Abstract
Initial discovery on sphingosine 1-phosphate (S1P) as an intracellular second messenger was faced unexpectedly with roles of S1P as a first messenger, which subsequently resulted in cloning of its G protein-coupled receptors, S1P1–5. The molecular identification of S1P receptors opened up a new avenue for pathophysiological research on this lipid mediator. Cellular and molecular in vitro studies and in vivo studies on gene deficient mice have elucidated cellular signaling pathways and the pathophysiological meanings of S1P receptors. Another unexpected finding that fingolimod (FTY720) modulates S1P receptors accelerated drug discovery in this field. Fingolimod was approved as a first-in-class, orally active drug for relapsing multiple sclerosis in 2010, and its applications in other disease conditions are currently under clinical trials. In addition, more selective S1P receptor modulators with better pharmacokinetic profiles and fewer side effects are under development. Some of them are being clinically tested in the contexts of multiple sclerosis and other autoimmune and inflammatory disorders, such as, psoriasis, Crohn’s disease, ulcerative colitis, polymyositis, dermatomyositis, liver failure, renal failure, acute stroke, and transplant rejection. In this review, the authors discuss the state of the art regarding the status of drug discovery efforts targeting S1P receptors and place emphasis on potential clinical applications.
Keywords: Sphingosine 1-phosphate, G protein-coupled receptor, Fingolimod, FTY720, Drug discovery, S1P agonist
GPCR IN DRUG DISCOVERY
G protein-coupled receptors (GPCRs) constitute the largest superfamily of receptors for signaling molecules, ligands, and currently comprise some 865 receptors (Im, 2002, 2013; Fredriksson et al., 2003; Kihara et al., 2015). GPCRs are also known as 7TM receptors, because they have seven transmembrane domains. These receptors may signal though G proteins but they also initiate signals via other entities (Davenport et al., 2013). Many ligands, including hormones, autacoids, neurotransmitters, and very small molecules to large proteins can bind and activate GPCRs, and their activations lead to a multitude of physiological processes (Howard et al., 2001; Overington et al., 2006; Im, 2013).
GPCRs represent a major drug target in all clinical areas. Currently, about 40% of drugs on the market target GPCRs and regulate their activities positively or negatively, because a variety of GPCRs offer selectivity and specificity for many human diseases (Im, 2013). Examples of their applications include Claritin a H1 histamine receptor antagonist, Cozaar an AT1 angiotensin receptor antagonist, Neurontin a GABAB γ-aminobutyric acid receptor agonist, Plavix a P2Y12 ADP receptor antagonist, Singulair a CysLT1 leukotriene D4 receptor antagonist, Zantac a H2 histamine receptor antagonist, and Zyprexa a mixed D2/D1/5-HT2 dopamine/serotonin receptors antagonist. About 55 GPCRs have been cloned and identified as receptors for intercellular lipid mediators, and recent studies have unearthed their functional roles under both physiological and pathological conditions (Im, 2004, 2009, 2013). Furthermore, the number of drug discovery studies being conducted on GPCRs have increased in many fields including cancer, cardiac dysfunction, central nervous system disorders, inflammatory diseases, metabolic disorders, and obesity, (Im, 2004, 2009; Mutoh et al., 2012; Pyne et al., 2012; Choi and Chun, 2013; Makide et al., 2014; Proia and Hla, 2015). Here, we summarize current knowledge on one specific aspect of drug discovery involving interactions between GPCRs and the intercellular lipid mediator, sphingosine 1 phosphate (S1P).
SPHINGOSINE 1-PHOSPHATE AND ITS GPCRs
S1P is a bioactive lysophospholipid metabolite that can act as an intercellular lipid mediator (Moolenaar and Hla, 2012). Initially, S1P was reported to be a second messenger that mediates increases in intracellular calcium levels as a result of PDGF and IgE signaling (Olivera and Spiegel, 1993; Choi et al., 1996). However, S1P was also unexpectedly found to function as an intercellular first messenger like autacoids. The presence of S1P GPCRs in the plasma membrane was suggested by the pertussis toxin sensitivity in S1P-induced actions (Bunemann et al., 1995; Goodemote et al., 1995; Im et al., 1997; Okajima et al., 1997; van Koppen et al., 1996). The discovery of S1P1 (formerly known as Edg-1) in 1998 along with four other S1P2–5 receptors represents a milestone in sphingolipid biology (An et al., 1997; Lee et al., 1998; Lynch and Im, 1999; Okamoto et al., 1999; Im et al., 2000; Van Brocklyn et al., 2000; Yamazaki et al., 2000), and leads to the identification of a variety of biological functions mediated by interactions between S1P and S1P receptors (Sanchez and Hla, 2004). In particular, S1P regulates the response and function of various cellular and organ systems, such as, cell differentiation, cell migration, cell proliferation, immune response, trafficking of T and B cells, and vascular stability (Gardell et al., 2006; Huwiler and Pfeilschifter, 2008). S1P receptors exhibit overlapping or distinct expression patterns in various cells and tissues, and as a result, the various cellular functions of S1P have been assigned to S1P receptor subtypes. Furthermore, because S1P plays critical roles in autoimmune diseases, cancer, and diseases related to the cardiovascular, immune, nervous, and reproductive systems, S1P receptors become important treatment targets (Kihara et al., 2015). In addition, the discovery of S1P receptors made the screening and development of S1P agonists and antagonists accessible. Furthermore, discoveries of S1P receptor subtype-selective agonists or antagonists could provide novel therapeutic candidates (Im, 2010).
The discovery that fingolimod (also known as FTY720) is an agonist of four S1P receptor subtypes by researchers in immune therapeutics field accelerated the drug discovery in this area. Fingolimod has been approved as a first-in-class drug targeting S1P1 and several selective S1P receptor modulators are being subjected to clinical trials. In this review, we focus on drug discovery involving S1P receptors, especially S1P1.
DEVELOPMENT OF S1P1 RECEPTOR MODULATORS
Fingolimod (FTY720, Gilenya®, Novartis)
Fingolimod is a well-known success of drug discovery in the S1P research field. In 1995, fingolimod was produced from the immunosuppressive natural product myriocin, which was isolated from the fungus Isaria sinclairii (Billich et al., 2003; Im, 2003; Paugh et al., 2003). In 2002, the mechanism responsible for the immunosuppressive activity of fingolimod was determined to be due to the regulation of lymphocyte trafficking in a rodent model of multiple sclerosis (Brinkmann et al., 2002). Fingolimod, through S1P1, was found to directly alter the trafficking of naïve and antigen-activated CD4+ T cells and to control egress of lymphocytes from secondary lymphoid tissues and endothelial barrier function (Xie et al., 2003; Brinkmann et al., 2004; Chiba, 2005). In 2010, the Food and Drug Administration (FDA) approved fingolimod as the first oral disease-modifying drug to treat relapses of multiple sclerosis (Kihara et al., 2015). Multiple sclerosis is a demyelinating disease that damages axonal myelin sheaths in the brain and spinal cord. The primary action mechanism of fingolimod is to reduce lymphocyte egress from secondary lymphoid organ, thymus, and bone marrow, resulting in lymphopenia (Adachi and Chiba, 2008). Thereby, lymphopenia contributes to inhibit axon myelin sheath damage.
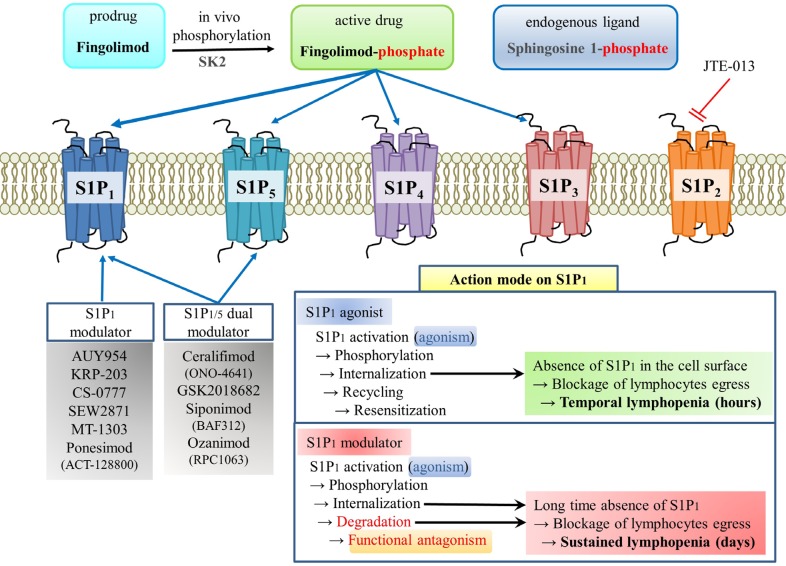
Fingolimod is a unique drug in two respects. First, fingolimod is a prodrug (Fig. 1). In vivo fingolimod is phosphorylated by sphingosine kinases (SK1 and SK2) and then phosphorylated fingolimod acts as an agonist on four S1P receptor subtypes (S1P1, S1P3, S1P4 and S1P5) (Billich et al., 2003). Second, effects of fingolimod on lymphocyte egress involve not agonistic but rather functional antagonistic activity against S1P1 (Fig. 1) (Matloubian et al., 2004). Both S1P and fingolimod-phosphate have been reported to induce lymphopenia via the agonistic activation of S1P1 and subsequent internalization of S1P1 in the lymphocytes (Brinkmann et al., 2004; Thangada et al., 2010). In fact, the absence of S1P1 on the cell surface blocks re-circulation of lymphocytes from secondary lymphoid organs to blood, because lymphocytes egress by chemotactic response to S1P concentration gradient (high in blood and low in lymph node) through S1P1 (Brinkmann et al., 2004; Chiba, 2009). In the case of S1P1 agonist like S1P, internalized S1P1 recycled back to the cell surface within several hours. However, in the case of S1P1 modulators like fingolimod-phosphate, internalized S1P1 undergoes proteosomal degradation, resulting in long-time absence of S1P1 until de novo synthesized (Oo et al., 2007). This kind of functional antagonism of fingolimod means it has a long action time, which can sometimes be disadvantageous (Subei and Cohen, 2015). The immune modulatory action of fingolimod has also been reported in autoimmune diseases other than multiple sclerosis, such as spontaneous autoimmnune polyneuropathy and experimental autoimmune neuritis (Kim et al., 2009; Zhang et al., 2009a).
Fig. 1.
Action mechanism of fingolimod and other S1P receptor modulators. Fingolimod is transformed to fingolimod-phosphate in vivo by sphingosine kinases. Fingolimod-phosphate can activate S1P1, S1P3, S1P4, and S1P5, and the fingolimod activation of S1P1 in lymphocytes leads to GRK2-mediated phosphorylation of C-terminal tail of S1P1, which recruits β-arrestin and induces S1P1 internalization. This internalization exposes S1P1 to proteosomal degradation, which prevents the recycling of S1P1 and results in the loss of S1P1 from the plasma membrane. This absence of S1P1 blocks lymphocyte egression from secondary lymphoid organs and reduces T and B cell counts in the blood. Lymphopenia is presumed to be the main mechanism whereby fingolimod causes immune suppression in autoimmune diseases like relapsing multiple sclerosis.
Other applications of fingolimod have also been suggested, such as, for the treatment of ischemia/reperfusion injury, which is the cellular damage that results from ischemia and re-supply of blood to infarcted tissues. In fact, it has been estimated that systemic inflammatory response after ischemia/reperfusion may account for 30–40% of intensive care unit mortalities (Eltzschig and Collard, 2004). In one animal study, fingolimod significantly inhibited leukocyte infiltration, peripheral blood lymphocyte counts, and vascular permeability in renal ischemia/reperfusion injury (Awad et al., 2006). In other studies, fingolimod attenuated arterial pressure and improved organ function when it was applied to heart or lung ischemia/reperfusion (Hofmann et al., 2009; Stone et al., 2015). Recently, fingolimod was reported to inhibit hypoxia/reperfusion-induced cardiomyocyte apoptosis by inhibiting caspase 3 activation, and by activating Akt and Erk signaling through S1P1/3 activation (Wang et al., 2014). These preclinical studies indicate that fingolimod has potential to be used for the treatment of ischemia/reperfusion injury and autoimmune disorders.
Fingolimod has been reported to attenuate neuroinflammation by regulating the activation and neuroprotective effects of microglia mainly via S1P1 (Jackson et al., 2011; Noda et al., 2013; Kolahdooz et al., 2015). In addition, fingolimod is known to inhibit allergen-induced airway inflammation and hyper-reactivity in mice (Ble et al., 2009; Marsolais et al., 2011; Trifilieff and Fozard, 2012). Furthermore, in low-density lipoprotein (LDL) receptor-deficient mice, fingolimod significantly attenuated atherosclerotic lesion formation, necrotic core formation, and lymphocyte function (Nofer et al., 2007). And peritoneal macrophages isolated from fingolimod-treated mice showed more of the M2 macrophage phenotype and less of the M1 macrophage phenotype (Nofer et al., 2007). However, Poti et al. (2012) reported in LDL receptor-deficient mice fed a Western diet, fingolimod reduced macrophage function, weight gain, and white adipose tissue amount, but failed to affect atherosclerosis.
A phase 3 clinical trial on fingolimod for primary progressive multiple sclerosis (INFORMS; NCT00731692) showed that it has anti-inflammatory effects but fails to reduce disease progression (Lublin et al., 2016). In addition, a phase 2 clinical trial for its effects on pulmonary function in moderate asthma patients was completed in 2009 (NCT00785083).
In a pilot study conducted in patients with acute ischemic stroke, fingolimod/alteplase combination therapy was well tolerated, attenuated reperfusion injury, and improved clinical outcomes (Fu et al., 2014; Li et al., 2015; Zhu et al., 2015). Fingolimod is now under a phase 2 clinical trial for acute stroke (NCT02002390). In addition, fingolimod is currently under phase 2 clinically testing in Rett’s syndrome (NCT02061137), phase 2 schizophrenia (NCT01779700), phase 4 neurodegeneration (NCT02575365), and multiple sclerosis (Table 1).
Table 1.
Summary of S1P receptor modulators currently undergoing or completed in clinical trials (based on data at http://www.clinicaltrials.gov/ in July
| Chemical | Target | Condition | Stage | Status | NLM ID |
|---|---|---|---|---|---|
| Fingolimod (FTY-720) | S1P1/3/4/5 | Relapsing remitting multiple sclerosis (RRMS) | Approved (2010) | ||
| Neurodegeneration | Phase IV | Active | NCT02575365 | ||
| Schizophrenia | Phase II | Active | NCT01779700 | ||
| Rett Syndrome | Phase II | Active | NCT02061137 | ||
| Acute Stroke | Phase II | Active | NCT02002390 | ||
| Amyotrophic lateral sclerosis | Phase II | Completed (2015) | NCT01786174 | ||
| Primary progressive multiple sclerosis | Phase III | Completed (2014) | NCT00731692 | ||
| Renal insufficiency | Phase I | Completed (2011) | NCT00731523 | ||
| Moderate asthma | Phase II | Completed (2009) | NCT00785083 | ||
| Renal/Kidney transplantation | Phase III | Completed (2006) | NCT00099801 | ||
| KRP-203 | S1P1 | Subacute cutaneous lupus erythematosus | Phase II | Active | NCT01294774 |
| Hematological malignancies | Phase I | Active | NCT01830010 | ||
| Siponimod (BAF312) | S1P1/5 | Secondary progressive multiple sclerosis | Phase III | Active | NCT01665144 |
| RRMS | Phase II | Completed (2012) | NCT00879658 | ||
| - (Extension) - | - Active - | NCT01185821 | |||
| Polymyositis | Phase II | Active | NCT01801917 | ||
| Active dermatomyositis | Phase II | Completed (2016) | NCT02029274 | ||
| Hepatic impairment | Phase I | Completed (2014) | NCT01565902 | ||
| Renal impairment | Phase I | Completed (2014) | NCT01904214 | ||
| CS-0777 | S1P1 | Multiple sclerosis | Phase I | Completed (2010) | NCT00616733 |
| Ponesimod (ACT-128800) | S1P1 | RRMS | Phase II | Active | NCT01093326 |
| Ponesimod vs teriflunomide in RRMS | Phase III | Active | NCT02425644 | ||
| Chronic GVHD | Phase II | Active | NCT02461134 | ||
| Psoriasis | Phase II | Completed (2012) | NCT01208090 | ||
| Ozanimod (RPC1063) | S1P1/5 | Multiple sclerosis | Phase III | Active | NCT02047734 |
| Ulcerative colitis | Phase III | Active | NCT02435992 | ||
| Crohn’s disease | Phase II | Active | NCT02531113 | ||
| Ceralifimod (ONO-4641) | S1P1/5 | RRMS | Phase II | Completed (2011) | NCT01081782 |
| - (Extension) - | Phase II | Terminated (2015) | NCT01226745 | ||
| GSK2018682 | S1P1/5 | RRMS | Phase I | Completed (2011) | NCT01466322 |
| MT-1303 | S1P1 | Crohn’s disease | Phase II | Active | NCT02378688 |
| Systemic lupus erythematosus | Phase I | Active | NCT02307643 | ||
| RRMS | Phase II | Completed (2014) | NCT01742052 | ||
| Plaque psoriasis | Phase II | Completed (2014) | NCT01987843 | ||
| Inflammatory bowel disease | Phase I | Completed (2014) | NCT01666327 |
RRMS, relapsing remitting multiple sclerosis.
Several side effects of fingolimod have been reported in three phase 3 trials (FREEDOMS, FREEDOM II, and TRANSFORMS) (Kappos et al., 2010; Calabresi et al., 2014; Cohen et al., 2016b). The common adverse effects were bradycardia at the first dose or atrioventricular block, macular edema, hypertension, headache, cough, dyspnea, back pain, influenza, and diarrhea (Subei and Cohen, 2015). First dose bradycardia is believed to be mediated via transient S1P1 activation in atrial myocytes, which would disappear by down-regulation of S1P1 (Camm et al., 2014). Unlike in man, S1P3 in atrial myocytes causes bradycardia in mice (Forrest et al., 2004; Sanna et al., 2004). Other adverse effects of fingolimod may be due to off-target effects via other S1P receptors, as it is a non-selective S1P agonist (S1P1, 3–5) (Brinkmann et al., 2002). Therefore, currently several S1P1 selective agonists/modulators are being developed as drug candidates in the wake of fingolimod (Fig. 1).
SEW2871
SEW2871 is a highly selective S1P1 agonist, which does not act on S1P2–5. As was expected, SEW2871 has been reported to reduce lymphocyte numbers in blood (Wei et al., 2005; Kim et al., 2009). As like fingolimod, SEW2871 also ameliorated ischemic acute renal failure after ischemia/reperfusion injury in mice (Lien et al., 2006). SEW2871 was also found to protect heart and liver tissues after myocardial or hepatic ischemia/reperfusion injury and these protective effects were ascribed to S1P1 activation (Hofmann et al., 2009; Park et al., 2010).
Several preclinical studies have shown SEW2871 has therapeutic implications in contexts of diabetes, Alzheimer’s disease, liver fibrosis, and inflammatory responses. In a non-obese mouse model of type 1 diabetes, SEW2871 inhibited monocyte adhesion to diabetic aortas and prevented monocyte/endothelial interactions, which suggests SEW2871-induced S1P1 signaling has potential for treatment of the vascular complications of type 1 diabetes (Whetzel et al., 2006). In a rat model of Alzheimer’s disease, chronic SEW2871 administration inhibited β amyloid (Aβ1–42)-induced spatial memory impairment and hippocampal neuronal loss, indicating the S1P1 signaling pathway offers a novel therapeutic target for the prevention of neurodegenerative disorders (Asle-Rousta et al., 2013).
SEW2871 was also found to modulate liver fibrosis by directly regulating the migration of human hepatic myofibroblasts into the damaged areas (Li et al., 2011). In LX-2 cells (a human hepatic stellate cell line), SEW2871 exerted a powerful migratory effect by increasing smooth muscle α-actin, procollagen αI and αIII, and total hydroxyproline contents (Liu et al., 2011). It has also been reported SEW2817 has the following anti-inflammatory effects; it inhibits dendritic cell chemotaxis and migration to lymph nodes, causes switching to the M2 macrophage phenotype, and decreases proinflammatory cytokine levels under inflammatory conditions (Gollmann et al., 2008; Hughes et al., 2008). The intravenous administration of SEW2871 was found to attenuate LPS-induced acute inflammatory lung injury (Sammani et al., 2010). Therefore, multiple applications have been suggested for S1P1 agonists.
KRP-203
KRP-203 is an immunosuppressive S1P1 agonist with a molecular structure similar to that of fingolimod (Shimizu et al., 2005). Like fingolimod and SEW2871, KRP-203 can regulate lymphocyte homing and has an immunosuppressive activity (Shimizu et al., 2005). The action mechanism of KRP-203 is identical to that of fingolimod, that is, it involves the in vivo phosphorylation and functional antagonism of S1P1. Furthermore, KRP-203-phosphate has an agonistic effect on S1P5 like S1P1, but not S1P2–4 (Lukas et al., 2014). KRP-203 has been developed for use in organ transplantation. In 2005, KRP-203 was reported to prolong graft survival significantly and to reduce chronic rejection and graft vasculopathy in rat skin and heart allografts (Shimizu et al., 2005; Takahashi et al., 2005), and in 2006, it was suggested to be a potential immune modulator after rat renal transplantation (Fujishiro et al., 2006).
KRP-203 has also been applied in several experimental models for autoimmune disorders and inflammatory bowel diseases. In a rat experimental autoimmune myocarditis model, KRP-203 significantly inhibited the infiltrations of macrophages and CD4+ T cells into myocardium, and reduced areas of inflammation (Ogawa et al., 2007). In a murine model of concanavalin A-induced autoimmune hepatitis, KRP-203 increased lymphocyte sequestration in secondary lymph nodes and decreased numbers of CD4+ lymphocytes in liver (Kaneko et al., 2006).
The effects of KRP-203 have also been examined in inflammatory disorders, such as, Crohn’s disease and atherosclerosis. In an interleukin (IL)-10 gene-deficient (IL-10−/−) mouse model of chronic colitis, KRP-203 inhibited body weight loss and proinflammatory cytokine production, suppressed lymphocyte infiltration at inflammatory sites, and prevented chronic colitis (Song et al., 2008). In LDL receptor-deficient mice on cholesterol-rich diet, KRP-203 dramatically suppressed atherosclerotic lesion formation and induced lymphopenia, and in vitro, inhibited tumor necrosis factor-α, IL-6, and interferonγ-induced protein-10 (Poti et al., 2013).
Currently, KRP-203 is undergoing on a phase 2 clinical trial for subacute lupus erythematosus (NCT01294774), and a phase 1 clinical trial to evaluate its safety, tolerability, pharmacokinetics, and efficacy in patients undergoing stem cell transplantation for hematological malignancies (NCT01830010) (Table 1).
AUY954
AUY954 is an aminocarboxylate analogue of fingolimod and a potent and selective S1P1 agonist (Pan et al., 2006). AUY954 has been demonstrated to have beneficial effects after rat heart transplantation, in experimental autoimmune neuritis, and on lung inflammation. In a stringent rat transplantation model, AUY954 decreased circulating lymphocytes and prolonged cardiac allograft survival (Pan et al., 2006). In addition, in an animal model of experimental autoimmune neuritis (a T cell-mediated autoimmune inflammatory demyelinating disease of nervous system), AUY954 sequestered lymphocytes into secondary lymphoid tissues and significantly inhibited inflammatory demyelination, immune cell infiltration, and expressions of IL-17 and metalloproteinase-9 in rat sciatic nerves (Zhang et al., 2009b).
The actions of AUY954 in respiratory disorders are complicated. In an allergen-induced airway inflammation model, intranasal administration of AUY954 inhibited lymphocyte accumulation in bronchoalveolar lavage fluid, but no effect on eosinophils (Ble et al., 2009). On the other hand, AUY954 inhibited airway chemokine release and accumulations of activated T cells and eosinophils in ovalbumin-induced eosinophilic airway inflammation (Marsolais et al., 2011). However, prolonged exposure of AUY954 dramatically worsened lung injury, vascular leak, and mortality in a mouse model of bleomycin-induced lung injury, and repeated AUY954 administration increased pulmonary fibrosis by inducing vascular leak (Shea et al., 2010). This cautions the effects of S1P1 modulators on respiratory disorders require careful interpretation.
Siponimod (BAF312)
Siponimod (also known as BAF312) is a novel alkoxyimino derivative and an agonist of S1P1 and S1P5 (Gergely et al., 2012). Siponimod is being investigated in the context of multiple sclerosis (Subei and Cohen, 2015). Like fingolimod, siponimod induces lymphopenia by preventing lymphocyte egress from lymph nodes (Fryer et al., 2012). In addition, siponimod was found to completely suppress experimental autoimmune encephalomyelitis in a rat model (Gergely et al., 2012), and in another study, to inhibit LPC-induced demyelination in organotypic slice cultures and attenuate LPS or TNFα/IL-17-induced IL-6 production in astrocytes and microglia (O’Sullivan et al., 2016). These findings suggest that siponimod and fingolimod may act directly through brain cells as well as through lymphopenia (Choi et al., 2011; O’Sullivan et al., 2016).
In healthy individuals, siponimod reduced T and B cell numbers in blood within 4–6 h, and numbers recovered to basal levels within a week after stopping treatment (Gergely et al., 2012). Siponimod may be an effective treatment for immune-mediated diseases. Initial and extended phase 2 clinical trials in patients with relapsing-remitting multiple sclerosis (BOLD) have been successfully completed (NCT 00879658, NCT01185821) (Selmaj et al., 2013; Kappos et al., 2016).
Currently, siponimod is undergoing a phase 3 efficacy and safety clinical trial in patients with secondary progressive multiple sclerosis (NCT01665144) along with mechanistic studies of phase 3 trial (NCT02330965). Also a phase 2 efficacy and tolerability clinical trial for polymyositis is undergoing (NCT01801917). A phase 2 clinical trial for dermatomyositis (NCT02029274), and two phase 1 pharmacokinetic trials for renal and hepatic impairments (NCT01904214 and NCT01565902) have been completed (Table 1).
CS-0777
CS-0777 is a selective S1P1 modulator that is currently being developed for the treatment of autoimmune diseases such as multiple sclerosis (Moberly et al., 2012a). CS-0777 is phosphorylated in vivo, and the phosphorylated CS-0777 acts as a selective S1P1 agonist like fingolimod (Nishi et al., 2011). CS-0777 has also been investigated in multiple sclerosis, like almost all other S1P1 agonists, it induces lymphopenia and suppresses experimental autoimmune encephalomyelitis (Nishi et al., 2011). In an open-label, pilot phase 1, clinical trial on healthy individuals and multiple sclerosis patients, oral CS-0777 decreased numbers of lymphocytes and CD4+ T cells in blood, and levels returned to normal condition within 4 weeks of discontinuation (NCT00616733) (Moberly et al., 2012a, 2012b) (Table 1).
Ponesimod (ACT-128800)
Ponesimod is an orally active, selective S1P1 agonist that induces sequestration of lymphocytes into lymphoid organs (Bolli et al., 2010). In contrast to the long half-life and slow elimination of fingolimod, ponesimod is eliminated within 1 week after discontinuation and its pharmacological effects are rapidly reversible (D’Ambrosio et al., 2016).
The clinical pharmacology of ponesimod has been described in several studies. In lymphocyte-mediated inflammatory diseases, ponesimod reduced several types of inflammation response, including edema formation, inflammatory cell infiltration, and proinflammatory cytokine levels (Piali et al., 2011). In addition, in a non-obese diabetic mouse model of autoimmune diabetes, ponesimod protected against disease development by reducing numbers of B and T cells in blood and spleen (You et al., 2013). Ponesimod is viewed as a potential new therapeutic strategy for autoimmune disorders. Once-daily treatment (10, 20 or 40 mg) significantly reduced the number of new T1 Gd+ lesions and had beneficial effects on clinical endpoints in relapsing-remitting multiple sclerosis (NCT01006265) (Olsson et al., 2014). Currently, a long-term safety phase 2 clinical trial in relapsing-remitting multiple sclerosis (NCT01093326) and a phase 3 oral ponesimod vs teriflunomide trial in relapsing multiple sclerosis (NCT02425644) are being conducted.
Psoriasis is a long-lasting autoimmune disease characterized by patches of abnormal skin, which are typically itchy, red, and scaly. Because psoriasis is a T cell-mediated inflammatory skin disease, studies on ponesimod have been conducted in this context. In particular, a randomized, double-blind, placebo-controlled phase 2 clinical trial showed the efficacy, safety, and tolerability of oral ponesimod in chronic plaque psoriasis (NCT01208090) (Vaclavkova et al., 2014).
Ponesimod is also under phase 2 clinical trial for chronic graft-versus-host disease (GVHD) (NCT02461134), which is a complication encountered after stem cell or bone marrow transplantation. GVHD has autoimmune-like features and chronic GVHD involves both autoreactive and alloreactive T and B cells. In GVHD, newly transplanted donor cells attack the transplant recipient’s body. As S1P1 modulators suppress vascular damage and immune cell accumulation in skin, and thus, reduce immune response (Huu et al., 2013), they offer potential means to targeting GVHD.
Ozanimod (RPC1063)
Ozanimod is an oral selective S1P1/5 dual modulator, and induces lymphopenia and regulates immune response (Scott et al., 2016). In three models of autoimmune diseases that is, experimental autoimmune encephalitis, TNBS-induced colitis, and CD4+ CD45RBhi T cell adaptive transfer colitis, oral ozanimod diminished inflammation parameters. This finding supports the clinical development of ozanimod for multiple sclerosis (Cohen et al., 2016a; Scott et al., 2016).
Inflammatory bowel disease is a disease of the small intestine and colon and is classified as Crohn’s disease or ulcerative colitis. Crohn’s disease affects the entire gastrointestinal tract, whereas ulcerative colitis usually affects colon and rectum. Because inflammatory bowel disease is a type of autoimmune disease, it is considered immunosuppression may allow control of its symptoms. Ozanimod induces peripheral lymphocyte sequestration and reduces circulating lymphocyte counts in the gastrointestinal tract (Rivera-Nieves, 2015). In a double-blind, placebo-controlled phase 2 trial in ulcerative colitis, ozanimod induced a significantly higher rate of clinical remission than a placebo (NCT01647516) (Sandborn et al., 2016), which suggests S1P1 modulation offers a means of treating inflammatory bowel disease. Currently, ozanimod is under phase 3 trials in relapsing multiple sclerosis (NCT02047734) and moderate to severe ulcerative colitis (NCT02435992), and a phase 2 trial in moderate to severe Crohn’s disease (NCT02531113) (Table 1).
Ceralifimod (ONO-4641)
Ceralifimod is a selective S1P1/5 dual agonist (Ohno et al., 2010). Like fingolimod, it suppresses peripheral blood lymphocyte counts in rats by inhibiting lymphocyte egress from secondary lymphoid tissues (Ohno et al., 2010). Ceralifimod was also found to prevent relapsing-remitting experimental autoimmune encephalomyelitis in a non-obese diabetic mouse model (Ohno et al., 2010; Komiya et al., 2013). A phase 2 clinical trial of ceralifimod was completed for relapsing-remitting multiple sclerosis (NCT01081782) in 2011. However, a phase 2 safety and efficacy extension study of ceralifimod was terminated by developers (NCT01226745) (Subei and Cohen, 2015).
GSK2018682
GSK2018682 is a potent S1P1 and S1P5 agonist (Xu et al., 2014). In an experimental autoimmune encephalomyelitis mouse model, GSK2018682 and fingolimod exhibited similar efficacy. A phase 1 clinical trial on GSK2018682 for relapsing-remitting multiple sclerosis was completed in 2011 (NCT01466322).
MT-1303
MT-1303 is a selective S1P1 modulator (a functional antagonist) that is undergoing development. MT-1303 has been subjected to a phase 2 study in moderate to severe chronic plaque psoriasis (NCT01987843), a phase 2 study of MT-1303 in relapsing-remitting multiple sclerosis (NCT01742052), and a phase 1 for inflammatory bowel disease (NCT01666327). Currently, phase 2 clinical trials are being conducted for Crohn’s disease (NCT02378688) and systemic lupus erythematosus (NCT02307643) (Table 1 and www.clinicaltrials.gov).
DEVELOPMENT OF S1P RECEPTOR ANTAGONIST
JTE-013
JTE-013 is a potent, selective S1P2 antagonist that has no effect on the other four S1P receptors (Osada et al., 2002), and has been mainly used to study the roles and functions of S1P2 in different cell types and diseases.
It has been established that S1P affects various cellular responses in endothelial cells, such as, cytoskeletal re-structuring and cell-extracellular/intracellular matrix interactions. S1P2 signaling is involved in microvascular permeability, and it has been reported JTE-013 inhibition of S1P2 significantly inhibited microvascular permeability in an in vivo animal model and regulated endothelial tight junctions and barrier function in vitro (Lee et al., 2009). In a recent preclinical study, JTE-013 modulated the responses of brain endothelium by inhibiting cerebrovascular permeability, the development of intracerebral heamorrhage, and neurovascular injury in an experimental model of stroke (Kim et al., 2015). After injecting the myotoxic drug notexin to induce muscle degeneration, JTE-013 treatment delayed regeneration of muscle and reduced levels of myogenin (a muscle differentiation marker), and the phosphorylation of Akt (a key marker of muscle growth) (Germinario et al., 2012). Therefore, S1P2 signaling plays an important role in microvascular permeability and muscle regeneration.
During allergic response in lung, mast cells contain many granules rich in histamine and heparin, and thus, play central roles in various allergic diseases and anaphylaxis. S1P2 expressed in mast cells was found to be involved in S1P-induced RBL-2H3 mast cell migration (Yokoo et al., 2004). JTE-013 also inhibited responses to ovalbumin and allergen-induced mast cell activation in rats (Trifilieff et al., 2009). Treatment with JTE-013 attenuated IgE-stimulated anaphylactic responses and pulmonary edema in mice (Oskeritzian et al., 2010), and in in vitro and in vivo studies, JTE-013 reduced mast cell activation, airway infiltration, and the serum levels of histamine and several cytokines (Oskeritzian et al., 2015). In addition, JTE-013 has been reported to inhibit S1P-induced fibroblast chemotaxis, Rho activation, and focal adhesion kinase phosphorylation, which all affect tissue repair after injury (Hashimoto et al., 2008). JTE-013 also blocked S1P-induced inhibition of migration and Rac1-dependent signaling pathway in human bronchial smooth muscle cells (Kawata et al., 2005). These findings suggest that S1P2 antagonism offers a novel means of treating airway allergic diseases.
Pancreatic β cell dysfunction contributes to the development of insulin resistance and of type 2 diabetes, and several authors have reported relations between S1P2 and type 2 diabetes. In a murine model, JTE-013 suppressed streptozotocin-induced blood glucose increases, pancreatic β cell apoptosis, and the incidence of diabetes (Imasawa et al., 2010). Furthermore, JTE-013 protected pancreatic β cells in a New Zealand obese diabetic mouse model under high-fat diet conditions (Japtok et al., 2015). Levels of plasminogen activator inhibitor-1, which is produced from adipocytes, are increased in obese individuals. Interestingly, JTE-013 suppressed plasminogen activator inhibitor-1 increases in mouse 3T3-L1 adipocytes (Ito et al., 2013), and S1P2 deficient mice fed a high-fat diet had better glucose/insulin tolerance test results and smaller epididymal adipocytes (Kitada et al., 2016). These results suggest JTE-013 might be useful for treating obesity and type 2 diabetes.
Treatment with JTE-013 also reduced plasma levels of IL-1β and IL-18 (endotoxin-induced inflammatory cytokines) in ApoE−/− mice and S1P2 gene deficiency reduced atherosclerosis (Skoura et al., 2011). Furthermore, JTE-013 modulated the permeability and inflammatory responses of the vascular endothelium during endotoxemia (Zhang et al., 2013), and S1P2 was suggested to be a critical receptor in macrophages due to its impairment of phagocytosis and antimicrobial defense during the pathogenesis of sepsis (Hou et al., 2015). These findings indicate JTE-013 offers a novel means of treating inflammatory disorders, such as, atherosclerosis and sepsis.
CLOSING REMARK
The discovery of S1P receptors and subsequent finding of fingolimod as a modulator of S1P1 receptor successfully linked to the FDA approval of fingolimod as a first orally active drug treating relapsing multiple sclerosis. The commercial release of fingolimod stimulated pharmaceutical researchers to develop better drugs targeting S1P1 in terms of efficacy and safety, and these efforts have resulted in multiple drug candidates for clinical trials (Table 1). Much effort has been made to overcome the non-selectivity of fingolimod, which acts on S1P1 and S1P3–5, and as a result S1P1 monoselective agonists, such as AUY954, CS-0777, KRP-203, SEW2871, ponesimod, and MT-1303, have been developed. Dual agonists on S1P1 and S1P5 have also been produced, such as, ceralifimod, siponimod, ozanimod, and GSK2018682. The first clinical approval issued for fingolimod was for the treatment of relapsing multiple sclerosis. Currently, fingolimod and other S1P1 modulators are being developed for autoimmune disease and for inflammatory disorders, such as, plaque psoriasis, dermatomyositis, Crohn’s disease, ulcerative colitis, polymyositis, liver failure, renal failure, acute stroke, GVHD, and transplant rejection. Because these exhibit greater selectivity for S1P1 than fingolimod, it is hoped that they will cause fewer adverse effects and be more effective. We are confident that in near future, more S1P receptor modulators will be approved for the treatment of disorders associated with autoimmune and inflammation.
Acknowledgments
This research was supported by the Basic Science Research Program of the Korean National Research Foundation funded by the Korean Ministry of Education, Science and Technology (NRF-2016R1D1A1A009917086) and by the Korean National Research Foundation funded by the Korean government (MSIP) (Grant no. 2009-0083538).
REFERENCES
- Adachi K, Chiba K. FTY720 story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspect Medicin Chem. 2008;1:11–23. [PMC free article] [PubMed] [Google Scholar]
- An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/S0014-5793(97)01301-X. [DOI] [PubMed] [Google Scholar]
- Asle-Rousta M, Oryan S, Ahmadiani A, Rahnema M. Activation of sphingosine 1-phosphate receptor-1 by SEW2871 improves cognitive function in Alzheimer’s disease model rats. EXCLI J. 2013;12:449–461. [PMC free article] [PubMed] [Google Scholar]
- Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Billich A1, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Ble FX, Cannet C, Zurbruegg S, Gerard C, Frossard N, Beckmann N, Trifilieff A. Activation of the lung S1P1 receptor reduces allergen-induced plasma leakage in mice. Br J Pharmacol. 2009;158:1295–1301. doi: 10.1111/j.1476-5381.2009.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli MH, Abele S, Binkert C, Bravo R, Buchmann S, Bur D, Gatfield J, Hess P, Kohl C, Mangold C, Mathys B, Menyhart K, Muller C, Nayler O, Scherz M, Schmidt G, Sippel V, Steiner B, Strasser D, Treiber A, Weller T. 2-iminothiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem. 2010;53:4198–4211. doi: 10.1021/jm100181s. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Bunemann M, Brandts B, zu Heringdorf DM, van Koppen CJ, Jakobs KH, Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J Physiol. 1995;489:701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, Li B, Cappiello L, von Rosenstiel P, Lublin FD. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- Camm J, Hla T, Bakshi R, Brinkmann V. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am Heart J. 2014;168:632–644. doi: 10.1016/j.ahj.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Chiba K. New therapeutic approach for autoimmune diseases by the sphingosine 1-phosphate receptor modulator, fingolimod (FTY720) Yakugaku zasshi. 2009;129:655–665. doi: 10.1248/yakushi.129.655. [DOI] [PubMed] [Google Scholar]
- Choi JW, Chun J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta. 2013;1831:20–32. doi: 10.1016/j.bbalip.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Arnold DL, Comi G, Bar-Or A, Gujrathi S, Hartung JP, Cravets M, Olson A, Frohna PA, Selmaj KW. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016a;15:373–381. doi: 10.1016/S1474-4422(16)00018-1. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Khatri B, Barkhof F, Comi G, Hartung HP, Montalban X, Pelletier J, Stites T, Ritter S, von Rosenstiel P, Tomic D, Kappos L. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatr. 2016b;87:468–475. doi: 10.1136/jnnp-2015-310597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio D, Freedman MS, Prinz J. Ponesimod, a selective S1P1 receptor modulator: a potential treatment for multiple sclerosis and other immune-mediated diseases. Ther Adv Chronic Dis. 2016;7:18–33. doi: 10.1177/2040622315617354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, Liew WC, Mpamhanga CP, Bonner TI, Neubig RR, Pin JP, Spedding M, Harmar AJ. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev. 2013;65:967–986. doi: 10.1124/pr.112.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, Hale J, Keohane C, Meyers C, Milligan J, Mills S, Nomura N, Rosen H, Rosenbach M, Shei GJ, Singer II, Tian M, West S, White V, Xie J, Proia RL, Mandala S. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J Pharmacol Exp Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Muthukumarana A, Harrison PC, Nodop Mazurek S, Chen RR, Harrington KE, Dinallo RM, Horan JC, Patnaude L, Modis LK, Reinhart GA. The clinically-tested S1P receptor agonists, FTY720 and BAF312, demonstrate subtype-specific bradycardia (S1P1) and hypertension (S1P3) in rat. PLoS ONE. 2012;7:e52985. doi: 10.1371/journal.pone.0052985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, Yan Y, Huang D, Yu C, Shi FD. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71:1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- Fujishiro J, Kudou S, Iwai S, Takahashi M, Hakamata Y, Kinoshita M, Iwanami S, Izawa S, Yasue T, Hashizume K, Murakami T, Kobayashi E. Use of sphingosine-1-phosphate 1 receptor agonist, KRP-203, in combination with a subtherapeutic dose of cyclosporine A for rat renal transplantation. Transplantation. 2006;82:804–812. doi: 10.1097/01.tp.0000232687.78242.cd. [DOI] [PubMed] [Google Scholar]
- Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, Pan S, Gray NS, Hinterding K, Cooke NG, Groenewegen A, Vitaliti A, Sing T, Luttringer O, Yang J, Gardin A, Wang N, Crumb WJ, Jr, Saltzman M, Rosenberg M, Wallstrom E. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167:1035–1047. doi: 10.1111/j.1476-5381.2012.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germinario E, Peron S, Toniolo L, Betto R, Cencetti F, Donati C, Bruni P, Danieli-Betto D. S1P2 receptor promotes mouse skeletal muscle regeneration. J Appl Physiol. 2012;113:707–713. doi: 10.1152/japplphysiol.00300.2012. [DOI] [PubMed] [Google Scholar]
- Gollmann G, Neuwirt H, Tripp CH, Mueller H, Konwalinka G, Heufler C, Romani N, Tiefenthaler M. Sphingosine-1-phosphate receptor type-1 agonism impairs blood dendritic cell chemotaxis and skin dendritic cell migration to lymph nodes under inflammatory conditions. Int Immunol. 2008;20:911–923. doi: 10.1093/intimm/dxn050. [DOI] [PubMed] [Google Scholar]
- Goodemote KA, Mattie ME, Berger A, Spiegel S. Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem. 1995;270:10272–10277. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Wang X, Mao L, Kobayashi T, Kawasaki S, Mori N, Toews ML, Kim HJ, Cerutis DR, Liu X, Rennard SI. Sphingosine 1-phosphate potentiates human lung fibroblast chemotaxis through the S1P2 receptor. Am J Respir Cell Mol Biol. 2008;39:356–363. doi: 10.1165/rcmb.2006-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, Ritter O, Bonz A. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- Hou J, Chen Q, Zhang K, Cheng B, Xie G, Wu X, Luo C, Chen L, Liu H, Zhao B, Dai K, Fang X. Sphingosine 1-phosphate receptor 2 signaling suppresses macrophage phagocytosis and impairs host defense against sepsis. Anesthesiology. 2015;123:409–422. doi: 10.1097/ALN.0000000000000725. [DOI] [PubMed] [Google Scholar]
- Howard AD, McAllister G, Feighner SD, Liu Q, Nargund RP, Van der Ploeg LH, Patchett AA. Orphan G-protein-coupled receptors and natural ligand discovery. Trends Pharmacol Sci. 2001;22:132–140. doi: 10.1016/S0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu DL, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, Fujimoto M. FTY720 ameliorates murine sclerodermatous chronic graft-versus-host disease by promoting expansion of splenic regulatory cells and inhibiting immune cell infiltration into skin. Arthritis Rheum. 2013;65:1624–1635. doi: 10.1002/art.37933. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Pfeilschifter J. New players on the center stage: sphingosine 1-phosphate and its receptors as drug targets. Biochem Pharmacol. 2008;75:1893–1900. doi: 10.1016/j.bcp.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Im DS. Orphan G protein-coupled receptors and beyond. Jpn J Pharmacol. 2002;90:101–106. doi: 10.1254/jjp.90.101. [DOI] [PubMed] [Google Scholar]
- Im DS. Linking Chinese medicine and G-protein-coupled receptors. Trends Pharmacol Sci. 2003;24:2–4. doi: 10.1016/S0165-6147(02)00012-3. [DOI] [PubMed] [Google Scholar]
- Im DS. Discovery of new G protein-coupled receptors for lipid mediators. J Lipid Res. 2004;45:410–418. doi: 10.1194/jlr.R300006-JLR200. [DOI] [PubMed] [Google Scholar]
- Im DS. New intercellular lipid mediators and their GPCRs: an update. Prostaglandins Other Lipid Medat. 2009;89:53–56. doi: 10.1016/j.prostaglandins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Im DS. Pharmacological tools for lysophospholipid GPCRs: development of agonists and antagonists for LPA and S1P receptors. Acta Pharmacol Sin. 2010;31:1213–1222. doi: 10.1038/aps.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS. Intercellular lipid mediators and GPCR drug discovery. Biomol. Ther. (Seoul) 2013;21:411–422. doi: 10.4062/biomolther.2013.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS, Fujioka T, Katada T, Kondo Y, Ui M, Okajima F. Characterization of sphingosine 1-phosphate-induced actions and its signaling pathways in rat hepatocytes. Am J Physiol. 1997;272:G1091–G1099. doi: 10.1152/ajpgi.1997.272.5.G1091. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Ancellin N, O’Dowd BF, Shei GJ, Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, George SR, Lynch KR. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J Biol Chem. 2000;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- Imasawa T, Koike K, Ishii I, Chun J, Yatomi Y. Blockade of sphingosine 1-phosphate receptor 2 signaling attenuates streptozotocin-induced apoptosis of pancreatic b-cells. Biochem Biophys Res Commun. 2010;392:207–211. doi: 10.1016/j.bbrc.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Iwaki S, Koike K, Yuda Y, Nagasaki A, Ohkawa R, Yatomi Y, Furumoto T, Tsutsui H, Sobel BE, Fujii S. Increased plasma sphingosine-1-phosphate in obese individuals and its capacity to increase the expression of plasminogen activator inhibitor-1 in adipocytes. Coron Artery Dis. 2013;24:642–650. doi: 10.1097/MCA.0000000000000033. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Giovannoni G, Baker D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflammation. 2011;8:76. doi: 10.1186/1742-2094-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japtok L, Schmitz EI, Fayyaz S, Kramer S, Hsu LJ, Kleuser B. Sphingosine 1-phosphate counteracts insulin signaling in pancreatic β-cells via the sphingosine 1-phosphate receptor subtype 2. FASEB J. 2015;29:3357–3369. doi: 10.1096/fj.14-263194. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Murakami T, Kawana H, Takahashi M, Yasue T, Kobayashi E. Sphingosine-1-phosphate receptor agonists suppress concanavalin A-induced hepatic injury in mice. Biochem Biophys Res Commun. 2006;345:85–92. doi: 10.1016/j.bbrc.2006.04.067. [DOI] [PubMed] [Google Scholar]
- Kappos L, Li DK, Stuve O, Hartung HP, Freedman MS, Hemmer B, Rieckmann P, Montalban X, Ziemssen T, Hunter B, Arnould S, Wallstrom E, Selmaj K. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: dose-blinded, randomized extension of the phase 2 BOLD study. JAMA Neurol. 2016;73:1089–1098. doi: 10.1001/jamaneurol.2016.1451. [DOI] [PubMed] [Google Scholar]
- Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P, Group FS. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Kawata T, Ishizuka T, Tomura H, Hisada T, Dobashi K, Tsukagoshi H, Ishiwara M, Kurose H, Mori M, Okajima F. Sphingosine 1-phosphate inhibits migration and RANTES production in human bronchial smooth muscle cells. Biochem Biophys Res Commun. 2005;331:640–647. doi: 10.1016/j.bbrc.2005.03.223. [DOI] [PubMed] [Google Scholar]
- Kihara Y, Mizuno H, Chun J. Lysophospholipid receptors in drug discovery. Exp Cell Res. 2015;333:171–177. doi: 10.1016/j.yexcr.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GS, Yang L, Zhang G, Zhao H, Selim M, McCullough LD, Kluk MJ, Sanchez T. Critical role of sphingosine-1-phosphate receptor-2 in the disruption of cerebrovascular integrity in experimental stroke. Nat Commun. 2015;6:7893. doi: 10.1038/ncomms8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Jung CG, Dukala D, Bae H, Kakazu R, Wollmann R, Soliven B. Fingolimod and related compounds in a spontaneous autoimmune polyneuropathy. J Neuroimmunol. 2009;214:93–100. doi: 10.1016/j.jneuroim.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada Y, Kajita K, Taguchi K, Mori I, Yamauchi M, Ikeda T, Kawashima M, Asano M, Kajita T, Ishizuka T, Banno Y, Kojima I, Chun J, Kamata S, Ishii I, Morita H. Blockade of sphingosine 1-phosphate receptor 2 signaling attenuates high-fat diet-induced adipocyte hypertrophy and systemic glucose intolerance in mice. Endocrinology. 2016;157:1839–1851. doi: 10.1210/en.2015-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahdooz Z, Nasoohi S, Asle-Rousta M, Ahmadiani A, Dargahi L. Sphingosin-1-phosphate receptor 1: a potential target to inhibit neuroinflammation and restore the sphingosin-1-phosphate metabolism. Can J Neurol Sci. 2015;42:195–202. doi: 10.1017/cjn.2015.19. [DOI] [PubMed] [Google Scholar]
- Komiya T, Sato K, Shioya H, Inagaki Y, Hagiya H, Kozaki R, Imai M, Takada Y, Maeda T, Kurata H, Kurono M, Suzuki R, Otsuki K, Habashita H, Nakade S. Efficacy and immunomodulatory actions of ONO-4641, a novel selective agonist for sphingosine 1-phosphate receptors 1 and 5, in preclinical models of multiple sclerosis. Clin Exp Immunol. 2013;171:54–62. doi: 10.1111/j.1365-2249.2012.04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D, Lee MJ. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009;296:H33–H42. doi: 10.1152/ajpheart.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Li C, Zheng S, You H, Liu X, Lin M, Yang L, Li L. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J Hepatol. 2011;54:1205–1213. doi: 10.1016/j.jhep.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Li YJ, Chang GQ, Liu Y, Gong Y, Yang C, Wood K, Shi FD, Fu Y, Yan Y. Fingolimod alters inflammatory mediators and vascular permeability in intracerebral hemorrhage. Neurosci Bull. 2015;31:755–762. doi: 10.1007/s12264-015-1532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P1-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- Liu X, Yue S, Li C, Yang L, You H, Li L. Essential roles of sphingosine 1-phosphate receptor types 1 and 3 in human hepatic stellate cells motility and activation. J Cell Physiol. 2011;226:2370–2377. doi: 10.1002/jcp.22572. [DOI] [PubMed] [Google Scholar]
- Lublin F, Miller DH, Freedman MS, Cree BA, Wolinsky JS, Weiner H, Lubetzki C, Hartung HP, Montalban X, Uitdehaag BM, Merschhemke M, Li B, Putzki N, Liu FC, Haring DA, Kappos L. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1075–1084. doi: 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- Lukas S, Patnaude L, Haxhinasto S, Slavin A, Hill-Drzewi M, Horan J, Modis LK. No differences observed among multiple clinical S1P1 receptor agonists (functional antagonists) in S1P1 receptor down-regulation and degradation. J Biomol Screen. 2014;19:407–416. doi: 10.1177/1087057113502234. [DOI] [PubMed] [Google Scholar]
- Lynch KR, Im DS. Life on the edg. Trends Pharmacol Sci. 1999;20:473–475. doi: 10.1016/S0165-6147(99)01401-7. [DOI] [PubMed] [Google Scholar]
- Makide K, Uwamizu A, Shinjo Y, Ishiguro J, Okutani M, Inoue A, Aoki J. Novel lysophosphoplipid receptors: their structure and function. J Lipid Res. 2014;55:1986–1995. doi: 10.1194/jlr.R046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Yagi S, Kago T, Leaf N, Rosen H. Modulation of chemokines and allergic airway inflammation by selective local sphingosine-1-phosphate receptor 1 agonism in lungs. Mol Pharmacol. 2011;79:61–68. doi: 10.1124/mol.110.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Moberly JB, Ford DM, Zahir H, Chen S, Mochizuki T, Truitt KE, Vollmer TL. Pharmacological effects of CS-0777, a selective sphingosine 1-phosphate receptor-1 modulator: results from a 12-week, open-label pilot study in multiple sclerosis patients. J Neuroimmunol. 2012a;246:100–107. doi: 10.1016/j.jneuroim.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Moberly JB, Rohatagi S, Zahir H, Hsu C, Noveck RJ, Truitt KE. Pharmacological modulation of peripheral T and B lymphocytes by a selective sphingosine 1-phosphate receptor-1 modulator. J Clin Pharmacol. 2012b;52:996–1006. doi: 10.1177/0091270011408728. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH, Hla T. SnapShot: Bioactive lysophospholipids. Cell. 2012;148:378–378.e2. doi: 10.1016/j.cell.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T, Rivera R, Chun J. Insights into the pharmacological relevance of lysophospholipid receptors. Br J Pharmacol. 2012;165:829–844. doi: 10.1111/j.1476-5381.2011.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi T, Miyazaki S, Takemoto T, Suzuki K, Iio Y, Nakajima K, Ohnuki T, Kawase Y, Nara F, Inaba S, Izumi T, Yuita H, Oshima K, Doi H, Inoue R, Tomisato W, Kagari T, Shimozato T. Discovery of CS-0777: a potent, selective, and orally active S1P1 agonist. ACS Med Chem Lett. 2011;2:368–372. doi: 10.1021/ml100301k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J Neuroimmunol. 2013;256:13–18. doi: 10.1016/j.jneuroim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- O’Sullivan C, Schubart A, Mir AK, Dev KK. The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J. Neuroinflammation. 2016;13:31. doi: 10.1186/s12974-016-0494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa R, Takahashi M, Hirose S, Morimoto H, Ise H, Murakami T, Yasue T, Kuriyama K, Hongo M, Kobayashi E, Ikeda U. A novel sphingosine-1-phosphate receptor agonist KRP-203 attenuates rat autoimmune myocarditis. Biochem Biophys Res Commun. 2007;361:621–628. doi: 10.1016/j.bbrc.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Ohno T, Hasegawa C, Nakade S, Kitagawa J, Honda N, Ogawa M. The prediction of human response to ONO-4641, a sphingosine 1-phosphate receptor modulator, from preclinical data based on pharmacokinetic-pharmacodynamic modeling. Biopharm Drug Dispos. 2010;31:396–406. doi: 10.1002/bdd.719. [DOI] [PubMed] [Google Scholar]
- Okajima F, Tomura H, Sho K, Kimura T, Sato K, Im DS, Akbar M, Kondo Y. Sphingosine 1-phosphate stimulates hydrogen peroxide generation through activation of phospholipase C-Ca2+ system in FRTL-5 thyroid cells: possible involvement of guanosine triphosphate-binding proteins in the lipid signaling. Endocrinology. 1997;138:220–229. doi: 10.1210/endo.138.1.4883. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Yatomi Y, Gonda K, Shigematsu H, Takuwa Y. EDG3 is a functional receptor specific for sphingosine 1-phosphate and sphingosylphosphorylcholine with signaling characteristics distinct from EDG1 and AGR16. Biochem Biophys Res Commun. 1999;260:203–208. doi: 10.1006/bbrc.1999.0886. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Olsson T, Boster A, Fernandez O, Freedman MS, Pozzilli C, Bach D, Berkani O, Mueller MS, Sidorenko T, Radue EW, Melanson M. Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial. J Neurol Neurosurg Psychiatr. 2014;85:1198–1208. doi: 10.1136/jnnp-2013-307282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and antiangiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun. 2002;299:483–487. doi: 10.1016/S0006-291X(02)02671-2. [DOI] [PubMed] [Google Scholar]
- Oskeritzian CA, Hait NC, Wedman P, Chumanevich A, Kolawole EM, Price MM, Falanga YT, Harikumar KB, Ryan JJ, Milstien S, Sabbadini R, Spiegel S. The sphingosine-1-phosphate/sphingosine-1-phosphate receptor 2 axis regulates early airway T-cell infiltration in murine mast cell-dependent acute allergic responses. J Allergy Clin Immunol. 2015;135:1008–1018.e1. doi: 10.1016/j.jaci.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, Ryan JJ, Milstien S, Spiegel S. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–474. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Pan S, Mi Y, Pally C, Beerli C, Chen A, Guerini D, Hinterding K, Nuesslein-Hildesheim B, Tuntland T, Lefebvre S, Liu Y, Gao W, Chu A, Brinkmann V, Bruns C, Streiff M, Cannet C, Cooke N, Gray N. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem Biol. 2006;13:1227–1234. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Park SW, Kim M, Chen SW, Brown KM, D’Agati VD, Lee HT. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P1 receptor activation. Lab Invest. 2010;90:1209–1224. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/S0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- Piali L, Froidevaux S, Hess P, Nayler O, Bolli MH, Schlosser E, Kohl C, Steiner B, Clozel M. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther. 2011;337:547–556. doi: 10.1124/jpet.110.176487. [DOI] [PubMed] [Google Scholar]
- Poti F, Costa S, Bergonzini V, Galletti M, Pignatti E, Weber C, Simoni M, Nofer JR. Effect of sphingosine 1-phosphate (S1P) receptor agonists FTY720 and CYM5442 on atherosclerosis development in LDL receptor deficient (LDL-R−/−) mice. Vascul Pharmacol. 2012;57:56–64. doi: 10.1016/j.vph.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Poti F, Gualtieri F, Sacchi S, Weissen-Plenz G, Varga G, Brodde M, Weber C, Simoni M, Nofer JR. KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R−/− mice. Arterioscler Thromb Vasc Biol. 2013;33:1505–1512. doi: 10.1161/ATVBAHA.113.301347. [DOI] [PubMed] [Google Scholar]
- Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125:1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Tonelli F, Lim KG, Long JS, Edwards J, Pyne S. Sphingosine 1-phosphate signalling in cancer. Biochem Soc Trans. 2012;40:94–100. doi: 10.1042/BST20110602. [DOI] [PubMed] [Google Scholar]
- Rivera-Nieves J. Strategies that target leukocyte traffic in inflammatory bowel diseases: recent developments. Curr Opin Gastroenterol. 2015;31:441–448. doi: 10.1097/MOG.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, Mathew B, Husain A, Moitra J, Sun X, Nunez L, Jacobson JR, Dudek SM, Natarajan V, Garcia JG. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43:394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Feagan BG, Wolf DC, D’Haens G, Vermeire S, Hanauer SB, Ghosh S, Smith H, Cravets M, Frohna PA, Aranda R, Gujrathi S, Olson A. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374:1754–1762. doi: 10.1056/NEJMoa1513248. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- Scott FL, Clemons B, Brooks J, Brahmachary E, Powell R, Dedman H, Desale HG, Timony GA, Martinborough E, Rosen H, Roberts E, Boehm MF, Peach RJ. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778–1792. doi: 10.1111/bph.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Li DK, Hartung HP, Hemmer B, Kappos L, Freedman MS, Stuve O, Rieckmann P, Montalban X, Ziemssen T, Auberson LZ, Pohlmann H, Mercier F, Dahlke F, Wallstrom E. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol. 2013;12:756–767. doi: 10.1016/S1474-4422(13)70102-9. [DOI] [PubMed] [Google Scholar]
- Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol. 2010;43:662–673. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Takahashi M, Kaneko T, Murakami T, Hakamata Y, Kudou S, Kishi T, Fukuchi K, Iwanami S, Kuriyama K, Yasue T, Enosawa S, Matsumoto K, Takeyoshi I, Morishita Y, Kobayashi E. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation. 2005;111:222–229. doi: 10.1161/01.CIR.0000152101.41037.AB. [DOI] [PubMed] [Google Scholar]
- Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, Hla T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Matsuda C, Kai Y, Nishida T, Nakajima K, Mizushima T, Kinoshita M, Yasue T, Sawa Y, Ito T. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharmacol Exp Ther. 2008;324:276–283. doi: 10.1124/jpet.106.119172. [DOI] [PubMed] [Google Scholar]
- Stone ML, Sharma AK, Zhao Y, Charles EJ, Huerter ME, Johnston WF, Kron IL, Lynch KR, Laubach VE. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1245–L1252. doi: 10.1152/ajplung.00302.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subei AM, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNS Drugs. 2015;29:565–575. doi: 10.1007/s40263-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Shimizu H, Murakami T, Enosawa S, Suzuki C, Takeno Y, Hakamata Y, Kudou S, Izawa S, Yasue T, Kobayashi E. A novel immunomodulator KRP-203 combined with cyclosporine prolonged graft survival and abrogated transplant vasculopathy in rat heart allografts. Transplant Proc. 2005;37:143–145. doi: 10.1016/j.transproceed.2004.12.107. [DOI] [PubMed] [Google Scholar]
- Thangada S, Khanna KM, Blaho VA, Oo ML, Im DS, Guo C, Lefrancois L, Hla T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff A, Baur F, Fozard JR. Role of sphingosine-1-phosphate (S1P) and the S1P2 receptor in allergen-induced, mast cell-dependent contraction of rat lung parenchymal strips. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:303–309. doi: 10.1007/s00210-009-0438-4. [DOI] [PubMed] [Google Scholar]
- Trifilieff A, Fozard JR. Sphingosine-1-phosphate-induced airway hyper-reactivity in rodents is mediated by the sphingosine-1-phosphate type 3 receptor. J Pharmacol Exp Ther. 2012;342:399–406. doi: 10.1124/jpet.112.191585. [DOI] [PubMed] [Google Scholar]
- Vaclavkova A, Chimenti S, Arenberger P, Hollo P, Sator PG, Burcklen M, Stefani M, D’Ambrosio D. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384:2036–2045. doi: 10.1016/S0140-6736(14)60803-5. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Graler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood. 2000;95:2624–2629. [PubMed] [Google Scholar]
- van Koppen C, Meyer zu Heringdorf M, Laser KT, Zhang C, Jakobs KH, Bunemann M, Pott L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- Wang M, Lu L, Liu Y, Gu G, Tao R. FTY720 attenuates hypoxia-reoxygenation-induced apoptosis in cardiomyocytes. Exp Mol Pathol. 2014;97:218–224. doi: 10.1016/j.yexmp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- Whetzel AM, Bolick DT, Srinivasan S, Macdonald TL, Morris MA, Ley K, Hedrick CC. Sphingosine-1 phosphate prevents monocyte/endothelial interactions in type 1 diabetic NOD mice through activation of the S1P1 receptor. Circ Res. 2006;99:731–739. doi: 10.1161/01.RES.0000244088.33375.52. [DOI] [PubMed] [Google Scholar]
- Xie JH, Nomura N, Koprak SL, Quackenbush EJ, Forrest MJ, Rosen H. Sphingosine-1-phosphate receptor agonism impairs the efficiency of the local immune response by altering trafficking of naive and antigen-activated CD4+ T cells. J Immunol. 2003;170:3662–3670. doi: 10.4049/jimmunol.170.7.3662. [DOI] [PubMed] [Google Scholar]
- Xu J, Gray F, Henderson A, Hicks K, Yang J, Thompson P, Oliver J. Safety, pharmacokinetics, pharmacodynamics, and bioavailability of GSK2018682, a sphingosine-1-phosphate receptor modulator, in healthy volunteers. Clin Pharmacol Drug Dev. 2014;3:170–178. doi: 10.1002/cpdd.98. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Kon J, Sato K, Tomura H, Sato M, Yoneya T, Okazaki H, Okajima F, Ohta H. Edg-6 as a putative sphingosine 1-phosphate receptor coupling to Ca2+ signaling pathway. Biochem Biophys Res Commun. 2000;268:583–589. doi: 10.1006/bbrc.2000.2162. [DOI] [PubMed] [Google Scholar]
- Yokoo E, Yatomi Y, Takafuta T, Osada M, Okamoto Y, Ozaki Y. Sphingosine 1-phosphate inhibits migration of RBL-2H3 cells via S1P2: cross-talk between platelets and mast cells. J Biochem. 2004;135:673–681. doi: 10.1093/jb/mvh081. [DOI] [PubMed] [Google Scholar]
- You S, Piali L, Kuhn C, Steiner B, Sauvaget V, Valette F, Clozel M, Bach JF, Chatenoud L. Therapeutic use of a selective S1P1 receptor modulator ponesimod in autoimmune diabetes. PLoS ONE. 2013;8:e77296. doi: 10.1371/journal.pone.0077296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Yang L, Kim GS, Ryan K, Lu S, O’Donnell RK, Spokes K, Shapiro N, Aird WC, Kluk MJ, Yano K, Sanchez T. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood. 2013;122:443–455. doi: 10.1182/blood-2012-11-467191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Zhang Z, Schluesener HJ. FTY720 attenuates lesional interleukin-17+ cell accumulation in rat experimental autoimmune neuritis. Neuropathol Appl Neurobiol. 2009a;35:487–495. doi: 10.1111/j.1365-2990.2009.01016.x. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Zhang Z, Zug C, Nuesslein-Hildesheim B, Leppert D, Schluesener HJ. AUY954, a selective S1P1 modulator, prevents experimental autoimmune neuritis. J Neuroimmunol. 2009b;216:59–65. doi: 10.1016/j.jneuroim.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, Shi FD. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]