Abstract
The widespread use of platinum in high-tech and catalytic applications has led to the production of diverse Pt loaded wastewaters. Effective recovery strategies are needed for the treatment of low concentrated waste streams to prevent pollution and to stimulate recovery of this precious resource. The biological recovery of five common environmental Pt-complexes was studied under acidic conditions; the chloro-complexes PtCl42- and PtCl62-, the amine-complex Pt(NH3)4Cl2 and the pharmaceutical complexes cisplatin and carboplatin. Five bacterial species were screened on their platinum recovery potential; the Gram-negative species Shewanella oneidensis MR-1, Cupriavidus metallidurans CH34, Geobacter metallireducens, and Pseudomonas stutzeri, and the Gram-positive species Bacillus toyonensis. Overall, PtCl42- and PtCl62- were completely recovered by all bacterial species while only S. oneidensis and C. metallidurans were able to recover cisplatin quantitatively (99%), all in the presence of H2 as electron donor at pH 2. Carboplatin was only partly recovered (max. 25% at pH 7), whereas no recovery was observed in the case of the Pt-tetraamine complex. Transmission electron microscopy (TEM) revealed the presence of both intra- and extracellular platinum particles. Flow cytometry based microbial viability assessment demonstrated the decrease in number of intact bacterial cells during platinum reduction and indicated C. metallidurans to be the most resistant species. This study showed the effective and complete biological recovery of three common Pt-complexes, and estimated the fate and transport of the Pt-complexes in wastewater treatment plants and the natural environment.
Introduction
The growing importance and use of platinum in clean and high-tech products in the last 30 years have induced the production of Pt loaded waste streams and the accumulation of platinum in the environment [1, 2]. For example, deterioration of automotive catalysts leads to the emission of Pt particles into the environment, part of which gets drained by stormwater into sewers [3]. Platinum is also the crucial building block of chemotherapeutic drugs such as cisplatin and carboplatin, and the excreted human metabolites contaminate both hospital and municipal wastewaters [1]. Finally, liquid waste streams (often diluted) containing platinum are also produced from the application of industrial catalysts, the manufacturing of jewelry and electronics, and both primary mining and precious metal recovery activities [2, 3].
The resulting residual platinum appears in different complexes in wastewater, with inorganic or organic ligands, such as cisplatin (cis-PtCl2[NH3]2), carboplatin (cis-(Pt[NH3]2[1,1-cyclobutanedicarboxylato])), and their metabolites, chloro-complexes Pt(II)Cl42- and Pt(IV)Cl62- or amine-complexes such as Pt(NH3)4Cl2, resulting from leaching or metal refinery processes [4–6]. The metal’s fate in a wastewater treatment plant or in the receiving environment depends largely on the metal’s speciation and the matrix composition of the waste stream [2]. An effective removal of the precious metal is advised to both lower the pollutant load in the environment, based on the pollution prevention principle, and since the behavior and impact of species such as cisplatin is mainly unknown in the environment [7]. Moreover, platinum’s high market value (av. 34.7 $ g-1 in 2015 [8]) and criticality stimulate the effective recovery and valorization of critical resources [9, 10]. Case by case, it should be questioned if the targeted Pt-complex could be removed and recovered from the waste stream, and whether this recovery could be interesting from an economical point of view [2].
Biotechnologies based on living biomass can serve as low-cost and green treatment techniques to recover platinum at low concentrations [11, 12]. The effective removal of platinum by different axenic cultures has been demonstrated before; PtCl42- and PtCl62- were sorbed by Shewanella putrefaciens [12], PtCl62- was reduced by Shewanella algae [13] and an undefined Pt-complex was reduced by Cupriavidus metallidurans [14]. However, the metal speciation can hamper an effective metal removal [2]. Complex waste streams such as highly acidic saline streams originating from metal refinery processes can be considered too challenging for conventional biological wastewater treatment plants (WWTP). They require specialized mixed cultures adapted to the prevalent conditions [11, 15].
The aim of this study was to further elaborate the biological recovery of different synthetic platinum complexes, representative for diluted Pt containing wastewaters of interest. It is important to explore the fate of these common Pt-complexes once they have entered a wastewater treatment plant or the environment. Therefore, this study investigates the relationship between Pt-speciation and the observed recovery by axenic cultures and the effect of the different Pt-complexes on the cell viability. The studied Pt-complexes include; chloro-complexes PtCl42- and PtCl62-, present in e.g. run-off waters or industrial process streams, cisplatin, carboplatin, and a Pt-amine complex Pt(NH3)4Cl2, being found in metal refinery streams. A series of axenic bacterial species (Shewanella oneidensis MR-1, Cupriavidus metallidurans CH34, Geobacter metallireducens, Bacillus toyonensis, and Pseudomonas stutzeri), commonly present in wastewater plants treating metal contaminated effluents, was examined. In this research, it was evaluated which bacteria recovered platinum, under which conditions (with and without electron donor) and how this was affected by the metal speciation. The morphology and metal speciation of the precipitated Pt particles were investigated, as well as the viability of the axenic cultures during Pt recovery. The results of this study can be used as a prediction of the fate and transport of Pt salts in wastewater treatment plants.
Materials and Methods
Bacterial cultures and growth conditions
Shewanella oneidensis MR-1 was obtained from the BCCM/LMG Bacteria Collection (Gent, Belgium; LMG 19005) and Cupriavidus metallidurans CH34 was obtained from SCK•CEN (Mol, Belgium). Both species were grown aerobically in Lysogeny broth (LB-Lennox) medium overnight at 28°C. Geobacter metallireducens was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ; ATCC 53774) and was cultivated anaerobically in DSMZ medium 579 at 28°C for 7 days. Bacillus toyonensis and Pseudomonas stutzeri were isolated from a wastewater treatment plant which processes metal streams, and were grown aerobically in Lysogeny broth (LB-Lennox) medium for 48 h at 28°C.
Platinum recovery experiments
The five different bacterial species were used to study the recovery of five different Pt-complexes; K2PtCl4 (Sigma-Aldrich, USA), K2PtCl6 (Sigma-Aldrich, USA), Pt(NH3)4.2HCO3 (Alfa Aesar, Germany), cisplatin (Alfa Aesar, Germany), and carboplatin (Alfa Aesar, Germany). Cells from the cultures S. oneidensis, C. metallidurans, B. toyonensis, and P. stutzeri were harvested by centrifugation (7000 g, 7 min) and were then washed twice with 25 mL phosphate buffer (8.5 g L-1 Na2HPO4.7H2O and 3 g L-1 KH2PO4). The washed cells were suspended in the phosphate buffer to a final optical density of 1 (OD610nm) and added to 120 mL glass serum bottles (50 mL cell suspensions). The glass bottles were flushed by 20 repeated cycles of N2 overpressure and vacuum underpressure. In the case H2 was used as electron donor, the headspace was replaced with 100% H2-gas. In the case of formate and acetate, 50 mM formate or 12.5 mM acetate was dosed to the biomass suspension. Subsequently, platinum was dosed to a final concentration of 100 mg L-1 Pt (2.5 g L-1 Pt 1 M HCl stock). The glass bottles were incubated and continuously mixed at 100 rpm and 28°C during the experiment.
The protocol was slightly modified for experiments based on G. metallireducens. The cells were centrifuged at 8000 g for 7 min, washed and suspended in 30 mM NaHCO3 to a final optical density of 0.31. All steps were executed in an anaerobic closet (37°C, 80% N2/20% CO2).
The washed biomass suspensions were all characterized by pH 7.0–7.1 prior to Pt dosage. The pH was adjusted to pH 1.8–2.2 at the start of the experiment. Samples were analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES) and Pt recovery efficiencies were calculated after 48 h.
Inductively coupled plasma optical emission spectrometry (ICP-OES)
Experimental details on ICP-OES analysis were previously described by Maes et al. [11]. The platinum concentrations were determined with a Spectro Arcos ICP-OES (Spectro Analytical Instruments GmbH, Kleve, Germany).
X-ray absorption spectroscopy
Biomass pellet samples of cultures S. oneidensis, C. metallidurans and G. metallireducens were investigated by X-ray absorption spectroscopy after the recovery of the platinum chloro-complexes Pt(II)Cl42- and Pt(IV)Cl62-. The indication “aerobic” or “anaerobic” refers to the atmospheric condition during cultivation. Anaerobic S. oneidensis was cultivated according to Schuetz et al. [16] with 50 mM ferric citrate as terminal electron acceptor. All recovery experiments were executed under anaerobic conditions. H2-gas was always applied as electron donor, except for the aerobic S. oneidensis sample with Pt(IV)Cl62- (with formate).
Experimental details on X-ray absorption spectroscopy were previously described by Maes et al. [11]. All μXAS spectroscopy measurements were performed using the microprobe beamline X27A at the National Synchrotron Light Source (NSLS), Upton, NY. Aliquots of fully hydrated Pt-biomass samples were transferred to an air-tight polypropylene bag to prevent drying. Samples were secured to an x, y, z motorized stage 45 degrees to the incident beam and 13-element HGe Canberra fluorescence detector. The beam spot-size on the sample was maintained at ca. 15 μm.
By means of X-ray Absorption Near Edge Structure (XANES) and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopies, the oxidation state and first-shell coordination environment of the Pt phase associated with the biomass was examined. All spectra were collected at room temperature. X-ray fluorescence was measured from 150 eV below to 800 eV above the Pt L3-edge. Absolute x-ray energy calibration was based on the first inflection point of standard Pt (11 919 eV) metal foil, which was collected in transmission mode as an internal calibration during each scan. Normalization, calibration and averaging of the XAS spectra and ab initio fitting of the EXAFS region of the spectra were performed using Athena and Artemis software [17].
Transmission electron microscopy (TEM)
After the recovery experiments were finished, the bacterial suspensions were washed twice with the according washing buffer; 30 mM NaHCO3 for G. metallireducens, phosphate buffer for all other species. Samples were stored overnight and the supernatants were removed. The TEM analysis was then performed as previously described by Maes et al. [11], using a Zeiss TEM900 transmission electron microscope (Carl Zeiss, Oberkochen, Germany).
Cell viability by flow cytometry analysis
Partial viability of the strains was assessed during recovery experiments by dual stain flow cytometry as described elsewhere [18]. Briefly, bacterial cells were stained with a mixed SYBR® Green I (SG, 10 000x concentrate, Invitrogen) and propidium iodide (PI, 20 μM, Invitrogen) staining solution which resolves membrane damaged from intact bacterial cells. At the start of the recovery experiments the suspension was acidified to pH 2 and the studied Pt-complex and H2-gas were added. Samples taken during batch experiments were diluted immediately 100 times in sterile, 0.22 μm filtered phosphate buffer and stored at +4°C until further analysis. Prior to analysis, samples were if necessary further diluted to approximately 106 cells mL-1 and stained with 10 μL mL-1 of the staining solution (final concentration of 1x SG and 4 μM PI). The stained samples were incubated for 20 minutes in the dark at 37°C and immediately analyzed on a BD FACSVerse (BD Biosciences, Erembodgem, Belgium) equipped with a 20 mW 488 nm blue laser, 40 mW 405 nm violet laser and a 640 nm red laser. Green and red fluorescence intensities corresponding to respectively the SG and PI emission wavelengths were collected through a 527 ± 32 nm band pass and 700 ± 54 nm band pass filter. All samples were collected and analyzed in triplicate within 24 hours of sampling. Cell counts were extracted from manually drawn gates on the green vs. red fluorescence intensity plots as described elsewhere [19]. The limit of detection was 33.3 x 103 cells mL-1.
Results and Discussion
Recovery of platinum complexes by axenic cultures
The recovery of five different platinum complexes was tested using five axenic bacterial cultures. The selection of Pt-complexes consisted of two Pt-chloro complexes (Pt(II)Cl42-, Pt(IV)Cl62-), the Pt-chemotherapy complexes cisplatin (cis-PtCl2[NH3]2) and carboplatin (cis-(Pt[NH3]2[1,1-cyclobutanedicarboxylato])), and a Pt-tetraamine complex (Pt(NH3)4Cl2). The biological platinum recovery was studied using three Gram-negative (G-) species (Shewanella oneidensis MR-1, Cupriavidus metallidurans CH34 and Geobacter metallireducens), each known for their ability to reduce metals, as shown for palladium [14, 20, 21], and extended with the Gram-positive (G+) species Bacillus toyonensis and the G- species Pseudomonas stutzeri, both isolated from a wastewater treatment plant which processes metal streams.
Influence of Pt-speciation on microbial recovery
The recovery of the five Pt-complexes was tested at pH 2 by using each of the cultures, in the presence of H2, formate or acetate as electron donor to study dissimilatory metal reduction, and without electron donor to evaluate sorption. The Pt-chloro complex Pt(II)Cl42- was recovered completely with H2 (Table 1) and a black microbial Pt suspension was formed (indicative for Pt reduction) [22]. The addition of formate or acetate as electron donor was less effective; 56–79% and 6–19% PtCl42- was recovered with formate and acetate, respectively (S1 Table). In experiments studying sorption, 6–25% of the dosed Pt was recovered on the biomass, however no color change was observed.
Table 1. An overview of the platinum recovery efficiencies (%) at pH 2 is given; the Pt recovery was investigated with and without (sorption control) the addition of H2-gas.
The platinum recovery was studied using five different bacterial species and five Pt-complexes (n = 1). All recoveries were measured after 48 h, except for: * 68 h, ** 107 h and *** 168 h, and **** 320 h. The chemical reduction was studied for all Pt-species using H2-gas.
| Pt-species | Pt(II)Cl42- | Pt(IV)Cl62- | Pt(II)(NH3)4Cl2 | Cisplatin | Carboplatin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Culture | Sorption | H2 | Sorption | H2 | Sorption | H2 | Sorption | H2 | Sorption | H2 | |
| S. oneidensis | 25 | 99 | 8 | 99*** | 0 | 0 | 8 | 99 | 3 | 6 | |
| C. metallidurans | 24 | 99 | 8 | 99 | 0 | 0 | 3 | 99 | 1 | 3 | |
| G. metallireducens | 6* | 99 | 5 | 98** | - | ||||||
| B. toyonensis | 15 | 98 | 3 | 99**** | 0 | 0 | 0 | 5 | 7 | 1 | |
| P. stutzeri | 18 | 99 | 2 | 99 | 0 | 0 | 8 | 10 | 10 | 9 | |
| Chemical reduction | - | 99 | - | 0 | - | 0 | - | 99 | - | 0 | |
The kinetics of the microbial reduction of Pt(IV)Cl62- were slower compared to Pt(II)Cl42-; whereas almost complete recovery was observed in the case of H2 and 49–87% recovery with formate, no substantial Pt recovery was obtained using acetate (Table 1, S1 and S2 Tables). The sorption control showed a limited removal of 2–8%. Based on these Pt recovery efficiencies, hydrogen gas was preferred as sole electron donor for further experiments.
Cisplatin and carboplatin showed a very different behavior. Although both species sorbed on the biomass to a very little amount, only cisplatin was reduced successfully by the bacteria. Finally, the Pt-tetraamine complex was neither sorbed on any of the studied microbial species, nor reduced by these same species.
Effect of the bacterial species on Pt recovery
Whereas all studied bacterial species recovered Pt(II)Cl42- quickly (within 2–4 h), with the G+ species B. toyonensis showing the slowest kinetics, none of them could recover Pt-tetraamine. Differences in recovery efficiency and kinetics were mainly observed for Pt(IV)Cl62- and cisplatin. P. stutzeri reduced PtCl62- remarkably quickly (< 24 h), whereas S. oneidensis and B. toyonensis were only able to recover the complex over an extended time period (≥ 1 week). A non-active biological control, i.e. heat-killed Shewanella oneidensis cells in the presence of H2-gas, removed 99% PtCl42- and 13% PtCl62-, but at a slower rate (mainly between 24–48 h).
In the case of cisplatin, S. oneidensis and C. metallidurans could fully recover the complex, while B. toyonensis and P. stutzeri recovered at maximum 10%. In contrast, all bacterial species showed limited recovery of carboplatin.
To explore the recovery potential of the Pt-complexes under circumneutral conditions as a proxy for their fate in wastewater treatment plants or the environment, their recovery was also examined at pH 7 in the absence of an electron donor (S1 Table). In general, lower recovery efficiencies were noted under sorptive conditions compared to the dissimilatory reduction, showing the need for an electron donor to obtain full recovery. By using S. oneidensis and C. metallidurans, the platinum recovery at neutral pH was observed to be very similar to the sorptive removal at acidic pH. For B. toyonensis and P. stutzeri, a better sorption was noted under neutral conditions for mainly cisplatin, carboplatin, and PtCl42- on the long term (28–40% PtCl42- was recovered after 117–144 h) (S1 Table).
Platinum speciation analysis
A characterization of the bacteria-metal interaction using X-ray absorption spectroscopy, was executed on the species Shewanella oneidensis MR-1, Cupriavidus metallidurans CH34 and Geobacter metallireducens removing the platinum chloro-complexes Pt(II)Cl42- and Pt(IV)Cl62-. Anaerobically grown S. oneidensis was included in this speciation analysis for comparison but was not further investigated in this study.
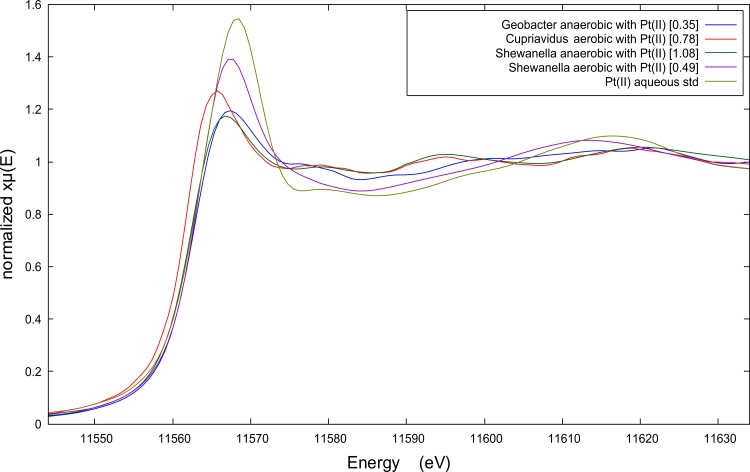
The X-ray energy of peak fluorescence along with the overall shape of the Pt K-edge XANES spectra provides measures of the average oxidation state and bonding environment of Pt associated with the biomass. The peak fluorescence of all the spectra in Fig 1has shifted to lower energies relative to the Pt(II) aqueous spectrum indicating that the Pt associated with the biomass underwent reduction during the recovery process. The spectral patterns between peak fluorescence and 11 600 eV for Pt(II) recovered by aerobic C. metallidurans and anaerobic S. oneidensis are consistent with metallic Pt(0) particles [11], while the spectral patterns of anaerobic G. metallireducens and aerobic S. oneidensis are similar to the Pt(II) aqueous spectrum suggesting only partial reduction of the available Pt. The EXAFS spectra and Fourier Transforms (FTs) shown in the Supporting Information provide further evidence for these interpretations of the XANES spectra (see S1 Fig).
Fig 1. X-ray absorption near edge spectroscopy (XANES) spectra of biomass pellet samples after Pt(II)Cl42- recovery (100 mg L-1 Pt; 50 mg L-1 Pt in case of anaerobic S. oneidensis), by three bacterial species: Geobacter metallireducens, Cupriavidus metallidurans CH34 and Shewanella oneidensis MR-1.
The XANES spectra also show how the level of Pt recovery (amount of Pt incorporated into the biomass) is correlated with each organism’s ability to reduce Pt(II) to metallic Pt(0) nanoparticles. Prior to normalization the edge jump in the XANES spectra reflects the number of Pt atoms fluorescing within the x-ray beam, and therefore, the height of the edge jump provides a measure of the relative amount of Pt per unit of biomass assuming equivalent biomass densities and sample thickness for all biomass samples. These assumptions are reasonable given that all underwent the same biomass separation method and were loaded in identical sample holders. The edge jump values (values shown in square brackets in Fig 1) increase as the Pt XANES spectra more closely resemble the XANES spectrum of metallic Pt(0) nanoparticles [11]. Without independent measures of Pt:biomass, the XANES edge jump only provides a relative measure of Pt recovery.
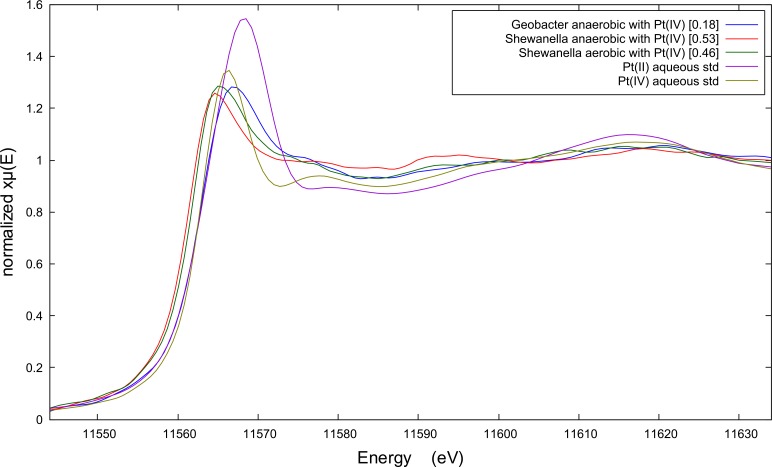
All bacterial strains are less efficient at reducing and recovering aqueous Pt(IV) ions under anaerobic conditions according to the spectral edge jumps reported in Fig 2(see values in square brackets). C. metallidurans was below detection and therefore its spectrum is not shown. Anaerobic G. metallireducens and S. oneidensis reduced approximately half as much Pt relative to when Pt(II) is the starting aqueous species. It needs to be noted that the mismatch between the amount of Pt that was reduced to Pt(0) (XANES spectra) and the total amount of Pt that was recovered from solution (ICP data) corresponds with unreduced or partly reduced Pt. The energy of peak fluorescence in the XANES spectrum is a less reliable diagnostic given that the peak fluorescence of Pt(IV) aqueous standard occurs at a similar energy to reduced Pt(0) in biomass. However, the EXAFS spectra indicate that only the anaerobically grown S. oneidensis is capable of reducing significant amounts of Pt(IV) to Pt(0). The FT’s of these EXAFS spectra do suggest that a minor fraction of the Pt(IV) could have been reduced by anaerobic G. metallireducens and aerobic S. oneidensis (see S2 Fig).
Fig 2. X-ray absorption near edge spectroscopy (XANES) spectra of biomass pellet samples after Pt(IV)Cl62- recovery (100 mg L-1 Pt; 50 mg L-1 Pt in case of anaerobic Shewanella), by two bacterial species: Geobacter metallireducens and Shewanella oneidensis MR-1.
Platinum particle morphology
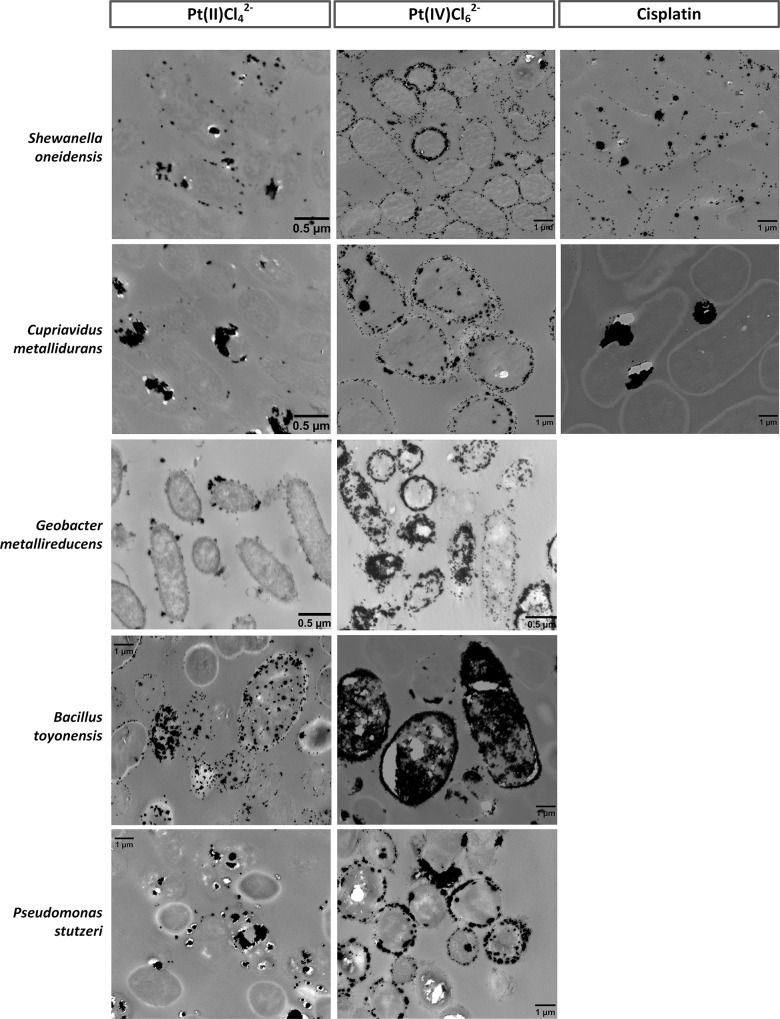
The characteristics of the Pt precipitates in and on the microbial biomass were studied using transmission electron microscopy (TEM). All bacterial suspensions that showed visible signs of Pt reduction were analyzed (i.e. black discoloration). The morphology of the platinum particles differed considerably according to the studied bacterial species and the recovered Pt-complex (Fig 3and S3 Fig).
Fig 3. Transmission electron microscopy (TEM) images of thin sections of the five different bacterial species, loaded with platinum particles.
The precipitation of platinum was induced by the presence of hydrogen gas. No Pt particles were observed during the recovery of cisplatin by Bacillus toyonensis and Pseudomonas stutzeri, while Geobacter metallireducens was not studied for this complex.
PtCl62- generally induced the formation of larger particles compared to PtCl42-, as could be concluded from the particle size distributions (S4 and S5 Figs). For example, 24% (with formate) to 80% (with H2) of the total particle surface area was allocated to particles larger than 100 nm for the reduction of Pt(IV)Cl62- by G. metallireducens, compared to 15–23% for the reduction of Pt(II)Cl42- by the same bacterial species. Precipitated cisplatin also formed mainly larger particles (min. 71% of the particles were larger than 100 nm).
The Pt-speciation influenced the location of the formed precipitates as well, as observed for B. toyonensis. Whereas PtCl42- precipitated as dispersed, small intra- and extracellular particles, large Pt clusters completely filled the cells in the case of PtCl62- (Fig 3). Overall, both intra- and extracellular particles were observed for all three Pt-complexes, depending on the bacterial species. The increased importance of large particles in the particle surface area also corresponds to the increase in edge jump value for Pt(II)Cl42- (see Fig 1and S4 Fig).
S. oneidensis precipitated particles mainly on the cell wall and in the periplasmic space. Depending on the conditions (Pt-complex, electron donor), C. metallidurans tended to precipitate platinum into larger clusters, which can be observed in the case of cisplatin where very large clusters were located near the bacterial cells. Numerous small particles can be observed in the presence of G. metallireducens, which can cluster together to bigger particles such as in case of PtCl62-. A similar clustering was observed after the reduction of PtCl62- by B. toyonensis; cells were completely filled with precipitated platinum. P. stutzeri reduced the Pt-chloro complexes into larger particles as well.
Next to the applied bacterial species and recovered Pt-complex, also the choice of electron donor influenced the precipitation of platinum. In general, larger particles were formed in the presence of H2-gas, compared to the presence of formate. Small uniformly dispersed particles were only observed in case of formate induced reduction (with S. oneidensis and G. metallireducens) (S3 Fig), which is in contrast with the palladium (Pd) study from De Windt et al. [20], that observed more small Pd particles in case H2 was used compared to formate.
Platinum precipitates formed in a previous study by Shewanella algae with Pt(IV) and lactate showed similarities with our results [13]. Pt particles of about 5 nm were observed in the periplasmic space, which is similar to the case of formate induced PtCl62- reduction by S. oneidensis, which resulted in particles of 4.8 nm (mean size), mainly formed in the periplasmic space and on the cell wall of the cells. In general, more intracellular Pt particles were formed in the case of the platinum(IV) chloro-complex, as was previously observed by Maes et al. when using halophilic mixed cultures [11].
Membrane integrity of axenic cultures during platinum recovery
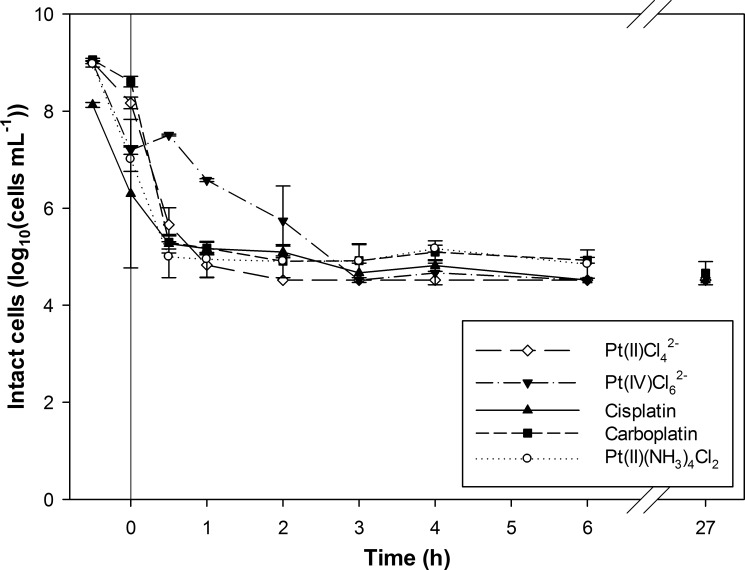
The recovery of Pt-complexes and the prevalent conditions of the target wastewater will influence the viability of the present microorganisms and could affect their metal recovery potential. Furthermore, bacteria might behave differently towards the studied Pt-complexes according to the different intrinsic and chemical characteristics. Therefore, the cell viability of the axenic cultures was investigated during Pt-complex recovery experiments through flow cytometry based membrane integrity staining [23]. This staining makes it possible to distinguish cells with an intact cellular membrane, referred to as intact cells, from damaged cells. The influence of the Pt-speciation upon recovery was investigated using S. oneidensis, as the model organism (Fig 4). The viability of the Shewanella culture decreased from approximately 109 intact cells mL-1 to below the detection limit (33.3 x 103 cells mL-1) within 2 hours after dosing of PtCl42-. For PtCl62- and cisplatin, the number of intact cells decreased to the detection limit within 4 and 6 hours, respectively. The addition of the Pt-tetraamine complex and carboplatin lowered the amount of intact cells until approximately 105 cells mL-1. These complexes were not reduced during the experiment, suggesting a damaging effect of the Pt reduction and the formation of intra- and extracellular precipitates. Furthermore, carboplatin is less toxic than cisplatin in chemotherapy treatments since it is more stable (due to a more stable leaving group), which might explain the more limited interaction in this study as well [24]. Although this study showed a slower decrease in intact cells in the case of PtCl42- compared to PtCl62- (based on the S. oneidensis culture), PtCl62- was previously found to be more toxic to C. metallidurans than PtCl42-; minimal inhibitory concentrations of 39 mg L-1 and 3.4 mg L-1 were determined for respectively the Pt(II) and Pt(IV)-chloro complex [25]. In this study, the cell viability was however mainly linked to the precipitation of Pt particles instead of the intrinsic toxicity of the metal salts.
Fig 4. Membrane integrity of Shewanella oneidensis MR-1 cells during platinum recovery as a function of time.
Five different Pt-complexes were dosed to investigate the effect of the Pt-speciation on the cell viability. The recovery experiment was initiated at t0 by the addition of 100 mg L-1 Pt and H2-gas as electron donor. The pH was initially set at pH 2.0.
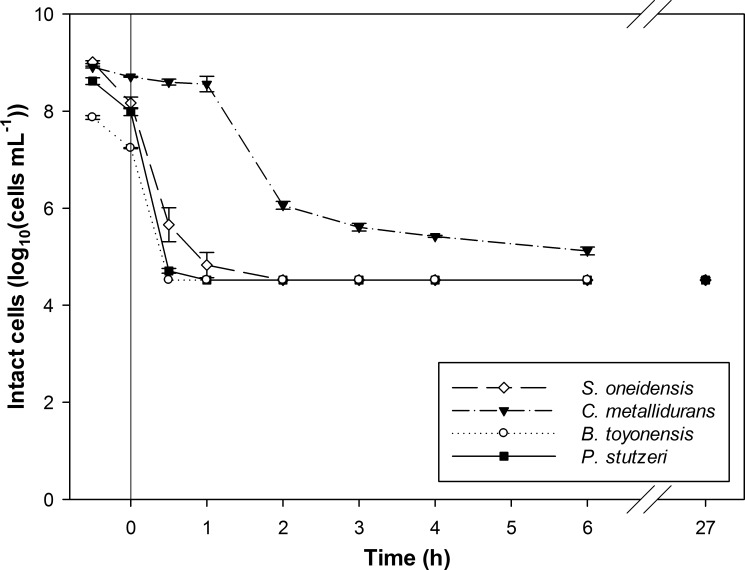
The PtCl42—complex was selected as a toxic and industrially relevant Pt-complex to study the vulnerability of the different bacterial organisms towards Pt-complex toxicity (Fig 5). Among the screened cultures, differences could be observed. The decrease in intact cells was almost identical for B. toyonensis and P. stutzeri; the detection limit was reached within 1 hour, indicating a severe damaging effect during Pt recovery. C. metallidurans appeared to be the most resistant species; 1.2 x 106 intact cells mL-1 were still measured after 2 hours and 1.3 x 105 intact cells mL-1 after 6 hours. This might be explained by the presence of metal resistance gene clusters in this species, enabling cell detoxification, as was demonstrated before for precious metals gold and silver [26, 27]. Still, the amount of intact cells of all bacterial suspensions decreased finally until the detection level (< 27 hours after the initiation of the experiment). Overall, the affected viability of the cultures will have been caused by the combined effect of (1) the acidic pH, (2) the exposure to the Pt-complexes, as an increased cell membrane permeability by Pt(IV) ions was observed before [22] and (3) the precipitation of Pt particles, previously shown for an Acinetobacter species [28]. The substantial effect of the acidic pH on the viability is shown for the S. oneidensis suspension containing cisplatin; 3.7 x 108 intact cells mL-1 were still detected after 6 h when working at pH 5, while only 3.3 x 104 intact cells mL-1 were measured at pH 2 (i.e. limit of detection). Next to metal toxicity, the pH of the metal containing wastewaters will be another important challenge to obtain an effective biological metal recovery.
Fig 5. The effect of the addition of Pt(II)Cl42- on the membrane integrity of different bacterial cells during platinum recovery as a function of time.
Four different bacterial cultures were studied; Shewanella oneidensis MR-1, Cupriavidus metallidurans CH34, Bacillus toyonensis and Pseudomonas stutzeri. The recovery experiment was started at t0 by the addition of 100 mg L-1 Pt and H2-gas as electron donor. The pH was initially set at pH 2.0.
Interaction mechanisms between bacteria and Pt-species
The bacterial interaction in these biorecovery processes is believed to consist of two concomitant steps; (1) the initial sorption of Pt to the cell, which is followed by (2) the microbial reduction of the sorbed Pt-molecules [29, 30].
Differences observed during the first sorptive step might be partly related to the different cell wall structure of G+ and G—species. In general, the recovery of deprotonated complexes is favored by a low pH as functional groups on the bacterial surface become protonated at low pH [31]. Furthermore, the speciation and valence of Pt-complexes is highly dependent on the pH and chloride concentration. Chloro, hydroxyl or hydrated complexes can be formed depending on the conditions, characterized by a complicated chemistry [32–35]. For example, the Pt-chloro complexes PtCl42- and PtCl62-, which are important species under acidic and saline conditions, are characterized by a negative charge at low pH [36]. This enables electrostatic interactions with positively charged binding sites such as amine groups and presumably results in effective recovery [12, 36]. The observed slower recovery and precipitation of Pt(IV) can be explained by the sequential transformation of Pt(IV) through Pt(II) to Pt(0) [37]. Additionally, PtCl62- is expected to be more difficult to reduce, based on the slightly lower standard reduction potential of this Pt-complex: E0(Pt(IV)Cl62- ⇔ PtCl42-) = + 0.726 V vs. SHE compared to E0(PtCl42-) = + 0.758 V vs. SHE [38]. The first reduction step of Pt(IV) to Pt(II) will have slowed down and limited the platinum recovery performance. Riddin et al. [37] proposed a dislocated two-step reduction of Pt(IV) by sulphate-reducing bacteria, in which Pt(IV) was reduced to Pt(II) by a cytoplasmic hydrogenase and Pt(II) further to Pt(0) by a periplasmic hydrogenase.
Furthermore, none of the bacterial species was able to recover the Pt-tetraamine complex. Since any sorption was lacking, the neutral complex Pt(NH3)4Cl2 will probably have formed, preventing any interaction with the bacterial surface and making a biological treatment ineffective. The uncharged cisplatin partitions (partially) into the hydrated complexes cis-[PtCl(NH3)2(H2O)]+ and cis-[Pt(NH3)2(H2O)2]2+ when low chloride concentrations are present [33]. The formation of these positively charged Pt-complexes might explain the limited sorption by protonated functional groups at low pH. Still, complete recovery was possible at low pH in the presence of H2-gas. The limited sorption of, for example, a residual amount of the uncharged mother compound can thus be sufficient to induce the full reduction of this Pt-complex, as long as a capable microbial species and an electron donor are present. The second chemotherapy complex carboplatin has been observed to mainly remain stable in wastewater and was characterized in our study by a limited recovery under all tested conditions [39]. The formation of Pt precipitates was only observed for cisplatin and not for carboplatin. The sorptive recovery of both chemotherapy complexes was studied before by using activated sludge, revealing the least sorption for carboplatin (70% vs. 96% for cisplatin) [32].
Different mechanisms might be responsible for the recovery potential of the studied bacterial species. The recovery of platinum was investigated before using the related marine species Shewanella algae, which was able to recover 90% PtCl62- within 1 hour using lactate as electron donor (C0 = 200 mg L-1 Pt at pH 7) [13]. To our knowledge, the recovery of platinum by the anaerobic species Geobacter metallireducens has not been studied yet, but the reduction of palladium was demonstrated recently by the related Geobacter sulfurreducens [21, 40]. The dissimilatory metal reducing bacteria Shewanella and Geobacter were found to use different hydrogenases and cytochromes, being present in the outer membrane, periplasm or cytoplasm, to transfer electrons to reduce the metals [40, 41]. The heavy metal-resistant and metallophilic species Cupriavidus metallidurans is well-studied for the reduction and precipitation of palladium and gold, initiated by the expression of different metal resistance genes [26, 42, 43]. Gauthier et al. [14] demonstrated the recovery of platinum and palladium by Cupriavidus metallidurans and Cupriavidus necator species in the presence of hydrogen gas; 70–74% Pt and 96–100% Pd were recovered from a mixed metal acidic leachate (pH 1.4; 24 h). The Pseudomonas stutzeri species has been shown to reduce selenate and selenite and to produce silver nanoparticles [44, 45]. It is hypothesized that siderophores, produced by the Pseudomonas species, are involved in a detoxification strategy of the species, by extracellularly complexing and reducing various metals [46]. The only studied G+ species, Bacillus toyonensis, has not been utilized yet in metal recovery studies, although Bacillus species have been shown to reduce palladium [47]. The recovery potential of these axenic cultures should be further explored under real stream conditions.
Supporting Information
(A) Extended X-ray Absorption Fine Structure (EXAFS) spectra and their (B) Fourier Transforms (FT) of biomass pellet samples after Pt(II)Cl42- recovery by three bacterial species: Geobacter metallireducens, Cupriavidus metallidurans CH34 and Shewanella oneidensis MR-1.
(TIF)
(A) Extended X-ray Absorption Fine Structure (EXAFS) spectra and their (B) Fourier Transforms (FT) of biomass pellet samples after Pt(IV)Cl62- recovery by two bacterial species: Geobacter metallireducens and Shewanella oneidensis MR-1.
(TIF)
The precipitation of platinum was induced by the presence of formate as electron donor.
(TIF)
The different Pt-complexes were precipitated in the presence of hydrogen gas. No Pt particles were observed during the recovery of cisplatin by Bacillus toyonensis and Pseudomonas stutzeri, while Geobacter metallireducens was not studied for this complex.
(TIF)
The Pt(II)Cl42- and Pt(IV)Cl62- complexes were precipitated in the presence of formate as electron donor.
(TIF)
All recovery efficiencies were measured after 48 h, except for: * 68 h, ** 107 h, *** 117 h and **** 144 h. The chemical reduction using formate was studied for Pt(II)Cl42- and Pt(IV)Cl62-.
(TIF)
(TIF)
Acknowledgments
The authors thank Asha Debrabandere and Thibaut Van Acker for the ICP measurements and Sam Van Nevel for critically reading the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
SM is supported by the Agency for Innovation by Science and Technology (IWT Flanders). TH was supported by a postdoctoral fellowship from the Research Foundation Flanders (FWO Flanders) (http://www.fwo.be). RP was supported by Ghent University (BOFDOC2015000601; http://www.ugent.be/en) and the Belgian Nuclear Research Centre (SCK•CEN) (http://sckcen.be). This work was also supported by the Metgrow Plus project (Contract No. 690088; H2020-SC5-2014-2015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ravindra K, Bencs L, Van Grieken R. Platinum group elements in the environment and their health risk. Sci Total Environ. 2004;318(1–3):1–43. 10.1016/S0048-9697(03)00372-3 [DOI] [PubMed] [Google Scholar]
- 2.Zhuang W-Q, Fitts JP, Ajo-Franklin CM, Maes S, Alvarez-Cohen L, Hennebel T. Recovery of critical metals using biometallurgy. Curr Opin Biotechnol. 2015;33:327–35. 10.1016/j.copbio.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westerhoff P, Lee S, Yang Y, Gordon GW, Hristovski K, Halden RU, et al. Characterization, Recovery Opportunities, and Valuation of Metals in Municipal Sludges from U.S. Wastewater Treatment Plants Nationwide. Environ Sci Technol. 2015;49(16):9479–88. 10.1021/es505329q [DOI] [PubMed] [Google Scholar]
- 4.Vyas N, Turner A, Sewell G. Platinum-based anticancer drugs in waste waters of a major UK hospital and predicted concentrations in recipient surface waters. Sci Total Environ. 2014;493:324–9. 10.1016/j.scitotenv.2014.05.127 [DOI] [PubMed] [Google Scholar]
- 5.Won SW, Kotte P, Wei W, Lim A, Yun Y-S. Biosorbents for recovery of precious metals. Bioresource Technology. 2014;160:203–12. 10.1016/j.biortech.2014.01.121 [DOI] [PubMed] [Google Scholar]
- 6.Umeda H, Sasaki A, Takahashi K, Haga K, Takasaki Y, Shibayama A. Recovery and Concentration of Precious Metals from Strong Acidic Wastewater. Materials Transactions. 2011;52(7):1462–70. [Google Scholar]
- 7.Curtis L, Turner A, Vyas N, Sewell G. Speciation and Reactivity of Cisplatin in River Water and Seawater. Environ Sci Technol. 2010;44(9):3345–50. 10.1021/es903620z [DOI] [PubMed] [Google Scholar]
- 8.Mineral commodity summaries 2016. United States Geological Survey, 2016.
- 9.Graedel TE, Harper EM, Nassar NT, Nuss P, Reck BK. Criticality of metals and metalloids. Proceedings of the National Academy of Sciences. 2015;112(14):4257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennebel T, Boon N, Maes S, Lenz M. Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives. New Biotechnology. 2015;32(1):121–7. 10.1016/j.nbt.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 11.Maes S, Props R, Fitts JP, De Smet R, Vilchez-Vargas R, Vital M, et al. Platinum Recovery from Synthetic Extreme Environments by Halophilic Bacteria. Environ Sci Technol. 2016;50(5):2619–26. 10.1021/acs.est.5b05355 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Watanabe N. Study on the Coordination Structure of Pt Sorbed on Bacterial Cells Using X-Ray Absorption Fine Structure Spectroscopy. PLoS One. 2015;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S, Hishida H, et al. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol. 2007;128(3):648–53. 10.1016/j.jbiotec.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 14.Gauthier D, Søbjerg LS, Jensen KM, Lindhardt AT, Bunge M, Finster K, et al. Environmentally Benign Recovery and Reactivation of Palladium from Industrial Waste by Using Gram-Negative Bacteria. ChemSusChem. 2010;3(9):1036–9. 10.1002/cssc.201000091 [DOI] [PubMed] [Google Scholar]
- 15.Maes S, Claus M, Verbeken K, Wallaert E, De Smet R, Vanhaecke F, et al. Platinum recovery from industrial process streams by halophilic bacteria: Influence of salt species and platinum speciation. Water Research. 2016;105:436–43.4 10.1016/j.watres.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 16.Schuetz B, Schicklberger M, Kuermann J, Spormann AM, Gescher J. Periplasmic Electron Transfer via the c-Type Cytochromes MtrA and FccA of Shewanella oneidensis MR-1. Appl Environ Microbiol. 2009;75(24):7789–96. 10.1128/AEM.01834-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravel B, Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. Journal of Synchrotron Radiation. 2005;12(4):537–41. [DOI] [PubMed] [Google Scholar]
- 18.Van Nevel S, Koetzsch S, Weilenmann H-U, Boon N, Hammes F. Routine bacterial analysis with automated flow cytometry. J Microbiol Methods. 2013;94(2):73–6. 10.1016/j.mimet.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 19.SLMB. Determining the total cell count and ratios of high and low nucleic acid content cells in freshwater using flow cytometry. Analysis method 3331: Swiss food book. Federal Office of Public Health, Swiss Confederation; 2012.
- 20.De Windt W, Aelterman P, Verstraete W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol. 2005;7(3):314–25 10.1111/j.1462-2920.2005.00696.x [DOI] [PubMed] [Google Scholar]
- 21.Pat-Espadas A, Razo-Flores E, Rangel-Mendez JR, Cervantes F. Reduction of palladium and production of nano-catalyst by Geobacter sulfurreducens. Appl Microbiol Biotechnol. 2013;97(21):9553–60. 10.1007/s00253-012-4640-9 [DOI] [PubMed] [Google Scholar]
- 22.Rashamuse KJ, Whiteley CG. Bioreduction of Pt(IV) from aqueous solution using sulphate-reducing bacteria. Appl Microbiol Biotechnol. 2007;75(6):1429–35. 10.1007/s00253-007-0963-3 [DOI] [PubMed] [Google Scholar]
- 23.Berney M, Hammes F, Bosshard F, Weilenmann H-U, Egli T. Assessment and Interpretation of Bacterial Viability by Using the LIVE/DEAD BacLight Kit in Combination with Flow Cytometry. Appl Environ Microbiol. 2007;73(10):3283–90. 10.1128/AEM.02750-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alderden RA, Hall MD, Hambley TW. The Discovery and Development of Cisplatin. J Chem Educ. 2006;83(5):728. [Google Scholar]
- 25.Brugger J, Etschmann B, Grosse C, Plumridge C, Kaminski J, Paterson D, et al. Can biological toxicity drive the contrasting behavior of platinum and gold in surface environments? Chem Geol. 2013;343:99–110. [Google Scholar]
- 26.Reith F, Etschmann B, Grosse C, Moors H, Benotmane MA, Monsieurs P, et al. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proceedings of the National Academy of Sciences. 2009;106(42):17757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen PJ, Van Houdt R, Moors H, Monsieurs P, Morin N, Michaux A, et al. The Complete Genome Sequence of Cupriavidus metallidurans Strain CH34, a Master Survivalist in Harsh and Anthropogenic Environments. PLoS One. 2010;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaidhani SV, Yeshvekar RK, Shedbalkar UU, Bellare JH, Chopade BA. Bio-reduction of hexachloroplatinic acid to platinum nanoparticles employing Acinetobacter calcoaceticus. Process Biochemistry. 2014;49(12):2313–9. [Google Scholar]
- 29.De Corte S, Hennebel T, Verschuere S, Cuvelier C, Verstraete W, Boon N. Gold nanoparticle formation using Shewanella oneidensis: a fast biosorption and slow reduction process. J Chem Technol Biotechnol. 2011;86(4):547–53. [Google Scholar]
- 30.Rotaru A-E, Jiang W, Finster K, Skrydstrup T, Meyer RL. Non-enzymatic palladium recovery on microbial and synthetic surfaces. Biotechnol Bioeng. 2012;109(8):1889–97. 10.1002/bit.24500 [DOI] [PubMed] [Google Scholar]
- 31.Guibal E, Larkin A, Vincent T, Tobin JM. Chitosan Sorbents for Platinum Sorption from Dilute Solutions. Ind Eng Chem Res. 1999;38(10):4011–22. [Google Scholar]
- 32.Lenz K, Hann S, Koellensperger G, Stefanka Z, Stingeder G, Weissenbacher N, et al. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci Total Environ. 2005;345(1–3):141–52. 10.1016/j.scitotenv.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 33.Michalke B. Platinum speciation used for elucidating activation or inhibition of Pt-containing anti-cancer drugs. J Trace Elem Med Biol. 2010;24(2):69–77. 10.1016/j.jtemb.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 34.Azaroual M, Romand B, Freyssinet P, Disnar JR. Solubility of platinum in aqueous solutions at 25 degrees C and pHs 4 to 10 under oxidizing conditions. Geochim Cosmochim Acta. 2001;65(24):4453–66. [Google Scholar]
- 35.Cotton S. Chemistry of Precious Metals: Springer Netherlands; 2012. [Google Scholar]
- 36.Colombo C, Oates CJ, Monhemius AJ, Plant JA. Complexation of platinum, palladium and rhodium with inorganic ligands in the environment. Geochemistry: Exploration, Environment, Analysis. 2008;8(1):91–101. [Google Scholar]
- 37.Riddin TL, Govender Y, Gericke M, Whiteley CG. Two different hydrogenase enzymes from sulphate-reducing bacteria are responsible for the bioreductive mechanism of platinum into nanoparticles. Enzyme Microb Technol. 2009;45(4):267–73. [Google Scholar]
- 38.Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. 2nd ed: John Wiley & Sons; 2001. [Google Scholar]
- 39.Hann S, Stefanka Z, Lenz K, Stingeder G. Novel separation method for highly sensitive speciation of cancerostatic platinum compounds by HPLC-ICP-MS. Anal Bioanal Chem. 2005;381(2):405–12.4 10.1007/s00216-004-2839-z [DOI] [PubMed] [Google Scholar]
- 40.Yates MD, Cusick RD, Logan BE. Extracellular Palladium Nanoparticle Production using Geobacter sulfurreducens. Acs Sustainable Chemistry & Engineering. 2013;1(9):1165–71 [Google Scholar]
- 41.Ng CK, Cai Tan TK, Song H, Cao B. Reductive formation of palladium nanoparticles by Shewanella oneidensis: role of outer membrane cytochromes and hydrogenases. RSC Advances. 2013;3(44):22498–503. [Google Scholar]
- 42.Nies HD. Heavy metal-resistant bacteria as extremophiles: molecular physiology and biotechnological use of Ralstonia sp. CH34. Extremophiles. 2000;4(2):77–82. [DOI] [PubMed] [Google Scholar]
- 43.Yong P, Mikheenko IP, Deplanche K, Redwood MD, Macaskie LE. Biorefining of precious metals from wastes: an answer to manufacturing of cheap nanocatalysts for fuel cells and power generation via an integrated biorefinery? Biotechnol Lett. 2010;32(12):1821–8. 10.1007/s10529-010-0378-6 [DOI] [PubMed] [Google Scholar]
- 44.Lortie L, Gould WD, Rajan S, McCready RGL, Cheng KJ. Reduction of Selenate and Selenite to Elemental Selenium by a Pseudomonas stutzeri Isolate. Appl Environ Microbiol. 1992;58(12):4042–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine-Nanotechnology Biology and Medicine. 2010;6(2):257–62. [DOI] [PubMed] [Google Scholar]
- 46.Zawadzka AM, Crawford RL, Paszczynski AJ. Pyridine-2,6-Bis(Thiocarboxylic Acid) Produced by Pseudomonas stutzeri KC Reduces and Precipitates Selenium and Tellurium Oxyanions. Appl Environ Microbiol. 2006;72(5):3119–29. 10.1128/AEM.72.5.3119-3129.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosseinkhani B, Hennebel T, Van Nevel S, Verschuere S, Yakimov MM, Cappello S, et al. Biogenic Nanopalladium Based Remediation of Chlorinated Hydrocarbons in Marine Environments. Environ Sci Technol. 2014;48(1):550–7. 10.1021/es403047u [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Extended X-ray Absorption Fine Structure (EXAFS) spectra and their (B) Fourier Transforms (FT) of biomass pellet samples after Pt(II)Cl42- recovery by three bacterial species: Geobacter metallireducens, Cupriavidus metallidurans CH34 and Shewanella oneidensis MR-1.
(TIF)
(A) Extended X-ray Absorption Fine Structure (EXAFS) spectra and their (B) Fourier Transforms (FT) of biomass pellet samples after Pt(IV)Cl62- recovery by two bacterial species: Geobacter metallireducens and Shewanella oneidensis MR-1.
(TIF)
The precipitation of platinum was induced by the presence of formate as electron donor.
(TIF)
The different Pt-complexes were precipitated in the presence of hydrogen gas. No Pt particles were observed during the recovery of cisplatin by Bacillus toyonensis and Pseudomonas stutzeri, while Geobacter metallireducens was not studied for this complex.
(TIF)
The Pt(II)Cl42- and Pt(IV)Cl62- complexes were precipitated in the presence of formate as electron donor.
(TIF)
All recovery efficiencies were measured after 48 h, except for: * 68 h, ** 107 h, *** 117 h and **** 144 h. The chemical reduction using formate was studied for Pt(II)Cl42- and Pt(IV)Cl62-.
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.