Abstract
G protein-coupled receptors (GPCRs) are a family of cell-surface proteins that play critical roles in regulating a variety of pathophysiological processes and thus are targeted by almost a third of currently available therapeutics. It was originally thought that GPCRs convert extracellular stimuli into intracellular signals through activating G proteins, whereas β-arrestins have important roles in internalization and desensitization of the receptor. Over the past decade, several novel functional aspects of β-arrestins in regulating GPCR signaling have been discovered. These previously unanticipated roles of β-arrestins to act as signal transducers and mediators of G protein-independent signaling have led to the concept of biased agonism. Biased GPCR ligands are able to engage with their target receptors in a manner that preferentially activates only G protein- or β-arrestin-mediated downstream signaling. This offers the potential for next generation drugs with high selectivity to therapeutically relevant GPCR signaling pathways. In this review, we provide a summary of the recent studies highlighting G protein- or β-arrestin-biased GPCR signaling and the effects of biased ligands on disease pathogenesis and regulation.
Keywords: β-arrestin, biased signaling, G protein-coupled receptor, G protein
INTRODUCTION
G protein-coupled receptors (GPCRs) represent the largest family of cell surface molecules involved in signal transduction. More than 1000 receptors for sensory (e.g., odor and light) and chemical stimuli (e.g., catecholamines, amino acids, peptides, and ions) have been identified based on their common structural and biochemical properties (Muller, 2000). Because GPCRs represent 1–5% of the total cell surface proteins in mammals, it is not surprising that nearly 30% of United States Food and Drug Administration (FDA)-approved drugs target GPCRs (Overington et al., 2006).
Historically, GPCRs were assumed to exist in equilibrium between active and inactive states, and thus activation of GPCRs would equally affect all downstream signaling pathways. However, accumulating evidences indicate that GPCRs exist in multiple conformational states where each conformation confers different downstream effects. In this context, some ligands are able to induce a differential receptor conformation which activates a different subset of signaling events, causing bias receptor signaling (Liu et al., 2012). Biased GPCR signaling has been mainly studied on adrenergic and angiotensin receptors, but accumulating evidences indicate that this phenomenon may be extended to a wide variety of GPCRs currently targeted by pharmacological agents. This notion further complicates drug discovery efforts, but also holds the promise to design specific biased ligands that antagonize detrimental signaling pathways while stimulating beneficial downstream processes. Here, we seek to summarize the current knowledge of biased signaling on GPCRs and to discuss how identified biased ligands of selected receptors modulate disease outcomes.
ACTIVATION OF GPCRs
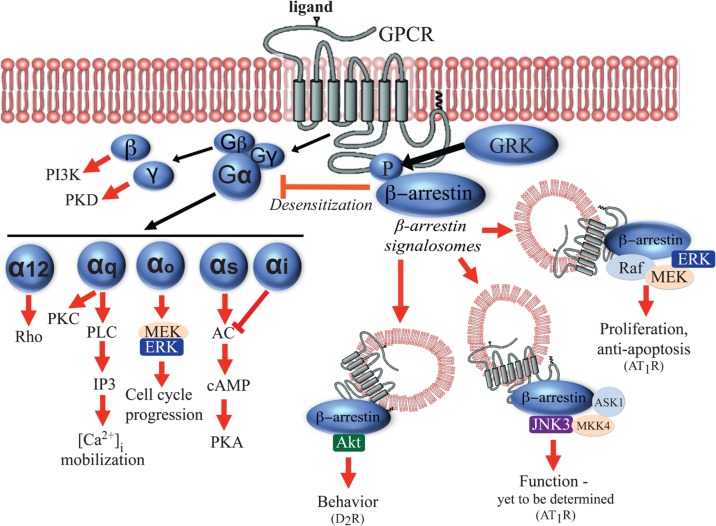
GPCRs are often referred to as seven-transmembrane receptors (7TMRs) because their structures are characterized by the presence of seven α-helices crossing the plasma membrane. GPCRs are consisted of intracellular and extracellular loops. The NH2 terminus is exposed to the extracellular environment and the COOH terminus is located in the intracellular part. The intracellular domains and loops mediate the interaction between the receptor and intracellular signaling partners such as G proteins (Gether, 2000; Hermans, 2003). The binding of exogenous ligands alters the conformation of critical domains of the seven-transmembrane helix pocket, which in turn causes the conformation changes of intracellular domains of the receptor. These changes promote the association of the receptor with a variety of heterotrimeric G proteins. They are composed of an α-subunit interacting with a βγ complex. Activation of the receptor promotes the exchange of a molecule of GDP by a molecule of GTP within the active site of the α-subunit. The binding of GTP to α-subunit causes the dissociation of the heterotrimeric complex, and both the GTP-bound α-subunit and the released βγ complex are then able to interact with intracellular or membrane effectors (e.g., enzymes or ion channels). The intrinsic GTPase activity of the α-subunit hydrolyses GTP into GDP, restoring its initial inactive conformation and its affinity for the βγ complex [for detailed reviews, see (Wess, 1997; Bockaert and Pin, 1999; Gether, 2000; Hermans, 2003)]. Up to now, at least 23 α-subunits derived from 17 different genes have been identified and are classified into four families (Gαi/o, Gαs, Gαq/11, and Gα12). At least 6 different β-subunits and 12 γ-subunits have been also discovered (Gautam et al., 1998; Vanderbeld and Kelly, 2000). Upon dissociation from the heterotrimeric complex, the various Gα subunits interact with the well-studied and classical effector enzymes in a highly specific manner. For instance, Gαs activates (and Gαi inhibits) adenylyl cyclase (AC), Gαt activates photoreceptor cGMP phosphodiesterase (PDE), and Gαq activates phospholipase C (PLC)-β (Skiba et al., 1996; Hamm, 1998). On the other hand, various Gβ subunits can activate or deactivate AC, activate PLCs or phosphatidylinositol 3-kinase (PI3K). Gγ subunits can activate various kinases including protein kinase D (PKD) (Morris and Malbon, 1999; Vanderbeld and Kelly, 2000) (Fig. 1).
Fig. 1.
Examples of G protein- and β-arrestin-mediated downstream signaling pathways on GPCRs. Upon agonist binding to GPCRs, both G proteins (Gα12, Gαq/11, Gαi/o, Gαs, Gβ and Gγ subunits) and β-arrestin are activated to mediate a variety of distinct downstream signaling pathways. Stimulation of Gβ subunit can activate PI3Kγ and Gγ subunit can activate PKD. Gα12 can activate Rho kinase signaling pathways and Gαq can induce the mobilization of calcium from intracellular stores through activation of PLC/IP3. Gαo signaling activates MEK/ERK pathway to mediate cell cycle progression. Gαs proteins promote AC-induced PKA activation. Phosphorylation of GPCRs by GRK results in the recruitment of β-arrestin, which in turn desensitizes G protein signaling, mediates receptor trafficking to endosomes, and activates β-arrestin-dependent signaling.
In addition to signaling through G proteins, GPCRs can also activate G protein-independent signaling pathways mainly through multi-functional adaptor proteins called arrestins. The arrestins are a small family of proteins originally discovered in the visual system. Arrestins, which include arrestin-1 and -4 (expressed in retinal rods and cones) and ubiquitously expressed arrestin-2 (β-arrestin1) and arrestin-3 (β-arrestin2), were initially characterized for their roles in GPCR desensitization (uncoupling of the G protein from the cognate receptor) (Shukla et al., 2011; Lefkowitz, 2013). Homologous desensitization is initiated by stimulation of the receptor with high concentrations of its agonist, resulting in a change in the receptor conformation to its active state. G protein-coupled receptor kinases (GRKs) can then phosphorylate the specific serine/threonine residues at C-terminus or intracellular loops of the activated receptor, which increases the affinity of β-arrestin for the receptor, thus resulting in the uncoupling of the Gα subunit from the receptor. By interacting with components of the endocytic machinery such as clathrin and the adaptor protein 2 (AP2) complex, β-arrestins also target the GPCRs for clathrin-mediated endocytosis and internalization (Lefkowitz, 1998; Ferguson, 2001). In addition to receptor endocytosis, β-arrestins were found to play prominent roles in signaling, trafficking, and ubiquitination of receptors (Shenoy et al., 2001). It has been shown that internalized receptor-β-arrestin complexes can form a scaffold for mitogen-activated protein kinases (MAPKs) including ERK1/2, p38 and c-Jun N terminal kinase-3 (JNK3) to generate signalosomes, which may mediate long-lasting cell signaling in the cytosol (Luttrell et al., 1999; McDonald et al., 2000; Gong et al., 2008; Song et al., 2009). β-Arrestins have been also reported to scaffold AKT, PI3K and PDE4 in the context of various specific receptors both in vitro and in vivo [reviewed in (DeWire et al., 2007)]. Lastly, β-arrestin1 has been recently proposed to mediate nuclear signaling such as microRNA (miR) processing after activation of β1-adrenergic receptors (β1-ARs) by biased ligands [(Kim et al., 2014), see chapter 3.1]. The examples of G protein- and β-arrestin-mediated downstream signaling pathways on GPCRs are summarized in Fig. 1 and 2.
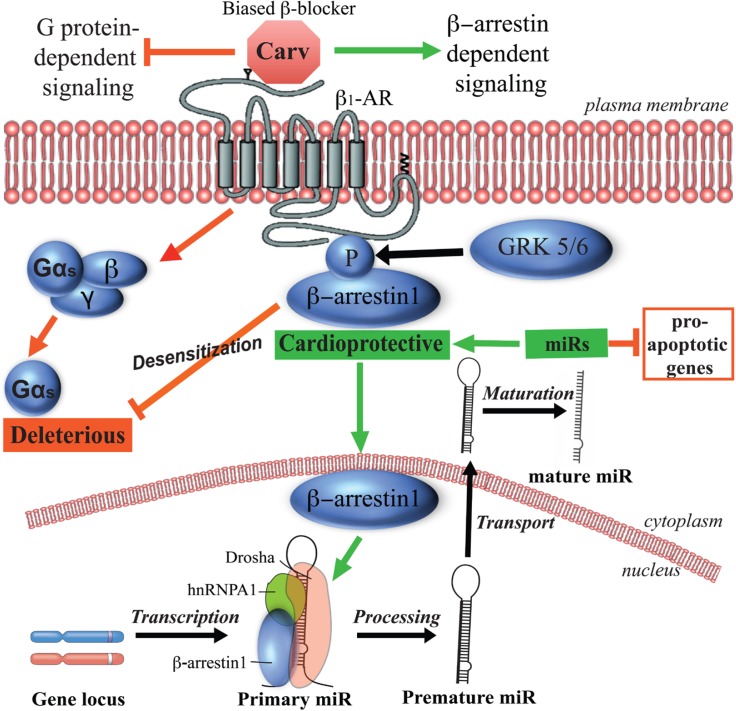
Fig. 2.
Carvedilol-mediated β-arrestin biased signaling on β1-adrenergic receptors in cardiomyocytes and hearts. Carvedilol selectively stimulates GRK5/6- and β-arrestin-dependent cardioprotective signaling without activating deleterious G protein signaling. Carvedilol-mediated GRK5/6 phosphorylation of β1-adrenergic receptors leads to β-arrestin1’s translocation into nucleus where β-arrestin1 interacts with a subset of primary miRs and components of the Drosha microprocessor complex. This results in an increased level of a subset of miRs, which act as cardioprotective miRs by repressing pro-apoptotic genes in cardiomyocytes and hearts.
MOLECULAR BASIS OF BIASED GPCR SIGNALING
As our understanding on in vivo effects of GPCR-targeting drugs becomes more profound, it has become clear that therapeutic strategies often require the modulation of a single specific downstream signaling pathway (i.e. G protein- v.s. β-arrestin-mediated signaling) in a given cell type or tissue. Most drugs targeting GPCRs often lack the specificity in this regard, and as such produce undesirable side effects. In recent years, there have been significant efforts to develop ligands that preferentially activate one beneficial GPCR signaling pathway, not another detrimental pathway. These functionally selective biased ligands have demonstrated the potential to use as novel therapies and have opened new possibilities in GPCR drug discovery (Rankovic et al., 2016).
Although the detailed molecular mechanism of biased signaling is not yet well understood, it has been reported that biased GPCR ligands induce a unique receptor conformation, activating a particular signaling pathway. It is understood that the GPCR conformation stabilized by a G protein-biased ligand is distinct from the conformation stabilized by a β-arrestin-biased ligand. For example, a fluorescence-based study on activation of the arginine-vasopressin type 2 receptor by biased and unbiased ligands provided an interesting experimental notion, suggesting that the transmembrane helix 6 (TM6) and third intracellular loop at the receptor are associated with selective G protein signaling, whereas the TM7 and helix 8 (H8) regions at the receptor are required for selective β-arrestin recruitment (Rahmeh et al., 2012).
Another recent study used site-specific fluorine-19 nuclear magnetic resonance (19F-NMR) labels in the β2-AR to unveil conformational changes of the receptor upon activation by biased and unbiased ligands. It was shown that unbiased ligand’s binding to the receptor primarily shifts the equilibrium toward the G protein-specific active state of helix 6, while β-arrestin-biased ligands predominantly regulate the conformational states of helix 7 (Liu et al., 2012). Also, Woo et al. (2014) showed that phosphorylation of the receptor itself does not necessarily lead to a switching of the receptor coupling to different G proteins as once proposed (Daaka et al., 1997). It was also shown that the biased ligand-mediated signaling depends on the specific interaction between the ligand and the β2-AR’s tyrosine 308 residue positioned on transmembrane helix 7 (Woo et al., 2014). Despite these advances in biased signaling field, further structural studies are needed to unravel how the interaction between GPCR and ligand translates into the receptor conformation for selective coupling to different G proteins and β-arrestins. Such progress would move us closer to structure-based design of drugs specific to a particular signaling pathway.
It is well appreciated that the G protein-independent effector on GPCRs, β-arrestin, is recruited upon phosphorylation of GPCRs by GRKs specifically on their C terminal and intracellular loops. An interesting “barcode” hypothesis suggests that different GRKs phosphorylate distinct sites on the C terminus and internal loops of the receptor, thereby establishing a “barcode” that would instruct or determine the conformation for different β-arrestin functions. This would in turn determine the differential roles of GRKs and β-arrestins (Butcher et al., 2011). A detailed mapping of the β2AR phosphorylation by GRKs supported this hypothesis by demonstrating that the β-arrestin-biased ligand carvedilol recruited different GRKs and induced a different phosphorylation pattern from that of a full agonist isoproterenol (Nobles et al., 2011). The experimental evidence also indicates that the biased ligands can stabilize both the receptor and β-arrestin in conformations that are distinct from those associated with unbiased ligands. For example, Shukla et al. (2008) used an intramolecular bioluminescence resonance energy transfer (BRET)-based biosensor of β-arrestin2 and a combination of biased ligands and/or biased mutants of three different GPCRs to show that β-arrestin can adopt multiple “active” conformations. These findings suggest the possibility that multiple distinct β-arrestin conformations can form various complexes with different binding partners, and thereby engage in different downstream signaling pathways.
Another interesting phenomenon in biased signaling observed for multiple GPCRs in vitro is the ability of biased ligands to recruit different subtypes of β-arrestins within a single receptor. Such differential involvement of β-arrestins can determine ligands’ downstream effects on receptor such as trafficking, ubiquitination, or signaling. For example, it is known that the recruitment of β-arrestin1 and 2 can mediate internalization of mu-opioid receptor (MOR). Interestingly, morphine, an agonist with the low rate of MOR internalization primarily recruits β-arrestin2, whereas another agonist DAMGO can recruit either β-arrestin1 or 2, which leads to the high level of receptor internalization. DAMGO was also shown to induce receptor ubiquitination in a β-arrestin1-dependent manner (Groer et al., 2011). Similarly, in delta-opioid receptor (DOR), an agonist SNC80, which causes the high internalization of the receptor, was shown to preferentially recruit β-arrestin1 over β-arrestin2. In contrast, agonists ARM390 and JNJ20788560 preferentially engage with β-arrestin2, resulting in the low rate of receptor internalization (Pradhan et al., 2016). Endogenous ligands for the C-C chemokine receptor 7 also showed differential internalizing properties. The ligand CCL19 with high-internalizing capacity preferentially recruits β-arrestin2 over 1, whereas CCL21 with low-internalizing capacity engages with neither (Byers et al., 2008). In addition, binding of ATP induces the greater interaction between P2Y2 receptor and β-arrestin1, whereas UTP nonselectively recruits both β-arrestin1 and 2 (Hoffmann et al., 2008). These studies support a novel interesting notion that different ligands for the same receptor can form distinct receptor-arrestin complexes.
Altogether, recent advances on GPCR biased signaling field suggest that biased GPCR ligands may have an important therapeutic potential in various diseases including cardiovascular diseases, neurological diseases, and cancers. As summarized in the following chapter and Table 1, we seek to provide recent scientific progress on identifying novel biased ligands on selected highly-profiled GPCRs.
Table 1.
Overview of biased ligands on selected GPCRs
| Receptor | Biased ligand | Pathway | Therapeutic area | References |
|---|---|---|---|---|
| Angiotensin I receptor | SII | β-arrestin | Cardiovascular & Renal | Ahn et al., 2009; Boerrigter et al., 2011; Violin et al., 2010 |
| TRV120027 | ||||
| Apelin receptor | MM07 | Gαi | Cardiovascular | Brame et al., 2015 |
| Arginine-vasopressin | MCF14 | Gαs | Renal | Jean-Alphonse et al., 2009 |
| V2 receptor | MCF18 | |||
| MCF57 | ||||
| β1-adrenergic receptor | Carvedilol | β-arrestin | Cardiovascular | Kim et al., 2008, 2014 |
| Alprenolol | ||||
| β2-adrenergic receptor | Fenoterol | Gαs | Pulmonary | Woo et al., 2009; Xiao et al., 2003 |
| Carvedilol | β-arrestin | Cardiovascular | Wisler et al., 2007 | |
| Dopamine D1 receptor | SKF83959 | Gαq | Neurology & Behavior | Conroy et al., 2015; Rashid et al., 2007 |
| SKF38393 | ||||
| SKF82957 | ||||
| SKF75670 | ||||
| Dopamine D2 receptor | UNC9975 | β-arrestin2 | Neurology & Behavior | Chen et al., 2012; Park et al., 2016 |
| UNC9994 | ||||
| MLS1547 | Gαi/o | Neurology & Behavior | Free et al., 2014 | |
| Histamine H2 receptor | Famotidine | Gαs | Gastrointestinal | Alonso et al., 2015 |
| Histamine H4 receptor | JNJ7777120 | β-arrestin2 | Inflammatory | Rosethorne and Charlton, 2011 |
| κ-Opioid receptor (KOR) | Isoquinoline 2.1 | Gαi/o | Neurology & Behavior | Zhou et al., 2013 |
| 6′-GNTI | Rives et al., 2012 | |||
| RB-64 | White et al., 2015 | |||
| μ-Opioid receptor (MOR) | TRV130 | Gαi/o | Neurology & Behavior | Chen et al., 2013; DeWire et al., 2013 |
| NAP | Gαi/o | Neurology & Behavior | Zhang et al., 2016 | |
| Serotonin 5-HT2B receptor | Ergotamin | β-arrestin | Neurology & Behavior | Wacker et al., 2013 |
BIASED SIGNALING ON SELECTED GPCRS
β-adrenergic receptors and their biased ligands
β-adrenergic receptors (β-ARs), prototypical members of GPCR superfamily, are known for their regulation of contractile function in the heart. The stimulation of cardiac β1-and β2-AR by catecholamines such as adrenaline and noradrenaline activates the canonical Gs-AC-cAMP-PKA signaling cascade, which increases calcium mobilization across different cellular compartments and sensitizes contractile proteins to cytosolic calcium. The overall physiological effect of cardiac β-AR stimulation is an increase in heart contractility (inotropic effect) and heart rate (chronotropic effect) (Rodefeld et al., 1996). The major subtype, β1-AR couples to the Gαs protein, whereas β2-AR is able to couple to both Gαs and Gαi proteins (Kilts et al., 2000; Xiang and Kobilka, 2003; Perrino and Rockman, 2007). Physiologically, the inotropic response to catecholamine stimulation is mediated mainly by β1-AR because the β2-AR-Gαs-mediated AC-cAMP-PKA response is inhibited by the co-activated β2-AR-Gαi signaling (Xiao et al., 1995). However, β2-AR can regulate the effect of β1-AR on excitation-contraction coupling by activating Gαi signaling. It is also known that activation of the β2-AR-Gαi signaling protects the cardiomyocytes from the pro-apoptotic stimuli of excessive β1-AR stimulation and activates a pro-survival PI3K-Akt signaling cascade (Chesley et al., 2000; Zhu et al., 2001). However, prolonged activation of Gαi through a synthetic receptor construct has been shown to lead to a depressed cardiac function and eventually the development of dilated cardiomyopathy in mice (McCloskey et al., 2008). Switching of β2-AR coupling from Gαs to Gαi was found to play an important role in ischemic preconditioning-induced cardioprotection in the mouse heart (Tong et al., 2005). In addition, the enhanced β2-AR-Gαi signaling contributes to the dysfunction of both β1-AR and β2-AR in the failing heart (Xiao et al., 2003; Xiao and Balke, 2004). In 2007, Noma et al. showed that β1-AR-mediated β-arrestin signaling confers cardioprotection independent on G protein-mediated second messenger signaling (Noma et al., 2007), bringing up a concept of biased signaling. Here, we summarize recent advances on β-AR biased signaling and highlight some of biased ligands which are used in clinic for a therapy.
Fenoterol: Racemic fenoterol is unique among the β2-AR agonists because it has been identified as a biased β2-AR ligand selectively coupling β2-AR to Gαs protein. In the study by Xiao et al., (2003) dysfunction of β2-AR but not β1-AR in the model of failing spontaneous hypertensive rats was induced by enhanced Gαi signaling. Disruption of Gαi signaling by pertussin toxin restored the blunted β2-AR contractile response in the failing heart. Interestingly, β2-AR agonist fenoterol had similar beneficial effects, which were due to selective activation of β2-AR-Gαs signaling (Xiao et al., 2003). Functional selectivity of fenoterol was shown to depend on the stoichiometry at its two chirality centers (Woo et al., 2009). Based on these observations, at least two other fenoterol derivatives (S, R′)-4′-methoxy-fenoterol and (S, R′)-4′-methoxy-1-naphthyl-fenoterol have been identified to selectively activate only Gαs signaling pathways on β2-AR. These biased β2-AR ligands are therapeutically used for bronchial asthma and chronic obstructive lung disease (Reinartz et al., 2015). These studies suggest that identifying novel Gαs-biased β2-AR ligands, which display a better efficacy, may provide a new therapy with the sustained efficacy by switching off β-arrestin-dependent receptor desensitization and down-regulation.
Carvedilol: Carvedilol is a well-known neurohormonal antagonist with multiple activities efficiently used in patients with congestive heart failure, stable angina pectoris (Dunn et al., 1997), myocardial infarction (Doughty et al., 2001), and myocardial ischemia-reperfusion injury (Brunvand et al., 1998). It blocks both the β1-and β2-AR, resulting in the improved myocardial function and attenuation (or reversal) of adverse myocardial remodeling in heart failure. It also reduces the peripheral vascular resistance via vasodilation caused by antagonism of α1-AR. In addition to these well-known properties, carvedilol has a number of ancillary activities including antioxidant, anti-inflammatory, and antiapoptotic actions (Ohtsuka et al., 2001; Dulin and Abraham, 2004; Mochizuki et al., 2007). Carvedilol was shown to confer a range of cardioprotective effects, which were hypothesized to stem from its antioxidant effects and/or from direct inhibition of proapoptotic pathways (Schwarz et al., 2003).
More importantly, carvedilol has been identified as a biased ligand on β1-AR and β2-AR, which selectively stimulates GRK5/6-and β-arrestin-dependent cardioprotective signaling without activating G proteins (Wisler et al., 2007; Kim et al., 2008). Our group also showed that after stimulation of β1-AR by carvedilol, β-arrestin1 promotes the processing of five miRs (miR-125a-5p, miR-125b-5p, miR-150, miR-199a-3p and miR-214) in murine hearts and human cells (Kim et al., 2014). MiRs, a class of ∼22 nucleotide small noncoding RNAs governing post-transcriptional repression of target mRNAs, were found to play important roles in normal cardiac physiology including the control of myocyte growth, contractility, and maintenance of cardiac rhythm as well as the pathogenesis of various heart diseases [reviewed in (Quiat and Olson, 2013)]. The hypothesis for the mechanism by which carvedilol promotes miR processing is that carvedilol-induced GRK5/6 phosphorylation of β1AR mediates the recruitment of β-arrestin1 to the ligand-occupied receptor, resulting in the translocation of β-arrestin1 to the nucleus where it interacts with a subset of primary miRs and components of the Drosha microprocessor complex. Formation of a nuclear complex of β-arrestin1 with the heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1) and Drosha, which are crucial nuclear RNA-binding proteins involved in miR processing (Lee et al., 2003; Guil and Caceres, 2007), leads to activation of RNA helicase-independent miR processing (Kim et al., 2014). In the mouse hearts, 7-day carvedilol infusion induced the upregulation of miR-150, miR-214, miR-125b-5p and miR-199a-3p, which were shown to be cardioprotective in the mouse models of heart failure (Salloum et al., 2010; Aurora et al., 2012; Wang et al., 2014; Tang et al., 2015). Moreover, our group also showed that 7-day infusion of carvedilol induced a unique gene signature on multiple genes related to cardiac disease. Genes upregulated by carvedilol included those encoding proteins in the tight junctions, malaria and viral myocarditis pathway, while downregulated genes were those encoding proteins in the glycosaminoglycan biosynthesis and arrhythmogenic right ventricular cardiomyopathy (Teoh et al., 2015). These findings make us speculate that carvedilol-responsive miRs can regulate the expression of various detrimental genes to confer cardioprotection (Fig. 2). We postulate that β1AR-mediated β-arrestin1 biased signaling provides an additional mechanism for the clinical efficacy of this β-blocker (Table 1).
Insulin signaling: Interestingly, a recent study suggests that insulin signaling can also mimic the effects of a biased β2AR ligand and selectively activate a Gαi-biased signaling pathway (Fu et al., 2014). Insulin and adrenergic stimulation represent two divergent regulatory systems that interact with overlapping downstream signaling pathways in adipocytes, liver, and skeletal and cardiac muscle. Stimulation of insulin receptor (IR) as well as βARs increases the glucose uptake in cardiac and skeletal muscle cells (Nevzorova et al., 2006; Ciccarelli et al., 2011). In the heart, IR and β2AR form a complex and the stimulation of both receptors shares common downstream signaling components including GRK2 (Cipolletta et al., 2009; Ciccarelli et al., 2011), Gαi (Song et al., 2001) and β-arrestin (Luan et al., 2009). Stimulation with either insulin or adrenergic receptors antagonizes the ability of the other to activate glucose transport (Morisco et al., 2006) and to modulate myocyte survival (Rane et al., 2010).
In both diabetes and heart failure, circulating insulin levels are chronically elevated, leading to persistent stimulation of IRs. Despite the insulin resistance of adipocytes and skeletal muscle cells, the heart retains its insulin sensitivity to activate IR signaling cascades in type 2 diabetes (Wright et al., 2009; Cook et al., 2010). Hyperactive insulin signaling was shown to significantly accelerate adverse left ventricular remodeling in pressure overload-induced hypertrophy in rodents (Shimizu et al., 2010). In the animal model of ischemia/reperfusion, insulin inhibited β-AR action in the hearts (Yu et al., 2008). It was shown that in the animal hearts, insulin could directly impair adrenergic signaling pathways for contractile function via an IR-β2AR signaling complex. Insulin stimulation promotes crosstalk with β2AR pathways via insulin receptor substrate (IRS) and GRK2-mediated phosphorylation of the β2AR, which selectively activates a Gαi-biased β2AR signaling cascade to inhibit cAMP/PKA activities. Consequently, this IR-β2AR cross-talk leads to impaired β-AR-induced contractile function in cardiomyocytes and perfused mouse hearts (Fu et al., 2014). However, these findings still need to be confirmed in humans, as it is known that diabetes is one of the risk factors for heart failure (Nichols et al., 2001), and heart failure is an insulin-resistant state (Cook et al., 2010). If the hyperinsulinemia can indeed inhibit β1AR signaling via Gαi-biased β2AR signaling in humans, it is possible that hyperinsulinemic subjects with type 2 diabetes and heart failure might have increased sensitivity to the cardio-depressive effects of nonselective or β1-blockade, which is the standard care for managing patients with heart failure (Fu et al., 2014).
Biased ligands on angiotensin receptors
Angiotensin II (AngII) type I receptor (AT1R), a primary regulator of blood pressure, is a prototype GPCR in the study of biased agonism. Upon binding of its natural ligand AngII to the receptor, Gαq proteins are activated, resulting in intracellular inositol triphosphate (IP3) production, calcium mobilization, protein kinase C (PKC) activation, which altogether mediate the physiological effects of AT1R such as vasoconstriction and fluid retention. Moreover, the conformational rearrangement of 7 transmembrane α helices of the receptor also leads to the recruitment of β-arrestins, which mediates G protein-independent signaling, leading to overall positive inotropic and cardioprotective effects (Ikeda et al., 2015). Angiotensin receptor blockers (ARBs) are clinically used for their anti-hypertensive activity, and some ARBs also show variable efficacies toward the protection against organ damage in diabetic nephropathy, cardiac hypertrophy, arrhythmia, and renal failure (Burnier and Brunner, 2000). Multiple studies suggest that such additional tissue-protective benefits of ARBs may be mediated by β-arrestin signaling (Kim et al., 2005; Miura et al., 2013), thus the development of biased ligands has resulted in a promising therapy.
SII: One of the first β-arrestin-biased ligands to be described was the peptide [Sar1, Ile4, Ile8]-Ang (SII). This peptide was reported to exert anti-apoptotic cytoprotective effects in rat vascular smooth muscle cells (Ahn et al., 2009) and induce positive inotropic and lusitropic effects in rat primary cardiomyocytes by stimulating endogenous AT1R-β-arrestin signaling (Rajagopal et al., 2006). SII was also able to activate MAPK signaling in perfused rodent hearts (Aplin et al., 2007).
TRV120027: Utilizing SII as a pharmacological probe in vitro and ex vivo was helpful in elucidating downstream AT1R-mediated β-arrestin signaling. However, due to its low-affinity for the receptor, it has been difficult to study SII’s potential pharmacological benefit in in vivo models. To overcome this limitation, custom-synthetic peptides have been developed based on SII sequence. Among the identified ligands, TRV120027 (TRV027) exhibited an improved potency compared to AngII in stimulating β-arrestin signaling, but no detectable G protein activation. TRV027 was shown to stimulate β-arrestin recruitment with subsequent activation of several kinase pathways such as p42/44 mitogen-activated protein kinase, Src, and Akt-endothelial nitric-oxide synthase pathways. TRV027 was also shown to increase cardiomyocyte contractility in vitro and decrease mean arterial pressure in rats in vivo, similar as unbiased ARBs such as losartan. However, unlike the unbiased ARBs, which decrease cardiac performance, TRV027 increased cardiac performance and preserved cardiac stroke volume (Violin et al., 2010). Moreover, in healthy and heart failure canines, TRV027 also reduced pulmonary capillary wedge pressure, systemic and renal vascular resistance while preserving renal functions (Boerrigter et al., 2011). Because of this unique pharmacological profile, TRV027 has already entered phase II clinical trial as a novel therapeutic agent for acute heart failure (Ikeda et al., 2015) (Table 1).
Biased signaling in apelin receptor
The apelin receptor (also known as APJ, APLNR, AGTRL1) is a class A GPCR discovered in 1993 based on its sequence similarity with the AT1R (O’Dowd et al., 1993). APJ is widely expressed in brain and peripheral organs including heart, lung, kidney, or placenta (Hosoya et al., 2000; Pope et al., 2012). Since its discovery, a number of physiological and pathophysiological roles for the receptor have been identified, including regulation of cardiovascular function, fluid homeostasis, and the adipoinsular axis [reviewed in (Pitkin et al., 2010)]. APJ does not bind AngII but its natural ligand, apelin is a potent inotropic and vasodilatory agent. Apelin induces coupling of APJ to Gαi (Habata et al., 1999) with additional evidence for the involvement of Gαq-coupled activation of PLC and PKC (Japp and Newby, 2008). Interestingly, the apelin receptor in the heart may act as a mechanosensor for stretch in an apelin-independent/G protein-independent manner through recruitment of β-arrestin (Scimia et al., 2012).
Apelin receptor system represents an attractive target in pathologies such as pulmonary hypertension and heart failure (Chong et al., 2006; Chandra et al., 2011), which encourages to the development of its synthetic ligands. However, the limitation in translation to clinic is that chronic administration of an agonist would likely cause receptor desensitization with subsequent β-arrestin-mediated downregulation and loss of therapeutic efficacy. Thus, the development of agonists biased toward G protein signaling is essential. MM07, a cyclic apelin peptide, was shown to preferentially activate G protein responses with the low potency toward β-arrestin and receptor internalization. In rats, systemic infusions of this peptide caused a dose-dependent increase in cardiac output greater than apelin. Moreover, MM07 was an effective vasodilator in human forearm without loss of effects on repeat dosing, providing proof-of-concept of a clinical potential for biased ligands (Brame et al., 2015). Another APJ ligand, K17P was also reported to be G protein-biased and displayed the strong impairment of β-arrestin-dependent signaling. This molecule lacks the vasodilator capacity of its mother molecule, which is due to the deletion of single C-terminal phenylalanine (Ceraudo et al., 2014). This reflects the impact of the specific structure of the ligand in determining the signaling pathway activated in the receptor (Table 1).
Biased signaling in histamine receptors
The human histamine H4 receptor (H4R) belongs to the GPCR family and is considered as an important receptor in immune and inflammatory processes (Leurs et al., 2009). H4R was originally thought to signal only through Gαi proteins and recently shown to also recruit and signal via β-arrestin2. This discovery made by its antagonist JNJ7777120, which was identified as a biased ligand in a β-arrestin2 recruitment assay (Rosethorne and Charlton, 2011). The therapeutic potential of JNJ7777120 has been successfully studied in the mouse model of chronic dermatitis, where it inhibited pruritus and skin inflammation when used in combination with H1R antagonist (Ohsawa and Hirasawa, 2012). Based on its indolecarboxamide structure, various JNJ7777120 analogues have been recently developed with the similar biased affinity toward β-arrestin2 (Nijmeijer et al., 2013). The potentially improved therapeutic efficacy of these substances is yet to be evaluated.
Currently, one of the most clinically relevant therapies for histamine receptors is achieved through the regulation of H2R, which is pathologically implicated in gastric acid-related diseases but widely expressed in most tissues. The H2R stimulation increases adenylate cyclase activity and induces cAMP accumulation. The most commonly used H2R blocker, famotidine was shown to act as an “inverse agonist” by diminishing G protein-mediated increase of cAMP. Interestingly, famotidine also mimicked the effect of histamine, and induced receptor desensitization and internalization along with increased ERK phosphorylation in gastric epithelial cells (Alonso et al., 2015) (Table 1).
Biased signaling in dopamine receptors
Dopamine receptors are another well-studied family of GPCRs largely because dopamine neurotransmission is important in multiple neuropsychiatric disorders. Among the dopamine receptors, dopamine receptor D2 (D2R) is one of the most validated drug targets in neurology and psychiatry. However, most drugs targeting the D2R are problematic, either being less efficacious than desired or possessing adverse side effects due to the activation or blockade of a subset of downstream signaling pathways.
D2R couples Gαi/o to negatively regulate cAMP-PKA pathways and modulate intracellular Ca2+ levels by acting on ion channels or by triggering the release of Ca2+ from intracellular stores. In addition, more recent discoveries showed that dopamine receptors exert their in vivo effects through β-arrestin2-mediated protein kinase B (Akt)-glycogen synthase kinase 3 (GSK3) signaling cascades (Beaulieu et al., 2005). In humans, the Akt1/GSK3β signaling pathways are implicated in schizophrenia as evidenced by the low levels of Akt1 protein and reduced phosphorylation of GSK3β in the brain and lymphocytes of schizophrenic patients (Emamian et al., 2004). As Akt and GSK3 responses are mediated through ligands that are biased for β-arrestin signaling (Beaulieu et al., 2005), this may represent a novel approach to develop drugs with fewer side effects, greater therapeutic selectivity, and enhanced efficacy for treating schizophrenia. Indeed, two newly synthetized β-arrestin-biased ligands for D2R, UNC9975 and UNC9994 have already shown some promise by displaying robust antipsychotic drug-like activities in wild-type mice, which were abolished in β-arrestin2 knockout mice (Chen et al., 2012). These two biased ligands also reduced schizophrenia-like behaviors in phencyclidine-treated or NR1-knockdown hypoglutamatergic mice, where they increased performance in various neurobehavioral tests, and elicited a lower level of catalepsy than standard antipsychotic drug and D2R antagonist haloperidol (Park et al., 2016).
Interestingly, biased ligands with the opposing pharmacology for the D2R, that is, the stimulation of G protein signaling pathways without activation of β-arrestin recruitment have been recently identified. The first example is MLS1547, which was shown to robustly activate G proteins while antagonizing of β-arrestin recruitment to the D2R (Free et al., 2014). Identification of such functionally selective ligands should help to dissect the roles of both signaling arms of the D2R in physiology and pathology. Moreover, functionally selective G protein-biased ligands may also result in improved therapies for certain neuropsychiatric disorders such as Parkinson’s disease, in which D2R stimulation is desired (Table 1). Several recent advances on D1R-biased signaling are also summarized in Table 1.
Biased signaling in opioid receptors
Opioid receptors are GPCRs, which are widely studied due to their crucial roles in pain management, drug abuse/addiction, and mood disorders. There are three major subtypes of opioid receptors: δ-receptor (DOR), κ-receptor (KOR), and μ-receptor (MOR). Majority of opioids exert their analgesic activities primarily via activating MOR. Upon activation, MOR predominately couples to Gαi/o, which orchestrates downstream signaling cascades including those contributing to antinociception. On the other hand, activation of β-arrestins, especially β-arrestin2 induces receptor internalization and desensitization, diminishing G protein-mediated signaling. Recent studies have shown that some MOR agonists such as fentanyl and [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) have the high efficacy to recruit β-arrestin, whereas other opioids such as morphine are biased toward G protein signaling (McPherson et al., 2010; Molinari et al., 2010). MOR-mediated β-arrestin activation has been associated with the adverse effects of opioids such as dependence, and gastrointestinal and respiratory dysfunction. Indeed, in the absence of β-arrestin2, morphine produced less tolerance, dependence, constipation, and respiratory suppressive side effects. Moreover, a significantly enhanced and prolonged analgesic effect of morphine was observed in loss-of-function of β-arrestin2 (Thompson et al., 2015). Therefore, the development of biased G protein ligands for MOR holds a greater therapeutic potential compared to an unbiased one. In fact, a G protein-biased MOR ligand TRV130 (made by Trevena Inc., King of Prussia, PA, USA) with little β-arrestin recruitment activity has recently been reported to show potent analgesic effects with reduced respiratory depression and constipation compared to morphine (DeWire et al., 2013). TRV130 was delivered intravenously and has successfully completed phase I trials for safety in healthy volunteers (Soergel et al., 2014). The first encouraging results from phase II trials were reported in a randomized and double-blinded study in patients experiencing moderate-to-severe post-operative pain after bunionectomy. The results showed that TRV130 rapidly produces profound analgesia with no serious adverse effects, suggesting that G protein-biased MOR activation is a promising target for novel analgesics (Viscusi et al., 2016).
Interestingly, biased ligands that are selective agonists at one desired pathway could also act as biased competitive antagonists for the undesired pathway. In general, MOR ligands with low efficacy for G protein activation also have low efficacy for β-arrestin2 recruitment, and in fact partial agonists such as buprenorphine do not significantly recruit β-arrestin2 in cell models (McPherson et al., 2010). In clinic, partial MOR agonists with bias toward antagonism of the β-arrestin2 would be beneficial for the treatment of opioid-induced constipation, which often complicates an analgesic therapy in patients. The pursuit of highly selective and potent non-peptide MOR ligands has yielded several more promising compounds including NAP, which acts as a peripherally selective MOR partial agonist. Being a P-glycoprotein substrate, NAP has limited access to the central nervous system. Moreover, it has no apparent analgesic effect due to its low efficacy in activating G protein-mediated signaling with no apparent effect on β-arrestin2 recruitment. However, its therapeutic potential lies in its ability to antagonize MOR full agonist-induced intracellular calcium flux and β-arrestin2 recruitment (Zhang et al., 2016). NAP dose-dependently restored the morphine-impaired intestinal motility without precipitating significant withdrawal of symptoms and thus held great promise in the treatment of opioid-induced constipation (Yuan et al., 2012) (Table 1). In addition to multiple GPCRs as aforementioned, we summarize recent advances on serotonin 5-HT2B receptor-and arginine-vasopressin V2 receptor-mediated biased signaling and highlight some biased ligands in Table 1.
Biased GPCR signaling in cancer
In contrast to the successful implementation of GPCR biased signaling concept for clinical benefit in the cardiovascular, neurological, behavior fields, there have been no reports demonstrating the utility of GPCR biased signaling for the treatment of cancer. However, a few recent studies reported the scientific progress in the potential use of biased signaling on endothelin receptors in cancer treatment.
Endothelin-1 (ET-1) is a peptide belonging to a family of the most potent vasoconstrictors. In addition to this function in the circulation, it has been implicated in various physiological and pathological conditions such as development, cell proliferation, differentiation, cardiac function and cancer (Schorlemmer et al., 2008; Rosano et al., 2013b). ET-1 is a well-recognized growth factor that is present in plasma and is produced by stromal and tumor cells. The ET-1 receptors, endothelin type A receptor (ETAR) and endothelin type B receptor (ETBR) are members of the GPCR family. ETBR is coupled to Gαq and Gαi, and expressed mainly in endothelial cells, while ETAR, which is coupled to Gαq, Gαs and Gα12/13, is expressed in vascular smooth muscle cells and cardiomyocytes as well as solid tumors (Sakurai et al., 1990; Williams et al., 1991; Bagnato et al., 1999). In ovarian cancer, ETAR/ET-1 axis has been shown to promote tumorigenesis by promoting anti-apoptosis, invasion, and neoangiogenesis (Spinella et al., 2004; Rosano et al., 2005). Indeed, ETAR overexpression is associated with poor survival in patients with ovarian carcinoma (Teoh et al., 2014). However, a recent clinical study demonstrated that specific ETAR antagonists are ineffective as auxiliary anti-cancer treatment (Cognetti et al., 2013). This might be explained by signaling bias of ETAR, which mediates both oncogenic and tumor suppressive properties. The known oncogenic downstream effects of ETAR are mediated by Gαq-coupled or β-arrestin-dependent signaling pathways (Spinella et al., 2004; Rosano et al., 2013a). It was shown that GRK5/6-mediated phosphorylation of the receptor leads to the recruitment and nuclear translocation of β-arrestin, which in turn functions as an epigenetic regulator of several angiogenic/metastatic genes including β-catenin, thus promoting cell invasion (Rosano et al., 2013a; Teoh et al., 2014). On the other hand, ETAR-mediated Gαs activation induces AC/cAMP/PKA signaling, which can confer tumor suppressive effects as reported in several carcinoma-derived cell lines (Takahashi et al., 2009; Follin-Arbelet et al., 2013; Teoh et al., 2014). Given that stimulation of the ET-1/ETAR axis can activate both tumor suppressive and oncogenic properties in cancer cells, ligands biased toward Gαs/cAMP/PKA signaling might represent a novel potential therapy of various malignancies. Unfortunately, such therapeutic agents are yet to be developed.
Other GPCRs, which are involved in the progression of cancer and have been suggested as potential targets for yet-to-be-identified biased ligands, are CXC chemokine receptor 4 (CXCR4) and protease activated receptor 2 (PAR2). The enhanced expression of CXCR4 and aberrant downstream signaling are implicated in several cancers, where it is involved in tumor growth, vascularization, and metastasis (Guleng et al., 2005; Rubin, 2009). Also, PAR2, a GPCR with distinct biased signaling, has emerged as one of the promising therapeutic targets to inhibit rapidly metastasizing breast cancer cells (Morris et al., 2006). Therefore, the development of novel biased ligands for CXCR4 and PAR2 may open new opportunities for cancer treatment.
DISCOVERING BIASED SIGNALING ON GPCR
Over the past decade, there has been a surge in publications to describe the identification of biased ligands at a wide variety of GPCRs. Accordingly, quantifying ligand bias has been an active area of research. One way to quantify ligand bias is to plot β-arrestin activity against G protein activity. For biased ligands, there would be different levels of β-arrestin-and G protein-mediated efficacies. Such data can also be represented as a matrix that incorporates data from multiple assays, or ligand bias factors that compare β-arrestin activity against G protein activity in different assays (Rajagopal et al., 2010). The quantification of the relative levels of bias is important in the identification of lead compounds and in the optimization of drug screening for biased ligands. Despite this effort, there are still considerable gaps in our understanding of bias. The conventional high throughput screening methodology used in the pharmaceutical industry is inadequate for the needs of novel biased ligand discovery. The drug discovery and experimental studies of biased signaling mechanisms require the use of highly sensitive assays and a standardized methodology to quantify responses related to the signaling pathways. For example, assays and analyses must be configured to remove any apparent bias resulting from the biological assay system and thus correctly identify true ligand bias. In addition, at least one of the endpoints requires the accurate quantification of a poor response. Lastly, the analysis must be compatible for use with a large number of compounds, and the data must be presented to enable medicinal chemistry to derive the relationship between structure and activity (Winpenny et al., 2016).
In the past years, most groups have relied on comparing the maximal effects (Emax) and potencies (EC50) of ligands for different signaling pathways. However, they are prone to errors in the interpretation in the setting of receptor reserve. For example, these parameters failed to account for the differences in the receptor reserve and amplification of different assays (Rajagopal et al., 2010). In assays with significant amplification such as second-messenger assays (e.g., cAMP formation), both full and partial agonists can reach the same maximal response, whereas in assays with little amplification such as assays that monitor the recruitment of β-arrestin to a receptor by enzyme complementation (Eglen et al., 2007), partial agonists have significantly lower maximal responses than full agonists (Rajagopal et al., 2010). Therefore, a partial agonist that reaches the maximal effect in one assay and halfmaximal effect in another assay would be incorrectly identified as being biased compared with a full agonist, which reaches the maximal response in both assays. Also, the difference in potencies between the full agonist and partial agonist may be smaller in assays with less receptor reserve (Rajagopal et al., 2010, 2011). The current best practice in the identification and quantification of biased agonism is thus complex and an ongoing topic of debate.
The key requirements for measuring bias signaling are a common reference compound to overcome observational and systemic bias as well as a scale, which accounts for both potency and maximal response of ligands. They allow the relative activity of ligands to be compared across assays. The current ‘gold standard’ method is the operational model of agonism that allows for the systematically independent quantification of agonist activity via the relative transduction ratio coefficient Δlog (τ/KA). The term τ incorporates agonist efficacy, receptor density and coupling within the system. The dissociation constant (KA) is the reciprocal of the conditional affinity of the agonist in the functional system [for detailed reviews, see (Kenakin and Christopoulos, 2013)]. The alternative to this method is to use the bias factor βlig, which is calculated by the ratios of the efficacy of agonists for a given signaling pathway in a cell. This method was first used to quantify ligand bias and to identify weak biased compounds in β2-AR and AT1R. This method differs from the previous one by assuming that a single estimate of KA for the receptor (obtained from biochemical binding studies) should be used to fit the data with the operational model [for detailed reviews, see (Rajagopal et al., 2011)]. However, the model to use the bias factor has limitations such as a possible error in the calculation of bias if the affinity of the agonist is changed when different signaling proteins are coupled to the receptor (Kenakin and Christopoulos, 2013). Another method for quantifying agonist bias offers the comparison of ratio of the maximal response (Emax) to the EC50 value for an agonist [Δlog (Emax/EC50)]. The equation has been employed to identify new biased MOR ligands. In this method, the relative signaling bias was quantified for each compound by calculating the difference in activity between β-arrestin recruitment assays (e.g., fluorescent labeling of β-arrestins and GPCRs) and G protein activation assays (e.g., cAMP assay). In such quantification, 0 represented no bias and +1 or -1 represented 10-fold bias for one signaling pathway [for detailed reviews, see (Winpenny et al., 2016)]. These methods for the identification and quantification of bias across large compound numbers have been essential for biased drug discovery.
One of increasingly recognized techniques for identification of GPCR signaling bias is BRET, which is a sensitive and non-destructive method commonly used in live cells to investigate protein-protein interactions or changes. BRET is a naturally occurring phenomenon resulting from the nonradioactive transfer of energy between luminescent donor and fluorescent acceptor proteins. In the sea pansy Renilla reniformis, the luminescence resulting from the catalytic degradation of coelenterazine by luciferase (Rluc, donor) is transferred to the green fluorescent protein (GFP, acceptor), which in turn emits fluorescence upon dimerization of the two proteins. The BRET response depends on the distance and the relative orientation of the donor and acceptor. In many studies, donor and acceptor are tagged individually on two proteins that potentially assemble in signaling complexes. In other cases, subtle changes in the protein conformation upon complex assembly or disassembly can be studied using modified proteins that are labeled with both BRET donor and acceptor moieties engineered into the same protein in an intramolecular setting. Because of its fast reaction kinetics, this method allows for the real time detection of complexes or conformational changes that may be transient (Angers et al., 2000; Milligan, 2004). BRET has been successfully applied to study GPCR dimerization (Angers et al., 2000), and more recently to study proximal interactions of GPCRs with different signaling effectors including G proteins and β-arrestins (Shukla et al., 2008; Molinari et al., 2010; Ceraudo et al., 2014). Recent new advances in BRET technology include the discovery of biosensors, which do not involve the labeling of receptor as either a BRET donor or acceptor, and allow the identification of biased signaling from unknown compounds for any GPCR of interest (Namkung et al., 2016).
In addition to BRET, fluorescence resonance energy transfer (FRET) proximity and conformation assays as well as signaling assays such as MAPK activation have been widely used for the discovery of biased ligands (Rajagopal et al., 2010).
CONCLUSIONS
The drug discovery strategy targeting only the primary ligand binding sites of GPCRs is becoming more and more difficult. As our understanding on in vivo effects of GPCR-targeting drugs becomes more profound, therapeutic strategies have often required the modulation of only a part of downstream effector pathways of a GPCR in a specific cell or tissue. Biased ligands with functional selectivity in specific GPCRs thus represent a new generation of drugs with increased specificity and fewer adverse effects. Much has been speculated regarding the potential advantages of the pharmacological feature of biased signaling and there are several biased ligands entering clinical studies. However, many gaps still exist. For example, few large animal and human studies have been performed. To completely translate existing basic research outcomes into clinical therapeutic options, further collaborative research should be pursued. Moreover, it is important for physicians and pharmacists to be continuously informed about which currently used drugs belong to the category of biased GPCR ligands and in which cases their use is beneficial or should be avoided due to the known adverse/unwanted effects.
Acknowledgments
We thank the editors for inviting us to write this review. Due to space restrictions, the authors cannot cite many important literatures on this field. The authors apologize to all colleagues whose work contributed significantly. This work was supported by the American Heart Association Postdoctoral Fellowship 16POST26990020 to Zuzana Bologna, American Heart Association Predoctoral Fellowship 16PRE30210016 to Jianpeng Teoh, and American Physiological Society Shih-Chun Wang Young Investigator Award, American Heart Association Grant-in-Aid 12GRNT12100048 and Scientist Development Grant 14SDG18970040, and National Institutes of Health R01 HL124251 to Il-man Kim.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors were involved either in the primary writing process or substantial revision of the manuscript. Il-mam Kim gave the final approval of the version to be published. All authors read and approved the final manuscript.
REFERENCES
- Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. β-arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem. 2009;284:8855–8856. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso N, Zappia CD, Cabrera M, Davio CA, Shayo C, Monczor F, Fernandez NC. Physiological implications of biased signaling at histamine H2 receptors. Front Pharmacol. 2015;6:45. doi: 10.3389/fphar.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M. Detection of β2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci USA. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin M, Christensen GL, Schneider M, Heydorn A, Gammeltoft S, Kjolbye AL, Sheikh SP, Hansen JL. The angiotensin type 1 receptor activates extracellular signal-regulated kinases 1 and 2 by G protein-dependent and -independent pathways in cardiac myocytes and Langendorff-perfused hearts. Basic Clin Pharmacol Toxicol. 2007;100:289–295. doi: 10.1111/j.1742-7843.2007.00063.x. [DOI] [PubMed] [Google Scholar]
- Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, Venuti A, Natali PG. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: Evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–727. [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerrigter G, Lark MW, Whalen EJ, Soergel DG, Violin JD, Burnett JC., Jr Cardiorenal actions of TRV120027, a novel β-arrestin-biased ligand at the angiotensin II yype I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4:770–778. doi: 10.1161/CIRCHEARTFAILURE.111.962571. [DOI] [PubMed] [Google Scholar]
- Brame AL, Maguire JJ, Yang PR, Dyson A, Torella R, Cheriyan J, Singer M, Glen RC, Wilkinson IB, Davenport AP. Design, characterization and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension. 2015;65:834–840. doi: 10.1161/HYPERTENSIONAHA.114.05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunvand H, Liu GL, Ma XL, Yue TL, Ruffolo RR, Jr, Feuerstein GZ. SB 211475, a metabolite of carvedilol, reduces infarct size after myocardial ischemic and reperfusion injury in rabbits. Eur J Pharmacol. 1998;356:193–198. doi: 10.1016/S0014-2999(98)00494-4. [DOI] [PubMed] [Google Scholar]
- Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645. doi: 10.1016/S0140-6736(99)10365-9. [DOI] [PubMed] [Google Scholar]
- Butcher AJ, Prihandoko R, Kong KC, McWilliams P, Edwards JM, Bottrill A, Mistry S, Tobin AB. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J Biol Chem. 2011;286:11506–11518. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers MA, Calloway PA, Shannon L, Cunningham HD, Smith S, Li F, Fassold BC, Vines CM. Arrestin 3 mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J Immunol. 2008;181:4723–4732. doi: 10.4049/jimmunol.181.7.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceraudo E, Galanth C, Carpentier E, Banegas-Font I, Schonegge AM, Alvear-Perez R, Iturrioz X, Bouvier M, Llorens-Cortes C. Biased signaling favoring Gi over β-arrestin promoted by an apelin fragment lacking the C-terminal phenylalanine. J Biol Chem. 2014;289:24599–24610. doi: 10.1074/jbc.M113.541698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra SM, Razavi H, Kim J, Agrawal R, Kundu RK, Perez VD, Zamanian RT, Quertermous T, Chun HJ. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2011;31:814–820. doi: 10.1161/ATVBAHA.110.219980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sassano MF, Zheng LY, Setola V, Chen M, Bai X, Frye SV, Wetsel WC, Roth BL, Jin J. Structure-functional selectivity relationship studies of β-arrestin-biased dopamine D-2 receptor agonists. J Med Chem. 2012;55:7141–7153. doi: 10.1021/jm300603y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XT, Pitis P, Liu GD, Yuan C, Gotchev D, Cowan CL, Rominger DH, Koblish M, DeWire SM, Crombie AL, Violin JD, Yamashita DS. Structure-activity relationships and discovery of a G protein biased μ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[ 4.5]decan-9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56:8019–8031. doi: 10.1021/jm4010829. [DOI] [PubMed] [Google Scholar]
- Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The β2-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through Gi-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.RES.87.12.1172. [DOI] [PubMed] [Google Scholar]
- Chong KS, Gardner RS, Morton JJ, Ashley EA, McDonagh TA. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur J Heart Fail. 2006;8:355–360. doi: 10.1016/j.ejheart.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei ZY, Peroutka RJ, Gold JI, Gumpert A, Chen M, Otis NJ, Dorn GW, Trimarco B, Iaccarino G, Koch WJ. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011;123:1953–1962. doi: 10.1161/CIRCULATIONAHA.110.988642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, Trimarco B, Iaccarino G. The G protein coupled receptor kinase 2 plays an essential role in β-adrenergic receptor-induced insulin resistance. Cardiovasc Res. 2009;84:407–415. doi: 10.1093/cvr/cvp252. [DOI] [PubMed] [Google Scholar]
- Cognetti F, Bagnato A, Colombo N, Savarese A, Scambia G, Sehouli J, Wimberger P, Sorio R, Harter P, Mari E, McIntosh S, Nathan F, Pemberton K, Baumann K. A Phase II, randomized, double-blind study of zibotentan (ZD4054) in combination with carboplatin/paclitaxel versus placebo in combination with carboplatin/paclitaxel in patients with advanced ovarian cancer sensitive to platinum-based chemotherapy (AGO-OVAR 2.14) Gynecol Oncol. 2013;130:31–37. doi: 10.1016/j.ygyno.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Conroy JL, Free RB, Sibley DR. Identification of G protein-biased agonists that fail to recruit β-arrestin or promote internalization of the D1 dopamine receptor. ACS Chem Neurosci. 2015;6:681–692. doi: 10.1021/acschemneuro.5b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, Punjabi P, Camici PG. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–111. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu GD, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD. A G proteinbiased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- Doughty RN, Whalley GA, Walsh H, Gamble G, Sharpe N, Investigat CES. Effects of carvedilol on left ventricular remodelling in patients following acute myocardial infarction: The CAPRICORN echo substudy. Circulation. 2001;104:517. doi: 10.1161/01.CIR.0000108928.25690.94. [DOI] [PubMed] [Google Scholar]
- Dulin B, Abraham WT. Pharmacology of carvedilol. Am J Cardiol. 2004;93:3B–6B. doi: 10.1016/j.amjcard.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Dunn CJ, Lea AP, Wagstaff AJ. Carvedilol. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disorders. Drugs. 1997;54:161–185. doi: 10.2165/00003495-199754010-00015. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Bosse R, Reisine T. Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening. Assay Drug Dev Technol. 2007;5:425–451. doi: 10.1089/adt.2007.062. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Follin-Arbelet V, Torgersen ML, Naderi EH, Misund K, Sundan A, Blomhoff HK. Death of multiple myeloma cells induced by cAMP-signaling involves downregulation of Mcl-1 via the JAK/STAT pathway. Cancer Lett. 2013;335:323–331. doi: 10.1016/j.canlet.2013.02.042. [DOI] [PubMed] [Google Scholar]
- Free RB, Chun LS, Moritz AE, Miller BN, Doyle TB, Conroy JL, Padron A, Meade JA, Xiao JB, Hu X, Dulcey AE, Han Y, Duan LH, Titus S, Bryant-Genevier M, Barnaeva E, Ferrer M, Javitch JA, Beuming T, Shi L, Southall NT, Marugan JJ, Sibley DR. Discovery and characterization of a G protein-biased agonist that inhibits β-arrestin recruitment to the D2 dopamine receptor. Mol Pharmacol. 2014;86:96–105. doi: 10.1124/mol.113.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Xu B, Liu YM, Parikh D, Li J, Li Y, Zhang Y, Riehle C, Zhu Y, Rawlings T, Shi Q, Clark RB, Chen XW, Abel ED, Xiang YK. Insulin inhibits cardiac contractility by inducing a Gi-biased β2-adrenergic signaling in hearts. Diabetes. 2014;63:2676–2689. doi: 10.2337/db13-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N, Downes GB, Yan K, Kisselev O. The G-protein β gamma complex. Cell Signal. 1998;10:447–455. doi: 10.1016/S0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- Gong KZ, Li ZJ, Xu M, Du JH, Lv ZZ, Zhang YY. A novel protein kinase A-independent, β-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by β2-adrenergic receptors. J Biol Chem. 2008;283:29028–29036. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer CE, Schmid CL, Jaeger AM, Bohn LM. Agonist-directed interactions with specific β-arrestins determine muopioid receptor trafficking, ubiquitination and dephosphorylation. J Biol Chem. 2011;286:31731–31741. doi: 10.1074/jbc.M111.248310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil S, Caceres JF. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat Struct Mol Biol. 2007;14:591–596. doi: 10.1038/nsmb1250. [DOI] [PubMed] [Google Scholar]
- Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi F, Tanaka Y, Washida M, Morikane K, Fukushima Y, Yamori T, Tsuruo T, Kawabe T, Miyagishi M, Taira K, Sata M, Omata M. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005;65:5864–5871. doi: 10.1158/0008-5472.CAN-04-3833. [DOI] [PubMed] [Google Scholar]
- Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta. 1999;1452:25–35. doi: 10.1016/S0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther. 2003;99:25–44. doi: 10.1016/S0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Ziegler N, Reiner S, Krasel C, Lohse MJ. Agonist-selective, receptor-specific interaction of human P2Y receptors with β-arrestin-1 and -2. J Biol Chem. 2008;283:30933–30941. doi: 10.1074/jbc.M801472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, Nishimura O, Fujino M. Molecular and functional characteristics of APJ - Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000;275:21061–21067. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Kumagai H, Motozawa Y, Suzuki J, Komuro I. Biased agonism of the angiotensin II type I receptor a potential strategy for the rreatment of acute heart failure. Int Heart J. 2015;56:485–488. doi: 10.1536/ihj.15-256. [DOI] [PubMed] [Google Scholar]
- Japp AG, Newby DE. The apelin-APJ system in heart failure pathophysiologic relevance and therapeutic potential. Biochem Pharmacol. 2008;75:1882–1892. doi: 10.1016/j.bcp.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Jean-Alphonse F, Perkovska S, Frantz MC, Durroux T, Mejean C, Morin D, Loison S, Bonnet D, Hibert M, Mouillac B, Mendre C. Biased agonist pharmacochaperones of the AVP V2 receptor may treat congenital nephrogenic diabetes insipidus. J Am Soc Nephrol. 2009;20:2190–2203. doi: 10.1681/ASN.2008121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- Kilts JD, Gerhardt MA, Richardson MD, Sreeram G, Mackensen GB, Grocott HP, White WD, Davis RD, Newman MF, Reves JG, Schwinn DA, Kwatra MM. β2-adrenergic and several other G protein-coupled receptors in human atrial membranes activate both Gs and Gi. Circ Res. 2000;87:705–709. doi: 10.1161/01.RES.87.8.705. [DOI] [PubMed] [Google Scholar]
- Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. β-blockers alprenolol and carvedilol stimulate β-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IM, Wang YC, Park KM, Tang YP, Teoh JP, Vinson J, Traynham CJ, Pironti G, Mao L, Su HB, Johnson JA, Koch WJ, Rockman HA. β-arrestin1-biased β1-adrenergic receptor signaling regulates microRNA processing. Circ Res. 2014;114:833–844. doi: 10.1161/CIRCRESAHA.114.302766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei HJ, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han JJ, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. Arrestins come of age: a personal historical perspective. Prog Mol Biol Transl Sci. 2013;118:3–18. doi: 10.1016/B978-0-12-394440-5.00001-2. [DOI] [PubMed] [Google Scholar]
- Leurs R, Chazot PL, Shenton FC, Lim HD, de Esch IJ. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol. 2009;157:14–23. doi: 10.1111/j.1476-5381.2009.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Horst R, Katritch V, Stevens RC, Wuthrich K. Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335:1106–1110. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan B, Zhao J, Wu HY, Duan BY, Shu GW, Wang XY, Li DS, Jia WP, Kang JH, Pei G. Deficiency of a β-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin FT, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. β-arrestin-dependent formation of β(2) adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- McCloskey DT, Turcato S, Wang GY, Turnbull L, Zhu BQ, Bambino T, Nguyen AP, Lovett DH, Nissenson RA, Karliner JS, Baker AJ. Expression of a Gi-coupled receptor in the heart causes impaired Ca2+ handling, myofilament injury and dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H205–H212. doi: 10.1152/ajpheart.00829.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. β-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, Henderson G, Kelly E. μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol. 2010;78:756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Applications of bioluminescence-and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur J Pharm Sci. 2004;21:397–405. doi: 10.1016/j.ejps.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Miura S, Okabe A, Matsuo Y, Karnik SS, Saku K. Unique binding behavior of the recently approved angiotensin II receptor blocker azilsartan compared with that of candesartan. Hypertens Res. 2013;36:134–139. doi: 10.1038/hr.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M, Yano M, Oda T, Tateishi H, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J Am Coll Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- Molinari P, Vezzi V, Sbraccia M, Gro C, Riitano D, Ambrosio C, Casella I, Costa T. Morphine-like opiates selectively antagonize receptor-arrestin interactions. J Biol Chem. 2010;285:12522–12535. doi: 10.1074/jbc.M109.059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisco C, Lembo G, Trimarco B. Insulin resistance and cardiovascular risk: new insights from molecular and cellular biology. Trends Cardiovasc Med. 2006;16:183–188. doi: 10.1016/j.tcm.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration and invasion of breast cancer cells. Cancer Res. 2006;66:307–314. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- Muller G. Towards 3D structures of G protein-coupled receptors: a multidisciplinary approach. Curr Med Chem. 2000;7:861–888. doi: 10.2174/0929867003374534. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Radresa O, Armando S, Devost D, Beautrait A, Le Gouill C, Laporte SA. Quantifying biased signaling in GPCRs using BRET-based biosensors. Methods. 2016;92:5–10. doi: 10.1016/j.ymeth.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Nevzorova J, Evans BA, Bengtsson T, Summers RJ. Multiple signalling pathways involved in β(2)-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells. Br J Pharmacol. 2006;147:446–454. doi: 10.1038/sj.bjp.0706626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence and risk factors. Diabetes Care. 2001;24:1614–1619. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- Nijmeijer S, Vischer HF, Sirci F, Schultes S, Engelhardt H, de Graaf C, Rosethorne EM, Charlton SJ, Leurs R. Detailed analysis of biased histamine H4 receptor signalling by JNJ 7777120 analogues. Br J Pharmacol. 2013;170:78–88. doi: 10.1111/bph.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles KN, Xiao KH, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, Shenoy SK, Gygi SP, Lefkowitz RJ. Distinct phosphorylation sites on the β2-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. B-arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi XM, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-O. [DOI] [PubMed] [Google Scholar]
- Ohsawa Y, Hirasawa N. The antagonism of histamine H1 and H4 receptors ameliorates chronic allergic dermatitis via antipruritic and anti-inflammatory effects in NC/Nga mice. Allergy. 2012;67:1014–1022. doi: 10.1111/j.1398-9995.2012.02854.x. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Hamada M, Hiasa G, Sasaki O, Suzuki M, Hara Y, Shigematsu Y, Hiwada K. Effect of β-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:412–417. doi: 10.1016/S0735-1097(00)01121-9. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Park SM, Chen M, Schmerberg CM, Dulman RS, Rodriguiz RM, Caron MG, Jin J, Wetsel WC. Effects of β-arrestin-biased dopamine D2 receptor ligands on schizophrenia-like behavior in hypoglutamatergic mice. Neuropsychopharmacology. 2016;41:704–715. doi: 10.1038/npp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino C, Rockman HA. Reversal of cardiac remodeling by modulation of adrenergic receptors: a new frontier in heart failure. Curr Opin Cardiol. 2007;22:443–449. doi: 10.1097/HCO.0b013e3282294d72. [DOI] [PubMed] [Google Scholar]
- Pitkin SL, Maguire JJ, Bonner TI, Davenport AP. International union of basic and clinical pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology and function. Pharmacol Rev. 2010;62:331–342. doi: 10.1124/pr.110.002949. [DOI] [PubMed] [Google Scholar]
- Pope GR, Roberts EM, Lolait SJ, O’Carroll AM. Central and peripheral apelin receptor distribution in the mouse: species differences with rat. Peptides. 2012;33:139–148. doi: 10.1016/j.peptides.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Perroy J, Walwyn WM, Smith ML, Vicente-Sanchez A, Segura L, Bana A, Kieffer BL, Evans CJ. Agonist-specific recruitment of arrestin isoforms differentially modify delta opioid receptor function. J Neurosci. 2016;36:3541–3551. doi: 10.1523/JNEUROSCI.4124-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013;123:11–18. doi: 10.1172/JCI62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmeh R, Damian M, Cottet M, Orcel H, Mendre C, Durroux T, Sharma KS, Durand G, Pucci B, Trinquet E, Zwier JM, Deupi X, Bron P, Baneres JL, Mouillac B, Granier S. Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc Natl Acad Sci USA. 2012;109:6733–6738. doi: 10.1073/pnas.1201093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. β-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA. 2006;103:16284–16289. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, DeWire SM, Violin JD, Lefkowitz RJ. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S, He MZ, Sayed D, Yan L, Vatner D, Abdellatif M. An antagonism between the AKT and β-adrenergic signaling pathways mediated through their reciprocal effects on miR-199a-5p. Cell Signal. 2010;22:1054–1062. doi: 10.1016/j.cellsig.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic Z, Brust TF, Bohn LM. Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorg Med Chem Lett. 2016;26:241–250. doi: 10.1016/j.bmcl.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MMC, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinartz MT, Kalble S, Littmann T, Ozawa T, Dove S, Kaever V, Wainer IW, Seifert R. Structure-bias relationships for fenoterol stereoisomers in six molecular and cellular assays at the β2-adrenoceptor. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:51–65. doi: 10.1007/s00210-014-1054-5. [DOI] [PubMed] [Google Scholar]
- Rives ML, Rossillo M, Liu-Chen LY, Javitch JA. 6′-Guanidinonaltrindole (6′-GNTI) is a G protein-biased κ-opioid receptor agonist that inhibits arrestin recruitment. J Biol Chem. 2012;287:27050–27054. doi: 10.1074/jbc.C112.387332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodefeld MD, Beau SL, Schuessler RB, Boineau JP, Saffitz JE. β-adrenergic and muscarinic cholinergic receptor densities in the human sinoatrial node: identification of a high β2-adrenergic receptor density. J Cardiovasc Electrophysiol. 1996;7:1039–1049. doi: 10.1111/j.1540-8167.1996.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Rosano L, Cianfrocca R, Tocci P, Spinella F, Di Castro V, Spadaro F, Salvati E, Biroccio AM, Natali PG, Bagnato A. β-arrestin-1 is a nuclear transcriptional regulator of endothelin-1-induced β-catenin signaling. Oncogene. 2013a;32:5066–5077. doi: 10.1038/onc.2012.527. [DOI] [PubMed] [Google Scholar]
- Rosano L, Spinella F, Bagnato A. Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer. 2013b;13:637–651. doi: 10.1038/nrc3546. [DOI] [PubMed] [Google Scholar]
- Rosano L, Spinella F, Di Castro V, Nicotra MR, Dedhar S, de Herreros AG, Natali PG, Bagnato A. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65:11649–11657. doi: 10.1158/0008-5472.CAN-05-2123. [DOI] [PubMed] [Google Scholar]
- Rosethorne EM, Charlton SJ. Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits β-arrestin without activating G proteins. Mol Pharmacol. 2011;79:749–757. doi: 10.1124/mol.110.068395. [DOI] [PubMed] [Google Scholar]
- Rubin JB. Chemokine signaling in cancer: One hump or two? Semin Cancer Biol. 2009;19:116–122. doi: 10.1016/j.semcancer.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a nonisopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Salloum FN, Yin C, Kukreja RC. Role of microRNAs in cardiac preconditioning. J Cardiovasc Pharmacol. 2010;56:581–588. doi: 10.1097/FJC.0b013e3181f581ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer A, Matter ML, Shohet RV. Cardioprotective signaling by endothelin. Trends Cardiovasc Med. 2008;18:233–239. doi: 10.1016/j.tcm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz ER, Kersting PH, Reffelmann T, Meven DA, Al-Dashti R, Skobel EC, Klosterhalfen B, Hanrath P. Cardioprotection by Carvedilol: antiapoptosis is independent of β-adrenoceptor blockage in the rat heart. J Cardiovasc Pharmacol Ther. 2003;8:207–215. doi: 10.1177/107424840300800306. [DOI] [PubMed] [Google Scholar]
- Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang JM, Woods CE, Purcell NH, Catalucci D, Akasaka T, Bueno OF, Vlasuk GP, Kaliman P, Bodmer R, Smith LH, Ashley E, Mercola M, Brown JH, Ruiz-Lozano P. APJ acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488:394–398. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, Tateno K, Moriya J, Yokoyama M, Nojima A, Koh GY, Akazawa H, Shiojima I, Kahn CR, Abel ED, Komuro I. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest. 2010;120:1506–1514. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. Distinct conformational changes in β-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA. 2008;105:9988–9993. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Xiao KH, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiba NP, Bae H, Hamm HE. Mapping of effector binding sites of transducin α-subunit using Gαt/Gαi1 chimeras. J Biol Chem. 1996;271:413–424. doi: 10.1074/jbc.271.1.413. [DOI] [PubMed] [Google Scholar]
- Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL, Violin JD, Lark MW. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014;54:351–357. doi: 10.1002/jcph.207. [DOI] [PubMed] [Google Scholar]
- Song XF, Coffa S, Fu HA, Gurevich VV. How does arrestin assemble MAPKs into a signaling complex? J Biol Chem. 2009;284:685–695. doi: 10.1074/jbc.M806124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XS, Zheng XL, Malbon CC, Wang HY. Gαi2 enhances in vivo activation of and insulin signaling to GLUT4. J Biol Chem. 2001;276:34651–34658. doi: 10.1074/jbc.M105894200. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A. Inhibition of cyclooxygenase-1 and-2 expression by targeting the endothelin a receptor in human ovarian carcinoma cells. Clin Cancer Res. 2004;10:4670–4679. doi: 10.1158/1078-0432.CCR-04-0315. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kato K, Kuboyama A, Inoue T, Tanaka Y, Kuhara A, Kinoshita K, Takeda S, Wake N. Induction of senescence by progesterone receptor-B activation in response to cAMP in ovarian cancer cells. Gynecol Oncol. 2009;113:270–276. doi: 10.1016/j.ygyno.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Tang YP, Wang YC, Park KM, Hu QP, Teoh JP, Broskova Z, Ranganathan P, Jayakumar C, Li J, Su HB, Tang YL, Ramesh G, Kim IM. MicroRNA-150 protects the mouse heart from ischaemic injury by regulating cell death. Cardiovasc Res. 2015;106:387–397. doi: 10.1093/cvr/cvv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh JP, Park KM, Broskova Z, Jimenez FR, Bayoumi AS, Archer K, Su HB, Johnson J, Weintraub NL, Tang Y, Kim IM. Identification of gene signatures regulated by carvedilol in mouse heart. Physiol. Genomics. 2015;47:376–385. doi: 10.1152/physiolgenomics.00028.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh JP, Park KM, Wang YC, Hu QP, Kim S, Wu GY, Huang S, Maihle N, Kim IM. Endothelin-1/Endothelin A receptor-mediated biased signaling is a new player in modulating human ovarian cancer cell tumorigenesis. Cell Signal. 2014;26:2885–2895. doi: 10.1016/j.cellsig.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GL, Kelly E, Christopoulos A, Canals M. Novel GPCR paradigms at the μ-opioid receptor. Br J Pharmacol. 2015;172:287–296. doi: 10.1111/bph.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Bernstein D, Murphy E, Steenbergen C. The role of β-adrenergic receptor signaling in cardioprotection. FASEB J. 2005;19:983–985. doi: 10.1096/fj.04-3067fje. [DOI] [PubMed] [Google Scholar]
- Vanderbeld B, Kelly GM. New thoughts on the role of the β gamma subunit in G protein signal transduction. Biochem Cell Biol. 2000;78:537–550. doi: 10.1139/o00-075. [DOI] [PubMed] [Google Scholar]
- Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335:572–579. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- Viscusi E, Minkowitz H, Webster L, Soergel D, Burt D, Subach R, Skobieranda F. Rapid reduction in pain intensity with oliceridine (TRV130), a novel μ receptor G protein Pathway Selective modulator (μ-GPS), vs. Morphine: an analysis of two phase 2 randomized clinical trials. J. Pain. 2016;17:S82–S83. doi: 10.1016/j.jpain.2016.01.410. [DOI] [Google Scholar]
- Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y, Chu MH, Siu FY, Liu W, Xu HE, Cherezov V, Roth BL, Stevens RC. Structural features for functional selectivity at serotonin receptors. Science. 2013;340:615–619. doi: 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Ha TZ, Zou JH, Ren DY, Liu L, Zhang X, Kalbfleisch J, Gao X, Williams D, Li CF. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102:385–395. doi: 10.1093/cvr/cvu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL. The G protein-biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Jr, Jones KL, Colton CD, Nutt RF. Identification of high affinity endothelin-1 receptor subtypes in human tissues. Biochem Biophys Res Commun. 1991;180:475–480. doi: 10.1016/S0006-291X(05)81089-7. [DOI] [PubMed] [Google Scholar]
- Winpenny D, Clark M, Cawkill D. Biased ligand quantification in drug discovery: from theory to high throughput screening to identify new biased opioid receptor agonists. Br J Pharmacol. 2016;173:1393–1403. doi: 10.1111/bph.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo AY, Jozwiak K, Toll L, Tanga MJ, Kozocas JA, Jimenez L, Huang Y, Song Y, Plazinska A, Pajak K, Paul RK, Bernier M, Wainer IW, Xiao RP. Tyrosine 308 is necessary for ligand-directed Gs protein-biased signaling of β2-adrenoceptor. J Biol Chem. 2014;289:19351–19363. doi: 10.1074/jbc.M114.558882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo AY, Wang TB, Zeng XK, Zhu WZ, Abernethy DR, Wainer IW, Xiao RP. Stereochemistry of an agonist determines coupling preference of β2-adrenoceptor to different G proteins in cardiomyocytes. Mol Pharmacol. 2009;75:158–165. doi: 10.1124/mol.108.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res. 2009;82:351–360. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Balke CW. Na+/Ca2+ exchange linking β2-adrenergic Gi signaling to heart failure: associated defect of adrenergic contractile support. J Mol Cell Cardiol. 2004;36:7–11. doi: 10.1016/j.yjmcc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Ji XW, Lakatta EG. Functional coupling of the β2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- Xiao RP, Zhang SJ, Chakir K, Avdonin P, Zhu WZ, Bond RA, Balke CW, Lakatta EG, Cheng HP. Enhanced Gi signaling selectively negates β2-adrenergic receptor (AR)-but not β1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation. 2003;108:1633–1639. doi: 10.1161/01.CIR.0000087595.17277.73. [DOI] [PubMed] [Google Scholar]
- Yu QJ, Si R, Zhou N, Zhang HF, Guo WY, Wang HC, Gao F. Insulin inhibits β-adrenergic action in ischemic/reperfused heart: a novel mechanism of insulin in cardioprotection. Apoptosis. 2008;13:305–317. doi: 10.1007/s10495-007-0169-2. [DOI] [PubMed] [Google Scholar]
- Yuan YY, Stevens DL, Braithwaite A, Scoggins KL, Bilsky EJ, Akbarali HI, Dewey WL, Zhang Y. 6β-N-heterocyclic substituted naltrexamine derivative NAP as a potential lead to develop peripheral mu opioid receptor selective antagonists. Bioorg Med Chem Lett. 2012;22:4731–4734. doi: 10.1016/j.bmcl.2012.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Williams DA, Zaidi SA, Yuan YY, Braithwaite A, Bilsky EJ, Dewey WL, Akbarali HI, Streicher JM, Selley DE. 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-(4′-pyridylcarboxamido)morphinan (NAP) modulating the mu opioid receptor in a biased fashion. ACS Chem Neurosci. 2016;7:297–304. doi: 10.1021/acschemneuro.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AM, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aube J, Bohn LM. Development of functionally selective, small molecule agonists at kappa opioid receptors. J Biol Chem. 2013;288:36703–36716. doi: 10.1074/jbc.M113.504381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]