Abstract
Epithelial-mesenchymal transition (EMT) is a process essential to wound healing and tissue remodeling after a thermal burn or other injury. EMT is characterized by phenotypic changes in epithelial cells that render them apolar, with decreased cell-cell adhesions, increased motility, and changes in cytoskeletal architecture similar to mesenchymal stem cells. With regard to healing a thermal burn wound, many facets of wound healing necessitate cells to undergo these phenotypic changes; two will be described in the following review. The first is the differentiation of epithelial cells into myofibroblasts that rebuild the extracellular matrix and facilitate wound contraction. The second is reepithelialization by keratinocytes. The primary cytokine signal identified in the literature that triggers EMT is transforming growth factor (TGF)-β. In addition to its vital role in the induction of EMT, TGF-β has many other roles in the wound healing process. The following review will provide evidence that EMT is a central event in wound healing. It will also show the importance of a regulated amount of TGF-β for proper wound healing. Finally, osteopontin will be briefly discussed with its relation to wound healing and its connections to EMT and TGF-β.
The majority of literature centered on epithelial-mesenchymal transition (EMT) is in the field of oncology, where EMT is thought to play an important role in rendering epithelial tumor cells more invasive and metastatic resulting in a highly aggressive phenotype of cancer. EMT was first described, however, in chick embryology by Trelstad et al1 in the 1960s, where a single-layered embryo reorganizes into a three-layer formation, also called gastrulation. The first experiments to demonstrate EMT were done by Hay2 about 20 years later, where she was able to transform epithelial cells into mesenchymal cells by suspending them in collagen gels. Since then, EMT has been classified into three types: type 1 EMT in embryogenesis and organ development; type 2 EMT in wound healing and organ fibrosis; and type 3 EMT in tumorigenesis and cancer metastasis.3,4 All three types have many similarities and play significant roles. For example, transforming growth factor (TGF)-β is the primary cytokine classified as the inducer of EMT. However, although TGF-β is the most well studied cytokine in the induction of EMT, it is not the only cytokine or growth factor capable of causing this transition from epithelial to mesenchymal-like cell. Other molecules involved in this complex process include hepatocyte growth factor, epidermal growth factors, insulin-like growth factor, connective tissue growth factor, tumor necrosis factor-α, and fibroblast growth factor.3 The relative contribution of each of these cytokines in the induction of EMT in a thermal burn wound is unknown.
Wound healing in all tissues undergoes three phases: 1) inflammatory phase, 2) proliferative phase, and 3) maturation phase.5,6 A healing thermal burn wound is no exception. The goal of the inflammatory phase is to obtain hemostasis and to provide an influx of neutrophils and macrophages into the wound bed that will limit further tissue damage and remove necrotic tissue, foreign debris, and bacteria through phagocytosis.7,8 At the cessation of the inflammatory phase, the wound is a milieu of cytokines and growth factors as well as some remaining immune cells. The second phase, the proliferative phase, is characterized by the formation of granulation tissue, angiogenesis, deposition of new extracellular matrix (ECM), and reepithelialization. The keratinocytes involved in reepithelialization rely on an EMT-like process to impart migratory ability to the epithelial cells localized to the wound edges. A provisional ECM consisting of fibrin, fibronectin, and vitronectin is established that provides a scaffold for cellular migration into the wound bed. Proteoglycans and glycosaminoglycans such as hyaluronan, heparan sulfate, keratan sulfate, and chondroitin sulfate are added next to provide cushioning and protection. Platelets and macrophages in the wound release TGF-β, a chemotactic factor for fibroblasts, which secrete collagen to provide strength to the healing wound. The third phase, the maturation phase, involves wound contraction and remodeling.7 It is during this final phase of wound healing that the differentiation of myofibroblasts takes places, exemplifying the need for EMT to occur in order for the end result to be a wound with minimal scarring.
TGF-β-MEDIATED EMT
As in any example of EMT, the epithelial cell must lose epithelial markers; gain mesenchymal markers such as vimentin, fibroblast-specific protien 1 (FSP-1), Tenascin-C, N-cadherin, and α-smooth muscle actin (SMA); lose their apical-basal polarity; and develop a migratory phenotype. They must also disassemble their cell-cell connections, specifically the occludins and claudins found in tight junctions that are localized at the apical aspect of the cell and cadherins in adherens junctions found basal to tight junctions (see Figure 1 for phenotypic example of EMT). For epithelial cells undergoing EMT in a thermal burn wound, this means losing epithelial markers such as E-cadherins, calcium-dependent adhesion molecules that connects epithelial cells to each other via adherens junctions.9 This downregulation of E-cadherin expression and the other adhesion molecules, such as occludin, claudins, and ZO-1, is mediated by TGF-β through either Smad-dependent or Smad-independent pathways. More specifically, the isoform TGF-β1 is the main isoform involved in these complex pathways (see Figure 2).
Figure 1.
The phenotypic changes that occur when a cell undergoes epithelial-mesenchymal transition. The epithelial cell, characterized by apical-basal polarity and cell-cell connections such as tight junctions and adherens junctions, changes phenotype to a mesenchymal cell that is spindle shaped with no cell-cell connections. TGF, transforming growth factor.
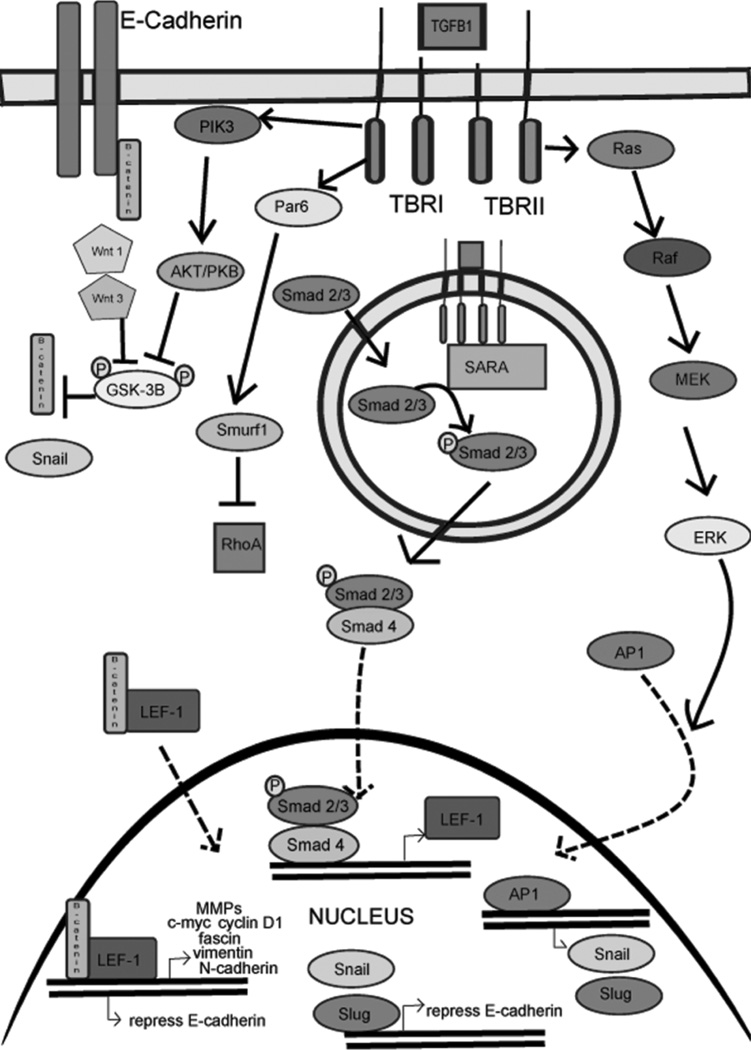
Figure 2.
This figure depicts the transforming growth factor-β pathways, both Smad-dependent and Smad-independent, that result in either repression of epithelial genes or expression of mesenchymal genes.
The well-defined TGF-β1 Smad-dependent pathway is initiated by the binding of TGF-β1 to TβRII (serine/threonine ligand binding receptor kinase for TGF-β1 or TGF-β1 receptor type II), which then combines with TβRI (signaling receptor for TGF-β1 or TGF-β1 receptor type I or ALK5). The hetero-tetramer receptor/ligand complex is then endocytosed by means of clarithin-coated pits by the epithelial cell. The kinase domain of TβRII phosphorylates TβRI. A Smad 2 or Smad 3 protein is then associated with TβRI by Smad anchor for receptor activation followed by phosphorylation of the R-Smad domain by TβRI. Smad 2 or Smad 3 then dissociates from the receptor complex and binds to a co-Smad, generally Smad4.10 As a complex, Smad2/3-Smad4 are then able to enter the nucleus of the epithelial cell and increase transcription of LEF-1, a member of the T-cell family of transcription factors that binds with β-catenin suppressing transcription of epithelial markers such as E-cadherin and promoting transcription of mesenchymal markers such as vimentin and N-cadherin.11,12
In addition to the cell markers and adhesion molecules, the LEF-1/β-catenin complex targets genes involved in cell proliferation such as c-myc and cyclin-D1, as well as genes involved in cell migration/invasion.13 For example, matrix metalloproteinases, a family of enzymes that degrade the ECM providing a pathway for epithelial cells to migrate, are upregulated by the LEF-1/β-catenin complex. Fascin expression is also increased; it is involved in bundling actin to form filopodia, cytoplasmic extensions, that impart increased motility to the cells.14,15 β-catenin is a protein that is actually a member of the adherens junction as it binds with E-cadherin and is released when E-cadherin is downregulated during EMT.16 The cytoplasmic concentration of β-catenin is important as it needs to be present in sufficient concentration to bind with LEF-1 to promote transcription of many EMT genes. Regulation of the cytoplasmic concentration of β-catenin is controlled by the Wnt pathway, whereby Wnt-1 and Wnt-3 inhibit the glycogen synthase kinase 3β (GSK-3β)/ubiquitin degradation of β-catenin.11,17
There have been several TGF-β1 Smad-independent pathways described that result in downregulation of epithelial markers and upregulation of mesenchymal markers. Some of these pathways then interact with the Smad pathway.18 One pathway involves the direct binding of TGF-β1 with TβRI resulting in the activation of PI3K, a kinase that phosphorylates AKT (also known as protein kinase B [PKB]). AKT then phosphorylates GSK-3β resulting in the inhibition of β-catenin degradation and increases the cytoplasmic concentration able to bind with LEF-1. Another TGF-β1Smad-independent pathway that decreases E-cadherin expression is the canonical or mitogen-activated protein kinase (MAPK)-dependent pathway (Ras-Raf-MEK-Erk), which results in translocation of the transcription factor activator protein 1 (AP-1) to the nucleus of the epithelial cell.12 AP-1 is a heterodimer composed of c-fos and c-jun proteins that causes transcription of the Snail family of transcription factors. The Snail family is a group of zinc finger proteins that function as transcriptional repressors of epithelial genes. Two members, Snail (SNAI1) and Slug (SNAI2) are known to bind to E-boxes in the promoter region of the E-cadherin gene repressing E-cadherin expression.19,20 Other less studied transcriptional repressors of E-cadherin include ZEB-1, SIP-1, E12, E47, and Twist. The relative involvement of these transcription factors in thermal burn wounds is unclear at this point. Other less described TGF-β1 Smad-independent pathways include phosphorylation of Par6 by TβRI, which then combines with Smurf1 (E3 ubiquitin ligase) to target RhoA for degradation. The ubiquitination of RhoA is necessary for the loss of tight junctions and breakdown of the basement membrane, as RhoA is an inhibitor of EMT.21 Another member of this GTPase family, RhoC, is actually upregulated in EMT.22
The other isoforms of TGF-β also play an important and necessary role in the induction of EMT as well. Generally, TGF-β2 has similar effects as TGF-β1. The upregulation of Snail and Slug results in an increased expression of β-catenin T-cell factor 4, which then binds to the promoter region of TGF-β3 gene and increases transcription of this third isoform of TGF-β. The downstream effects of TGF-β3 signaling causes LEF-1 gene transcription which then associates with β-catenin and results in either suppression or repression of EMT genes as described above.23 Thus, a complex interplay of the three isoforms is involved in EMT. In addition, an imbalance of these three isoforms would likely result in abnormal wound healing in response to a thermal burn wound or other injury.
OTHER ROLES OF TGF-β IN WOUND HEALING
TGF-β is not only an important cytokine by inducing EMT but also plays many other essential roles in wound healing. As previously mentioned, it is a chemotactic protein for fibroblasts. In addition to stimulating all the pathways described earlier, TGF-β1 also stimulates TGF-β1 mRNA expression in an autocrine fashion in fibroblasts, acting to potentiate its own actions even further. The expression of TGF-β receptors by fibroblasts involved in the wound healing process has been examined in both normal skin and healing skin. It was shown that the fibroblasts from normal uninjured skin have very low expression of the TGF-β1 receptor. However, in granulation tissue from healing thermal burn wounds, the fibroblasts showed a very high expression of both type I and type II TGF-β1 receptors, which returns to the low levels of normal skin once a healed scar has formed. Also of note, hypertrophic scars chronically expressed high levels of both types of receptors, implicating TGF-β1 as a central player in hypertrophic scar formation after thermal burn wound injury.24
One study further revealed that TGF-β has an important role in thermal burn wound healing by demonstrating that it is the cytokine released by circulating fibrocytes that activates resident dermal fibroblasts to synthesize collagen and differentiate into myofibroblasts. The circulating number of fibrocytes increased with the severity of the thermal burn injury.25 Fibrocytes play a role in wound healing by secreting ECM proteins and synthesizing collagen; however, the capacity to synthesize collagen is far less than that of dermal fibroblasts. The authors of this study treated dermal fibroblasts with media containing fibrocytes from burn patients and found that by adding the fibrocytes, the dermal fibroblasts upregulated production of collagen and increased their differentiation into myofibroblasts.26 As a result, TGF-β is the most likely cytokine being released by fibrocytes which causes increased fibroblast chemotaxis, proliferation, and function.
TGF-β AND HYPERTROPHIC SCARRING
As with many processes, more is not always better. The same is true with TGF-β. Studies have shown that although TGF-β1 and TGF-β2 are required to heal wounds and can actually improve wound healing in impaired wound healing models, it is also true that excess TGF-β1 or TGF-β2 becomes associated with fibrosis and hypertrophic scarring (with TGF-β3 antagonizing the profibrotic effects).27 One study in the burn literature looked at the longitudinal changes in plasma TGF-β1 concentration in postburn scars from children who suffered thermal burns with the interest of delineating the relationship between TGF-β1 concentration and scar formation.28 They found that the plasma levels of TGF-β1 were influenced by the extent and depth of burn injury. Children who healed with good quality scars showed a sharp increase and then resolution in plasma TGF-β1 concentration in the 2 weeks after the thermal burn wound injury. In contrast, children who developed hypertrophic scars did not show this rise in plasma TGF-β1. They also found that less severe burns (<10% TBSA) showed a larger increase in plasma TGF-β1 concentration than larger burn wounds (>10% TBSA). At first glance, their results seem contrary to the theory that excess TGF-β is associated with fibrosis and hypertrophic scarring. However, they reasoned that the children who developed hypertrophic scars had lower plasma levels of TGF-β1, because the TGF-β1 was being recruited into the tissues promoting a fibrotic response in the tissues. Thus, the measured low concentration of TGF-β1 in the plasma may not reflect the concentration of TGF-β1 in the wound bed itself. It was also postulated that increased levels of TGF-β1 may cause an increase in the production of TGF-β3 in the wound bed, which has an antifibrotic effect, explaining why children with well-healed scars had high plasma concentrations of TGF-β1.28 Overall, they concluded that monitoring plasma levels of TGF-β isoforms could provide a means for determining the quality of scar after a thermal burn wound.
TWO EXAMPLES OF EMT IN THE THERMAL BURN WOUND
Myofibroblast Differentiation
Myofibroblasts are crucial for the maturation phase of wound healing. The sources of myofibroblasts in a healing wound are numerous. For example, dermal fibroblasts from adjacent uninjured skin undergo differentiation into myofibroblasts in a TGF-β-dependent fashion. In addition, pericytes and smooth muscle cells from the surrounding vessels contribute to the population of myofibroblasts by dedifferentiation. Bone marrow-derived stem cells and circulating fibrocytes also differentiate into myofibroblasts.29,30 An important local source that is available in abundance is the epithelial cell population already present in the wound bed. These transform into myofibroblasts via EMT.31,32 It is believed that the relative hypoxia of the healing wound helps drive this EMT process. Also, many cytokines such as TGF-β (specifically isoform 1), connective tissue growth factor, IL1, oncostatin M, angiotensin II, proteases, plasminogen activator inhibitor 1, and glycation end products released by inflammatory cells have been implicated in assisting the transformation of epithelial cells into myofibroblasts. This model has been well characterized in renal fibrosis, where tubular epithelial cells have been observed to undergo this transformation to mesenchymal-like cells.21 It has also been observed in liver and lung fibrosis.33
To mediate wound contraction, or the centripetal movement of the entire thickness of the wound bed, myofibroblasts need to have contractile ability. This is founded through de novo synthesis of α-SMA, resulting in the formation of stress fibers.34 The myofibroblast develops a contractile apparatus in their cytoskeleton consisting of α-SMA bundles that organize longitudinally to connect the cytoplasm to the ECM via adapter proteins and cell adhesion molecules. Integrin cell surface molecules, specifically integrin β1 (which can associate with 12 different α subunits to form the heterodimer integrin receptor), have been identified as an important link between the myofibroblast cytoskeleton and ECM components such as collagen and fibronectin. In the skin, fibroblasts have been shown to bind to collagen through three specific β1 integrin receptors—α1β1, α2β1, and α11β1.35 Dermal fibroblasts exhibit slower migration, reduced proliferation, and impaired differentiation into myofibroblasts when integrin β1 is deleted. The healing wounds show less α-SMA and reduced contraction in this deletion model, indicating that integrin β1 is integral for adequate tissue repair, specifically repair facilitated by the myofibroblast.36
The reorganization of the cytoskeleton that occurs during the formation of the contractile apparatus results in a cell that is elongated and spindle shaped with front to back polarity instead of apical-basal polarity typical of epithelial cell precursors. The contractile ability of myofibroblasts α-SMA stress fibers has been shown to be twice that of α-SMA-negative myofibroblasts.37 In addition to anchoring to the ECM, myofibroblasts also anchor to each other through cell-cell adhesions of the N-cadherin type. The end result is the ability to generate tensile force to pull the wound edges together and limit scar size.
As discussed above with TGF-β, more is not always better. The same is true of myofibroblasts in a healing wound. Once the wound has undergone contraction and inflammation ceases, the myofibroblasts receives signals to undergo apoptosis. If the myofibroblasts persists in the wound, however, hypertrophic scarring or organ fibrosis occurs.38 This can result in a severely contracted scar that is thick with delayed reepithelialization. In scars from thermal burn wounds, hypertrophic scars have been shown to have higher expression of α-SMA than well-healed mature scars. In addition, higher α-SMA levels were found in more severely contracted and thicker scars.39
Reepithelialization
After a thermal burn wound, the body seeks to restore the skin barrier against the external environment by rapidly regenerating its epithelium. The epithelial cells or keratinocytes at the edges of the burn wound form epithelial tongues that move and interact with the dermal cells and ECM to reestablish coverage of the wound bed. This helps the body control fluid and electrolyte losses and protects vital underlying tissues and organs from further injury. To migrate, keratinocytes need to undergo a transition and change in phenotype which allows them to shed their cell-cell adhesions, lose their apical-basal polarity, dissolve the basement membrane, and reorganize their cytoskeletal structure to generate cytoplasmic extensions such as lamellipodia. Studies have shown that epithelial cells lose some epithelial markers and gain some mesenchymal markers such as FSP-1 and vimentin, indicating that an EMT-like process has occurred.40 Of note, keratinocytes do not appear to undergo a complete EMT, as they tend to migrate in sheets to cover a wound in the skin. This induction of EMT in the keratinocytes is also attributable to TGF-β stimulation, and in one study, tumor necrosis factor-α also appeared to induce EMT changes in keratinocytes of acute cutaneous wounds.41
Several studies have examined Slug expression in skin injury models to provide evidence for an EMT-like process in cutaneous wound healing.42,43 They produced in vivo and in vitro results showing that the keratinocytes in the actively migrating reepithelializing tongues and the keratinocytes at the wound margins exhibited increased Slug expression in their nuclei. In addition, keratinocytes displaying cytoplasmic actin projections such as lamellipodia also had increased Slug expression. The downstream results of this increased Slug expression were keratinocytes with decreased cell-cell adhesions and change to a more migratory phenotype. Furthermore, in Slug-null mice, a 1.7-fold decrease in reepithelialization was seen in an excisional wound model. These changes were described as EMT-like, because not all changes characteristic of EMT occurred. For example, some keratinocytes still expressed E-cadherin, and there was no upregulation of Snail expression. Other studies have replicated this increase of Slug in migrating keratinocytes and have shown upregulation of Twist as well. Of note, in Slug-null mice, there was an increased E-cadherin expression. Overall, these studies indicate that an EMT-like process occurs in the reepithelialization process of healing a thermal burn wound.
ROLE OF OSTEOPONTIN IN WOUND HEALING AND EMT
Osteopontin (OPN) is a secreted glycoprotein also known as secreted phosphoprotein 1 that has recently been implicated in all three processes that involve EMT: embryogenesis, wound healing/fibrosis, and tumorigenesis. OPN is known to bind to multiple integrin receptors (including five β1 receptors) as well as to CD44. Ligands for OPN include ECM components such as fibronectin, fibrinogen, vitronectin, collagen, laminin, and hyaluronic acid.44 Several transcription factors activated by TGF-β1, including LEF-1/β-catenin and AP-1, have been implicated in regulatory control of OPN expression.45
With regard to wound healing, OPN is upregulated during the inflammatory phase and plays several key roles in wound healing. As with TGF-β and myofibroblasts, even though OPN is needed for proper wound healing, it does lead to fibrosis and excess scar formation if it persists for too long or in too high of concentration. For example, OPN has been implicated in the progression of renal interstitial fibrosis and glomerular fibrosis. Studies have shown upregulation of OPN at the mRNA and protein level in kidneys that have progressed to glomerular fibrosis.46 One study looked at the progression to interstitial fibrosis in a unilateral ureteral obstruction model. They found that OPN null mice developed interstitial fibrosis to a lesser degree.47
One of the main roles of OPN is the recruitment, regulation, and differentiation of fibroblasts and myofibroblasts.48 It acts as a cytokine that is chemoattractant for fibroblasts and appears to be necessary for their proper deposition of ECM components and collagen. In studies of mice that were deficient in OPN, the healed wounds exhibited a less organized matrix consisting of collagen fibers present in reduced number, less organized fashion, and with decreased fibril diameter.49 The wound beds were characterized by an ECM with more vacant space. In addition, these OPN null mice showed reduced expression of collagen type I mRNA, matrix metalloproteinase 9, fibronectin, and TGF-β mRNA.50 The fibroblasts in OPN null mice showed no response to stimulation by TGF-β1; however, it did still transform into myofibroblasts expressing α-SMA. One study showed faster reepithelialization and wound closure in OPN null mice,50 whereas another utilizing corneal injury showed that wound closure was actually delayed.51 Therefore, it is likely that the role of OPN is tissue dependent. Several studies have shown no difference in tensile strength of the healed wound with or without the presence of OPN.
Overall, OPN plays an important role in regulating TGF-β-mediated processes and likely also regulates TGF-β-mediated EMT. Recently, our laboratory sought to determine whether OPN does indeed play a role in the induction of EMT by TGF-β. We cocultured breast cancer cells that express high levels of OPN (MDA-MB231) with mesenchymal stem cells (MSCs). We found that the OPN-stimulated MSCs expressed high levels of TGF-β. TGF-β then acted in a paracrine fashion to cause EMT in the tumor cells, resulting in cells with high expression of vimentin, tenascin-C, FSP-1, and α-SMA. When breast cancer cells that do not express OPN (MCF7) were cocultured with MSCs, there was no observed increase in expression of TGF-β and no evidence of EMT occurring. Thus, we hypothesize that tumor-derived OPN causes an increase in expression of TGF-β, which then induces EMT in epithelial cells through a paracrine path. Our future studies will seek to delineate how OPN regulates the transcription of TGF-β (Kuo et al, unpublished data).
CONCLUSION
In conclusion, EMT plays an important function in the wound healing process. In a thermal burn wound, myofibroblast formation and keratinocyte reepithelialization both rely on EMT. TGF-β is a key cytokines involved in many elements of wound healing, including the induction EMT via many pathways. Finally, OPN appears to play an important role in TGF-β-dependent processes and likely is involved in TGF-β-mediated EMT.
REFERENCES
- 1.Trelstad RL, Hay ED, Revel JD. Cell contact during early morphogenesis in the chick embryo. Dev Biol. 1967;16:78–106. doi: 10.1016/0012-1606(67)90018-8. [DOI] [PubMed] [Google Scholar]
- 2.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 6.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(2A Suppl):26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 7.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 8.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 12.Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jesse S, Koenig A, Ellenrieder V, et al. Lef-1 isoforms regulate different target genes and reduce cellular adhesion. Int J Cancer. 2010;126:1109–1120. doi: 10.1002/ijc.24802. [DOI] [PubMed] [Google Scholar]
- 14.Vignjevic D, Schoumacher M, Gavert N, et al. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- 15.Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol. 2006;174:863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller T, Bain G, Wang X, Papkoff J. Regulation of epithelial cell migration and tumor formation by beta-catenin signaling. Exp Cell Res. 2002;280:119–133. doi: 10.1006/excr.2002.5630. [DOI] [PubMed] [Google Scholar]
- 17.Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- 18.Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- 19.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 20.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 21.Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis—evidence for and against. Int J Exp Pathol. 2011;92:143–150. doi: 10.1111/j.1365-2613.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellovin DI, Simpson KJ, Danilov T, et al. Reciprocal regulation of RhoA and RhoC characterizes the EMT and identifies RhoC as a prognostic marker of colon carcinoma. Oncogene. 2006;25:6959–6967. doi: 10.1038/sj.onc.1209682. [DOI] [PubMed] [Google Scholar]
- 23.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid P, Itin P, Cherry G, Bi C, Cox DA. Enhanced expression of transforming growth factor-beta type I and type II receptors in wound granulation tissue and hypertrophic scar. Am J Pathol. 1998;152:485–493. [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 26.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 27.Chalmers RL. The evidence for the role of transforming growth factor-beta in the formation of abnormal scarring. Int Wound J. 2011;8:218–223. doi: 10.1111/j.1742-481X.2011.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rorison P, Thomlinson A, Hassan Z, Roberts SA, Ferguson MW, Shah M. Longitudinal changes in plasma Transforming growth factor beta-1 and post-burn scarring in children. Burns. 2010;36:89–96. doi: 10.1016/j.burns.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 30.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 35.Blumbach K, Zweers MC, Brunner G, et al. Defective granulation tissue formation in mice with specific ablation of integrin-linked kinase in fibroblasts—role of TGFbeta1 levels and RhoA activity. J Cell Sci. 2010;123(Pt 22):3872–3883. doi: 10.1242/jcs.063024. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Xu SW, Blumbach K, et al. Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123(Pt 21):3674–3682. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 37.Hinz B, Celetta G, Tomasak JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarrazy V, Billet F, Micallef L, Coulomb B, Desmoulière A. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10–s5. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang XQ, Kravchuk O, Winerford C, Kimble RM. The correlation of in vivo burn scar contraction with the level of alpha-smooth muscle actin expression. Burns. 2011;37:1367–1377. doi: 10.1016/j.burns.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura M, Tokura Y. Epithelial-mesenchymal transition in the skin. J Dermatol Sci. 2011;61:7–13. doi: 10.1016/j.jdermsci.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Yan C, Grimm WA, Garner WL, et al. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol. 2010;176:2247–2258. doi: 10.2353/ajpath.2010.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudson LG, Newkirk KM, Chandler HL, et al. Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2) J Dermatol Sci. 2009;56:19–26. doi: 10.1016/j.jdermsci.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savagner P, Kusewitt DF, Carver EA, et al. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- 44.Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- 45.El-Tanani M, Platt-Higgens A, Rudland PS, Campbell FC. Ets gene PEA3 cooperates with beta-catenin-Lef-1 and c-Jun in regulation of osteopontin transcription. J Biol Chem. 2004;279:20794–20806. doi: 10.1074/jbc.M311131200. [DOI] [PubMed] [Google Scholar]
- 46.Merszei J, Wu J, Torres L, et al. Osteopontin overproduction is associated with progression of glomerular fibrosis in a rat model of anti-glomerular basement membrane glomerulonephritis. Am J Nephrol. 2010;32:262–271. doi: 10.1159/000319238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo KH, Thornhill BA, Forbes MS, et al. Osteopontin regulates renal apoptosis and interstitial fibrosis in neonatal chronic unilateral ureteral obstruction. Kidney Int. 2006;70:1735–1741. doi: 10.1038/sj.ki.5000357. [DOI] [PubMed] [Google Scholar]
- 48.Lenga Y, Koh A, Perera AS, McCulloch CA, Sodek J, Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ Res. 2008;102:319–327. doi: 10.1161/CIRCRESAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 49.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyazaki K, Okada Y, Yamanaka O, et al. Corneal wound healing in an osteopontin-deficient mouse. Invest Ophthalmol Vis Sci. 2008;49:1367–1375. doi: 10.1167/iovs.07-1007. [DOI] [PubMed] [Google Scholar]