Abstract
Background
Although it has been demonstrated that visceral adipose tissue content and serum levels of adiponectin are associated with metabolic syndrome, their predictive potential for the development of metabolic syndrome remains to be elucidated.
Methods
We studied 1,130 participants of the Seoul Metabolic Syndrome cohort. A total of 337 subjects without metabolic syndrome underwent the follow-up evaluation and finally analyzed. Visceral fat area (VFA) was measured using dual bioelectrical impedance analysis. We compared the 1-year incidence rate of metabolic syndrome among four different groups: Group 1 (high adiponectin level and low VFA), Group 2 (low adiponectin level and low VFA), Group 3 (high adiponectin level and high VFA) and Group 4 (low adiponectin level and high VFA).
Results
Median follow-up duration was 17 months. Cut-off points of adiponectin level and VFA for metabolic syndrome were 7.34 ng/ml and 84 cm2 for men, and 12.55 and 58 cm2 ng/ml for women, respectively. The incidence of metabolic syndrome was the highest in Group 4 (Group 1; 16.47%, Group 2; 22.08%, Group 3; 25%, and Group 4; 46.15%, p<0.001). Adjusted logistic regression analyses for metabolic syndrome prediction demonstrated that Group 4 exhibited the highest odds ratio compared with Group 1 (4.918 [2.05–11.795]), which was predominantly affected by waist circumference and serum triglyceride levels. Notably, triglyceride/high-density lipoprotein cholesterol (TG/HDL) ratio was significantly higher in Group 4 (p = 0.017).
Conclusion
Incidence rate of metabolic syndrome was the highest in subjects with low serum adiponectin levels and high visceral fat area. Higher TG/HDL ratio in these subjects suggested insulin resistance may contribute to the development of metabolic syndrome.
Background
Prevalence of metabolic syndrome, which is composed of abdominal obesity, high blood pressure, hyperglycemia, and dyslipidemia, is increasing worldwide and is known to cause cardiovascular diseases [1–3]. From this perspective, it is essential that risk factors that cause metabolic syndrome are elucidated.
Obesity has long been studied with metabolic syndrome, and it is associated with adiposity and insulin resistance. Insulin resistance is an important factor that can predict the cause of metabolic syndrome. Several studies have shown that visceral adipose tissue pertaining to insulin resistance is correlated with obesity-related disease [4–8]. Visceral adipose tissue is also associated with metabolic syndrome [9–11].
There were also several longitudinal studies on the impact of visceral adiposity on metabolic syndrome. The MESA study showed that visceral adiposity significantly increased the metabolic syndrome risk during the median 3.3-year follow-up period [12]. The Hitachi Health Study revealed that more than 50 cm2 increase in visceral fat area during 3-year follow-up period was significantly associated with the incidence of metabolic risk factors, especially high triglyceride and low high-density lipoprotein levels [13]. These suggested that visceral adipose tissue might play an important role in the progression to metabolic syndrome.
Previous studies have suggested that serum levels of adiponectin are associated with metabolic syndrome [14–19] and metabolic syndrome development may be associated with obesity, adipose tissue content, and hormonal levels. Adipokines, such as adiponectin, are secreted by the adipose tissue or as a result of glucose and lipid metabolism.[20–22]. Accordingly, the level of adiponectin is associated with obesity-related disorders and metabolic risk factors [23–26]. Adiponectin levels are inversely correlated with visceral obesity; therefore, high levels of adiponectin are negative correlated with obesity whereas low adiponectin levels exhibit a positive correlation9.
Although visceral adipose tissue and serum levels of adiponectin have been associated with metabolic syndrome, their predictive potential for the development of metabolic syndrome remains unknown. The present study aimed to investigate the predictability of serum adiponectin levels and visceral adipose tissue for the development of metabolic syndrome.
Methods
Study population
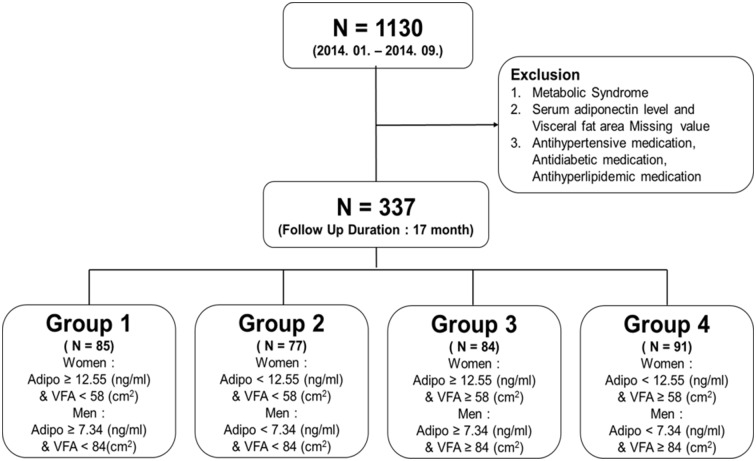
This is a prospective cohort study of the Seoul Metabolic Syndrome (S-MAS) study researched by Korea University Anam hospital in Seoul, Korea. 1,130 participants of both sexes between 30 and 64 years of age suspected of having metabolic syndrome were enrolled from 25 public healthcare centers from January 2014 to September 2014. Subjects with a previous history of angina pectoris, myocardial infarction, stroke, or any revascularization were excluded for the enrollment. At each visit, demographic characteristics including age, underlying diseases and medications were collected through a standardized questionnaire. Basic medical examinations, including a physical examination, were performed by physicians. Abdominal fat content measurements and other laboratory examinations were also performed. From May 2015 to November 2015, 756 participants performed the follow-up evaluation. The follow-up loss rate was 33.1% (n = 374). Among the remaining 756 participants, 419 subjects was excluded due to the following reasons: metabolic syndrome at baseline (n = 258), missing serum adiponectin levels (n = 84), missing visceral adipose tissue area (n = 179), in addition to being treated with antihypertensive medication (n = 67), antidiabetic medication (n = 17), and antihyperlipidemic medication (n = 63). 249 subjects had concurrently two reasons or more than two. The present study was approved by the institutional review board of Korea University Anam Hospital (IRB NO. ED13087) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Abdominal fat content measurement
Abdominal fat content was measured by a trained nurse by using the Visceral Fat scan device (Omron HDS-2000) according to the manufacturer’s protocol. Briefly, visceral fat area (VFA) and subcutaneous fat area (SFA) were measured at the umbilicus level according to the dual bioelectrical impedance analysis method. The correlation between abdominal fat areas measured by this method and computed tomography was good, with a correlation coefficient of 0.888 [27].
Laboratory tests
Blood samples were obtained from each subject after at least 8 hours of fasting. Serum glucose levels were determined using a UV assay. Serum levels of total cholesterol, low density lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol and triglycerides were measured using the homogeneous enzymatic colorimetric assay. Serum adiponectin levels, high-sensitivity C-reactive protein (hsCRP), apolipoprotein A1, and apolipoprotein B were gauged from the immunoturbidimetry assay.
Definitions
Subjects were categorized into four groups according to the combinations of the median of VFA and serum adiponectin levels for each sex. The median serum adiponectin level was 9.83 ng/mL in the total population, 12.55 ng/mL in women, and 7.34 ng/mL in men. The median of VFA was 68 cm2 in the total population, 58 cm2 in women, and 84 cm2 in men. The definition of the four groups was as follows (Fig 1): i) adiponectin level ≥ 12.55 ng/mL and VFA < 58 cm2 for women and adiponectin level ≥ 7.34 ng/mL and VFA < 84 cm2 for men (Group 1); ii) adiponectin level < 12.55 ng/mL and VFA < 58 cm2 for women and adiponectin level < 7.34 ng/mL and VFA < 84 cm2 for men (Group 2); iii) adiponectin level ≥ 12.55 ng/ml and VFA ≥ 58 cm2 for women and adiponectin level ≥ 7.34 ng/ml and VFA ≥ 84 cm2 for men (Group 3); and iv) adiponectin level < 12.55 ng/mL and VFA ≥ 58 cm2 for women and adiponectin level < 7.34 ng/mL and VFA ≥ 84 cm2 for men (Group 4).
Fig 1. Study population.
Adipo, Serum adiponectin level; VFA, Visceral fat area.
Metabolic syndrome was defined according to the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Adult Treatment Panel III and the Korean Society for the Study of Obesity. Therefore, a person who exhibited any at least three of the following five factors was considered to have metabolic syndrome: i) waist circumference ≥90 cm for men and ≥80 cm for women; ii) blood pressure ≥ 130/85 mmHg; iii) fasting glucose level ≥ 100 mg/dL; iv) triglycerides level ≥150 mg/dL; and v) HDL cholesterol level <40 mg/dL for men and <50 mg/dL for women.
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median (25th percentile, 75th percentile). Categorical variables were presented as counts (percentages) and were analyzed by chi-square test and Fisher’s exact test for group comparisons. Normality was examined by the Kolmogorov-Smirnov test for the analysis of each variable. For continuous variables, the comparison of four groups was performed using one way analysis of variance for normal distributions or by the Kruskal-Wallis test for non-normal distributions. In addition, post-hoc Bonferroni contrast analysis was performed.
The logistic regression model was used to elucidate the association between four groups or the quartile of each factor (adiponectin level and VFA) and metabolic syndrome or five components of it. Associations were presented as an odds ratio (OR) with 95% confidence intervals (CI). In order to investigate the association, Model 1 was adjusted for age and sex. Model 2 was adjusted for Model 1 adjusted factors, smoking status, alcohol intake, education level, and physical activity. Model 3 was further adjusted for five continuous components of metabolic syndrome. Additionally, Model 4 was adjusted for hsCRP, LDL-cholesterol, apolipoprotein A1 and apolipoprotein B. A two-sided p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA) software.
Results
Baseline characteristics
The association between the level of adiponectin and VFA exhibited a negative correlation (r = -0.1371, p <.0001). The initial demographic features are presented in Table 1. Study subjects consisted of 189 women (56.08%) and 148 men (43.92%). The median age of subjects was 56. In Group 1 (n = 85), there were 34 (40%) men with a median age of 56. Group 2 (n = 77) consisted of 38 (49.35%) men, with a median age of 56. Group 3 (n = 84) was composed of 40 (47.62%) men, and subjects were median aged 58 years. In Group 4 (n = 91), there were 36 (39.56%) men aged 56 years. Body mass index (BMI) and waist circumference were demonstrated to be significantly different among the four groups (p <.001). These factors were increased in Groups 3 and 4, as compared with Groups 1 and 2. Laboratory findings demonstrated that triglyceride levels were increased in Group 4 and high-density lipoprotein (HDL) cholesterol levels were increased in Group 1. Therefore, the triglyceride to HDL-cholesterol ratio was significantly different among the groups (p = 0.017) and was the highest in Group 4. Total cholesterol, fasting glucose, LDL-cholesterol levels and blood pressure were similar among the groups.
Table 1. Baseline characteristics.
| Variable | Group 1 (N = 85) | Group 2 (N = 77) | Group 3 (N = 84) | Group 4 (N = 91) | p-value |
|---|---|---|---|---|---|
| Adiponectin (ng/mL) ₀ | 17.07±8.03a | 6.80±3.10b | 15.37±5.01a | 6.38±2.90b | <.001 |
| Visceral fat area (cm2) ₀ | 50(38,64)a | 55(45,69)a | 91(66,108)b | 87(69,102)b | <.001 |
| Men (%) | 34(40) | 38(49.35) | 40(47.62) | 36(39.56) | 0.454 |
| Age (years) | 56(52,60) | 56(51,59) | 58(54,61) | 56(52,60) | 0.101 |
| Waist circumference (cm) ₀ | 82.11±8.00a | 82.87±5.94a | 88.8±7.91b | 88.35±6.79b | <.001 |
| BMI (kg/m2) ₀ | 23.85±2.67a | 23.88±2.13a | 26.05±2.61b | 26.37±2.63b | <.001 |
| Current Smoker (%) | 16(19.05) | 15(19.48) | 13(15.48) | 13(14.44) | 0.767 |
| Systolic blood pressure (mmHg) | 118.65±13.34 | 117.95±13.21 | 118.16±12.34 | 119.86±12.72 | 0.768 |
| Diastolic blood pressure (mmHg) | 73.71±9.17 | 74.29±9.15 | 73.17±9.46 | 75.03±9.5 | 0.594 |
| Pulse pressure (mmHg) | 72(66,79.5) | 70.5(66,78.5) | 69(62.5,77.5) | 72(66.5,81) | 0.263 |
| Total Cholesterol (mg/dL) | 201.92±32.36 | 197.29±32.63 | 205.83±32.09 | 202.79±34.34 | 0.429 |
| LDL-cholesterol (mg/dL) | 133.91±28.21 | 132.95±31.99 | 138.86±29.37 | 136.74±32.37 | 0.590 |
| HDL-cholesterol (mg/dL) ₀ | 57.66±13.64a | 51.27±11.97b | 53.94±11.46a,b | 52.7±10.97b | 0.006 |
| Triglyceride (mg/dL) ₀ | 111(76,138)a | 108(85,130)a | 116(92.5,138)a,b | 124(98,152)b | 0.020 |
| TG / HDL ratio ₀ | 1.88(1.24,2.69)a | 2.2(1.5,2.89)a,b | 2.24(1.76,2.84)b | 2.4(1.75,3.16)b | 0.017 |
| Glucose (mg/dL) | 93(88,100) | 84(89,99) | 94.5(90,100) | 84(89,98) | 0.462 |
| High sensitivity CRP (mg/dL) | 0.6(0.3,1.1) | 0.4(0.3,1.0) | 0.6(0.3,1.0) | 0.7(0.4,1.4) | 0.061 |
| Apolipoprotein B (mg/dL) | 98(85.4,114.1) | 99.7(86.7,116.3) | 101.85(90.35,116.7) | 104(90,117.7) | 0.352 |
| Apolipoprotein AI (mg/dL) | 143.3(128.9,162.7) | 135(121.7,151.8) | 143.05(128.6,156.65) | 139.4(127.9,15.8) | 0.086 |
| Apolipoprotein B / A1 ratio | 0.56(0.33,0.64) | 0.73(0.60,0.88) | 0.73(0.60,0.89) | 0.76(0.63,0.86) | 0.062 |
Data are presented as the mean ± standard deviation or (25 percentile, 75 percentile) for continuous variables and the number (%) for categorical variables.
BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG/HDL, Triglyceride/HDL-cholesterol; CRP, C-reactive protein.
₀ Multiple comparison (post-hoc) results, same letter means no difference.
Incidence of metabolic syndrome and follow-up
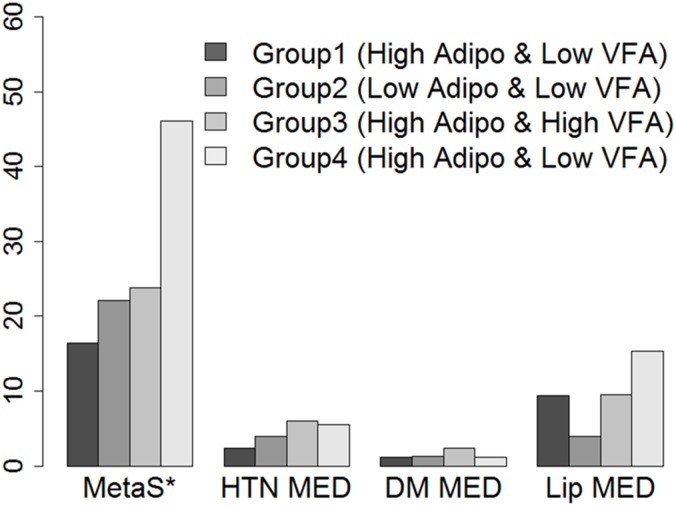
During the median 17-month follow-up, metabolic syndrome was newly developed in 94 (27.89%) subjects. Incidence of metabolic syndrome was 16.47% in Group 1, 22.08% in Group 2, 25% in Group 3 and 46% in Group 4 (Group 4 vs other groups, p <.001) (Fig 2). However, the initiation rates of anti-hypertensive, anti-diabetic, and anti-hyperlipidemic medications were not significantly different among the groups.
Fig 2. Incidence of metabolic syndrome at follow-up.
Adipo, Serum adiponectin level; VFA, Visceral fat area; MetaS, Metabolic syndrome; HTN MED, Antihypertensive Medication; DM MED, Antidiabetic Medication; Lip MED, Antihyperlipidemic Medication. * Result of comparison between Groups, p <.0001.
Table 2 shows the follow-up data and their changes respective to the baseline. As compared with the baseline characteristics, follow-up waist circumference and BMI were significantly different (p <.001), and were demonstrated to be the highest in Groups 3 and 4. Triglyceride, HDL-cholesterol and the Triglyceride to HDL-cholesterol ratio also exhibited significant differences among the groups (all, p <.05). HDL cholesterol levels were the highest in Group1 and triglyceride levels and the Triglyceride to HDL-cholesterol ratio were lowest in Group 1 and highest in Group 4. Among these variables, only the alterations in triglyceride levels were significantly different among the groups (p = 0.042). Group 4 exhibited the largest increase in triglyceride levels between the baseline and follow-up. Notably, hsCRP levels at the follow-up were significantly different (p = 0.002) and were the highest in Group 4, although there were no significant differences detected at baseline (p = 0.061).
Table 2. Follow-up characteristics and their respective variations from the baseline.
| Variable | Group 1 (N = 85) | Group 2 (N = 77) | Group 3 (N = 84) | Group 4 (N = 91) | p-value |
|---|---|---|---|---|---|
| Waist circumference (cm) ₀ | 84(80,90)a | 85(81,89)a | 89.5(85,94.5)b | 90(87,93.5)b | <.001 |
| Δ Waist circumference (cm) | 1.5(-2,4) | 1.5(0,5) | 1(-1,3.5) | 2(-1,5) | 0.690 |
| BMI (kg/m2) ₀ | 23.34(22.04,24.65)a | 23.37(22.06,24.8)a | 25.16(23.94,27.54)b | 25.95(24.53,27.31)b | <.001 |
| Δ BMI (kg/m2) | -0.34(-0.89,0.29) | -0.3(-0.97,0.32) | -0.32(-0.95,0.36) | -0.32(-0.98,0.32) | 0.971 |
| Systolic blood pressure (mmHg) | 119.4±13.51 | 120.7±13.06 | 119.2±14.56 | 122.25±12.98 | 0.414 |
| Δ Systolic blood pressure (mmHg) | 0.83±13.65 | 2.75±12.67 | 1.63±12.77 | 2.4±12.47 | 0.780 |
| Diastolic blood pressure (mmHg) | 78.43±8.61 | 80.77±8.54 | 78.65±10.41 | 80.47±7.87 | 0.203 |
| Δ Diastolic blood pressure (mmHg) | 4.75±6.19 | 6.48±8.07 | 5.68±7.32 | 5.44±8.49 | 0.547 |
| Pulse pressure (mmHg) | 71.5(64,78.5) | 72(66,84) | 70.75(62,76.75) | 71(65.5,79) | 0.328 |
| Δ Pulse pressure (mmHg) | -0.5(-7,7) | 2.5(-3,7.5) | 0(-6.5,6) | -1(-9,7) | 0.189 |
| Total Cholesterol (mg/dL) | 206(186,235) | 199(180,219) | 200(183,226) | 205(189,233) | 0.371 |
| Δ Total Cholesterol (mg/dL) | 11(-13,28) | 8(-9,27) | 2(-19,15) | 6(-10,30) | 0.148 |
| LDL-cholesterol (mg/dL) | 140.07±33.35 | 135.1±32.07 | 136.25±28.78 | 139.29±35.78 | 0.727 |
| Δ LDL-cholesterol (mg/dL) | 10(-9,27) | 3(-11,17) | 1(-15,16) | 2(-13,23) | 0.244 |
| HDL-cholesterol (mg/dL) ₀ | 55(45,67)a | 46(41,55)b | 51(43,58)a | 49(43,59)b | 0.002 |
| Δ HDL-cholesterol (mg/dL) | -1(-7,5) | -2(-8,2) | -2(-8,3) | -2(-7,2) | 0.727 |
| Triglyceride (mg/dL) ₀ | 109(88,144)a | 125(98,175)b | 121(91,155)a | 144(103,188)b | 0.001 |
| Δ Triglyceride (mg/dL) ₀ | 6(-21,34)a | 16(-5,44)b | 7(-17,29)a | 19(-15,56)b | 0.042 |
| TG / HDL ratio ₀ | 1.92(1.29,3.1)a | 2.76(2,4.3)b | 2.36(1.66,3.28)a | 2.81(1.82,4.13)b | <.001 |
| Δ TG / HDL ratio | 0.19(-0.35,0.64) | 0.35(-0.13,1.43) | 0.12(-0.4,0.86) | 0.43(-0.29,1.44) | 0.075 |
| Glucose (mg/dL) | 88(79,97) | 89(78,95) | 87(80,97) | 89(78,96) | 0.961 |
| Δ Glucose (mg/dL) | -6(-17,3) | -7(-15,-1) | -8(-16,2) | -6(-18,1) | 0.694 |
| High-sensitivity CRP (mg/dL) ₀ | 0.3(0.2,0.6)a | 0.3(0.2,0.6)a | 0.4(0.3,1)a,b | 0.6(0.3,1.4)b | 0.002 |
| Δ High-sensitivity CRP (mg/dL) | -0.2(-0.6,0) | -0.1(-0.5,0.1) | -0.1(-0.4,0.1) | -0.1(-0.5,0.1) | 0.535 |
Data are presented as the mean ± standard deviation or (25 percentile, 75 percentile) for continuous variables and the number (%) for categorical variables.
Δ, variation from baseline; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG/HDL, Triglyceride/HDL-cholesterol; CRP, C-reactive protein.
₀ Multiple comparison (post-hoc) results, same letter means no difference.
Although Group 2 had low serum adiponectin level and Group 3 had high VFA, Table 2 showed the similar results between Group 2 and Group 3. Further comparison between Group 2 and Group 3 was showed in the S1 Table. The most significant differences between Group 2 and Group 3 were waist circumference and body mass index. Group 3 had larger waist circumference and BMI compared to Group 2 (waist circumference; 85 vs 89.5 cm, BMI; 23.37 vs 25.16 kg/m2, both p <.001). Other blood pressure, lipid profile, glucose and high-sensitivity CRP were similar except that the follow-up HDL-cholesterol were lower (46 vs 51 mg/dL, p = 0.049) and the increase of triglyceride level was bigger (16 vs 7 mg/dL, p = 0.024) in Group 2 compared to Group 3.
For the further delineation of the spectrum between higher VFA and lower serum adiponectin level, we analyzed the changes of metabolic syndrome risk factors dependent to the tertile of VFA and the tertile of serum adiponectin level (S2 and S3 Tables). Both higher VFA and lower serum adiponectin level showed significant unfavorable effect with the metabolic syndrome risk factors at the follow-up (waist circumference, BMI, systolic and diastolic blood pressure, HDL-cholesterol, triglyceride and triglyceride/HDL-cholesterol ratio). High-sensitivity CRP level has greater value in the higher VFA. Interestingly, VFA was associated with waist circumference change, and serum adiponectin level was associated with the changes of triglyceride and the triglyceride/HDL-cholesterol ratio. Those data suggested that adiponectin and VFA might contribute to metabolic syndrome with somewhat different mechanisms although adiponectin and VFA were significantly associated with each other.
Risk of metabolic syndrome and its components
The effects of adiponectin level and VFA at baseline were analyzed using multivariable-adjusted models of metabolic syndrome and its five components (Table 3). Model 1 included age and sex. Model 2 included the lifestyle-related factors (smoking, alcohol, education level and physical activity). Model 3 included the 5 metabolic syndrome risk factors (waist circumference, triglyceride, HDL-cholesterol, blood pressure, and glucose). Model 4 included the other precise biomarkers (high sensitivity CRP, LDL-cholesterol, apolipoprotein AI, and apolipoprotein B). In Model 4, in which all the factors were adjusted, higher VFA was significantly associated with the development of metabolic syndrome (T3 vs T1; OR, 2.920; 95% CI, 1.101–7.747; p = 0.031). Among the metabolic syndrome risk factors, high waist circumference was significantly associated with higher VFA (OR, 4.188; 95% CI, 1.528–11.479; p = 0.005). Serum adiponectin level was also significantly associated with the development of metabolic syndrome(OR, 0.419; 95% CI, 0.199–0.884; p = 0.022). The combination of higher VFA and lower adiponectin levels (Groups 4 vs 1) was also demonstrated to be significantly associated with the development of metabolic syndrome (OR, 4.918; 95% CI, 2.05–11.795; p <.001). Among the metabolic syndrome risk factors, high waist circumference and high triglyceride levels were significantly associated with Group 4. For high waist circumference, OR was 2.827 (95% CI, 1.154–6.925; p = 0.026) and for high triglyceride, OR was 3.508 (95% CI, 1.537–8.007; p = 0.007). Subgroup analysis showed that the disfavoring effect of Group 4 for metabolic syndrome appeared consistent across sex (S4 Table).
Table 3. Associations among adiponectin, visceral fat area and their groups for metabolic syndrome and its risk factors.
| Adiponectin (T3 vs T1) |
Visceral fat area (T3 vs T1) |
Group (Group 4 vs Group 1) |
||
|---|---|---|---|---|
| Metabolic syndrome | Crude Model | 0.545(0.305,0.972) | 1.725(0.924,3.218) | 4.347(2.146,8.806) |
| Model 1 | 0.365(0.188,0.706) | 2.661(1.307,5.417) | 4.464(2.193,9.088) | |
| Model 2 | 0.324(0.159,0.659) | 3.392(1.565,7.354) | 5.907(2.753,12.676) | |
| Model 3 | 0.426(0.203,0.893) | 2.883(1.107,7.507) | 4.72(2.012,11.072) | |
| Model 4 | 0.419(0.199,0.884) | 2.92(1.101,7.747) | 4.918(2.05,11.795) | |
| High waist circumference | Crude Model | 0.662(0.389,1.126) | 7.381(4.089,13.324) | 5.463(2.846,10.488) |
| Model 1 | 0.688(0.383,1.236) | 10.816(5.361,21.823) | 5.513(2.862,10.619) | |
| Model 2 | 0.677(0.367,1.249) | 16.745(7.678,36.52) | 6.584(3.284,13.2) | |
| Model 3 | 0.661(0.288,1.517) | 4.123(1.542,11.021) | 2.717(1.14,6.477) | |
| Model 4 | 0.623(0.269,1.447) | 4.188(1.528,11.479) | 2.827(1.154,6.925) | |
| High blood pressure | Crude Model | 0.694(0.401,1.2) | 1.371(0.801,2.345) | 1.104(0.591,2.061) |
| Model 1 | 0.933(0.509,1.712) | 1.011(0.547,1.869) | 1.117(0.594,2.099) | |
| Model 2 | 0.97(0.515,1.829) | 1.026(0.534,1.972) | 1.15(0.596,2.221) | |
| Model 3 | 0.901(0.434,1.87) | 1.159(0.468,2.867) | 1.237(0.552,2.77) | |
| Model 4 | 0.896(0.431,1.865) | 1.155(0.456,2.927) | 1.252(0.547,2.868) | |
| High glucose | Crude Model | 0.848(0.428,1.681) | 0.899(0.444,1.818) | 0.677(0.313,1.463) |
| Model 1 | 1.015(0.476,2.166) | 0.648(0.291,1.44) | 0.672(0.31,1.458) | |
| Model 2 | 1.072(0.479,2.396) | 0.784(0.334,1.841) | 0.691(0.305,1.566) | |
| Model 3 | 1.438(0.493,4.194) | 0.659(0.189,2.294) | 0.516(0.157,1.691) | |
| Model 4 | 1.441(0.488,4.255) | 0.665(0.188,2.344) | 0.527(0.158,1.755) | |
| High triglyceride | Crude Model | 0.56(0.327,0.959) | 1.492(0.867,2.57) | 2.619(1.415,4.848) |
| Model 1 | 0.544(0.301,0.985) | 1.511(0.818,2.79) | 2.659(1.434,4.932) | |
| Model 2 | 0.507(0.271,0.951) | 1.46(0.757,2.814) | 3.06(1.593,5.875) | |
| Model 3 | 0.584(0.291,1.172) | 1.461(0.618,3.452) | 3.649(1.621,8.215) | |
| Model 4 | 0.601(0.297,1.217) | 1.328(0.555,3.176) | 3.508(1.537,8.007) | |
| Low HDL-cholesterol | Crude Model | 1.147(0.666,1.978) | 0.634(0.362,1.109) | 1.611(0.869,2.986) |
| Model 1 | 0.659(0.355,1.224) | 1.004(0.531,1.901) | 1.628(0.866,3.06) | |
| Model 2 | 0.642(0.33,1.249) | 1.124(0.562,2.247) | 1.788(0.919,3.482) | |
| Model 3 | 0.814(0.382,1.738) | 1.665(0.638,4.344) | 1.962(0.84,4.583) | |
| Model 4 | 0.807(0.374,1.743) | 1.831(0.689,4.866) | 2.024(0.847,4.833) |
Data are presented as odd ratio (95% confidence intervals).
Model 1. Adjusted variable: age, sex.
Model 2. Adjusted variable: Model 1 + smoking, alcohol, education level, physical activity.
Model 3. Adjusted variable: Model 2 + waist circumference, triglyceride, HDL-cholesterol, systolic blood pressure, diastolic blood pressure, glucose.
Model 4. Adjusted variable: Model 3 + high sensitivity CRP, LDL-cholesterol, apolipoprotein AI, apolipoprotein B.
Discussion
The main finding of the present study was that subjects with higher VFA and lower serum adiponectin levels exhibited a significantly higher risk for the development of metabolic syndrome. Notably, the combination of higher VFA and lower serum adiponectin levels was an independent predictor for metabolic syndrome following adjustment for the other important risk factors including waist circumference, blood pressure, glucose, lipid profile, and other biomarkers.
Previously, visceral fat has been shown to be associated with a greater cardiometabolic risk, as compared with subcutaneous fat [28] and other obesity measurements, including BMI [12]. The present study demonstrated that the highest quartiles of visceral adipose tissue exhibited a higher risk of metabolic syndrome, as compared with the lowest quartiles after covariate adjustments (Table 3). Subgroup analyses of the metabolic syndrome component also indicated that visceral fat was independently associated with waist circumference rather than the other metabolic syndrome components. Therefore, increased VFA potentiated the risk of metabolic syndrome predominantly driven by a higher waist circumference.
Several studies have demonstrated that serum adiponectin levels are inversely correlated with visceral adipose tissue [29]. Baseline characteristics in the present study also showed the inverse relationship between VFA and serum adiponectin levels in the community-based general population without metabolic syndrome (S1 Fig). Notably, these associations were differently affected by sex. Women exhibited reduced VFA and increased serum adiponectin levels compared to men (p <.001). Furthermore, women had a strong negative correlation between VFA and serum adiponectin levels (r = -0.328, p <.001); whereas men had a weak positive correlation without statistical significance (r = 0.071, p = 0.391). These findings were similar to the results from the western country [30].
Previous studies reported that serum adiponectin was decreased in obese subjects and associated with metabolic syndrome components [31]. High serum adiponectin levels have also been reported to be associated with reduced insulin resistance and other favorable effects including anti-inflammatory and anti-atherogenic properties [32]. In the present study, serum adiponectin level exhibited a significant association with metabolic syndrome development. Although there was no statistical significance, high triglyceride showed the lowest odd ratio among 5 metabolic syndrome risk factors. There may be several explanations for this. First, the effect of serum adiponectin levels may be potentiated in a manner that is dependent on visceral adiposity. Adiponectin levels have been reported to increase or remain constant with aging [33,34]. Recently, Li JB et al demonstrated that adiponectin levels and the visceral fat ratio decreased with aging in an animal model [35]. This finding suggested that relatively insufficient amounts of adiponectin on visceral adipose tissue may be associated with an increased risk of the progression of metabolic syndrome. Second, there might be some functional differences among the adiponectins secreted from different adipose tissues. Adiponectin circulates in different multimeric forms [36]. High molecular weight adiponectin exhibited a greater association with insulin sensitivity, as compared with total adiponectin [37]. Different forms of adiponectin have been reported to exhibit binding properties with other proteins including C1q, and these protein complexes may reflect the risk of metabolic syndrome [38]. Notably, visceral adipose tissue has been associated with C1q-adiponecitn complex levels.
The present study has some limitations. Although this study was based on a community-based cohort, it was performed in a single center and the follow-up loss rate was up to 33.1%. Therefore, our study population may not represent the whole general population in the community, and the selection bias may limit our interpretation. Moreover, the small sample size and the short follow-up duration (median 17 months) also abate the precision of risk estimation in the present study. In addition, we only evaluated a single measurement of serum adiponectin levels and VFA at baseline. Follow-up measurements may explain the mechanism of their independent roles for the development of metabolic syndrome. Despite these limitations, the major strength of the present study is that the data were collected from a community-based cohort study. The present findings may contribute to the establishment of causality between the indices (visceral fat and adiponectin) and metabolic syndrome.
In addition, considering that the prediction and prevention of metabolic syndrome are now considered of great importance for public health, our findings may contribute to assess the high risk population for metabolic syndrome. A future intervention trials should evaluate the benefits of a screening the high risk group for metabolic syndrome.
Conclusion
In a community-based population without overt metabolic risks, the combination of low serum adiponectin levels and high VFA significantly predicted the development of metabolic syndrome, which was predominantly driven by an increase in triglyceride level. Higher insulin resistance and systemic inflammation, indicated by a higher triglyceride to HDL cholesterol ratio and higher hsCRP level, may also contribute to the development of metabolic syndrome in subjects with low serum adiponectin levels and high VFA.
Supporting Information
(TIF)
Data are presented as the mean ± standard deviation or (25 percentile, 75 percentile) for continuous variables and the number (%) for categorical variables. Δ, variation from baseline; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG/HDL, Triglyceride/HDL-cholesterol; CRP, C-reactive protein.
(DOCX)
Data are presented as the mean ± standard deviation or (25 percentile, 75 percentile) for continuous variables and the number (%) for categorical variables. Δ, variation from baseline; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG/HDL, Triglyceride/HDL-cholesterol; CRP, C-reactive protein.
(DOCX)
Data are presented as the mean ± standard deviation or (25 percentile, 75 percentile) for continuous variables and the number (%) for categorical variables. Δ, variation from baseline; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG/HDL, Triglyceride/HDL-cholesterol; CRP, C-reactive protein.
(DOCX)
Data was presented as odd ratio (95% confidence interval). OR, odd ratio; 95% CI, 95% confidence interval.
(DOCX)
Acknowledgments
The authors thank Hyeon Seo Shin, Jung Min Park, Nana Kim and Min Ju Hong for their technical assistance.
Data Availability
Data are available from the database at the Metabolic Syndrome Research Center for researchers who meet the criteria for access to confidential data according to the regulation implemented by the Seoul Metropolitan Government and the institutional review board of Korea University Anam Hospital. Interested researchers may submit requests to Professor Do-Sun Lim, MD, PhD. Phone: +82-2-920-5445; Fax: +82-2-927-1478; E-mail: dslmd@kumc.or.kr.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005; 28: 1769–1778. [DOI] [PubMed] [Google Scholar]
- 2.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004; 110: 1245–1250. 10.1161/01.CIR.0000140677.20606.0E [DOI] [PubMed] [Google Scholar]
- 3.Bao W, Srinivasan SR, Valdez R, Greenlund KJ, Wattigney WA, Berenson GS. Longitudinal changes in cardiovascular risk from childhood to young adulthood in offspring of parents with coronary artery disease: the Bogalusa Heart Study. Jama. 1997; 278: 1749–1754. [PubMed] [Google Scholar]
- 4.Druet C, Baltakse V, Chevenne D, Dorgeret S, Zaccaria I, Wang Y, et al. Independent effect of visceral adipose tissue on metabolic syndrome in obese adolescents. Horm Res. 2008; 70: 22–28. 10.1159/000129674 [DOI] [PubMed] [Google Scholar]
- 5.Pouliot MC, Despres JP, Nadeau A, Moorjani S, Prud'Homme D, Lupien PJ, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992; 41: 826–834. [DOI] [PubMed] [Google Scholar]
- 6.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004; 89: 108–113. 10.1210/jc.2003-031188 [DOI] [PubMed] [Google Scholar]
- 7.Lim KI, Yang SJ, Kim TN, Yoo HJ, Kang HJ, Song W, et al. The association between the ratio of visceral fat to thigh muscle area and metabolic syndrome: the Korean Sarcopenic Obesity Study (KSOS). Clin Endocrinol (Oxf). 2010; 73: 588–594. [DOI] [PubMed] [Google Scholar]
- 8.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005; 19: 547–566. 10.1016/j.beem.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 9.Valsamakis G, Chetty R, Anwar A, Banerjee AK, Barnett A, Kumar S. Association of simple anthropometric measures of obesity with visceral fat and the metabolic syndrome in male Caucasian and Indo-Asian subjects. Diabet Med. 2004; 21: 1339–1345. 10.1111/j.1464-5491.2004.01361.x [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Chung JH, Song SW, Jung WS, Lee YA, Kim HN. Relationship between deep subcutaneous abdominal adipose tissue and metabolic syndrome: a case control study. Diabetol Metab Syndr. 2016; 8: 10 10.1186/s13098-016-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Kuk JL, Kim Y, Arslanian SA. Measurement site of visceral adipose tissue and prediction of metabolic syndrome in youth. Pediatr Diabetes. 2011; 12: 250–257. 10.1111/j.1399-5448.2010.00705.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014; 7: 1221–1235. 10.1016/j.jcmg.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushita Y, Nakagawa T, Yamamoto S, Takahashi Y, Yokoyama T, Mizoue T, et al. Effect of longitudinal changes in visceral fat area on incidence of metabolic risk factors: the Hitachi health study. Obesity (Silver Spring). 2013; 21: 2126–2129. [DOI] [PubMed] [Google Scholar]
- 14.Ding YS, Guo SX, Ma RL, Li SG, Guo H, Zhang JY, et al. Association of Metabolic Syndrome with the Adiponectin to Homeostasis Model Assessment of Insulin Resistance Ratio. Mediators Inflamm. 2015; 2015: 607364 10.1155/2015/607364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahonen TM, Saltevo JT, Kautiainen HJ, Kumpusalo EA, Vanhala MJ. The association of adiponectin and low-grade inflammation with the course of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2012; 22: 285–291. 10.1016/j.numecd.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Baldasseroni S, Mannucci E, Orso F, Di Serio C, Pratesi A, Bartoli N, et al. Adiponectin in outpatients with coronary artery disease: independent predictors and relationship with heart failure. Nutr Metab Cardiovasc Dis. 2012; 22: 292–299. 10.1016/j.numecd.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 17.Koh SB, Yoon J, Kim JY, Yoo BS, Lee SH, Park JK, et al. Relationships between serum adiponectin with metabolic syndrome and components of metabolic syndrome in non-diabetic Koreans: ARIRANG study. Yonsei Med J. 2011; 52: 234–241. 10.3349/ymj.2011.52.2.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006; 8: 264–280. 10.1111/j.1463-1326.2005.00510.x [DOI] [PubMed] [Google Scholar]
- 19.Pyrzak B, Ruminska M, Popko K, Demkow U. Adiponectin as a biomarker of the metabolic syndrome in children and adolescents. Eur J Med Res. 2010; 15 Suppl 2: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abiko A, Makita S, Naganuma Y, Nagai M, Nakamura M. Association between metabolic syndrome and carotid atherosclerosis: relevance of combined criteria including the serum adiponectin level for the general population. Intern Med. 2011; 50: 381–387. [DOI] [PubMed] [Google Scholar]
- 21.Timar R, Timar B, Degeratu D, Serafinceanu C, Oancea C. Metabolic syndrome, adiponectin and proinflammatory status in patients with type 1 diabetes mellitus. J Int Med Res. 2014; 42: 1131–1138. 10.1177/0300060514541829 [DOI] [PubMed] [Google Scholar]
- 22.Won H, Kang SM, Shin MJ, Oh J, Hong N, Park S, et al. Plasma adiponectin concentration and its association with metabolic syndrome in patients with heart failure. Yonsei Med J. 2012; 53: 91–98. 10.3349/ymj.2012.53.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JY, Yadav D, Ahn SV, Koh SB. A prospective study of serum adiponectin and regression of metabolic syndrome: The ARIRANG study. Biochem Biophys Res Commun. 2015; 466: 201–205. 10.1016/j.bbrc.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 24.Persson J, Folkersen L, Ekstrand J, Helleberg J, Gabrielsen A, Lundman P, et al. High plasma adiponectin concentration is associated with all-cause mortality in patients with carotid atherosclerosis. Atherosclerosis. 2012; 225: 491–496. 10.1016/j.atherosclerosis.2012.09.036 [DOI] [PubMed] [Google Scholar]
- 25.Shafiee G, Ahadi Z, Qorbani M, Kelishadi R, Ziauddin H, Larijani B, et al. Association of adiponectin and metabolic syndrome in adolescents: the caspian- III study. J Diabetes Metab Disord. 2015; 14: 89 10.1186/s40200-015-0220-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh SB, Park JK, Yoon JH, Chang SJ, Oh SS, Kim JY, et al. Preliminary report: a serious link between adiponectin levels and metabolic syndrome in a Korean nondiabetic population. Metabolism. 2010; 59: 333–337. 10.1016/j.metabol.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 27.Ryo M, Maeda K, Onda T, Katashima M, Okumiya A, Nishida M, et al. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care. 2005; 28: 451–453. [DOI] [PubMed] [Google Scholar]
- 28.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990; 10: 497–511. [DOI] [PubMed] [Google Scholar]
- 29.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001; 86: 1930–1935. 10.1210/jcem.86.5.7463 [DOI] [PubMed] [Google Scholar]
- 30.Bidulescu A, Liu J, Hickson DA, Hairston KG, Fox ER, Arnett DK, et al. Gender differences in the association of visceral and subcutaneous adiposity with adiponectin in African Americans: the Jackson Heart Study. BMC Cardiovasc Disord. 2013; 13: 9 10.1186/1471-2261-13-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santaniemi M, Kesaniemi YA, Ukkola O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur J Endocrinol. 2006; 155: 745–750. 10.1530/eje.1.02287 [DOI] [PubMed] [Google Scholar]
- 32.Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011; 18: 629–639. [DOI] [PubMed] [Google Scholar]
- 33.Koh SJ, Hyun YJ, Choi SY, Chae JS, Kim JY, Park S, et al. Influence of age and visceral fat area on plasma adiponectin concentrations in women with normal glucose tolerance. Clin Chim Acta. 2008; 389: 45–50. 10.1016/j.cca.2007.11.017 [DOI] [PubMed] [Google Scholar]
- 34.Takenouchi Y, Kobayashi T, Matsumoto T, Kamata K. Gender differences in age-related endothelial function in the murine aorta. Atherosclerosis. 2009; 206: 397–404. 10.1016/j.atherosclerosis.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 35.Li JB, Nishida M, Kaimoto K, Asakawa A, Chaolu H, Cheng KC, et al. Effects of aging on the plasma levels of nesfatin-1 and adiponectin. Biomed Rep. 2014; 2: 152–156. 10.3892/br.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008; 149: 2270–2282. 10.1210/en.2007-1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sulistyoningrum DC, Gasevic D, Lear SA, Ho J, Mente A, Devlin AM. Total and high molecular weight adiponectin and ethnic-specific differences in adiposity and insulin resistance: a cross-sectional study. Cardiovasc Diabetol. 2013; 12: 170 10.1186/1475-2840-12-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakatsuji H, Kobayashi H, Kishida K, Nakagawa T, Takahashi S, Tanaka H, et al. Binding of adiponectin and C1q in human serum, and clinical significance of the measurement of C1q-adiponectin / total adiponectin ratio. Metabolism. 2013; 62: 109–120. 10.1016/j.metabol.2012.06.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data are presented as the mean ± standard deviation or (25 percentile, 75 percentile) for continuous variables and the number (%) for categorical variables. Δ, variation from baseline; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG/HDL, Triglyceride/HDL-cholesterol; CRP, C-reactive protein.
(DOCX)
Data are presented as the mean ± standard deviation or (25 percentile, 75 percentile) for continuous variables and the number (%) for categorical variables. Δ, variation from baseline; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG/HDL, Triglyceride/HDL-cholesterol; CRP, C-reactive protein.
(DOCX)
Data are presented as the mean ± standard deviation or (25 percentile, 75 percentile) for continuous variables and the number (%) for categorical variables. Δ, variation from baseline; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG/HDL, Triglyceride/HDL-cholesterol; CRP, C-reactive protein.
(DOCX)
Data was presented as odd ratio (95% confidence interval). OR, odd ratio; 95% CI, 95% confidence interval.
(DOCX)
Data Availability Statement
Data are available from the database at the Metabolic Syndrome Research Center for researchers who meet the criteria for access to confidential data according to the regulation implemented by the Seoul Metropolitan Government and the institutional review board of Korea University Anam Hospital. Interested researchers may submit requests to Professor Do-Sun Lim, MD, PhD. Phone: +82-2-920-5445; Fax: +82-2-927-1478; E-mail: dslmd@kumc.or.kr.