Abstract
Objective
This study aimed to compare the efficacy of local anaesthetic infiltration to trocar wounds and intraperitoneally on postoperative pain as a part of a multimodal analgesia method after laparoscopic cholecystectomies.
Methods
The study was performed on 90 ASA I–III patients aged between 20 and 70 years who underwent elective laparoscopic cholecystectomy. All patients had the same general anaesthesia drug regimen. Patients were randomized into three groups by a closed envelope method: group I (n=30), trocar site local anaesthetic infiltration (20 mL of 0.5% bupivacaine); group II (n=30), intraperitoneal local anaesthetic instillation (20 mL of 0.5%) and group III (n=30), saline infiltration both trocar sites and intraperitoneally. Postoperative i.v. patient controlled analgesia was initiated for 24 h. In total, 4 mg of i.v. ondansetron was administered to all patients. Visual analogue scale (VAS), nausea and vomiting and shoulder pain were evaluated at 1., 2., 4., 8., 12., 24. hours. An i.v. nonsteroidal anti-inflammatory drug (NSAID) (50 mg of dexketoprofen) as a rescue analgesic was given if the VAS was ≥5.
Results
There were no statistical significant differences between the clinical and demographic properties among the three groups (p≥0.005). During all periods, VAS in group I was significantly lower than that in groups II and III (p<0.001). Among the groups, although there was no significant difference in nausea and vomiting (p=0.058), there was a significant difference in shoulder pain. Group III (p<0.05) had more frequent shoulder pain than groups I and II. The total morphine consumption was higher in groups II and III (p<0.001 vs p<0.001) than in group I. The requirement for a rescue analgesic was significantly higher in group III (p<0.05).
Conclusion
Trocar site local anaesthetic infiltration is more effective for postoperative analgesia, easier to apply and safer than other analgesia methods. Morphine consumption is lesser and side effects are fewer; therefore, this method can be used as a part of common practice.
Keywords: Multimodal analgesia, bupivacaine, peritrocar, intraperitoneal local anaesthetic
Introduction
Laparoscopic interventions that have been commonly used in recent years have significant advantages over conventional surgery, such as fewer surgical traumas, shorter hospital stay and faster functional recovery. Laparoscopic cholecystectomy is the most important of these interventions. Pain observed after laparoscopy is quite different from that observed after laparotomy. While pain is primarily observed as parietal type (abdominal wall) after laparotomy, patients also complain of visceral pain after laparoscopic operations. In laparoscopic interventions, in addition to surgical trauma, the local irritation of carbon dioxide intraperitoneally administered and the increase in intra-abdominal pressure causes the pain to increase even more in the postoperative period. Multimodal analgesia techniques are generally used to relieve pain caused by the laparoscopic cholecystectomy. Nonsteroidal anti-inflammatory drugs, epidural analgesia, opioids (oral, intravenous, PCA), incision-site local anaesthetic infiltration and intraperitoneal local anaesthetic application (1–3) are among the multimodal analgesia options.
The main reason for using multimodal analgesia techniques is to avoid possible side effects by limiting the utilisation of commonly used opioids to provide postoperative analgesia (4).
For postoperative analgesia in laparoscopic interventions, administering local anaesthesia at the trocar site or in an intraperitoneal area may be considered to provide lower postoperative pain scores (5, 6).
This study aimed to compare the effects of local anaesthesia at the trocar site and intraperitoneal local anaesthetic application on postoperative pain in laparoscopic cholecystectomy cases.
Methods
This study was conducted with the approval of the Ethics Committee, dated 27.04.2015 and numbered 22/13, that was received from Ankara Dışkapı Yıldırım Beyazıt Research and Education Hospital and with the written consents of the patients.
A total of 90 patients with ASA physiological scoring I–III and aged between 20 and 70 years who were scheduled to undergo elective laparoscopic cholecystectomy were included. Patients who were in the risk group of ASA IV and above, had acute pancreatitis, were undergoing chronic pain treatment and antiepileptic treatment, had alcohol or drug addiction, had severe liver or kidney failure, had allergies to local anaesthetics, were pregnant or lactating, had communication problems and cognitive dysfunction and were transferred to open surgery were excluded.
During the preoperative visit, physical examinations of all patients were performed, and the laboratory findings were evaluated. VAS was explained to all the patients, and information was given regarding the pain scoring system, which ranged from 0 to 10, for the determination of pain severity. Patients were asked to indicate pain conditions on the scale by marking 0 for no pain and 10 for the most severe pain. Patients were informed about the patient-controlled analgesia device (CADD-Legacy PCA Pump, Smiths Medical International, Inc., St. Paul, MN 55112, USA) and instructed on its use.
The patients were randomly divided into three groups with 30 people in each by the closed envelope method.
Group I (n=30): Patients in whom local anaesthetic infiltration (20 mL of 0.5% bupivacaine-Bustesin; Vem Pharmaceuticals Inc., Istanbul, Turkey) was administered in the trocar sites and saline infusion through the intra-abdominal catheter.
Group II (n=30): Patients in whom intraperitoneal local anaesthetic (20 mL of 0.5% bupivacaine-Bustesin, Vem Pharmaceuticals Inc., Istanbul, Turkey) was administered with a separate catheter that passed through one of the trocars and in whom saline infusion was applied in the trocar sites.
Group III (n=30): Patients in whom saline was administered both in the trocar sites and intraperitoneal area.
After the patients were taken to the operating room, 0.9% NaCl infusion was initiated by establishing a peripheral vascular access with a 20 G cannula. The patients were monitored with noninvasive arterial blood pressure, electrocardiography, peripheral oxygen saturation (SpO2) and end-tidal capnography (EtCO2). After administering 1 μg kg−1 fentanyl for anaesthesia induction, 2–3 mg kg−1 propofol and 0.5–0.6 mg kg−1 rocuronium IV were administered for muscle relaxation. Anaesthesia was maintained with sevoflurane in 50% O2 and 50% air mixture (1.5–2 minimal alveolar concentration) and remifentanil 0.05–0.2 μg kg−1 min−1 via an intravenous infusion. Mechanical ventilator settings were set to ensure that the EtCO2 value was between 32 and 40 mmHg. Systolic, diastolic and mean arterial pressure (SAP, DAP and MAP), heart rate and SpO2 values of the patients were recorded at baseline, 1 min after inducing anaesthesia, 1 min after intubation and subsequently at 15-min intervals.
Four trocars (a 10-mm trocar was placed in the infraumbilical region, 10-mm trocar in the mid-epigastrium 5 mm below the xyphoid, 5 mm trocar in the right subcostal region in the midclavicular line and 5 mm trocar in the anterior axillary line) were used in patients who were positioned by the surgical team. Intra-abdominal pressure was maintained between 12 and 15 mmHg.
At the end of the operation, 20 mL saline was administered in both the intraperitoneal subdiaphragmatic space and in the gallbladder bed with a separate catheter passed through one of the trocars (DuploSpray MIS Applicator, Micromedics, St Paul, USA). Furthermore, 20 mL of 0.5% bupivacain was applied to the skin, fascial muscle and preperitoneal area according to the rules of infiltration in trocar sites in group I patients by the surgical team, i.e. 6 mL in each 10 mm trocar site and 4 mL in each 5 mm trocar site. After removing the gallbladder in group II patients, 20 mL of 0.5% bupivacaine was applied in the intraperitoneal subdiaphragmatic space and the gall bladder bed with a separate catheter passed through one of the trocars (DuploSpray MIS Applicator, Micromedics, St Paul, USA), and 20 mL saline was applied to the skin, fascial muscle and preperitoneal area according to the infiltration rules in the trocar sites. Saline was administered both to the trocar sites and intraperitoneal area in group III patients by the same technique as discussed for the other two groups.
The inhalation agents were halted 5 min before the end of the operation and the patients were made to breathe 100% oxygen. When spontaneous respiratory movement began, the effect of the muscle relaxant was reversed with neostigmine 0.04 mg kg−1 and atropine 0.01 mg kg−1, and the patients were extubated after their airway reflexes returned. After adequate ventilation was provided with 100% oxygen, the patients were taken to the postoperative anaesthesia care unit. Postoperative analgesia (morphine sulphate loading dose of 1 mg, lock-out time 10 min and bolus dose of 1 mg) was initiated with intravenous PCA for 24 h. Ondansetron (4 mg) was intravenously administered to each patient before extubation.
After the patients were taken into the postoperative anaesthetic care unit, by taking the extubation time as the 0 min, the patients were asked about their pain levels according to VAS, as previously mentioned. Of the patients, nausea and vomiting scores (vomiting. 2; nausea, 1; none, 0) and Ramsay sedation scores (7) were also recorded in the same period of time. When the Aldrete score (8) was over nine in the postoperative anaesthetic care unit, the patients were transferred to the general surgery service unit.
Nausea, vomiting and shoulder pain were evaluated in reference to VAS (while resting, coughing, during mobilization) at the 1, 2, 4, 8 and 12 h. NSAIDs (Dexketoprofen-Arveles; Ufsa Pharmaceutical, Spain) were intravenously administered as an additional analgesic in patients with a VAS score of ≥5.
Statistical analysis
In the power analysis performed before the study, 29 cases were planned for each group in order to test the statistical significance of at least a two-unit difference of VAS levels from the baseline between at least two of the groups at any monitoring time on the 80% power and 5% error level. The knowledge of two-unit difference has been obtained from the pilot study. In the possibility of excluding any patients from the study, 30 patients were taken into each group.
Data analysis was performed on the SPSS package program (Statistical Package for Social Science Inc., Chicago, IL, USA) for Windows 11.5. The Kolmogorov–Smirnov test was used to determine whether the distribution of continuous and intermittent numerical variables was close to normal, and the homogeneity of variances was investigated by Levene’s test. Descriptive statistics were expressed as mean±standard deviation or median (minimum–maximum) for the continuous and intermittent numerical variables and the nominal variables as case numbers and percentages. The significance of the difference in terms of the averages among the groups was investigated with one-way analysis of variance (ANOVA) and with the Kruskal–Wallis test in terms of the median values. When the results of one-way ANOVA and Kruskal–Wallis test statistics were found significant, the post-hoc Tukey HSD or Conover’s nonparametric multiple comparison test was used to identify the cases causing the difference. The nominal variables were examined by Pearson’s chi-square, Fisher’s exact, or likelihood ratio tests. ANOVA in repeated measures was used to assess whether there was a statistically significant change in haemodynamic measurements in the groups according to the monitoring time. When the results were significant, the corrected Bonferroni multiple comparison or Wilcoxon Sign test was used to determine the follow-up durations that caused the difference. A p values of <0.05 was considered statistically significant.
Results
There was no statistically significant difference among the groups in terms of the demographic and clinical characteristics (p>0.05) (Table 1).
Table 1.
Demographic and clinical characteristics among the groups
| Variables | Group I (n=30) | Group II (n=30) | Group III (n=30) | p |
|---|---|---|---|---|
| Age (years) | 47.6±12.8 | 49.2±11.9 | 48.4±11.1 | 0.874 |
| Sex | 0.510 | |||
| Male | 11 (36.7%) | 7 (23.3%) | 10 (33.3%) | |
| Female | 19 (63.3%) | 23 (76.7%) | 20 (66.7%) | |
| ASA | 0.217 | |||
| I | 14 (46.7%) | 12 (40.0%) | 9 (30.0%) | |
| II | 16 (53.3%) | 18 (60.0%) | 19 (63.3%) | |
| III | - | - | 2 (6.7%) | |
| Operation duration (min) | 45 (35–55) | 45 (35–50) | 45 (30–50) | 0.557 |
| Nausea/vomiting | 7 (23.3%) | 12 (40.0%) | 16 (53.3%) | 0.058 |
| Shoulder pain | 8 (26.7%)a | 6 (20.0%)b | 17 (56.7%)a,b | <0.05† |
| Total morphine dose (mg) | 15 (9–18)a,c | 22 (17–33)b,c | 32.5 (23–47)a,b | <0.001‡ |
| Additional analgesic requirement (mg) | 24±20.9 | 35±23.3 | 46.6±12.6 | <0.05† |
Pearson’s chi-square test;
Kruskal–Wallis test; the difference is statistically significant between agroups I and III (p<0.05); the difference is statistically significant between bgroups II and III (p<0.05); the difference is statistically significant between cgroups I and II (p<0.05).
No significant difference was found among the groups in terms of the haemodynamic variables during any of the follow-up times.
There was no statistically significant difference among the groups in terms of EtCO2 at any monitoring time (p>0.0125). The VAS level of group I at all follow-up times was found to be statistically significantly lower than that of groups II and III (p<0.001). The VAS level of group III was also statistically significantly higher than that of group II at all follow-up times, except for the initial value (p<0.0071) (Figure 1).
Figure 1.
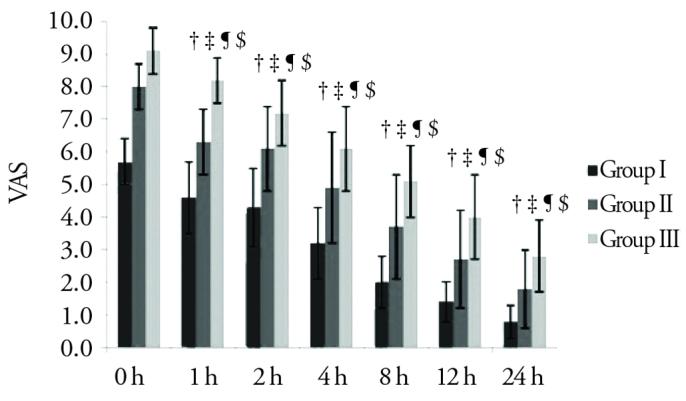
The VAS changes among the groups
†: Grup I ile Grup II arasındaki fark istatistiksel olarak anlamlı (p<0,001).
‡: Grup I ile Grup III arasındaki fark istatistiksel olarak anlamlı (p<0,001).
¶: Grup II ile Grup III arasındaki fark istatistiksel olarak anlamlı (p<0,0071).
$: Grup I ve II’de 1. ile 2.Saat arası hariç gruplar içerisinde tüm izlem zamanlarının birbirleri arasındaki fark istatistiksel olarak anlamlı (p<0,00079).
While no statistically significant difference was found among the groups in terms of frequency of nausea and vomiting (p=0.058), there was a statistically significant difference in terms of shoulder pain; group III had more frequent shoulder pain than groups I and II (p<0.05).
Total morphine consumption was statistically significantly higher in groups II and III than in group I (p<0.001 and p<0.001, respectively). Furthermore, total morphine consumption was statistically significantly higher in group III than in group II (p<0.001). The need for additional analgesic was also higher in group III than in groups I and II (p<0.05).
Discussion
Although laparoscopic cholecystectomy is superior to open cholecystectomy in terms of postoperative analgesia, patients continue to have moderate and severe pain. Pain after laparoscopy is quite different from that after laparotomy. While patients primarily experience parietal type pain (abdominal wall) after laparotomy, patients complain of visceral pain after laparoscopic operations. Many studies showed that pain after laparoscopic cholecystectomy arises from different components such as parietal, visceral and shoulder pain with different intensities and time (5, 9, 10). Parietal type pain observed after laparoscopic cholecystectomy is a sudden onset, well-localized and sharp pain. Previous studies have shown that local anaesthetic infiltration into the incision site significantly reduces the analgesic requirement and parietal pain in the postoperative period (11, 12).
Visceral pain observed after laparoscopic cholecystectomy is a blunt, diffuse and midline pain that grows slowly, cannot be easily localized and spreads to the reflection areas. Chemical irritants, sudden stretching of organs, excessive contractions and reduced blood flow can be considered among the causes of visceral pain. Reflected pain (shoulder pain) can be experienced in a place different from the stimulus site. The irritation of the diaphragmatic muscle and phrenic nerve with CO2 gas and exposure to the pressure manifest as postoperative shoulder pain (13).
The efficacy of intraperitoneal local anaesthetic administration, particularly for visceral and shoulder pain, was investigated in many studies to provide analgesia in the postoperative period after laparoscopic interventions (14, 15). In a meta-analysis performed by Moiniche et al. (15), the effectiveness of intraperitoneal local anaesthetic administration for postoperative analgesia in laparoscopic surgery was investigated. They reported that very accurate results regarding the effectiveness of intraperitoneal local anaesthetic administration in laparoscopic cholecystectomy for postoperative analgesic could not be obtained and that it is difficult to explain the reason for the different results from the randomized controlled trials. Different local anaesthetic agents were intraperitoneally administered at different concentrations with different adjuvant agents; however, a definitive conclusion could not be reached on postoperative analgesic efficacy (16–19). The limited efficacy of intraperitoneal local anaesthetic administration can be explained by the rapid dilution of the local anaesthetics or adjuvant agents in the intraperitoneal area (20). In this study, we also observed that the use of intraperitoneal local anaesthetics was less effective than local anaesthetic infiltration in the trocar sites in terms of postoperative analgesia.
In our study, the VAS scores, shoulder pain and morphine consumption were lower in patients who underwent local anaesthetic infiltration in the trocar insertion sites than those treated with intraperitoneal local anaesthetic and control patients. In the application of intraperitoneal local anaesthesia, the VAS scores, shoulder pain and morphine consumption were lower than the control group but higher than the administration in the trocar sites. The higher incidence of shoulder pain in the group in which we intraperitoneally administered a local anaesthesia can be explained by the fact that the local anaesthesia was diluted and that a drain was used to observe potential bile leakages. The difference in the VAS scores among the groups is more evident, particularly after 4 h postoperative. When we evaluated the VAS scores as mild (0–3), moderate (4–7) and severe (>7), pain was mild in all 30 (100%) patients in the group of trocar site after 8 h. In the intraperitoneal group, mild pain was detected in 18 (60%) patients and moderate pain in 12 (40%) patients. In the control group, seven (23.3%) patients had mild pain and 23 (76.7%) patients had moderate pain. Total morphine consumption was 1.5 times lower in patients in whom local anaesthesia was administered in the trocar incision site than patients in whom intraperitoneal local anaesthetic was administered and was 2 times lower than the control patients. This suggests that intraperitoneal local anaesthetic administration is partially effective, and local anaesthetic infiltration in the trocar sites is more effective than intraperitoneal local anaesthetic administration. While we evaluated the postoperative VAS scores of the patients in this study, similar to previously reported results, we observed that pain was more severe, particularly in the trocar insertion sites. In our study, the fact that the local anaesthetic application method in the trocar insertion site was more advantageous than intraperitoneal application can be explained by the fact that the pain of patients was mostly incisional–parietal pain.
Side effects such as hypotension because of opioid use for postoperative analgesia, impaired carbon dioxide respiratory response, suppression of the cough reflex and reduction of mucus excretion may occur (4). Laparoscopic cholecystectomy operations are in the high risk group in terms of nausea and vomiting (21). When we compared the frequency of nausea and vomiting among the groups, nausea and vomiting were found in seven (23.3%) patients in the local anaesthetic infiltration group, 12 (40%) patients in the intra-abdominal local anaesthetic group and 16 (53.3%) patients in the control group. The difference among the groups in terms of the incidence of nausea and vomiting was not significant. Because we believed that both opioid use and laparoscopic cholecystectomy could increase the frequency of nausea and vomiting, our intravenous administration of 4 mg ondansetron to every patient prophylactically may explain the lack of difference among the groups in terms of nausea and vomiting.
The side effects that may be observed because of the local anaesthetic administration intraperitoneally or in the trocar insertion site may be local irritation at the injection site or local anaesthetic systemic toxicity (22). In our study, the dose of bupivacaine was well below the dose that would result in a toxic effect, and we did not observe any regional side effects because of the local anaesthetic administration.
In our study, assistive methods in maintaining analgesia, such as heating of the CO2 and washing the abdominal cavity with saline, were not applied. While applying the analgesia methods to be performed after laparoscopic cholecystectomy in the future, considering the heating of the CO2 gas and routine intra-abdominal saline irrigation may be useful to increase analgesic efficiency. The limitation of our work is that a drain was applied in order to identify possible bile leakages. Drain application may have caused local anaesthetic loss and decreased the analgesic effect in patients receiving intraperitoneal local anaesthetic.
Conclusion
We believe that local anaesthetic infiltration in a trocar site can be used in laparoscopic cholecystectomy cases more commonly because it is easy, reliable and effective on postoperative analgesia and because it is a method with low morphine consumption and low side effect frequencies.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Dışkapı Yıldırım Beyazıt Training and Research Hospital.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.A., Ş.B.; Design - G.A., D.Ö., M.M.S., Ö.T.A.; Supervision - D.Ö., M.M.S., Ö.T.A.; Resources - G.A., D.Ö.; Data Collection and/or Processing - G.A., D.A., M.M.S., E.Ö.; Analysis and/or Interpretation - G.A., M.M.S., D.Ö., Ö.T.A.; Literature Search - G.A.; Writing Manuscript - G.A., Ö.T.A., D.Ö., M.M.S.; Critical Review - Ö.T.A., M.M.S., D.Ö.; Other - G.A., E.Ö., Ş.B., Ö.T.A., M.M.S., D.Ö.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Wilson YG, Rhodes M, Ahmed R, Daugherty M, Cawthorn SJ, Armstrong CP. Intramuscular diclofenac sodium for postoperative analgesia after laparoscopic cholecystectomy: a randomised, controlled trial. Surg Laparosc Endosc. 1994;4:340–4. https://doi.org/10.1097/00019509-199410000-00003. [PubMed] [Google Scholar]
- 2.Pasqualucci A, Angelis V, Contardo R, Colò F, Terrosu G, Donini A, et al. Preemptive analgesia: intraperitoneal local anesthetic in laparoscopic cholecystectomy. A randomized, double-blind, placebo-controlled study. Anesthesiology. 1996;85:11–20. doi: 10.1097/00000542-199607000-00003. https://doi.org/10.1097/00000542-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Erol DD, Yilmaz S, Polat C, Arikan Y. Efficacy of thoracic epidural analgesia for laparoscopic cholecystectomy. Adv Ther. 2008;25:45–52. doi: 10.1007/s12325-008-0005-2. https://doi.org/10.1007/s12325-008-0005-2. [DOI] [PubMed] [Google Scholar]
- 4.Berkenbosch A, Teppemb J, Olivier CN, Dahan A. Influences of morphine on the ventilatory response to isocapnic hypoxia. Anesthesiology. 1997;86:1342–9. doi: 10.1097/00000542-199706000-00016. https://doi.org/10.1097/00000542-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Avtan L, Berber E, Avcı C. Laparoskopik cerrahide postoperatif analjezi. Ağrı. 1996;8:22–25. [Google Scholar]
- 6.Kılıç A, Başgül E, Özdemir A, Erdem MK. Laparoskopik kolesistektomilerde, intraperitoneal bupivakain uygulamasının erken postoperatif ağrı tedavisindeki yeri ve postoperatif kan gazlarına etkisi. Ağrı. 1996;8:20–26. [Google Scholar]
- 7.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. https://doi.org/10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. https://doi.org/10.1016/0952-8180(94)00001-K. [DOI] [PubMed] [Google Scholar]
- 9.Bisgaard T, Klarskov B, Kristiansen VB, Callesen T, Schulze S, Kehlet H, et al. Multi-regional local anesthetic infiltration during laparoscopic cholecystectomy in patients receiving prophylactic multi-modal analgesia: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 1999;89:1017–24. doi: 10.1097/00000539-199910000-00036. https://doi.org/10.1097/00000539-199910000-00036. [DOI] [PubMed] [Google Scholar]
- 10.Ure BM, Troidl H, Spangenberger W, Dietrich A, Lefering R, Neugebauer E. Pain after laparoscopic cholecystectomy. Intensity and localization of pain and analysis of predictors in preoperative symptoms and intraoperative events. Surg Endosc. 1994;8:90–6. doi: 10.1007/BF00316616. https://doi.org/10.1007/BF00316616. [DOI] [PubMed] [Google Scholar]
- 11.Başgül E, Kaynak Ş, Öcal T, Erçelen Ö, Şahin A. İnsizyon bölgesine bupivakain infiltrasyonunun geniş karın insizyonuna bağlı postoperatif ağrı ve narkotik analjezik gereksinimi üzerine etkisi. Ağrı. 1992;4:32–35. [Google Scholar]
- 12.Alessandri F, Lijoi D, Mistrangelo E, Nicoletti A, Ragni N. Effect of presurgical local infiltration of levobupivacaine in the surgical field on postsurgical wound pain in laparoscopic gynegological surgery. Acta Obstet Gynecol Scand. 2006;85:844–9. doi: 10.1080/00016340500494846. https://doi.org/10.1080/00016340500494846. [DOI] [PubMed] [Google Scholar]
- 13.Kandil TS, El Hefnawy E. Shoulder pain following laparoscopic cholecystectomy: factors affecting the incidence and severity. J Laparoendosc Adv Surg Tech A. 2010;20:677–82. doi: 10.1089/lap.2010.0112. https://doi.org/10.1089/lap.2010.0112. [DOI] [PubMed] [Google Scholar]
- 14.Pasqualucci A, de Angelis V, Contardo R, Colò F, Terrosu G, Donini A. Preemptive analgesia: intraperitoneal local anesthetic in laparoscopic cholecystectomy. A randomized, double-blind, placebo-controlled study. Anesthesiology. 1996;85:11–20. doi: 10.1097/00000542-199607000-00003. https://doi.org/10.1097/00000542-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Moiniche S, Jørgensen H, Wetterslev J, Dahl JB. Local anesthetic infiltration for postoperative pain relief after laparoscopy: a qualitative and quantitative systematic review of intraperitoneal, port-site infiltration and mesosalpinx block. Anesth Analg. 2000;90:899–912. doi: 10.1097/00000539-200004000-00024. https://doi.org/10.1213/00000539-200004000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Özyılmaz MA, Ölmez G, Şimşek E. Laparoskopik kolesistektomilerde preempitif ketamin ile intraperitoneal ropivakain kombinasyonunun postoperatif analjezik etkinliklerinin karşılaştırılması. Fırat Tıp Dergisi. 2006;11:116–20. [Google Scholar]
- 17.Sozbilen M, Yeniay L, Unalp M, Makay O, Pirim A, Ulukaya S, et al. Effects of ropivacaine on pain after laparoscopic cholecystectomy: a prospective, randomized study. Adv Ther. 2007;24:247–57. doi: 10.1007/BF02849892. https://doi.org/10.1007/BF02849892. [DOI] [PubMed] [Google Scholar]
- 18.Alexander DJ, Ngoi SS, Lee L, So J, Mak K, Chan S, et al. Randomized trial of periportal peritoneal bupivacaine for pain relief after laparoscopic cholecystectomy. Br J Surg. 1996;83:1223–5. https://doi.org/10.1002/bjs.1800830914. [PubMed] [Google Scholar]
- 19.Alkhamesi NA, Peck DH, Lomax D, Darzi AW. Intraperitoneal aerosolization of bupivacaine reduces postoperative pain in laparoscopic surgery: a randomized prospective controlled double-blinded clinical trial. Surg Endosc. 2007;21:602–6. doi: 10.1007/s00464-006-9087-6. https://doi.org/10.1007/s00464-006-9087-6. [DOI] [PubMed] [Google Scholar]
- 20.Ng A, Smith G. I: Intraperitoneal administration of analgesia: is this practice of any utility? Br J Anaesth. 2002;89:535–7. doi: 10.1093/bja/aef219. https://doi.org/10.1093/bja/aef219. [DOI] [PubMed] [Google Scholar]
- 21.Iitomi T, Toriumi S, Kondo A, Akazawa T, Nakahara T. Incidence of nausea and vomiting after cholecystectomy performed via laparotomy or laparoscopy. Masui. 1995;44:1627–31. [PubMed] [Google Scholar]
- 22.Spielman FJ, Hulka JF, Ostheimer GW, Mueller RA. Pharmacokinetics and pharmacodynamics of local analgesia for laparoscopic tubal ligations. Am J Obstet Gynecol. 1983;146:821–4. doi: 10.1016/0002-9378(83)91085-2. https://doi.org/10.1016/0002-9378(83)91085-2. [DOI] [PubMed] [Google Scholar]


