Abstract
Endocytosis is a process by which cells absorb extracellular materials via the inward budding of vesicles formed from the plasma membrane. Receptor-mediated endocytosis is a highly selective process where receptors with specific binding sites for extracellular molecules internalize via vesicles. G protein-coupled receptors (GPCRs) are the largest single family of plasma-membrane receptors with more than 1000 family members. But the molecular mechanisms involved in the regulation of GPCRs are believed to be highly conserved. For example, receptor phosphorylation in collaboration with β-arrestins plays major roles in desensitization and endocytosis of most GPCRs. Nevertheless, a number of subsequent studies showed that GPCR regulation, such as that by endocytosis, occurs through various pathways with a multitude of cellular components and processes. This review focused on i) functional interactions between homologous and heterologous pathways, ii) methodologies applied for determining receptor endocytosis, iii) experimental tools to determine specific endocytic routes, iv) roles of small guanosine triphosphate-binding proteins in GPCR endocytosis, and v) role of post-translational modification of the receptors in endocytosis.
Keywords: G protein-coupled receptor, Endocytosis, Ral, ARF6, Glycosylation, Palmitoylation
INTRODUCTION
Cells are able to take up materials from their environment through endocytic processes. In receptor-mediated endocytosis, the engulfed materials (cargo; ligand and receptor) move to a specialized region of the plasma membrane, such as clathrin-coated pits (Mukherjee et al., 1997). In the clathrin-coated pits, the cargo is wrapped by the plasma membrane, which is coated with clathrins, leading to the formation of nascent vesicle buds (Pearse, 1976). As newly formed vesicles mature, they separate from the plasma membrane to form intracellular vesicles (van der Bliek et al., 1993). Receptor-mediated endocytosis is characterized by the highly selective sorting of molecules to be ingested by the cell. This selectivity results from the specific interaction between a receptor on the plasma membrane and its extracellular ligand. The functional role of receptor-mediated endocytosis is interpreted as the regulation of receptor functions rather than ingestion of ligands from the cell exterior.
Following agonistic stimulation, G protein-coupled receptors (GPCRs) undergo conformational changes that allow binding to G proteins (Gilman, 1987), leading to the activation of various signaling pathways and initiation of intracellular trafficking. After agonist stimulation, the receptor is phosphorylated by GPCR kinases (GRKs) (Pitcher et al., 1998), enhancing the binding of β-arrestins, which connect to adaptors, such as adaptor protein (AP)-2 and clathrin (Ferguson et al., 1996; Goodman et al., 1996; Laporte et al., 1999). These cellular processes are classified as homologous (agonist-induced, GRK-mediated) regulation of receptor responsiveness. By contrast, heterologous regulation of receptor responsiveness occurs regardless of agonists occupying the receptor. Receptor phosphorylation mediated by second-messenger-mediated kinases [protein kinase A (PKA) or protein kinase C (PKC)] is a key cellular event that leads to heterologous regulation.
The molecular mechanisms involved in GPCR endocytosis are highly conserved. GRKs/β-arrestins and PKA/PKCs are two protein families that mediate homologous and heterologous regulation, respectively. However, as described in the following sections, various cellular environments and components are involved in the regulation of GPCR endocytosis. Among these multiple factors, this review focused on the functional interactions between homologous and heterologous pathways and the roles of small guanosine triphosphate (GTP)-binding proteins and receptor post-translational modification on the regulation of receptor endocytosis.
FUNCTIONAL CROSSTALK BETWEEN HOMOLOGOUS AND HETEROLOGOUS REGULATION OF GPCRs
Although not mandatory, a major mechanism underlying GPCR regulation is receptor phosphorylation (Stadel et al., 1983; Barak et al., 1994; Ferguson, 2007; Cho et al., 2010). Second-messenger-dependent protein kinases, such as PKA and PKC, are responsible for agonist-nonspecific heterologous regulation. Alternatively, GRK2/3 is responsible for agonist- specific homologous regulation. Initially, GPCR phosphorylation by PKA or PKC was regarded as the sole mechanism of GPCR regulation (Benovic et al., 1985); however, it was found that β2 adrenoceptor (β2AR) could be phosphorylated in S49 lymphoma cells, which lack functional PKA (Strasser et al., 1986), suggesting the existence of additional protein kinases capable of phosphorylating GPCRs and leading to identification of the novel protein kinase family of GRKs (Benovic et al., 1986, 1989). Subsequent studies showed that β-arrestins (Lohse et al., 1990; Attramadal et al., 1992), analogues of visual arrestin, were required for receptor desensitization (Benovic et al., 1987) and potentiation of receptor endocytosis (Ferguson et al., 1996; Goodman et al., 1996).
Second-messenger-dependent protein kinases such as PKA and PKC can phosphorylate GPCRs at consensus phosphorylation sites and interfere with receptor-G protein coupling. Since PKA and PKC can phosphorylate agonist-unoccupied receptors, as well as agonist-occupied receptors, they inhibit not only ongoing signaling, but also prevent subsequent signaling activation. A complete understanding of the selective involvement of second-messenger-dependent protein kinases in heterologous regulatory processes remains elusive. Perhaps Gs- or Gq-coupled receptors, which are phosphorylated by GRK2/3, could be subjected to feedback regulation by activated PKA or PKC, suggesting that receptor phosphorylation mediated by second-messenger-dependent protein kinases could also contribute to homologous desensitization (Clark et al., 1988; Kelly et al., 2008).
Along with ligand selectivity, the main difference between homologous and heterologous regulation is the concentration of agonist required to induce receptor phosphorylation. Agonists in the nanomolar range are sufficient for PKA/PKC-mediated GPCR phosphorylation and desensitization (Jimenez-Baranda et al., 2007; Kim et al., 2008). In the case of PKA/PKC-mediated receptor phosphorylation, the signaling cascade initiated from the agonist-activated receptor is strongly amplified i.e., G protein→cyclic adenosine monophosphate (cAMP)/diacylglycerol (DAG)→PKA/PKC. By contrast, GRK2/3-mediated receptor phosphorylation, which depends upon agonist occupancy, requires higher concentrations of agonist as compared with those required for PKA/PKC-mediated phosphorylation. Additionally, higher levels of receptor expression are needed to facilitate β-arrestin translocation to mediate receptor desensitization and endocytosis. Therefore, GRK2/3-mediated receptor phosphorylation plays important roles in synaptic nerve terminals where high concentrations of neurotransmitters are attainable. By contrast, PKA/PKC-mediated receptor phosphorylation might affect tissues with low concentrations of circulating agonists (Arriza et al., 1992; Tran et al., 2004; Pollok-Kopp et al., 2007; Kelly et al., 2008).
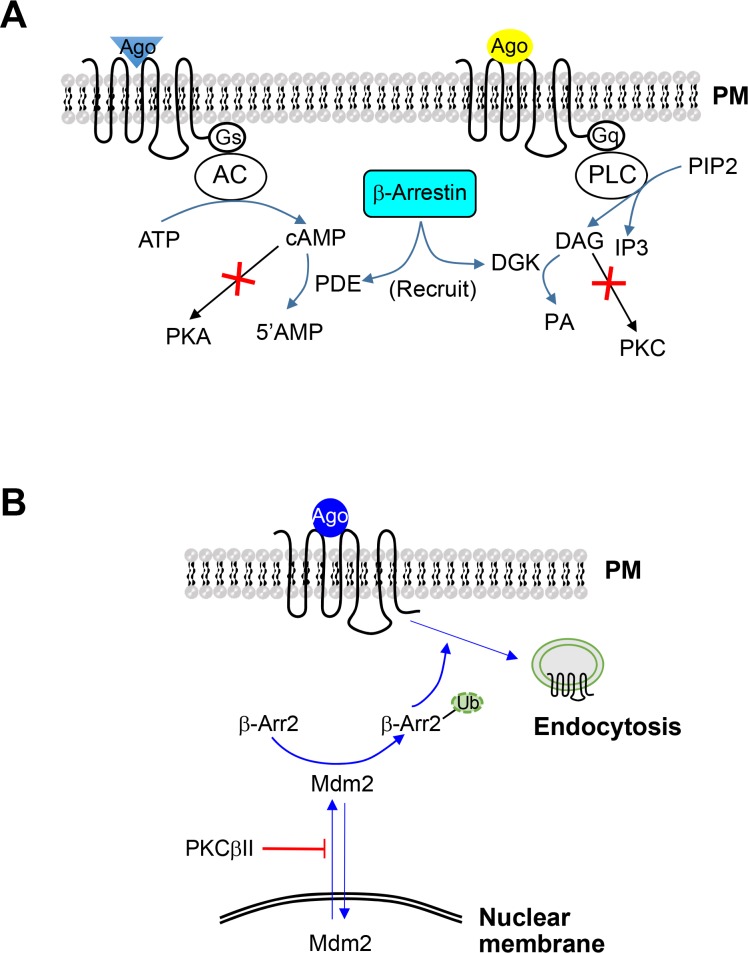
Understanding the functional interaction between homologous and heterologous pathways of GPCR regulation is an important field of study. Reports implicated the involvement of GRK2 and β-arrestins on PKC/PKA-mediated regulatory pathways. For example, activated GRK2 binds to PKCβ through its pleckstrin homology domain, resulting in PKC inhibition (Ji et al., 2003). β-Arrestins inhibit PKC-mediated regulatory pathways by activating DAG kinase, which subsequently mediates conversion of DAG to phosphatidic acid (PA) (Nelson et al., 2007). Another study showed that β-arrestins recruit cAMP phosphodiesterases to ligand-activated receptors, promoting the degradation of cAMP and thus inhibiting PKA activation (Perry et al., 2002) (Fig. 1A).
Fig. 1.
Functional interactions between homologous and heterologous regulatory pathways of GPCRs. (A) Functional antagonism of βarrestins on Gs- or Gq-coupled receptors downstream of receptor-G protein coupling. For Gs-coupled receptors, β-arrestins recruit phosphodiesterases to inhibit cAMP accumulation. For Gq-coupled receptors, β-arrestins recruit DGK to convert DAG to phosphatidic acid. (B) Effects of PKCβII on β-arrestin2 ubiquitination. PKCβII inhibits β-arrestin2 ubiquitination by interfering with the nuclear export of Mdm2, which occurs in response to agonist stimulation of GPCRs that have a tendency to undergo endocytosis. Ago, agonist; PM, plasma membrane; AC, adenylyl cyclase; PKA, protein kinase A; PDE, phosphodiesterase; PIP2, phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; DGK, diacylglycerol kinase; PA, phosphatidic acid; PKC, protein kinase C; β-Arr, β-arrestin; Ub, ubiquitin; Mdm2, Mouse double-minute-2 homolog; cAMP, cyclic adenosine monophosphate; GPCR, G protein-coupled receptor.
There have been various and sometimes contradictory reports regarding the effects of PKA or PKC on the homologous regulatory pathway. For example, PKA or PKC activates GRK2 by enhancing its translocation to the plasma membrane (Winstel et al., 1996; Cong et al., 2001). A subsequent study showed that GRK2 is phosphorylated by PKCα, PKCδ, and PKCδ in vitro, relieving the tonic inhibition by calmodulin (Krasel et al., 2001). By contrast, other studies showed that Rafkinase-inhibitor protein is phosphorylated on Ser153 by PKC activation, leading to its association with GRK2 and the inhibition of GRK2 activity (Lorenz et al., 2003; Huang et al., 2007). Furthermore, a recent study showed that PKCβII inhibits the homologous regulatory pathway by inhibiting β-arrestin-2 ubiquitination (Zheng et al., 2015) (Fig. 1B). Ubiquitination involves addition of ubiquitin, a small (8.5 kDa) regulatory protein and is a frequent cue for the degradation of the acceptor protein by the proteasome (Glickman and Ciechanover, 2002). In addition to protein degradation, ubiquitination is required for other functions, including β-arrestin2-mediated endocytic activities (Shenoy et al., 2001).
Therefore, it is suggested that functional interactions between homologous and heterologous pathways occur in two layers: between the major players of each endocytic pathway (PKA/PKC, GRK2/β-arrestins) or through manipulation of second-messenger levels. Further studies are needed to clarify the contradictory reports regarding the functional roles of PKC in the homologous regulation of GPCRs.
METHODOLOGIES USED FOR DETERMINATION OF RECEPTOR ENDOCYTOSIS
The terms ‘endocytosis’, ‘internalization’, and ‘sequestration’ are usually used interchangeably to describe the inward movement of extracellular material across the plasma membrane through endocytic processes. Strictly speaking, sequestration describes the isolation of ligands from receptors located on the cell surface; thus, it does not necessarily represent inward movement of materials from the exterior of the cell into the cytosol. Various experimental approaches have been utilized to assess GPCR internalization (endocytosis) or sequestration.
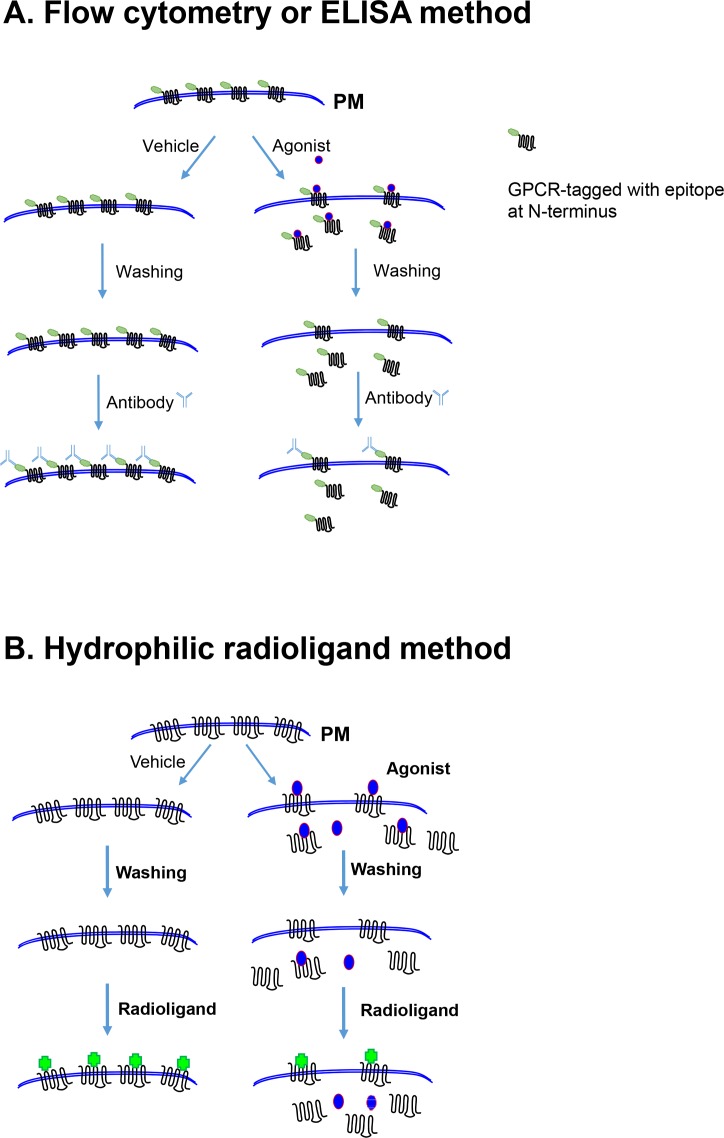
In the case of receptor endocytosis or internalization, the most universally utilized methodology is tagging of receptors at the N-terminus with specific epitopes, usually hemagglutinin (HA) or FLAG (Kim et al., 2001; Zhang and Kim, 2016) (Fig. 2A). Antibody labeled receptors on the cell surface can be detected using either enzyme-linked immunosorbent assay (ELISA) or fluorescence-activated cell sorting (FACS). For the ligand-binding methodology, radiolabeled hydrophilic ligands are commonly used to directly label receptors on the cell surface (Fig. 2B). Radiolabeled hydrophobic ligands are sometimes combined with hydrophilic ligands (to compete with surface binding of hydrophobic radioligands) to measure total receptor levels, as well as intracellular receptor levels (Itokawa et al., 1996; Kim et al., 2001; Zhang et al., 2016b). In our personal experience, the radioligand-binding assay is more convenient and accurate when compared with other methodologies. Receptor internalization can also be measured by fluorescence (Thompson and Whistler, 2011) or cell-surface biotinylation (Vickery and von Zastrow, 1999); however, these two methods can result in either difficultly in selectively quantifying receptors on the cell membrane and in the cytosol or the necessity to supplement with immunoprecipitation and immunoblot assays, which could introduce large variations in the measurements. When receptor sequestration accompanies conformational changes and short-distance trafficking within the plasma membrane, resulting in failure to bind hydrophilic ligands (pharmacological sequestration; Fig. 3), hydrophilic radioligands are used to label receptors on the cell surface.
Fig. 2.
Common methodologies used for determining GPCR endocytosis. (A) Determination of receptor endocytosis using epitope-tagged GPCRs at the N-terminus. This process involves induction of receptor internalization through agonist treatment for a desired period of time, washing, and labeling with primary and secondary antibodies. Receptors located on the cell surface are determined by flow cytometry or ELISA. According to the diagram, 60 percent of cell surface receptors are internalized. (B) Determination of receptor endocytosis using hydrophilic radioligands. This process involves induction of receptor endocytosis through agonist treatment for a desired period of time, thorough washing, and labeling with hydrophilic radioligands. Due to the hydrophilicity of radioligands, only receptors located on the cell surface are counted. According to the diagram, 60 percent of cell surface receptors are internalized. GPCR, G protein-coupled receptor; ELISA, enzyme-linked immunosorbent assay.
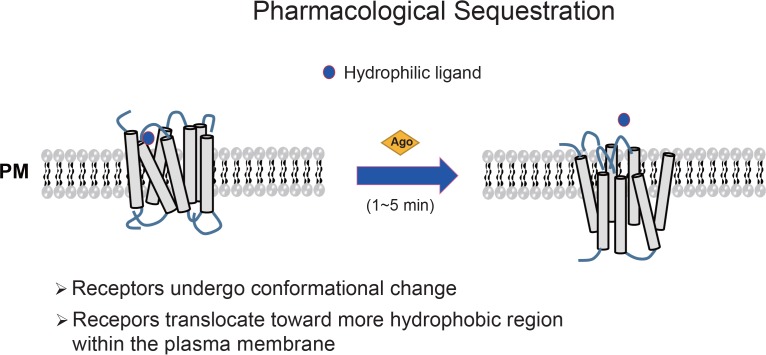
Fig. 3.
Diagram showing the pharmacological sequestration of GPCRs. In pharmacological sequestration, receptors undergo conformational changes and translocate toward more hydrophobic regions within the plasma membrane. Pharmacologically sequestered receptors do not actually translocate to the cytosol, but cannot bind to hydrophilic ligands, because they are located within more hydrophobic regions of the plasma membrane. GPCR, G protein-coupled receptor; PM, plasma membrane.
FACS analysis
Detection of GPCR endocytosis by FACS requires an N-terminal epitope-tagged GPCR. Because the location of the N-terminus changes from the exterior to the interior of the cell following GPCR internalization, FACS can be used to quantify the decrease in GPCR localization at the exterior surface of the plasma membrane (Kim et al., 2001). In this procedure, cells are transfected with a GPCR containing an epitope-tagged N-terminus. Cells are distributed in 6-well plates, stimulated with a vehicle or agonist for a desired period, and labeled with antibodies specific to the corresponding epitope. The mean cell-surface fluorescence and the number of fluorescent cells are then determined by FACS, and the percentage of receptor endocytosis is calculated from the FACS values of vehicle- or agonist-treated cells [i.e., (vehicle-treated−agonist-treated)/(vehicle-treated)].
ELISA
The principle behind determining receptor internalization by ELISA is essentially the same as that for FACS analysis: presence of an N-terminal epitope tag on the receptor (Zhang et al., 2016a); the epitope is no longer recognized by the cognate antibody once the receptor is internalized. For ELISA, cells are transfected with a receptor tagged at the N-terminus with a specific epitope, stimulated with agonist for a period of time, and labeled with antibodies corresponding to the specific epitope, followed by labeling with horseradish peroxidase-conjugated secondary antibodies. o-Phenylenediamine, a horseradish-peroxidase substrate, is then added, and the optical density (OD) of the supernatants is read using an ELISA reader. The background reading obtained from mock-transfected cells is subtracted to calculate the percentage of internalization. It is important to have a sufficient margin between the OD values of the cells transfected with receptor cDNA and mock plasmid. The percentage of receptor endocytosis is calculated from the OD values of vehicle- and agonist-treated cells [i.e., (vehicle-treated−agonist-treated)/(vehicle-treated)].
Radioligand binding
Hydrophilic radiolabeled ligands can be used to selectively label receptors expressed on the cell surface (Kim et al., 2001; Zheng et al., 2016). Cells are transfected with a GPCR, distributed in a 24-well plate, and stimulated with an agonist for a desired period of time. Cells need to be thoroughly washed with ice-cold serum-free media or low-pH buffer to stop further intracellular trafficking and to completely remove the agonist bound to the receptors. During the initial stage of the assay protocol, it is important to confirm that all of the agonists are removed under washing conditions. Cells are labeled with a hydrophilic radioligands on ice, during which time, further intracellular trafficking is blocked. Half of the experimental group needs to be labeled with a radioligand along with an excess concentration of non-labeled ligand to determine non-specific binding. Cells are washed thoroughly, lysed with detergent, and counted using a liquid-scintillation counter. The percentage of receptor endocytosis is calculated from the specific binding values of vehicle- and agonist-treated cells [i.e., (vehicle- treated−agonist-treated)/(vehicle-treated)].
Pharmacological sequestration
Agonist-induced intracellular trafficking of GPCRs typically involves the actual movement of receptors from the exterior surface of the plasma membrane into the cytosol (Moore et al., 2007). Both the radioligand-binding method and epitope-tagged receptor approaches can be applied to measure this internalization. By contrast, pharmacological sequestration involves conformational changes of the receptors accompanied by a shift toward more hydrophobic domains within the plasma membrane without movement into other intracellular compartments (Mostafapour et al., 1996). A recent study showed that pharmacological sequestration can be performed to predict the acute tolerance (desensitization) of the dopamine D3 receptor (Min et al., 2013). Since pharmacological sequestration does not accompany actual translocation of receptors across the plasma membrane, the ligand-binding method, but not flow cytometry or ELISA, can be applied to determine the extent of pharmacological sequestration (failure of hydrophilic ligand binding). In this procedure, cells are transfected with a GPCR and stimulated with vehicle or agonist for 1 to 5 min, during which time, typical receptor endocytosis does not occur. The reaction is stopped by placing the cells on ice, followed by washing with ice-cold low-pH buffer to completely remove all of the agonist bound to the receptor on the cell surface. Cells are then incubated with hydrophilic radioligands on ice in the absence or presence of excess unlabeled ligand. The cells are washed and lysed, and the remaining radioactivity is counted using a liquid scintillation counter. The percentage of pharmacological sequestration is calculated from the binding values of vehicle-treated and agonist-treated cells [i.e., (vehicle-treated−agonist-treated)/(vehicle-treated)].
SELECTIVE REGULATION OF CLATHRIN-MEDIATED AND CAVEOLAE-DEPENDENT ENDOCYTIC PATHWAYS
Clathrin-mediated and caveolae-dependent pathways are the best-characterized internalization routes of GPCRs (Hansen et al., 1993; Doherty and McMahon, 2009; Guo et al., 2015). Clathrin-mediated endocytosis (CME) is initiated by the formation of specialized membrane regions called clathrin-coated pits, into which cell-surface receptors concentrate and form clusters. Through a series of highly regulated steps, the pits bud off to form clathrin-coated vesicles with the help of dynamin, a protein that separates the newly-formed vesicles from the plasma membrane (Schmid, 1997; Marchese et al., 2003; Cho et al., 2006). A number of adaptor and accessory molecules are involved in this process, including the AP-2 complex, amphiphysin, GRK2/3, and β-arrestins that phosphorylate and connect receptors to clathrin and the AP-2 complex (Claing et al., 2002; Wolfe and Trejo, 2007; Ivanov, 2008; Romer et al., 2010).
Caveolae are flask-shaped invaginations of the plasma membrane and contain caveolin1 as a main component (Rothberg et al., 1992; Anderson, 1998; Parton and Simons, 2007). Caveolae are involved in mediating receptor signaling (Parton and Simons, 2007) and clathrin-independent, raft-dependent receptor endocytosis (Nabi and Le, 2003; Lajoie and Nabi, 2010). Caveolar endocytosis is sensitive to cholesterol depletion and requires dynamin (Henley et al., 1998; Rodal et al., 1999).
A number of molecular biological tools and pharmacological agents have been used to selectively inhibit CME and caveolar endocytosis. Molecular biology approaches include the use of dominant-negative mutants or RNA-interference technology to compete with or downregulate the expression of endogenous proteins involved in CME. With the stipulations that they do not exhibit serious cytotoxicity and that their selectivity be established, pharmacological agents might be more convenient than molecular biological approaches, because they are easy to use and influence the entire cell population equally (Ivanov, 2008).
There have been a number of reports suggesting selectivity of various cellular environments and chemical inhibitors of CME. For example, clathrin-coated pits can be blocked by decreasing cytosolic pH (Sandvig et al., 1987; Cosson et al., 1989). Monodansylcadaverine (MDC), an inhibitor of tissue transglutaminase (Mishra and Murphy, 2004), was used to block CME of insulin-like growth factor-1 and the α2 adrenergic receptor (Chow et al., 1998; Pierce et al., 2000). Transglutaminase is involved in protein cross-linking (Davies et al., 1980), which mediates clathrin clustering and internalization (Budd et al., 1999). Pitstop2 (N-[5-(4-bromobenzylidene)-4-oxo-4,5-dihydro-1,3-thiazol-2-yl]naphthalene-1-sulfonamide) is a recently developed CME inhibitor of transferrin, with an IC50 value of 12 μM to 15 μM. Pitstop2 inhibits the association between the terminal domain of clathrin and amphiphysin (von Kleist et al., 2011). However, the selectivity of these inhibitors was not clearly established, because criteria used to evaluate their selectivity were not properly implemented.
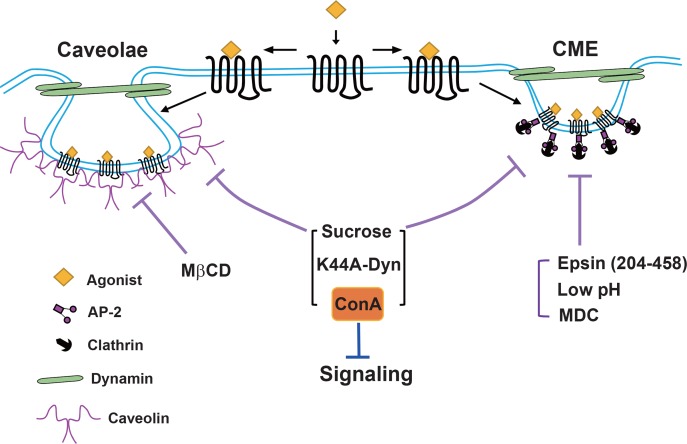
In a recent study, clathrin heavy chain- or caveolin1-knockdown cells were employed to determine the specificity of various chemical and molecular biological tools for CME and caveolar endocytosis (Guo et al., 2015). The study showed that sucrose, concanavalin A (Con A), and dominant-negative mutants of dynamin blocked other endocytic pathways, as well as the clathrin-mediated pathway. In particular, Con A non-specifically interfered with the signaling of several GPCRs tested in the study. Decreased pH, MDC, and dominant-negative epidermal growth factor (EGF)-receptor-pathway substrate 15 (Eps15)-interacting protein (Epsin) mutants are specific for CME when used properly, whereas Pitstop2 is marginally selective for CME (Dutta et al., 2012; Guo et al., 2015). These results are summarized in Fig. 4.
Fig. 4.
Selectivity of endocytic inhibitors on clathrin-mediated and caveolae-dependent endocytosis. Epsin (204–458), low pH, and MDC can be used to block CME. MβCD is selective for caveolar endocytosis. Careful dosage adjustment is needed for MDC and MβCD. Sucrose, K44A-dynamin, and Con A do not show selectivity. Con A inhibits GPCR signaling independent of its effects on endocytosis. Epsin, epidermal growth factor receptor-pathway substrate 15-interacting protein; AP-2, adaptor protein 2; CME, clathrin-mediated endocytosis; Con A, concanavalin A; MDC, monodansylcadaverine; MβCD, methyl-β-cyclodextrin.
ROLES OF SMALL G PROTEINS IN GPCR ENDOCYTOSIS
GTP-binding proteins (GTPases) are classified into two families: heterotrimeric large G proteins, which are composed of three subunits (α, β, and γ), and small G proteins. Based on the amino acid sequence of the Gα subunit, heterotrimeric G proteins are divided into Gs, Gi, Gq/11, and G12/13. The small G-protein superfamily is generally classified into five subfamilies: the Ras family (Ras, Rap, and Ral), the Rho family (Rho, Rac, and cdc42), the adenosine diphosphate ribosylation factor (Arf) family (Arf1-6, Arl1-Arl7, and Sar), the Rab family, and the Ran family (Burgoyne, 1989; Takai et al., 2001). All of these small GTPases control cell function by cycling between a GDP-bound ‘inactive’ state and a GTP bound ‘active’ state (Bos, 1998; Takai et al., 2001). An increasing number of studies show that GPCRs crosstalk with small G proteins. For example, small G proteins and related regulators [e.g., RhoA, Rabs, Arfs, and Arf-guanine nucleotide-exchange factors (GEFs)] can associate directly with GPCRs, and GPCRs may also function as GEFs for small GTPases (Bhattacharya et al., 2004a). In this review, we focused on the roles of Ral, Rab5, and Arf in GPCR endocytosis.
Effects of Ral on GPCR endocytosis
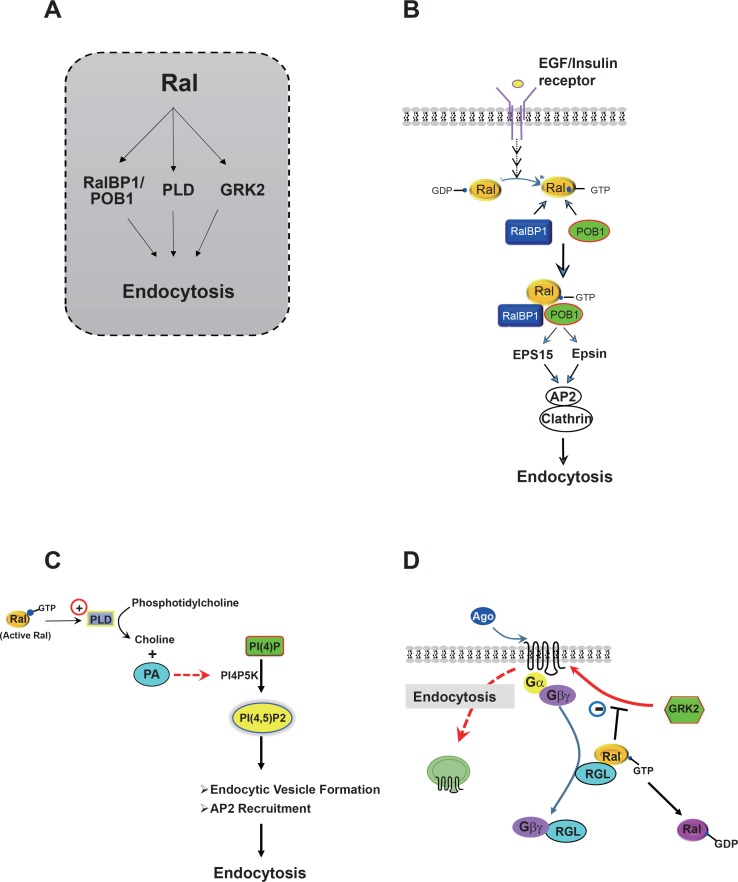
Ral, a member of the Ras family, possesses multiple regulatory roles, such as gene transcription, cytoskeletal regulation, and cell differentiation and migration (Chardin and Tavitian, 1986; Feig et al., 1996; Bos, 1998; Takai et al., 2001). Additionally, Ral is implicated in the endocytosis of receptors containing single transmembrane domains (van Dam and Robinson, 2006). Three downstream components of Ral have been reported: Ral-binding protein 1 (RalBP1) or Ral-interacting protein-76 kDa, phospholipase D (PLD), and GRK2 (Fig. 5A). First, Ral may regulate receptor endocytosis through RalBP1. RRalBP1 contains a GTPase-activating protein domain for the Rho family proteins and a Ral-binding domain. Thus, RalBP1 could play a role in connecting Rho- and Ras-family signaling (Mott and Owen, 2014). Active Ral and a dominant-negative mutant of RalBP1 inhibit endocytosis of insulin receptor, transferrin receptor, EGF receptor, and activin type II receptor (Nakashima et al., 1999; Jullien-Flores et al., 2000; Matsuzaki et al., 2002). RalBP1 associates with POB1, which forms a complex with Eps15, Epsin, AP-2, and clathrin to regulate endocytosis of transmembrane receptors (Fig. 5B). Second, Ral appears to regulate receptor endocytosis through PLD activation (Jiang et al., 1995; Kim et al., 1998) (Fig. 5C). For example, endocytosis of EGF receptor requires Ral-dependent PLD activation (Shen et al., 2001), and both metabotropic glutamate receptor (mGluR)-1a and -5a can be internalized constitutively by a Ral/PLD2-mediated endocytic mechanism (Bhattacharya et al., 2004b), which requires PLD2-dependent phosphatidic acid (PA) formation. Additionally, PA plays a regulatory role in clathrin-coated vesicle formation and receptor-mediated endocytosis by activating phosphatidylinositol 4-phosphate 5-kinase [PI(4)P5K] (Jenkins et al., 1994; Antonescu et al., 2010). PI(4)P5K is a type I lipid kinase that generates the lipid second messenger phospholipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], which is critically important in clathrin-coated pit dynamics (Zoncu et al., 2007; Nakatsu et al., 2010). Furthermore, AP-2 can bind clathrin in collaboration with PI(4,5)P2 (Kelly et al., 2014). Third, Ral might regulate receptor endocytosis through a functional interaction with GRK2 (Fig. 5D). Ral regulates the signaling or endocytosis of lysophosphatidic acid receptor-1 by modulating the interaction between the receptor and GRK2 (Aziziyeh et al., 2009). Zheng et al. (2016) recently showed that GTP-bound RalA inhibits the GPCR endocytosis by sequestering GRK2 from the activated receptors. Agonist-induced conversion of GTP-to GDP-bound RalA, which presumably releases sequestered GRK2, was observed selectively with the GPCRs, which have a tendency to undergo endocytosis. According to this study, agonist-induced Gβγ-mediated conversion of GTP-RalA to GDP-RalA is suggested as a critical cellular event that allows receptor-mediated endocytosis to occur.
Fig. 5.
Different modes of Ral regulation of receptor endocytosis. (A) Three downstream effectors of Ral in the regulation of receptor endocytosis. (B) RalBP1 (formerly known as RLIP76 or Ral-interacting protein) is involved in Ral-mediated regulation of endocytosis of receptors, such as EGF receptor and insulin receptor. Agonist-activated receptors transmit signals through Ral, RalBP1, and POB1 to Epsin and Eps15. Epsin and Eps15 bind to the AP-2/clathrin complex, leading to formation of clathrin-coated vesicles, which contain transmembrane receptors. This diagram was modified based on a previous publication (Nakashima et al., 1999). (C) Ral-mediated activation of PLD is involved in the endocytosis of EGF receptor and metabotropic glutamate receptor. PI(4,5)P2 is synthesized from PI(4)P by PI(4)P5K, which is activated by PA, a product of PLD. (D) RalA regulates GPCR endocytosis in an activity dependent manner. GTP-bound RalA sequesters GRK2 from binding to its receptor, resulting in the inhibition of receptor endocytosis. When cells are treated with agonist, Gβγ translocates to the cytosol as a complex with RGL, resulting in the dissociation of RGL from RalA and conversion of GTP-RalA to GDP-RalA, to which GRK2 has low affinity. GRK2 dissociated from GTP-RalA is the prepared for interaction with a receptor or other endocytic regulators. Ral-BP1, Ral-binding protein; EGF, epidermal growth factor receptor, Eps15, EGF-pathway substrate 15; Epsin, Eps15-interacting protein; AP-2, adaptor protein 2; PLD, phospholipase D; PI(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PI(4)P, phosphatidylinositol 4-phosphate; PI(4) P5K, phosphatidylinositol 4-phosphate 5-kinase; PA, phosphatidic acid; GPCR, G protein-coupled receptor; GRK, GPCR kinase; GDP, guanosine diphosphate; RGL, Ral-GDP-dissociation-stimulator-like protein; GTP, guanosine triphosphate.
Effects of Rab5 on GPCR endocytosis
The Rab GTPase family regulates multiple steps of vesicular-membrane trafficking, including vesicle budding, docking, and fusion. Thus, Rab is referred to as a master regulator of intracellular transport (Pfeffer, 1994; Olkkonen and Stenmark, 1997; Hutagalung and Novick, 2011).
Rab5 is associated with the plasma membrane and early endosomes, and regulates multiple steps involved in vesicular trafficking (Bucci et al., 1992; Novick and Zerial, 1997). Evidence for the roles of Rab5 were obtained using the Rab5 mutants Rab5-S34N and Rab5-Q79L, a dominant-negative mutant and a constitutively active mutant, respectively.
Rab5 mediates the formation of clathrin-coated vesicles at the cell surface. Rab5 is a component of clathrin-coated vesicles, and a complex of Rab5 and Rab-guanine nucleotide-dissociation inhibitor is necessary for the invagination of clathrin-coated pits (Bucci et al., 1992; McLauchlan et al., 1998; Seachrist et al., 2000; Weir et al., 2014). A possible isotypespecific interaction of Rab5 with clathrin was recently reported in Leishmania donovani (Rastogi et al., 2016).
Furthermore, Rab5 mediates the transport and fusion of endocytic vesicles with early endosomes. The Rab5-Q79L mutant stimulates endosome fusion and endocytosis, whereas the Rab5-S34N mutant blocks these processes. These effects are observed with transferrin receptor, endothelin receptors, neurokinin-1 receptor, and β2AR (Stenmark et al., 1994; Bremnes et al., 2000; Seachrist et al., 2000; Schmidlin et al., 2001). Rab5 was also reported to be phosphorylated by protein kinases, such as leucine-rich repeat kinase 2 or PKCε (Ong et al., 2014; Yun et al., 2015), suggesting intricate functional interactions with other signaling pathways.
Effects of Arf in GPCR endocytosis
Mammalian Arfs are divided into three classes: class I (Arf1-3), class II (Arf4-5), and class III (Arf6) (Moss and Vaughan, 1995). Arf1 and Arf6 are the best-characterized Arf subtype in terms of their roles in the intracellular trafficking of membrane proteins. Arf1 can be recruited to the plasma membrane on activation of some GPCRs (Mitchell et al., 2003), and Arf1 activation promotes the recruitment of components needed for the formation of trafficking vesicles, such as coats for non-clathrin (coatomer for COP1 vesicles) (Donaldson et al., 1992; Orcl et al., 1993) and clathrin (AP-1 and AP-3) (Traub et al., 1993; Ooi et al., 1998). Additionally, Arf1-mediated PLD activation is required for endocytosis of M3 muscarinic receptors and μ-opioid receptor (Luo et al., 1998; Koch et al., 2003; Mitchell et al., 2003).
Arf6 is mainly found on the plasma membrane, but not within clathrin-coated vesicles (D’Souza-Schorey et al., 1995; Cavenagh et al., 1996). A previous study proposed that the GDP-bound form of Arf6 localizes to the plasma membrane, the GDP-GTP cycle of Arf6 occurs at the plasma membrane, and activated Arf6 triggers clathrin translocation onto the membrane (Macia et al., 2004). Two Arf6 mutants (Q67L and T27N), despite unclear application of T27N (Macia et al., 2004), are considered to mimic the GTP- and GDP-bound forms of Arf6, respectively, and have been used extensively to elucidate Arf6 localization and function.
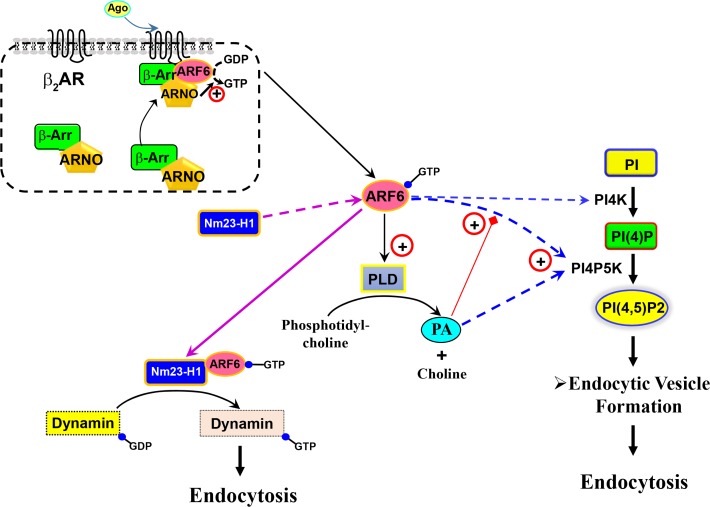
Arf6 plays an essential role in the internalization of several GPCRs, regardless of their endocytic route (Houndolo et al., 2005). For example, in response to agonist stimulation of β2AR, β-arrestins recruit Arf nucleotide-binding-site opener (ARNO; Arf6-GEF) to a position near the plasma membrane to form a complex comprising β-arrestin, Arf6, and ARNO. These processes result in Arf6 activation, which is essential for β2AR internalization (Claing et al., 2001; Lawrence et al., 2005) (Fig. 6, upper panel). Similar to Arf1, Arf6 regulates GPCR endocytosis via PLD2 activation, which leads to the hydrolysis of phosphatidylcholine to PA and choline (West et al., 1997; Rankovic et al., 2009). Additionally, Arf6 is capable of increasing PI(4,5)P2 concentration and facilitating clathrincoated pit assembly (Krauss et al., 2003; Posor et al., 2015). PI(4)P5K, which catalyzes the conversion of PI(4)P to PI(4,5) P2, is a downstream effector of Arf6 (Godi et al., 1999; Honda et al., 1999). Arf6 requires PA, the product of PLD, to activate PI(4)P5K (Czech, 2000), and PI(4)P5K can also be directly activated by PA. PI(4,5)P2 plays a regulatory role in a wide variety of cellular functions, including exocytosis, endocytosis, endosomal recycling, and membrane-ruffle formation (Honda et al., 1999; Funakoshi et al., 2011). PI(4,5)P2 is also required for the initial targeting of AP-2 to the plasma membrane, as well as for cargo recognition, which stabilize nascent coated pits during clathrin-mediated endocytosis (Wenk et al., 2001; Loerke et al., 2009).
Fig. 6.
Roles of Arf6 in regulating GPCR endocytosis. Studies of β2AR indicated β-arrestin involvement in recruiting ARNO to convert GDP-bound Arf to the GTP-bound form. GTP-bound Arf6 regulates different routes of receptor endocytosis. For example, by recruiting NM23-H1, Arf6 converts GDP-dynamin to GTP-dynamin to enhance receptor endocytosis. GTP-Arf6 activates PI(4)P5K to convert PI(4)P to PI(4,5)P2. Arf6 protein also can activate PI4K to convert PI to PI(4)P. Additionally, GTP-Arf6 activates PLD2 to produce PA. ADP, adenosine diphosphate; Arf, ADP-ribosylation factor; ARNO, Arf nucleotide-binding-site opener; GDP, guanosine diphosphate; GTP, guanosine triphosphate; PI, phosphatidylinositol; PI(4)P, phosphatidylinositol 4-phosphate; PI(4)P5K, phosphatidylinositol 4-phosphate 5-kinase; PI(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PI4K, phosphatidylinositol 4-kinase; NM23-H1, nucleoside diphosphate kinase; PA, phosphatidic acid; GPCR, G protein-coupled receptor; GTP, guanosine triphosphate; PLD, phospholipase D.
Alternatively, Arf6-GTP might recruit other proteins, such as Nm23-H1, a nucleoside diphosphate kinase that provides a source of GTP for dynamin-dependent fission of coated vesicles (Palacios et al., 2002). These hypotheses are summarized in Fig. 6.
ROLES OF POST-TRANSLATIONAL MODIFICATIONS IN GPCR ENDOCYTOSIS
GPCRs are post-translationally modified in a number of ways, including phosphorylation, ubiquitination, glycosylation, and palmitoylation. Receptor phosphorylation has been extensively investigated, and its roles in receptor endocytosis are well established (McCaffrey et al., 1984; Sibley et al., 1987), whereas the roles of ubiquitination in receptor endocytosis are clear only for yeast GPCRs (Hicke and Riezman, 1996; Shenoy et al., 2001; Zhang et al., 2016c). In this review, we focused on the roles of ubiquitination, glycosylation, and palmitoylation in receptor endocytosis.
Roles of ubiquitination in receptor endocytosis
During ubiquitination, ubiquitin, a small (8.5 kDa) regulatory protein, is added to a substrate protein. Ubiquitination requires three enzymes: activating, conjugating, and ubiquitin ligases (E1, E2, and E3, respectively). The carboxyl group of Gly76, the terminal amino acid of ubiquitin, is bound to the epsilon group of the target lysine residue on the substrate (Pickart, 2001; Marotti et al., 2002). Of the several lysine residues located within the ubiquitin molecule, Lys48 is involved in the formation of polyubiquitin chains that signal proteins for proteasomal processing. Alternatively, Lys63-linked chains are involved in endocytic processes (Boname et al., 2010). While the major role of ubiquitination involves mediation of protein degradation (Glickman and Ciechanover, 2002), ubiquitination also mediates various other cellular functions, such as gene transcription, cell division, differentiation, signal transduction, protein trafficking, and protein interactions (Alaluf et al., 1995; Conaway et al., 2002; Adlanmerini et al., 2014).
A role of ubiquitination in GPCR trafficking was originally reported from studies in yeast α-mating factor pheromone receptor (Ste2p). In 1993, it was found that Lys337 is required for Ste2p endocytosis (Rohrer et al., 1993). Using a yeast mutant lacking multiple ubiquitin-conjugating enzymes, it was shown that ubiquitination is required for Ste2p endocytosis and subsequent lysosomal degradation (Hicke and Riezman, 1996; Kim et al., 2005).
Several studies demonstrated diverse roles for ubiquitination in the regulation of mammalian GPCR trafficking. An essential role of ubiquitination was proposed in post-endocytic lysosomal sorting rather than in endocytosis (Koch et al., 1994; Holtmann et al., 1996; Jacob et al., 2005; Kim et al., 2007; Lahaie et al., 2016; Zhang et al., 2016c). By contrast, some GPCRs, including δ-opioid receptor, protease-activated receptor 1 (PAR1), and the P2Y1 purinergic receptor, are delivered to the lysosome independent of receptor ubiquitination (Tanowitz and Von Zastrow, 2002; Dores et al., 2012, 2016).
Roles of glycosylation in receptor endocytosis
N-linked glycosylation is a highly conserved post-translational modification that occurs on the Asn-X-Ser/Thr motif on GPCR extracellular domains (Kim et al., 1997; Ulloa-Aguirre et al., 1999; Filipek et al., 2003). Glycosylation aids the association of proteins with specific plasma-membrane microdomains (Rands et al., 1990; Boer et al., 2000; Kohno et al., 2002; Lichnerova et al., 2015). A recent study showed that N-linked glycosylation of the N-terminus of dopamine D2 and D3 receptors determines the endocytic pathways used by these receptors via their interactions with specific microdomains (Min et al., 2015).
Glycosylation also plays an important role in the ligand-binding affinity of many receptors, such as the EP3α receptor (Huang and Tai, 1998), human urokinase receptor (Moller et al., 1993), and human transferrin receptor (Williams and Enns, 1993). Deglycosylation of these receptors causes significant decreases in ligand-binding affinity.
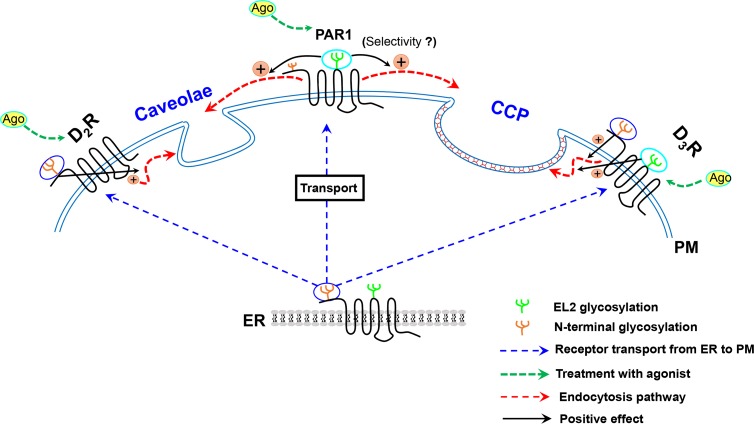
Additionally, the roles of glycosylation could be specific to the receptor region where glycosylation occurs. For example, N-linked glycosylation of the D3 receptor on the N-terminus is responsible for surface expression, desensitization, and inter nalization, whereas N-linked glycosylation within the second extracellular loop is exclusively responsible for internalization (Min et al., 2015). As in D3R, glycosylation on the N-terminus and within the second extracellular loop of PAR1 is important for its transport to the cell surface and internalization, respectively (Soto and Trejo, 2010) (Fig. 7). A similar effect was reported for the prostacyclin receptor (Zhang et al., 2001).
Fig. 7.
Glycosylation in the regulation of receptor endocytosis. Glycosylation on the N-terminus of GPCRs is involved in GPCR localization on the plasma membrane. Studies on dopamine D3 receptor and PARs suggest that glycosylation on different regions of the receptor is involved in the regulation of different receptor functions. Glycosylation on the N-terminus of D2R, D3R, or PAR1 is required for cell-membrane localization. Glycosylation on the N-terminus of D2R and D3R mediates their endocytosis within caveolae and CCP microdomains of the plasma membrane, respectively. Glycosylation within the second extracellular loop of D3R and PAR1 is important for internalization. (Selectivity?) next to PAR1 represents that it is not known whether palmitoylation on the second extracellular loop of PAR1 mediates CME or caveolar endocytosis. GPCR, G protein-coupled receptor; D3R, dopamine D3 receptor; D2R, dopamine D2 receptor; PAR, protease activator receptor; CCP, clathrin-coated pit.
The extent of glycosylation and its influence on receptor function seem to vary according to receptor type and cellular environment. Therefore, carefully controlled experiments are necessary to understand the broader functional roles of glycosylation and the underlying regulatory mechanisms.
Roles of palmitoylation in receptor endocytosis
Palmitoylation is mediated by palmitoyl transferase, which contains a characteristic Cys-rich Asp-His-His-Cys domain (Fukata et al., 2004; Greaves and Chamberlain, 2011). Palmitoylation is reversible and usually occurs on either Cys residues of membrane proteins or, less frequently, on Ser and Thr residues (Bizzozero, 1997; Qanbar and Bouvier, 2003). Palmitoylation exhibits various effects on receptor localization on the plasma membrane. For example, palmitoylation is needed for the proper localization of receptors, such as dopamine D3 receptor (Zhang et al., 2016b), CB1 cannabinoid receptor (Oddi et al., 2012), and PAR2 (Adams et al., 2011). Palmitoylation of the NR2 subunit of the N-methyl-D-aspartate receptor in the C-terminal region differentially affects surface expression, depending on the location of specific palmitoylation sites (Hayashi et al., 2009). Plasma membrane versus nuclear localization of estrogen receptors is also controlled by differential palmitoylation, which may promote the interaction between these estrogen receptors and caveolins (Acconcia et al., 2005; Boulware et al., 2005; Meitzen et al., 2013; Adlanmerini et al., 2014). In caveolae, estrogen receptors associate with mGluRs and activate them (Meitzen et al 2013).
Palmitoylation regulates both G proteins and their receptors (Wedegaertner et al., 1993; Ross, 1995), and is required for efficient signaling by most GPCRs, including β2AR (O’Dowd et al., 1989; Moffett et al., 1993), endothelin receptor type B (Okamoto et al., 1997), CB1 cannabinoid receptor (Oddi et al., 2012), PAR 2 (Adams et al., 2011), and μ-opioid receptor (Zheng et al., 2012). β2AR palmitoylation on Cys341 inhibits PKA access, allowing for more efficient coupling with G proteins (Moffett et al., 1996). By contrast, palmitoylation is not required for normal signaling by some GPCRs, such as α2AAR (Kennedy and Limbird, 1993; Eason et al., 1994) and thyrotropin receptor (Kosugi and Mori, 1996). In the case of tumor necrosis factor (TNF)α receptor, a member of the cytokinereceptor family, the affinity of the receptor for TNF decreases when the TNF ligand is palmitoylated (Poggi et al., 2013), suggesting that palmitoylation of ligand rather than receptor could regulate signaling. Palmitoylation-mediated redistribution of GPCRs between lipid raft and non-raft microdomains on the plasma membrane indirectly implicates palmitoylation in biased signaling (Zheng et al., 2008, 2013). The concept of biased signaling involves the agonists of one particular receptor activating downstream signaling pathways with different efficacies. The μ-opioid receptor can activate extracellular signal-regulated kinase (ERK) phosphorylation through either G protein- or β-arrestin-dependent pathways, depending on the association of the receptor with lipid raft or non-lipid raft microdomains, respectively (Zheng et al., 2008).
Similar to GPCRs, the α subunits of G proteins are palmitoylated (Linder et al., 1993; Parenti et al., 1993), with palmitoylation regulated by agonist stimulation of GPCRs, such as β2AR (Mumby et al., 1994) or 5-hydroxytryptamine1A receptor (Chen and Manning, 2000). Palmitoylation also influences membrane association, subcellular localization, and protein-protein interactions of Gα subunits. For example, palmitoylation regulates Gαq and Gαs attachment to the membrane and signaling by controlling interactions with cognate receptors or Gβγ (Wedegaertner et al., 1993; Edgerton et al., 1994; Iiri et al., 1996; Sikarwar et al., 2014). A recent study involving Gαi showed that palmitoylation regulates selective association with membrane microdomains having different compositions of fatty acids (Alvarez et al., 2015).
Palmitoylation exhibits various effects on receptor endocytosis. First, palmitoylation is required for the endocytosis of thyrotropin-releasing hormone receptor (Groarke et al., 2001), somatostatin receptor 5 (Hukovic et al., 1998), PAR2 (Adams et al., 2011), and dopamine D3 receptor (Zhang et al., 2016b). Second, palmitoylation has minimal or no effects on endocytosis of some GPCRs, such as β2AR (Moffett et al., 1993), α1AR (Gao et al., 1999), and C-C chemokine receptor type 5 (Blanpain et al., 2001). Third, palmitoylation has inhibitory effects on the endocytosis of luteinizing hormone/human choriogonadotropin receptor (Kawate and Menon, 1994) and V1A vasopressin receptor (Hawtin et al., 2001).
Interestingly, mutation of palmitoylation sites in the α2AAR does not affect receptor endocytosis, but completely inhibits agonist-induced downregulation (Eason et al., 1994). More diverse functional roles and palmitoylation sites were reported for β2AR, including mutation of the previously established palmitoylation site Cys341, which does not affect receptor endocytosis, but alters the endocytic route to a β-arrestin-independent and caveolae-dependent pathway (Liu et al., 2012). A recent study showed that β2AR, in response to agonist treatment, is palmitoylated at Cys265 via palmitate transferase, which is localized within the Golgi complex (Adachi et al., 2016).
As discussed, GPCR post-translational modifications affect various receptor functions, including cell-surface expression, signaling, endocytosis, and agonist affinity. Caution is needed when interpreting the functional consequences of post-translational modifications, given the possibility that the effects of palmitoylation could be indirect and secondary to those on receptor surface expression.
FUNCTIONAL ROLES OF GPCR ENDOCYTOSIS
Defects in GPCR signaling have been implicated in multiple human diseases, including autoimmunity, vascular diseases, and cancer (Rosenthal et al., 1993; O’Hayre et al., 2014). Because receptor trafficking is crucial for the temporal and spatial control of GPCR signaling, GPCR endocytosis could exhibit various degrees of physiological and pathological importance (Roth et al., 1998; Shapiro et al., 2000; von Zastrow, 2001; Booden et al., 2004).
Receptor endocytosis, which results in a decreased number of receptors on the cell surface, can be perceived as a mechanism of negative feedback to protect cells from agonistic overstimulation (Sibley and Lefkowitz, 1985). It is generally accepted that receptors are desensitized (uncoupled with effectors) within seconds to minutes after agonist stimulation via receptor phosphorylation and association with β-arrestins (Sibley et al., 1987; Dang and Christie, 2012). According to this molecular paradigm, most receptors undergoing endocytosis are already desensitized (phosphorylated and bound to β-arrestins), and are dephosphorylated by protein phosphatase type A2 in acidic environments. Thereafter, receptors recycle back to the cell surface in a resensitized state or are degraded in the lysosome (Menard et al., 1996; McDonald et al., 2000). Therefore, combined with receptor recycling, the main functional role of receptor endocytosis is considered to be a restoration of receptor responsiveness rather than a decrement in signaling (Yu et al., 1993; Pippig et al., 1995; Cho et al., 2010). However, endocytosis/recycling might not be mandatory for the resensitization of receptor responsiveness, because receptors can be rapidly dephosphorylated on the cell surface without the need for endocytosis and recycling (Nelson et al., 2007). Thus, interpretations of experimental results related to receptor endocytosis need to be handled with precaution.
The uncertainty in the consequences of receptor endocytosis might arise from limitations in experimental design (Connor et al., 2004), especially when experiments involve receptor overexpression or employ assays in which intense amplification of signaling processes occurs. Under these conditions, the effects of a decrease in the number of receptors on the cell surface would be underestimated. Another problem is employment of endocytic inhibitors or mutant receptors to make conclusions about the functional meaning of receptor endocytosis without their endocytic properties having been fully characterized (Yu et al., 1993; Pippig et al., 1995; Hanley and Hensler, 2002; Cho et al., 2010).
Although it is difficult to completely overcome the obstacles in selective regulation of specific endocytic routes, several points need to be carefully considered. First, the selectivity of inhibitors for specific endocytic routes (e.g., CME or caveolar endocytosis) is always relative (Guo et al., 2015), and at the same time there could be mutual interactions between endocytic pathways (Rodal et al., 1999; Subtil et al., 1999). Second, pharmacological and molecular biological blockade of endocytic processes can inhibit endocytosis of a GPCR, but simultaneously affect other membrane proteins. For example, it was initially concluded that ERK activation requires GPCR endocytosis (Luttrell et al., 1997). Later, it was suggested that the endocytosis of epidermal growth factor receptor (EGFR), which crosstalks with GPCRs, is required for ERK activation (Vieira et al., 1996; DeGraff et al., 1999; Pierce et al., 2000). There still exist some conflicting issues. For example, studies on protease-activated receptor (PAR)1, the neurokinin-1 receptor, and the angiotensin 1A receptor showed that internalized GPCRs form complexes on internal membranes via β-arrestin, with downstream components of the mitogen-activated protein kinase-signaling pathway, including Raf1, meiosisspecific serine/threonine-protein kinase (MEK)1, and ERK2 (DeFea et al., 2000; Luttrell et al., 2001; Teis et al., 2002). Based on these observations, the authors suggested that endocytosis of other signaling components, such as phosphorylated MEK rather than activated GPCRs or EGFRs might be required for ERK activation. Third, some experiments inevitably need to be conducted using indirect approaches that may alter protein function. For example, determination of roles associated with receptor phosphorylation through site-directed mutagenesis of potential and consensus phosphorylation sites or through abolishment of endogenous protein kinases. The introduction of point mutations could affect other aspects of receptor function in conjunction with receptor phosphorylation. Moreover, knockdown or knockout of endogenous protein kinases not only affects receptor phosphorylation, but also other cellular proteins, which could directly or indirectly affect receptor functions. Finally, studies using HEK-293 cells suggested that heterogeneity within the same cell line or among different cells can introduce response diversity and add to the complexity of the regulatory mechanisms of each receptor (Lefkowitz et al., 2002).
CONCLUSIONS
GPCRs are the largest group of cell membrane receptors, with as many as 800–1000 different human genes predicted to encode GPCRs, resulting in highly variable structural features, signaling mechanisms, and tissue distribution. Nevertheless, the molecular mechanisms involved in the regulation of GPCR functions are perceived as being highly conserved, with only several protein kinases and a couple of arrestin isotypes playing central roles in receptor endocytosis. However, as described here, complicated regulatory mechanisms are involved in GPCR endocytosis through a multitude of cellular components and processes, including heterogeneity of endocytic routes, functional interactions between different endocytic processes and pathways, post-translational modifications of receptor and endocytic vehicles, as well as other regulatory components, such as small G proteins. Beside the intrinsic complexity of endocytic processes, a primary obstacle in this research area involves the lack of powerful experimental tools and techniques to dissect the critical events associated with endocytosis.
Acknowledgments
This work was funded by KRF-2014R1A2A2A01002547.
REFERENCES
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor α membrane localization: regulation by 17β-estradiol. Mol. Biol. Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Hess DT, McLaughlin P, Stamler JS. Spalmitoylation of a novel site in the β2-adrenergic receptor associated with a novel intracellular itinerary. J Biol Chem. 2016;291:20232–20246. doi: 10.1074/jbc.M116.725762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MN, Christensen ME, He Y, Waterhouse NJ, Hooper JD. The role of palmitoylation in signalling, cellular trafficking and plasma membrane localization of protease-activated receptor-2. PLoS ONE. 2011;6:e28018. doi: 10.1371/journal.pone.0028018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessières E, Kim SH, Lière P, Fontaine C, Krust A, Chambon P, Katzenellenbogen JA, Gourdy P, Shaul PW, Henrion D, Arnal JF, Lenfant F. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci USA. 2014;111:E283–E290. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaluf S, Mulvihill ER, McIlhinney RA. Palmitoylation of metabotropic glutamate receptor subtype 4 but not 1 α expressed in permanently transfected BHK cells. Biochem Soc Trans. 1995;23:87S. doi: 10.1042/bst023087s. [DOI] [PubMed] [Google Scholar]
- Alvarez R, López DJ, Casas J, Lladó V, Higuera M, Nagy T, Barceló M, Busquets X, Escribá PV. G protein-membrane interactions I: Gαi1 myristoyl and palmitoyl modifications in protein-lipid interactions and its implications in membrane microdomain localization. Biochim. Biophys. Acta. 2015;1851:1511–1520. doi: 10.1016/j.bbalip.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Antonescu CN, Danuser G, Schmid SL. Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis. Mol. Biol. Cell. 2010;21:2944–2952. doi: 10.1091/mbc.E10-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, Dawson TM, Simerly RB, Martin LJ, Caron MG, Snyder SH, Lefkowitz RJ. The G-protein-coupled receptor kinases β ARK1 and β ARK2 are widely distributed at synapses in rat brain. J Neurosci. 1992;12:4045–4055. doi: 10.1523/JNEUROSCI.12-10-04045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ. β-arrestin2, a novel member of the arrestin/β-arrestin gene family. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- Aziziyeh AI, Li TT, Pape C, Pampillo M, Chidiac P, Possmayer F, Babwah AV, Bhattacharya M. Dual regulation of lysophosphatidic acid (LPA1) receptor signalling by Ral and GRK. Cell Signal. 2009;21:1207–1217. doi: 10.1016/j.cellsig.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Barak LS, Tiberi M, Freedman NJ, Kwatra MM, Lefkowitz RJ, Caron MG. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated β2- adrenergic receptor sequestration. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- Benovic JL, DeBlasi A, Stone WC, Caron MG, Lefkowitz RJ. β-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989;246:235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- Benovic JL, Kühn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ. Functional desensitization of the isolated β-adrenergic receptor by the β-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci USA. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic JL, Pike LJ, Cerione RA, Staniszewski C, Yoshimasa T, Codina J, Caron MG, Lefkowitz RJ. Phosphorylation of the mammalian β-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985;260:7094–7101. [PubMed] [Google Scholar]
- Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. β-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Babwah AV, Godin C, Anborgh PH, Dale LB, Poulter MO, Ferguson SS. Ral and phospholipase D2-dependent pathway for constitutive metabotropic glutamate receptor endocytosis. J Neurosci. 2004b;24:8752–8761. doi: 10.1523/JNEUROSCI.3155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Babwah AV, Ferguson SS. Small GTP-binding protein-coupled receptors. Biochem Soc Trans. 2004a;32:1040–1044. doi: 10.1042/BST0321040. [DOI] [PubMed] [Google Scholar]
- Bizzozero OA. The mechanism and functional roles of protein palmitoylation in the nervous system. Neuropediatrics. 1997;28:23–26. doi: 10.1055/s-2007-973660. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Wittamer V, Vanderwinden JM, Boom A, Renneboog B, Lee B, Le Poul E, El Asmar L, Govaerts C, Vassart G, Doms RW, Parmentier M. Palmitoylation of CCR5 is critical for receptor trafficking and efficient activation of intracellular signaling pathways. J Biol Chem. 2001;276:23795–23804. doi: 10.1074/jbc.M100583200. [DOI] [PubMed] [Google Scholar]
- Böer U, Neuschäfer-Rube F, Möller U, Püschel GP. Requirement of N-glycosylation of the prostaglandin E2 receptor EP3β for correct sorting to the plasma membrane but not for correct folding. Biochem. J. 2000;350 Pt 3:839–847. doi: 10.1042/bj3500839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boname JM, Thomas M, Stagg HR, Xu P, Peng J, Lehner PJ. Efficient internalization of MHC I requires lysine-11 and lysine-63 mixed linkage polyubiquitin chains. Traffic. 2010;11:210–220. doi: 10.1111/j.1600-0854.2009.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booden MA, Eckert LB, Der CJ, Trejo J. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol Cell Biol. 2004;24:1990–1999. doi: 10.1128/MCB.24.5.1990-1999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 1998;17:6776–6782. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem. 2000;275:17596–1604. doi: 10.1074/jbc.M000142200. [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-W. [DOI] [PubMed] [Google Scholar]
- Budd DC, Rae A, Tobin AB. Activation of the mitogen-activated protein kinase pathway by a Gq/11-coupled muscarinic receptor is independent of receptor internalization. J Biol Chem. 1999;274:12355–12360. doi: 10.1074/jbc.274.18.12355. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. Small GTP-binding proteins. Trends Biochem Sci. 1989;14:394–396. doi: 10.1016/0968-0004(89)90281-8. [DOI] [PubMed] [Google Scholar]
- Cavenagh MM, Whitney JA, Carroll K, Zhang CJ, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- Chardin P, Tavitian A. The ral gene: a new ras related gene isolated by the use of a synthetic probe. EMBO J. 1986;5:2203–2208. doi: 10.1002/j.1460-2075.1986.tb04485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Manning DR. Regulation of gα i palmitoylation by activation of the 5-hydroxytryptamine-1A receptor. J Biol Chem. 2000;275:23516–23522. doi: 10.1074/jbc.M003439200. [DOI] [PubMed] [Google Scholar]
- Cho D, Zheng M, Min C, Ma L, Kurose H, Park JH, Kim KM. Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D2 receptors. Mol Endocrinol. 2010;24:574–586. doi: 10.1210/me.2009-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Beom S, Van Tol HH, Caron MG, Kim KM. Characterization of the desensitization properties of five dopamine receptor subtypes and alternatively spliced variants of dopamine D2 and D4 receptors. Biochem Biophys Res Commun. 2006;350:634–640. doi: 10.1016/j.bbrc.2006.09.090. [DOI] [PubMed] [Google Scholar]
- Chow JC, Condorelli G, Smith RJ. Insulin-like growth factor-I receptor internalization regulates signaling via the Shc/mitogen- activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J Biol Chem. 1998;273:4672–4680. doi: 10.1074/jbc.273.8.4672. [DOI] [PubMed] [Google Scholar]
- Claing A, Chen W, Miller WE, Vitale N, Moss J, Premont RT, Lefkowitz RJ. β-Arrestin-mediated ADP-ribosylation factor 6 activation and β 2-adrenergic receptor endocytosis. J Biol Chem. 2001;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and β-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/S0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Clark RB, Kunkel MW, Friedman J, Goka TJ, Johnson JA. Activation of cAMP-dependent protein kinase is required for heterologous desensitization of adenylyl cyclase in S49 wildtype lymphoma cells. Proc Natl Acad Sci USA. 1988;85:1442–1446. doi: 10.1073/pnas.85.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway RC, Brower CS, Conaway JW. Emerging roles of ubiquitin in transcription regulation. Science. 2002;296:1254–1258. doi: 10.1126/science.1067466. [DOI] [PubMed] [Google Scholar]
- Cong M, Perry SJ, Lin FT, Fraser ID, Hu LA, Chen W, Pitcher JA, Scott JD, Lefkowitz RJ. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J Biol Chem. 2001;276:15192–15199. doi: 10.1074/jbc.M009130200. [DOI] [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ. Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol. 2004;143:685–696. doi: 10.1038/sj.bjp.0705938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, de Curtis I, Pouysségur J, Griffiths G, Davoust J. Low cytoplasmic pH inhibits endocytosis and transport from the trans-Golgi network to the cell surface. J Cell Biol. 1989;108:377–387. doi: 10.1083/jcb.108.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/S0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol. 2012;165:1704–1716. doi: 10.1111/j.1476-5381.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ, Davies DR, Levitzki A, Maxfield FR, Milhaud P, Willingham MC, Pastan IH. Transglutaminase is essential in receptor-mediated endocytosis of α2-macroglobulin and polypeptide hormones. Nature. 1980;283:162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Déry O, Mullins RD, Bunnett NW. β-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGraff JL, Gagnon AW, Benovic JL, Orsini MJ. Role of arrestins in endocytosis and signaling of α2-adrenergic receptor subtypes. J Biol Chem. 1999;274:11253–11259. doi: 10.1074/jbc.274.16.11253. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores MR, Chen B, Lin H, Soh UJ, Paing MM, Montagne WA, Meerloo T, Trejo J. ALIX binds a YPX3L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J Cell Biol. 2012;197:407–419. doi: 10.1083/jcb.201110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores MR, Grimsey NJ, Mendez F, Trejo J. ALIX Regulates the Ubiquitin-Independent Lysosomal Sorting of the P2Y1 Purinergic Receptor via a YPX3L Motif. PLoS ONE. 2016;11:e0157587. doi: 10.1371/journal.pone.0157587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Williamson CD, Cole NB, Donaldson JG. Pitstop 2 is a potent inhibitor of clathrin-independent endocytosis. PLoS ONE. 2012;7:e45799. doi: 10.1371/journal.pone.0045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- Eason MG, Jacinto MT, Theiss CT, Liggett SB. The palmitoylated cysteine of the cytoplasmic tail of α2A-adrenergic receptors confers subtype-specific agonist-promoted downregulation. Proc Natl Acad Sci USA. 1994;91:11178–11182. doi: 10.1073/pnas.91.23.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton MD, Chabert C, Chollet A, Arkinstall S. Palmitoylation but not the extreme amino-terminus of Gq α is required for coupling to the NK2 receptor. FEBS Lett. 1994;354:195–199. doi: 10.1016/0014-5793(94)01101-X. [DOI] [PubMed] [Google Scholar]
- Feig LA, Urano T, Cantor S. Evidence for a Ras/Ral signaling cascade. Trends Biochem Sci. 1996;21:438–441. doi: 10.1016/S0968-0004(96)10058-X. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Phosphorylation-independent attenuation of GPCR signalling. Trends Pharmacol Sci. 2007;28:173–179. doi: 10.1016/j.tips.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Ménard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Filipek S, Stenkamp RE, Teller DC, Palczewski K. G protein-coupled receptor rhodopsin: a prospectus. Annu Rev Physiol. 2003;65:851–879. doi: 10.1146/annurev.physiol.65.092101.142611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Funakoshi Y, Hasegawa H, Kanaho Y. Regulation of PIP5K activity by Arf6 and its physiological significance. J Cell Physiol. 2011;226:888–895. doi: 10.1002/jcp.22482. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ni Y, Szabo G, Linden J. Palmitoylation of the recombinant human A1 adenosine receptor: enhanced proteolysis of palmitoylation-deficient mutant receptors. Biochem J. 1999;342:387–895. doi: 10.1042/bj3420387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-arrestin acts as a clathrin adaptor in endocytosis of the β2- adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho) physiology. Trends Biochem Sci. 2011;36:245–253. doi: 10.1016/j.tibs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Groarke DA, Drmota T, Bahia DS, Evans NA, Wilson S, Milligan G. Analysis of the C-terminal tail of the rat thyrotropin-releasing hormone receptor-1 in interactions and cointernalization with beta-arrestin 1-green fluorescent protein. Mol Pharmacol. 2001;59:375–385. doi: 10.1124/mol.59.2.375. [DOI] [PubMed] [Google Scholar]
- Guo S, Zhang X, Zheng M, Zhang X, Min C, Wang Z, Cheon SH, Oak MH, Nah SY, Kim KM. Selectivity of commonly used inhibitors of clathrin-mediated and caveolaedependent endocytosis of G protein-coupled receptors. Biochim. Biophys. Acta. 2015;1848:2101–2010. doi: 10.1016/j.bbamem.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Hanley NR, Hensler JG. Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine(2A) receptor. J Pharmacol Exp Ther. 2002;300:468–477. doi: 10.1124/jpet.300.2.468. [DOI] [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtin SR, Tobin AB, Patel S, Wheatley M. Palmitoylation of the vasopressin V1a receptor reveals different conformational requirements for signaling, agonist-induced receptor phosphorylation, and sequestration. J Biol Chem. 2001;276:38139–38146. doi: 10.1074/jbc.M106142200. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64:213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/S0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Holtmann MH, Roettger BF, Pinon DI, Miller LJ. Role of receptor phosphorylation in desensitization and internalization of the secretin receptor. J Biol Chem. 1996;271:23566–23571. doi: 10.1074/jbc.271.38.23566. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/S0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Houndolo T, Boulay PL, Claing A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J Biol Chem. 2005;280:5598–5604. doi: 10.1074/jbc.M411456200. [DOI] [PubMed] [Google Scholar]
- Huang C, Tai HH. Prostaglandin E2 receptor EP3α subtype: the role of N-glycosylation in ligand binding as revealed by site-directed mutagenesis. Prostaglandins Leukot. Essent. Fatty Acids. 1998;59:265–271. doi: 10.1016/S0952-3278(98)90140-5. [DOI] [PubMed] [Google Scholar]
- Huang J, Mahavadi S, Sriwai W, Grider JR, Murthy KS. Cross-regulation of VPAC(2) receptor desensitization by M(3) receptors via PKC-mediated phosphorylation of RKIP and inhibition of GRK2. Am J Physiol Gastrointest Liver Physiol. 2007;292:G867–G874. doi: 10.1152/ajpgi.00326.2006. [DOI] [PubMed] [Google Scholar]
- Hukovic N, Panetta R, Kumar U, Rocheville M, Patel YC. The cytoplasmic tail of the human somatostatin receptor type 5 is crucial for interaction with adenylyl cyclase and in mediating desensitization and internalization. J Biol Chem. 1998;273:21416–21422. doi: 10.1074/jbc.273.33.21416. [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiri T, Backlund PS, Jr, Jones TL, Wedegaertner PB, Bourne HR. Reciprocal regulation of Gsα by palmitate and the βγ subunit. Proc Natl Acad Sci USA. 1996;93:14592–14597. doi: 10.1073/pnas.93.25.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itokawa M, Toru M, Ito K, Tsuga H, Kameyama K, Haga T, Arinami T, Hamaguchi H. Sequestration of the short and long isoforms of dopamine D2 receptors expressed in Chinese hamster ovary cells. Mol Pharmacol. 1996;49:560–566. [PubMed] [Google Scholar]
- Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008;440:15–33. doi: 10.1007/978-1-59745-178-9_2. [DOI] [PubMed] [Google Scholar]
- Jacob C, Cottrell GS, Gehringer D, Schmidlin F, Grady EF, Bunnett NW. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J Biol Chem. 2005;280:16076–16087. doi: 10.1074/jbc.M500109200. [DOI] [PubMed] [Google Scholar]
- Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994;269:11547–11554. [PubMed] [Google Scholar]
- Ji S, Liu X, Li S, Shen L, Li F, Wang J, Han J, Yao L. PH domain of G protein-coupled receptor kinase-2 binds to protein kinase C (PKC) and negatively regulates activity of PKC kinase. Front Biosci. 2003;8:a34–a39. doi: 10.2741/987. [DOI] [PubMed] [Google Scholar]
- Jiang H, Luo JQ, Urano T, Frankel P, Lu Z, Foster DA, Feig LA. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature. 1995;378:409–412. doi: 10.1038/378409a0. [DOI] [PubMed] [Google Scholar]
- Jimenez-baranda S, Gómez-Moutón C, Rojas A, Martínez-Prats L, Mira E, Ana Lacalle R, Valencia A, Dimitrov DS, Viola A, Delgado R, Martínez-A C, Mañes S. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol. 2007;9:838–846. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- Jullien-Flores V, Mahé Y, Mirey G, Leprince C, Meunier-Bisceuil B, Sorkin A, Camonis JH. RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J Cell Sci. 2000;113:2837–2844. doi: 10.1242/jcs.113.16.2837. [DOI] [PubMed] [Google Scholar]
- Kawate N, Menon KM. Palmitoylation of luteinizing hormone/human choriogonadotropin receptors in transfected cells. Abolition of palmitoylation by mutation of Cys-621 and Cys-622 residues in the cytoplasmic tail increases ligand-induced internalization of the receptor. J Biol Chem. 1994;269:30651–30658. [PubMed] [Google Scholar]
- Kelly BT, Graham SC, Liska N, Dannhauser PN, Höning S, Ungewickell EJ, Owen DJ. Clathrin adaptors. AP2 controls clathrin polymerization with a membrane-activated switch. Science. 2014;345:459–463. doi: 10.1126/science.1254836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol. 2008;153(Suppl 1):S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy ME, Limbird LE. Mutations of the α2A-adrenergic receptor that eliminate detectable palmitoylation do not perturb receptor-G-protein coupling. J Biol Chem. 1993;268:8003–8011. [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Cho EY, Min C, Park JH, Kim KM. Characterization of functional roles of DRY motif in the 2nd intracellular loop of dopamine D2 and D3 receptors. Arch Pharm Res. 2008;31:474–481. doi: 10.1007/s12272-001-1181-x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SD, Han JM, Lee TG, Kim Y, Park JB, Lambeth JD, Suh PG, Ryu SH. Activation of phospholipase D1 by direct interaction with ADP-ribosylation factor 1 and RalA. FEBS Lett. 1998;430:231–235. doi: 10.1016/S0014-5793(98)00661-9. [DOI] [PubMed] [Google Scholar]
- Kim JY, Soede RD, Schaap P, Valkema R, Borleis JA, Van Haastert PJ, Devreotes PN, Hereld D. Phosphorylation of chemoattractant receptors is not essential for chemotaxis or termination of G-protein-mediated responses. J Biol Chem. 1997;272:27313–27318. doi: 10.1074/jbc.272.43.27313. [DOI] [PubMed] [Google Scholar]
- Kim KM, Pae HO, Zheng M, Park R, Kim YM, Chung HT. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res. 2007;101:919–927. doi: 10.1161/CIRCRESAHA.107.154781. [DOI] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and β-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Schulz S, Liang Y, Klein J, Hollt V. ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced mu-opioid receptor endocytosis. J Biol Chem. 2003;278:9979–9985. doi: 10.1074/jbc.M206709200. [DOI] [PubMed] [Google Scholar]
- Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates Gβγ-mediated signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- Kohno T, Wada A, Igarashi Y. N-glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. FASEB J. 2002;16:983–992. doi: 10.1096/fj.01-0809com. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Mori T. Cysteine-699, a possible palmitoylation site of the thyrotropin receptor, is not crucial for cAMP or phosphoinositide signaling but is necessary for full surface expression. Biochem Biophys Res Commun. 1996;221:636–640. doi: 10.1006/bbrc.1996.0648. [DOI] [PubMed] [Google Scholar]
- Krasel C, Dammeier S, Winstel R, Brockmann J, Mischak H, Lohse MJ. Phosphorylation of GRK2 by protein kinase C abolishes its inhibition by calmodulin. J Biol Chem. 2001;276:1911–1915. doi: 10.1074/jbc.M008773200. [DOI] [PubMed] [Google Scholar]
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Iγ. J Cell Biol. 2003;162:113–124. doi: 10.1083/jcb.200301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaie N, Kralikova M, Prézeau L, Blahos J, Bouvier M. Post-endocytotic deubiquitination and degradation of the metabotropic γ-aminobutyric acid receptor by the ubiquitin-specific protease 14. J Biol Chem. 2016;291:7156–7170. doi: 10.1074/jbc.M115.686907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The β2-adrenergic receptor/βarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J, Mundell SJ, Yun H, Kelly E, Venkateswarlu K. Centaurin-α1, an ADP-ribosylation factor 6 GTPase activating protein, inhibits β2-adrenoceptor internalization. Mol Pharmacol. 2005;67:1822–1828. doi: 10.1124/mol.105.011338. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Pierce KL, Luttrell LM. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol. 2002;62:971–974. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- Lichnerova K, Kaniakova M, Park SP, Skrenkova K, Wang YX, Petralia RS, Suh YH, Horak M. Two N-glycosylation sites in the GluN1 subunit are essential for releasing N-methyl-d-aspartate (NMDA) receptors from the endoplasmic reticulum. J Biol Chem. 2015;290:18379–18390. doi: 10.1074/jbc.M115.656546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder ME, Middleton P, Hepler JR, Taussig R, Gilman AG, Mumby SM. Lipid modifications of G proteins: α subunits are palmitoylated. Proc Natl Acad Sci USA. 1993;90:3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang D, Shi Q, Fu Q, Hizon S, Xiang YK. Palmitoylation regulates intracellular trafficking of β2 adrenergic receptor/arrestin/phosphodiesterase 4D complexes in cardiomyocytes. PLoS ONE. 2012;7:e42658. doi: 10.1371/journal.pone.0042658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G, Schmid SL. Cargo and dynamin regulate clathrin- coated pit maturation. PLoS Biol. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. β-Arrestin: a protein that regulates β-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574–579. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
- Luo JQ, Liu X, Frankel P, Rotunda T, Ramos M, Flom J, Jiang H, Feig LA, Morris AJ, Kahn RA, Foster DA. Functional association between Arf and RalA in active phospholipase D complex. Proc Natl Acad Sci USA. 1998;95:3632–3637. doi: 10.1073/pnas.95.7.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Della Rocca GJ, Lefkowitz RJ. G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J Biol Chem. 1997;272:31648–31656. doi: 10.1074/jbc.272.50.31648. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Luton F, Partisani M, Cherfils J, Chardin P, Franco M. The GDP-bound form of Arf6 is located at the plasma membrane. J Cell Sci. 2004;117:2389–2398. doi: 10.1242/jcs.01090. [DOI] [PubMed] [Google Scholar]
- Marchese A, Chen C, Kim YM, Benovic JL. The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci. 2003;28:369–376. doi: 10.1016/S0968-0004(03)00134-8. [DOI] [PubMed] [Google Scholar]
- Marotti LA, Jr, Newitt R, Wang Y, Aebersold R, Dohlman HG. Direct identification of a G protein ubiquitination site by mass spectrometry. Biochemistry. 2002;41:5067–5074. doi: 10.1021/bi015940q. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Hanai S, Kishi H, Liu Z, Bao Y, Kikuchi A, Tsuchida K, Sugino H. Regulation of endocytosis of activin type II receptors by a novel PDZ protein through Ral/Ral-binding protein 1-dependent pathway. J Biol Chem. 2002;277:19008–19018. doi: 10.1074/jbc.M112472200. [DOI] [PubMed] [Google Scholar]
- McCaffrey PG, Friedman B, Rosner MR. Diacylglycerol modulates binding and phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984;259:12502–12507. [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. β-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8:34–45. doi: 10.1016/S0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154:4293–4304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard L, Ferguson SS, Barak LS, Bertrand L, Premont RT, Colapietro AM, Lefkowitz RJ, Caron MG. Members of the G protein-coupled receptor kinase family that phosphorylate the β2-adrenergic receptor facilitate sequestration. Biochemistry. 1996;35:4155–4160. doi: 10.1021/bi952961+. [DOI] [PubMed] [Google Scholar]
- Min C, Zheng M, Zhang X, Caron MG, Kim KM. Novel roles for β-arrestins in the regulation of pharmacological sequestration to predict agonist-induced desensitization of dopamine D3 receptors. Br J Pharmacol. 2013;170:1112–1129. doi: 10.1111/bph.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C, Zheng M, Zhang X, Guo S, Kwon KJ, Shin CY, Kim HS, Cheon SH, Kim KM. N-linked Glycosylation on the N-terminus of the dopamine D2 and D3 receptors determines receptor association with specific microdomains in the plasma membrane. Biochim. Biophys. Acta. 2015;1853:41–51. doi: 10.1016/j.bbamcr.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Mishra S, Murphy LJ. Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem. 2004;279:23863–23868. doi: 10.1074/jbc.M311919200. [DOI] [PubMed] [Google Scholar]
- Mitchell R, Robertson DN, Holland PJ, Collins D, Lutz EM, Johnson MS. ADP-ribosylation factor-dependent phospholipase D activation by the M3 muscarinic receptor. J Biol Chem. 2003;278:33818–33830. doi: 10.1074/jbc.M305825200. [DOI] [PubMed] [Google Scholar]
- Moffett S, Adam L, Bonin H, Loisel TP, Bouvier M, Mouillac B. Palmitoylated cysteine 341 modulates phosphorylation of the β2-adrenergic receptor by the cAMP-dependent protein kinase. J Biol Chem. 1996;271:21490–21497. doi: 10.1074/jbc.271.35.21490. [DOI] [PubMed] [Google Scholar]
- Moffett S, Mouillac B, Bonin H, Bouvier M. Altered phosphorylation and desensitization patterns of a human β2-adrenergic receptor lacking the palmitoylated Cys341. EMBO J. 1993;12:349–356. doi: 10.1002/j.1460-2075.1993.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller LB, Pöllänen J, Rønne E, Pedersen N, Blasi F. N-linked glycosylation of the ligand-binding domain of the human urokinase receptor contributes to the affinity for its ligand. J Biol Chem. 1993;268:11152–11159. [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Moss J, Vaughan M. Structure and function of ARF proteins: activators of cholera toxin and critical components of intracellular vesicular transport processes. J Biol Chem. 1995;270:12327–12330. doi: 10.1074/jbc.270.21.12327. [DOI] [PubMed] [Google Scholar]
- Mostafapour S, Kobilka BK, von Zastrow M. Pharmacological sequestration of a chimeric β3/β2 adrenergic receptor occurs without a corresponding amount of receptor internalization. Recept Signal Transduct. 1996;6:151–163. [PubMed] [Google Scholar]
- Mott HR, Owen D. Structure and function of RLIP76 (RalBP1): an intersection point between Ras and Rho signalling. Biochem Soc Trans. 2014;42:52–58. doi: 10.1042/BST20130231. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Kleuss C, Gilman AG. Receptor regulation of G-protein palmitoylation. Proc Natl Acad Sci USA. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S, Morinaka K, Koyama S, Ikeda M, Kishida M, Okawa K, Iwamatsu A, Kishida S, Kikuchi A. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 1999;18:3629–3642. doi: 10.1093/emboj/18.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu F, Perera RM, Lucast L, Zoncu R, Domin J, Gertler FB, Toomre D, De Camilli P. The inositol 5-phosphatase SHIP2 regulates endocytic clathrin-coated pit dynamics. J Cell Biol. 2010;190:307–315. doi: 10.1083/jcb.201005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by β-arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/S0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Oddi S, Dainese E, Sandiford S, Fezza F, Lanuti M, Chiurchiù V, Totaro A, Catanzaro G, Barcaroli D, De Laurenzi V, Centonze D, Mukhopadhyay S, Selent J, Howlett AC, Maccarrone M. Effects of palmitoylation of Cys(415) in helix 8 of the CB(1) cannabinoid receptor on membrane localization and signalling. Br J Pharmacol. 2012;165:2635–2651. doi: 10.1111/j.1476-5381.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd BF, Hnatowich M, Caron MG, Lefkowitz RJ, Bouvier M. Palmitoylation of the human β2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989;264:7564–7569. [PubMed] [Google Scholar]
- O’Hayre M, Degese MS, Gutkind JS. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr Opin Cell Biol. 2014;27:126–135. doi: 10.1016/j.ceb.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Ninomiya H, Tanioka M, Sakamoto A, Miwa S, Masaki T. Palmitoylation of human endothelinB. Its critical role in G protein coupling and a differential requirement for the cytoplasmic tail by G protein subtypes. J Biol Chem. 1997;272:21589–21596. doi: 10.1074/jbc.272.34.21589. [DOI] [PubMed] [Google Scholar]
- Olkkonen VM, Stenmark H. Role of Rab GTPases in membrane traffic. Int Rev Cytol. 1997;176:1–85. doi: 10.1016/S0074-7696(08)61608-3. [DOI] [PubMed] [Google Scholar]
- Ong ST, Freeley M, Skubis-Zegadło J, Fazil MH, Kelleher D, Fresser F, Baier G, Verma NK, Long A. Phosphorylation of Rab5a protein by protein kinase C is crucial for T-cell migration. J Biol Chem. 2014;289:19420–19434. doi: 10.1074/jbc.M113.545863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CE, Dell’Angelica EC, Bonifacino JS. ADP-Ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcl L, Palmer DJ, Amherdt M, Rothman JE. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993;364:732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol. 2002;4:929–936. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- Parenti M, Vigano MA, Newman CM, Milligan G, Magee AI. A novel N-terminal motif for palmitoylation of G-protein α subunits. Biochem J. 1993;291:349–353. doi: 10.1042/bj2910349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Pearse BM. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci USA. 1976;73:1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to β 2-adrenergic receptors by β-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: master regulators of membrane trafficking. Curr Opin Cell Biol. 1994;6:522–526. doi: 10.1016/0955-0674(94)90071-X. [DOI] [PubMed] [Google Scholar]
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Maudsley S, Daaka Y, Luttrell LM, Lefkowitz RJ. Role of endocytosis in the activation of the extracellular signal-regulated kinase cascade by sequestering and nonsequestering G protein-coupled receptors. Proc Natl Acad Sci USA. 2000;97:1489–1494. doi: 10.1073/pnas.97.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ. Sequestration and recycling of β2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Poggi M, Kara I, Brunel JM, Landrier JF, Govers R, Bonardo B, Fluhrer R, Haass C, Alessi MC, Peiretti F. Palmitoylation of TNF α is involved in the regulation of TNF receptor 1 signalling. Biochim. Biophys. Acta. 2013;1833:602–612. doi: 10.1016/j.bbamcr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Pollok-Kopp B, Hüttenrauch F, Rethorn S, Oppermann M. Dynamics of protein kinase C-mediated phosphorylation of the complement C5a receptor on serine 334. J Biol Chem. 2007;282:4345–4353. doi: 10.1074/jbc.M601317200. [DOI] [PubMed] [Google Scholar]
- Posor Y, Eichhorn-Grünig M, Haucke V. Phosphoinositides in endocytosis. Biochim. Biophys. Acta. 2015;1851:794–804. doi: 10.1016/j.bbalip.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Qanbar R, Bouvier M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol Ther. 2003;97:1–33. doi: 10.1016/S0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- Rands E, Candelore MR, Cheung AH, Hill WS, Strader CD, Dixon RA. Mutational analysis of β-adrenergic receptor glycosylation. J Biol Chem. 1990;265:10759–10764. [PubMed] [Google Scholar]
- Rankovic M, Jacob L, Rankovic V, Brandenburg LO, Schröder H, Höllt V, Koch T. ADP-ribosylation factor 6 regulates mu-opioid receptor trafficking and signaling via activation of phospholipase D2. Cell Signal. 2009;21:1784–1793. doi: 10.1016/j.cellsig.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Rastogi R, Verma JK, Kapoor A, Langsley G, Mukhopadhyay A. Rab5 Isoforms Specifically Regulate Different Modes of Endocytosis in Leishmania. J Biol Chem. 2016;291:14732–14746. doi: 10.1074/jbc.M116.716514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Bénédetti H, Zanolari B, Riezman H. Identification of a novel sequence mediating regulated endocytosis of the G protein-coupled α-pheromone receptor in yeast. Mol. Biol. Cell. 1993;4:511–521. doi: 10.1091/mbc.4.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer W, Pontani LL, Sorre B, Rentero C, Berland L, Chambon V, Lamaze C, Bassereau P, Sykes C, Gaus K, Johannes L. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell. 2010;140:540–553. doi: 10.1016/j.cell.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Rosenthal W, Antaramian A, Gilbert S, Birnbaumer M. Nephrogenic diabetes insipidus. A V2 vasopressin receptor unable to stimulate adenylyl cyclase. J Biol Chem. 1993;268:13030–13033. [PubMed] [Google Scholar]
- Ross EM. Protein modification. Palmitoylation in G-protein signaling pathways. Curr Biol. 1995;5:107–109. doi: 10.1016/S0960-9822(95)00026-1. [DOI] [PubMed] [Google Scholar]
- Roth BL, Willins DL, Kroeze WK. G protein-coupled receptor (GPCR) trafficking in the central nervous system: relevance for drugs of abuse. Drug Alcohol Depend. 1998;51:73–85. doi: 10.1016/S0376-8716(98)00067-2. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-Z. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S, Petersen OW, van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105:679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schmidlin F, Dery O, DeFea KO, Slice L, Patierno S, Sternini C, Grady EF, Bunnett NW. Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. J Biol Chem. 2001;276:25427–25437. doi: 10.1074/jbc.M101688200. [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Anborgh PH, Ferguson SS. β 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem. 2000;275:27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- Shapiro MJ, Weiss EJ, Faruqi TR, Coughlin SR. Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J Biol Chem. 2000;275:25216–25221. doi: 10.1074/jbc.M004589200. [DOI] [PubMed] [Google Scholar]
- Shen Y, Xu L, Foster DA. Role for phospholipase D in receptor-mediated endocytosis. Mol Cell Biol. 2001;21:595–602. doi: 10.1128/MCB.21.2.595-602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β 2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987;48:913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Lefkowitz RJ. Molecular mechanisms of receptor desensitization using the β-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985;317:124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sikarwar AS, Hinton M, Santhosh KT, Chelikani P, Dakshinamurti S. Palmitoylation of Gαq determines its association with the thromboxane receptor in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2014;50:135–143. doi: 10.1165/rcmb.2013-0085OC. [DOI] [PubMed] [Google Scholar]
- Soto AG, Trejo J. N-linked glycosylation of protease-activated receptor-1 second extracellular loop: a critical determinant for ligand-induced receptor activation and internalization. J Biol Chem. 2010;285:18781–18793. doi: 10.1074/jbc.M110.111088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel JM, Nambi P, Shorr RG, Sawyer DF, Caron MG, Lefkowitz RJ. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the β-adrenergic receptor. Proc Natl Acad Sci USA. 1983;80:3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser RH, Sibley DR, Lefkowitz RJ. A novel catecholamine-activated adenosine cyclic 3′,5′-phosphate independent pathway for β-adrenergic receptor phosphorylation in wild-type and mutant S49 lymphoma cells: mechanism of homologous desensitization of adenylate cyclase. Biochemistry. 1986;25:1371–1377. doi: 10.1021/bi00354a027. [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrincoated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Tanowitz M, Von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J Biol Chem. 2002;277:50219–50222. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- Teis D, Wunderlich W, Huber LA. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell. 2002;3:803–814. doi: 10.1016/S1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- Thompson D, Whistler JL. Dopamine D(3) receptors are down-regulated following heterologous endocytosis by a specific interaction with G protein-coupled receptor-associated sorting protein-1. J Biol Chem. 2011;286:1598–1608. doi: 10.1074/jbc.M110.158345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TM, Friedman J, Qunaibi E, Baameur F, Moore RH, Clark RB. Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the β2-adrenergic receptor using phosphoserine-specific antibodies. Mol Pharmacol. 2004;65:196–206. doi: 10.1124/mol.65.1.196. [DOI] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Timossi C, Damián-Matsumura P, Dias JA. Role of glycosylation in function of follicle-stimulating hormone. Endocrine. 1999;11:205–215. doi: 10.1385/ENDO:11:3:205. [DOI] [PubMed] [Google Scholar]
- van Dam EM, Robinson PJ. Ral: mediator of membrane trafficking. Int J Biochem Cell Biol. 2006;38:1841–1847. doi: 10.1016/j.biocel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery RG, von Zastrow M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Kräusslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146:471–484. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- von Zastrow M. Role of endocytosis in signalling and regulation of G-protein-coupled receptors. Biochem Soc Trans. 2001;29:500–504. doi: 10.1042/bst0290500. [DOI] [PubMed] [Google Scholar]
- Wedegaertner PB, Chu DH, Wilson PT, Levis MJ, Bourne HR. Palmitoylation is required for signaling functions and membrane attachment of Gqα and Gsα. J Biol Chem. 1993;268:25001–25008. [PubMed] [Google Scholar]
- Weir DL, Laing ED, Smith IL, Wang LF, Broder CC. Host cell virus entry mediated by Australian bat lyssavirus G envelope glycoprotein occurs through a clathrin-mediated endocytic pathway that requires actin and Rab5. Virol J. 2014;11:40. doi: 10.1186/1743-422X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Iγ is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/S0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- West MA, Bright NA, Robinson MS. The role of ADP-ribosylation factor and phospholipase D in adaptor recruitment. J Cell Biol. 1997;138:1239–1254. doi: 10.1083/jcb.138.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Enns CA. A region of the C-terminal portion of the human transferrin receptor contains an asparagine-linked glycosylation site critical for receptor structure and function. J Biol Chem. 1993;268:12780–12786. [PubMed] [Google Scholar]
- Winstel R, Freund S, Krasel C, Hoppe E, Lohse MJ. Protein kinase cross-talk: membrane targeting of the β-adrenergic receptor kinase by protein kinase C. Proc Natl Acad Sci USA. 1996;93:2105–2109. doi: 10.1073/pnas.93.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Yu SS, Lefkowitz RJ, Hausdorff WP. β-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem. 1993;268:337–341. [PubMed] [Google Scholar]
- Yun HJ, Kim H, Ga I, Oh H, Ho DH, Kim J, Seo H, Son I, Seol W. An early endosome regulator, Rab5b, is an LRRK2 kinase substrate. J Biochem. 2015;157:485–495. doi: 10.1093/jb/mvv005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Choi BG, Kim KM. Roles of dopamine D2 receptor subregions in interactions with β-Arrestin2. Biomol. Ther. (Seoul) 2016a;24:517–522. doi: 10.4062/biomolther.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Le HT, Zhang X, Zheng M, Choi BG, Kim KM. Palmitoylation on the carboxyl terminus tail is required for the selective regulation of dopamine D2 versus D3 receptors. Biochim. Biophys. Acta. 2016b;1858:2152–2162. doi: 10.1016/j.bbamem.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sun N, Zheng M, Kim KM. Clathrin-mediated endocytosis is responsible for the lysosomal degradation of dopamine D3 receptor. Biochem Biophys Res Commun. 2016c;476:245–251. doi: 10.1016/j.bbrc.2016.05.104. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kim KM. Palmitoylation of the carboxyl-terminal tail of dopamine D4 receptor is required for surface expression, endocytosis, and signaling. Biochem Biophys Res Commun. 2016;479:398–403. doi: 10.1016/j.bbrc.2016.09.094. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Austin SC, Smyth EM. Glycosylation of the human prostacyclin receptor: role in ligand binding and signal transduction. Mol Pharmacol. 2001;60:480–487. [PubMed] [Google Scholar]
- Zheng H, Chu J, Qiu Y, Loh HH, Law PY. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci USA. 2008;105:9421–9426. doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. Posttranslation modification of G protein-coupled receptor in relationship to biased agonism. Meth Enzymol. 2013;522:391–408. doi: 10.1016/B978-0-12-407865-9.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Pearsall EA, Hurst DP, Zhang Y, Chu J, Zhou Y, Reggio PH, Loh HH, Law PY. Palmitoylation and membrane cholesterol stabilize mu-opioid receptor homodimerization and G protein coupling. BMC Cell Biol. 2012;13:6. doi: 10.1186/1471-2121-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Zhang X, Guo S, Zhang X, Choi HJ, Lee MY, Kim KM. PKCβII inhibits the ubiquitination of β-arrestin2 in an autophosphorylation-dependent manner. FEBS Lett. 2015;589:3929–3937. doi: 10.1016/j.febslet.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Zheng M, Zhang X, Guo S, Zhang X, Min C, Cheon SH, Oak MH, Kim YR, Kim KM. Agonist-induced changes in RalA activities allows the prediction of the endocytosis of G protein-coupled receptors. Biochim. Biophys. Acta. 2016;1863:77–90. doi: 10.1016/j.bbamcr.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]