Abstract
Whisker hair follicles are sensory organs that sense touch and perform tactile discrimination in animals, and they are sites where sensory impulses are initiated when whisker hairs touch an object. The sensory signals are then conveyed by whisker afferent fibers to the brain for sensory perception. Electrophysiological property and chemical sensitivity of whisker afferent fibers, important factors affecting whisker sensory processing, are largely not known. In the present study, we performed patch-clamp recordings from pre-identified whisker afferent neurons in whole-mount trigeminal ganglion preparations and characterized their electrophysiological property and sensitivity to ATP, serotonin and glutamate. Of 97 whisker afferent neurons examined, 67% of them are found to be large-sized (diameter ≥45 µm) cells and 33% of them are medium- to small-sized (diameter <45 µm) cells. Almost every large-sized whisker afferent neuron fires a single action potential but many (40%) small/medium-sized whisker afferent neurons fire multiple action potentials in response to prolonged stepwise depolarization. Other electrophysiological properties including resting membrane potential, action potential threshold, and membrane input resistance are also significantly different between large-sized and small/medium-sized whisker afferent neurons. Most large-sized and many small/medium-sized whisker afferent neurons are sensitive to ATP and/or serotonin, and ATP and/or serotonin could evoke strong inward currents in these cells. In contrast, few whisker afferent neurons are sensitive to glutamate. Our results raise a possibility that ATP and/or serotonin may be chemical messengers involving sensory signaling for different types of rat whisker afferent fibers.
Keywords: Mechanotransduction; mechanoreceptor, whisker hair follicle, serotonin, 5-HT receptors, ATP
Introduction
Whisker hair follicles in animals are important for tactile tasks including environmental exploration, social interaction, and tactile discrimination. These sensory tasks are accomplished by whisker hair follicle mechanoreceptors which are activated when whiskers touch an object. Several types of mechanoreceptors have been identified within whisker hair follicles of different species, including Merkel discs, lanceolate endings, reticular endings and other endings (Rice et al., 1993, Ebara et al., 2002). These mechanoreceptors are located at different parts of whisker hair follicles. Most Merkel discs are located in the outer root sheath in the enlargement section of a whisker hair follicle, and a small number of Merkel discs are present in the epidermal layer around the collar of a whisker hair follicle (Ebara et al., 2002). In contrast, both lanceolate endings and reticular endings are located in mesenchymal sheath layer, and lanceolate endings are present in front part and reticular endings in rear part of whisker hair follicle enlargement section (Rice et al., 1993). Structurally, Merkel discs consist of epidermal Merkel cells and their associated large caliber Aβ-afferent endings (Merkel, 1875, Iggo and Muir, 1969). Lanceolate endings in whisker hair follicles are Aβ-afferent endings with associated terminal Schwann cells and reticular endings appear to be simple nerve endings derived from Aβ-afferent fibers (Ebara et al., 2002). In addition to Aβ-afferent fibers that are large calibers in diameters, whisker hair follicles are innervated by smaller caliber afferents that are likely to be C-fibers and/or Aδ-fibers (Rice et al., 1993). Neonatal animals treated with capsaicin resulted in substantial loss of smaller caliber afferent fibers within whisker hair follicles (Waite and Li, 1993), suggesting that capsaicin-sensitive afferent fibers innervate whisker hair follicles and may play a role in nociception.
When whisker hairs are stimulated by a hair displacement, sensory impulses could be recorded from whisker afferent bundles (Ikeda et al., 2014). The tactile-evoked afferent impulses show continual but irregular firing with a feature of slow adaptation during tactile stimulation. The term slowly adapting type 1 response (SA1) has been used to describe this type of electrophysiological response to tactile stimulation (Iggo and Muir, 1969). SA1 responses were long thought to be a result of mechanical transduction at Merkel discs (Iggo and Muir, 1969, Halata et al., 2003). However, only recently have the mechanisms underlying mechanical transduction at Merkel discs been uncovered (Ikeda et al., 2014, Maksimovic et al., 2014, Woo et al., 2014). Merkel cells have now been established as an essential site of tactile transduction and Piezo2 channels are mechanotransducers in Merkel cells (Ikeda et al., 2014, Maksimovic et al., 2014, Woo et al., 2014). Since sensory information transduced by mechanoreceptors (e.g. Merkel discs, lanceolate endings, and reticular endings) and nociceptors (free nerve endings of C- and/or Aδ-afferent fibers) is encoded as action potential firing, a study on the intrinsic electrophysiological properties of whisker afferent neurons would provide essential information to help understand sensory encoding in whisker hair follicles.
In addition to the intrinsic electrophysiological properties, chemical messengers may either directly excite whisker afferent endings or significantly modify their excitability. Therefore, a study on the chemical sensitivity of whisker afferent neurons would also provide useful information to understand sensory processing at whisker afferent endings. Previously studies have hypothesized that ATP, serotonin, and glutamate may be transmitters mediating tactile signaling from Merkel cells to their associated Aβ-afferent endings (Maksimovic et al., 2013). However, it is not known whether these candidate chemical messengers may excite rat whisker afferent neurons. In the present study, we retrogradely labeled rat whisker afferent neurons and performed patch-clamp recordings from them, and we characterized their intrinsic electrophysiological properties and their sensitivity to ATP, serotonin, and glutamate.
Materials and Methods
Sprague Dawley rats aged 4-9 weeks were used. Animal care and use conformed to NIH guidelines for care and use of experimental animals. Experimental protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee. To retrogradely label whisker afferent neurons, DiI (20 mg/ml) was injected into whisker hair follicles 1-4 weeks before patch-clamp recordings. In brief, animals were anesthetized by isoflurane, a long whisker hair (D2 whisker or adjacent whisker) was lifted vertically by a forceps, and a sharp glass electrode containing DiI solution was inserted along the whisker hair into the hair follicle. The glass electrode was affixed on an injection holder of a stereotaxic apparatus to assist a precise insertion of the electrode tip into whisker hair follicles (distance from follicle opening: 1 mm). A small volume of DiI solution (1.5–2.0 µl) was injected into the follicle over 2.5 min using a micro-injection system.
Seven to 27 days after DiI injection, animals were sacrificed and trigeminal ganglions (TGs) were dissected out from the animals. Under a dissection microscope, connective tissues on the surface of TGs were removed by a pair of forceps. The TGs were then fixed in a recording chamber with a tissue anchor and submerged in a Krebs solution that contained (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES and 10 glucose; the solution was saturated with 95 % O2 and 5% CO2, had pH of 7.3 and osmolarity of 325 mOsm, and was maintained at the room temperature of 23oC - 24oC. The recording chamber was mounted on the stage of an Olympus IX50 microscope that was equipped with IR-DIC and fluorescent imaging systems. The TGs were exposed to 0.2-0.25% dispase II plus 0.05-0.1% collagenase in the Krebs solution for 5-15 min, and the enzymes were then washed off with the Krebs solution. Under a 40X objective, the labeled TG neurons were identified using the fluorescent imaging system.
Patch-clamp recordings were made at the room temperature from DiI-labeled neurons situated in whole-mount TGs. In brief, recording electrodes were fabricated using a P-97 Brown-Flaming Micropipette Puller, and the resistance of each electrode was ∼5 MΩ after filling recording internal solution. Recording internal solution was a K+-based solution containing (in mM): 135 K-gluconate, 5 KCl, 0.5 CaCl2, 2.4 MgCl2, 5 EGTA, 10 HEPES, 5 Na2ATP and 0.33 GTP-TRIS salt; the pH of the solution was adjusted to 7.3 with KOH. For making a seal onto the membrane of a TG neuron, a positive pressure was delivered into patch-clamp recording electrodes before and during approaching the cell. The positive pressure was produced by compressing ∼5 ml air in the 50-ml syringe that was connected to the recording electrode. Under the positive pressure from the electrode tip, the electrode tip was lowered until it penetrated through the satellite cell that wrapped on the targeted TG neuron. The positive pressure in the recording electrode was then reduced and the position of the electrode tip adjusted to allow it to directly contact the membranes of the targeted TG neuron. Once the recording electrode touched TG neuron membranes, the positive pressure was gradually reduced and a negative pressure was applied through the syringe until the formation of gigaohm seal (usually 2 to 4 GΩ) between the recording electrode and the TG neuron membranes. Unless otherwise stated, signals were amplified and filtered at 2 kHz using the Multiclamp 700 A amplifier and sampled at 4 kHz using pCLAMP10 software (Molecular Devices). To determine membrane and action potential properties of DiI-labeled TG neurons, under the whole-cell current-clamp mode, step current pulses were injected into cells through patch-clamp electrodes from −200 pA to 2900 pA in increments of 100 pA per step and the duration of each pulse was 200 ms. Under the voltage-clamp mode, voltage-activated outward currents were recorded with the K+-based internal solution. Unless otherwise indicated, TG neurons were held at −76 mV in voltage-clamp experiments, and voltage steps were applied from −136 mV to 64 mV with increments of 20 mV each step and a step duration of 100 ms. To determine chemical sensitivity of DiI-labeled TG neurons to ATP, serotonin, and glutamate, ATP (250 µM), serotonin (100 µM), or glutamate (500 µM) was bath applied onto the recorded TG neurons under either voltage-clamp configuration with cells held at −76 mV or under the current-clamp configuration. Y25130 (Tocris, USA), a 5-HT3 receptor blocker, was tested at 200 nM for its inhibitory effect on serotonin-evoked responses. It was pre-applied 1 min prior to serotonin application and then co-applied with serotonin. In all electrophysiology experiments, unless otherwise indicated, membrane voltages mentioned in the texts have been corrected for the calculated junction potentials of −16 mV.
Results
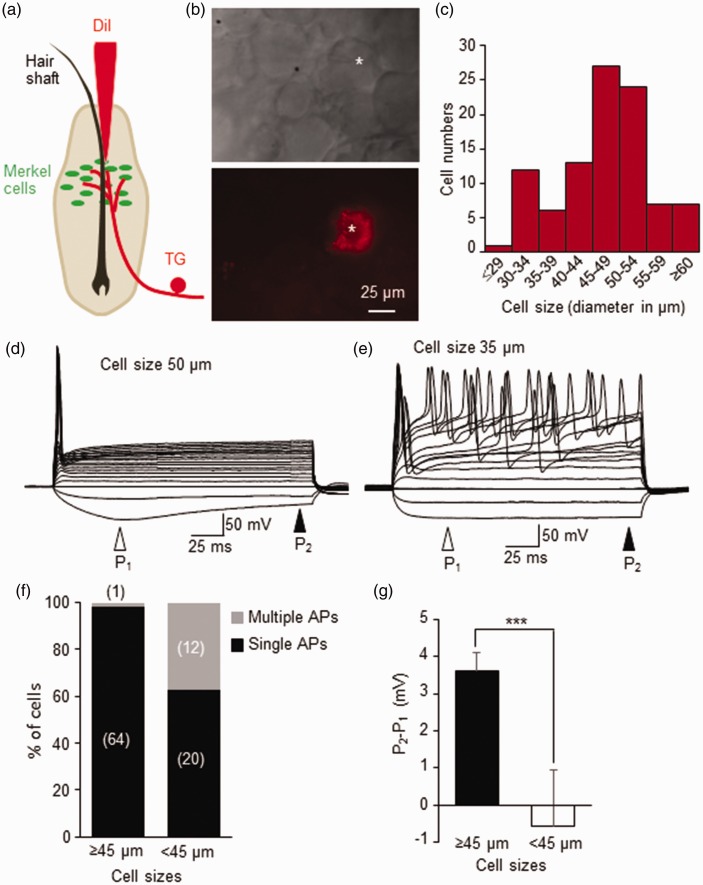
Whisker afferent neurons in trigeminal ganglion (TG) were retrogradely labeled by micro-injection of fluorescent tracer DiI into individual hair follicles (Figure 1a, also see (Ikeda and Gu, 2014)). DiI-labeled whisker afferent neurons could be observed in TGs 7 days or longer following the DiI-microinjection (Figure 1b). Most (67%, 65/97) of DiI-labeled neurons were large-sized cells with diameters ≥ 45 µm, and some DiI-labeled neurons were medium- to small-sized cells with diameters < 45 µm (33%, 32/97, Figure 1c). All patch-clamp recordings were performed from DiI-labeled whisker afferent neurons in situ in whole-mount TGs. Basic electrophysiological parameters of membrane properties for these whisker afferent neurons were obtained under the current-clamp mode (Table 1). These parameters including membrane capacitance, input resistance, resting membrane potential, action potential (AP) rheobase, AP threshold, AP width, and AP amplitude. All these parameters were significantly different between large-sized whisker afferent neurons (n = 65) and small/medium-sized neurons (n = 32, P < 0.01 to P < 0.001). For example, resting membrane potential was ∼74 mV for large-sized whisker afferent neurons but was ∼66 mV for small/medium-sized whisker afferent neurons (Table 1). Action potential firing induced by the injection of stepwise depolarizing currents showed two main patterns, single AP firing (Figure 1d) and multiple AP firing (Figure 1e). Almost every large-sized whisker afferent neuron (64/65 cells) fired only a single action potential in response to supra-threshold stepwise membrane depolarization (Figure 1d & f). In contrast, as many as 40% (12/32) of small/medium-sized whisker afferent neurons fired multiple action potentials and the remaining (20/32 cells) small/medium-sized whisker afferent neurons fired single action potentials in response to supra-threshold stepwise membrane depolarization (Figure 1e&f). Another significant different feature between the two populations of whisker afferent neurons was that large-sized whisker afferent neurons (n = 65, Figure 1d&g) displayed sag potentials in response to hyperpolarizing current steps but the sag potentials were not clearly observed in small/medium-sized whisker afferent neurons (n = 32, Figure 1e&g)
Figure 1.
Membrane responses and action potential firing patterns of retrogradely labeled whisker afferent neurons. (a) Schematic diagram shows intra-follicle microinjection of DiI to retrogradely label whisker afferent neurons in trigeminal ganglia (TG). The intra-follicle microinjection was performed in vivo on anesthetized rats. (b) Images show a DiI-labeled whisker afferent neuron (star indicated) in a trigeminal ganglion viewed under bright field (top panel) and fluorescent microscopy (bottom panel). (c) Cell size distribution of 97 DiI-labeled whisker afferent neurons that were randomly selected for patch-clamp recordings in this study. (d) A set of sample traces shows a single action potential firing pattern in a large-sized (50 µm in diameter) DiI-labeled neuron. A sag potential can be seen in this cell during the application of hyperpolarizing current steps. P1 and P2 are potentials at the position indicated by an open and a closed arrow, respectively. (e) A set of sample traces shows a multiple action potential firing pattern in a small/medium-sized (35 µm) DiI-labeled neuron. There is no change in hyperpolarizing membrane potentials in this cell during the application of hyperpolarizing current steps. (f) Percent of DiI-labeled neurons that fire single or multiple action potentials. Left bar, large-sized cells with diameter ≥45 µm. Right bar, cells with diameter <45 µm. (g) Summary data of sag potentials (P2-P1) in large-sized (≥45 µm) and small/medium-sized (<45 µm) cells. Data represent Mean ± SEM, ***P < 0.001.
Table 1.
Basic membrane properties of rat whisker afferent neurons
| Membrane capacitance (pF) | Input resistance (MΩ) | RMP (mV) | AP rheobase (pA) | AP threshold (mV) | AP width (ms) | AP amplitude (mV) | |
|---|---|---|---|---|---|---|---|
| Cell size ≥ 45 µm (n = 65) | 107.4 ± 3.5*** | 45.9 ± 2.4*** | −74.3 ± 0.6*** | 1586.2 ± 77.8*** | −33.1 ± 1.3*** | 1.4 ± 0.1** | 94.9 ± 1.4*** |
| Cell size < 45 µm (n = 32) | 59.9 ± 3.9 | 88.3 ± 8.1 | −66.4 ± 1.8 | 665.6 ± 68.6 | −24.7 ± 1.9 | 2.0 ± 0.2 | 82.5 ± 3.0 |
Note. Whisker afferent neurons were recorded under the current-clamp mode. Abbreviations. RMP, resting membrane potential; AP, action potential. Data represent Mean ± SEM, **P < 0.01, ***P < 0.001.
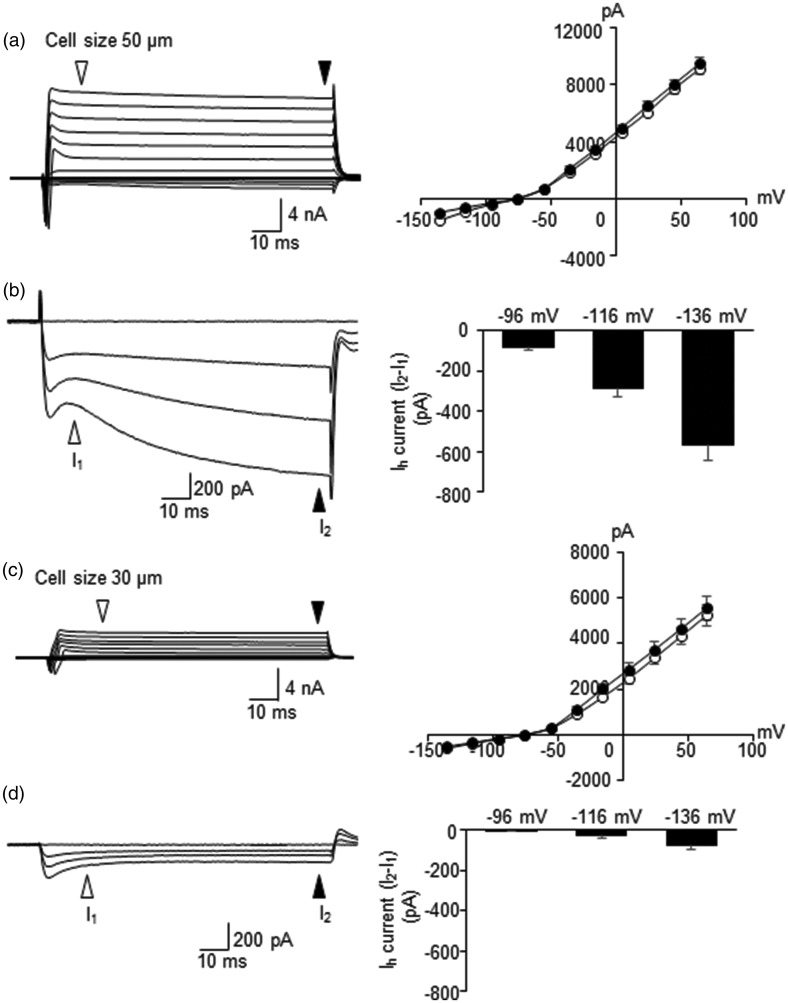
Under voltage-clamp mode, transient inward currents and sustained outward currents could be elicited by depolarizing voltage steps (Figure 2a&c), which represent mainly voltage-gated Na+ currents and voltage-gated K+ currents, respectively. In both large-sized and small/medium-sized whisker afferent neurons, the outward currents were predominantly mediated by the activation of non-inactivating voltage-gated K+ channels (Figure 2a&c). A unique feature of large-sized whisker afferent neurons was the presence of strong inward currents in response to hyperpolarizing voltage steps (Ih currents, Figure 2b, n = 29), a result consistent with the presence of sag potentials in these neurons as shown in Figure 1d&g. In contrast, Ih currents were absent or very small in small/medium-sized whisker afferent neurons (Figure 2d, n = 29).
Figure 2.
Voltage-activated currents in retrogradely labeled whisker afferent neurons. (a) Left panel shows a set of sample traces of voltage-activated currents recorded from a large-sized (50 µm in diameter) DiI-labeled whisker afferent neuron. Right panel shows I-V relationship of the same cell at the places indicated by white and black arrows in A. (b) Right panel is at an expanded scale of A to show hyperpolarization-activated inward currents (Ih currents), which is the difference between the currents at the two arrow-indicated sites. Left panel is summary data of Ih currents at −96, −116, and −136 mV for large-sized cells (≥45 µm). (c&d) Similar to A&B except data are from small/medium-sized cells (<45 µm). Data represent Mean ± SEM.
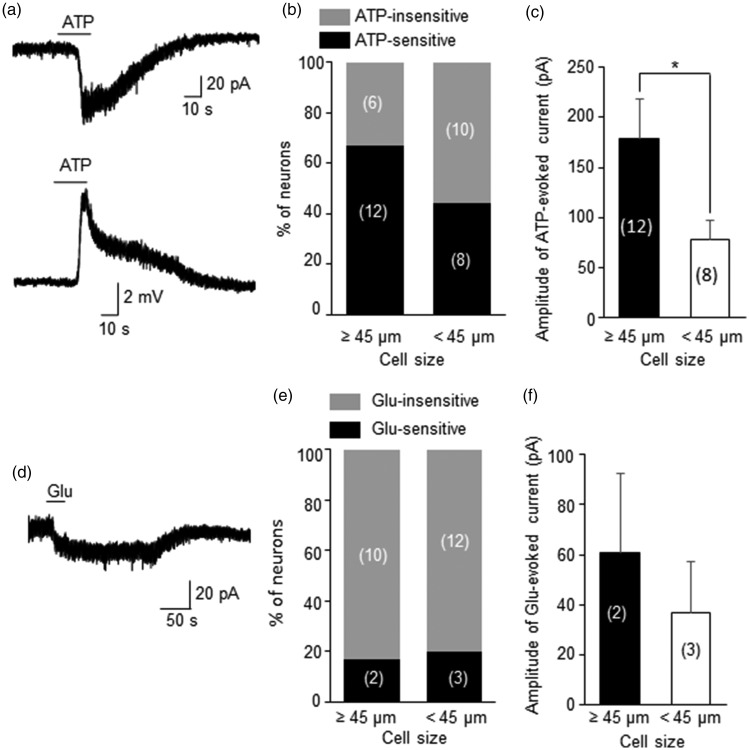
We tested ATP sensitivity of DiI-labeled whisker afferent neurons by bath application of ATP (250 μM, Figure 3a–c). A relatively high concentration of ATP was used in the present study in considering factors such as diffusion barrier and ATP degradation by ecto-ATPase in whole-amount TG preparation. For similar reasons, relatively high concentrations of glutamate and serotonin were used in the present study as well. Many DiI-labeled whisker afferent neurons responded to 250 μM ATP with inward currents under the voltage-clamp mode and membrane depolarization under the current-clamp mode (Figure 3a). Of 18 large-sized whisker afferent neurons, 12 of them (67%) were ATP-sensitive and 6 (33%) were ATP-insensitive as determined by inward currents recorded under the voltage-clamp mode (Figure 3b). Of 18 small/medium-sized whisker afferent neurons, 8 of them (44%) were ATP-sensitive and 56% (10/18) were ATP-insensitive (Figure 3b). For ATP-sensitive cells, the amplitudes of ATP-evoked inward currents were significantly higher in large-sized ATP-sensitive neurons than in small/medium-sized ones (Figure 3c).
Figure 3.
Sensitivity to ATP or glutamate of retrogradely labeled whisker afferent neurons. (a) Two sample traces show the inward current (top) and membrane depolarization (bottom) recorded from a DiI-labeled whisker afferent neuron (cell size 50 µm) following the applications of 250 µM ATP. (b) Bar graph shows percent of neurons that are ATP-sensitive and ATP-insensitive. Left bar, cell size ≥45 µm; Right bar, cell size <45 µm. Cell numbers of each category are indicated in the bars. (c) Bar graph shows the amplitudes of ATP-evoked inward currents in ATP-sensitive neurons. Left bar, cell size ≥45 µm (n = 12); Right bar, cell size <45 µm (n = 8). (d) A sample trace shows an inward current recorded from a DiI-labeled whisker afferent neuron (cell size 45 µm) following the application of 500 µM glutamate (Glu). (e) Bar graph shows percent of neurons that are Glu-sensitive and Glu-insensitive. Left bar, cell size ≥45 µm; Right bar, cell size <45 µm. Cell numbers of each category are indicated in the bars. (f) Bar graph shows the amplitude of Glu-evoked inward currents in Glu-sensitive neurons. Left bar, cell size ≥45 µm (n = 2); Right bar, cell size <45 µm (n = 3). Data represent Mean ± SEM, *P < 0.05.
Glutamate-sensitivity of DiI-labeled whisker afferent neurons was tested by bath application of a high concentration of glutamate (Glu, 500 µM, Figure 3d–e). A very small numbers of DiI-labeled neurons (5/22) responded to glutamate with very small inward currents (Figure 3d&e), and most large-sized (10/12) as well as small/medium-sized (12/15) whisker afferent neurons were completely insensitive to 500 µM glutamate (Figure 3e). For the whisker afferent neurons that responded to glutamate, the inward-currents evoked by glutamate (Figure 3f) were very small in both large-sized (n = 2) and small/medium-sized glutamate-sensitive cells (n = 3). No attempt was made to statistically compare glutamate-evoked currents between large-sized and small/medium-sized cells because very small numbers of whisker afferent neurons responded to glutamate with detectable inward currents (Figure 3f).
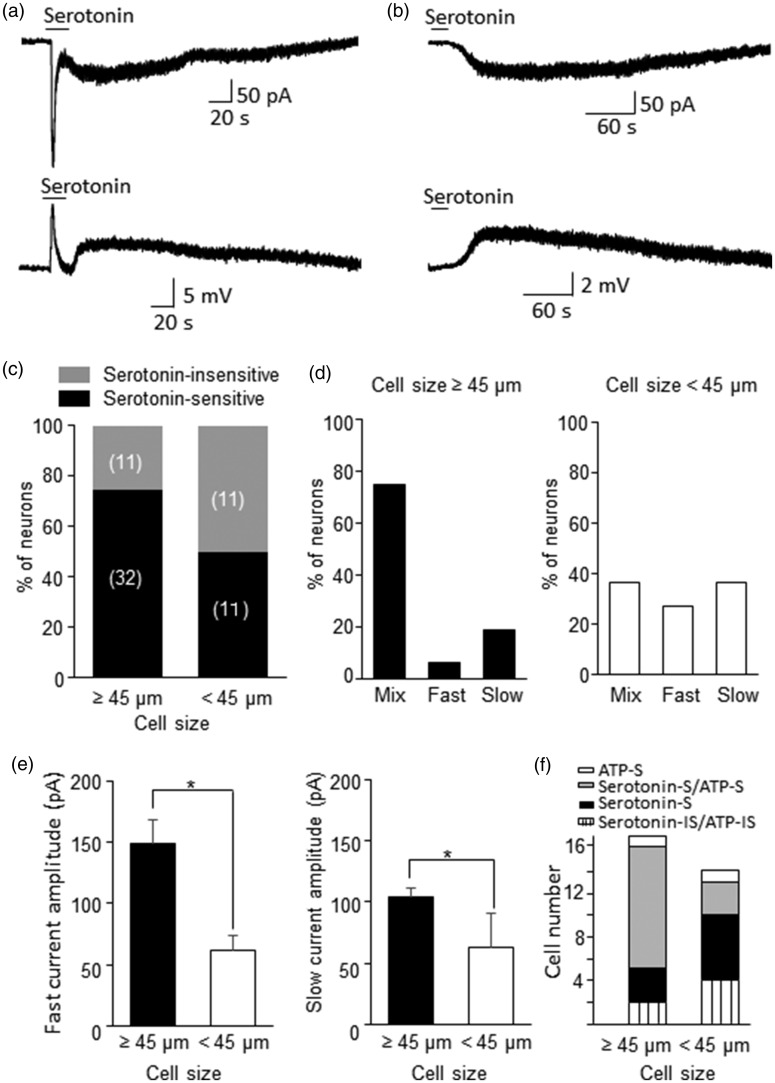
We determined whether whisker afferent neurons were sensitivity to serotonin by bath application of 100 µM serotonin (Figure 4). Many whisker afferent neurons responded to serotonin with inward currents recorded under voltage-clamp mode and membrane depolarization recorded under current clamp mode (Figure 4a–c). The serotonin-evoked inward current often displayed a mixture of a fast and a slow current (Figure 4a), but some cells only showed slow (Figure 4b&d) or fast current type (Figure 4d). Of 43 large-sized whisker afferent neurons tested, most of them (32/43, 74%) responded to serotonin with inward currents; of 22 small/medium-sized whisker afferent neurons tested, 11 of them (50%) were serotonin-sensitive (Figure 5c). In 32 serotonin-sensitive large-sized whisker afferent neurons, most of them (75%, 24/32) showed mixed fast and slow inward currents in response to serotonin (Figure 5a&d). About 6% (2/32) of large-sized serotonin-sensitive whisker afferent neurons only showed fast inward currents in response to bath application of serotonin (Figure 4d), and 19% (6/32) of large-sized serotonin-sensitive whisker afferent neurons only showed slow inward currents (Figure 4b&d). In contrast, the fractions of cells with mixed currents (4/11), fast currents only (3/11) of slow currents only (4/11) were similar in the small/medium-sized serotonin-sensitive whisker afferent neurons (Figure 4d). For the fast currents, their amplitudes were higher in large-sized whisker afferent neurons (n = 26) than in small/medium-sized whicker afferent neurons (n = 7) (Figure 4e). Similarly, the slow currents had greater amplitude in large-sized whisker afferent neurons than in small/medium-sized whisker afferent neurons (Figure 4e). In some DiI-labeled cells ATP (250 µM) and serotonin (100 µM) were tested sequentially in the same cells to determine whether they were sensitive to both ATP and serotonin (Figure 4f). Most of the large-sized whisker afferent neurons (11/17, 65%) were found to be ATP-sensitive as well as serotonin-sensitive. The remaining small numbers of large-sized whisker afferent neurons were sensitive to either serotonin (3/17), or to ATP (1/17), or to neither (2/17). In contrast, only small fraction of small/medium-sized whisker afferent neurons (3/13, 23%) were sensitive to both ATP and serotonin, and about half of small/medium-sized whisker afferent neurons (6/13, 46%) were sensitive to serotonin but not ATP.
Figure 4.
Serotonin-evoked inward currents in retrogradely labeled whisker afferent neurons. (a) Two sample traces show the inward current (top) and membrane depolarization (bottom) recorded from a DiI-labeled whisker afferent neuron (cell size 50 µm) following the applications of 100 µM serotonin. The inward current (top) shows an immediate fast current followed by a slow current. The membrane depolarization (bottom) also shows both fast and slow responses. (b) Sample traces recorded from a different cell (Cell size 50 µm) that only shows a slow inward current (top) and a slow membrane depolarization following the applications of serotonin. (c) Bar graph shows percent of whisker afferent neurons that are serotonin-sensitive and serotonin-insensitive. Left bar, cell size ≥45 µm; Right bar, cell size <45 µm. Cell numbers of each category are indicated in the bars. (d) Bar graphs show percent of serotonin-sensitive cells that display mixed fast and slow currents, fast current only, and slow current only. Left panel, cell size ≥45 µm; Right panel, cell size <45 µm. (e) Bar graphs show the amplitudes of serotonin-evoked fast (left panel) and slow (right panel) inward currents. Solid bars, cell size ≥45 µm; Open bars, cell size <45 µm. (f) Bar graph shows whisker afferent neurons that are sensitive to serotonin and ATP (serotonin-S/ATP-S), to serotonin only (serotonin-S), to ATP only (ATP-S), or to neither (serotonin-IS/ATP-IS). Data represent Mean ± SEM, *P < 0.05.
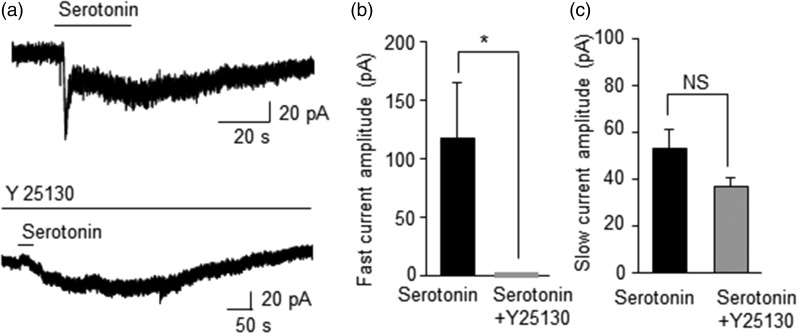
Figure 5.
Effects of 5-HT3 receptor antagonist Y25130 on serotonin-evoked inward currents in retrogradely labeled whisker afferent neurons. (a) Two sample traces show serotonin-evoked inward currents (mixture of fast and slow currents) in a large DiI-labeled neuron in the absence (top) and presence (bottom) of the 5HT3 antagonist Y25130 (200 nM). (b) Summary data show the inhibition of fast currents by Y25130 (n = 6). (c) Summary date show the lack of significant inhibition by Y25130 on serotonin-evoked slow inward currents (n = 6). Data represent Mean ± SEM, NS, no significant difference, *P < 0.05.
We tested effects of Y25130, a 5-HT3 receptor selective antagonist, on serotonin-evoked currents in large-sized whisker afferent neurons (Figure 5). For cells showing mixed fast and slow currents in response to serotonin (100 µM, Figure 5a), the fast current was completely inhabited in the presence of Y25130 (200 nM, n = 6, Figure 5a&b). In contrast, serotonin-evoked slow current was not significantly affected by Y25130 (n = 6, Figure 5c).
Discussion
Microinjection of DiI into individual whisker hair follicles allows us to retrogradely label whisker afferent neurons for a functional study using the patch-clamp recording technique. We show that whisker afferent neurons are heterogeneous with cell calibers ranging from small to large sizes. We have found that the intrinsic electrophysiological properties including action potential firing patterns are different between small/medium-sized and large-sized whisker afferent neurons. We show that most large-sized and also many small/medium-sized whisker afferent neurons are sensitive to ATP and serotonin but most of whisker afferent neurons are insensitive to glutamate. The present study to our knowledge is the first patch-clamp recordings from rat whisker afferent neurons to characterize their electrophysiological properties and chemical sensitivity.
The cell size distribution of whisker afferent neurons shown in the present study suggests that while whisker hair follicles are mainly innervated by large caliber Aβ-afferent fibers, they also receive smaller caliber C- and Aδ-afferent fiber innervation. This cell-size distribution is consistent with previous studies showing that within whisker hair follicles about 2/3 of afferent fibers are large caliber Aβ-afferent fibers and remaining 1/3 are smaller caliber afferent fibers (Rice et al., 1993, Waite and Li, 1993, Ebara et al., 2002). The endings of Aβ-afferent fibers are believed to be mechanoreceptors of Merkel discs, lanceolate endings, reticular endings and other low threshold mechanoreceptor endings (Rice et al., 1993, Ebara et al., 2002). Large-sized whisker afferent neurons and small/medium-sized whisker afferent neurons in the present study show significant differences in many of their electrophysiological properties such as resting membrane potentials, action potential firing patterns, and sag potentials. Almost every large-sized whisker afferent neuron fires a single action potential in response to supra-threshold membrane depolarization while many small/medium-sized whisker afferent neurons fire multiple action potentials. The action potential firing pattern of large-sized afferent neurons is consistent with the electrophysiological feature of rapidly adapting mechanoreceptors such as lanceolate endings (Abraira and Ginty, 2013). However, the single action potential firing pattern also raises an interesting question as how Aβ-endings of Merkel discs may sustain their slowly adapting impulses. For small/medium-sized afferent neurons, the differences in action potential firing patterns may suggest that these are functionally heterogeneous populations of sensory neurons. It is possible that some small/medium-sized whisker afferent fibers are nociceptors. This idea is supported by previous studies showing that many small caliber whisker afferent fibers could be selectively ablated by treating neonatal animals with capsaicin (Waite and Li, 1993). There is also a possibility that some small caliber whisker afferents may be C- or Aδ-low threshold mechanoreceptors (Abraira and Ginty, 2013). Another significant difference in electrophysiological properties between small/medium-sized and large-sized whisker afferent neurons is that the former usually don’t have sag potentials but the latter show prominent sag potentials. Sag potentials are usually due to hyperpolarization-activated inward currents (Ih). Consistently, significant Ih currents can be elicited in large-sized whisker afferent neurons. The function of Ih currents in these sensory neurons remains to be investigated.
An interesting finding of the present study is that a large number of whisker afferent neurons are sensitive to ATP. ATP-sensitivity is seen in most of large-sized and also many small/medium-sized whisker afferent neurons. ATP-sensitive whisker afferent neurons responded to ATP with inward currents, suggesting the involvement of P2X receptor activation (Burnstock, 2012) in these whisker afferent neurons. Previous studies in dorsal root ganglion (DRG) neurons have shown that several different subtypes of P2X receptors (P2X1, P2X2, P2X3, P2X4, P2X6 and P2X7) are expressed on DRG neurons; P2X4 and P2X7 receptors are found to be expressed in both small- to large-sized DRG neurons and other P2X subtypes mainly in small/medium-sized neurons (Ruan et al., 2005). Future studies will be needed to determine which P2X-receptor subtypes are involved in ATP-evoked inward currents in large-sized and small/medium-sized whisker afferent neurons.
Similar to ATP-sensitivity, we show that most large-sized and many small/medium-sized whisker afferent neurons are sensitive to serotonin. Interestingly, the majority of large-sized whisker neurons are sensitive to both ATP and serotonin. Serotonin-sensitive whisker afferent neurons show strong inward currents in response to serotonin. In most of large-sized whisker afferent cells, serotonin-evoked inward currents feature a mixed current type with a fast current followed by a slow/long-lasting current. In a small number of whisker afferent neurons, either fast current alone or slow current alone was evoked by serotonin. In contrast, serotonin-evoked currents in small/medium-sized whisker afferent neurons are not predominated by mixed current type. Fast currents evoked by serotonin could be inhibited by the selective 5-HT3 receptor antagonist Y25130, indicating that the fast currents are mediated by the ionotropic 5-HT3 receptors. On the other hand, slow currents are not significantly affected by Y25130, which may suggest that they are mediated by metabotropic 5-HT receptors (Hoyer et al., 1994). Although metabotropic 5-HT receptors do not directly permit current flowing through, previous studies have shown that activation of 5-HT2A/2C and 5-HT7 receptors could elicit inward currents through the potentiation of Ih currents (Tang and Trussell, 2015). We have recently shown in mouse whisker afferent neurons that serotonin evoke fast and slow currents by activating 5-HT3 and 5-HT2A/2B receptors, respectively (Chang et al., 2016).
Merkel discs in whisker hair follicles are main sites innervated by whisker Aβ-afferent fibers. Our present study of chemical sensitivity in large-sized whisker afferent neurons raises a possibility that rat Merkel disc Aβ-afferent endings may be ATP-sensitive and/or serotonin-sensitive. If ATP and/or serotonin are released from rat Merkel cells in responses to mechanical stimulation, they may serve as chemical messengers to transmit or modulate tactile signaling at rat Merkel discs. Recently we have identified serotonin as a transmitter at mouse Merkel discs (Chang et al., 2016). The sensitivity to ATP and/or serotonin of small/medium-sized whisker afferent neurons shown in our present study raises a possibility that ATP and serotonin may also play roles in nociceptive signaling in rat whisker hair follicles.
Acknowledgements
Experiments in this study were performed at the University of Cincinnati and other work was done at the University of Alabama at Birmingham.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH Grants DE018661 and DE023090 to J.G.G.
References
- 1.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 2013; 79: 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays 2012; 34: 218–225. [DOI] [PubMed] [Google Scholar]
- 3.Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc Natl Acad Sci USA 2016; 113: E5491–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebara S, Kumamoto K, Matsuura T, Mazurkiewicz JE, Rice FL. Similarities and differences in the innervation of mystacial vibrissal follicle-sinus complexes in the rat and cat: a confocal microscopic study. J Comp Neurol 2002; 449: 103–119. [DOI] [PubMed] [Google Scholar]
- 5.Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol 2003; 271: 225–239. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 1994; 46: 157–203. [PubMed] [Google Scholar]
- 7.Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 1969; 200: 763–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive abeta-afferent impulses. Cell 2014; 157: 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda R, Gu JG. Piezo2 channel conductance and localization domains in Merkel cells of rat whisker hair follicles. Neurosci Lett 2014; 583: 210–215. [DOI] [PubMed] [Google Scholar]
- 10.Maksimovic S, Baba Y, Lumpkin EA. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann N Y Acad Sci 2013; 1279: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014; 509: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merkel F. Tastzellen and Tastkoerperchen bei den Hausthieren und beim Menschen. Arch Mikrosc Anat 1875; 11: 636–652. [Google Scholar]
- 13.Rice FL, Kinnman E, Aldskogius H, Johansson O, Arvidsson J. The innervation of the mystacial pad of the rat as revealed by PGP 9.5 immunofluorescence. J Comp Neurol 1993; 337: 366–385. [DOI] [PubMed] [Google Scholar]
- 14.Ruan HZ, Birder LA, de Groat WC, Tai C, Roppolo J, Buffington CA, Burnstock G. Localization of P2X and P2Y receptors in dorsal root ganglia of the cat. J Histochem Cytochem 2005; 53: 1273–1282. [DOI] [PubMed] [Google Scholar]
- 15.Tang ZQ, Trussell LO. Serotonergic regulation of excitability of principal cells of the dorsal cochlear nucleus. J Neurosci 2015; 35: 4540–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waite PM, Li L. Unmyelinated innervation of sinus hair follicles in rats. Anat Embryol (Berl) 1993; 188: 457–465. [DOI] [PubMed] [Google Scholar]
- 17.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014; 509: 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]