Abstract
An estimated third of the world’s population is latently infected with Mycobacterium tuberculosis (Mtb), with no clinical signs of tuberculosis (TB), but lifelong risk of reactivation to active disease. The niches of persisting bacteria during latent TB infection remain unclear. We detect Mtb DNA in peripheral blood selectively in long-term repopulating pluripotent hematopoietic stem cells (LT-pHSCs) as well as in mesenchymal stem cells from latently infected human donors. In mice infected with low numbers of Mtb, that do not develop active disease we, again, find LT-pHSCs selectively infected with Mtb. In human and mouse LT-pHSCs Mtb are stressed or dormant, non-replicating bacteria. Intratracheal injection of Mtb-infected human and mouse LT-pHSCs into immune-deficient mice resuscitates Mtb to replicating bacteria within the lung, accompanied by signs of active infection. We conclude that LT-pHSCs, together with MSCs of Mtb-infected humans and mice serve as a hitherto unappreciated quiescent cellular depot for Mtb during latent TB infection.
Introduction
Tuberculosis (TB) is a major infectious disease in humans, caused by Mycobacterium tuberculosis (Mtb), with 9.6 million active cases reported in 2014. A much larger part of the world’s population is, in addition, latently TB-infected (LTBI), with no clinical signs of disease, but lifelong risk of reactivation. Mtb can enter dormancy, thereby persisting in the human host despite a strong immune response [1, 2]. How, where, and under what circumstances Mtb is retained in the host during LTBI, and how it could undergo resuscitation and cause active TB, remain important but unresolved questions [3–5]. We test the hypothesis that in LTBI Mtb bacteria acquire a non-replicating state inside resting, long-term repopulating pluripotent hematopoietic stem cells (LT-pHSCs) [6]. LT-pHSCs tolerate hypoxia [7, 8], lack tuberculocidal activity mediated by endogenous reactive oxygen and nitrogen species [9, 10], and tune down immune surveillance mechanisms of the host [11]. In addition, they tune down immune-stimulating mechanisms of Mtb, if Mtb are able to acquire dormancy from the host [1, 4, 6].
Here, we detect Mtb DNA in human peripheral blood, selectively accumulated in a portion of CD34+CD90+CD38- pHSCs [12, 13], as well as previously reported CD271+CD45- mesenchymal stem cells (MSCs) [14, 15], from individuals with LTBI determined by IFN-γ release assay (IGRA) [16]. In mice that harbor Mtb, but do not develop TB, we, again, identified a portion of LT-pHSCs in bone marrow [17, 18] selectively infected with Mtb. Mtb in human and mouse pHSCs express stress or dormancy genes and do not form colonies on agar [19, 20]. Intratracheal application of human and mouse pHSCs harboring Mtb into immune-deficient mice resuscitates Mtb replication accompanied by increased cellularity indicative of an inflammatory infiltrate, typically observed when these mice are infected with replicating Mtb.
LT-pHSCs are thought to reside in hypoxic niches of bone marrow in close contact with other hematopoietic and non-hematopoietic cells, e.g. mesenchymal stem cells (MSCs) [7, 8, 21], that provide the supportive microenvironmental niche for HSCs [22]. Das et al. have reported that human bone marrow CD271+CD45− MSCs in vitro as well as an equivalent population of bone marrow MSCs in the mouse may provide a long-term protective intracellular niche in the host in which Mtb can reside [23, 24]. The quiescence of the hypoxic niche of mesenchymal and hematopoietic cells could provide stress and induce dormancy genes of Mtb [25–27].
Our findings suggest that Mtb-infected bone marrow-derived hematopoietic stem cells of mice and humans, together with Mtb-infected MSCs serve as a hitherto unappreciated cellular niche during LTBI, capable of resuscitating active TB disease. Mtb-infected, blood-borne LT-pHSCs can be used to diagnose LTBI, and to monitor drug treatments. Their existence challenges current hypotheses of TB pathogenesis and epidemiology.
Results
Human peripheral blood SP+ and Lin–CD34+CD90+CD38lo pHSCs of IGRA+ donors carry Mtb DNA
We searched for Mtb DNA in human pHSCs of donors with LTBI. To this end we purified Lin–CD34+ progenitors, and within them Lin–CD34+CD90+CD38- pHSCs (S1A Fig), as well as, CD1c+ dendritic cells, CD14+CD16low and CD14lowCD16+ monocytes, CD15+ granulocytes, CD4+ or CD8+ T cells, CD19+ cells identifying B as well as dendritic cells and CD56+ NK cells by FACS from blood of IGRA+ and IGRA−donors (Table 1). We also isolated pHSCs by their drug efflux properties as Hoechst low/ negative side population (SP) phenotype cells (S1A Fig), since pHSCs are highly enriched in SP cells [28, 29]. DNA of all these potentially Mtb-infected cells was used to PCR-amplify DNA fragments of Mtb-encoded sequences to search for Mtb infection and Bacille Calmette-Guérin (BCG)-encoded sequences to score for possible remnants of a BCG-vaccination (Fig 1A–1E) [30, 31].
Table 1. Patient characteristics.
| # | Age | Sex | IGRA | Comorbidities | Medication |
|---|---|---|---|---|---|
| 1 | 22 | m | neg | Psoriasis | - |
| 2 | 53 | f | neg | - | - |
| 3 | 38 | m | neg | - | - |
| 4 | 33 | m | neg | allerg RC | - |
| 5 | 45 | f | neg | - | - |
| 6 | 38 | f | neg | - | - |
| 7 | 41 | f | neg | - | - |
| 8 | 29 | m | 0.87 | Psoriasis | - |
| 9 | 43 | m | 1.8 | Psoriasis | - |
| 10 | 27 | f | 10 | Psoriasis | - |
| 11 | 57 | m | 3.64 | - | - |
| 12 | 55 | m | 4.2 | chronic Urticaria | Antihistamines |
| 13 | 37 | f | 0.65 | Eczema | - |
| 14 | 46 | f | 2.71 | - | - |
| 15 | 48 | m | 9 | - | - |
Tablenotes: #: patient number; Sex—m: male; Sex—f: female; neg: negative; allerg RC: allergic rhinoconjunctivitis
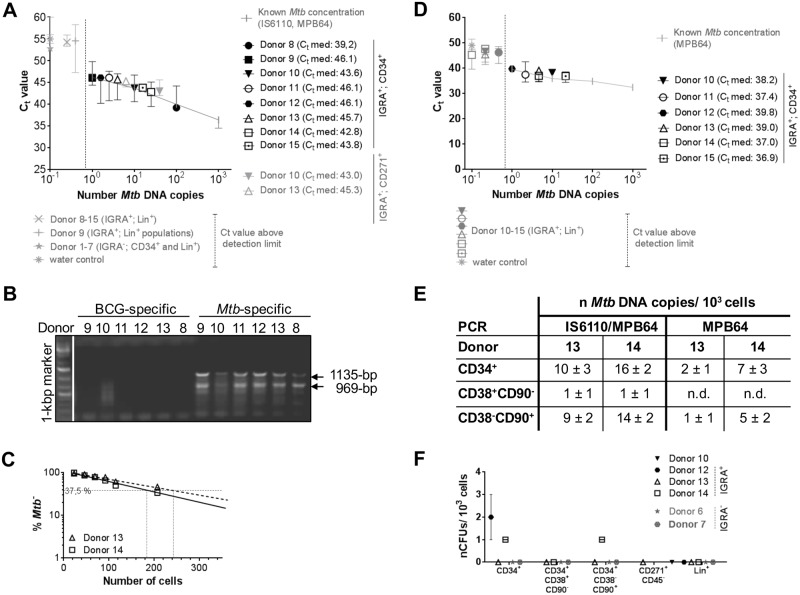
Fig 1. Human peripheral Lin–CD34+, Lin–CD34+CD38lowCD90+, SP+ pHSCs as well as CD271+CD45- MSCs of IGRA+ donors harbour Mtb DNA.
Lin+, Lin–CD34+, Lin–CD34+CD38lowCD90+, Lin–CD34+CD38+CD90–, Lin–SP+ and Lin+ SP−cells (Donors 1, 6, 8, 9) were purified from blood of IGRA+ (n = 8) and IGRA−donors (n = 7; S1A Fig). CD271+CD45- MSCs (Donors 10 and 13) were purified from blood of IGRA+ donors (n = 2; S1B Fig). CD1c+, CD14+, CD16+, CD4+/8+, CD15+, CD19+, and CD56+ cells were prepared from blood of IGRA+ donors (n = 3). Genomic DNA prepared from 103 hematopoietic progenitors and MSCs as well as 105 Lin+ cells from IGRA+ and IGRA- donors were tested for the presence of Mtb DNA by PCR. (A, E) Quantification of Mtb-specific DNA by real-time TaqMan PCR using probes that target MPB64 and IS6110 together (S4 Fig). (B) Genomic DNA of 103 hematopoietic progenitors from IGRA+ and IGRA- donors were tested by PCR for a DNA fragment present in Mtb, but not in BCG. (C) Quantification of Mtb-specific DNA by limiting dilutions using a single-target PCR for IS6110 (Donor 13, 14; S2A Fig). (D, E) Quantification of Mtb-specific DNA by real-time SYBR green PCR using primers that target MPB64 alone (S4 Fig). Real-time PCRs were performed in 2 independent runs in technical triplicates and normalized to human GAPDH. Known Mtb concentrations were used as reference. (F) CFU Mtb growth on Middlebrook 7H11 agar plates (n = 2–3). Data are shown as median + interquartile.
Eight of eight IGRA+ donors scored positive in blood cells for Mtb (Fig 1A–1E), and none of them positive for BCG (Donors 8–13; Fig 1B), selectively in ~2×103 SP+ and Lin–CD34+ pHSCs. Quantitation of Mtb-specific DNA was done by real-time TaqMan PCR targeting two Mtb-specific genes, the single copy MPB64 and the multiple copy IS6110 sequence (Fig 1A and 1E), as well as in limiting dilution PCRs targeting IS6110 alone (Fig 1C; S2A Fig). In PCR tests detecting multiple IS6110 elements in a single Mtb genome [32–35], in SP+ and Lin–CD34+ pHSCs from IGRA+ donors we detected between seven and twenty copies of Mtb-specific DNA within lysates of 103 cells (Fig 1A, 1C and 1E; S4 Fig). Using primers that target the single copy MPB64 alone, we detected between one and seven copies of Mtb DNA within lysates of 103 cells (Fig 1D; S4 Fig). In the genome of individual IGRA+ Mtb+ donors two to 10-fold higher IS6110 copy numbers were detected than the one MPB64 copy.
We also analyzed CD271+CD45- MSCs (S1B Fig) from selected donors (Donors 10 and 13) for presence of Mtb. Thereby, we detected between one and ten copies of Mtb-specific DNA within lysates of 103 MSCs by real-time TaqMan PCR targeting MPB64 and IS6110 from blood of human donors with LTBI (Fig 1A), in confirmation of previous results by Das et al. [23, 24]
Moreover, none of the IGRA−donors harbored detectable Mtb DNA in any of the Lin−and Lin+ blood cells tested by both PCRs (Fig 1A and 1D). From two IGRA+ donors, CD34+ progenitors were further FACS-purified as Lin–CD34+CD90+CD38lo pHSCs (S1A Fig) [13]. 103 of these harbored between 9 and 14 Mtb DNA copies in the qPCR targeting MPB64 and IS6110, and between one and five in the MPB64 qPCR, while 103 of the more differentiated Lin–CD34+CD38+CD90– cells harbored none (Fig 1E). Also, 103 of the pool of Lin+ cells of IGRA+ donors, as well as FACS-purified dendritic cells, monocytes, granulocytes, T cells, B cells and NK cells, scored negative in all of these qPCR assays (Fig 1A and 1D).
We conclude that within human peripheral Lin-SP+ and CD34+ cells in blood of IGRA+ donors, the Lin–CD34+CD90+CD38- pHSCs, as well as CD271+CD45- MSCs, selectively carried Mtb DNA while their peripheral Lin+ cells were consistently Mtb–.
Replication-competent Mtb can form colonies on agar. Thus, we tested the different cell populations from three IGRA+ donors for growth measured by enumerating colony-forming units. Thereby, only one CFU was formed from lysates of 103 Lin–CD34+ and Lin–CD34+CD90+CD38- pHSCs isolated from two of the donors, which, in the MPB64 PCR assays, contained between one and five Mtb DNA copies (Fig 1A–1F). Thus, potentially only one of five Mtb DNA copies detected in Lin–CD34+ and Lin–CD34+CD90+CD38- cells was replication-competent, the majority were non-replicative in quiescent hematopoietic cells. Furthermore, from lysates of 103 CD271+CD45- MSCs no CFUs were detected (Fig 1F). Therefore, in the donors tested the majority of Mtb DNA copies detected in CD271+CD45- MSCs cells were similarly not replication-competent.
A portion of CD150+ LT-pHSCs, but neither ST-pHSCs nor MPPs in bone marrow of Mtb-infected mice harbour Mtb DNA
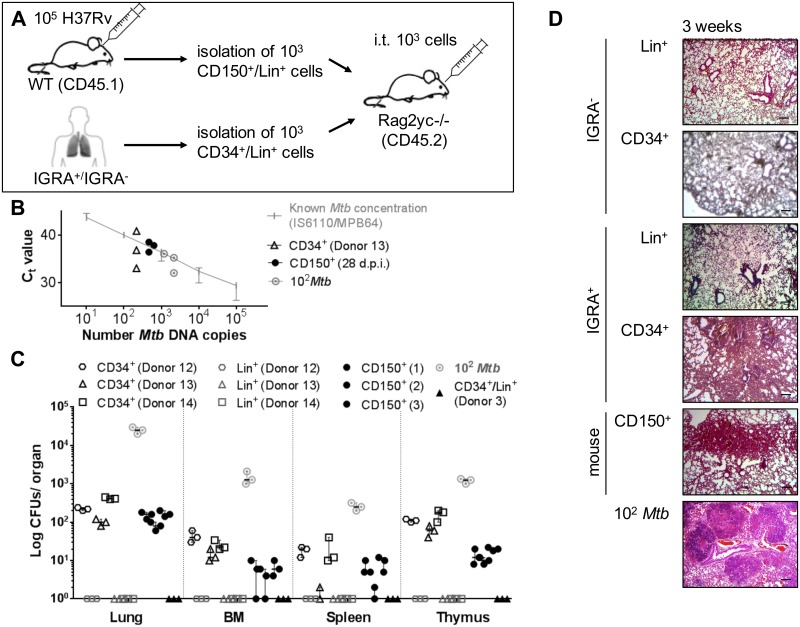
Next, we infected mice with Mtb to see whether bone marrow-derived LT-pHSCs could become carriers of the bacterium too. We used a mouse model of intradermal ear infection, where low numbers of Mtb persist systemically without developing active TB typically seen after aerosol infection of mice with Mtb [5]. A variety of organs, such as the lung, and the spleen, but not the thymus, and hematopoietic cells in them were found to be infected 28 days post-infection (p.i.). DNA purified from 105 lung cells harboured between one and ten copies of Mtb DNA (Fig 2A and 2C).
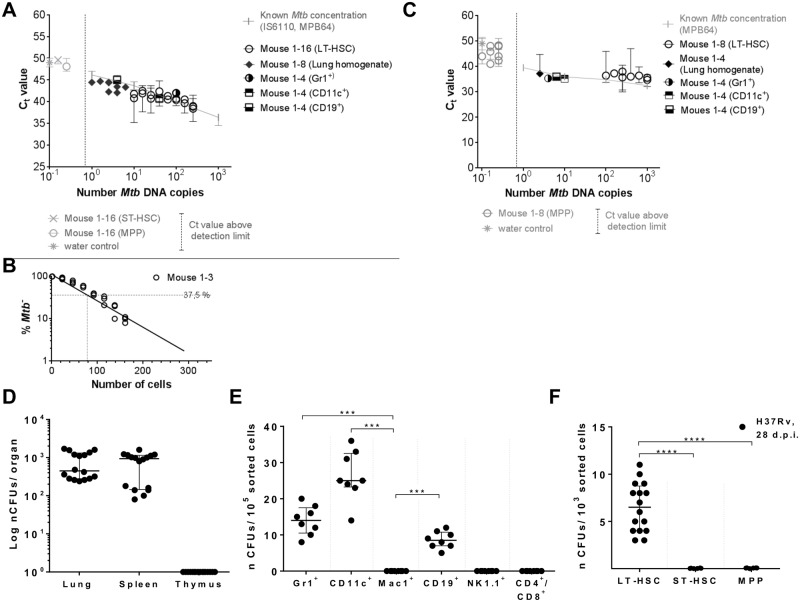
Fig 2. Detection of Mtb infection in different organs and hematopoietic cells of mice day 28 p.i. by Mtb DNA PCR and Mtb CFU.
C57BL/6 mice were infected with 105 CFUs Mtb (H37Rv). (A) Quantification of Mtb-specific DNA by real-time TaqMan PCR using probes targeting MPB64 and IS6110 (S4 Fig) on genomic DNA of 105 lung cells (n = 8), 105 Gr1+, CD11c+, CD19+, Mac1+, NK1.1+, CD4+/8+ cells (n = 4; S1C Fig), and 103 LT-pHSCs, ST-pHSCs and MPPs (n = 16; S1B Fig). (B) Quantification of Mtb-specific DNA by limiting dilutions using a single-target PCR for IS6110 (n = 3; S2B Fig). (C) Real-time SYBR green PCR using primers targeting MPB64 (n = 4–8; S4 Fig). Real-time PCRs were performed in 2 independent runs in technical triplicates and normalized to murine GAPDH. Known Mtb concentrations were used as reference. (D) CFU enumeration on Middlebrook 7H11 agar in cells of lung, spleen and thymus (n = 16). (E) CFU enumeration on Middlebrook 7H11 agar for Lin+ cell populations (n = 8). (F) CFU enumeration on Middlebrook 7H11 agar for hematopoietic progenitors (n = 16). Shown are data of 4 independent experiments. Data are shown as median + interquartile. *P ˂ 0.05, **P ˂ 0.005, ***P ˂ 0.0005, ****P ˂ 0.00005 by Mann-Whitney test.
103 FACS-purified Lin–Sca1+c-Kit+CD150+CD48– LT-pHSCs (S1C Fig) [36] were found to harbor between 40 and 100 copies of Mtb DNA, as detected by qPCR targeting MPB64 and IS6110 and in limiting dilution analyses targeting IS6110 alone (Fig 2A–2B; S2B Fig). In MPB64 qPCRs LT-pHSCs were found to harbor between five and 90 copies of Mtb DNA (Fig 2C). By contrast, in 103 Lin–Sca1+c-Kit+CD150+CD48+ short-term repopulating pluripotent hematopoietic stem cells (ST-pHSCs) and Lin–Sca1+c-Kit+CD150-CD48+ multipotent progenitors (MPPs) (S1C Fig) [36], no Mtb DNA could be detected using any of these PCR analyses (Fig 2A and 2C).
Furthermore, no Mtb DNA was found in 105 FACS-enriched Mac1+ macrophages, NK1.1+ NK cells, and CD4+ as well as CD8+ T cells (Fig 2A; S1D Fig), while between eight and 60 copies of Mtb DNA were found in qPCR analyses of lysates of 105 FACS-enriched CD11c+ dendritic cells, Gr1+ granulocytes and CD19+ cells identifying B as well as dendritic cells, representing 10 to 100-fold lower numbers of Mtb DNA copies than in LT-pHSCs (Fig 2A and 2C). We have not attempted to purify MSCs from bone marrow of infected mice.
We conclude that intradermal infection of mice resulted in Mtb-infected LT-pHSCs in bone marrow 28 days p.i., and that during this infection, similarly to our observations in human LTBI, only LT-pHSCs harbored Mtb DNA among specific pHSC populations. Nevertheless, in this mouse model, the lung and more mature Lin+ cells in spleen and bone marrow of mice are infected with Mtb detected by qPCR and CFU assays, which is in contrast with our observations in human LTBI. Accordingly, we do not suggest that this mouse infection model, at day 28 p.i., is comparable to the pathophysiological status of latent infections in LTBI. Nevertheless, the selective Mtb infection of LT-pHSCs over ST-pHSCs and MPPs was recapitulated in our mouse model.
Numbers of replication-competent, colony-forming Mtb in Mtb-infected mouse cells
We tested the different Mtb- DNA+ cell populations for replication-competent, active Mtb measured by enumerating CFUs (Fig 2D–2F). The vast majority, if not all, of the Mtb bacteria detected by PCR in 105 lung cells (Fig 2A, 2C and 2D), as well as in 105 FACS-enriched CD11c+ dendritic cells, Gr1+ granulocytes and CD19+ dendritic or B-lineage cells in bone marrow (Fig 2A, 2C and 2E) produced CFUs. Hence, most Mtb bacteria inside these cells were replication-competent. These results also document, that the two assays, for Mtb DNA and for CFUs of active Mtb, detect comparable numbers of bacteria.
By contrast, only 10 CFUs were formed from lysates of 103 LT-pHSCs isolated from Mtb-infected mice, which, in MPB64 PCR assays, contained between five and 90 Mtb DNA copies (Fig 2C and 2F). Thus, up to 80% of Mtb DNA in LT-pHSCs were not replication-competent as assayed by CFUs.
Our results also suggest that not all, but only a part, of the LT-pHSC pool is infected by Mtb, almost all of which were in a quiescent stage. However, these analyses do not reveal the number of infected cells in the pHSC pool.
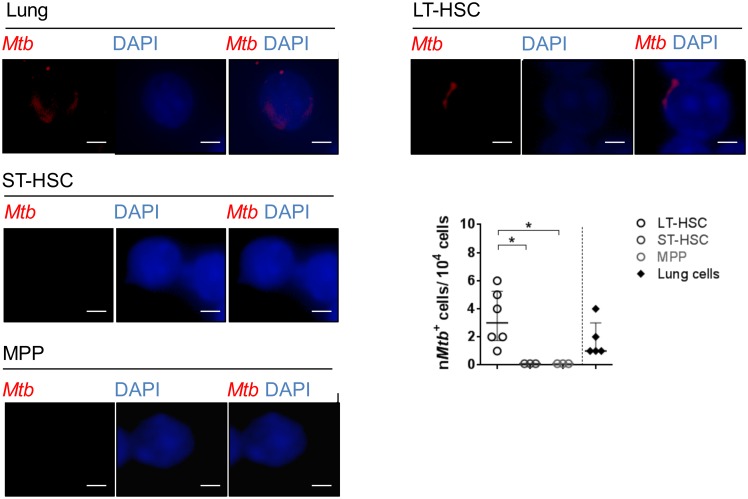
Next we directly visualized LT-pHSCs carrying Mtb by rhodamine-auramin staining [37]. In LT-pHSCs, Mtb was readily detectable, whereas ST-pHSCs and MPPs did not show positive staining (Fig 3). Approximately one to six of ~ 103 analyzed LT-pHSCs stained positive for Mtb (Fig 3). As a control Mtb-infected lung cells were analyzed. In these cells the number of Mtb positive cells as revealed by rhodamine-auramin staining, qPCR and CFU were showing a good correlation (Figs 3 and 4).
Fig 3. Detection of Mtb in cells of the lung and hematopoietic cells of Mtb-infected mice by histology.
Rhodamin-auramin stainings of representative LT-pHSCs (n = 6), ST-pHSCs (n = 3), MPPs (n = 3) as well as cells of the lung (n = 5) at day 28 p.i. For each sample (cell sort) 10,000 cells were screened per slide for Mtb positive cells. At least 3 images were taken from each slide of each sample. Rhodamin-auramin stainings were screened on high power (100×) and verified under oil immersion using a fluorescent microscope. Analyses were carried out using ProGres Capture Pro 2.8.8. (Mtb, red; nuclei, blue). Shown are representative data (cropping of images) for staining of LT-pHSCs, ST-pHSCs, MPPs and cells of the lung. scale bar: 10 μm. *P ˂ 0.05 by Mann-Whitney test.
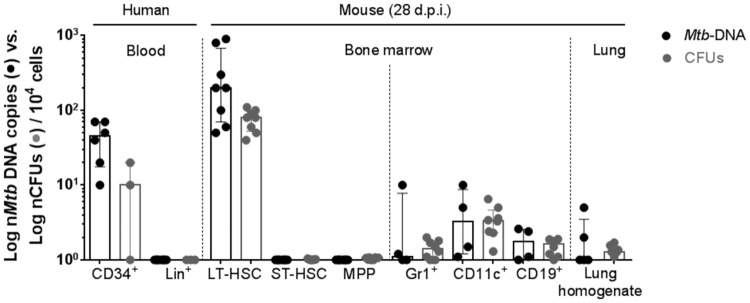
Fig 4. Numbers of Mtb DNA copies (MPB64 qPCR) and CFUs in 104 cells of different cell populations.
Data are shown as median + interquartile (n = 4–8).
However, this staining method was not as sensitive as qPCR in determining the actual number of LT-pHSCs harbouring Mtb [38].
Mtb residing within human CD34+ as well as mouse CD150+ pHSCs express genes of the dormancy regulon
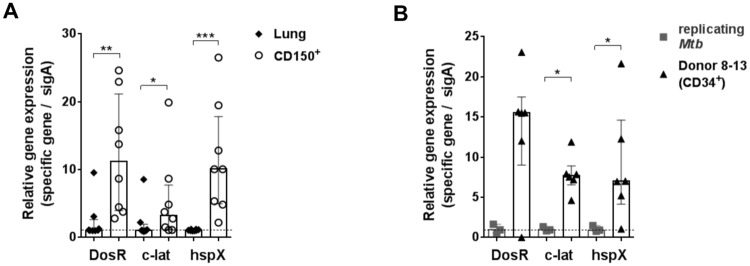
Since 103 mouse LT-pHSCs were found to contain between five and 90 copies of Mtb genomes, but generated only 10 CFUs (Figs 2C, 2F and 4), we concluded that the vast majority, i.e. around 80%, of Mtb bacterial genomes persist in a non-replicating form. Dormancy or stress of Mtb is induced under hypoxic conditions and is reflected in a change of gene expression [25–27]. It is controlled by the dormancy regulon and involves transcription of approximately 50 so-called dormancy genes, among them DosR, c-lat and hspX [19, 25–27]. SigA is expressed in non-replicating as well as in replicating, CFU-forming Mtb and thus, can be used as a housekeeping gene [20].
We hypothesized that the hypoxic niche of LT-pHSCs, by being stressed, could induce dormancy of Mtb [7, 8]. To test for this hypothesis, we performed quantitative RNA expression analyses for Mtb dormancy genes in mouse LT-pHSCs as well as in Mtb-infected lung cells 28 days p.i., in which the vast majority of Mtb organisms form CFUs (Fig 4).
SigA was detected in both cell types. By contrast, Mtb DNA+ LT-pHSCs did express DosR, c-lat and hspX RNA, while Mtb from infected lung cells did not express dormancy genes (Fig 5A). We conclude that Mtb resides within mouse CD150+ LT-pHSCs in a non-replicating state, expressing dormancy genes.
Fig 5. Murine and human pHSCs are infected with Mtb expressing dormancy genes.
(A) Expression analyses on RNA isolated from Mtb-infected mouse lung cells and purified LT-pHSCs (n = 8). (B) Expression analyses on RNA isolated from Lin–CD34+ pHSCs from IGRA+ donors (n = 6) and Mtb-infected human monocytic leukemia cell line 96 h p.i. (n = 3). Expression analyses were done by real-time TaqMan PCR for SigA, DosR, c-lat and hspX. SigA was used as reference for Mtb. Real-time TaqMan PCRs were performed in 3 independent runs in technical triplicates. Data are shown as median + interquartile. *P ˂ 0.05, **P ˂ 0.005, ***P ˂ 0.005 by Mann-Whitney test.
In human Mtb-infectedSP+ and Lin–CD34+ pHSCs we similarly detected both SigA expression and the expression of the dormancy regulator genes, DosR, c-lat and hspX, while replicating Mtb isolated from an infected human monocytic leukemia cell line did not express these genes (Fig 5B). Collectively, our results show that pHSCs act as an intracellular niche for stressed or dormant non-replicating Mtb in mice and humans.
Intratracheal transfer of Mtb-infected human and murine pHSCs leads to resuscitation and expansion of active TB in transplanted hosts
Finally, we tested the capacity of Mtb-infected pHSCs of human LTBI and of mice 28 days p.i. to resuscitate active infection upon intratracheal application into the trachea of Rag2–/–Il2rg–/–mice [39]. One thousand Lin–CD34+ cells from blood of latently infected, IGRA+, Mtb DNA+ human donors, containing between 1 and 7 copies of Mtb-DNA, and of IGRA−donors (Fig 1A and 1D; S4 Fig), Lin–CD150+CD48– LT-pHSCs from bone marrow of infected mice (Fig 2A and 2C), containing between 5 and 90 (Fig 2C) copies of Mtb-DNA as well as 100 pure, replication-competent Mtb as control, were administered into the trachea of Rag2–/–Il2rg–/–mice and organs were analyzed 3 weeks later (Fig 6A). Mtb DNA as well as active, replicating Mtb CFUs were detected in the lungs (Fig 6B and 6C). Between 100 and 400 CFUs were detected in total lung cells of mice receiving human LT-pHSCs, while in lungs of mice receiving mouse LT-pHSCs contained between 50 and 200 CFUs. Hence, Mtb contained in pHSCs had expanded between 10 and 100-fold as active, replicating bacteria. Active, replicating Mtb was also detected in spleen, thymus, and bone marrow in mice recieving pHSCs from IGRA+ donors and Mtb-infected mice, but not in mice recieving Lin+ cells from IGRA+ donors or cells from IGRA−donors (Fig 6B–6C).
Fig 6. Intratracheal transfer of Mtb infected human and murine pHSCs leads to Mtb growth and increased cellularity into the lungs in transplanted hosts.
(A) Injection of Lin-CD34+ and Lin+ cells from blood of IGRA+ human donors (Donor 12–14) and mouse LT-pHSCs from bone marrow 28 days p.i. into the trachea of Rag2–/–Il2rg/–mice (3 mice/population). Transfer of 102 CFUs Mtb was used as positive (n = 3), uninfected pHSCs and Lin+ cells of an IGRA−donor (Donor 3; n = 1) as negative, control. Recipients were analyzed after 3 weeks. (B) Monitoring of Mtb infection by TaqMan PCR using probes that target MPB64 and IS6110 together on genomic DNA of 105 lung cells 3 weeks upon transfer. PCRs were performed in technical triplicates and normalized to murine GAPDH. (C) CFU Mtb growth on Middlebrook 7H11 agar in cells of lung, spleen, thymus and non-separated, 105 bone marrow cells 3 weeks upon transfer (n = 3/population). Shown are data from 3 independent experiments. (D) Histopathology of representative lung sections 3 weeks upon transfer. Lungs were stained with hematoxylin/eosin, screened with 5×objectives and verified using a light microscope. Shown are representative data from 3 independent experiments. Data are shown as median + interquartile. Scale bar: 100 μm.
We conclude from these results, that Mtb replicated in the lungs of mice, into which Mtb-infected human or mouse pHSCs had been transferred. These expanded Mtb were replication—competent and had spread systemically. However, while in total bone marrow cells of the recipient mice Mtb DNA and replicating Mtb CFUs were detectable (Fig 6C), pHSCs of the donors (human or mice) could not be detected by FACS in the bone marrow of the recipient. This suggests, that bone marrow infection was caused by a dissemination of replication-competent Mtb from the lung resulting from a primary infection, rather than by Mtb-infected donor pHSCs homing from lung to bone marrow.
In histological sections of lungs of the Rag2–/–Il2rg–/–mice transplanted with either human pHSCs from LTBI or mouse pHSCs from Mtb-infected mice, we observed increased cellularity in the lungs indicative of an inflammatory infiltrate in response to an active infection 3 weeks after pHSC transfer (Fig 6D). Transfer of Lin+ cells from IGRA+ donors and cells from IGRA−donors did not induce these histological changes in the lung of recipients.
The observed increase in cellularity in the lung could result from the expansion of replication-competent Mtb or from both replication-competent and stressed dormant bacteria. In the latter case, stressed dormant Mtb could be resuscitated to active replicating bacteria. In any case, we conclude that Mtb-infected human and mouse pHSCs can reproduce an active infection after introduction into recipient mice by intratracheal transfer.
Discussion
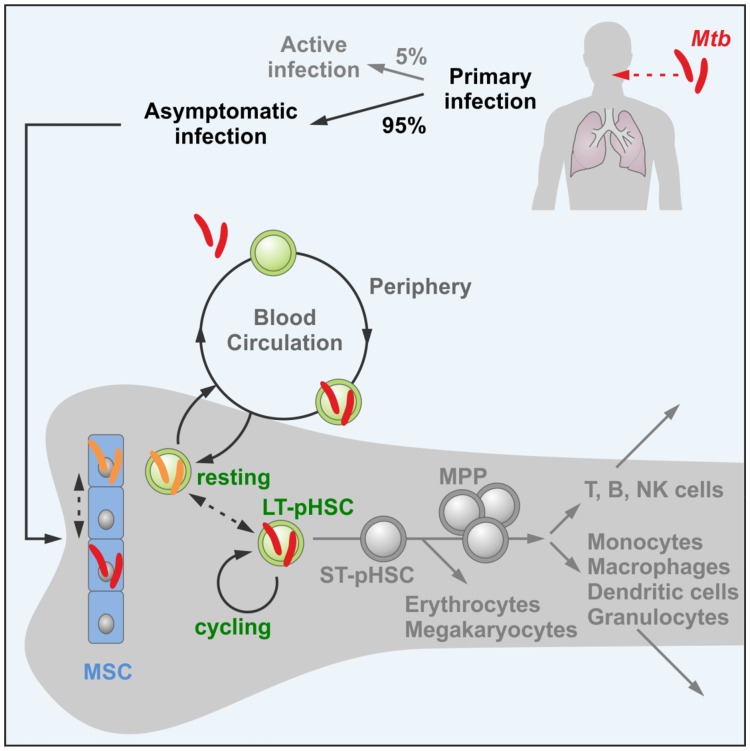
Our results suggest that, in individuals with LTBI, dormant Mtb bacteria reside, and perhaps transit, between long-lived, resting hematopoietic and non-hematopoietic cells in hypoxic niches (Fig 7) [6–8, 40]. We have interpreted the expression of DosR, hspX and c-Lat genes as a sign of either stress or dormancy of Mtb, both induced in hypoxic areas of bone marrow that are thought to promote energy saving, and thus, could favor long-term rest of both LT-pHSCs and Mtb. If so, do the energy-saving gene expression programs of both Mtb and host pHSCs interact with each other [4, 19]?
Fig 7. A model of a long-term persisting niche for non-replicating Mtb bearing the risk of resuscitation of active Mtb.
Model of LTBI where non-replicating Mtb reside, and perhaps move, between long-lived, resting hematopoietic and non-hematopoietic cells in hypoxic niches in bone marrow and in which actively replicating Mtb can be resuscitated leading to TB.
Future whole transcriptome next-generation sequencing of Mtb- as well as of LT-pHSC-encoded genes expressed in single LT-pHSCs will not only allow monitoring of Mtb- but also of LT-pHSC-controlled gene expression programs and their potential for interactions in hypoxic stem cell niches of bone marrow. It will also provide more precise information on the number of LT-pHSCs infected by either replicative or dormant Mtb. If Mtb and LT-pHSCs adapt to each other by dormancy, it is conceivable that other facultative intracellular bacteria could find the same long-term quiescent niche for long-term persistence in a dormant state.
The possibility of a transfer of Mtb from infected bone marrow donors has been made likely in several case reports that have described incidence of Mtb infections between 120 days and 20 months post allogenic bone marrow transfer. However, these reports only refer to the induction of an Mtb infection as a consequence of the administration of immunosuppressive drugs to the recipients [41–45]. While patients that receive an allogeneic pHSC transfer, or that are scheduled to be treated with anti-inflammatory agents such as anti-TNFα antibodies, are usually tested for their IGRA-status prior immunosuppressive treatment, pHSCs are not screened for possible infections. Our results suggest that bone marrow donors should be screened for Mtb infection, so that they can be cured of the infection prior to bone marrow transplantation.
Within the limited numbers of human donors with LTBI, that were available to us, all of them carried Mtb exclusively in pHSCs. Two of them were also analysed for the presence of Mtb in MSCs, and both were positive. However, a much larger number of LTBI donors should be screened to evaluate, whether a small percentage of LTBI donors could be free of Mtb in their pHSCs. Such a larger analysis could also test the possibility, that a low percentage of LTBI donors could carry Mtb in the progeny of pHSCs, e.g. in MPPs, CMPs or CLPs.
A host with LTBI has immunological memory for Mtb [2]. Therefore, resuscitation of TB will only be successful if the immune system of the LTBI host fails to eliminate cells, in which actively replicating Mtb have been resuscitated from a stressed or dormant state. This can be readily observed in patients with inborn or acquired immunodeficiencies, e.g. in HIV-infected patients or patients treated with TNFα-inhibitors. The precise stimuli leading to reactivation require further investigation. However, the consequences of pHSCs spreading active Mtb throughout the body, either directly or after differentiation, can be detrimental. Our data thereby provide an additional explanation for the possible occurrence of reactivated TB in other bodily organs after primary infection and encapsulation in granulomas.
Materials and Methods
Human samples
Latently Mtb infected subjects included in the study were from a Western country, had not been treated previously for tuberculosis (TB), had normal chest radiography and were not suffering from active TB. Hence, LTBI individuals were routinely identified by positive IGRA (Quantiferon-TB Gold® test, Cellestis, Qiagen) and exclusion of active TB. IGRA testing was performed either because of a scheduled treatment with TNF-α inhibitors or because of occupational contact with patients suffering from active pulmonary TB.
Collection of blood samples was approved by the Ethics Committee of the Medical University of Vienna (EK 071/2005) and conducted according to the Declaration of Helsinki. Informed written consent was obtained from all patients.
Mice
C57BL/6 wild-type mice were purchased from Charles River Laboratories. CD45.1 C57BL/6 and Rag2–/–Il2rg–/–mice were bred in our facilities. Infected mice were maintained at biosafety level 3. All animal experiments were approved by the local ethics committee of the German authorities (State Office of Health and Social Affairs Berlin; Landesamtes für Gesundheit und Soziales Berlin, # G0009-14).
Infection with Mtb
Mtb strain H37Rv was cultured in Middlebrook 7H9 broth (BD) supplemented with 0.05% (v/v) Tween 80 and Middlebrook AODC Enrichment (BD) to mid-log phase (OD 600 nm 0.6–0.8). Bacteria were harvested, resuspended in PBS (GIBCO), and frozen at –80°C until use. For dermal infections, 8- to 10-week-old female C57BL/6 wild-type mice were anesthestized by i.p. administration of ketamine (50 mg/kg) and Rompun (5 mg/kg; Bayer), and 105 Mtb in 50 μl PBS were administered into the ear dermis. Mice were monitored daily regarding their health, body condition and well-being. Specifically, we monitored mice for loss of body weight, abdominal respiration and lesions of ear dermis. Once a week mice were weighed. At the end of experiment mice were sacrificed by cervical dislocation. For the infection of human monocytic leukemia cells in vitro, THP-1 cells (ATCC®TIB-202™, ATCC cell lines, UK) were used, that were authenticated by STR profiling and tested for mycoplasma contamination. We have not used any cell line from the list of commonly misidentified cell lines (ICLAC). THP-1 cells were seeded in T75 flasks (TPP) in complete RPMI-1640 (cRPMI, RPMI-1640 medium supplemented with 1% L-glutamine, 1% Hepes, 0.1% 2-ME and fetal bovine serum to a final concentration of 10%; GIBCO, Life Technologies). For proper viability of cells, a concentration of 1 × 106 cells/ml was not exceeded. Cells were incubated at 37°C and 5% CO2. Differentiation to macrophages was triggered by overnight incubation with PMA (50 ng/ml), followed by two washes in RPMI-1640 and addition of cRPMI-1640 over 48 h post-differentiation. For infection, 107 differentiated macrophages were seeded into T150 flasks in 25 ml cRPMI and 1 ml of medium containing 105 Mtb was added. Non-internalized bacteria were washed away 4 h p.i. using PBS and cells were placed back in cRPMI. Cells were harvested for RNA isolation 48 and 96 hours p.i.
Antibodies
For the purification of 2–40 × 103 circulating human hematopoietic precursor cells as well as mesenchymal stem cells from 90 ml of peripheral blood, PBMCs were obtained by Ficoll-Paque density gradient centrifugation (Ficoll-Paque Plus; GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and the following antibodies were used: CD1c (clone AD5-8E7, Miltenyi Biotec), CD3 (UCHT1, Beckman Coulter), CD11c (Bu15, Beckman Coulter), CD14 (RMO52, Beckman Coulter), CD15 (HI98, BioLegend), CD16 (3G8, Beckman Coulter), CD20 (2H7, BioLegend), CD41 (SZ22, Beckman Coulter), CD56 (C218, Beckman Coulter), CD203c (NP4D6, BioLegend), CD235a (KC16, Beckman Coulter), BDCA2 (AC144, Miltenyi Biotec), CD34 (8G12, BD Biosciences), CD38 (HIT2, Biolegend), CD90 (5E10, Biolegend), CD45 (H130, BD Bioscience) and CD271 (ME20.4-1.H4, BD Bioscience). Secondary staining was done with goat anti-mouse IgG Alexa Fluor 488 (MolecularProbes). Immunomagnetic depletion was performed using anti-mouse IgG (magnetic cell sorting; Miltenyi Biotec). Hematopoietic progenitor cells were sorted to a purity >98% on a FACS Aria (BD Biosciences).
For purification of 103 cells of mouse bone marrow hematopoietic progenitor cells and 105 Lin+ cells, the following antibodies were used: Mac1 (M1/70), Gr1 (RB6-8C5), Ter119 (TER-119), CD19 (1D3), B220 (RA3-6B2), CD5 (53–7.3), CD3ε (145-2C11), CD11c (N418), CD4 (GK1.5), CD8 (53–6.7), NK1.1 (PK136), c-Kit (2B8), Sca1 (D7), CD150 (TC15-12F12.2) and CD48 (HM48-1). Antibodies were obtained from eBioscience. Cells were sort purified to a purity of >98% on an LSRII flow cytometer (Aria II, BD Biosciences).
Hoechst staining
Human PBMC were resuspended in SP buffer (HBSS, 2% FCS, 2mM HEPES buffer; GIBCO, Life Technologies), prewarmed to 37°C and incubated with Hoechst 33342 (Molecular Probes, Life Technologies) at 5 μg/ml for 2 h at 37°C. All subsequent steps were carried out on ice. Cells were stained with antibodies against lineage markers as described above. 7-AAD (5 μg/ml, Calbiochem) was added for live—dead cell discrimination. As negative control, PBMCs were preincubated with verapamil (100 μM, Sigma Aldrich). Cells showing a dim staining in the Hoechst blue (450/50 nm band pass filter) and Hoechst red (660/20 nm) channels were sorted to a purity >98% on a FACS ARIA (BD Biosciences).
Mtb DNA detection
We used the mycobacterial DNA extraction procedure first described by van Soolingen et al. 50 ng (pHSCs)—1 μg (Lin+ and lung cells) of DNA was analyzed by real-time TaqMan® PCR using gene-specific probes targeting two Mtb sequences, namely MPB64 and IS6110 together (Path-M.tuberculosis_MPB64/IS6110, Integrated Science). Each sample was assayed in technical triplicates. TaqMan probes for GAPDH were used as endogenous controls for eukaryotic cells (Human: Hs99999905_m1, Mouse: Mm99999915_g1, Invitrogen). H37Rv DNA was used to construct a standard curve for the probes.
In addition, DNA was analyzed by quantitative PCR using the primers 5′-CAGGCATCGTCGTCAGCAGC-3′ and 5′-GTGATTGGCTTGCGATAGGC-3′ targeting MPB64 alone (543-bp DNA fragment) [46], using the SYBR green system of detection. Primers for human 5′-CTCCCCACACACATGCACTTA-3′ and 5′- CCTAGTCCCAGGGCTTTGATT-3′ and mouse GAPDH 5′-CATGTTCCAATATGATTCCAC-3′ and 5′-CCTGGAAGATGGTGATG-3′ were used as endogenous controls for eukaryotic cells. H37Rv DNA was used to construct a standard curve for primers used. PCR products were detected as an increase in fluorescence with the ABI PRISM 7700 instrument and quantified using the SDS software, version 2.2.2.
In order to reduce amplification backgrounds with primers, we have performed quantitative PCR analyses using a “no template = water control” for every run. Exponential amplification in the “water control” with Ct values of 51–55 was taken as the detection limit for Mtb in PCRs targeting IS6110 and MPB64 together. In PCRs targeting MPB64 alone, “water control” Ct values of 48–50 was taken as the detection limit. To ensure that this background did not result from a contamination by genomic Mtb DNA, the MPB64 qPCR product was analyzed by gel electrophoresis. While the expected PCR product size was detectable in the Mtb+ samples, such a distinct PCR band were not found in the “water control” (S3 Fig). In PCRs targeting IS6110 together with MPB64 we considered samples as positive for Mtb with a Ct value of 48 (equivalent to 1 Mtb DNA copy) or lower (equivalent to several Mtb DNA copies). In PCR tests targeting MPB64 alone, we considered samples as Mtb positive with a Ct of 39–40 (equivalent to 1 Mtb DNA copy) or lower (equivalent to several Mtb DNA copies).
For limiting dilution analyses on DNA the primers 5′-CGTGAGGGCATCGAGGTGGC-3′ and 5′-GCGTAGGCGTCGGTGACAAA-3′ were used to amplify a 245-bp DNA fragment encoded by the IS6110 insertion sequence in the Mtb genome [30]. At the point where the PCR signal was lost in serial dilutions, limiting dilution analyses were performed.
Mtb-specific and BCG-specific DNA was also detected using primers previously described [31]. PCR reactions were performed in a thermal cycler at 95°C for 15 min, followed by 50 cycles at 95°C for 30 s, 45 s at different annealing temperatures and 45 s at 72°C (DNA Engine® PTC2000, Biozym DiagnosticRad). For every reaction uninfected DNA and DNA from H37Rv Mtb were included. PCR products were analyzed by electrophoresis on 2% agarose gels.
Colony-forming units
Mice were sacrificed at 28 days p.i., and organs (spleen, thymus, lung and bone marrow) were aseptically removed and homogenized in 1 ml PBS containing 0.05% Tween 80 (v/v). For pulmonary CFU determination, the lung was removed and incubated in 1 mg/ml collagenase type VIII (Sigma-Aldrich) and 30 μg/ml DNase I (Roche) at 37°C for 30 min. One half of each of the lung homogenate, the spleen, and the thymus were diluted in PBS containing 0.05% v/v Tween 80 and plated onto Middlebrook 7H11 agar plates supplemented with Middlebrook OADC Enrichment (Dibco). In addition, purified human and mouse pHSCs were plated. CFUs were enumerated after 4–6 weeks of incubation at 37°C and 5% CO2.
RNA/qRT-PCR
Cells were homogenized in TRIzol (Invitrogen) and RNA was isolated via chloroform extraction (Life Technologies), treated with ethanol and dissolved in RNase-free water. RNA from Mtb infected THP-1 cells was isolated as previously described [47, 48]. One hundred ng of total RNA was reverse-transcribed by SuperScript III (Invitrogen) primed with oligodT. The cDNA for the specific target assays was then amplified by pre-amplification reaction using pooled gene-specific primers according to the manufacturer`s protocol (Invitrogen). The pre-amplification product was diluted [1:20) and finally analyzed by real-time TaqMan® PCR using the following TaqMan probes: DosR, c-lat, hspX and SigA (Design Batch ID: w1406535517000, order number: 2106064SO, Invitrogen). DosR, c-lat and hspX RNA abundances were normalized to SigA as endogenous controls for Mtb. Each sample was assayed in triplicate. H37Rv DNA was used to construct a standard curve for all inspected genes. The PCR product was detected as an increase in fluorescence with the ABI PRISM 7700 instrument. RNA was quantified using the SDS software, version 2.2.2.
Cytology
Sorted cells were fixed in PBS containing 4% w/v PFA for 24 h at 4°C. Thereafter, cells were immobilized by cytospin on a solid support (Shandon Centrifuge, Modell Cytospin 3). Slides were flooded with auramine-rhodamine for 15 min, fluorescent decolorizer for 2–3 min and potassium permanganate for 3–4 min. A 2-μg/ml working solution of DAPI was used for nuclear visualization.
Cover slips were mounted in ProLong Gold anti-fade reagent (Cat. No. P36934; Invitrogen) and sealed using adhesives. Slides were screened with 100× (for images) objectives under oil immersion using a fluorescence microscope (DMRB Fluorescence Microscope, Leica Microsystems). Analyses using the fluorescence microscope were done using ProGres Capture Pro 2.8.8. (Optical Systems, Jenoptick AG). For confocal microscopy image acquisition was performed using Zen 2010 Version 6.0 and images were analyzed by Zen 2012 Light Edition software (Carl Zeiss MicroImaging). For each sample 10,000 cells were analyzed per slide for the number of Mtb positive cells. For each slide at least three images of representative cells were taken.
Histology
For histology, lung caudal lobes were preserved in PBS containing 4% w/v PFA for 24 h at 4°C, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Slides were screened with 5× objectives on a light microscope (Leica DMLB, Leica Microsystems). Analyses were done using ProGres Capture Pro 2.8.8. (Optical Systems, Jenoptick AG).
Intratracheal administration of Mtb-infected pHSCs
For challenge, 8- to 10-week-old female Rag2–/–Il2rg–/–mice were anesthetized by i.p. administration of ketamine (50 mg/kg) and Rompun (5 mg/kg). Thereafter, using a micropipette, 100 CFU of Mtb H37Rv, 1,000 pHSCs of uninfected and Mtb-infected mice at day 28 p.i., or 1,000 Lin+ cells and pHSCs of human IGRA+ as well as IGRA−donors diluted in 50 μl of sterile PBS were gently placed in the trachea of each mouse. Mice were monitored daily for loss of body weight and abdominal respiration. Mice were sacrificed by cervical dislocation 3 weeks post-transfer and lungs were analyzed for Mtb-specific DNA, CFUs and histologically.
Statistics
For all statistical analyses, PRISM (Version 6, GraphPad, San Diego) software was used. Dispersion is presented as the median + interquartile, unless stated otherwise. Statistical analysis was performed with Mann-Whitney two-tailed test. P values <0.05 were considered significant.
Supporting Information
(A) Purification of Lin+, Lin–CD34+, Lin–CD34+CD38–CD90+, Lin–CD34+CD38+CD90– as well as Lin–SP+ and Lin+ SP−cells by FACS from blood cells from IGRA+ and IGRA−donors. (B) Purification of CD271+CD45- mesenchymal stem cells by FACS from blood cells from IGRA+ donors. (C) Purification of Lin−hematopoietic progenitors and (D) Lin+ Gr1+ granulocytes, CD11c+ dendritic cells, Mac1+ macrophages, NK 1.1+ NK cells, CD4+/8+ T cells and CD19+/B220+ B cells by FACS from bone marrow of infected mice day 28 p.i. Representative FACS blots are shown. The data contained herein relate to both main Figs 1 and 2.
(DOC)
(A) Representative example (Donor 14) of a gel analysis of single Mtb DNA samples expanded by limiting dilution to a single-target IS6110 PCR. (B) Representative example (Mouse 2) of a gel analysis of single Mtb DNA samples expanded by limiting dilution to a single-target IS6110 PCR. Note: as expected from Poisson’s distribution not all, but in the analysis of human pHSC, only 5 of 23 individual PCR tests (left), and of mouse LT-pHSC only 6 of 23 individual PCR tests (right) yielded a PCR product (see arrow). The data contained herein relate to both main Figs 1 and 2.
(DOC)
(A) Analysis of SYBR green qPCR products by gel electrophoresis, to ensure that qPCR background did not result from a contamination by genomic DNA, thus the amplification of a Mtb specific DNA fragment. (B) Amplification plot for the “water control” (1) and for 1 Mtb DNA copy (2). (C) Melt curve for the “water control” (1) and for 1 Mtb DNA copy (2). The data contained herein relate to both main Figs 1 and 2.
(DOC)
Genomic DNA was prepared and DNA of 103 hematopoietic progenitors from IGRA+ donors was tested by PCR. Quantification of Mtb-specific DNA was done by real-time TaqMan PCR using probes that target MPB64 and IS6110 together as well as real-time SYBR green PCR using primers that target MPB64 alone. PCRs were performed in technical triplicates and normalized to human GAPDH (median + interquartile). Due to a lack of sufficient DNA material we were not able to include single-target qPCRs on donors 8 and 9.
(DOC)
Acknowledgments
The Max Planck Institute for Infection Biology (Lymphocyte Development, F.M., and Immunology, S.H.E.K.) and the Division of Immunology, Allergy and Infectious Diseases, Medical University of Vienna cooperated in this work.
We thank Anca Dorhoi, MPIIB Berlin, Jörg Vogel, University of Würzburg, Jan Anderson, University of Basel and Andreas Radbruch, DRFZ Berlin for critical reading of our manuscript and Mary Louise Grossman for editorial assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the European Union’s Seventh Framework Programme (EU FP7) project “ADITEC” (HEALTH-F4-2011-280873); and EU Horizon 2020 project “TBVAC 2020” (grant 643381) to SHEK. In addition, the work was supported in parts by a Reinhard Koselleck-Grant of the Deutsche Forschungsgemeinschaft ME 2764/1-1 to FM.
References
- 1.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 2004;84: 29–44. [DOI] [PubMed] [Google Scholar]
- 2.Young JS, Gormley E, Wellington EME. Molecular Detection of Mycobacterium bovis and Mycobacterium bovis BCG. Appl. Environ. Microbiol. 2005;71: 1946–1952. 10.1128/AEM.71.4.1946-1952.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom BR, McKinney JD. The death and resurrection of tuberculosis. Nat Med. 1999;5: 872–874. 10.1038/11309 [DOI] [PubMed] [Google Scholar]
- 4.Gengenbacher M, Kaufmann SHE. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev. 2012;36(3): 514–532. 10.1111/j.1574-6976.2012.00331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reece ST, Loddenkemper Ch, Askew DJ, Zedler U, Schommer-Leitner S, Stein M, et al. Serine protease activity contributes to control of Mycobacterium tuberculosis in hypoxic lung granulomas in mice. J Clin Invest. 2010;120(9): 3365–3376. 10.1172/JCI42796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96: 3120–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7: 380–390. 10.1016/j.stem.2010.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD, Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104: 5431–5436. 10.1073/pnas.0701152104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4): 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7: 1028–1034. 10.1038/nm0901-1028 [DOI] [PubMed] [Google Scholar]
- 11.Fujisaki J, Juwell W, Carlson AL, Silberstein L, Putheti P, Larocca R, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474: 216–219. 10.1038/nature10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbein G, Sovalat H, Wunder E, Baerenzung M, Bachorz J, Lewandowski H, et al. Isolation and identification of two CD34+ cell subpopulations from normal human peripheral blood. Stem Cells. 1994;12(2): 187–197. 10.1002/stem.5530120207 [DOI] [PubMed] [Google Scholar]
- 13.Majeti R, Park CJ, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1: 635–645. 10.1016/j.stem.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuçi S, Kuçi Z, Kreyenberg H, Deak E, Pütsch K, Huenecke S, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010; 95: 651–659. 10.3324/haematol.2009.015065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quirici N, Soligo D, Bossolasco P, Servida F, Lumini, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002;30: 783–791. [DOI] [PubMed] [Google Scholar]
- 16.Pai M, Riley LW, Colford JM. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4(12): 761–776. 10.1016/S1473-3099(04)01206-X [DOI] [PubMed] [Google Scholar]
- 17.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89(4): 1502–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada S, Nakaauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80(12): 3044–3050. [PubMed] [Google Scholar]
- 19.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell M I, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc Natl Acad Sci USA. 2001;98: 7534–7539. 10.1073/pnas.121172498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manganelli R, Dubnau E, Tyagi S, Kramer FR, Smith I. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol Microbiol. 1999;31(2): 715–724. [DOI] [PubMed] [Google Scholar]
- 21.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macathur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308): 829–34. 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5): 641–50. 10.1002/jor.1100090504 [DOI] [PubMed] [Google Scholar]
- 23.Das B, Kashino SS, Pulu I, Kalita D, Swami V, Yeger H, et al. CD271+ Bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med. 2013;5(170): 170ra13 10.1126/scitranslmed.3004912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garhyan J, Bhuyan S, Pulu I, Kalita D, Das B, Bhatnagar R. Preclinical and clinical evidence of Mycobacterium tuberculosis persistence in the hypoxic niche of bone marrow mesenchymal stem cells after therapy. Am J Pathol. 2015;185(7): 1924–1934. 10.1016/j.ajpath.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 25.Leistikov RL, Morton RA, Bartek IL, IFrimpong I, Wagner K, Voskuil MI. The Mycobacterium tuberculosis DosR Regulon Assists in Metabolic Homeostasis and Enables Rapid Recovery from Nonrespiring Dormancy. J. Bacteriol. 2009;192(6): 1662–1670. 10.1128/JB.00926-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deb Ch, Lee Ch-M, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, et al. A Novel In Vitro Multiple-Stress Dormancy Model forMycobacterium tuberculosis Generates a Lipid-Loaded, Drug-Tolerant, Dormant Pathogen. PLoS ONE. 2009;4(6): e6077 10.1371/journal.pone.0006077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakob U, Reichmann D. Oxidative Stress and Redox Regulation. Springer Verlag; 2013. ISBN 978-94-007-5787-5. [Google Scholar]
- 28.Goodell MA. Multipotential stem cells and ‘side population’ cells. Cytotherapy. 1994;4: 507–518. [DOI] [PubMed] [Google Scholar]
- 29.Lin KK, Goodell MA. Purification of hematopoietic stem cells using the side population. Methods Enzymol. 2006;420: 255–264. 10.1016/S0076-6879(06)20011-9 [DOI] [PubMed] [Google Scholar]
- 30.Thierry D, Cave MD, Eisenach KD, Crawford JT, Bates JH, Gicquel B, et al. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990;18: 188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zumárraga M, Bigi F, Alito A, Romano MI, Cataldi AA. 12.7 kb fragment of the Mycobacterium tuberculosis genome is not present in Mycobacterium bovis. Microbiology. 1999;145(Pt 4): 893–897. [DOI] [PubMed] [Google Scholar]
- 32.Brosch RG, Gordon SV, Eiglmeier K, Garnier T, Tekaia F, Yeramian E, et al. Genomics, biology, and evolution of the Mycobacterium tuberculosis complex Molecular Genetics of Mycobacteria. American Society for Microbiology Press; 2000;19–36. ISBN 1-55581-191-4. [Google Scholar]
- 33.Fomukong NG, Tang TH, al-Maamary S, Ibrahim WA, Ramayah S, Yates M, et al. Insertion sequence typing of Mycobacterium tuberculosis: characterization of a widespread subtype with a single copy of IS6110. Tuber Lung Dis. 1994;75: 435–440. 10.1016/0962-8479(94)90117-1 [DOI] [PubMed] [Google Scholar]
- 34.Lok KH, Benjamin WH, Kimerling ME, Pruit V, Lathan M, Razeq J, et al. Molecular differentiation of Mycobacterium tuberculosis strains without IS6110 insertions. Emerg Infect Dis. 2002;8: 1310–1313. 10.3201/eid0811.020291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steensels D, Fauville-Dufaux M, Boie J, De Beenhouwer H. Failure of PCR-Based IS6110 Analysis To Detect Vertebral Spondylodiscitis Caused by Mycobacterium bovis. J Clin Microbiol. 2012;51: 366–368. 10.1128/JCM.02524-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107: 924–930. 10.1182/blood-2005-05-2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis RC, Zabrowarny LA. Safer staining method for acid fast bacilli. J Clin Pathol. 1993;46: 559–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seiler P, Ulrichs T, Bandermann S, Pradl L, Jörg S, Krenn V, et al. Cell-wall alterations as an attribute of Mycobacterium tuberculosis in latent infection. J Infect Dis. 2003;188(9): 1326–1331. 10.1086/378563 [DOI] [PubMed] [Google Scholar]
- 39.Woolhiser L, Tamayo MH, Wang B, Gruppo V, Belisle JT, Lenaerts AJ, et al. In vivo adaptation of the Wayne model of latent tuberculosis. Infect Immun. 2007;75(5): 2621–5. 10.1128/IAI.00918-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7): 1109–1121. 10.1016/j.cell.2005.05.026 [DOI] [PubMed] [Google Scholar]
- 41.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL, Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294: 1933–1936. 10.1126/science.1064081 [DOI] [PubMed] [Google Scholar]
- 42.Aljurf M, Gyger M, Alrajhi A, Sahovic E, Chaudhri N, Musa M, et al. Mycobacterium tuberculosis infection in allogeneic bone marrow transplantation patients. Bone Marrow Transplant. 1999;24(5): 551–4. 10.1038/sj.bmt.1701930 [DOI] [PubMed] [Google Scholar]
- 43.Kindler T, Schindel C, Brass U, Fischer T. Fatal sepsis due to Mycobacterium tuberculosis after allogeneic bone marrow transplantation. Bone Marrow Transplantation. 2001;27: 217–18. 10.1038/sj.bmt.1702737 [DOI] [PubMed] [Google Scholar]
- 44.Kerridge I, Ethell M, Potter M, Prentice HG. Mycobacterium tuberculosis infection following allogeneic peripheral blood stem cell transplantation. Intern Med J. 2003;33(12): 619–20. [DOI] [PubMed] [Google Scholar]
- 45.Russo RL, Dulley FL, Suganuma L, França IL, Yasuda MA, Costa SF. Tuberculosis in hematopoietic stem cell transplant patients: case report and review of the literature. Int J Infect Dis. 2010;14(Suppl 3): e187–91. [DOI] [PubMed] [Google Scholar]
- 46.Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends Microbiol. 2009;17: 183–188. 10.1016/j.tim.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 47.Dietrich G, Schaible UE, Diehl KD, Mollenkopf H, Wiek S, Hess J J, et al. Isolation of RNA from mycobacteria grown under in vitro and in vivo conditions. FEMS Microbiol Lett. 2000;186: 177–80. [DOI] [PubMed] [Google Scholar]
- 48.Rienksma A, Suarez-Diez M, Mollenkopf HJ, Dolganov GM, Dorhoi A, Schoolnik GK, et al. Comprehensive insights into transcriptional adaptation of intracellular mycobacteria by microbe-enriched dual RNA sequencing. BMC Genomics. 2015;16: 34 10.1186/s12864-014-1197-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Purification of Lin+, Lin–CD34+, Lin–CD34+CD38–CD90+, Lin–CD34+CD38+CD90– as well as Lin–SP+ and Lin+ SP−cells by FACS from blood cells from IGRA+ and IGRA−donors. (B) Purification of CD271+CD45- mesenchymal stem cells by FACS from blood cells from IGRA+ donors. (C) Purification of Lin−hematopoietic progenitors and (D) Lin+ Gr1+ granulocytes, CD11c+ dendritic cells, Mac1+ macrophages, NK 1.1+ NK cells, CD4+/8+ T cells and CD19+/B220+ B cells by FACS from bone marrow of infected mice day 28 p.i. Representative FACS blots are shown. The data contained herein relate to both main Figs 1 and 2.
(DOC)
(A) Representative example (Donor 14) of a gel analysis of single Mtb DNA samples expanded by limiting dilution to a single-target IS6110 PCR. (B) Representative example (Mouse 2) of a gel analysis of single Mtb DNA samples expanded by limiting dilution to a single-target IS6110 PCR. Note: as expected from Poisson’s distribution not all, but in the analysis of human pHSC, only 5 of 23 individual PCR tests (left), and of mouse LT-pHSC only 6 of 23 individual PCR tests (right) yielded a PCR product (see arrow). The data contained herein relate to both main Figs 1 and 2.
(DOC)
(A) Analysis of SYBR green qPCR products by gel electrophoresis, to ensure that qPCR background did not result from a contamination by genomic DNA, thus the amplification of a Mtb specific DNA fragment. (B) Amplification plot for the “water control” (1) and for 1 Mtb DNA copy (2). (C) Melt curve for the “water control” (1) and for 1 Mtb DNA copy (2). The data contained herein relate to both main Figs 1 and 2.
(DOC)
Genomic DNA was prepared and DNA of 103 hematopoietic progenitors from IGRA+ donors was tested by PCR. Quantification of Mtb-specific DNA was done by real-time TaqMan PCR using probes that target MPB64 and IS6110 together as well as real-time SYBR green PCR using primers that target MPB64 alone. PCRs were performed in technical triplicates and normalized to human GAPDH (median + interquartile). Due to a lack of sufficient DNA material we were not able to include single-target qPCRs on donors 8 and 9.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.