Abstract
Viruses continue to evolve a new strategy to take advantage of every aspect of host cells in order to maximize their survival. Due to their central roles in transducing a variety of transmembrane signals, GPCRs seem to be a prime target for viruses to pirate for their own use. Incorporation of GPCR functionality into the genome of herpesviruses has been demonstrated to be essential for pathogenesis of many herpesviruses-induced diseases. Here, we introduce US28 of human cytomegalovirus (HCMV) as the best-studied example of virally-encoded GPCRs to manipulate host GPCR signaling. In this review, we wish to summarize a number of US28-related topics including its regulation of host signaling pathways, its constitutive internalization, its structural and functional analysis, its roles in HCMV biology and pathogenesis, its proliferative activities and role in oncogenesis, and pharmacological modulation of its biological activities. This review will aid in our understanding of how pathogenic viruses usurp the host GPCR signaling for successful viral infection. This kind of knowledge will enable us to build a better strategy to control viral infection by normalizing the virally-dysregulated host GPCR signaling.
Keywords: G protein-coupled receptor, Human cytomegalovirus, US28, Virally-encoded G protein-coupled receptor, Chemokine receptor, Antiviral target
G PROTEIN-COUPLED RECEPTORS
G protein-coupled receptors (GPCRs) constitute one of the largest receptor families encrypted in the human genome. Presence of the signature seven-transmembrane domain and a number of highly conserved amino acid residues in the transmembrane domains or the second intracellular loop are two well-known features of this kind of receptor families. However, their extreme diversity in selecting binding partners as well as executing signaling functions based on common structural platforms has been their another unique trait (Lefkowitz, 2007; Kolar et al., 2016). Due to their central roles in initiation and regulation of many physiologically-relevant signaling events that occur through plasma membranes, they have proved as one of the most effective targets for numerous pharmacological interventions to improve a variety of pathological conditions.
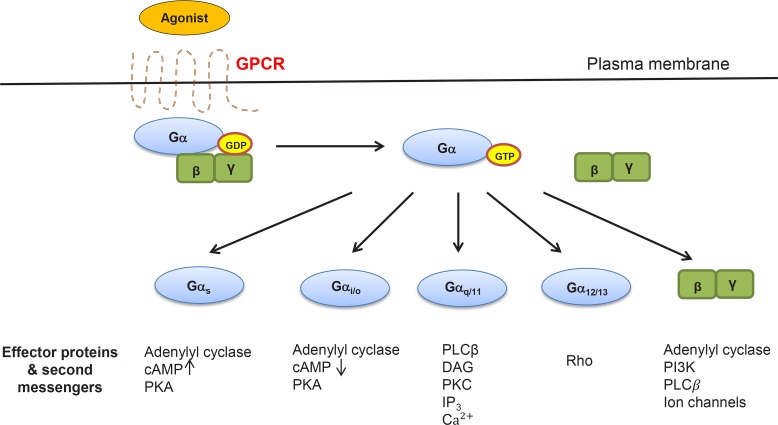
Several biomolecules including peptides, fatty acids, amino acids, steroids, nucleotides have been shown to act as ligands for GPCRs. In general, their engagement with corresponding GPCRs initiates a series of molecular events starting with dissociation of pre-assembled G protein complex into two separate Gα and Gβγ components (Fig. 1). This leads to stimulation of different kinds of multiple downstream signaling pathways depending on interacting ligands and their receptors. Conversion of a guanosine diphosphate (GDP)-bound form of Gα to a guanosine triphosphate (GTP)-bound represents another critical event necessary to jump-start overall GPCRs signaling. This GDP-to-GTP switch gives rise to separation of Gα and Gβγ from trimeric G proteins complex, allowing each of them to activate independent downstream signaling cascades through engagement of various second messenger (Kolar et al., 2016). Based on their specificities for second messengers, G protein α subunits can be further classified into four different members including Gαs, Gαi/o, Gαq/11, and Gα12/13 (Cabrera-Vera et al., 2003; Bongers et al., 2010) (Fig. 1). In general, both Gαs and Gαi/o interact and regulate enzymatic activity of adenylyl cyclase (AC) in the opposite direction, resulting in an increased and decreased production of cyclic adenosine monophosphate (cAMP), respectively. Changes in the level of cAMP affect the enzymatic activity of protein kinase A (PKA). On the other hand, Gαq/11 activates phospholipase C beta (PLCβ), contributing to hydrolysis of phosphatidylinositol 4, 5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol triphosphates (IP3). Production of DAG and IP3 ultimately lead to stimulation of protein kinase C (PKC) and up-regulation of cytoplasmic calcium level. In turn, activation of PKC and cytoplasmic mobilization of calcium modulates a variety of downstream signaling pathways via phosphorylation of a number of PKC substrate proteins and stimulation of several calcium-binding proteins, respectively. Activation of Gα12/13 is associated with rearrangement of actin filament and modulation of chemotaxis through interaction with small G protein Rho and focal adhesion kinase (FAK). Dissociated Gβγ complex was also shown to regulate a number of downstream effector proteins including AC, phosphoinositide 3-kinase (PI3K), PLCβ and ion channels.
Fig. 1.
Schematic diagram of general GPCR signaling pathways and key molecular players. Signaling pathways were drawn based on specific functions of four different members of activated Gα and Gβγ complex upon ligand binding. Major effector proteins mediating downstream signaling were also depicted. Acronyms used are as follows. GDP: guanosine diphosphate, GTP: guanosine triphosphate, cAMP: cyclic adenosine monophosphate, PKA: protein kinase A, PLCβ: phospholipase C beta, DAG: diacyl glycerol, IP3: inositol triphosphates, PI3K: phosphoinositide 3-kinase.
CHEMOKINE AND CHEMOKINE RECEPTORS
Chemokines are a large family of small size cytokines required for recruitment of immune cells to site of inflammation (Vomaske et al., 2009). They are subdivided into four different chemokine classes (CC-, CXC-, CX3C-, and XC-) based on location of their signature cysteine residues found in amino terminus (Vomaske et al., 2009). Chemokines serves as ligands for chemokine receptors (CCRs), which comprise a subfamily of the large class A rhodopsin-like GPCRs (Murphy et al., 2000). Both chemokines and their corresponding receptors play a fundamental role in control of various immune responses in many pathological conditions. Recently, one of chemokines, CXCR4 was found to be in a deep involvement with modulation of cancer metastasis. This further highlights not only their functional linkage to regulation of many pathological events but also their significance as druggable targets to manipulate a number of immune-related diseases (Balkwill, 2004).
VIRALLY ENCODED G-PROTEIN COUPLED RECEPTORS
Viruses establish an inseparable relationship with host cells for their survival. In this regard, they need to take advantage of every aspect of host cellular signaling for successful completion of their life cycle. In accordance with this requirement, they have adopted many strategies to maximize their survival. They include hijacking a number of host GPCRs into their genome through co-evolutionary relationship with diverse viral hosts. Interestingly, these so-called virally encoded GPCRs (vGPCRs) turned out to be highly homologous with several immunologically-important CCRs. Among many viruses with pathological significance, herpesviruses have been a frontrunner in developing these extraordinary abilities to utilize CCRs-dependent signaling pathways as their survival strategies. As shown in Table 1 (which was edited from Vischer et al., 2014), six CCR homologues were found to be encoded in herpesvirus genomes. They include ORF74 for Kaposi’s sarcoma-associated herpesvirus (KSHV), BILF1 for Epstein-Barr virus (EBV) and four CCR homologues such as UL33, US27, US28 and UL78 for human cytomegalovirus (HCMV) (Chee et al., 1990; Vischer et al., 2014). Indeed, these vGPCRs were also demonstrated to execute very efficient couplings to diverse host GPCR signaling networks in order to achieve the most virally-favorable environment for their efficient propagation inside the host cell (Boomker et al., 2005). However, in spite of many common features shared by both host and viral GPCRs, intrinsic differences between them were also striking. In contrast to predominant coupling of human CCRs to Gαi/o, vGPCRs tend to transmit their signal by using a diverse range of Gα proteins including Gαs, Gαi/o, Gαq/11, and Gα12/13 (Slinger et al., 2011) (Fig. 1). In some cases, ligand-independent constitutive signaling though a subset of vGPCRs was also reported to be intimately linked to virus-induced pathogenesis, especially oncogenesis (Kralj et al., 2011). Therefore, study of these vGPCR signaling in host cells will help us to better understand the molecular mechanism for viral pathogenesis by these vGPCR-utilizing viruses such as herpesviruses. This will enable us to better design various inhibitors for vGPCR in order to develop them as antiviral drugs by interfering with these key vGPCR signaling necessary for virus-induced pathogenesis.
Table 1.
Human herpesvirus-encoded GPCRs
| Virus | GPCRs | Ligand | G protein-coupling | Activated signaling pathways |
|---|---|---|---|---|
| KSHV | ORF74 |
|
|
|
| EBV | BILF1 |
|
|
|
| HCMV | US28 |
|
|
|
| UL33 |
|
|
|
|
| US27 |
|
|
|
|
| UL78 |
|
|
|
HUMAN CYTOMEGALOVIRUS
HCMV is a member of β-herpesvirus family. It is a double-stranded DNA virus whose genome is composed of approximately 250,000 base pairs (Vomaske et al., 2009). It is thought to be a very ubiquitous virus since it can infect up to 90% of the human population (Vomaske et al., 2009; Vischer et al., 2014). Once infected by HCMV, a life-long persistent and latent infection is believed to be established even in healthy hosts. One of the most important hallmarks for HCMV infection is its absolute dependency on latent phase in the viral life cycle. During its latency, HCMV genome is maintained to a minimal level with little or no major viral protein synthesis or production of progeny virus. Despite its high infection specificity for species, productive infection can be established in nearly every tissue of the human body. In addition, HCMV-infected cells were shown to secrete a wide array of soluble factors necessary for its pathogenesis although their exact roles in HCMV-induced diseases are yet to be elucidated.
HCMV-INDUCED DISEASES
Although HCMV infection is involved in various acute and chronic diseases (Maussang et al., 2009b), most of HCMV-infected people show no symptom (asymptomatic) due to its unique ability to maintain latency in the immunocompetent persons (Zhang et al., 2016). However, once reactivated, HCMV infection can be responsible for development of many severe inflammatory diseases including pneumonitis, hepatitis, and retinitis especially in immunocompromised patients and infants (Zhang et al., 2016). Congenital HCMV infection through vertical transmission from pregnant women to immunocompromised fetuses was also shown to cause a developmental defect like hearing loss (Kenneson and Cannon, 2007). In the United States alone, up to 80,000 children suffer from various forms of HCMV-induced birth defects annually. Among immunocompromised individuals such as organ transplant recipients and AIDS patients, graft rejection, loss, and end-organ diseases were also casually linked with HCMV infection (Vischer et al., 2014). Even in immunocompetent hosts, development of autoimmune disease, cardiovascular disease and proliferative diseases have been consistently attributed to HCVM infection (Maussang et al., 2009b; Vomaske et al., 2009; Vischer et al., 2014; Zhang et al., 2015). Therefore, effective control of HCMV infection by using anti-herpes drugs would confer a remarkable positive impact on overall health of HCMV patients around the world.
US28, AN HCMV vGPCR
Among six CCR homologues encoded by herpesviruses, US28 is by far the best characterized CCR homologue identified so far. Many studies have demonstrated a key role for US28 in initiation and maintenance of various vascular as well as proliferative diseases induced by HCMV infection (Maussang et al., 2006). Especially, US28-triggered constitutive GPCR signaling has been shown to be essential not only for its vascular-activating function but also for its cancer-modulating activity (Vischer et al., 2014). Therefore, US28 has been regarded as one of the best antiviral targets for intervention of a variety of herpesviruses-induced diseases.
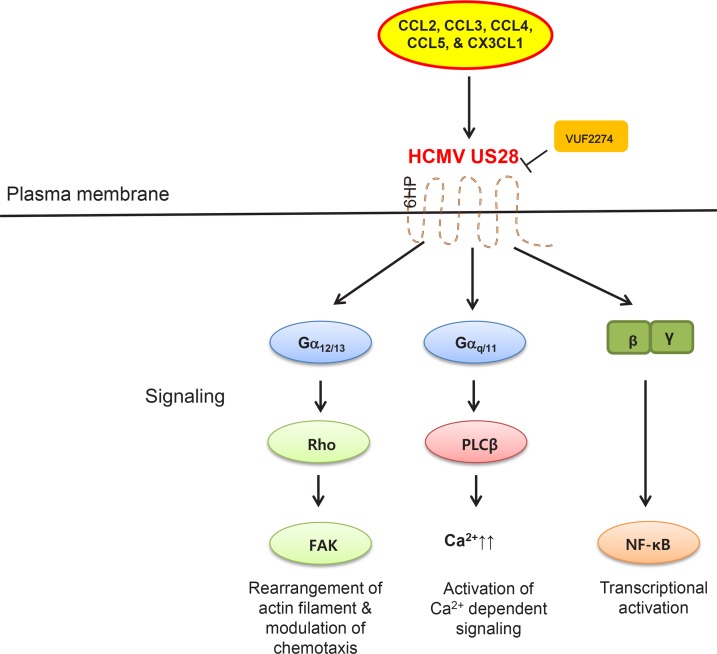
US28 turns out to be a homologue of three CCRs such as CCR1, CCR2 and CX3CR1. Therefore, it possesses a very unique ability to engage with diverse class of CCR ligands. This ligand promiscuity of US28 is regarded as one of the most important characteristics to explain its leading role in HCMV-induced pathogenesis. Cytokines that have been shown to work as endogenous agonists for US28 include CC chemokines such as CCL2/monocyte chemo-attractant protein-1 (MCP-1), CCL3/macrophage inflammatory protein-1α (MIP-1α), CCL4/macrophage inflammatory protein-1 β (MIP-1β), CCL5/regulated on activation, normal T cell expressed and secreted (RANTES), and membrane-tethered CX3C chemokine ligand 1 (CX3CL1)/Fractalkine (Gao and Murphy, 1994; Kuhn et al., 1995; Vischer et al., 2014) (Fig. 2). Especially, CX3CL1 was unique among them since it was demonstrated to function as an inverse agonist for US28 (Waldhoer et al., 2003). This multiple ligands-binding activity of US28 seems to be necessary for modulation of diverse GPCRs-coupled signaling pathways in a very context-dependent manner. Therefore, a pharmacological intervention of US28 activities based on understanding of its molecular functions in HCMV infection is thought to be a highly realistic strategy for development of effective anti-HCMV therapeutics.
Fig. 2.
Schematic diagram of GPCR signaling pathways activated by HCMV US28 receptor upon endogenous ligand binding. Agonist-dependent signaling pathways by US28 were drawn based on specific functions of two different members of activated Gα and Gβγ complex upon ligand binding. Major effector proteins mediating downstream signaling, upregulated calcium concentration and an activated transcription factor were also depicted. Acronyms used are as follows. Rho: Ras homolog gene family member, FAK: focal adhesion kinase, PLCβ: phospholipase C beta, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, 6HP: hexapeptide. VUF2274 is an inverse agonist for US28.
HOST SIGNALING PATHWAYS REGULATED BY US28
Due to its unusual ability to participate in promiscuous coupling selectivity, US28 is capable of exerting a variety of ligand-specific and ligand-nonspecific signaling in a highly cell type-specific manner (Fig. 3). For this reason, US28 has different sequences requirements for binding and activation of diverse G proteins. In addition, HCMV seems to utilize this context-specific US28 stimulation of ligand-dependent and ligand-independent signaling pathways to maximize their chances for cellular survival (Molleskov-Jensen et al., 2015). In this regards, US28’s ability to execute this “functional selectivity” could serve as a master switch for HCMV to fine-tune its host cellular environment to its utmost favor (Urban et al., 2007). Therefore, it is of prime interest to understand nature of various host signaling pathways regulated by HCMV US28 in order to develop the most effective strategy to target this multi-faceted molecular player which is absolutely required to maintain overall “healthy” HCMV infection in affected hosts.
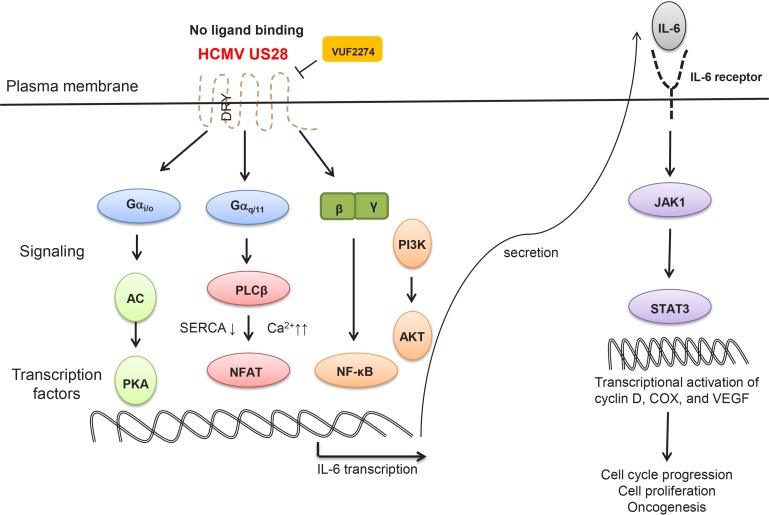
Fig. 3.
Schematic diagram of GPCR signaling pathways activated by HCMV US28 receptor without any ligand binding. Agonist-independent signaling pathways by US28 were drawn based on specific functions of two different members of activated Gα and Gβγ complex without ligand binding. Major effector proteins mediating downstream signaling and activated transcription factors were also depicted. Acronyms used are as follows. AC: adenylyl cyclase, PKA: protein kinase A, SERCA: sarcoplasmic reticulum calcium ATPase, NFAT: nuclear factor of activated T-cells, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, IL-6: interleukin 6, JAK1: Janus kinase 1, STAT3: signal transducer and activator of transcription 3, COX: cyclic oxygenase, VEGF: vascular epidermal growth factor, DRY: dry motif. VUF2274 is an inverse agonist for US28.
One of the most heavily-explored biological functions of US28 is its ability to maintain constitutive activation of PLCβ and one of the most famous inflammatory transcription factors, nuclear factor kappa B (NF-κB) via positive regulation of Gαq/11 and Gα in the absence of any appropriate binding ligands (Fig. 3) (Casarosa et al., 2001). This US28-induced agonist-independent constitutive signaling to PLCβ is also shown to work as a trigger for extended calcium signaling (Sherrill and Miller, 2006). In regards to its ligand-dependent functions, US28 was reported to be able to interact with CCL5 and CX3CL1 and promote calcium release in smooth muscle cells (SMCs) (Fig. 2). However, CC chemokines such as CCL2 and CCL5 do not seem to affect the basal activity of US28 signaling. They rather seem to act as neutral antagonists for GPCRs-mediated signaling (Arvanitakis et al., 1997; Hulshof et al., 2005). It was also found that activation of Rho and FAK only occurs via Gα12/13 following chemokine binding to US28 (Billstrom et al., 1998; Gao and Murphy, 1994; Melnychuk et al., 2004; Streblow et al., 2003) (Fig. 2). On the other hand, activation of nuclear factor of activated T-cells (NFAT) by US28 seems to occur through inhibition of the sarcoplasmic reticulum calcium ATPase (SERCA) and subsequent mobilization of calcium (Zhang et al., 2015) (Fig. 3). Importantly, this interaction of US28 with SERCA and subsequent NFAT activation was shown to be necessary for US28-induced tumor formation (Zhang et al., 2015).
CONSTITUTIVE INTERNALIZATION OF US28
Unlike typical vGPCRs which are found in the plasma membrane, US28 was mainly localized in perinuclear endosomes. This unusual localization pattern of US28 turned out to be due to its consistent internalization from the plasma membrane (Margulies et al., 1996; Mokros et al., 2002; Miller et al., 2003) (Fig. 4). A ligand-dependent and -independent phosphorylation of its carboxyl terminal domain by GPCRs-associated kinases (GRKs) turned out to be required for consistent internalization of US28. In addition, this requirement of carboxyl terminal phosphorylation of US28 by GRKs as a critical determinant for its subcellular localization was further verified by following two separate observations. First, deletion of the carboxyl terminal domain of US28 resulted in reduced constitutive endocytosis and consequent enhanced signaling capacity of US28 (Melnychuk et al., 2004). Second, host and other viral GPCRs showed constitutive endocytosis and subsequent perinuclear localization when fused with the carboxyl terminal domain of US28 (Waldhoer et al., 2003; Molleskov-Jensen et al., 2015). These two reports further emphasize the critical role of GRKs-dependent phosphorylation on carboxyl terminal domain of US28 in determination of the subcellular localization of US28.
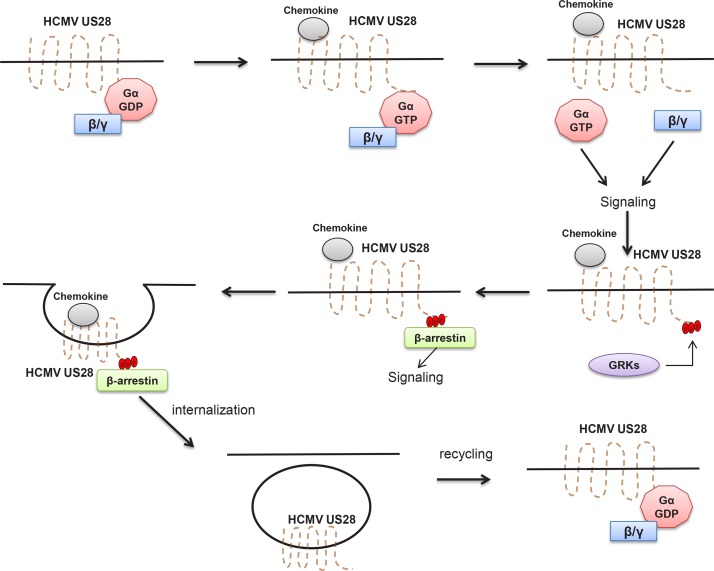
Fig. 4.
Schematic diagram of internalization and recycling of HCMV US28. Major effector proteins involved in internalization and recycling of HCMV US28 upon chemokine binding were also depicted. Acronyms used are as follows. GDP: guanosine diphosphate, GTP: guanosine triphosphate, GRK: GPCR-associated kinase.
Then, why the phosphorylated form of US28 is more susceptible to constitutive internalization and intracellular localization? It turns out that the phosphorylated form of US28 is better able to recruit β-arrestin from cytoplasm and this recruitment of β-arrestin by US28 leads to facilitation of its internalization (Fig. 4). In addition, this association of β-arrestin with US28 can be further stimulated by overexpression of one member of GRKs, GPCR kinase 2 (GRK2) (Vomaske et al., 2009). In general, carboxyl terminal phosphorylation of an activated CCR by GRKs mediates recruitment of β-arrestin proteins to the receptor carboxyl terminus (Vomaske et al., 2009). β-Arrestin recruitment was shown to be able to start the clathrin-mediated internalization of CCRs (Fig. 4). In turn, this results in desensitization and inactivation of numerous CCRs (Vomaske et al., 2009). The 40-amino-acid carboxyl-terminal region of CCRs contains several clusters of Ser/Thr residues that can be constitutively phosphorylated by GRKs. These phosphorylated Ser/Thr residues are responsible for recruitment of the β-arrestin proteins to CCRs (Krupnick and Benovic, 1998). Therefore, phosphorylation by GRKs and recruitment of β-arrestin proteins represent key steps in the desensitization or down-regulation of GPCRs-mediated signal transduction and this process seems to be utilized by virtually all cellular GPCRs to control the magnitude and duration of GPCR signaling (Violin et al., 2006). In this regard, US28 appears to be no exception in a sense that it also faithfully follows this mode of negative regulation of GPCR signaling through β-arrestin-dependent constitutive internalization. Of note, the GPCR associated sorting protein, called as GASP-1, was also identified as another key regulator in the post-endocytic sorting and the signaling capacities of US28 (Tschische et al., 2010).
Then, why HCMV needs to down-regulate molecular concentration of US28 at cell surface through its consistent internalization via carboxyl terminal phosphorylation by GRKs? Some groups suggest that consistent internalization of US28 results in scavenging of normal cellular chemokines through their direct binding with US28. This might work as a potential immune evasion mechanism to physically remove chemokines from the extracellular environment (Bodaghi et al., 1998; Billstrom et al., 1999; Fraile-Ramos et al., 2001; Randolph-Habecker et al., 2002; Tschische et al., 2010). However, this kind of description of US28 as consistent remover of cellular chemokines seems not to be true since physiological concentration of chemokines in reality is thought to be too high to be effectively scavenged by US28 at its actual concentration (Vomaske et al., 2009). Therefore, physiological relevance of this chemokines removal theory by US28 still needs to be reconsidered in the future.
STRUCTURAL AND FUNCTIONAL ANALYSIS OF US28
Although chemokine ligands interact with multiple sites on the extracellular domain of chemokine receptors, they first need to bind with amino terminal domain of receptor with high-affinity (Monteclaro and Charo, 1996). For this reason, a hexapeptide sequence found in the amino terminus of US28 has been shown to be critical for high-affinity binding of chemokine ligands to US28 (Casarosa et al., 2005). This region is highly conserved between US28 and the endogenous human chemokine receptors CCR1 and CCR2 and is known to be a critical determinant for CCL2 binding to CCR2. However, CC-chemokine and CX3CL1 binding to US28 require different residues within this hexapeptide region for high affinity binding (Casarosa et al., 2005). In addition, mutation of the tyrosine at position 16 of the US28 amino terminus negatively affects high affinity binding of both classes of chemokine.
The DRY-motif found in the transmembrane 3 (TM3) proximal end of intracellular loop on most CCRs is believed to be a critical determinant of heterotrimeric G-protein binding and activation (Murphy et al., 2000) (Fig. 3). Therefore, the R to A mutant in the DRY motif of US28 turned out to be unable to activate PLCβ, and fails to induce COX-2 or vascular epidermal growth factor (VEGF) production, ultimately demonstrating much weaker oncogenic activity in vivo (Maussang et al., 2006). In addition, the recently solved crystal structure of US28 in complex with the chemokine domain of human CX3CL1 suggests that the presence of Glu1243 seems to be unique for US28 (Rosenkilde et al., 2000; Burg et al., 2015). According to this US28 crystal structure model, a potential reason for the constitutive activity of US28 could be attributed to the presence of the small hydrophobic residue (A114) in position of the transmembrane helix TM3 that does not support the sufficient TM2/TM3/TM7 inter-helical interactions (Montaner et al., 2013).
ROLE OF US28 IN HCMV BIOLOGY
US28 is expressed during both the latent and lytic cycles. US28 was found to be able to up-regulate expression of the major immediate early promoter of HCMV. This suggests that US28 might be able to mediate potential enhancement of HCMV gene expression/replication at transcriptional level. In addition, all of the HCMV GPCR homologs (UL33, UL78, US27, and US28) have been detected in the viral envelope. This proposes a new role for US28 to facilitate delivery of a viral particle to the host cell and cell-to-cell spread of virus infection immediately after viral entry. This proposal was further strengthened by fact that the US28 is able to serve as a co-receptor for HIV entry. Since CCR5 and CXCR4 are the primary HIV-1 co-receptors, US28 seems to be able to augment HIV infection by acting as a co-receptor for HIV-1 entry into cells. When the mutant HCMV was constructed, in which a FLAG-YFP cassette replaces the US28 coding region, this mutant HCMV displayed a significant defect in virus growth, suggesting a potential role for US28 in replication and assembly of HCMV DNA and particle. On the other hand, there are some reports suggesting that HCMV US28 may contribute at a late stage of the viral life cycle to cell-to-cell dissemination of virus since a US28 mutant HCMV produced increased levels of extracellular virus.
ROLE OF US28 IN HCMV PATHOGENESIS
Many studies found a strong association between HCMV infection and vascular diseases such as atherosclerosis, restenosis, and transplant vascular sclerosis. HCMV also has been suspected as a candidate cofactor for atherosclerosis (Rosenfeld and Campbell, 2011; Frostegard, 2013). US28 was demonstrated to promote the migration of HCMV-infected cells towards CC-chemokine-secreting tissues, thereby assisting virus dissemination (Streblow et al., 1999). US28 was also shown to assist the chemotactic infiltration of HCMV-infected SMCs into blood vessels. Thus, this US28-mediated chemotaxis appears to recruit SMCs into atherosclerotic lesions, thereby accelerating the formation of an SMC-enriched atherosclerotic plaque. Especially, the activation of FAK, Src, and small GTPase Rho are demonstrated to be absolutely required for pro-migratory signaling functions for US28 (Fig. 2). However, in contrast to its positive role in induction of chemotaxis of SMCs, US28 expression in HCMV-infected fibroblasts was also shown to be sufficient to inhibit the monocyte chemotactic activity of the HCMV-infected cell supernatants compared to supernatants from fibroblasts infected with a US28 knockout virus (Vomaske et al., 2009). Therefore, a role for US28 in modulation of development of cardiovascular diseases might be much more diverse depending on nature of HCMV-infected cells and context of HCMV infection in the cellular environment.
ONCOGENIC ACTIVITIES OF US28
US28 is causally linked to HCMV-associated inflammatory and proliferative diseases (Slinger et al., 2010). Typical examples for association of HCMV infection with cancer is colon cancer and glioblastoma (GBM). US28 has also been found to induce an invasive and angiogenic phenotype in GBM, (Maussang et al., 2006; Soroceanu et al., 2011). Especially, US28 expression is found to be positive in many GBM specimens from patients (Slinger et al., 2010; Bhattacharjee et al., 2012; Soderberg-Naucler et al., 2013). In regard of its oncogenic mechanism of action, US28 was shown to be able to induce IL-6 expression, thereby activating the Janus kinase 1 (JAK1)-signal transducer and activator of transcription 3 (STAT3) signaling axis to promote cell proliferation in both an autocrine and paracrine fashion (Slinger et al., 2010) (Fig. 3). US28 activates this IL-6-JAK1-STAT3 signaling axis through activation of the NF-κB and the consequent production of IL-6 (Slinger et al., 2010). Transgenic mice in which US28 expression was targeted to intestinal epithelial cells developed an intestinal neoplasia in vivo. Interestingly, pharmacological inhibition of cyclooxygenase-2 (COX-2) with Celecoxib was able to inhibit the angiogenic activity and tumorigenesis of US28-transfected NIH-3T3 cells fibroblasts (Maussang et al., 2009a). In addition, US28 induces various oncogenic responses, including increased cyclin D1 and COX2 production, as well as that of vascular endothelial growth factor (VEGF), when expressed in NIH3T3 fibroblasts (Slinger et al., 2010) (Fig. 3). Therefore, a pharmacological intervention of US28 also could be an attractive strategy to reduce the severity of various HCMV-induced cancers.
PHARMACOLOGICAL MODULATION OF US28
Recent advances in the identification of small-weight allosteric modulators for vGPCRs enabled us to study molecular interaction between endogenous chemokines and their corresponding receptors in more detailed manner. This progress in designing a new class of vGPCR inhibitors also has been applied to development of a variety of pharmacological modulators for US28. Here, we summarized several endogenous ligands for US28 as well as a number of identified small molecules with different chemical back-bones to regulate biological activities of US28.
ENDOGENOUS LIGANDS FOR US28
CX3CL1 has been proposed to act as an inverse agonist due to its ability to block the US28-dependent stimulation of PLCβ activity (Casarosa et al., 2001). CX3CL1 also works as a true agonist to promote US28-mediated calcium release and activation of FAK (Casarosa et al., 2001; Waldhoer et al., 2002; Casarosa et al., 2005) (Fig. 2). Similar phenomenon was also known for CCL5. CCL5 was demonstrated to work as inverse agonist on the wild type receptor and as true agonist on the US28 mutant receptor, which lacks the 55 amino acids on the carboxyl terminus, exhibits constitutive signaling and an increased expression on the cell surface due to blockage of β-arrestin-dependent internalization of US28 (Fig. 4) (Tschammer, 2014). Study of the this mutant receptor suggested that the inverse to true agonism switch in some US28 inhibitors is due to the differential coupling of the receptor to its downstream signaling partners. In some cases, the path from an agonist to an inverse agonist could be as short as a methyl group (Tschammer, 2014).
VUF2274
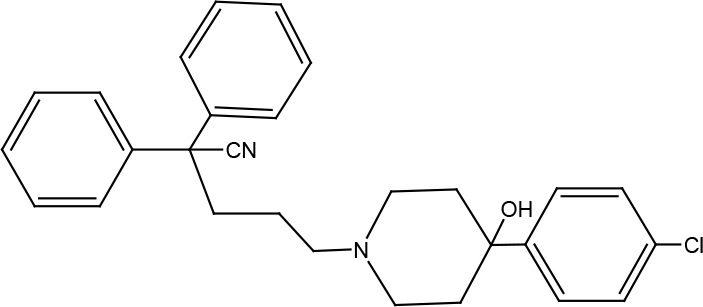
A nonpeptidergic CCR1 antagonist, VUF2274 [5-(4-(4-chlorophenyl)-4-hydroxy-piperidin-1-yl)-2, 2-diphenylpentanenitrile] was identified as a full inverse agonist for PLCβ activation by US28 (Casarosa et al., 2003b; Waldhoer et al., 2003; Vischer et al., 2010) (Table 2). Consequently, VUF2274 inhibited constitutive production of inositol phosphates by US28. VUF2274 was also able to displace CCL5 binding to US28 (Waldhoer et al., 2003). Its EC50 concentration required for this inverse inhibition turned out to be around 4.5 μM. According to mutational analysis of US28, VUF2274 binds US28 within the seven-transmembrane pocket and serve as an allosteric ligand to block chemokine binding (Casarosa et al., 2003a). Especially, a glutamic acid in transmembrane 7, which is highly homologous among chemokine receptors, was found as a key residue for VUF2274 binding to US28 (Casarosa et al., 2003a). A series of VUF2273 derivatives were synthesized to optimize their activities to inhibit CCL5 chemokine binding to US28 and production of inositol phosphates (Vischer et al., 2010). Especially, 4-phenylpiperidine moiety within VUF2274 turned out to be essential for inverse agonism (Hulshof et al., 2005).
Table 2.
Summary of small molecule US28 modulators. Their groups, names of compounds, types, mechanisms, EC50 values, chemical structures, and references were also included
| Group | Compound | Type | Mechanism | EC50 (μm) | Chemical structure | Reference |
|---|---|---|---|---|---|---|
| VUF2274 | 5-(4-(4-chlorophenyl)-4-hydroxy-piperidin-1-yl)-2,2-diphenylpentanenitrile | Inverse agonist | Constitutive PLCb activation CCL5 binding |
4.5 |
|
Tschammer, 2014 |
| Methiothepin | Antagonist | CCL5 binding | 0.4 |
|
Fraile-Ramos et al., 2001 | |
| Hydroisoquinoline | 3-(2-hydroxyethyl)-3-methyl-2-phenyl03,4-dihydroisoquinolin-1(2H)-one | Inverse agonist | Constitutive PLCb activation | 1.5 |
|
Kralj et al., 2011 |
| N-(2-aminoethyl)-N-((3-methyl-1-oxo-1,2,3,4-tetrahydroisoquinolin-3-yl)methyl)decanamide | Inverse agonist | Constitutive PLCb activation | 1.5 |
|
Kralj et al., 2011 | |
| 3-(2-hydroxyethyl)-3-methyl-2-phenyl-3,4-dihydroisoquinolin-1(2H)-one | Inverse agonist | Constitutive PLCb activation | 1.0 |
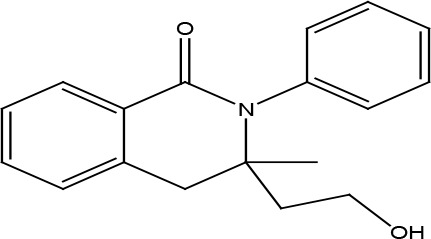
|
Kralj et al., 2011 | |
| (3-methyl-1-oxo-2-(2-phenylacetyl)-1,2,3,4-tetrahydroisoquinolin-3-yl)methyl 2-phenylacetate | Inverse agonist | Constitutive PLCb activation | 3.4 |
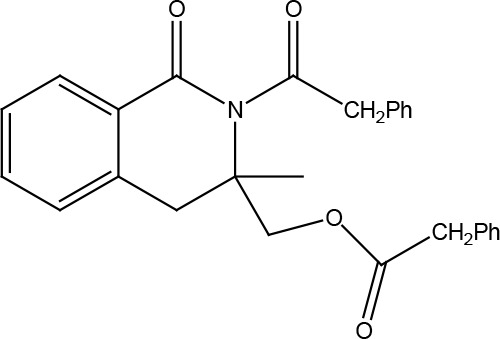
|
Vischer et al., 2010 | |
| Biphenyl amide | Decanoic acid (4′-chloro-6-methoxybiphen-3-ylmethyl)-(2-dimethylaminoethyl) amide | Inverse agonist | Constitutive PLCb activation | 2.4 |
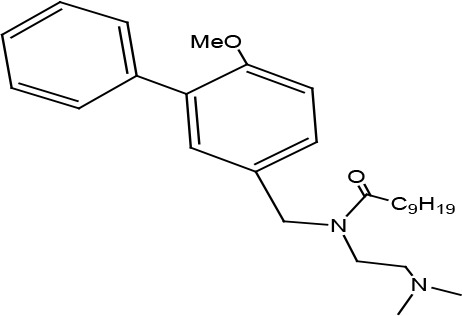
|
Kralj et al., 2014 |
| Flavonoid | 5-(benzyloxy)-2-(5-bromo-2-methoxyphenyl)-4H-chromen-4-one | Inverse agonist | Constitutive PLCb activation | 3.5 |
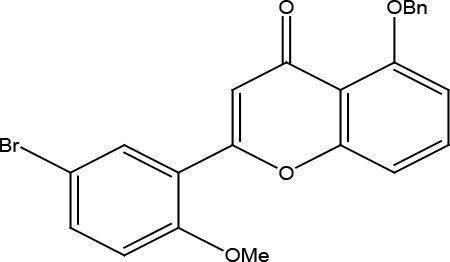
|
Kralj et al., 2013 |
| Other | (4-(4-chlorophenyl)-1-(4,4-diphenylbutyl)piperidin-4-yl) | Antagonist | CCL5 binding | 3.6 |
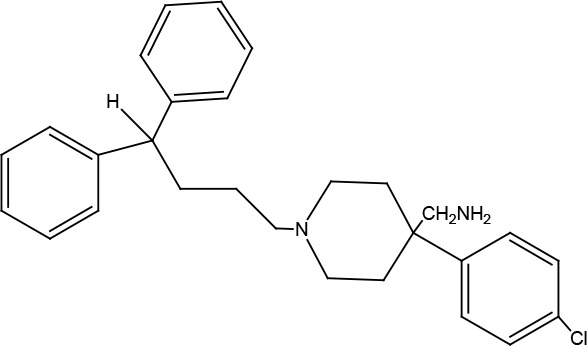
|
Hulshof et al., 2006 |
METHIOTHEPIN
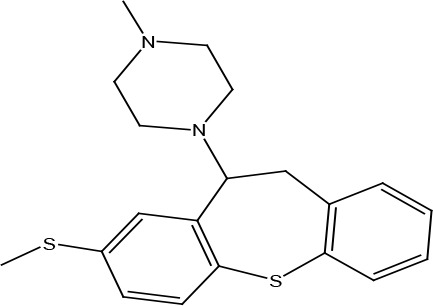
A series of piperazinyldibenzothiepine derivatives were synthesized to test their effects on binding of one of endogenous ligand, CCL5 to US28. Among them, methiothepin was shown to be able to block CCL5 binding to US28 with the EC50 value of 0.4 μM (Fraile-Ramos et al., 2001) (Table 2). A structurally related compound, octoclothepin was also reported to be an inhibitor for CCL5’s association with the US28 receptor (Vischer et al., 2010).
HYDROISOQUINOLINES
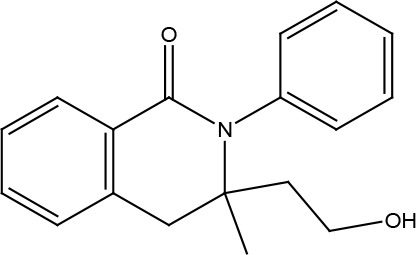
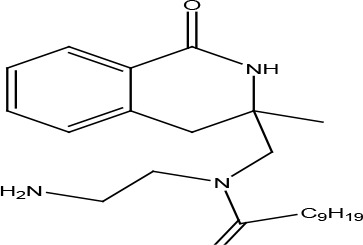
A series of isoquinoline compounds were constructed to test their effects on agonist-independent constitutive signaling of US28 to PLCβ. For this goal, the firefly luciferase-based PathDetect Elk1 gene reporter assay was used to readily detect modulation of US28 constitutive activity by small allosteric ligands. Di- and tetra-hydroisoquinolines were identified as promising candidate molecules for allosteric inverse agonists of US28 and several derivatives have been shown to reduce US28 signaling (Table 2) (Kralj et al., 2011). Among them, the substituted dihydroisoquinolinone and its spirocyclic derivative demonstrated an inverse agonism on US28, with the EC50 values of 1.50 and 4.80 μM (Table 2).
BIPHENYL AMIDES
Biphenyl-derived ligands turned out to be a preliminary pharmacophore model for US28 inhibitors. Four promising candidates with full inverse agonist properties were discovered within this group of compounds (Kralj et al., 2014). Of interest, only the derivatives containing a decanoyl side chain acted as inverse agonists with the EC50 value of 2.4 μM ((Kralj et al., 2014) (Table 2).
Flavonoid-Based inverse agonists
US28 ligands a series of halogen-substituted flavonoids were previously shown to inhibit constitutive US28 signaling (Kralj et al., 2013). A series of 31 chalcone- and flavonoid-based derivatives were synthesized and screened for their inverse agonist activity on the US28. Among them, 5-(benzyloxy)-2-(5-bromo-2-methoxyphenyl)-4H-chromen-4-one (11b) acted on the US28 receptor as a nontoxic, inverse agonist with the potency (EC50=3.5 μM) (Table 2).
OTHER NON-PEPTIDERGIC INHIBITORS
A number of novel non-peptidergic inhibitors were identified as neutral antagonists or inverse agonists on US28 that allosterically inhibit chemokine binding to US28 (Vischer et al., 2010). (4-(4-chlorophenyl)-1-(4,4-diphenylbutyl)piperidin-4-yl) methanamine was also reported as an antagonist on the human chemokine receptor CCR1 (EC50=3.6 μM) (Table 2) (Hulshof et al., 2006).
CONCLUSIONS
In this review, we summarized a number of GPCRs-related activities of HCMV US28, which are important for pathogenesis of many HCMV-induced diseases. They include its regulation of host signaling pathways, its constitutive internalization, its structural and functional analysis, its roles in HCMV biology and pathogenesis, its proliferative activity and role in oncogenesis, and pharmacological modulation of its biological activities. Better understanding of mechanism of action for dysregulation of GPCR signaling by pathogenic viruses will enable us to develop a novel strategy for pharmacological control of viral infection by correcting virally-dysregulated host GPCR signaling.
Acknowledgments
This work was supported by the GRRC program of Gyeonggi province [(GRRC-DONGGUK2016-A04), Development of therapeutics for hepatitis C].
REFERENCES
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee B, Renzette N, Kowalik TF. Genetic analysis of cytomegalovirus in malignant gliomas. J Virol. 2012;86:6815–6824. doi: 10.1128/JVI.00015-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstrom MA, Johnson GL, Avdi NJ, Worthen GS. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J Virol. 1998;72:5535–5544. doi: 10.1128/jvi.72.7.5535-5544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstrom MA, Lehman LA, Scott Worthen G. Depletion of extracellular RANTES during human cytomegalovirus infection of endothelial cells. Am J Respir Cell Mol Biol. 1999;21:163–167. doi: 10.1165/ajrcmb.21.2.3673. [DOI] [PubMed] [Google Scholar]
- Bodaghi B, Jones TR, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier JL, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998;188:855–866. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers G, Maussang D, Muniz LR, Noriega VM, Fraile-Ramos A, Barker N, Marchesi F, Thirunarayanan N, Vischer HF, Qin L, Mayer L, Harpaz N, Leurs R, Furtado GC, Clevers H, Tortorella D, Smit MJ, Lira SA. The cytomegalovirus-encoded chemokine receptor US28 promotes intestinal neoplasia in transgenic mice. J Clin Invest. 2010;120:3969–3978. doi: 10.1172/JCI42563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomker JM, van Luyn MJ, The TH, de Leij LF, Harmsen MC. US28 actions in HCMV infection: lessons from a versatile hijacker. Rev Med Virol. 2005;15:269–282. doi: 10.1002/rmv.468. [DOI] [PubMed] [Google Scholar]
- Burg JS, Ingram JR, Venkatakrishnan AJ, Jude KM, Dukkipati A, Feinberg EN, Angelini A, Waghray D, Dror RO, Ploegh HL, Garcia KC. Structural biology. Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science. 2015;347:1113–1117. doi: 10.1126/science.aaa5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, Leurs R, Smit MJ. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem. 2001;276:1133–1137. doi: 10.1074/jbc.M008965200. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Menge WM, Minisini R, Otto C, van Heteren J, Jongejan A, Timmerman H, Moepps B, Kirchhoff F, Mertens T, Smit MJ, Leurs R. Identification of the first non-peptidergic inverse agonist for a constitutively active viral-encoded G protein-coupled receptor. J Biol Chem. 2003a;278:5172–5178. doi: 10.1074/jbc.M210033200. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Menge WM, Minisini R, Otto C, van Heteren J, Jongejan A, Timmerman H, Moepps B, Kirchhoff F, Mertens T, Smit MJ, Leurs R. Identification of the first non-peptidergic inverse agonist for a constitutively active viral-encoded G protein-coupled receptor. J Biol Chem. 2003b;278:5172–5178. doi: 10.1074/jbc.M210033200. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Waldhoer M, LiWang PJ, Vischer HF, Kledal T, Timmerman H, Schwartz TW, Smit MJ, Leurs R. CC and CX3C chemokines differentially interact with the N terminus of the human cytomegalovirus-encoded US28 receptor. J Biol Chem. 2005;280:3275–3285. doi: 10.1074/jbc.M407536200. [DOI] [PubMed] [Google Scholar]
- Chee MS, Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, Horsnell T, Hutchison CA, 3rd, Kouzarides T, Martignetti JA, Preddie EP, Satchwell SC, Tomlinson P, Weston KM, Barrell BG. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Fraile-Ramos A, Kledal TN, Pelchen-Matthews A, Bowers K, Schwartz TW, Marsh M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell. 2001;12:1737–1749. doi: 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JL, Murphy PM. Human cytomegalovirus open reading frame US28 encodes a functional β chemokine receptor. J Biol Chem. 1994;269:28539–28542. [PubMed] [Google Scholar]
- Hulshof JW, Casarosa P, Menge WM, Kuusisto LM, van der Goot H, Smit MJ, de Esch IJ, Leurs R. Synthesis and structure-activity relationship of the first nonpeptidergic inverse agonists for the human cytomegalovirus encoded chemokine receptor US28. J Med Chem. 2005;48:6461–6471. doi: 10.1021/jm050418d. [DOI] [PubMed] [Google Scholar]
- Hulshof JW, Vischer HF, Verheij MH, Fratantoni SA, Smit MJ, de Esch IJ, Leurs R. Synthesis and pharmacological characterization of novel inverse agonists acting on the viral-encoded chemokine receptor US28. Bioorg Med Chem. 2006;14:7213–7230. doi: 10.1016/j.bmc.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- Kolar GR, Grote SM, Yosten GL. Targeting orphan G protein-coupled receptors for the treatment of diabetes and its complications: C-peptide and GPR146. J Intern Med. 2016 doi: 10.1111/joim.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj A, Kurt E, Tschammer N, Heinrich MR. Synthesis and biological evaluation of biphenyl amides that modulate the US28 receptor. ChemMedChem. 2014;9:151–168. doi: 10.1002/cmdc.201300369. [DOI] [PubMed] [Google Scholar]
- Kralj A, Nguyen MT, Tschammer N, Ocampo N, Gesiotto Q, Heinrich MR, Phanstiel O., 4th Development of flavonoid-based inverse agonists of the key signaling receptor US28 of human cytomegalovirus. J Med Chem. 2013;56:5019–5032. doi: 10.1021/jm4003457. [DOI] [PubMed] [Google Scholar]
- Kralj A, Wetzel A, Mahmoudian S, Stamminger T, Tschammer N, Heinrich MR. Identification of novel allosteric modulators for the G-protein coupled US28 receptor of human cytomegalovirus. Bioorg Med Chem Lett. 2011;21:5446–5450. doi: 10.1016/j.bmcl.2011.06.120. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Kuhn DE, Beall CJ, Kolattukudy PE. The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem Biophys Res Commun. 1995;211:325–330. doi: 10.1006/bbrc.1995.1814. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol. (Oxf.) 2007;190:9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- Margulies BJ, Browne H, Gibson W. Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles. Virology. 1996;225:111–125. doi: 10.1006/viro.1996.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van Walsum M, Dijkman R, Borg MK, Slinger E, Schreiber A, Michel D, Tensen CP, van Dongen GA, Leurs R, Smit MJ. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 2009a;69:2861–2869. doi: 10.1158/0008-5472.CAN-08-2487. [DOI] [PubMed] [Google Scholar]
- Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci USA. 2006;103:13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maussang D, Vischer HF, Schreiber A, Michel D, Smit MJ. Pharmacological and biochemical characterization of human cytomegalovirus-encoded G protein-coupled receptors. Methods Enzymol. 2009b;460:151–171. doi: 10.1016/S0076-6879(09)05207-0. [DOI] [PubMed] [Google Scholar]
- Melnychuk RM, Streblow DN, Smith PP, Hirsch AJ, Pancheva D, Nelson JA. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Gα12. J Virol. 2004;78:8382–8391. doi: 10.1128/JVI.78.15.8382-8391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WE, Houtz DA, Nelson CD, Kolattukudy PE, Lefkowitz RJ. G-protein-coupled receptor (GPCR) kinase phosphorylation and β-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J Biol Chem. 2003;278:21663–21671. doi: 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- Mokros T, Rehm A, Droese J, Oppermann M, Lipp M, Hopken UE. Surface expression and endocytosis of the human cytomegalovirus-encoded chemokine receptor US28 is regulated by agonist-independent phosphorylation. J Biol Chem. 2002;277:45122–45128. doi: 10.1074/jbc.M208214200. [DOI] [PubMed] [Google Scholar]
- Molleskov-Jensen AS, Oliveira MT, Farrell HE, Davis-Poynter N. Virus-encoded 7 transmembrane receptors. Prog Mol Biol Transl Sci. 2015;129:353–393. doi: 10.1016/bs.pmbts.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Montaner S, Kufareva I, Abagyan R, Gutkind JS. Molecular mechanisms deployed by virally encoded G protein-coupled receptors in human diseases. Annu Rev Pharmacol Toxicol. 2013;53:331–354. doi: 10.1146/annurev-pharmtox-010510-100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteclaro FS, Charo IF. The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1α receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. J Biol Chem. 1996;271:19084–19092. doi: 10.1074/jbc.271.32.19084. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Randolph-Habecker JR, Rahill B, Torok-Storb B, Vieira J, Kolattukudy PE, Rovin BH, Sedmak DD. The expression of the cytomegalovirus chemokine receptor homolog US28 sequesters biologically active CC chemokines and alters IL-8 production. Cytokine. 2002;19:37–46. doi: 10.1006/cyto.2002.0874. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011;106:858–867. doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Kledal TN, Holst PJ, Schwartz TW. Selective elimination of high constitutive activity or chemokine binding in the human herpesvirus 8 encoded seven transmembrane oncogene ORF74. J Biol Chem. 2000;275:26309–26315. doi: 10.1074/jbc.M003800200. [DOI] [PubMed] [Google Scholar]
- Sherrill JD, Miller WE. G protein-coupled receptor (GPCR) kinase 2 regulates agonist-independent Gq/11 signaling from the mouse cytomegalovirus GPCR M33. J Biol Chem. 2006;281:39796–39805. doi: 10.1074/jbc.M610026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinger E, Langemeijer E, Siderius M, Vischer HF, Smit MJ. Herpesvirus-encoded GPCRs rewire cellular signaling. Mol Cell Endocrinol. 2011;331:179–184. doi: 10.1016/j.mce.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, Fraile-Ramos A, Lira SA, Soderberg-Naucler C, Smit MJ. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci Signal. 2010;3:ra58. doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- Soderberg-Naucler C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med. 2013;369:985–986. doi: 10.1056/NEJMc1302145. [DOI] [PubMed] [Google Scholar]
- Soroceanu L, Matlaf L, Bezrookove V, Harkins L, Martinez R, Greene M, Soteropoulos P, Cobbs CS. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011;71:6643–6653. doi: 10.1158/0008-5472.CAN-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streblow DN, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, Ruchti F, Mattison K, Altschuler Y, Nelson JA. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99:511–520. doi: 10.1016/S0092-8674(00)81539-1. [DOI] [PubMed] [Google Scholar]
- Streblow DN, Vomaske J, Smith P, Melnychuk R, Hall L, Pancheva D, Smit M, Casarosa P, Schlaepfer DD, Nelson JA. Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J Biol Chem. 2003;278:50456–50465. doi: 10.1074/jbc.M307936200. [DOI] [PubMed] [Google Scholar]
- Tschammer N. Allosteric modulation of the G protein-coupled US28 receptor of human cytomegalovirus: are the small-weight inverse agonist of US28 ‘camouflaged’ agonists? Bioorg Med Chem Lett. 2014;24:3744–3747. doi: 10.1016/j.bmcl.2014.06.082. [DOI] [PubMed] [Google Scholar]
- Tschische P, Moser E, Thompson D, Vischer HF, Parzmair GP, Pommer V, Platzer W, Schwarzbraun T, Schaider H, Smit MJ, Martini L, Whistler JL, Waldhoer M. The G-protein coupled receptor associated sorting protein GASP-1 regulates the signalling and trafficking of the viral chemokine receptor US28. Traffic. 2010;11:660–674. doi: 10.1111/j.1600-0854.2010.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Violin JD, Ren XR, Lefkowitz RJ. G-protein-coupled receptor kinase specificity for β-arrestin recruitment to the β2-adrenergic receptor revealed by fluorescence resonance energy transfer. J Biol Chem. 2006;281:20577–20588. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- Vischer HF, Hulshof JW, Hulscher S, Fratantoni SA, Verheij MH, Victorina J, Smit MJ, de Esch IJ, Leurs R. Identification of novel allosteric nonpeptidergic inhibitors of the human cytomegalovirus-encoded chemokine receptor US28. Bioorg Med Chem. 2010;18:675–688. doi: 10.1016/j.bmc.2009.11.060. [DOI] [PubMed] [Google Scholar]
- Vischer HF, Siderius M, Leurs R, Smit MJ. Herpesvirus-encoded GPCRs: neglected players in inflammatory and proliferative diseases? Nat Rev Drug Discov. 2014;13:123–139. doi: 10.1038/nrd4189. [DOI] [PubMed] [Google Scholar]
- Vomaske J, Nelson JA, Streblow DN. Human Cytomegalovirus US28: a functionally selective chemokine binding receptor. Infect. Disord. Drug Targets. 2009;9:548–556. doi: 10.2174/187152609789105696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Casarosa P, Rosenkilde MM, Smit MJ, Leurs R, Whistler JL, Schwartz TW. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem. 2003;278:19473–19482. doi: 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Kledal TN, Farrell H, Schwartz TW. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J Virol. 2002;76:8161–8168. doi: 10.1128/JVI.76.16.8161-8168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Feng H, Xu S, Feng P. Hijacking GPCRs by viral pathogens and tumor. Biochem Pharmacol. 2016;114:69–81. doi: 10.1016/j.bcp.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, He S, Wang Y, Brulois K, Lan K, Jung JU, Feng P. Herpesviral G protein-coupled receptors activate NFAT to induce tumor formation via inhibiting the SERCA calcium ATPase. PLoS Pathog. 2015;11:e1004768. doi: 10.1371/journal.ppat.1004768. [DOI] [PMC free article] [PubMed] [Google Scholar]