = male; f = female). Prior to testing, adult males received site-specific administration of either aCSF or OTA into the NAc. Fig. 1C shows box-and-whisker plots of the time engaged in non-interaction, social investigation, mounting, intromission, and huddling among mated males (n = 11 aCSF; n = 12 OTA) during 30 min of sociosexual interaction with a female. There was no main effect of treatment (aCSF versus OTA) on time engaged in any of these behaviors (two-way repeated measures ANOVA; F1,109 < 0.01, p > 0.99; Eta-squared < 0.01).
2.3. Antagonist administration
After recovery, and 3 h prior to cohabitation (Fig. 1B), subjects received 1–2% isofluorane anesthesia during bi-lateral microinjection of either 500 nL of control aCSF (n = 14 exposed; n = 6 unexposed) or 2.5 ng/μL OTA, peptidergic ornithine vasotocin analog desGly–NH2,d(CH2)5[Tyr(Me)2, Thr4]OVT (Bachem, Torrance, CA), dissolved in 500 nL aCSF (n = 16 exposed) in accordance with previous experiments using site-specific OTA administration targeting forebrain nuclei (Burkett et al., 2016). Microinjections were performed using a 33-gauge internal cannula (C235I/SPC; Plastics One; Roanoke, VA) extending 0.5 mm beyond the end of the guide cannula. Injections were performed with a 10 μL Hamilton syringe (Hamilton; Reno, Nevada) connected to polyethylene-20 tubing (Plastics One), which in turn was connected to the internal cannula. Infusions were administered over 60 s, and the internal cannula was left in place for 3 min following infusion to prevent backflow. One subject (aCSF-treated, unexposed) was excluded due to significant backflow during infusion (final aCSF-unexposed group size, n = 5). All subjects received two consecutive infusions, one into each hemisphere. Larger group sizes were selected for socially exposed treatment groups to obtain sufficient samples of subjects that mated during cohabitation.
2.4. Cohabitation
As seen in Fig. 1B, 3 h after aCSF/OTA infusion, subjects underwent 30 min of video-recorded cohabitation with a sexually experienced, ovariectomized, estrogen-primed, adult stimulus female. In the three days preceding cohabitation, stimulus females were brought into behavioral estrus with daily subcutaneous injections of 4.0 μg 17β-estradiol benzoate dissolved in sesame oil (Sigma; St. Louis, MO; S3547). Cohabitation began with the introduction of the stimulus female into the male subject's home cage with food hoppers removed to facilitate video recording and behavioral analysis. Lab chow pellets and water bottles were left in the home cage to allow ad libitum access to food and water during the cohabitation. After 30 min of cohabitation, stimulus females were removed and subjects remained in their home cage for 45 min before transcardial perfusion (75 min following initial introduction of the stimulus female). For unexposed control subjects (n = 5), the cage was opened and a small amount of bedding was scooped and immediately returned by an experimenter at the beginning and end of the 30-minute session to control for experimenter activity during introduction/removal of stimulus females.
2.5. Perfusion, post-fixation, and sectioning
75 min after the initiation of cohabitation (or control sessions), subjects were administered an overdose of isofluorane and were immediately perfused transcardially at a rate of approximately 4 mL/min with 40 mL of 1x phosphate buffered saline (PBS, pH = 7.4; diluted to 1x in distilled water from 10x PBS stock; Teknova; Hollister, CA; P0401) followed by 40 mL of 4% paraformaldehyde (Polysciences; Warrington, PA; 00380) in 1x PBS using an Easy-Load II MASTERFLEX pump (Cole-Palmer; Vernon Hills, IL). Immediately following perfusion, brains were extracted and post-fixed in 4% paraformaldehyde dissolved in 1X PBS overnight before storage in 30% sucrose in 1x PBS solution until sectioning. Brains were cut into 40 μm sections using a Microm HM 440E freezing microtome and were stored in 1x PBS with 0.05% sodium azide until Nissl or immunohistochemical staining.
2.6. Behavioral scoring
Five mutually exclusive behaviors were quantified using Observer XT 10 behavioral scoring software (Noldus Information Technology Inc.; Leesburg, VA). These behaviors included non-interaction, social investigation, mounting, intromission, and huddling behavior. The operational definitions used for each behavior were as follows. Noninteraction included any male behavior not directed toward the female, including autogrooming, running, freezing, eating, drinking, sleeping, digging, and exploration. Investigation included social behavior directed toward the female, including physical pursuit, olfactory investigation, and allogrooming. Mounting was defined as placement of the forepaws on the female from the rear without intromission. Intromission was defined by stereotypical patterns of pelvic movement during copulation bouts with the female. Huddling behavior was defined as direct, side-by-side social contact between the male and female.
2.7. Nissl staining
Sections containing cannula implant tracks were mounted onto Superfrost Plus slides (Fisher Scientific; 12-550-150) and allowed to dry overnight before Nissl staining. Immediately prior to staining, sections were submerged in 100% ethanol (EtOH) for 2 min, 95% EtOH for 2 min, and 70% EtOH for 2 min. Sections were stained in 0.1% cresyl violet in distilled water for 2 min. After staining, sections were dehydrated in 95% EtOH twice for 2 min and then 100% EtOH twice for 2 min. Prior to coverslipping, slides were submerged in a 1:1 EtOH:HistoClear mixture for 2 min and were then submerged in fresh HistoClear twice for 2 min. Immediately after Histoclear treatment slides were coverslipped while still partially wet using Krystalon (EMD Chemicals Inc., Gibbstown, NJ).
2.8. Injection site validation
Anatomical placement of cannula implants was determined on Nissl-stained sections. Infusion sites were validated using a Nikon E800 microscope with a 10x objective. Subjects were included if the cannulation track terminated within 0.5 mm of the dorsal NAc. Two subjects were excluded; one (OTA-treated, exposed) due to an anterior miss, and a second (aCSF-treated, exposed) due to extensive unilateral leakage of blood through the cannula and into the NAc. Cannulation tracks in all remaining subjects terminated within 0.5 mm of the dorsal NAc and were included in subsequent behavioral analyses (Fig. 1A; n = 13 aCSF-treated, exposed; n = 15 OTA-treated, exposed; n = 5 aCSF-treated, unexposed).
2.9. Immunohistochemistry
Sections underwent three washes in 1x PBS, 10 min of incubation in 1% sodium hydroxide in 1x PBS, and three washes in 1x PBS with 0.5% Triton-X (PBST; Sigma) before treatment with 5% normal goat serum (Fitzgerald; Acton, MA) in PBST for 1 h at room temperature. Sections were then incubated for 48 h in primary rabbit polyclonal anti-Fos antibody (diluted in PBST to a final concentration of 1:20,000; Calbiochem ABE457) on an orbital shaker at 4 °C. Following primary incubation sections were washed five times in 1x PBS, once in 1x PBST, and were then incubated in secondary biotinylated goat anti-rabbit IgG antibody (diluted 1:500 in PBST; Vector Labs, BA-1000) for 2 h. After secondary incubation, sections were treated with an avidin-biotin peroxidase system (Vectastain Elite ABC System; Vector Labs: PK6100) for 1 h before staining with a Nickel-DAB peroxidase substrate kit (Vector Labs; SK4100). All sections were dehydrated in a series of increasingly concentrated ethanol solutions (5 min in 70% EtOH, 10 min in fresh 95% EtOH twice, 10 min in fresh 100% EtOH twice), bathed in Xylenes (15 min in Xylenes twice), mounted onto Superfrost Plus slides (Fisher Scientific; 12-550-150) while still partially wet, and coverslipped using Krystalon (EMD Chemicals Inc., Gibbstown, NJ).
2.10. Selection of SSN nuclei
Fos-ir was analyzed in seven SSN nuclei: the NAc shell, AON, PFC, PVN, medial amygdala (MeA), basolateral amygdala (BLA), and ventral tegmental area (VTA). All of the analyzed regions are innervated by OTergic fibers, reciprocally interconnected with other SSN nuclei, and express OTRs in male prairie voles (see Fig. 2C for a simplified schematic of the SSN). The NAc shell was selectively targeted with OTA because OTR density and activation within this nucleus are associated with social learning and behavior in prairie voles and other rodents (Barrett et al., 2015; Bosch et al., 2016; Dolen et al., 2013; Keebaugh et al., 2015; Mooney et al., 2015; Okuyama et al., 2016; Olazabal, 2014; Olazabal and Young, 2006a, 2006b; Ophir et al., 2012; Ophir et al., 2009; Ross et al., 2009b; Young et al., 2001; Yu et al., 2016; Zheng et al., 2013). Similarly, in the PFC, OTRs modulate multiple forms of social learning and behavior in rodents (Brill-Maoz and Maroun, 2016; Nakajima et al., 2014), including pair bonding in prairie voles (Young et al., 2001). The VTA also modulates pair bonding in male prairie voles (Curtis and Wang, 2005); projects to, releases dopamine (DA) within, and modulates IEG expression in multiple SSN nuclei (Argiolas and Melis, 2013); and contains OTR-expressing neurons that modulate both social behavior and dopamine release into the NAc shell (Peris et al., 2016; Shahrokh et al., 2010; Song et al., 2016; Stivers et al., 1988). The AON and MeA were selected due to their role in OTR-dependent social odor processing and social recognition (Ferguson et al., 2001; Gur et al., 2014; Oettl et al., 2016; Wacker and Ludwig, 2012). Direct projections from the BLA modulate both cue-evoked excitation of accumbal neurons and reward-seeking behavior; and this circuit is thought to relay sociosensory information from the amygdala to the NAc shell during formation of selective social attachments (Ambroggi et al., 2008; Numan and Young, 2016). Finally, the PVN is a major site of OT synthesis and somatodendritic OT release, and is the predominant source of OTergic fiber innervation to the NAc shell (Bosch et al., 2016; Ross et al., 2009a) and other SSN nuclei (Knobloch et al., 2012).
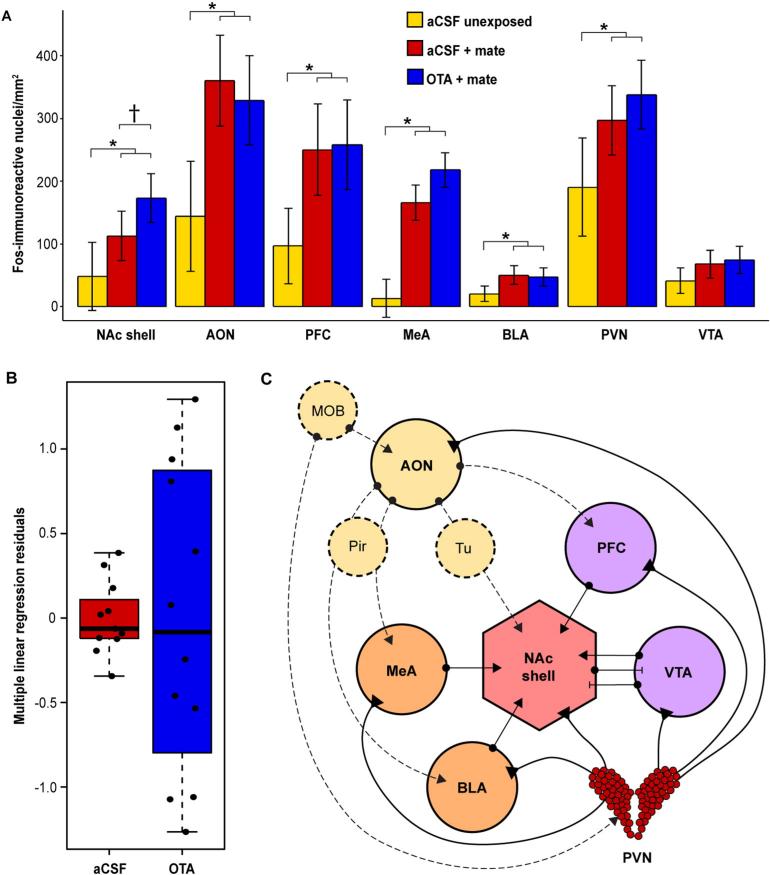
Fig. 2.
Effects of mating and accumbal OTR signaling on Fos-ir across the SSN. Fig. 2A shows Fos-ir, represented as least squared mean estimates of Fos-positive nuclei/mm2, in each SSN nucleus across treatment groups (aCSF-unexposed, n = 5; aCSF-mated, n = 11; OTA-mated, n = 12). Sociosexual interaction and mating resulted in a robust induction of Fos-ir across the SSN (linear mixed-effects model; t1,25 = 4.49, p < 0.001). The direction of this effect was consistent in all SSN nuclei and was significant (p < 0.05) in every region except the VTA (see Supplementary Table 1). Among mated subjects, in the NAc shell, aCSF-treated males exhibited a trend toward decreased Fos-ir relative to OTA-treated males, although this effect was not statistically significant (t1,20 = 1.85; p = 0.08). A multiple linear regression (MLR) model revealed that Fos-ir across non-accumbal SSN nuclei explained a large amount of the variance of Fos-ir in the NAc shell in aCSF- (R2 = 0.91) but not OTA- (R2 = 0.20) treated subjects. A permutation test indicated that this difference in variance explained (71%) was statistically significant (p = 0.02). Fig. 2B illustrates the distribution of residuals extracted from the MLR model. The variance of residuals was significantly greater in OTA- compared to aCSF-treated subjects (F-test; F1,20 = 17.25, p < 0.001). Fig. 2C illustrates neuroanatomical connectivity of the NAc shell with other SSN nuclei. In Fig. 2A, error bars indicate ± standard error of the mean, asterisks indicate p < 0.05, and daggers indicate p < 0.10. In Fig. 2C, dotted lines represent olfactory pathways; arrows represent excitatory input, perpendicular lines represent inhibitory input, and filled triangles represent innervation by OT fibers originating from the PVN. Abbreviations: AON = anterior olfactory nucleus; BLA = basolateral amygdala; MeA = medial amygdala; MOB = main olfactory bulb; NAc shell = nucleus accumbens shell; PFC = prefrontal cortex; Pir = piriform cortex; PVN = paraventricular nucleus of the hypothalamus; Tu = olfactory tubercle; VTA = ventral tegmental area.
2.11. Imaging and analysis
Because the aim of these experiments was to investigate the effects of region-specific OTR signaling on Fos-ir, analyses of Fos-ir were restricted to subjects that mated during cohabitation (aCSF, n = 11; OTA, n = 12) to maximize divergence in presumed OT signaling (mating induces OT release) between socially-exposed treatment groups, consistent with previous experiments (Johnson et al., 2016). Images were captured through a Nikon E800 microscope and a 4x objective using MCID Core imaging software (InterFocus Ltd.; Cambridge, UK). The Mouse Brain (Franklin and Paxinos; 3rd Edition) atlas was used as a reference to determine anatomical boundaries. For the NAc, PFC, and MeA, images were captured from the shell, prelimbic, and posterodorsal divisions, respectively. For each brain region, Fos-ir was quantified in both left and right hemispheres across three consecutive sections for all subjects. Sections or hemispheres with damaged or absent tissue in the target region were excluded. Fos-positive nuclei were quantified for each brain region using the MCID grain count function with constant size, density, and shape thresholds applied across all subjects. For each subject, Fos-ir counts within each region were averaged, yielding a single average Fos-ir count per region per subject.
2.12. Statistics
2.12.1. Immunohistochemistry batch effects and data transformation
Immunohistochemistry was performed in two batches for these experiments. To control for differences between batches, batch was included as a random effect in all linear mixed-effects regression analyses of raw Fos-ir count values. For multiple linear regression (MLR) and principle components analysis (PCA) of covariance, raw Fos-ir count values were transformed into standardized Fos-ir z-scores within each batch in order to allow pooling of data across batches.
2.12.2. Sociosexual behaviors
Behavioral data from cohabitation were analyzed using a two-way repeated-measures ANOVA with treatment (OTA versus aCSF) as a between subjects factor and behavior (non-interaction, investigation, mounting, intromission, and huddling) as a within subjects factor. Poisson regression was used for analysis of count outcome variables (e.g. mating bout frequency). For all ANOVAs, effect size (Eta-squared) was calculated by dividing the individual effect sum of squares by the total sum of squares.
2.12.3. Fos-ir across the SSN
To test whether levels of Fos-ir across the SSN were influenced by treatment, we used a linear mixed-effects model (the “lme” function in the statistical software package R) with number of Fos-positive nuclei per mm2 (hereafter referred to as “Fos-ir count”) as the dependent variable, treatment (aCSF-mated, OTA-mated, or aCSF-unexposed) and brain region (see “Selection of SSN nuclei” above) as fixed effects, and immunohistochemistry batch (see “Data transformation” above) and individual subject ID as random effects. To measure the effect of socio-sexual interaction and mating on Fos induction across the SSN, we first tested whether “treatment” was a significant predictor of Fos-ir count among only mated subjects. To do this, we excluded unexposed subjects and again used a linear mixed-effects model fitting Fos-ir count against treatment and brain region, with batch and individual subject ID included as random effects. Based on these results, treatment was removed as a predictor and Fos-ir count was fitted against “mating” (unexposed versus mated; with OTA-mated and aCSF-mated subjects pooled) and brain region (both fixed effects), with batch and individual subject ID included as random effects. We then measured the effect of sociosexual interaction and mating on Fos induction in each individual SSN nucleus using linear mixed-effect models that fit Fos-ir count in the region of interest against mating, with batch included as a random effect.
2.12.4. Effect of OTR antagonist administration on Fos-ir in the NAc shell
To test the hypothesis that site-specific manipulation of OTR signaling in the NAc influenced local Fos induction, we restricted analysis to mated subjects and used a linear mixed-effects model with Fos-ir count in the NAc shell as the dependent variable, treatment as a fixed effect, and batch as a random effect.
2.12.5. Fos-ir covariance between the NAc shell and other SSN nuclei
To investigate the hypothesis that blocking OTRs in the NAc specifically disrupts correlated Fos-ir between the NAc shell and the other SSN nodes, we transformed raw Fos-ir count values into standardized Fos-ir z-scores for each batch to enable pooling across batches (see “Data transformation” section above). We then used a multiple linear regression (MLR; the “lm” function in R) model with Fos-ir z-score in the NAc shell as the dependent variable and Fos-ir z-scores in the six other SSN nuclei as independent variables. Briefly, this analysis assessed the relationship between Fos-ir in the NAc shell and Fos-ir in the other SSN nuclei while simultaneously accounting for relationships among the other SSN nuclei. We extracted R2 values from these analyses and compared how much of the variance of Fos-ir in the NAc shell was explained by Fos-ir in the other SSN nuclei between groups treated with OTA versus aCSF. To test for statistical significance, we generated a permuted null distribution through multiple, random assignment of individuals to either of the investigated treatment groups, without replacement. For each permutation we calculated the multiple R2 for both groups and the absolute value of the difference of this value between treatment groups. This procedure was repeated 10,000 times and the p-value for the difference in variance explained was calculated as the proportion of permuted differences exceeding the initial observed difference value. We also extracted residuals from these analyses to test whether observed Fos-ir counts deviated from those predicted by the MLR model differentially by treatment (OTA versus aCSF). In order to test this, we first confirmed that these residuals did not significantly deviate from normality (Shapiro-Wilks test; p = 0.5822). We then used an F-test to determine whether variance of MLR residuals significantly differed between OTA- versus aCSF-treated groups.
2.12.6. Global Fos-ir covariance across the SSN
These analyses were performed as previously described and combined principal component analyses (PCA) to estimate effect size (difference in variance explained by the first principal component) and permutation testing to assess statistical significance of the effect (Johnson et al., 2016). For PCA analyses, we again used standardized Fos-ir z-scores to enable pooling across batches (see “Data transformation” section above).
3. Results
3.1. Mating frequency
We previously demonstrated that central OTR blockade reduces mating frequency in male prairie voles (Johnson et al., 2016), consistent with findings in rats (Argiolas et al., 1988). Here, we tested whether this effect is mediated specifically by OTRs within the NAc. aCSF-treated males (n = 13) engaged in an average frequency of 4.11 ± 1.05 intro-mission bouts per 30 min with the stimulus female compared to 5.56 ± 1.19 bouts per 30 min in OTA-treated males (n = 15). A regression analysis comparing Poisson distributions of mating bouts showed no significant differences in mating frequency between OTA- and aCSF-treated subjects (z = 1.328; p = 0.184), suggesting that OTRs in other nuclei modulate mating frequency in male prairie voles.
3.2. Sociosexual behaviors
We next tested whether OTR antagonism in the NAc was associated with differences in duration of sociosexual behavior (non-interaction, investigation, mounting, intromission, or huddling). Among subjects that mated with the stimulus female (aCSF, n = 11; OTA, n = 12), a two-way repeated-measures ANOVA revealed a significant main effect of behavior (non-interaction, investigation, mounting, intromission, or huddling; F1,109 = 68.00; p < 0.001; Eta-squared = 0.38), but not of treatment (aCSF versus OTA; F1,109 ≤ 0.01; p N 0.99; Eta-squared < 0.01) or the interaction between behavior and treatment (F1,109 = 0.01; p = 0.93; Eta-squared ≤ 0.01) on time engaged in the analyzed social behaviors during the 30 min period of cohabitation (Fig. 1C). Thus, among mated animals, OTA versus aCSF treatment had no detectable effect on any of the analyzed sociosexual behaviors, consistent with previous results following ICV administration (Johnson et al., 2016). Importantly, these data suggest that any observed differences in Fos-ir between OTA- and aCSF-treated subjects in these experiments were not due to differences in performance of the analyzed social behaviors.
3.3. Fos-ir across the SSN
We previously found that sociosexual interaction and mating with a female is associated with a robust increase in Fos-ir across SSN nuclei in male prairie voles, regardless of central aCSF versus OTA administration (Johnson et al., 2016). As a positive control, we tested whether sociosexual interaction and mating with the stimulus female was associated with increased Fos-ir across SSN nuclei in the present experiments. To test this, we first fit a linear mixed-effects model with Fos-ir count as the dependent variable, treatment (aCSF-unexposed, aCSF-mated, OTA-mated) and brain region as fixed effects, and batch and individual subject ID as random effects. These analyses revealed significantly increased Fos-ir in aCSF-mated (t2,24 = 3.74, p = 0.001) and OTA-mated (t2,24 = 4.47, p < 0.001) subjects compared to unexposed controls. To test whether Fos-ir differed by aCSF- versus OTA-treatment among mated animals, we restricted analysis to mated subjects and fit a linear mixed-effects model with Fos-ir count as the dependent variable, treatment (aCSF or OTA) and brain region as fixed effects, and batch and individual subject ID as random effects. This test showed that Fos-ir did not significantly differ between aCSF-mated and OTA-mated animals across brain regions (t1,20 = 0.87, p = 0.39). These results suggest that sociosexual interaction and mating, but not OTR blockade in the NAc, strongly influenced levels of Fos-ir across SSN nuclei. To validate this interpretation, we excluded treatment as a predictor and fit a linear mixed-effects model of Fos-ir count against “mating” (OTA-mated and aCSF-mated subjects pooled versus unex-posed controls) and brain region, with batch and individual subject ID included as random effects. This test confirmed that sociosexual interaction and mating with a female was associated with a significant increase in Fos-ir across the SSN (t1,25 = 4.49, p < 0.001), consistent with previous results (Johnson et al., 2016). To complement these analyses, we tested whether sociosexual interaction and mating was associated with increased Fos-ir in each SSN nucleus. For each individual brain region, we fit a linear mixed-effects model of Fos-ir count against mating (OTA-mated and aCSF-mated subjects pooled versus unexposed controls), with batch included as a random effect. These analyses confirmed that sociosexual interaction and mating with a female was associated with a significant (p < 0.05) increase in Fos-ir in all analyzed SSN nuclei with the exception of the VTA (Fig. 2A; Supplementary Table 1).
3.4. Fos-ir in the NAc shell
Because we used a selective OTR antagonist to manipulate OTR signaling specifically within the NAc, we tested whether this manipulation influenced local Fos-ir. To test this, we restricted analysis to mated animals and fit a linear mixed-effects model of Fos-ir count in the NAc shell against treatment (aCSF or OTA), with batch included as a random effect. This analysis revealed a trend toward increased Fos-ir in OTA-treated compared to aCSF-treated subjects, although this trend was not statistically significant (t1,20 = 1.85, p = 0.08).
3.5. Correlated Fos-ir between the NAc shell and other SSN nodes
We hypothesized that site-specific infusion of a selective OTA into the NAc would decrease correlated Fos-ir between the NAc shell and other SSN nodes. Consistent with this hypothesis, a MLR model fitting Fos-ir count in the NAc shell against Fos-ir count in every other SSN node revealed that Fos-ir count in non-accumbal SSN nodes explained a large amount of the variance in Fos-ir count in the NAc shell in aCSF-treated animals (R2 = 0.91) but not in animals treated with OTA (R2 = 0.20). In support of our hypothesis, permutation testing indicated that this difference in variance explained (71%) was statistically significant (p = 0.02). We also extracted residuals from the MLR model and tested whether the variance of the residuals differed between groups treated with OTA versus aCSF. In further support of our hypothesis, the variance of MLR residuals was significantly greater in OTA- (σ2 = 0.82) compared to aCSF- (σ2 = 0.05) treated subjects (F-test; F1,20 = 17.25, p < 0.001; Fig. 2B).
3.6. Global covariance of Fos-ir across the SSN
We previously found that blockade of central OTRs during sociosexual interaction and mating was associated with a “global” decrease in correlated Fos-ir across the SSN. We hypothesized that this effect was mediated by disruption of OTR signaling simultaneously across many interconnected forebrain nuclei during sociosexual interaction and mating. In these experiments, we hypothesized that blocking OTRs specifically in the NAc would result in disruption of covariance between the NAc shell and the other SSN nodes, in contrast to the more global effect we observed in our previous experiments following ICV administration of OTA (Johnson et al., 2016). To test this prediction, we combined PCA and permutation testing and tested whether OTR blockade in the NAc influenced global covariance of Fos-ir across the SSN as a whole. In line with our hypothesis, we found no effect of treatment on global covariance of Fos-ir across the SSN; the variance explained by the first principal component was virtually identical in animals treated with OTA and aCSF (difference in R2 < 0.01, p = 0.98).
4. Discussion
Site-specific blockade of OTRs in the NAc had no effect on mating frequency or any of the analyzed sociosexual behaviors in male prairie voles during 30 min of sociosexual interaction with a female. We previously demonstrated that ICV administration of OTA reduces mating frequency in male prairie voles (Johnson et al., 2016). Taken together, these data suggest that OTRs in other nuclei modulate sexual behavior in male prairie voles, consistent with findings in rats (Argiolas and Melis, 2013; Gil et al., 2011). Importantly, these results suggest that any significant differences in Fos-ir between treatment groups are not due to differences in any of the analyzed sociosexual behaviors. Additionally, because most of the analyzed behaviors are active behaviors, it is unlikely that differences in Fos-ir between treatment groups are accounted for by differences in movement during the cohabitation, although we could not directly analyze locomotion per se.
Here we use Fos-ir as an indicator of transcriptional activity and synaptic plasticity across the SSN. Briefly, Fos is the protein product of the immediate early gene C-fos. In response to synaptic input, Ca2+ influx triggers Ca2+-dependent kinase cascades that regulate transcription of C-fos across many, but not all, neuronal phenotypes (Kawashima et al., 2014). Following translation, Fos protein heterodimerizes with JUN to form AP-1 transcription factor complexes that regulate downstream expression of genes involved in a variety of intracellular processes, including synaptic plasticity (Lyons and West, 2011). Thus, central Fos-ir is thought to reflect rapid adaptation of neuronal and synaptic physiology in response to synaptic input through robust transcriptional regulation, and has been linked with depolarization in many, but not all, neuronal phenotypes.
Fos-ir across SSN nuclei was significantly higher in mated versus un-exposed subjects, regardless of OTA- versus aCSF-treatment, confirming that sociosexual interaction and mating was associated with a robust induction of Fos-ir across the network. This pattern was observed in all brain regions analyzed, with no region-specific differences in Fos-ir distinguishing aCSF-treated versus OTA-treated animals. Notably, however, we observed a statistical trend toward increased Fos-ir in OTA-treated versus aCSF-treated subjects in the NAc shell, similar to previous results following ICV administration of OTA (Johnson et al., 2016). Together these data raise the possibility that endogenous activation of OTRs during sociosexual interaction and mating attenuates Fos induction in populations of accumbal neurons, and encourage future investigation of these relationships.
We hypothesized that endogenous activation of accumbal OTRs modulates correlated Fos-ir between the NAc shell and other SSN nuclei during sociosexual interaction and mating. In support of this hypothesis, we observed a robust and significant effect of treatment on correlated Fos-ir between the NAc shell and other SSN nuclei; Fos-ir counts in non-accumbal SSN nuclei collectively explained the vast majority of variance of Fos-ir counts in the NAc shell in aCSF-treated (91% of variance explained) but not OTA-treated (20% of variance explained) subjects. In further support of our hypothesis, we found that the variance of residuals extracted from the MLR model was significantly greater among OTA-treated compared to aCSF-treated subjects, indicating that observed Fos-ir count values deviated further from MLR-predicted Fos-ir count values in OTA- compared to aCSF-treated subjects. The effect of treatment on correlated Fos-ir across SSN nuclei was not general-izable to the network as a whole, as PCA combined with permutation testing suggested that global patterns of covariance of Fos-ir across the SSN did not differ between aCSF- and OTA-treated subjects. Taken together these data provide strong evidence that endogenous accumbal OTR signaling during sociosexual interaction and mating robustly modulates how synaptic plasticity and transcriptional activity in the NAc shell are correlated with synaptic plasticity and transcriptional activity in other nodes of the SSN.
Given the anatomical connectivity between the NAc shell and other SSN nuclei, these data raise the possibility that activation of accumbal OTRs during sociosexual interaction and mating modulates functional coupling between neuronal populations embedded within the NAc shell and neuronal populations embedded across SSN nuclei. Considering the trend toward elevated accumbal Fos-ir in OTA-mated subjects, it is intriguing to speculate that endogenous activation of OTRs in the NAc shell attenuates excitability of some neuronal subpopulations while simultaneously promoting a “state” of enhanced transmission across SSN projections to the NAc shell. Such a mechanism would be consistent with hippocampal slice recordings in rats in which selective OTR agonists greatly improve signal-to-noise ratio and cortical information transfer by suppressing background noise (through inhibitory interneuronal networks) while increasing fidelity of spike transmission and spike timing onto CA1 pyramidal neurons (Owen et al., 2013).
The hypothesis that central OTR activation modulates correlated activity across distributed brain networks is consistent with continually emerging data from experiments in primates. For example, fMRI experiments in both rhesus macaques and humans have found that intranasal OT administration modulates correlated fluctuations in BOLD responses across limbic, striatal, cortical, and/or brainstem nuclei during a variety of social contexts (Bethlehem et al., 2013; Gorka et al., 2015; Kirsch et al., 2005; Liu et al., 2015; Riem et al., 2012; Rilling et al., 2012). However, investigations using intranasal OT should be interpreted with caution (Leng and Ludwig, 2016; Walum et al., 2016). Two studies have shown that naturally-occurring polymorphisms in human OXTR (the gene encoding OTR) are associated with correlated fluctuations in BOLD responses between hypothalamic, limbic, and/or cortical brain areas during social tasks (Tost et al., 2010; Tost et al., 2011), and one has shown that methylation of OXTR in peripheral blood cells is associated with decreased correlated fluctuations in BOLD responses among limbic and cortical regions while viewing fearful and angry faces (Puglia et al., 2015). However, it is unclear whether genetic/epigenetic effects are mediated directly (e.g. through differences in central OTR activation) or indirectly (e.g. through developmental differences in OTR expression or differences in peripheral OTR signaling). Additionally, in contrast to many non-primate species, hurdles in the development of specific ligands for primate OTRs have delayed mapping of central OTR distributions in the human brain (Freeman et al., 2014; Freeman and Young, 2016; Smith et al., 2012). It is therefore unclear whether the genetic and/or epigenetic polymorphisms investigated in these experiments reflect variation in OTR expression in the human brain. However, it is important to note that OXTR polymorphisms in the prairie vole robustly predict individual differences in OTR density in the NAc as well as pair bonding behavior (King et al., 2016).
There are several important limitations to our data. Firstly, the neuronal phenotypes and anatomical projections of Fos-positive neurons within each SSN nucleus are unknown, and therefore identifying anatomically interconnected neuronal subpopulations that become functionally connected during social contexts is a critical area for future investigation. Secondly, site-specific OTA administration targets all region-specific OTRs, obscuring any distinct functional roles that may depend on neuronal phenotype and cellular localization; future electrophysiological investigations are necessary to dissect the neuromodulatory roles of OTRs in different cellular contexts. Thirdly, these experiments focused on OTRs in a single SSN nucleus in male prairie voles. Future investigations of other steroid and neuropeptide systems, additional brain areas, female subjects, and alternative behavioral contexts and species are needed. Finally, site-specific OTR blockade does not likely reflect natural intra- and interspecific variation in levels of region-specific OTR expression and signaling (King et al., 2016). Future studies should investigate how natural variation in central OTR distribution influences distributed neural network function during social contexts within and across species.
Despite these limitations, these data advance our understanding of how neuropeptide systems modulate distributed network function. To our knowledge these are the first data to demonstrate that endogenous activation of a region-specific neuropeptide receptor population modulates distributed patterns of transcriptional activity across a neural network in a behaving vertebrate, linking OTR populations that are critical for social behavior with network properties that are hypothesized to be critical for social information processing. In contrast to previous experiments following central OTR blockade, here we show that site-specific blockade of OTRs in the NAc during sociosexual interaction does not modulate global patterns of correlated Fos-ir across nodes of the SSN. Instead, these data support the view that activation of accumbal OTRs selectively modulates coupling of the NAc shell with other SSN nodes. Taken together, these experiments are consistent with hypotheses that central and region-specific OTR signaling contribute to individual and species diversity in distributed neural network function during social contexts. This may occur in part through OTR-mediated promotion of transient network states that improve transmission from other nodes of the SSN to the NAc shell, facilitating attachment of salience and valence to sociosensory cues and thereby promoting social learning.
Supplementary Material
Acknowledgements
The authors would like to thank Asha Caslin for her contributions to imaging, Lorra Mathews for her assistance in maintaining the prairie vole colony, Jamie LaPrairie for her contributions to behavioral testing and discussion about these experiments, and Nicholas Johnson for insightful discussion regarding statistical methods. This research was supported by NIH grants R01MH096983, and 1P50MH100023 to LJY. Additional funding was provided by the NIH Office of Research Infrastructure Programs/OD P51OD11132 to YNPRC.
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.yhbeh.2016.10.009.
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Beery AK. Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci. 2013;7:185. doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. Neuropeptides and central control of sexual behaviour from the past to the present: a review. Prog. Neurobiol. 2013;108:80–107. doi: 10.1016/j.pneurobio.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Collu M, Gessa GL, Melis MR, Serra G. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur. J. Pharmacol. 1988;149:389–392. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm. Behav. 2013;63:518–526. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl. Psychiatry. 2015;5:e606. doi: 10.1038/tp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016;64:66–78. doi: 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill-Maoz N, Maroun M. Extinction of fear is facilitated by social presence: synergism with prefrontal oxytocin. Psychoneuroendocrinology. 2016;66:75–81. doi: 10.1016/j.psyneuen.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso SD, Teles MC, Oliveira RF. Neurogenomic mechanisms of social plasticity. J. Exp. Biol. 2015;218:140–149. doi: 10.1242/jeb.106997. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol. Behav. 2005;86:338–346. doi: 10.1016/j.physbeh.2005.08.022. [DOI] [PubMed] [Google Scholar]
- D'Cunha TM, King SJ, Fleming AS, Levy F. Oxytocin receptors in the nucleus accumbens shell are involved in the consolidation of maternal memory in postpartum rats. Horm. Behav. 2011;59:14–21. doi: 10.1016/j.yhbeh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH. Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology. 2016;64:79–88. doi: 10.1016/j.psyneuen.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J. Neuroendocrinol. 2016;28 doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science. 2012;338:540–543. doi: 10.1126/science.1226201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M, Bhatt R, Picotte KB, Hull EM. Oxytocin in the medial preoptic area facilitates male sexual behavior in the rat. Horm. Behav. 2011;59:435–443. doi: 10.1016/j.yhbeh.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog. Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front. Neuroendocrinol. 2009;30:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, Labuschagne I, Hosanagar A, Wood AG, Nathan PJ, Phan KL. Oxytocin modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacology. 2015;40:278–286. doi: 10.1038/npp.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol. Psychiatry. 2016;79:155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Gur R, Tendler A, Wagner S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol. Psychiatry. 2014;76:377–386. doi: 10.1016/j.biopsych.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Beery AK, Blumstein DT, Couzin ID, Earley RL, Hayes LD, Hurd PL, Lacey EA, Phelps SM, Solomon NG, Taborsky M, Young LJ, Rubenstein DR, Mo NWGI. An evolutionary framework for studying mechanisms of social behavior. Trends Ecol. Evol. 2014;29:581–589. doi: 10.1016/j.tree.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10712–10717. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. U. S. A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, Young LJ. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm. Behav. 2016;79:8–17. doi: 10.1016/j.yhbeh.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Bito H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front Neural Circuits. 2014;8:37. doi: 10.3389/fncir.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci. 2015;10:561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatry. 2016;80:160–169. doi: 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog. Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biol. Psychiatry. 2016;79:243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Liu N, Hadj-Bouziane F, Jones KB, Turchi JN, Averbeck BB, Ungerleider LG. Oxytocin modulates fMRI responses to facial expression in macaques. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E3123–E3130. doi: 10.1073/pnas.1508097112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog. Neurobiol. 2011;94:259–295. doi: 10.1016/j.pneurobio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. Contexts and catalysts: a resolution of the localization and integration of function in the brain. Neuroinformatics. 2004;2:175–182. doi: 10.1385/NI:2:2:175. [DOI] [PubMed] [Google Scholar]
- Mooney SJ, Coen CW, Holmes MM, Beery AK. Region-specific associations between sex, social status, and oxytocin receptor density in the brains of eusocial rodents. Neuroscience. 2015;303:261–269. doi: 10.1016/j.neuroscience.2015.06.043. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Gorlich A, Heintz N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell. 2014;159:295–305. doi: 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, Meyer-Lindenberg A, Grinevich V, Shusterman R, Kelsch W. Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron. 2016;90:609–621. doi: 10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–1541. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal DE. Comparative analysis of oxytocin receptor density in the nucleus accumbens: an adaptation for female and male alloparental care? J. Physiol. Paris. 2014;108:213–220. doi: 10.1016/j.jphysparis.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006a;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm. Behav. 2006b;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Zheng DJ, Eans S, Phelps SM. Social investigation in a memory task relates to natural variation in septal expression of oxytocin receptor and vasopressin receptor 1a in prairie voles (Microtus ochrogaster). Behav. Neurosci. 2009;123:979–991. doi: 10.1037/a0016663. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm. Behav. 2012;61:445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A, Tomaszycki ML. Oxytocin antagonist treatments alter the formation of pair relationships in zebra finches of both sexes. Horm. Behav. 2012;62:113–119. doi: 10.1016/j.yhbeh.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J. Comp. Neurol. 2016 doi: 10.1002/cne.24116. http://dx.doi.org/10.1002/cne.24116. [DOI] [PMC free article] [PubMed]
- Puglia MH, Lillard TS, Morris JP, Connelly JJ. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2015;112:3308–3313. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, van IMH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ. No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology. 2012;37:1257–1266. doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009a;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 2009b;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Coomber P, Gonzalez-Lima F, Crews D. Functional connectivity among limbic brain areas: differential effects of incubation temperature and gonadal sex in the leopard gecko, Eublepharis macularius. Brain Behav. Evol. 2000;55:139–151. doi: 10.1159/000006648. [DOI] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Stehouwer JS, Inoue K, Voll RJ, Young LJ, Goodman MM. Synthesis and evaluation of C-11, F-18 and I-125 small molecule radioligands for detecting oxytocin receptors. Bioorg. Med. Chem. 2012;20:2721–2738. doi: 10.1016/j.bmc.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Borland JM, Larkin TE, O'Malley M, Albers HE. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 2016;74:164–172. doi: 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stivers JA, Kaltwasser MT, Hill PS, Hruby VJ, Crawley JN. Ventral tegmental oxytocin induces grooming. Peptides. 1988;9(Suppl. 1):223–231. doi: 10.1016/0196-9781(88)90248-3. [DOI] [PubMed] [Google Scholar]
- Teles MC, Almeida O, Lopes JS, Oliveira RF. Social interactions elicit rapid shifts in functional connectivity in the social decision-making network of zebrafish. Proc. Biol. Sci. 2015;282:20151099. doi: 10.1098/rspb.2015.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Verchinski BA, Bilek E, Goldman AL, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Neurogenetic effects of OXTR rs2254298 in the extended limbic system of healthy Caucasian adults. Biol. Psychiatry. 2011;70:e37–e39. doi: 10.1016/j.biopsych.2011.06.034. author reply e41–32. [DOI] [PubMed] [Google Scholar]
- Wacker DW, Ludwig M. Vasopressin, oxytocin, and social odor recognition. Horm. Behav. 2012;61:259–265. doi: 10.1016/j.yhbeh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Walum H, Waldman ID, Young LJ. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol. Psychiatry. 2016;79:251–257. doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Wilczynski W. Social experience organizes parallel networks in sensory and limbic forebrain. Dev. Neurobiol. 2007;67:285–303. doi: 10.1002/dneu.20347. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm. Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Zhang SW, Tai FD. Effects of nucleus accumbens oxytocin and its antagonist on social approach behavior. Behav. Pharmacol. 2016 doi: 10.1097/FBP.0000000000000212. http://dx.doi.org/10.1097/FBP.0000000000000212. [DOI] [PubMed]
- Zheng DJ, Larsson B, Phelps SM, Ophir AG. Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behav. Brain Res. 2013;246:139–147. doi: 10.1016/j.bbr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.