Abstract
Insulin resistance is characterized by the reduced ability of insulin to stimulate tissue uptake and disposal of glucose including cardiac muscle. These conditions accelerate the progression of heart failure and increase cardiovascular morbidity and mortality in patients with cardiovascular diseases. It is noteworthy that some conditions of insulin resistance are characterized by up-regulation of the sympathetic nervous system, resulting in enhanced stimulation of β-adrenergic receptor (βAR). Over-stimulation of βARs leads to the development of heart failure and is associated with the pathogenesis of insulin resistance in the heart. However, pathological consequences of the cross-talk between the βAR and the insulin sensitivity and the mechanism by which βAR over-stimulation promotes insulin resistance remain unclear. This review article examines the hypothesis that βARs over-stimulation leads to induction of insulin resistance in the heart.
Keywords: β-adrenergic receptor, β-blockers, G protein-coupled receptor kinase, Heart diseases, Insulin resistance, Protein kinase A
ROLE OF G PROTEIN-COUPLED RECEPTORS ON INSULIN RESISTANCE
G protein-coupled receptors (GPCRs) are a conserved family of seven transmembrane receptors (Pierce et al., 2002) and are one of the largest receptor classes for drug targeting. β-Adrenergic receptors (βARs) belong to the GPCR family that activates intracellular Gαs protein after binding of catecholamines (Salazar et al., 2007). In the heart, acute stimulation of βARs physiologically augments cardiac contraction, whereas chronic stimulation of βARs promotes adverse cardiac remodeling, leading to cardiomyocyte apoptosis, myocardial hypertrophy, and heart failure (Salazar et al., 2007). In addition, sustained and overstimulation of myocardial βARs trigger a state of insulin resistance in the heart (Mangmool et al., 2016). Insulin resistance is associated with impairments in cardiac function and has been observed in patients with heart failure and dilated cardiomyopathy (Ginsberg, 2000; Shah and Shannon, 2003). Interestingly these conditions are characterized by the dysregulation of the sympathetic nervous system, which results in the up-regulation of βAR. However, a pathological consequence of the relationship between βAR, insulin sensitivity, and the exact mechanisms by which βAR overstimulation leads to impaired insulin activity have not fully described in the heart.
INSULIN RECEPTOR AND ITS SIGNALING
Insulin is a potent anabolic hormone that is synthesized and secreted from the β-cells of the islets of Langerhans in the pancreas, and then circulated throughout the blood stream. Insulin which consists of two polypeptide chains joined by disulfide bonds, play a vital role in cell growth and development, and maintenance of glucose homeostasis (White and Kahn, 1994; De Meyts, 2004). The insulin receptor (IR) belongs to the receptor tyrosine kinase family with intrinsic tyrosine kinase activity.
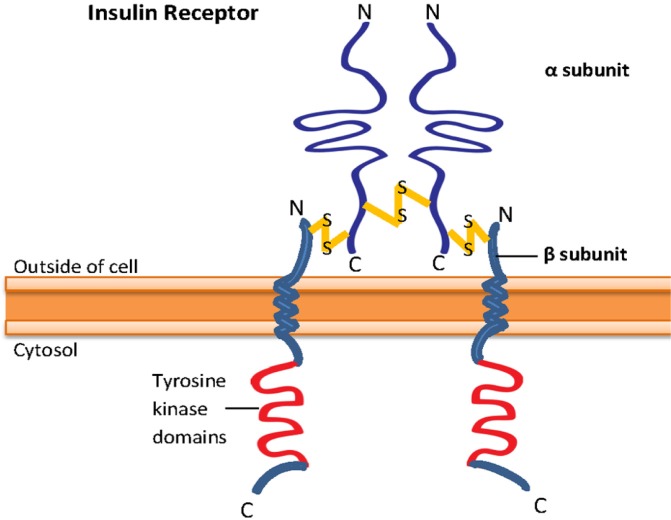
IR is a heterotetrameric receptor composed of two extracellular α subunits and two membrane-spanning β subunits that are disulfide linked into an α2β2 configuration (Fig. 1) (Becker and Roth, 1990; Lee and Pilch, 1994; Bevan, 2001). The α subunits span the extracellular portion that contains the ligand-binding domain of the receptor, while the β subunits span extracellular, transmembrane, and intracellular domains. The intracellular domain of the β subunits, which expresses intrinsic tyrosine kinase activity, is involved in signal transduction (Ullrich et al., 1985; Becker and Roth, 1990; De Meyts, 2004).
Fig. 1.
Structure of insulin receptor. Insulin receptor is a heterotetrameric receptor that contains two α subunits, which is extracellular and has the ligand-binding domain, and two β subunits, which consist of extracellular, transmembrane and intracellular domains. The tyrosine kinase domain of the receptor is present at the intracellular β subunits.
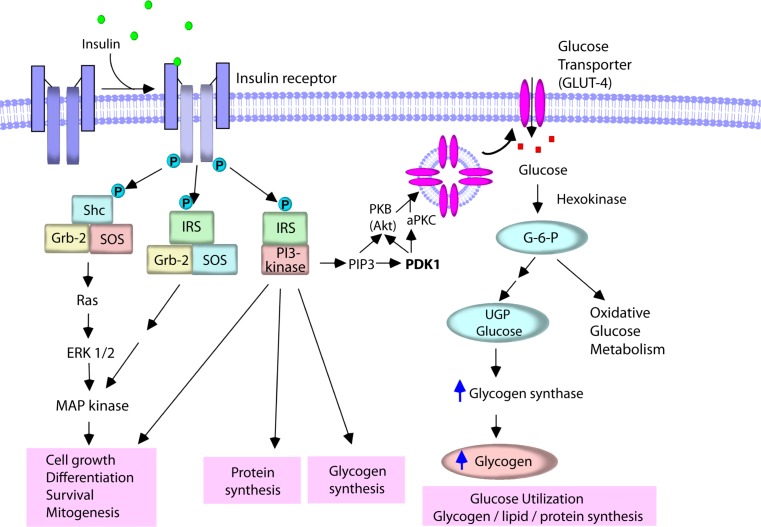
Binding of insulin to the α subunits of the receptor activates the intracellular tyrosine kinase activity of the β subunits, resulting in transphosphorylation of one β subunit by the other on specific tyrosine residues, and then autophosphorylation at specific sites in the intracellular tail of the receptor which is followed by tyrosine phosphorylation of cytoplasmic substrate proteins such as Src-homology-2-containing (Shc) proteins and insulin receptor substrate (IRS) proteins (Becker and Roth, 1990; Lee and Pilch, 1994; Bevan, 2001; Taniguchi et al., 2006). These substrate proteins interact with their effectors that amplify and extend the insulin signaling cascades (Fig. 2). For example, the phosphorylated Shc induces mitogenesis (Sasaoka and Kobayashi, 2000). Phosphorylation of IRS promotes the interaction of IRS with many signaling proteins including growth factor receptor-bound protein-2 (Grb-2) (Myers et al., 1994), Src homology 2 (SH2) domain-containing protein tyrosine phosphatase (SHP-2) (Hayashi et al., 2004), and phosphatidylinositol-3-kinase (PI3K) (Sarbassov and Peterson, 1998) to produce many biological responses. The binding of IRS proteins to Grb-2 leads to the activation of the extracellular regulated kinase-1 and -2 (ERK1/2) which promotes mitogenesis, cell growth, and differentiation.
Fig. 2.
Insulin signaling pathway. Upon binding of insulin to its receptor, a cascade of intracellular events in the cells is initiated. The activated insulin receptor phosphorylates and activates substrate proteins such as Shc and IRS. Phosphorylation of Shc promotes the formation of Shc/Grb-2/SOS complex which stimulates MAP kinase pathway, resulting in mitogenesis, cell growth, and differentiation. Phosphorylated IRS proteins interact with many other signaling proteins including Grb-2 and PI3K, and change cellular function. PI3K catalyzes the formation of PIP3 which, in turn, activates Akt and aPKC, and controls many aspects of insulin action, including protein synthesis, glycogen synthesis, and glucose transport via the translocation of GLUT4 to the plasma membrane. Glucose that enters the cells is rapidly phosphorylated by hexokinase enzymes to generate glucose-6-phosphate (G-6-P), and is subsequently utilized for metabolism and/or stored in the cells as glycogen or TG.
In particular, insulin action is primarily dependent on the activation of PI3K. IRS interacts with PI3K, leading to activate the PI3K activity (Backer et al., 1992). The activated PI3K then generates phosphatidylinositol 3,4,5-trisphosphate (PIP3) from the substrate phosphatidylinositol 4,5-bisphosphate (PIP2) on the plasma membrane, which regulates the activity of several protein effectors, including Akt (known as protein kinase B, PKB) and atypical isoforms of protein kinase C (aPKC) (Lee and Pilch, 1994; Bevan, 2001; Taniguchi et al., 2006) that control many aspects of cellular insulin action, including protein synthesis, glycogen synthesis, and glucose transporter (GLUT) translocation from intracellular to the cell surface and ultimately triggers glucose uptake into the cells (Czech and Corvera, 1999). Activation of Akt leads to an increased glucose transport and persistent translocation of GLUT subtype 4 (GLUT4) to the plasma membrane in adipocytes (Kohn et al., 1996, 1998). In addition, stimulation of PKCζ or PKCλ induces GLUT4 translocation, whereas inhibition of PKCλ suppresses GLUT4 translocation (Kitamura et al., 1998; Kotani et al., 1998). GLUT4 is widely expressed in various tissues such as skeletal muscle, cardiac muscle and adipose tissue that constitute important sites of glucose utilization after food intake (Zhao and Keating, 2007). In the basal condition, most GLUT4 is localized in the intracellular space. Upon stimulation of IRs, GLUT4 is rapidly translocated to the cell surface, where it facilitates inward transport of glucose from the circulation into the cells (Watson and Pessin, 2001). Glucose that enters the cell is rapidly phosphorylated by hexokinase to generate glucose-6-phosphate (G-6-P), and is subsequently used for metabolism or stored in the cell as glycogen or triglyceride (TG). Hexokinase II is found in association with GLUT4 in skeletal and cardiac muscle and in adipose tissue (Postic et al., 1993).
ACTIONS OF INSULIN
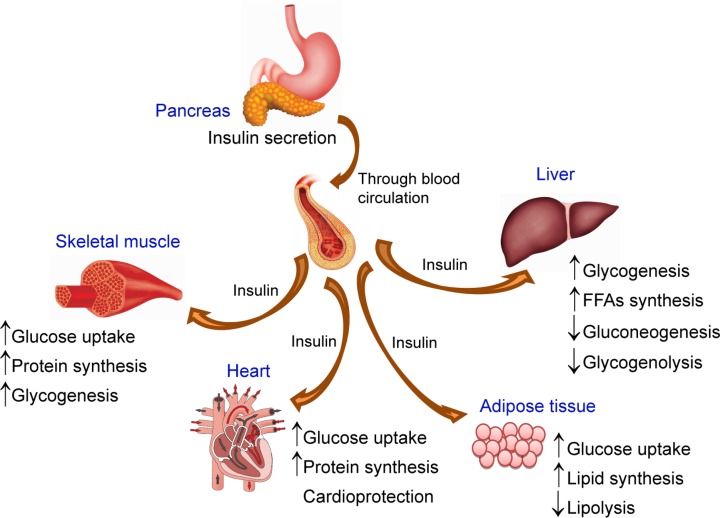
Downstream effects of insulin are initiated through the binding of insulin to the extracellular β subunits of its receptor and transmission of the signal across the plasma membrane that activates several insulin signaling pathway. Although the details linking between the second messengers to the metabolic effects of insulin are still under investigation, the metabolic effects of insulin action are well understood. IR is widely expressed on many cell types, implicating the broad array of biological responses to insulin (Fig. 3). The tissues that are considered important for control of glucose homeostasis are liver, skeletal muscle and adipose tissues. In addition, the brain, pancreatic islets, and cardiac muscle are also main targets for insulin action.
Fig. 3.
Insulin actions on many tissues. Insulin is secreted from the pancreas from the β-cells of the islets of Langerhans and circulated throughout the body via the bloodstream. The released insulin acts on insulin receptors located on the plasma membrane of target tissues (i.e., skeletal muscle, liver, adipose tissue and heart). In skeletal muscle, cardiac muscle, and liver, insulin promotes glucose uptake and storage as glycogen or TG. In adipose tissue, insulin promotes conversion of glucose to TG by increasing lipid synthesis.
The actions of insulin are anabolic, and insulin signaling is critical for promoting the uptake, utilization, and storage of the major nutrients (e.g., glucose, lipids, and amino acids), resulting in glycogenesis, lipogenesis, and protein synthesis. Insulin also inhibits the catabolism of these macromolecules. In the liver, insulin increases glucokinase activity, thereby mediating the phosphorylation and trapping of glucose in hepatocytes. The increased glucose uptake into the hepatocytes accelerates the glycogen synthesis, glycolysis, and fatty acid synthesis. Insulin also reduces hepatic glucose output through the reduction of gluconeogenesis and glycogenolysis. Moreover, insulin suppresses the production and secretion of very-low-density lipoprotein (VLDL) in the liver (Chirieac et al., 2000). In muscle, insulin increases amino acid uptake, stimulates the ribosomal protein synthesis machinery, and promotes glycogen synthase activity and subsequent glycogen storage. In adipose tissue, insulin promotes the expression of lipoprotein lipase, which hydrolyzes TG from circulating lipoproteins for uptake into fat cells. In addition, insulin inhibits the release of free fatty acids (FFAs) from adipose tissue, increases TG and FFAs synthesis and also trigger glucose uptake into adipose tissue (Eckel et al., 2005).
Furthermore, insulin has anti-atherogenic actions in blood vessels (Eckel et al., 2005). Insulin enhances production of the vasodilator nitric oxide (NO) (Montagnani et al., 2002), and exerts anti-inflammatory and anti-oxidative stress effects (Yu et al., 2011). Insulin also inhibits platelet aggregation (Trovati and Anfossi, 1998) and plasminogen activator inhibitor type 1 (PAI-1) activity (Juhan-Vague et al., 1996). Furthermore, insulin has been suggested to be a growth factor that induces vascular cell growth and synthesis of some matrix proteins (Feener and King, 1997; McFarlane et al., 2001).
INSULIN RESISTANCE
Insulin resistance is presented as an insulin function deficiency whereby normal circulating concentrations of insulin are insufficient to induce signals regulating normal glucose absorption and glucose homeostasis of tissues and organs (Jellinger, 2007). Thus, insulin resistance is a defect in insulin action. Insulin resistance is a key feature of type 2 diabetes and metabolic syndrome, and contributes to abnormalities in many tissues (Table 1). Insulin resistance in muscle and fat is generally marked by a decrease in glucose clearance from the circulation. Insulin resistance in the liver generally refers to a blunted ability of insulin to suppress glucose production, leading to an overproduction of glucose through gluconeogenesis and glycogenolysis, which contribute to hyperglycemia and development of diabetes (Matthaei et al., 2000; DeFronzo, 2004). In adipocytes, insulin resistance causes increased rates of lipolysis resulting in excessive breakdown of stored TG into FFAs, which is responsible for hyperlipidemia in the obese state (Boden and Shulman, 2002). In skeletal muscle, insulin resistance causes the reduction in both glucose transport and glycogen synthesis, which leads to inhibit glucose clearance (DeFronzo and Tripathy, 2009). At the cellular level, insulin resistance involves blunted steps in the signaling cascade from the IR tyrosine kinase to translocation of GLUT4, but the molecular mechanisms are incompletely defined.
Table 1.
The biological actions of insulin and insulin resistance in many tissues/organs
| Tissues/organs | Insulin actions | Insulin resistance |
|---|---|---|
| Liver | Increase glycogen synthesis1,2 Decrease hepatic glucose production1,2 Inhibit glycogenolysis1,2 Increase fatty acids synthesis2,3 Suppress the production of very-low-density lipoprotein (VLDL)4,5,6 |
Decrease glycogen synthesis and glycogen storage4,5 Increase glucose production4,5 Increase glycogenolysis6 |
| Skeletal muscle | Increase amino acid uptake and protein synthesis2 Increase glycogenesis2 Increase glucose uptake by stimulation of GLUT4 translocation4,7 |
Impair insulin-stimulated glucose uptake8,9 Impair GLUT4 translocation6,9 |
| Adipose tissue | Increase TG and fatty acids synthesis3 Inhibit lipolysis and the release of free fatty acids (FFAs) from adipose tissue3,4,6 Increase glucose uptake by stimulation of GLUT4 translocation4,7 |
Impair glucose uptake by altering GLUT4 translocation10,11 Decrease responsiveness of adipose tissue to normal levels of insulin to increase lipid synthesis and inhibit lipolysis6 Increase intracellular hydrolysis of TG and release of FFAs12,13 |
| Cardiac muscle | Increase protein synthesis4 Increase glucose uptake14,15 Elicit cardioprotective actions16,17 |
Impair glucose uptake14,15 Increase FFAs metabolism18 Impair cardiac functions19,20,21 |
Insulin resistance is associated with inflammation, oxidative stress, cardiac remodeling, and endothelial dysfunction that results in diminished endothelial NO synthase (eNOS) expression leading to an increase in vascular tone (Scherrer et al., 1994; Kuboki et al., 2000). Insulin resistance leading to hyperinsulinemia and hyperglycemia is a predictor and/or marker of cardiovascular diseases. Insulin resistance is a hallmark in diabetic patients (Olefsky et al., 1973) and is associated with hypertension (Reaven, 1991), obesity (Mingrone et al., 1997), heart failure (Swan et al., 1994, 1997), and dilated cardiomyopathy (Shah and Shannon, 2003). In non-ischemic heart failure patients, the prevalence of insulin resistance highly contributes to heart failure development (Witteles and Fowler, 2008).
A previous study reported that fatty acids compete with glucose for substrate oxidation in isolated rat heart muscle and rat diaphragm muscle (Randle et al., 1963). They speculated that an increase in fat oxidation might be responsible for insulin resistance in obese states. Moreover, lipid accumulation within skeletal muscle and liver inhibits tyrosine phosphorylation of IRS-1, resulting in blockade of IRS-1/PI3K interaction which, in turn, blunts PI3K activity (Savage et al., 2005). Therefore, there is an association between insulin resistance found in type2 diabetes and the development of heart disease. It is believed that the relationship between heart disease and diabetes is bidirectional whereby heart disease induces insulin resistance, and vice versa.
INSULIN RESISTANCE CAUSES HEART DISEASE
The relationship between diabetes and heart disease are well established. Diabetic patients who are diagnosed at younger ages are at much greater risk for cardiovascular diseases (Hillier and Pedula, 2003). The incidence rate of congestive heart failure in diabetic patients is much greater than patients without diabetes (Nichols et al., 2004), emphasizing the demand of early diagnosis and an intensive treatment of diabetes for preventing the progression of cardiac dysfunction and heart disease. Alström syndrome is a rare autosomal recessively inherited disorder that affects many organs. In patients with Alström syndrome, metabolic disturbances begin in childhood including severe insulin resistance, hyperinsulinemia and type 2 diabetes. These abnormalities result in a potentially fatal dilated cardiomyopathy and congestive heart failure (Marshall et al., 2005). Studies using isolated perfused heart preparations and cardiac myocytes have demonstrated insulin resistance in both human and animal models of the diabetic heart (Ohtake et al., 1995; Kolter et al., 1997). Insulin resistance develops in the hearts of mice as early as after high-fat feeding that involves reduction in glucose uptake and GLUT4 expression in the heart (Park et al., 2005). A condition of insulin resistance and dysregulation of glucose metabolism might induce pathological conditions such as systolic/diastolic dysfunction and cardiac remodeling (Park et al., 2005). Moreover, cardiac insulin resistance reduces the metabolic efficiency of the heart, which can lead to contractile dysfunction (Belke et al., 2000; Mazumder et al., 2004). In addition, impaired myocardial insulin signaling promotes oxidative stress and mitochondrial dysfunction in the heart (Boudina et al., 2009). Thus, a condition involving insulin resistance and the dysregulation of glucose metabolism might induce other pathological conditions, such as systolic/diastolic dysfunction and cardiac remodeling. Despite the importance of insulin resistance in the diabetic heart, the molecular mechanisms by which insulin resistance develops in the heart are not fully understood.
Heart failure is twice as high in diabetic men while five times as high in diabetic women. Furthermore, the prevalence of heart failure in elderly diabetic patients is high as 39% (Bell, 2003). The cardiomyopathic heart in the setting of insulin resistance is the worst scenario for energy metabolism, as insulin resistance causes down-regulation of genes regulating glucose homeostasis and metabolism that consequently leads to a state of energy starvation, which promotes further deterioration in cardiac functions (Witteles and Fowler, 2008). The reduction of GLUT4 expression in the myocardium can be found in patients who have insulin resistance with concomitant cardiac hypertrophy (Paternostro et al., 1999). Furthermore, reduction in GLUT4 and increase in FFAs concentrations have also been found in ischemic patients with mild chronic heart failure (Murray et al., 2004). Moreover, insulin resistance often precedes the development of heart disease due to the altered metabolic environment (e.g., altered ATP generation, increased FFA oxidation, and downregulation of genes for glucose oxidation). These abnormalities result in myocardial dysfunction and HF (Heck and Dutka, 2009). However, the pathophysiological consequences of insulin resistance to cause heart disease are incompletely understood.
HEART DISEASE INDUCES INSULIN RESISTANCE
The evidence for insulin resistance causing heart failure is more extensive than the reverse. Patients with coronary artery disease, heart failure, or a history of myocardial infarction are insulin resistant (Paternostro et al., 1996). Patients with severe heart failure have been reported to exhibit insulin resistance, and the degree of insulin resistance has been associated with the severity of heart failure (Swan et al., 1997). The mechanisms underlying the worsening of insulin resistance in patients with heart failure are not quite understood. The possible mechanisms include sympathetic overactivation, endothelial dysfunction, and increased circulating cytokines such as TNF-α (Coats and Anker, 2000; Heck and Dutka, 2009). The evidences that support the mechanisms for heart disease causing insulin resistance include sympathetic overactivation such as increased catecholamine secretion through βAR. Over-stimulation of βAR leads to the inhibition of insulin-stimulated glucose uptake in cardiomyocytes (Mangmool et al., 2016). It has been proposed that infarcted rat hearts have reduced rates of insulin-induced glucose uptake and GLUT4 protein levels (Murray et al., 2006). In addition, cardiac glucose uptake is defective after myocardial ischemia and after chronic βAR stimulation (Ciccarelli et al., 2011). Moreover, decreased GLUT4 expression has been observed in an animal model of cardiac hypertrophy (Paternostro et al., 1995). Increased nor-epinephrine concentration is found in heart failure (Thomas and Marks, 1978). Norepinephrine can cause the elevation of FFA through the stimulation of lipolysis (Paolisso et al., 1991). In addition, an increase of plasma FFA levels stimulates cardiac sympathetic activity (Paolisso et al., 2000) and adversely affects insulin signaling, thereby reducing glucose utilization by cardiac muscle. In the same way, the detrimental metabolic effects following sympathetic overstimulation extend further inhibition of pancreatic insulin secretion and increased glucose production, both of which worsen hyperglycemia (Nonogaki, 2000). Currently, the interrelationship between insulin resistance and heart disease has been an interest topic. Although many questions remain with regard to the relationship between insulin resistance and heart disease, a number of exciting future research and trial promise to contribute to the amelioration of heart disease prognosis.
PROLONGED STIMULATION OF βAR INDUCES CARDIAC INSULIN RESISTANCE
Previous studies have reported that up-regulation of βAR plays an important role in the pathogenesis of insulin resistance in several tissues, especially the heart (Morisco et al., 2005, 2007). Insulin resistance might be associated with sustained βAR stimulation and excessive sympathetic nerve activity leading to structural and functional abnormalities in the heart. Acute βAR stimulation physiologically augments cardiac contraction, whereas chronic stimulation of βAR promotes adverse cardiac remodeling leading to cardiac myocyte apoptosis, myocardial hypertrophy and heart failure (Rockman et al., 2002). In isolated perfused rat hearts, decreased insulin sensitivity was observed following isoproterenol (ISO) infusion and was accompanied by decreased GLUT4 levels (Heather et al., 2009). This study demonstrated that ISO infusion impaired in vivo cardiac functions, induced hypertrophy and decreased both fatty acid and glucose metabolism, which are similar to the alterations observed in animal models of myocardial infarction (Heather et al., 2009). In addition, sustained and overstimulation of myocardial βAR trigger a state of insulin resistance in cardiomyocytes (Morisco et al., 2005) and in heart tissue (Mangmool et al., 2016). Insulin resistance can be assessed by uptake of glucose, GLUT expression and translocation, insulin receptor-beta (IR-β) expression, and IRS-1 expression and phosphorylation. In our previous study (Mangmool et al., 2016), we reported that sustained βAR stimulation induces cardiac insulin resistance through attenuation of glucose uptake and inhibition of GLUT4 synthesis in the heart.
Stimulation of βARs has a biphasic effect on insulin-induced glucose uptake in cardiomyocytes (Morisco et al., 2005). Rapid stimulation of βAR (within minutes) has synergistic effects on insulin-induced glucose uptake. This effect of βAR is mediated by phosphorylation of Akt at threonine 308 (Thr308) through protein kinase A (PKA)/Ca2+-dependent and PI3K-independent pathway, whereas insulin-induced Thr phosphorylation of Akt exclusively depends on PI3K (Morisco et al., 2005). On the other hand, chronic stimulation of βAR inhibits both insulin-induced glucose uptake and autophosphorylation of the IRs which is mediated by serine 473 phosphorylation of Akt through PKA/Ca2+ and PI3K-dependent pathways (Morisco et al., 2005).
Catecholamines (i.e., epinephrine, norepinephrine) including glucagon, cortisol and growth hormone are up-regulated in patients with heart failure and likely play a role in the development of insulin resistance and alteration of glucose homeostasis (Lager, 1991; Nikolaidis et al., 2004). Up-regulation of sympathetic nervous system (e.g., elevation of catecholamines) activity not only enhances the development of insulin resistance but also directly contributes to the progression of heart failure. Elevation of catecholamine levels also stimulates lipolysis, increasing circulating FFA levels and exacerbating insulin resistance (Nonogaki, 2000). Treatment with carvedilol (βAR antagonist) could decrease the utilization of FFA with improved myocardial efficiency (Wallhaus et al., 2001; Nikolaidis et al., 2006).
In addition, insulin-induced glucose uptake might be suppressed by epinephrine, norepinephrine, or isoproterenol (ISO) in adipocytes. This inhibition was characterized by stimulation of several subtypes of βARs (Klein et al., 1999). Stimulation of βARs with epinephrine or norepinephrine reduced the amount of plasma membrane GLUT4 by inhibiting insulin-induced GLUT4 translocation in adipocytes. In the same way, stimulation of βAR in brown adipose tissue has the similar effects (Klein et al., 1999). The inhibitory effect of catecholamines on insulin-induced glucose uptake has also been observed in rat skeletal muscle (Chiasson et al., 1981; Lee et al., 1997).
The above results indicate that alterations of glucose homeostasis and insulin resistance are often associated with pathological conditions characterized by excessive activation of sympathetic nervous system and sustained stimulation of βARs. (Deibert and DeFronzo, 1980; Morisco et al., 2005; Cipolletta et al., 2009). However, the exact molecular mechanism by which βAR mediates cardiac insulin resistance and changes in its signaling have not been fully elucidated.
A few studies have demonstrated that βAR-mediated insulin resistance may be induced through cAMP- and PKA-dependent signaling pathways. For example, cAMP analogs inhibited insulin-stimulated glucose uptake in 3T3-L1 adipocytes and rat adipocytes (Kashiwagi et al., 1983; van Putten and Krans, 1985). Moreover, the activation of adenylyl cyclase (AC) by forskolin prevented insulin-stimulated glucose transport in rat adipocytes (Joost and Steinfelder, 1987). In 3T3-L1 adipocytes, treatment with either forskolin or 8-bromo-cAMP resulted in the down-regulation of GLUT4 mRNA (Kaestner et al., 1991). Similarly, blockade of AC abolishes the inhibitory effect of ISO on insulin-stimulated glucose uptake and GLUT4 mRNA expression in rat cardiomyocytes (Mangmool et al., 2016). Therefore, cAMP appears to be an important mediator of βAR-mediated insulin resistance in the heart. Cellular responses to cAMP might be associated with many cAMP effectors, including PKA and a cAMP-regulated guanine nucleotide exchange factor (Epac). Therefore, the divergent cellular responses induced by cAMP and their compartmentalization remain to be defined.
After agonist binding, the βAR can couple with the α subunit of heterotrimeric G protein (Gαs), which results in activation of AC, followed by elevation of cAMP levels (Pierce et al., 2002). There are at least two pathways induced by cAMP, a PKA-dependent and PKA-independent pathway including Epac (Bos, 2006; Oestreich et al., 2007). Chronic stimulation of βAR inhibited both insulin-induced glucose uptake and the autophosphorylation of IRs, which is mediated by PKA/Ca2+ and PI3K-dependent pathways (Morisco et al., 2005). In brown adipocytes, insulin-induced glucose uptake was inhibited by prestimulation of β3ARs (Klein et al., 1999). This effect was mediated via a PKA-dependent signaling pathway. In cardiomyocytes, sustained βAR stimulation-mediated inhibition of insulin-induced glucose uptake and GLUT4 expression was not affected by Epac depletion or inhibition of its activity, whereas blockade of either AC or PKA activity strongly inhibited these effects (Mangmool et al., 2016). Thus, the cAMP/PKA-dependent signaling pathway plays a major role in the pathogenesis of insulin resistance that is associated with an overactive sympathetic system in the heart.
Moreover, βAR stimulation impairs insulin signaling mechanism through an Akt-dependent pathway in the heart, demonstrating that Akt crucially contributes to the regulation of insulin sensitivity and plays a key role in βAR stimulated insulin resistance in cardiomyocytes (Morisco et al., 2005). There is therefore ample evidence to link insulin resistance to up-regulation of the sympathetic nervous system.
SUBTYPE SPECIFICITY OF βARS ON CARDIAC INSULIN RESISTANCE
Insulin resistance is an underlying common feature of heart disease, probably consequent to chronic βAR stimulation (Morisco et al., 2005, 2006). Although all β1-, β2- and β3-ARs can couple to the Gs protein and stimulate AC, to generate the second messenger cAMP, there are considerable differences in their ability to activate downstream signaling pathways. For instance, the CaMKII-dependent induction of fetal genes and apoptotic pathways in cardiac myocytes is specifically mediated by β1ARs, but not β2ARs (Zhu et al., 2003; Sucharov et al., 2006). In contrast, stimulation of β2ARs activates cell survival signals, whereas β1ARs elicit apoptotic pathways in cardiac myocytes (Communal et al., 1999).
The β2AR is widely expressed in several tissues including the vasculature, liver, skeletal muscle, adipocytes, and cardiac muscle, and therefore participates in cardiac function and body metabolism. Indeed, epidemiological studies have shown an association of β2AR genetic polymorphism with obesity, diabetes, and hyperlipidemia (Ishiyama-Shigemoto et al., 1999; Yamada et al., 1999; Iaccarino et al., 2005b). βAR (but not αAR) stimulation attenuates insulin-induced glucose uptake by inhibiting GLUT4 translocation to the plasma membrane in 3T3-L1 adipocytes (Mulder et al., 2005). This inhibitory effect on GLUT4 translocation is mediated at least by the β2- and β3-ARs. The insulin-induced tyrosine phosphorylation of IRs, IRS-1, and IRS-2 was reduced by stimulating β3ARs in brown adipose tissue (Klein et al., 1999). In addition, insulininduced glucose uptake was completely blocked by stimulating β3ARs (Klein et al., 1999). In HEK-293 cells that stably overexpressed β2AR, chronic β2AR stimulation resulted in impaired insulin-induced glucose uptake and IRS-1 phosphorylation (Cipolletta et al., 2009). Moreover, overstimulation of β2ARs inhibited insulin-induced GLUT4 translocation in β2AR-overexpressing HEK-293 cells and rat cardiomyocytes (Mangmool et al., 2016). Thus, β2AR is considered as the βAR subtype that interferes with insulin-mediated GLUT4 translocation in the heart. Moreover, the reduction of insulin-induced autophosphorylation of IRs in response to chronic βAR stimulation was associated with Thr phosphorylation of the β-subunit of IRs (Morisco et al., 2005). In rat cardiomyocytes, ISO-induced phosphorylation of the β-subunit of IRs on Thr residues was mediated by the β1AR subtype (Morisco et al., 2005). In contrast, our recent study showed that overstimulation of β2AR significantly reduced the ability of insulin to induce glucose uptake and the translocation of GLUT4 in cardiomyocytes and heart tissue (Mangmool et al., 2016). That previous study showed that ISO-induced phosphorylation of IR on Thr residues was inhibited by propranolol (a non-selective β-blocker) and betaxolol (a selective β1AR antagonist), whereas pretreatment with ICI118,551 (a specific β2AR antagonist) did not affect this response. These results suggest that β1AR selectively mediates Thr phosphorylation of IR after long term stimulation by ISO in rat neonatal cardiomyocytes (Morisco et al., 2005). Nonetheless, the mechanisms by which β1AR stimulation inhibits the insulin-mediated phosphorylation of IRs, and stimulation of β2AR suppresses insulin-induced glucose uptake and GLUT4 translocation in cardiomyocytes have not been fully resolved.
Even though β1AR and β2AR share 54% sequence identity, the C-terminus (CT) tail of these two subtypes play different roles in receptor endocytosis and signaling pathways. The binding affinity of β-arrestin1 and 2 to the CT tail of the β1AR is lower than that for the β2AR (Shiina et al., 2000). A chimeric β2AR containing the C-tail region of β1AR loses its ability to promote β-arrestin2-mediated ERK nuclear translocation (Kobayashi et al., 2005). In addition, β1ARs and β2ARs have different PDZ-binding motifs within their CT tail leading to the recruitment of unique regulatory proteins when stimulated by their agonists. For example, the Na+/H+ exchanger regulatory factor (NHERF) binds to a DSLL motif in the CT tail of β2ARs to stimulate Na+/H+ exchange (Hall et al., 1998), whereas N-methyl-D-aspartate receptor binds to an ESKV motif within the CT of β1ARs and promotes receptor internalization (Hu et al., 2000). The CT tail of β1AR, but not β2AR, mediates a unique conformation of β-arrestin that allows scaffolding of Epac and CaMKII to form a stable complex, leading to the subsequent cAMP/Epac-mediated activation of CaMKII (Yoo et al., 2009; Mangmool et al., 2010). It is still unknown how the CT tail of βARs (both β1- and β2-AR) regulates βAR-mediated insulin resistance in the heart.
THE ROLE OF GRK ON CARDIAC INSULIN RESISTANCE
βARs belong to the GPCR family. Agonist binding of βAR leads to a dissociation of the heterotrimeric G protein into Gα and Gβγ subunits, both of which activate several effectors (Pierce et al., 2002). In parallel, agonist stimulation also triggers the termination of GPCR signals with rapid attenuation of receptor responsiveness, termed as receptor desensitization. This process is initiated by GRKs that specifically bind to and phosphorylate the agonist-occupied receptors (Benovic et al., 1986). GRK-mediated phosphorylation promotes the binding of β-arrestins to the receptors, and β-arrestins sterically inhibit further interactions of the receptors with G proteins (Ferguson et al., 1996). The β-arrestin-bound receptors are then internalized from the plasma membrane via clathrin-coated vesicles (Krupnick and Benovic, 1998). Overexpression of GRK enhances the βAR-mediated β-arrestin translocation to the plasma membrane and βAR internalization (Mangmool et al., 2006).
Based on sequence and structural homology, the members of the GRK family can be subdivided into the following three groups: rhodopsin kinase subfamily (GRK1 and GRK7); βARK subfamily (GRK2 and GRK3); and GRK4 subfamily (GRK4, GRK5, and GRK6) (Penn et al., 2000). GRKs are composed of three distinct domains as follows: (1) a highly conserved centrally located catalytic domain flanked by (2) an amino-terminal domain that includes a region of homology to the regulator of G protein signaling (RGS) protein and (3) a carboxyl-terminal domain of various lengths (Penela et al., 2003).
GRK expression and activity has been found to be altered in heart disease. It has been shown that GRK2 synthesis and its activity are increased in the failing heart with consequent decrease in β1AR levels (Ungerer et al., 1993). Increased GRK2 expression and activity was found in cardiomyocytes from patients with heart failure (Ungerer et al., 1994). Moreover, overexpression of GRK2 reduces the response to βAR stimulation as a result of increased receptor desensitization (Koch et al., 1995). Inhibition of GRK2 activity enhanced cardiac contractility and βAR responsiveness (Koch et al., 1995; Akhter et al., 1998), confirming the essential role of GRK2 in mediating βAR desensitization. Beyond its classical function on receptor desensitization, GRK2 mediates the inhibitory effects of βAR on insulin signaling (Usui et al., 2004b; Cipolletta et al., 2009), through mechanisms that are still subject to debate. In animal models of diabetes (both Zucker diabetic fatty rats and db/db mice), inhibition of GRK2/3 through synthetic peptides (derived from a kinase-substrate interaction site in GRK2/3) rescues glucose tolerance and enhances insulin sensitivity (Anis et al., 2004), suggesting that GRK2 phosphorylates an unknown substrate to induce impaired glucose tolerance. It is interesting to note that GRKs are ubiquitously expressed in mammalian tissues, and their activities and production are increased in conditions characterized by chronic adrenergic activation, such as human congestive heart failure (Iaccarino et al., 2005a) and hypertension (Izzo et al., 2008). For example, GRK2 is up-regulated through chronic βAR activation (Iaccarino et al., 2001). All the above considerations support the hypothesis that GRK2 is involved in the progression of insulin resistance induced by chronic βAR activation.
Endothelin-1 (ET-1) treatment leads to an enhanced IRS-1 degradation, a decreased tyrosine phosphorylation of IRS-1 and a decreased insulin-stimulated glucose transport in 3T3-L1 adipocytes (Ishibashi et al., 2001). Insulin-induced GLUT4 translocation was inhibited by pretreatment with ET-1 for 24 h. This inhibitory effect was rescued by microinjection of anti-GRK2 antibody or GRK2 short interfering RNA (Usui et al., 2005), suggesting a potential role of GRK2 on insulin resistance. Overexpression of kinase dead GRK2 mutant, but not wild-type GRK2, inhibited ET-1-induced serine 612 phosphorylation of IRS-1 and restored activation of this pathway. Moreover, overexpression of the kinase dead GRK2 mutant suppressed ET-1-induced IRS-1 degradation, whereas wild-type-GRK2 did not, demonstrating a potential role for GRK2 kinase activity in this mechanism. Inhibition of GRK2 could also rescue the ET-1-induced insulin resistance (Usui et al., 2005). Taken together, these results suggest that GRK2 mediates ET-1-induced insulin resistance by its kinase activity on IRS-1 serine phosphorylation and degradation.
Treatment with insulin causes tyrosine phosphorylation of IRS-1 and induces glucose uptake in human kidney embryonic (HEK-293) cells (Cipolletta et al., 2009). Overexpression of β2AR increases GRK2 expression that is associated with significant deficit of IRS1 activation and glucose uptake by insulin (Cipolletta et al., 2009). Similarly, overexpression of GRK2 prevents insulin-induced tyrosine phosphorylation of IRS1 and insulin-induced glucose uptake. This study also found that GRK2 interacts with IRS1, but not IR (Cipolletta et al., 2009). These results provide a regulatory network between insulin signaling and GRK2 in which GRK2 kinase activity mediates βAR-induced insulin resistance and that inhibition of GRK2 activity leads to enhanced insulin sensitivity both in cell and animal models of insulin resistance.
Chronic stimulation of βAR signaling or marked over-expression of β2ARs causes the development of insulin resistance through an increase in GRK2 expression levels. GRK2 may function as a key negative regulator of insulin responsiveness. In endothelial cells, insulin stimulates a signaling pathway which leads to elevation of NO through the activation of endothelial NO synthase (eNOS) and subsequently promotes vasodilation (Kuboki et al., 2000). A recent study showed that the interaction between GRK2 and Akt inhibited the phosphorylation of Akt on Thr 308, resulting in decreased eNOS phosphorylation and a consequent reduction of NO production (Taguchi et al., 2012). However, the precise mechanism for GRK2 binding to IR and formation of the complex with IR and/or Akt remains to be elucidated. Recently, Luan et al. (2009) reported that insulin stimulates the formation of a new β-arrestin2 signal complex in which β-arrestin2 acts as a scaffold for the translocation of Akt to IR, even though insulin is not a GPCR. In contrast, Taguchi et al. (2012) demonstrated that up-regulation of GRK2 and a decrease in β-arrestin2 inhibited insulin-induced stimulation of Akt/eNOS signaling and that GRK2 overactivation may be induced by an increase in PKC activity in aortas from diabetic mice with hyperinsulinemia. In the normal aorta, β-arrestin2 binds to Akt under insulin stimulation. In contrast, insulin causes translocation of GRK2 to the plasma membrane in diabetes, where it binds to Akt and prevents β-arrestin2 from binding to Akt as GRK2 remains bound (Taguchi et al., 2012). As mention above, previous studies have described a relationship between insulin resistance and increased GRK2 levels in pathological conditions. GRK2 inhibition can improve glucose uptake and insulin resistance in the heart (Ciccarelli et al., 2011). Thus, GRK2 inhibitor (e.g., βARKct; C-terminus of βAR kinase) could be used for the possible treatment of insulin resistance in the heart.
THE ROLE OF β-ARRESTINS ON INSULIN RESISTANCE
β-Arrestins are key regulators of βAR (G protein-coupled receptor) endocytosis and trafficking, and G protein-independent signaling. β-Arrestins interact with agonist-occupied and phosphorylated receptors and inhibit further G protein activation (Ferguson et al., 1996). Beyond their classical function, β-arrestins also function as scaffolding proteins linking receptors to several effectors such as ERK1/2, JNK, Src and Mdm2, an ubiquitin ligase (Lefkowitz and Shenoy, 2005; Lefkowitz et al., 2006). The scaffolding of Ca2+/calmodulin kinase II (CaMKII) and Epac by β-arrestin with subsequent translocation of this multimeric complex to agonist-occupied β1ARs is an essential mechanism for β1AR-mediated CaMKII activation (Mangmool et al., 2010).
Insulin resistance is a central feature of type 2 diabetes and is caused by a deficiency in IR signaling. Luan et al. (2009) reported that the amount of β-arrestin2 was decreased either in a mouse model of type 2 diabetes (db/db) or in liver samples from diabetic patients. Deletion of β-arrestin2 induces insulin resistance, whereas overexpression of β-arrestin2 restores insulin sensitivity (Luan et al., 2009). They also found that β-arrestin 2 forms the complex with Akt and Src after insulin stimulation which is consistent with previous studies reporting that β-arrestin interacts with Akt (Beaulieu et al., 2005) and Src (Luttrell et al., 1999). This association between Src and Akt was reduced in liver samples from β-arrestin 2 KO mice, leading to the disturbance of insulin signaling and development of insulin resistance. In addition, insulin-stimulated phosphorylation of Akt, GSK3β and Foxo1 were reduced in livers of β-arrestin2 KO mice while increased in β-arrestin2 transgenic mice (Luan et al., 2009), emphasizing that β-arrrestin2 controls the insulin signaling pathway.
In addition to β-arrestin2, β-arrestin1 also plays a role in insulin signaling and insulin resistance. Overexpression of β-arrestin-1 attenuated insulin-induced IRS-1 degradation, leading to enhanced insulin signaling downstream of IRS-1. Whereas insulin-induced degradation of IRS-1 was increased in cells treated with β-arrestin1 siRNA, stimulation of IR promotes the formation of a complex between IRS-1 and Mdm2, an E3 ubiquitin ligase enzyme, and this complex could be inhibited by β-arrestin1, resulting in a decreased ubiquitin content of IRS-1 (Usui et al., 2004a). From these results, they postulated that both β-arrestin1 and IRS-1 competitively bind to Mdm2, which can ubiquitinate IRS-1. Moreover, dephosphorylation of S412 on β-arrestin and the amino terminus of β-arrestin1 are required for this effect of β-arrestin on IRS-1 degradation (Usui et al., 2004a). Inhibition of β-arrestin1 leads to enhanced IRS-1 degradation and accentuates cellular insulin resistance.
TREATMENT WITH β-BLOCKERS IMPROVES βAR-MEDIATED CARDIAC INSULIN RESISTANCE
β-Blockers are widely used to treat heart failure, angina and myocardial infarction. They act by blocking the over-stimulation of catecholamine to βAR in the heart (Bangalore et al., 2007). During heart failure, a large amount of catecholamine is released from synaptic ends and this exerts harmful effects on the heart. Considering that βAR mediates cardiac insulin resistance as shown in previous studies (Morisco et al., 2005; Ciccarelli et al., 2011; Mangmool et al., 2016), β-blockers are expected to exert beneficial effects by suppressing insulin resistance in the heart.
Propranolol and metoprolol were able to abolish ISO inhibition of insulin-induced glucose uptake, GLUT4 expression, and GLUT4 translocation, whereas atenolol had no effect. The differential potency of these β-blockers might be due to differences in their affinity to βAR subtypes. We show that ISO-mediated cardiac insulin resistance involves β2AR; hence, a β-blocker that has a higher affinity for β2AR could potentially exert an increased beneficial effect. Propranolol was found to antagonize both the β1AR and β2AR subtypes, while atenolol and metoprolol displayed a selectivity for β1AR (Hoffmann et al., 2004). Previous studies have investigated the Ki values by βAR binding assay and showed that various β-blockers have different Ki values for human β2AR: propranolol (0.8 nM), metoprolol (2,960 nM), and atenolol (8,140 nM) (Hoffmann et al., 2004). Moreover, the offset in activity induced by β-blockers in the heart is related to their lipophilicity (Doggrell and Henderson, 1998). The βAR blocking activity of atenolol, which exhibits low lipophilicity, was offset more quickly than that of propranolol, which exhibits high lipophilicity on contractile responses to ISO (Doggrell and Henderson, 1998). Collectively, propranolol showed a more superior effect than atenolol and metoprolol in suppressing β2AR-mediated insulin resistance.
Interestingly, in addition to blocking βAR effects, some β-blockers have been shown to evoke signal transduction through the βARs in G protein-independent and β-arrestin-dependent manner (Wisler et al., 2007; Nakaya et al., 2012). Long-term administration of metoprolol to mice induced cardiac fibrosis through β-arrestin2 and GRK5-dependent pathway (Nakaya et al., 2012). Although metoprolol induces cardiac fibrosis, it still has a beneficial effect on heart failure by inhibiting catecholamine overstimulation in heart failure. Thus, it will be worthwhile to determine G protein-independent signal transduction of β-blockers on cardiac insulin resistance. However, some β-blockers have additional effects in addition to βAR blockades, such as intrinsic sympathomimetic activity, and antioxidant and vasodilating properties. Thus, these additional effects of β-blockers require further study for their ability to attenuate βAR-mediated insulin resistance in the heart.
SUMMARY
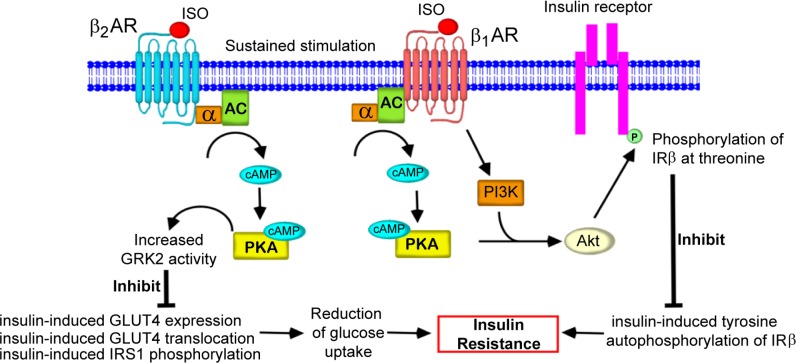
Based on data from previous studies, we propose that sustained and overstimulation of βARs leads to the development of heart failure and is associated with the pathogenesis of insulin resistance in the heart. Overstimulation of β2ARs enhances insulin resistance in the heart by inhibiting insulin-induced glucose uptake, GLUT4 synthesis, and translocation of GLUT4 to the plasma membrane via the cAMP/PKA/GRK2-dependent pathway (Fig. 4). As treatment with β-blockers such as propranolol and metoprolol could antagonize the effects of ISO-mediated cardiac insulin resistance in the heart, β-blockers may exert beneficial effects that can improve insulin resistance in the heart. Moreover, inhibition of GRK2 by βARKct gene therapy could be used for the improvement of glucose uptake in the heart.
Fig. 4.
Schematic diagram representing the signaling pathway for βAR-mediated cardiac insulin resistance. Agonist binding to β2ARs leads to the G protein-mediated activation of AC and cAMP generation. cAMP directly binds to and activates PKA. In β2AR signaling, PKA phosphorylates GRK2, which subsequently increases GRK2 activity. This leads to the inhibition of insulin-induced GLUT4 expression and the translocation of GLUT4 to the plasma membrane, thereby interfering with glucose uptake into cells and insulin-induced IRS1 phosphorylation. In β1AR signaling, PKA and PI3K activate Akt by phosphorylation at Ser 473, which in turn, phosphorylates the β subunit of insulin receptor (IRβ) at Thr residues. Tyrosine phosphorylation of IRβ inhibits insulin-induced tyrosine autophosphorylation of IRβ, leading to impairment of insulin signaling. These conditions lead to insulin resistance in the heart. Treatment with β-blockers antagonizes ISO-mediated insulin resistance in the heart.
Acknowledgments
This work was supported by National Science and Technology Development Agency (NSTDA) grant P-12-01409 and the Center of Excellence for Innovation in Drug Design and Discovery, Faculty of Pharmacy, Mahidol University to S. Mangmool, and by JSPS KAKENHI (25253011) and the Uehara Memorial Foundation to H. Kurose.
Footnotes
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
REFERENCES
- Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- Akikawa R, Nawano M, Gu Y, Katagiri H, Asano T, Zhu W, Nagai R, Komuro I. Insulin prevents cardiomyocytes from oxidative stress-induced apoptosis through activation of PI3 kinase/Akt. Circulation. 2000;102:2873–2879. doi: 10.1161/01.CIR.102.23.2873. [DOI] [PubMed] [Google Scholar]
- Anis Y, Leshem O, Reuveni H, Wexler I, Ben Sasson R, Yahalom B, Laster M, Raz I, Ben Sasson S, Shafrir E, Ziv E. Antidiabetic effect of novel modulating peptides of G-protein-coupled kinase in experimental models of diabetes. Diabetologia. 2004;47:1232–1244. doi: 10.1007/s00125-004-1444-1. [DOI] [PubMed] [Google Scholar]
- Backer JM, Myers MG, Jr, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore S, Messerli FH, Kostis JB, Pepine CJ. Cardiovascular protection using β-blockers: a critical review of the evidence. J Am Coll Cardiol. 2007;50:563–572. doi: 10.1016/j.jacc.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Becker AB, Roth RA. Insulin receptor structure and function in normal and pathological conditions. Annu Rev Med. 1990;41:99–115. doi: 10.1146/annurev.me.41.020190.000531. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. β-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan P. Insulin signalling. J Cell Sci. 2001;114:1429–1430. doi: 10.1242/jcs.114.8.1429. [DOI] [PubMed] [Google Scholar]
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur. J. Clin. Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson JL, Shikama H, Chu DT, Exton JH. Inhibitory effect of epinephrine on insulin-stimulated glucose uptake by rat skeletal muscle. J Clin Invest. 1981;68:706–713. doi: 10.1172/JCI110306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirieac DV, Chirieac LR, Corsetti JP, Cianci J, Sparks CE, Sparks JD. Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. Am J Physiol Endocrinol Metab. 2000;279:E1003–E1011. doi: 10.1152/ajpendo.2000.279.5.E1003. [DOI] [PubMed] [Google Scholar]
- Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, Gold JI, Gumpert A, Chen M, Otis NJ, Dorn GW, 2nd, Trimarco B, Iaccarino G, Koch WJ. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011;123:1953–1962. doi: 10.1161/CIRCULATIONAHA.110.988642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, Trimarco B, Iaccarino G. The G protein coupled receptor kinase 2 plays an essential role in β-adrenergic receptor-induced insulin resistance. Cardiovasc Res. 2009;84:407–415. doi: 10.1093/cvr/cvp252. [DOI] [PubMed] [Google Scholar]
- Coats AJ, Anker SD. Insulin resistance in chronic heart failure. J Cardiovasc Pharmacol. 2000;35:S9–S14. doi: 10.1097/00005344-200000004-00002. [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.CIR.100.22.2210. [DOI] [PubMed] [Google Scholar]
- Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- De Meyts P. Insulin and its receptor: structure, function and evolution. Bioessays. 2004;26:1351–1362. doi: 10.1002/bies.20151. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980;65:717–721. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggrell SA, Henderson CJ. The offset of β-adrenoceptor antagonism of the responses of the rat right ventricle to isoprenaline. J Auton Pharmacol. 1998;18:263–269. doi: 10.1046/j.1365-2680.1998.18592.x. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350(Suppl 1):SI9–SI13. doi: 10.1016/S0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, Grinstein S, Lefkowitz RJ. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Shibata K, Morita T, Iwasaki K, Watanabe M, Sobue K. Insulin receptor substrate-1/SHP-2 interaction, a phenotype-dependent switching machinery of insulin-like growth factor-I signaling in vascular smooth muscle cells. J Biol Chem. 2004;279:40807–40818. doi: 10.1074/jbc.M405100200. [DOI] [PubMed] [Google Scholar]
- Heather LC, Catchpole AF, Stuckey DJ, Cole MA, Carr CA, Clarke K. Isoproterenol induces in vivo functional and metabolic abnormalities: similar to those found in the infarcted rat heart. J Physiol Pharmacol. 2009;60:31–39. [PubMed] [Google Scholar]
- Heck PM, Dutka DP. Insulin resistance and heart failure. Curr Heart Fail Rep. 2009;6:89–94. doi: 10.1007/s11897-009-0014-8. [DOI] [PubMed] [Google Scholar]
- Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26:2999–3005. doi: 10.2337/diacare.26.11.2999. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human β-adrenergic receptor subtypes-characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:151–159. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- How OJ, Aasum E, Severson DL, Chan WY, Essop MF, Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes. 2006;55:466–473. doi: 10.2337/diabetes.55.02.06.db05-1164. [DOI] [PubMed] [Google Scholar]
- Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ, Hall RA. β1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of β1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem. 2000;275:38659–38666. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Barbato E, Cipolleta E, Esposito A, Fiorillo A, Koch WJ, Trimarco B. Cardiac βARK1 upregulation induced by chronic salt deprivation in rats. Hypertension. 2001;38:255–260. doi: 10.1161/01.HYP.38.2.255. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005a;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Trimarco V, Lanni F, Cipolletta E, Izzo R, Arcucci O, De Luca N, Di Renzo G. β-Blockade and increased dyslipidemia in patients bearing Glu27 variant of β2 adrenergic receptor gene. Pharmacogenomics J. 2005b;5:292–297. doi: 10.1038/sj.tpj.6500324. [DOI] [PubMed] [Google Scholar]
- Ishibashi KI, Imamura T, Sharma PM, Huang J, Ugi S, Olefsky JM. Chronic endothelin-1 treatment leads to heterologous desensitization of insulin signaling in 3T3-L1 adipocytes. J Clin Invest. 2001;107:1193–1202. doi: 10.1172/JCI11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama-Shigemoto S, Yamada K, Yuan X, Ichikawa F, Nonaka K. Association of polymorphisms in the β2-adrenergic receptor gene with obesity, hypertriglyceridaemia, and diabetes mellitus. Diabetologia. 1999;42:98–101. doi: 10.1007/s001250051120. [DOI] [PubMed] [Google Scholar]
- Izzo R, Cipolletta E, Ciccarelli M, Campanile A, Santulli G, Palumbo G, Vasta A, Formisano S, Trimarco B, Iaccarino G. Enhanced GRK2 expression and desensitization of βAR vasodilatation in hypertensive patients. Clin Transl Sci. 2008;1:215–220. doi: 10.1111/j.1752-8062.2008.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger PS. Metabolic consequences of hyperglycemia and insulin resistance. Clin. Cornerstone. 2007;8(Suppl 7):S30–S42. doi: 10.1016/S1098-3597(07)80019-6. [DOI] [PubMed] [Google Scholar]
- Joost HG, Steinfelder HJ. Forskolin inhibits insulin-stimulated glucose transport in rat adipose cells by a direct interaction with the glucose transporter. Mol Pharmacol. 1987;31:279–283. [PubMed] [Google Scholar]
- Juhan-Vague I, Alessi MC, Vague P. Thrombogenic and fibrinolytic factors and cardiovascular risk in non-insulin-dependent diabetes mellitus. Ann Med. 1996;28:371–380. doi: 10.3109/07853899608999095. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Flores-Riveros JR, McLenithan JC, Janicot M, Lane MD. Transcriptional repression of the mouse insulin-responsive glucose transporter (GLUT4) gene by cAMP. Proc Natl Acad Sci USA. 1991;88:1933–1937. doi: 10.1073/pnas.88.5.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi A, Huecksteadt TP, Foley JE. The regulation of glucose transport by cAMP stimulators via three different mechanisms in rat and human adipocytes. J Biol Chem. 1983;258:13685–13692. [PubMed] [Google Scholar]
- Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/MCB.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. β3-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999;274:34795–34802. doi: 10.1074/jbc.274.49.34795. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Narita Y, Nishida M, Kurose H. β-arrestin2 enhances β2-adrenergic receptor-mediated nuclear translocation of ERK. Cell Signal. 2005;17:1248–1253. doi: 10.1016/j.cellsig.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a β ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Barthel A, Kovacina KS, Boge A, Wallach B, Summers SA, Birnbaum MJ, Scott PH, Lawrence JC, Jr, Roth RA. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Kolter T, Uphues I, Eckel J. Molecular analysis of insulin resistance in isolated ventricular cardiomyocytes of obese Zucker rats. Am J Physiol. 1997;273:E59–E67. doi: 10.1152/ajpendo.1997.273.1.E59. [DOI] [PubMed] [Google Scholar]
- Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/MCB.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.CIR.101.6.676. [DOI] [PubMed] [Google Scholar]
- Lager I. The insulin-antagonistic effect of the counterregulatory hormones. J Intern Med Suppl. 1991;735:41–47. [PubMed] [Google Scholar]
- Lamounier-Zepter V, Ehrhart-Bornstein M, Bornstein SR. Insulin resistance in hypertension and cardiovascular disease. Best Pract Res Clin Endocrinol Metab. 2006;20:355–367. doi: 10.1016/j.beem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Lee AD, Hansen PA, Schluter J, Gulve EA, Gao J, Holloszy JO. Effects of epinephrine on insulin-stimulated glucose uptake and GLUT-4 phosphorylation in muscle. Am J Physiol. 1997;273:C1082–C1087. doi: 10.1152/ajpcell.1997.273.3.C1082. [DOI] [PubMed] [Google Scholar]
- Lee J, Pilch PF. The insulin receptor: structure, function, and signaling. Am J Physiol. 1994;266:C319–C334. doi: 10.1152/ajpcell.1994.266.2.C319. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for β-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G. Deficiency of a β-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. β-arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Mangmool S, Denkaew T, Phosri S, Pinthong D, Parichatikanond W, Shimauchi T, Nishida M. Sustained βAR stimulation mediates cardiac insulin resistance in a PKA-dependent manner. Mol Endocrinol. 2016;30:118–132. doi: 10.1210/me.2015-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangmool S, Haga T, Kobayashi H, Kim KM, Nakata H, Nishida M, Kurose H. Clathrin required for phosphorylation and internalization of β2-adrenergic receptor by G protein-coupled receptor kinase 2 (GRK2) J Biol Chem. 2006;281:31940–31949. doi: 10.1074/jbc.M602832200. [DOI] [PubMed] [Google Scholar]
- Mangmool S, Shukla AK, Rockman HA. β-Arrestin-dependent activation of Ca2+/calmodulin kinase II after β1-adrenergic receptor stimulation. J Cell Biol. 2010;189:573–587. doi: 10.1083/jcb.200911047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, Paisey RB, Carey C, Macdermott S, Russell-Eggitt I, Shea SE, Davis J, Beck S, Shatirishvili G, Mihai CM, Hoeltzenbein M, Pozzan GB, Hopkinson I, Sicolo N, Naggert JK, Nishina PM. New Alstrom syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165:675–683. doi: 10.1001/archinte.165.6.675. [DOI] [PubMed] [Google Scholar]
- Matthaei S, Stumvoll M, Kellerer M, Haring HU. Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev. 2000;21:585–618. doi: 10.1210/edrv.21.6.0413. [DOI] [PubMed] [Google Scholar]
- Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab. 2001;86:713–718. doi: 10.1210/jcem.86.2.7202. [DOI] [PubMed] [Google Scholar]
- Mingrone G, DeGaetano A, Greco AV, Capristo E, Benedetti G, Castagneto M, Gasbarrini G. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia. 1997;40:599–605. doi: 10.1007/s001250050721. [DOI] [PubMed] [Google Scholar]
- Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide–dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol. 2002;16:1931–1942. doi: 10.1210/me.2002-0074. [DOI] [PubMed] [Google Scholar]
- Morisco C, Condorelli G, Trimarco V, Bellis A, Marrone C, Condorelli G, Sadoshima J, Trimarco B. Akt mediates the cross-talk between β-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ Res. 2005;96:180–188. doi: 10.1161/01.RES.0000152968.71868.c3. [DOI] [PubMed] [Google Scholar]
- Morisco C, Lembo G, Trimarco B. Insulin resistance and cardiovascular risk: New insights from molecular and cellular biology. Trends Cardiovasc Med. 2006;16:183–188. doi: 10.1016/j.tcm.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Morisco C, Marrone C, Trimarco V, Crispo S, Monti MG, Sadoshima J, Trimarco B. Insulin resistance affects the cytoprotective effect of insulin in cardiomyocytes through an impairment of MAPK phosphatase-1 expression. Cardiovasc Res. 2007;76:453–464. doi: 10.1016/j.cardiores.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Mulder AH, Tack CJ, Olthaar AJ, Smits P, Sweep FC, Bosch RR. Adrenergic receptor stimulation attenuates insulin-stimulated glucose uptake in 3T3-L1 adipocytes by inhibiting GLUT4 translocation. Am J Physiol Endocrinol Metab. 2005;289:E627–E633. doi: 10.1152/ajpendo.00079.2004. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet. 2004;364:1786–1788. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Lygate CA, Cole MA, Carr CA, Radda GK, Neubauer S, Clarke K. Insulin resistance, abnormal energy metabolism and increased ischemic damage in the chronically infarcted rat heart. Cardiovasc Res. 2006;71:149–157. doi: 10.1016/j.cardiores.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Wang LM, Sun XJ, Zhang Y, Yenush L, Schlessinger J, Pierce JH, White MF. Role of IRS-1-GRB-2 complexes in insulin signaling. Mol Cell Biol. 1994;14:3577–3587. doi: 10.1128/MCB.14.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya M, Chikura S, Watari K, Mizuno N, Mochinaga K, Mangmool S, Koyanagi S, Ohdo S, Sato Y, Ide T, Nishida M, Kurose H. Induction of cardiac fibrosis by β-blocker in G protein-independent and G protein-coupled receptor kinase 5/β-arrestin2-dependent signaling pathways. J Biol Chem. 2012;287:35669–35677. doi: 10.1074/jbc.M112.357871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Poornima I, Parikh P, Magovern M, Shen YT, Shannon RP. The effects of combined versus selective adrenergic blockade on left ventricular and systemic hemodynamics, myocardial substrate preference, and regional perfusion in conscious dogs with dilated cardiomyopathy. J Am Coll Cardiol. 2006;47:1871–1881. doi: 10.1016/j.jacc.2005.11.082. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in β-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem. 2007;282:5488–5495. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- Ohtake T, Yokoyama I, Watanabe T, Momose T, Serezawa T, Nishikawa J, Sasaki Y. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–463. [PubMed] [Google Scholar]
- Olefsky J, Farquhar JW, Reaven G. Relationship between fasting plasma insulin level and resistance to insulin-mediated glucose uptake in normal and diabetic subjects. Diabetes. 1973;22:507–513. doi: 10.2337/diab.22.7.507. [DOI] [PubMed] [Google Scholar]
- Paolisso G, De Riu S, Marrazzo G, Verza M, Varricchio M, D’Onofrio F. Insulin resistance and hyperinsulinemia in patients with chronic congestive heart failure. Metabolism. 1991;40:972–977. doi: 10.1016/0026-0495(91)90075-8. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Manzella D, Rizzo MR, Ragno E, Barbieri M, Varricchio G, Varricchio M. Elevated plasma fatty acid concentrations stimulate the cardiac autonomic nervous system in healthy subjects. Am J Clin Nutr. 2000;72:723–730. doi: 10.1093/ajcn/72.3.723. [DOI] [PubMed] [Google Scholar]
- Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- Paternostro G, Camici PG, Lammerstma AA, Marinho N, Baliga RR, Kooner JS, Radda GK, Ferrannini E. Cardiac and skeletal muscle insulin resistance in patients with coronary heart disease. A study with positron emission tomography. J Clin Invest. 1996;98:2094–2099. doi: 10.1172/JCI119015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternostro G, Clarke K, Heath J, Seymour AM, Radda GK. Decreased GLUT-4 mRNA content and insulin-sensitive deoxyglucose uptake show insulin resistance in the hypertensive rat heart. Cardiovasc Res. 1995;30:205–211. doi: 10.1016/S0008-6363(95)00019-4. [DOI] [PubMed] [Google Scholar]
- Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res. 1999;42:246–253. doi: 10.1016/S0008-6363(98)00233-8. [DOI] [PubMed] [Google Scholar]
- Penela P, Ribas C, Mayor F., Jr Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003;15:973–981. doi: 10.1016/S0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Penn RB, Pronin AN, Benovic JL. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc Med. 2000;10:81–89. doi: 10.1016/S1050-1738(00)00053-0. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Postic C, Leturque A, Rencurel F, Printz RL, Forest C, Granner DK, Girard J. The effects of hyperinsulinemia and hyperglycemia on GLUT4 and hexokinase II mRNA and protein in rat skeletal muscle and adipose tissue. Diabetes. 1993;42:922–929. doi: 10.2337/diab.42.6.922. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/S0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Insulin resistance, hyperinsulinemia, and hypertriglyceridemia in the etiology and clinical course of hypertension. Am J Med. 1991;90:7S–12S. doi: 10.1016/0002-9343(91)90028-V. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Salazar NC, Chen J, Rockman HA. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim. Biophys. Acta. 2007;1768:1006–1018. doi: 10.1016/j.bbamem.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Peterson CA. Insulin receptor substrate-1 and phosphatidylinositol 3-kinase regulate extracellular signal-regulated kinase-dependent and -independent signaling pathways during myogenic differentiation. Mol Endocrinol. 1998;12:1870–1878. doi: 10.1210/mend.12.12.0205. [DOI] [PubMed] [Google Scholar]
- Sasaoka T, Kobayashi M. The functional significance of Shc in insulin signaling as a substrate of the insulin receptor. Endocr J. 2000;47:373–381. doi: 10.1507/endocrj.47.373. [DOI] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension. 2005;45:828–833. doi: 10.1161/01.HYP.0000163475.04421.e4. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin’s vascular effects in humans. J Clin Invest. 1994;94:2511–2515. doi: 10.1172/JCI117621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti G. Pathophysiology of insulin resistance. Best Pract. Res Clin Endocrinol Metab. 2006;20:665–679. doi: 10.1016/j.beem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Shah A, Shannon RP. Insulin resistance in dilated cardiomyopathy. Rev. Cardiovasc. Med. 2003;4(Suppl 6):S50–S57. [PubMed] [Google Scholar]
- Shiina T, Kawasaki A, Nagao T, Kurose H. Interaction with β-arrestin determines the difference in internalization behavior between β1- and β2-adrenergic receptors. J Biol Chem. 2000;275:29082–29090. doi: 10.1074/jbc.M909757199. [DOI] [PubMed] [Google Scholar]
- Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A β1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol Heart Circ Physiol. 2006;291:H1299–H1308. doi: 10.1152/ajpheart.00017.2006. [DOI] [PubMed] [Google Scholar]
- Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–532. doi: 10.1016/S0735-1097(97)00185-X. [DOI] [PubMed] [Google Scholar]
- Swan JW, Walton C, Godsland IF, Clark AL, Coats AJ, Oliver MF. Insulin resistance in chronic heart failure. Eur Heart J. 1994;15:1528–1532. doi: 10.1093/oxfordjournals.eurheartj.a060425. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Matsumoto T, Kamata K, Kobayashi T. G protein-coupled receptor kinase 2, with β-arrestin 2, impairs insulin-induced Akt/endothelial nitric oxide synthase signaling in ob/ob mouse aorta. Diabetes. 2012;61:1978–1985. doi: 10.2337/db11-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol. 1978;41:233–243. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- Trovati M, Anfossi G. Insulin, insulin resistance and platelet function: similarities with insulin effects on cultured vascular smooth muscle cells. Diabetologia. 1998;41:609–622. doi: 10.1007/s001250050958. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, Gray A, Coussens L, Liao YC, Tsubokawa M, Mason PH, Seeburg C, Grunfeld O, Rosen M, Ramachandran J. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.CIR.87.2.454. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Parruti G, Bohm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. Expression of β-arrestins and β-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74:206–213. doi: 10.1161/01.RES.74.2.206. [DOI] [PubMed] [Google Scholar]
- Usui I, Imamura T, Babendure JL, Satoh H, Lu JC, Hupfeld CJ, Olefsky JM. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19:2760–2768. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- Usui I, Imamura T, Huang J, Satoh H, Shenoy SK, Lefkowitz RJ, Hupfeld CJ, Olefsky JM. β-arrestin-1 competitively inhibits insulin-induced ubiquitination and degradation of insulin receptor substrate 1. Mol Cell Biol. 2004a;24:8929–8937. doi: 10.1128/MCB.24.20.8929-8937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui I, Imamura T, Satoh H, Huang J, Babendure JL, Hupfeld CJ, Olefsky JM. GRK2 is an endogenous protein inhibitor of the insulin signaling pathway for glucose transport stimulation. EMBO J. 2004b;23:2821–2829. doi: 10.1038/sj.emboj.7600297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten JP, Krans HM. Long-term regulation of hexose uptake by isoproterenol in cultured 3T3 adipocytes. Am J Physiol. 1985;248:E706–E711. doi: 10.1152/ajpendo.1985.248.6.E706. [DOI] [PubMed] [Google Scholar]
- Wallhaus TR, Taylor M, DeGrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103:2441–2446. doi: 10.1161/01.CIR.103.20.2441. [DOI] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001;56:175–193. doi: 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of β-blocker action: carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ishiyama-Shigemoto S, Ichikawa F, Yuan X, Koyanagi A, Koyama W, Nonaka K. Polymorphism in the 5′-leader cistron of the β2-adrenergic receptor gene associated with obesity and type 2 diabetes. J Clin Endocrinol Metab. 1999;84:1754–1757. doi: 10.1210/jcem.84.5.5822. [DOI] [PubMed] [Google Scholar]
- Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. β1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;297:H1377–H1386. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Gao F, Ma XL. Insulin says NO to cardiovascular disease. Cardiovasc Res. 2011;89:516–524. doi: 10.1093/cvr/cvq349. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr. Genomics. 2007;8:113–128. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI200316326. [DOI] [PMC free article] [PubMed] [Google Scholar]