Abstract
The successful application of dihydropyrido[1,2-a]indolone (DHPI) substrates in Pd-catalyzed asymmetric allylic alkylation chemistry facilitates rapid access to multiple alkaloid frameworks in an enantioselective fashion. Strategic bromination at the indole C3 position greatly improved the allylic alkylation chemistry and enabled a highly efficient Negishi cross-coupling downstream. The first catalytic enantioselective total synthesis of (−)-goniomitine, along with divergent formal syntheses of (+)-aspidospermidine and (−)-quebrachamine are reported herein.
Keywords: total synthesis, allylic alkylation, asymmetric catalysis, Aspidosperma alkaloids, quaternary centers
Graphical Abstract
Magnum (DH)PI: The successful application of dihydropyrido[1,2-a]indolone (DHPI) substrates in Pd-catalyzed asymmetric allylic alkylation chemistry facilitates rapid access to multiple alkaloid frameworks in an enantioselective fashion. The first catalytic enantioselective total synthesis of (−)-goniomitine, along with divergent formal syntheses of (+)-aspidospermidine and (−)-quebrachamine are reported herein.
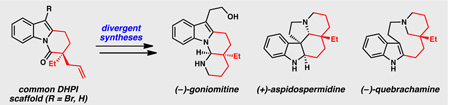
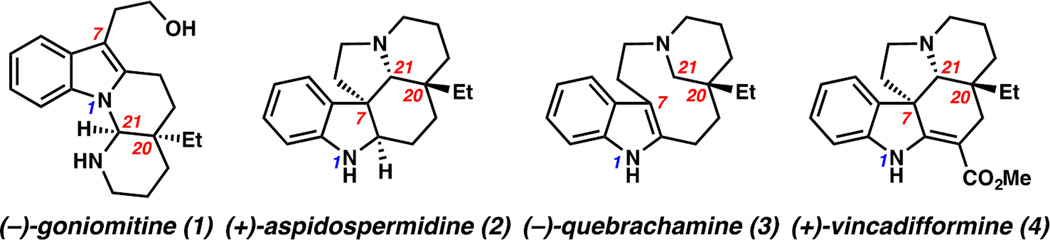
Monoterpene indole alkaloids have been extensively studied by chemists and biologists alike due to their vast structural diversity and broad biological activity.1 (−)-Goniomitine (1), isolated from the bark of Gonioma malagasy, is an Aspidosperma alkaloid with a unique octahydroindolo[1,2-a][1,8]naphthyridine core (Figure 1).2 The key structural differences between goniomitine (1) and many Aspidosperma alkaloids (e.g., 2–4, Figure 1)3 are the aminal functionality at C21 and the vestigial (2-hydroxy)ethyl moiety at C7.4
Figure 1.
Skeletally Diverse Aspidosperma alkaloids.
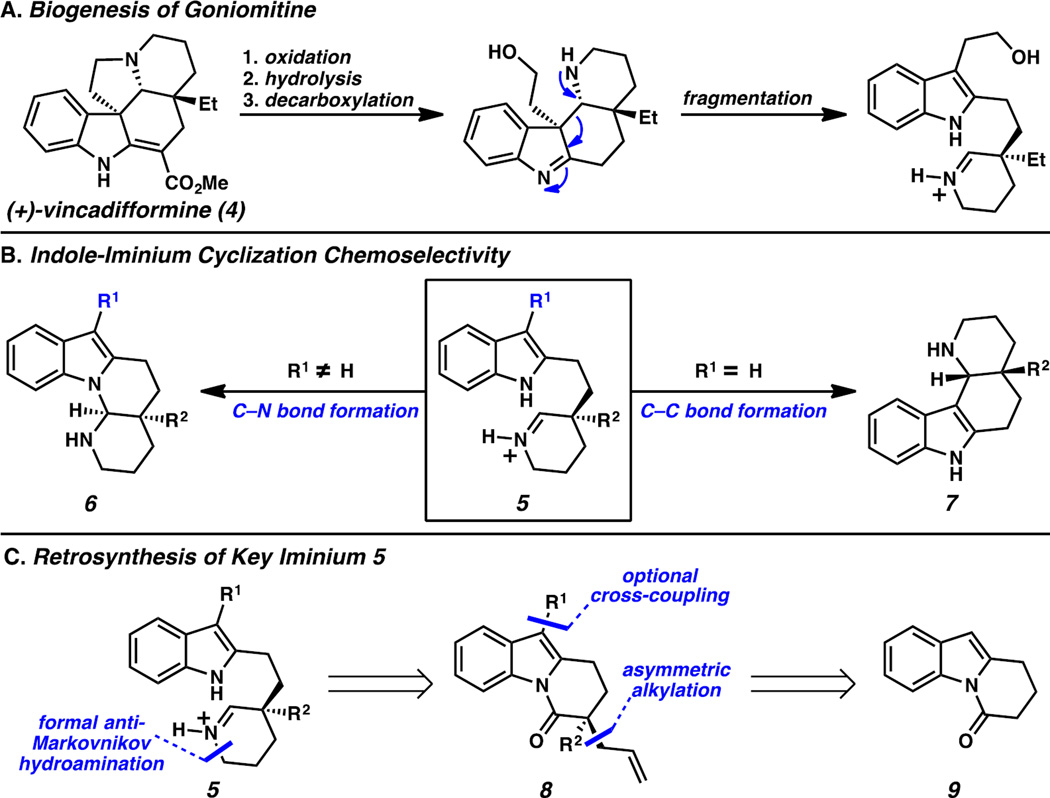
Biosynthetically, these features are believed to arise from oxidative degradation of the tryptamine fragment in vincadifformine (4, Scheme 1A) followed by fragmentation and N1–C21 recombination.5 Cyclizations between an indole and a C2-tethered iminium moiety (e.g., 5, Scheme 1B) are remarkably chemoselective. In the case of a C3-substituted indole fragment (e.g., 5, R1 ≠ H), cyclization proceeds via C–N bond formation to furnish aminal-containing tetracycle 6, as seen in previous syntheses of goniomitine (1).5–7 Conversely, a C3-unsubstituted indole fragment (e.g., 5, R1 = H) undergoes C–C bond formation followed by rearomatization to arrive at alternative tetracycle 7, a core that is present in numerous alkaloids (e.g., 2 & 4).8 We anticipated that iminium intermediates such as 5 could be accessed in straightforward fashion from compounds containing a dihydropyrido[1,2-a]indolone (DHPI) core (Scheme 1C). Retrosynthetically, we envisioned that the propylamine fragment in 5 could arise from an anti-Markovnikov hydroamination of the allyl functionality in α-quaternary lactam 8. Given our lab’s long-standing interest in the asymmetric synthesis of all-carbon quaternary centers, we believed that we could employ our Pd-catalyzed allylic alkylation chemistry to construct the quaternary stereocenter at C20 in an enantioselective fashion.9,10 We expected that a cross-coupling reaction could enable optional substitution at the C3 position of the DHPI scaffold, thereby providing selective routes to tetracycles 6 and 7. Therefore, development of this versatile substrate class in our Pd-catalyzed allylic alkylation chemistry would provide a powerful tool for divergent enantioselective syntheses of multiple Aspidosperma alkaloids.
Scheme 1.
A) Biogenesis of goniomitine from vincadifformine. B) Effects of C3 substitution on indole-iminium cyclization. C) Retrosynthetic analysis of 5.
Due to its antiproliferative activity and unusual structure, several groups have targeted goniomitine (1) for total synthesis.6,7 While modern approaches to this molecule have improved upon the seminal report by Takano and co-workers,7a a synthesis of goniomitine (1) that employs asymmetric catalysis to achieve stereocontrol has not yet been demonstrated. To date, asymmetric syntheses of goniomitine (1) have relied on either enzymatic resolutions or chiral pool materials.7 Furthermore, in previous syntheses of goniomitine, unless the (2-hydroxy)ethyl moiety was incorporated using a tryptophol-derived starting material, a multi-step sequence from an unsubstituted C7 position was required. We instead anticipated that a cross-coupling reaction between a C3-brominated DHPI and a suitable organometallic reagent would enable efficient access to the (2-hydroxy)ethyl fragment in the natural product. Realization of this synthetic plan would deliver the first catalytic enantioselective total synthesis of (−)-goniomitine (1).
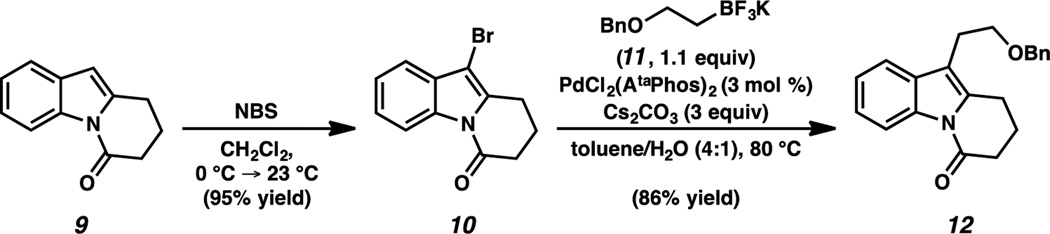
Our synthesis of (−)-goniomitine (1) commenced from known N-acyl indole 9,11 which underwent regioselective bromination to give heteroaryl bromide 10 in 95% yield (Scheme 2). Treatment of 10 with potassium (2-benzyloxy)ethyl trifluoroborate (11) and catalytic PdCl2(AtaPhos)2 afforded cross-coupled product 12 in 86% yield.12,13 Facile C-acylation and -alkylation of tricycles 9, 10 and 12 positioned us to investigate the heretofore untested asymmetric allylic alkylation of the dihydropyrido[1,2-a]indolone (DHPI) substrate class.14
Scheme 2.
Suzuki cross-coupling of a 3-bromoindole fragment. [a] AtaPhos = di-tert-butyl(4-dimethylamino)phenylphosphine.
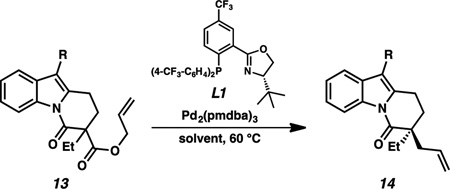
Exposure of (2-benzyloxy)ethyl-substituted DHPI 13a to standard Pd-catalyzed decarboxylative allylic alkylation conditions yielded the α-quaternary product 14a in 38% yield and 89% enantiomeric excess (Table 1, Entry 1). Switching from toluene to TBME as solvent greatly improved the reaction rate and yield, albeit with a minor decrease in enantioselectivity (Entry 2). Previous studies by our group have revealed that electron-withdrawing substituents on the lactam nitrogen atom provide the best results in the allylic alkylation chemistry.9b As the enolate intermediate would be in cross-conjugation with the arene π-system, we postulated that a bromide at the C3 position could provide both a beneficial electronic effect and a handle for cross-coupling downstream. While there are numerous reports of aryl bromides withstanding the conditions of Pd-catalyzed allylic alkylation reactions, their strategic implementation for cross-coupling events following the allylic alkylation is comparatively limited.15 Gratifyingly, brominated β-amidoester 13b reacted to give the desired quaternary alkylated product 14b (Entries 3 and 4), which in TBME was afforded in 83% yield and 96% ee with no observable interference from the C3 bromide (Entry 4). Given that the successful inclusion of a C3–H substrate in our allylic alkylation chemistry would enable divergent construction of additional alkaloids (vide infra), we were pleased to find that β-amidoester 13c could deliver α-quaternary lactam 14c in 71% yield and 94% ee (Entry 6).
Table 1.
Pd-catalyzed asymmetric allylic alkylation of DHPI substrates[a]
| |||||||
|---|---|---|---|---|---|---|---|
| entry | R (13 | solvent | Pd2(pmdba)3 [mol %] |
ligand [mol %] |
time [h] | yield (%)[b] | ee [%][c] |
| 1 | CH2CH2OBn (13a 14a) | toluene | 10 | 25 | 72 | 38 | 89 |
| 2 | CH2CH2OBn (13a 14a) | TBME | 10 | 25 | 24 | 59 | 87 |
| 3 | Br (13b 14b) | toluene | 5 | 12.5 | 24 | 21 | 93 |
| 4 | Br (13b 14b) | TBME | 5 | 12.5 | 8 | 83 | 96 |
| 5 | H (13c 14c) | toluene | 10 | 25 | 48 | 54 | 92 |
| 6 | H (13c 14c) | TBME | 5 | 12.5 | 24 | 71 | 94 |
Reactions were performed in stated solvent (0.033 M) at 60 °C.
Yield of isolated product.
Determined by chiral SFC.
pmdba = 4,4’-dimethoxydibenzylideneacetone.
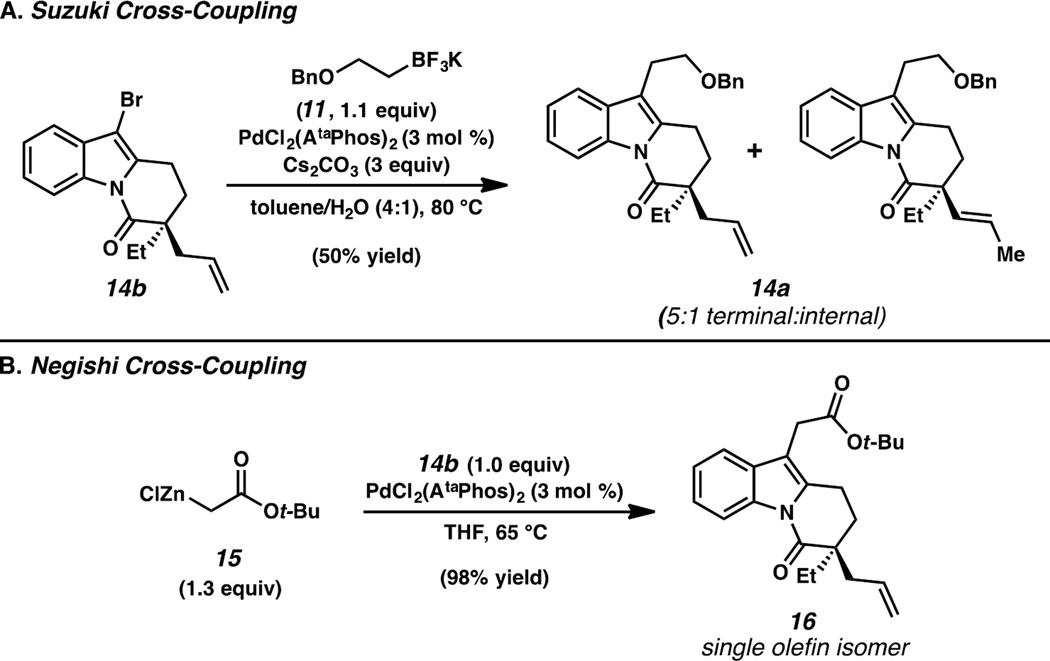
We next turned our attention toward the cross-coupling of brominated α-quaternary lactam 14b with a suitable hydroxyethyl surrogate. Unfortunately, we found that the Suzuki reaction between 14b and trifluoroborate 11 could not be improved beyond a 50% yield of inseparable olefin isomers 14a in a 5:1 ratio (Scheme 3A). We hypothesized that a Pd–H species was responsible for this undesired isomerization pathway, and sought to identify an alternative Csp3 nucleophilic coupling partner that would not allow for facile β-hydride elimination. Recognizing that a reduction would ultimately be required to convert the amide present in 14b to the aminal present in goniomitine (1), we decided to incorporate a substituent in a higher oxidation state via the cross-coupling, thereby allowing concomitant unveiling of the (2-hydroxy)ethyl moiety at a later stage. After investigating a multitude of Negishi conditions, we were thrilled to find that Reformatsky reagent 15 could be efficiently coupled with heteroaryl bromide 14b using catalytic PdCl2(AtaPhos)2 to deliver arylated product 16 in 98% yield without any detectable amount of undesired olefin isomers (Scheme 3B).16
Scheme 3.
Cross-coupling reactivity of α-quaternary DHPI 14b.
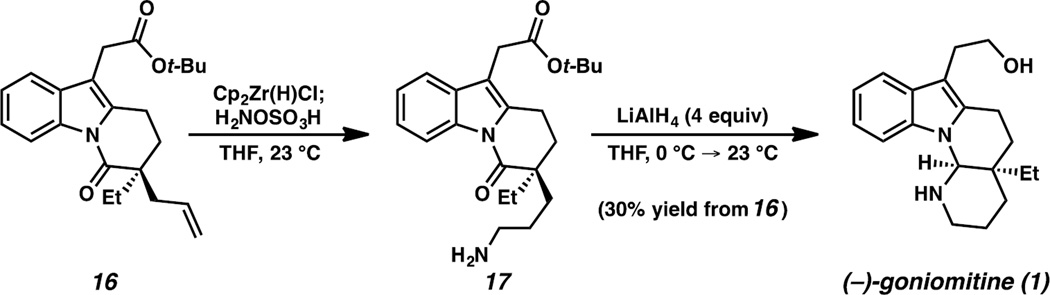
With the requisite carbon–carbon bonds established, we began investigating methods to effect an anti-Markovnikov hydroamination of the terminal olefin of 16. To this end, we employed a one-pot hydrozirconation/amination sequence reported by Hartwig and co-workers.17 To our knowledge, this is the first implementation of Hartwig’s hydrozirconation/amination protocol in the context of natural product synthesis. Following this formal hydroamination, we were pleased to find that complete reduction of the tert-butyl ester of 17 could be achieved alongside partial reduction of the amide carbonyl in one pot using a single reductant. In the event, primary amine 17 was subjected to LiAlH4 in THF, followed by acidic workup, to afford (−)-goniomitine (1) in 30% yield from 16 (Scheme 4).
Scheme 4.
Completion of the synthesis of (−)-goniomitine.
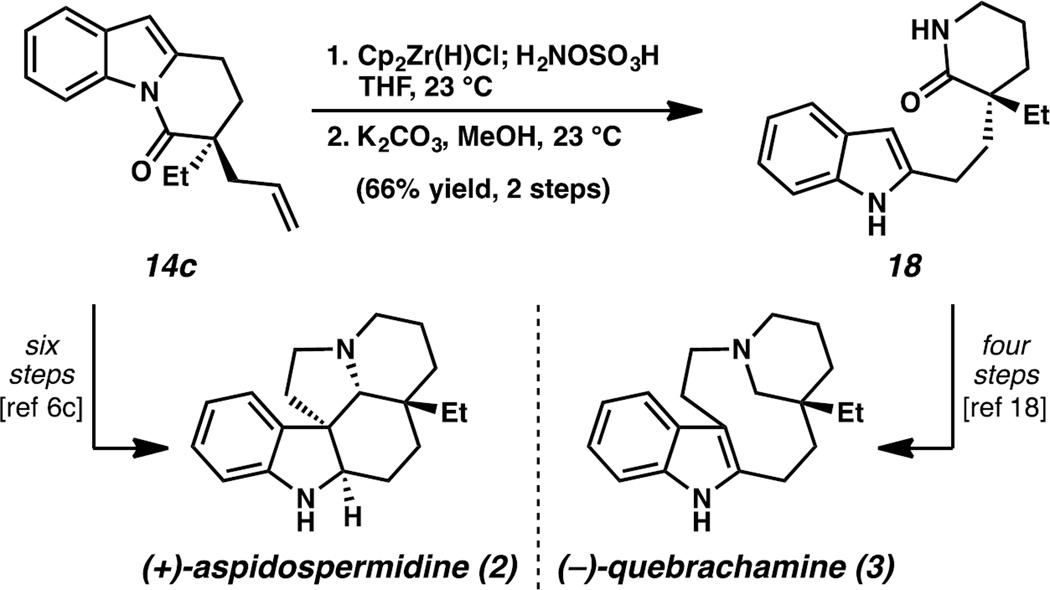
Having completed the total synthesis of (−)-goniomitine (1), we sought to leverage the flexibility of the DHPI scaffold by exploiting the chemoselectivity in cyclizations of an indole with a C2-tethered iminium functionality (Scheme 1B). Indeed, the synthesis of 14c completes an enantioselective formal synthesis of (+)-aspidospermidine (2) (Scheme 5).6c Furthermore, treatment of 14c with the aforementioned hydroamination conditions followed by a mild amide exchange furnishes free N–H α-quaternary δ-lactam 18 in 66% yield over two steps, constituting an asymmetric formal synthesis of (−)-quebrachamine (3).18
Scheme 5.
Asymmetric formal syntheses of other Aspidosperma alkaloids.
In summary, we have completed the first catalytic enantioselective total synthesis of (−)-goniomitine (1) in 11 steps and 8% overall yield from indole, or 7 steps and 17% overall yield from known DHPI 9. The redox efficiency and freedom from protecting-group manipulations is a marked improvement from previous nonracemic syntheses, which deliver the target in 10–28 steps and 0.25–3.2% overall yield from commercial materials. Rationally designed heteroaryl bromide 13b underwent Pd-catalyzed allylic alkylation to deliver the α-quaternary product (14b) in 83% yield and 96% ee. The surprisingly robust Caryl–Br bond served as a handle for a subsequent Negishi cross-coupling. The compatibility of aryl bromides in our allylic alkylation reactions, along with the identification of cross-coupling conditions that do not isomerize the allyl group, provide a powerful platform for the convergent synthesis of complex organic molecules. Additionally, by completing formal syntheses of (+)-aspidospermidine (2) and (−)-quebrachamine (3), we demonstrate the ability of the DHPI scaffold to provide divergent, enantioselective access to structurally diverse alkaloid frameworks. Efforts to expand upon the capabilities of allylic alkylation/cross-coupling sequences and to further exploit the utility of DHPIs in the context of alkaloid total synthesis will be reported in due course.
Supplementary Material
Acknowledgments
The authors wish to thank NIH-NIGMS (R01GM080269), Amgen, the Gordon and Betty Moore Foundation, and Caltech for financial support. B.P.P. thanks the NSF for a predoctoral fellowship (Grant DGE-1144469). Y. N. thanks Toray Industries Inc. for a postdoctoral fellowship. The authors thank Mona Shahgholi and Naseem Torian for mass spectrometry assistance, and Dr. Scott Virgil (Caltech) and the Caltech Center for Catalysis and Chemical Synthesis, for instrumentation assistance.
References
- 1.For reviews, see: Saxton JE. Alkaloids. 1998;51:1–197. O’Connor SE. J. J. Maresh, Nat. Prod. Rep. 2006;23:532–547. doi: 10.1039/b512615k.
- 2.For initial isolation of goniomitine (1) and proposed biosynthesis from vincadifformine (4), see: Randriambola L, Quirion J-C, Kan-Fan C, Husson H-P. Tetrahedron Lett. 1987;28:2123–2126.
- 3.a) Biemann K, Friedmann-Spiteller M, Spiteller G. Tetrahedron Lett. 1961;2:485–492. [Google Scholar]; b) Hesse O. Ber. Dtsch. Chem. Ges. 1880;13:2308–2309. [Google Scholar]; c) Djerassi C, Budzikiewicz H, Wilson JM, Gosset J, Le Men J, Janot M-M. Tetrahedron Lett. 1962;3:235–239. [Google Scholar]
- 4.For a uniform numbering system of monoterpene indole alkaloids, see: Le Men J, Taylor WI. Experientia. 1965;21:508–510. doi: 10.1007/BF02138961.
- 5.For a biomimetic semisynthesis of goniomitine (1) from vincadifformine (4), see: Lewin G, Bernadat G, Aubert G, Cresteil T. Tetrahedron. 2013;69:1622–1627.
- 6.For a total synthesis of (±)-goniomitine (1), as well as the evaluation of its antiproliferative activity, see: De Simone F, Gertsch J, Waser J. Angew. Chem. Int. Ed. 2010;49:5767–5770. doi: 10.1002/anie.201001853. Angew. Chem. 2010, 122, 5903–5906. For other nonenantioselective total syntheses, see: Morales CL, Pagenkopf BL. Org. Lett. 2008;10:157–159. doi: 10.1021/ol702376j. Jiao L, Herdtweck E, Bach T. J. Am. Chem. Soc. 2012;134:14563–14572. doi: 10.1021/ja3058138. Xu Z, Wang Q, Zhu J. Angew. Chem. Int. Ed. 2013;52:3272–3276. doi: 10.1002/anie.201209970. Angew. Chem. 2013, 125, 3354–3358. Zhou B, Du J, Yang Y, Li Y. Chem. Eur. J. 2014;20:12768–12772. doi: 10.1002/chem.201403973. Vellucci JK, Beaudry CM. Org. Lett. 2015;17:4558–4560. doi: 10.1021/acs.orglett.5b02277.
- 7.For asymmetric syntheses of goniomitine (1) see: Takano S, Sato T, Inomata K, Ogasawara K. J. Chem. Soc., Chem. Commun. 1991:462–464. Mizutani M, Inagaki F, Nakanishi T, Yanagihara C, Tamai I, Mukai C. Org. Lett. 2011;13:1796–1799. doi: 10.1021/ol200320z. Zhou S, Jia Y. Org. Lett. 2014;16:3416–3418. doi: 10.1021/ol501341b.
- 8.For selected examples of this type of cyclization (i.e., 5 – 7), see: Nicolaou KC, Dalby SM, Majumder U. J. Am. Chem. Soc. 2008;130:14942–14943. doi: 10.1021/ja806176w. Chen Z, Zhou S, Jia Y. J. Org. Chem. 2015;80:12545–12551. doi: 10.1021/acs.joc.5b02402. Mizutani M, Yasuda S, Mukai C. Chem. Commun. 2014;50:5782–5785. doi: 10.1039/c4cc01843e.
- 9.For examples of asymmetric allylic alkylation of nitrogen-containing substrates published by our group, see: Behenna DC, Mohr JT, Sherden NH, Marinescu SC, Harned AM, Tani K, Seto M, Ma S, Novák Z, Krout MR, McFadden RM, Roizen JL, Enquist JA, Jr, White DE, Levine SR, Petrova KV, Iwashita A, Virgil SC, Stoltz BM. Chem. Eur. J. 2011;17:14199–14223. doi: 10.1002/chem.201003383. Behenna DC, Liu Y, Yurino T, Kim J, White DE, Virgil SC, Stoltz BM. Nat. Chem. 2012;4:130–133. doi: 10.1038/nchem.1222. Bennett NB, Duquette DC, Kim J, Liu W-B, Marziale AN, Behenna DC, Virgil SC, Stoltz BM. Chem. Eur. J. 2013;19:4414–4418. doi: 10.1002/chem.201300030. Korch KM, Eidamshaus C, Behenna DC, Nam S, Horne D, Stoltz BM. Angew. Chem. Int. Ed. 2015;54:179–183. doi: 10.1002/anie.201408609. Angew. Chem. 2015, 127, 181–185. Numajiri Y, Jiménez-Osés G, Wang B, Houk KN, Stoltz BM. Org. Lett. 2015;17:1082–1085. doi: 10.1021/ol503425t. Numajiri Y, Pritchett BP, Chiyoda K, Stoltz BM. J. Am. Chem. Soc. 2015;137:1040–1043. doi: 10.1021/ja512124c.
- 10.For asymmetric allylic alkylation of carbazolone substrates, see: Gartshore CJ, Lupton DW. Angew. Chem. Int. Ed. 2013;52:4113–4116. doi: 10.1002/anie.201209069. Angew. Chem. 2013, 125, 4207–4210. Li Z, Zhang S, Wu S, Shen X, Zou L, Wang F, Li X, Peng F, Zhang H, Shao Z. Angew. Chem. Int. Ed. 2013;52:4117–4121. doi: 10.1002/anie.201209878. Angew. Chem. 2013, 125, 4211–4215.
- 11. Jiao L, Bach T. J. Am. Chem. Soc. 2011;133:12990–12993. doi: 10.1021/ja2055066. b) We developed a 4-step synthesis of 9 from indole that was more practical and scalable. For details, see the supporting information.
- 12.Fleury-Brégeot N, Presset M, Beaumard F, Colombel V, Oehlrich D, Rombouts F, Molander GA. J. Org. Chem. 2012;77:10399–10408. doi: 10.1021/jo3021665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guram AS, King AO, Allen JG, Wang X, Schenkel LB, Chan J, Bunel EE, Faul MM, Larsen RD, Martinelli MJ, Reider PJ. Org. Lett. 2006;8:1787–1789. doi: 10.1021/ol060268g. [DOI] [PubMed] [Google Scholar]
- 14.For details regarding the synthesis of 13a–c, see the supporting information.
- 15.For selected examples of Mizoroki–Heck and Suzuki cross-couplings, respectively, of allylic alkylation products, see: Mingoia F, Vitale M, Madec D, Prestat G, Poli G. Tetrahedron Lett. 2008;49:760–763. Zhuo C-X, You S-L. Angew. Chem. Int. Ed. 2013;52:10056–10059. doi: 10.1002/anie.201304591. Angew. Chem. 2013, 125, 10240–10243.
- 16.Multiple parameters were investigated for the Suzuki reaction, to no avail. Arylation using a Reformatsky reagent prepared in situ from tert-butyl bromoacetate proceeded in high yields using several palladium precatalysts, but as a 1:3–5:1 mixture of olefin isomers (terminal:internal). The reaction employing organozinc chloride 15, purchased from Rieke Metals, and PdCl2(AtaPhos)2 was singularly successful in this transformation.
- 17.Strom AE, Hartwig JF. J. Org. Chem. 2013;78:8909–8914. doi: 10.1021/jo401498w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajtos B, Pagenkopf BL. Eur. J. Org. Chem. 2009;7:1072–1077. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.