Abstract
Streptococcus pneumoniae is a major pathogen that causes different invasive pneumococcal diseases (IPD). The pneumococcal polysaccharide capsule is a main virulence factor. More than 94 capsule types have been described, but only a limited number of capsule types accounted for the majority of IPD cases before the introduction of pneumococcal vaccines. After the introduction of the conjugated pneumococcal vaccine PCV7, which covered the seven most frequent serotypes in IPD in the USA, an increase in IPD caused by non-vaccine serotypes was observed, and serotype 19A, which belongs to sequence type (ST) 199, was among the most prevalent STs. After the introduction of the extended vaccine PCV13, which includes serotype 19A, serogroup 15B/C increased in IPD. Therefore, whole genome sequences of 39 isolates of ST199 from Germany (collected between 1998 and 2011) with serotype 19A (n = 24) and serogroup 15B/C (n = 15) were obtained using an Illumina platform and were analysed to identify capsular switches within ST199. Two 19A to 15B/C serotype switch events were identified. Both events occurred before the introduction of PCV7, which indicates that a capsular switch from 19A to 15B among ST199 isolates is not unusual and is not directly linked to the vaccination. The observed serotype replacement appears to be the result of a vacant niche due to the displacement of vaccine serotypes that is now successfully occupied by ST199 clones.
Introduction
Streptococcus pneumoniae are encapsulated, facultative anaerobic, non-sporulating Gram-positive bacteria that usually occur as diplococci. S. pneumoniae, also known as pneumococcus, is a major pathogen that causes community-acquired pneumonia (CAP), acute exacerbations of chronic bronchitis, meningitis, sinusitis, otitis media, and sepsis. Pneumococcal infections usually involve infants, immunocompromised individuals and the elderly. Non-invasive diseases (i.e., sinusitis and otitis media) are frequent but not severe. Invasive pneumococcal diseases (IPD) refer to the isolation of S. pneumoniae from a normally sterile site, e.g., blood, cerebrospinal fluid, or pleural fluid, and have a lower incidence but are associated with a high case fatality rate [1]. The main reservoir of S. pneumoniae is the nasopharyngeal zone of healthy carriers, particularly infants. Up to 70% of infants attending day care centres and more than 90% of infants in some native communities [2], but less than 5% of immune-competent adults, are colonized [2–4]. However, in HIV-endemic regions, the prevalence among the parents might be significantly higher, with rates of 43.2% in HIV-infected parents and 26.8% in HIV-non-infected parents [5].
The genome of S. pneumoniae is relatively small and highly variable. Molecular epidemiology studies using multi-locus sequence typing (MLST) have classified 11,176 different pneumococcal sequence types (ST) that are subordinate to approximately 100 clonal complexes (CC) (http://pubmlst.org/spneumoniae/, accessed 2 March 2016). The polysaccharide capsule, the synthesis of which is encoded by the cps locus, is a major virulence factor. This capsule is poorly recognized by phagocytes and protects pneumococci from phagocytosis. Pneumococci can be discriminated into different serotypes according to their different capsule types using the Neufeld Quellung reaction [6]. To date, more than 94 pneumococcal polysaccharide capsule types have been described, but only a limited number of serotypes account for the majority of infections, some of which are associated with antibiotic resistance.
The introduction of the conjugated pneumococcal vaccine PCV7, which covers the seven most frequent serotypes (4, 6B, 9V, 14, 18C, 19F and 23F), for use in infants between 2000 (USA) and 2006 (Germany) led to a substantial decrease in vaccine-serotype associated IPD and in non-vaccinated children and adults via herd protection effects [7, 8]. Simultaneously, increasing rates of IPD caused by the non-PCV7 serotypes were reported in different regions. Globally, the most important PCV7 replacement serotype was 19A, which was also associated with penicillin and macrolide resistance [9–14]. Molecular analyses revealed that a substantial proportion of those serotype 19A isolates belong to ST199 [11, 12, 15].
To account for the observed replacement, a 13-valent vaccine (PCV13) was introduced in 2009 in Germany and in 2010 in the USA. In addition to the seven serotypes in PCV7, this vaccine contains the most frequent replacement serotypes [16, 17] and serotypes with high prevalence in the developing world [5, 18] (1, 3, 5, 7F, 6A and 19A), which has led to an additional 94% reduction in IPD caused by those 13 vaccine serotypes [7, 19]. Currently, increased rates of IPD by serogroup 15B/C, which is predominantly associated with ST199, can be observed in some countries [20]. Therefore, 39 IPD isolates of ST199 and serotypes 19A, 15B and 15C collected in Germany between 1998 and 2011 were sequenced to investigate whether the observed spread of ST199 can be explained by a successful clone that switched its serotype and whether this switch is related to selective pressure from the introduced vaccinations.
Results and Discussion
Draft genome details
The sequencing project included all available isolates with ST199, serotype 15B (n = 9) and serotype 15C (n = 6) and 24 randomly chosen isolates of serotype 19A collected by the German National Reference Centre for Streptococci (Aachen, Germany) (GNRCS) in Germany. The whole-genome sequence libraries were constructed using a transpososome-mediated fragmentation technique and were sequenced in both directions with at least 35-fold coverage for 37 isolates and a 21- or 30-fold coverage for the two remaining isolates. On average, 50% of the draft genomes were covered by at least 75±12 kb in the contigs and 130±12 kb in the scaffolds, resulting in 65±18-fold coverage of the draft genomes. All draft genomes were deposited in GenBank under the accession numbers listed in Table 1.
Table 1. Characterization of the S. pneumoniae isolates used in this study.
Resistance and susceptibility were determined based on EUCAST rules. MICs categorized as resistance# are indicated in bold letters.
| ID | serotype | Accession No. | Date of Sampling | Type of Infection | Sampling Material | Age in years | Age in months | MIC PCN | MIC CTX | MIC CLR | MIC CLI | MIC LVX | MIC TET | MIC VAN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13827 | 15B | FLUW01000001-FLUW01000068 | 04/1999 | Pneumonia | Tracheal secretion | 76 | 0.015 | 0.008 | 0.125 | 0.03 | 1 | 0.25 | 0.5 | |
| 1541 | 15B | FLMH01000001-FLMH01000070 | 06/2003 | Meningitis | CSF | 36 | 0.015 | 0.015 | 0.125 | 0.06 | 1 | 0.25 | 0.25 | |
| 20007 | 15B | FLMN01000001-FLMN01000059 | 03/2004 | Pneumonia | Puncture | 8 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.25 | 2 | |
| 21299 | 15B | FLNA01000001-FLNA01000060 | 10/2004 | Pneumonia | Blood | 67 | 0.015 | 0.015 | 0.125 | 0.06 | 1 | 0.5 | 0.25 | |
| 27401 | 15B | FLNL01000001-FLNL01000064 | 02/2006 | Meningitis | CSF | 11 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.12 | 2 | |
| 28838 | 15B | FLSY01000001-FLSY01000068 | 10/2006 | Meningitis | CSF | 6 | 0.015 | 0.015 | 4 | 0.12 | 1 | 0.25 | 0.5 | |
| 38301 | 15B | FLTA01000001-FLTA01000062 | 02/2009 | Carriage | Nasopharynx | 3 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.25 | 0.5 | |
| 41023 | 15B | FLNI01000001-FLNI01000059 | 09/2009 | Carriage | Nasopharynx | 11 | 0.015 | 0.015 | 0.125 | 0.12 | 1 | 0.5 | 0.5 | |
| 41844 | 15B | FLNG01000001-FLNG01000070 | 12/2009 | Carriage | Nasopharynx | 13 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.25 | 0.5 | |
| 27516 | 15C | FLUC01000001-FLUC01000075 | 01/2005 | Pneumonia | Sputum | 0.015 | 0.015 | 0.125 | 0.12 | 0.5 | 0.5 | 2 | ||
| 23543 | 15C | FLMZ01000001-FLMZ01000051 | 08/2005 | Sepsis | Blood | 11 | 0.015 | 0.015 | 0.125 | 0.12 | 1 | 0.25 | 2 | |
| 27700 | 15C | FLSW01000001-FLSW01000058 | 04/2006 | Sepsis | Blood | 36 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.25 | 2 | |
| 40086 | 15C | FLSX01000001-FLSX01000061 | 05/2009 | Carriage | Nasopharynx | 2 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 1 | 0.5 | |
| 37666 | 15C | FLNC01000001-FLNC01000060 | 10/2009 | Carriage | Nasopharynx | 11 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.5 | 0.5 | |
| 44233 | 15C | FLUD01000001-FLUD01000058 | 04/2010 | Otitis media | Middle ear fluid | 29 | 0.015 | 0.015 | 0.5 | 0.12 | 1 | 0.25 | 0.5 | |
| 246 | 19A | FLMI01000001-FLMI01000058 | 02/1998 | Blood | 19 | 0.06 | 0.03 | 0.125 | 0.06 | 0.5 | 0.25 | 0.12 | ||
| 376 | 19A | FLMJ01000001-FLMJ01000061 | 10/1998 | Meningitis | CSF | 6 | 0.015 | 0.015 | 0.03 | 0.06 | 1 | 0.25 | 0.25 | |
| 13514 | 19A | FLMK01000001-FLMK01000063 | 02/1999 | Sinusitis | Nasopharynx | 5 | 0.12 | 0.03 | 0.03 | 0.03 | 1 | 0.12 | 2 | |
| 762 | 19A | FLML01000001-FLML01000063 | 10/2000 | Blood | 14 | 0.015 | 0.015 | 0.125 | 0.06 | 1 | 0.5 | 0.5 | ||
| 893 | 19A | FLUU01000001-FLUU01000055 | 02/2001 | Blood | 8 | 0.015 | 0.015 | 0.125 | 0.06 | 0.5 | 0.5 | 0.5 | ||
| 20605 | 19A | FLNM01000001-FLNM01000060 | 06/2003 | Pneumonia | Blood | 81 | 0.015 | 0.015 | 0.125 | 0.06 | 1 | 0.25 | 0.25 | |
| 1720 | 19A | FLMM01000001-FLMM01000057 | 02/2004 | Meningitis | CSF | 7 | 0.06 | 0.25 | 0.125 | 0.12 | 1 | 0.5 | 2 | |
| 20253 | 19A | FLMO01000001-FLMO01000060 | 03/2004 | Blood | 12 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.25 | 2 | ||
| 21295 | 19A | FLMY01000001-FLMY01000060 | 06/2004 | Pneumonia | Blood | 82 | 0.015 | 0.015 | 0.125 | 0.06 | 0.5 | 0.25 | 0.25 | |
| 23150 | 19A | FLUJ01000001-FLUJ01000081 | 05/2005 | Meningitis, Sepsis | n.s. | 21 | 0.125 | 0.03 | 4 | 0.12 | 0.5 | 0.5 | 2 | |
| 36180 | 19A | FLUE01000001-FLUE01000061 | 11/2008 | Otitis media | Middle ear fluid | 17 | 0.125 | 0.06 | 0.125 | 0.12 | 1 | 0.5 | 1 | |
| 37119 | 19A | FLNJ01000001-FLNJ01000058 | 12/2008 | Otitis media | Middle ear fluid | 10 | 0.015 | 0.03 | 0.125 | 0.12 | 2 | 0.5 | 0.5 | |
| 44017 | 19A | FLNE01000001-FLNE01000057 | 04/2009 | Pneumonia | Blood | 0.125 | 0.03 | 0.125 | 0.12 | 1 | 0.5 | 0.5 | ||
| 37752 | 19A | FLNK01000001-FLNK01000068 | 09/2009 | Mastoiditis | Ear swab | 10 | 0.015 | 0.015 | 0.125 | 0.12 | 1 | 0.25 | 0.5 | |
| 41617 | 19A | FLNF01000001-FLNF01000060 | 11/2009 | Meningitis | CSF | 35 | 0.125 | 0.03 | 0.125 | 0.12 | 1 | 0.25 | 0.5 | |
| 42538 | 19A | FLSZ01000001-FLSZ01000058 | 01/2010 | Sinusitis | Wound swab | 13 | 0.03 | 0.06 | 0.125 | 0.12 | 2 | 0.25 | 0.5 | |
| 42694 | 19A | FLUI01000001-FLUI01000067 | 02/2010 | Otitis media | Middle ear fluid | 11 | 0.25 | 0.06 | 4 | 0.12 | 1 | 0.5 | 0.5 | |
| 43003 | 19A | FLNH01000001-FLNH01000070 | 02/2010 | Pneumonia | Blood | 17 | 0.015 | 0.015 | 0.125 | 0.12 | 1 | 0.5 | 0.5 | |
| 43985 | 19A | FLMW01000001-FLMW01000066 | 03/2010 | Meningitis | CSF | 19 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.5 | 0.5 | |
| 46048 | 19A | FLUG01000001-FLUG01000065 | 07/2010 | Blood | 22 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.5 | 0.5 | ||
| 46770 | 19A | FLNB01000001-FLNB01000056 | 10/2010 | Blood | 20 | 0.015 | 0.03 | 0.125 | 0.12 | 1 | 0.5 | 0.5 | ||
| 46464 | 19A | FLUF01000001-FLUF01000058 | 10/2010 | Sepsis | Blood | 14 | 0.015 | 0.015 | 0.125 | 0.12 | 1 | 0.25 | 0.5 | |
| 47301 | 19A | FLUH01000001-FLUH01000067 | 12/2010 | Meningitis | CSF | 7 | 0.015 | 0.015 | 0.125 | 0.12 | 1 | 0.25 | 0.5 | |
| 48565 | 19A | FLND01000001-FLND01000067 | 03/2011 | Otitis media | Middle ear fluid | 20 | 0.015 | 0.015 | 0.125 | 0.12 | 1 | 0.25 | 0.5 |
MIC—minimum inhibitory concentration in mg/L, # according to EUCAST, the low-level MIC of ≥ 0.125 mg/L penicillin is interpreted as nonsusceptible when meningitis is the focus of infection, OXA—oxacillin, PCN—penicillin, CTX—cefotaxime, CLR—clarithromycin, CLA—clindamycin, LVX—levofloxacin, TET—tetracycline, VAN—vancomycin, CSF—cerebrospinal fluid, n.s.—not specified
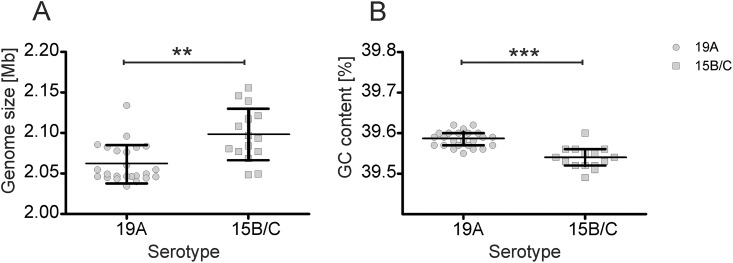
Because the serotypes 15B and 15C only differ in mutations in the wciZ gene of the cps locus, their genome data sets were merged, and the estimated mean of the serogroup 15B/C isolates was 2.098±0.033 MB. As presumed, the genome sizes of serotypes 15B and 15C did not differ significantly (p = 0.9839): In contrast to serogroup B/C (P = 0.6735), the genomes of the serotype 19A did not pass the normality test (P = 0.0022), and the stronger dispersion of the values indicated higher sequence variability within this serotype. The mean genome size of the serotype 19A isolates was 2.061 ± 0.023 MB and, despite the observed dispersion, was significantly smaller (P = 0.0007) than the mean genome size of serogroup 15B/C (Fig 1). Significant differences (P < 0.0001) in the GC content were observed between isolates of serotype 19A (39.59 ± 0.02%) and serogroup 15B/C (39.54 ± 0.02%). The GC content in the serogroups was normally distributed (P > 0.5). However, these data were obtained on scaffolds and must therefore be treated with caution.
Fig 1. Distribution of the genome sizes (A) and the GC contents (B) of the analysed ST199 isolates with serotype 19A or serogroup 15B/C.
Significance is indicated by asterisks (** P < 0.0013, *** P < 0.0001).
No plasmids were identified, but one to three prophages or related signatures were observed within the assembled genomes of all 39 isolates. In two serotype 15B isolates (>32,500 bp in isolate ID 41844 and >37,500 bp in isolate ID 13827), two scaffolds could not be unambiguously assembled into the chromosomes. A BLAST analysis revealed the phage-related origins of these scaffolds. In isolate ID 13827, the unknown scaffold was nearly identical to phage 11865 (FR671409.1) (99%, e-value 0) and phage 23782 (FR671408.1) (98%, e-value 0), both of which have been detected in various S. pneumoniae isolates with different serotypes. The unknown scaffold of isolate ID 41844 was nearly identical to parts of two other pneumococcal isolates with serotype 11 A (CP002121.1) (99%, e-value 0) and serotype 19A (CP000936.1) (99%, e-value 0).
The pan genome of these isolates consisted of 2,678 genes, with 888 genes that were not identified in all isolates. Among those genes, 241 (27%) were detected in serotype 19A and 280 (32%) were detected in serogroup 15B/C only. Thus, the core genome of the analysed isolates was estimated to consist of 1,790 genes.
The cps locus
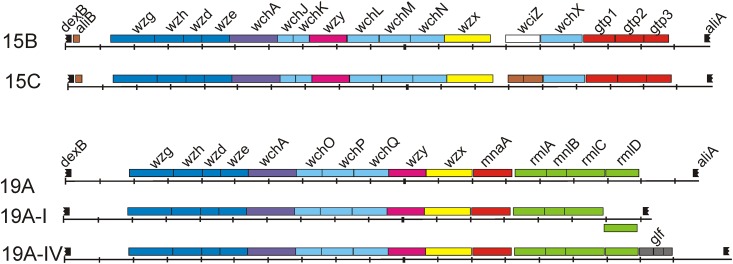
The cps cluster was approximately 20 kb and was located between the dexB and aliA region as previously described [21] (Fig 2). As expected, 8 genes were specific for serotype 19A and 10 genes were specific for serogroup 15B/C. The first four conserved genes, wzg, wzh, wzd and wze, which encode regulatory proteins, and the wchA gene, which encodes the initial transferase, were detected in all analysed strains.
Fig 2. The cps clusters of serotypes 15B and 15C and the variations in the cps clusters of serotype 19A detected in this study.
The genes are coloured using the scheme reported by Bentley et al. [21]: regulatory genes in dark blue, initial transferase in violet, glycosyl transferase in light blue, polymerase in pink, flippase in yellow, acetyl transferase in white, dTDP-L-rhamnose pathway genes in green, UDP-N-acetyl-D-mannosamine pathway genes in red, glf genes in grey, pseudogenes in brown, and flanking genes in black. The flanking transposons are not indicated.
In all strains of serogroup 15B/C, 12 genes downstream of wchA were identified that were conserved among the analysed isolates and identical to those annotated by Bentley et al [21]: wchJ, wchK, wzy, wchL, wchM, wchN, wzx, wciZ (2-bp frameshift of the TA tandem repeats in serotype 15C), wchX, gtp1, gtp2, and gtp3.
Downstream of wchA, strains of serotype 19A bear 10 genes organized in a serotype-specific manner: wchO, wchP, wchQ, wzy, wzx, mnaA, rmlA, rmlB, rmlC and rmlD. However, differences in gene composition were observed at the 5’-end compared to the annotation of Bentley et al [21], who sub-grouped the serotype 19A isolates into two cps variants. In one group (n = 8), the rmlD gene was divergently oriented and has been described as serotype 19A-I [22], which indicates that within serotype-specific cps clusters variations in the genes and their positions can occur without notable phenotypic changes. The other serotype 19A cluster (n = 17) was named 19A-IV and contained the rmlD gene, which was oriented as described by Bentley et al [21], but the glf pseudogenes with three overlapping open reading frames (ORFs) were also identified downstream of rmlD. In general, the glf pseudogenes were observed in different arrangements within the known cps clusters, mainly upstream of rmlD or within the central region upstream of ugd or wciG or wzy. A BLAST analysis revealed a similar arrangement of the glf gene in the serotype 19A strain TCH8431 isolated from the respiratory tract that was sequenced during the human microbiome project (NCBI accession number CP001993). The functional glf gene encodes UDP-galactopyranose mutase, a 387-amino acid protein involved in the conversion of UDP-α-galactose to UDP-α-galactofuranose and has been experimentally identified in some mycobacteria and Gram-negative species [23, 24]. The glf ORFs in the cps cluster encode three short putative proteins predicted for various serotype cps clusters, but the function of the pseudogenes remains elusive. Thus, the sequence variations that caused ORF rearrangements led to a loss of initial glf function. A short, 114-bp sequence was located directly downstream of the glf gene and was spread throughout some annotated S. pneumoniae genomes of various serotypes (6A, 6B, 7A, 7B, 7F, 13, 18B, 18C, 19A, 24A, 24F, 25A, 29, 33D, 38, 40), including clone TCH8431 and the PMEN clone Taiwan19F-14. This sequence is often located upstream or downstream of putative ORFs, and genes with known functions, such as the fatB gene that encodes a siderophore binding protein, the prsW gene that encodes a membrane proteinase of anti-sigma factor RsiW, certain gcnA-like genes that encode N-acetyl-β-D-glucosaminidases, a gene encoding the IIB subunits of the PTS, and the clpA gene that encodes the ClpX protease. This short sequence is also located upstream of aliA in various cps loci when the glf is missing [21], which suggests that the glf locus became a part of the recombination system and might be involved in rearranging the cps cluster.
Phylogenetic analysis and serotype switching
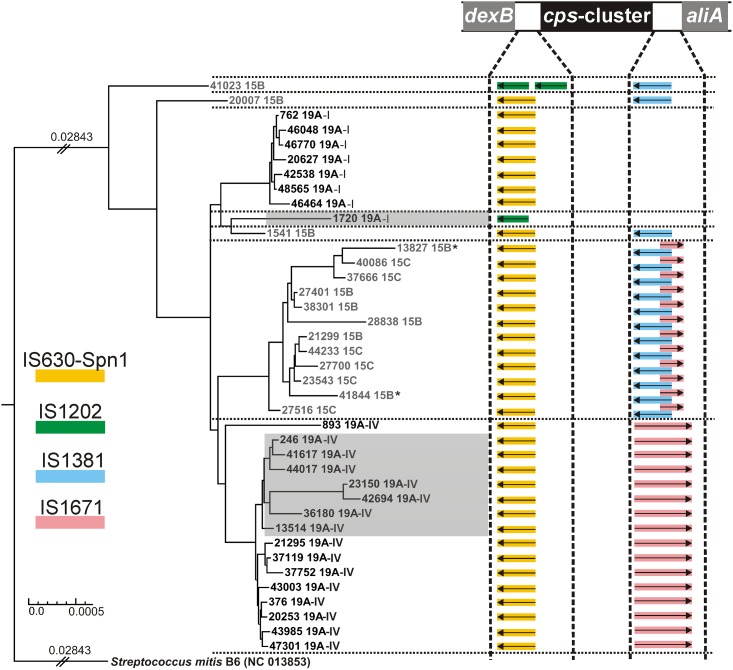
We performed a phylogenetic analysis to identify any serotype replacement within the 39 isolates of ST199 using Streptococcus mitis NC 013853 as the outgroup. In this manner, the phylogeny was estimated based on 1388 overlapping genes. Seven distinct clades could be identified, with 3 belonging to serotype 19A and 4 belonging to serogroup 15B/C (Fig 3). The analysis of the DNA sequences of the regions flanking the cps cluster (up to 5’ pbp2X upstream to 3’ pbp1a downstream) revealed differences in the transposase gene (tnp) pattern within the clades, suggesting that recombination events had occurred.
Fig 3. Phylogenetic relationship based on the core genome of 1388 genes derived from the 39 S. pneumoniae isolates with ST199 and Streptococcus mitis (as outgroup).
The two isolates with phage-specific sequences that could not be assembled into the chromosomes are indicated by asterisks. The low-level penicillin-resistant isolates are underlined in grey. The transposases (tnp) flanking the cps clusters are shown as coloured arrows, and the legend for the tpn sources is indicated on the left side. The different constellations of tpn genes correlated with the linages are separated by dotted lines.
The serotype 19A isolates showed two distant clades that corresponded to the variations identified in their cps clusters. The major clade 19A-IV contained 15 isolates and was related to isolate ID 893, which showed some phylogenetic distance to the other 19A-IV isolates. All serotype 19A-IV isolates carried the tnp of IS630-Spn1 upstream of wzg and tnp of IS1671 downstream of rmlD, as previously described [21]. The serotype 19A-I clade carries the tnp of IS630-Spn1 upstream of wzg, but the expected tnp of IS1671 downstream of rmlD was likely lost during the inversion process of the rmlD gene.
Two serotype 15B isolates (ID 41023 and ID 20007) represented two distinct clades, and their cps clusters were flanked by different tnp gene patterns. Both isolates contained a tnp gene of IS1381 downstream of gtp3, but two copies of the IS1202 tnp were identified upstream of wzg in ID 41023, which showed the longest distance to the other isolates, whereas isolate ID 20007 was flanked by the tnp of IS630-Spn1.
The 19A-I isolate ID 1720 showed an uncommon tnp pattern, with missing transposase structures downstream of the inverted rmlD gene but one IS1202 copy upstream of wzg, indicating some cross-interactions with the ID 41023 linage.
The major clade of serogroup 15B/C was more closely related to the serotype 19A-IV than it was to the two distant serotype 15B isolates, indicating a serotype switch and further diversification of both clades. All 15B/C isolates of this clade contained the tnp gene of IS630-Spn1 upstream of wzg that was common in all 19A and 15B/C serotypes. However, the tnp of IS1381 that was also present in the distinct serotype 15B isolates ID 41023 and ID 20007 was located downstream of gtp3, followed by an artefact of IS1671, the tnp common in 19A-IV. Considering the nature of the recombination process this tnp pattern supports the idea of a 19A-IV ancestor. The 15B/C clade contained 6 isolates of serotype 15B and 5 isolates of serotype 15C distributed throughout the lineages. The difference between both serotypes is a 2-bp insertion (AT) within a poly-(AT)-region. It has been shown that tandem repeats exhibit a highly increased rate of frameshift mutations due to deletions or insertions [25], which suggests that serotype 15C emerged via mutations rather than recombination processes.
A second serotype switch was observed for serotype 15B isolate ID 1541, which showed a genetic relation to serotype 19A-I isolate ID 1720. Because of their distances to each other and the other 19A-1 isolates, both are suggested to belong to two separate clades. Both regions flanking the cps of ID 1541 were similar to ID 20007 cps regions. ID 1541 shared identical point mutations in the wzg, wzh and wze genes with isolate ID 20007, indicating that the cps cluster recombined between the ancestors of these isolates. The different tnp pattern of serotype 19A-I isolate ID 1720 that showed only a IS1202-like sequence identified upstream of the cps locus of the serotype 15B isolate ID 41023 might also indicate a serotype switch, but it is difficult to interpret the event because no closer relatives of this isolate were sequenced in this study.
Resistance to antimicrobials
Serotype 19A is associated with antibiotic resistance; therefore, the phenotypic resistance profiles and possible genetic determinants for the most recommended antimicrobials for treating CAP (Table 1) were investigated: macrolides (clarithromycin), fluoroquinolones (levofloxacin) and β-lactams (penicillin and cefotaxime). Susceptibility to alternative antimicrobials, such as clindamycin, tetracycline, and vancomycin, was also investigated.
The isolates were susceptible to most of the antibiotics tested, except for certain isolates with resistance to clarithromycin (minimum inhibitory concentrations (MIC) > 0.5 mg/L). Here, one 15B (ID 28838) and two 19A-IV isolates (ID 23150 and ID 42694) showed a MIC of 4 mg/L clarithromycin. This macrolide resistance could be traced to the transposon-associated mefA and msrD genes that encode dual macrolide efflux protein A (MefA and Mel, respectively) [26] (100% identity, e-value 0).
Two isolates of serotype 19A-IV (ID 37119) and 19A-I (ID 42538) showed intermediate MICs of levofloxacin of 2 mg/L each. The increased fluoroquinolone MICs might be caused by substitutions in the quinolone resistance-determining region (QRDR) of the subunits of topoisomerase IV (ParE/ParC) and/or gyrase (GyrA/GyrB) [27] or by the increased expression of multidrug efflux pumps, primarily PmrA [28] and PatAB [29]. Substitutions were identified outside the QRDRs in the ORFs of ParC (A382T and A596V) and ParE (V162I, P165L, S216) and within the QRDR of ParE (I460V) in both isolates. However, these substitutions were also identified in other levofloxacin-susceptible isolates [30]; therefore, it remains unlikely that these substitutions outside the QRDR and the I460V substitution within the QRDR are associated with the increased MIC of levofloxacin. No differences were observed in the pmrA promoters [28] or the patAB terminator regions in the resistant isolates that might lead to enhanced expression or attenuation [31]. However, the transcriptional levels of the genes encoding the efflux pumps were not quantified. Moreover, it was suggested that other unknown efflux pumps might be involved in the drug resistance in pneumococci [32].
All isolates were susceptible to the cephalosporin cefotaxime, but low-level penicillin resistance (MICs > 0.06 to 0.25 mg/L) was observed in six isolates (ID 13514, ID 23150, ID 36180, ID 41617, ID 44017 and ID 42694). Those formed their own sub-linage within clade 19A-IV (labelled as 19A-IV’ in Fig 3), of which only isolate ID 246 showed the MIC of penicillin of 0.06 mg/L. According to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and based on the epidemiological cut-off (ECOFF) that separates the natural susceptible population, the MIC of penicillin > 0.06 mg/L) is interpreted as nonsusceptible and as resistant when meningitis is the focus of infection (http://www.eucast.org/clinical_breakpoints/). The main mechanism of high-level resistance (MIC > 4 mg/L) to β-lactams is related to amino acid substitutions in the active site (motifs SXXK, HSXN and K[ST]G) of penicillin binding proteins (PBPs), whereas low-level resistance is related to various other substitutions within PBPs, mainly in PBP1A, PBP2B and PBP2X [33, 34], and other proteins of peptidoglycan synthesis [35]. The cps cluster is proximally flanked by the genes pbp2x (8 kbp upstream) and pbp1a (7 kbp downstream), which have been shown to be involved in the recombination processes of the cps cluster and thus might lead to the transmission of penicillin resistance via serotype switching [36]. However, penicillin resistance might act as the driver and the switch might be the result of the gain in resistance.
To investigate whether the observed low-level resistance was caused by already described or new substitutions, translated ORFs of the penicillin binding proteins (PBPs) and other genes associated with increased MICs of β-lactams of the nonsusceptible isolates were compared to the other susceptible isolates.
Isolates belonging to the sub-lineage of clade 19A-IV’ showed the same amino acid substitutions within PBP2X and PBP2B, the class B monofunctional transpeptidases, and the corresponding gene sequences were identical, which indicated that these mutations were specific for this sub-lineage of 19A-IV. Some substitutions had not been previously described but were located within the N-terminally located non-penicillin-binding transmembrane domain (n-PB, up to residue 265) or the C-terminal domain (residues 635–750) [37, 38]. The substitutions within the n-TP-domain likely do not influence the phenotypes of either PBP2B or PBP2X. The C-terminal domain contains two PASTA (penicillin-binding protein and Ser/Thr kinase associated) motifs and is suggested to bind to uncross-linked murein; it was also shown to interact with a second β-lactam. The impact of the mutations found in this domain on PBP2X is unknown. Most of the identified substitutions within the transpeptidase domain (TP, residues 266 to 616) of PBP2X [37, 38] were previously identified in other nonsusceptible isolates (italic letters in Fig 4) [37, 39, 40]. Only three substitutions within the TP domain of PBP2X, E320K, A347S, and D567N were not described (leucine (L) at position 565 was found in the susceptible strain R6). Asparagine (N) at 567 was also identified in the susceptible isolate ID 1720, thus this mutation does not appear to be critical. However, substitutions of the negatively charged glutamic acid (E) to positively charged lysine (K) at position 320 and from hydrophobic alanine (A) to hydrophilic serine (S) at position 347, both closely located to the S337TMK (SXXK) motif, might have an impact on the penicillin nonsusceptibility. Interestingly, we found four substitutions within the TP domain of BPB2X, being related to nonsusceptible strains, also in isolate ID 1720 (V358Y, R384G, Q552E, and S576N) as well as some unknown mutations (Fig 3) that seem to be specific for this strain or lineage and suggest that these mutation might be not crucial for the phenotype.
Fig 4. Amino acid substitutions identified in the PBPs (1A, 2B and 2X) and MurE of the ST199 isolates in this study (bold letters) and of other isolates published elsewhere (italic letters).
‘Others’ indicates the protein sequence of all merged isolates of this study, except for those indicated by their ID. The nonsusceptibility bases on the epidemiological cut-off (ECOFF) > 0.06 mg/L that indicates the breakpoint between the susceptible (s) and nonsusceptible isolates (ns). The position of the conserved motif SXXK of the PBPs is indicated by asterisks. The amino acid positions, as well as the TP-domain of the respective PBPs shown as bars, are indicated above the sequences. Sequences published elsewhere (italic letters) refer to [35, 37, 41–44].
All substitutions found within the TP domain of the PBP2B in the sub-lineage 19A-IV’ have been previously described, primarily in nonsusceptible isolates (except for one susceptible isolate indicated by s/ns in Fig 4 for PBP2B). Therefore, it is likely that those mutations lead to an increased penicillin MIC. The susceptible phenotype of isolate ID 246 suggested additional alterations in other unknown genes that were not further investigated.
The isolates ID 23150 and ID 42694, both of which are located in a distant clade but are strongly related to each other, showed a similar substitution pattern outside the conserved motifs of the TP-domain (residues 337 to 681) of PBP1A [45] (Fig 4), the class A bifunctional transglycosylase-transpeptidases. All of these substitutions have already been described for intermediate and resistant isolates [41] and mutagenized clones [42], and thus might contribute to the increased MIC of penicillin in these isolates. Both isolates also exhibited identical substitutions within MurE, a UDP-N-acetylmuramyl tripeptide synthetase. However, in the case of MurE, mutations in the promoter region leading to increased transcription levels have been shown to increase β-lactam resistance in Gram-positive species, particularly resistance to oxacillin [43, 46]. Thus, it remains unclear whether those substitutions have any impact on the phenotype. Interestingly, isolate ID 42694 showed the highest MIC of penicillin that might be caused by these observed substitutions. However, because isolate ID 23150 showed a lower MIC, it cannot be excluded that unknown mechanism might be involved either in the increased MIC of ID 42694 or the lower MIC of ID 23150.
The isolate ID 1720 showed also two substitutions within PBP1A (V386I and T540S) and in PBP2A (A73V and A172G) with unknown effects. No amino acid changes in PBP1B and PBP3 or in non-PBP-targets (CpoA, CiaH, CiaR, MurN or MurM) that were associated with the increased MIC of β-lactams [35] were identified within the tested isolates.
Conclusions
The number of multidrug resistant pneumococcal clones, including those with penicillin resistance, was successfully reduced by the PCV7 [47–49] and the PCV13 vaccine programme for infants [50, 51]. Before 1987, the rate of penicillin nonsusceptible isolates (MIC of ≥ 0.1 mg/L) associated with IPD has been estimated at 5% in the USA, but increased to 25.1% in 1999, with PCV7 serotypes accounting for ∼80% of the cases [51]. These could be reduced to 17% by PCV7 and 14.4% by PCV13 in USA [50]. In some European countries, such as France (56.7%), Greece (36.4%) or Spain (54.5%), the prevalence of penicillin nonsusceptible isolates was greater before the introduction of the PCV7 in Europe (2001) [52]. After the introduction of PCV7, the incidence of non-PCV7 serotypes increased, and 19A was among the most frequent serotypes that were also associated with increased penicillin resistance [12, 36]. In this study, six isolates of serotype 19A, that could be phylogenetically sub-grouped to the sub-linage 19A-IV’, showed increased penicillin MIC values and were categorized as possessing low-level penicillin resistance. The sub-linage 19A-IV’ shared identical substitutions in the PBP2B and PBP2X. Isolate ID 13514 was obtained in 1999; therefore, these substitutions appear to have been established before the PCV7 vaccine was introduced. Interestingly, isolate ID 246 was isolated in 1998 and was susceptible to penicillin but bears the same substitution pattern in PBP2B and PBP2X. Thus, the low-level penicillin resistance in this sub-lineage might also be caused by other unknown mutations that spread clonally. In two strains that were isolated in 2005 (ID 23150) and in 2010 (ID 42694), the pattern of substitutions was extended to PBP1A and MurE, but the relevance of these substitutions to the increased MIC of penicillin in isolate ID 42694 remains unclear. The most recent clone, ID 42694, might have established additional unknown mutations in other targets, but these additional mutations were not analysed in this study.
An analysis of the serotype 19A isolates collected by the CDC Active Bacterial Core Surveillance programme in the USA revealed that the vast majority of the isolates could be assigned to 3 major clonal complexes: CC 199, CC3 20/271, and CC 695. These 3 clonal complexes collectively comprised approximately 87% of the serotype 19A isolates collected from 2005–2007 [53]. CC 199 was the most prevalent clonal complex of serotype 19A before the PCV7 vaccine, and the IPD caused by serotype 19A of CC 199 declined gradually from 72% (in 2003–2004) to 40% (in 2007) in the USA, indicating changes in the epidemiology of serotype 19A [54]. Simultaneously, IPDs caused by serotype 19A with increased penicillin MIC values belonging to CC 320/271 and CC 695 increased in USA. Before the introduction of PCV7, these clonal complexes were primary related to serotypes 19F and 4. Both serotypes are covered by PCV7, and their prevalence was strongly reduced between 1998/1999 and 2006/2007 from 11.1% to 1.2% (data for serotype 19F) and 6.8% to 0.2% (data for serotype 4). Because penicillin resistance in pneumococci is often associated with multi-drug resistant phenotype, it was suggested that the observed serotype switches of those clonal complexes to 19A were driven by selective antimicrobial pressure [54]. A transmission and recombination of the pbp2x-cps locus-pbp1a from CC 19919A (donor) to CC6954 (recipient) was proposed to result in CC69519A [36]. The pbp2x genes of the nonsusceptible strains of this study were also identical to the serotype CC 19919A donor strain cdc2 described by Bruegemann et al. [36]. In Germany, other than CC 199 (30.4%), the most prevalent clonal complex related to 19A was CC 230 (18.4%) until 2011 [15]. Before the introduction of PCV7, intermediate penicillin resistance was only observed in CC 230 [15], which indicated strong regional differences in the epidemiology of the clonal complexes.
In the present study, at least two serotype switches from 19A to 15B of ST199 belonging to CC 199 were identified. Based on the IS pattern flanking the cps-cluster, one of the switches was most likely from serotype 19A-IV to serogroup 15B/C. It remains unclear when the serotype switch occurred because almost all of the isolates were obtained after 2004, but one serotype 15B strain (ID 13827) was isolated in 1999; thus, this serotype switch may have occurred in or before 1999. The second serotype switch from 19A-I to 15B is more difficult to interpret because closer relatives are missing but must have occurred before 2003. Because both serotype switches occurred before the introduction of PCV13 in Germany in 2009, the vaccination did not directly provoke the serotype switch. Some ST199 isolates within serogroup 15B/C were already identified in the pre-PCV era, suggesting that the recombination of the cps-cluster from 19A to 15B among ST199 isolates is not unusual. Temporal changes in pneumococcal epidemiology already occurred before the introduction of the conjugated vaccines [55] and cannot always be sufficiently explained. It can be assumed that recombinations occur at random and that recombinants with advantageous capsules are selected. However, the likelihood for recombination with pneumococci bearing a non-vaccine serotype also increases with their prevalence. Therefore, the passive selective pressure of the vaccine not only selects recombinants with non-vaccine serotypes but might also increase the likelihood of such recombination.
This study shows that there is a dynamic flexibility in CC 199 population genomics. In particular, the capsular switch between serotypes 19A and serogroup 15B/C appears to be a rather frequent event and had already occurred in isolates collected before the PCV7 selective pressure. Therefore, it can be assumed that in response to the selective pressure from PCV13, which covers serotype 19A, the low hurdles for this cps recombination event will trigger a vaccine escape towards serogroup 15B/C due to the already existing serotype-switched clones that can successfully occupy the vacant niche. The switched serotype 15B isolates were penicillin susceptible, but as our results indicate, a serotype recombination with the penicillin nonsusceptible sub-lineage 19A-IV’ is highly possible. Indeed, there are recent reports describing an increased detection of serogroup 15B/C in IPD including those isolates with MIC of penicillin >0.6 mg/L [20, 56]. This issue is concerning because the current development of an extended PCV with 15 serotypes will not cover serogroup 15B/C.
In conclusion, the PCVs displaced the most prevalent strains, revealing the highly dynamic serotype changes that can lead to the successful spread of escaping serotypes. Those changes can only be reliably identified by NGS and may help predict the most probable trends in pneumococcal epidemiology. These techniques and studies should also be considered by retrospective epidemiological studies performed by the pharmaceutical industry when strategic decisions regarding the composition of a vaccine are made before beginning the resource- and time-consuming clinical development.
Materials and Methods
Bacterial strains and culture
The Streptococcus pneumoniae isolates were collected in Germany from 1998 to 2011 in various studies by the German National Reference Center for Streptococci (Aachen, Germany). The characterization of the sequence type (ST) was performed using multi locus sequence typing (MLST), and serotyping was performed using the Neufeld Quellung reaction, as previously described [15]. Antibiotic susceptibility was tested according to the recommendations of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) by the double-dilution method. In total, 39 isolates of ST199, consisting of 24 isolates of serotype 19A, 9 isolates of serotype 15B and 6 isolates of serotype 15C, were included in this study (for details, see Table 1).
The S. pneumoniae strains were inoculated into 10 ml Todd-Hewitt broth and grown for 8 to 10 hours at 35°C in a 5% CO2 atmosphere in 50 ml tubes. The cells were harvested by centrifugation at 4,000 rpm.
DNA preparation
The bacterial pellets were washed in buffer A (20 mM Tris-HCl (pH 8.0), 50 mM NaCl, and 10 mM EDTA (ethylenediaminetetraacetic acid)) and resuspended in 700 μl of buffer A supplemented with 4 mg/mL lysozyme. The cell suspension was chilled on ice for 10 min, followed by a 20-min incubation at 37°C. The cells were lysed by adding 25 μL of N-laurylsarcosyl (30%) and 10 μL of proteinase K (20 mg/mL) and were incubated at 70°C for 20 min. Chromosomal DNA was purified by three sequential extractions with a Tris-EDTA-saturated phenol/chloroform/isoamyl alcohol (25:24:1) mixture (Roth GmbH, Germany). The inorganic phase was separated by centrifugation at 10,000 rpm for 5 min and was treated with 5 μL of RNAse (100 mg/ml) for 15 min at 37°C. The DNA was precipitated for 10 min on ice by adding of 3 volumes of ice-cold ethanol (96%), and then pelleted by centrifugation at 13,000 rpm for 15 min at 4°C. The pellet was washed once with 300 μl of ice-cold ethanol (70%) and air-dried. The DNA was resuspended in DNase-free water (Gibco, Thermo Fisher Scientific, Waltham, MA USA) overnight and stored at 4°C.
Genome sequencing and assembly
For each sample, a whole-genome sequence library was prepared using the Illumina-Compatible Nextera DNA Sample Prep Kit (Epicentre, Madison, WI USA), according to the manufacturer's protocol. Each library was tagged with an individual tag combination and a library pool containing equimolar amounts of the individual libraries was prepared. The library pool was sequenced in 2x250 bp paired read runs on the MiSeq platform, yielding 21,928,122 total reads. After de-multiplexing, the individual sample reads were assembled using the Newbler assembler v2.8 (Roche, Branford, CT USA). Contigs of the initial Newbler assemblies (unordered drafts) were then aligned to the reference genome of S. pneumoniae ATCC 700669 using MUMmer [57], and ordered drafts were constructed using a combination of ad hoc Perl and shell scripts. Contigs that could not be assigned to a position were placed in separate FASTA files.
Gap closure of the cps locus
Gap closure of the flanking regions of the cps locus was performed by Sanger-sequencing. Primers that bound within dexA (GTTCCATGGGATGCTTTCTG) and wzg (TCGCTTCACTTTCTGTGAAC), and within aliA (AATAATGTCACGCCCGCAAG) and wciA (serogroup 15B/C) (AGGAGAAGCAACGGTGAATG-3) or glf (serotype 19A) (TGAGTTTGGGAGTCAAGCAAAG) were designed and purchased from Metabion GmbH (Germany) to amplify the regions. The PCR was performed in a 20 μl reaction using 3 ng of chromosomal DNA, 0.2 μM primers, 200 μM dNTPs, and 0.4 U of Phusion™ High-Fidelity DNA Polymerase in 1 x Phusion™ HF Buffers (New England BioLabs Inc., Ipswich, MA USA). The following PCR algorithm was applied: 98°C for 30 s; 29 cycles of 98°C for 10 s, 62°C for 20 s, and 72°C for 30 s; and 72°C for 10 min. The PCR products were extracted from agarose gels using the QIAquick Gel Extraction Kit (Qiagen GmbH, Hilden, Germany) or by ExoSAP-IT™ (Affymetrix Inc, Santa Clara, CA USA.), according to the manufacturers’ protocols. Sanger sequencing of the PCR products was performed on a 3730xl DNA-Analyzer (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA USA) by IIT Biotech GmbH using the capillary sequencing technique.
Genome annotation
The genomes were annotated using the platform GenDB [58]. The contigs of each draft sequence were concatenated into one contig using a 6-frame stop linker (ctagcatgctag), which was then annotated using the gene and function prediction pipelines in GenDB. If unassigned contigs were present in a genome, these contigs were concatenated separately and uploaded as an additional contig. The MLST strain types were confirmed by analysing the respective alleles using MLST 1.7 [59]. Phage-specific sequences were determined using PHAST [60]. Genes encoding resistance determinants were identified by ResFinder 2.1 [61], and the amino acid sequences were compared using MUSCLE 3.8 [62] and further analysed by sequence alignments using CLC Main Workbench (Qiagen).
Phylogenetic analysis
The calculation of the core genomes and construction of the phylogenetic tree was performed with the software tool EDGAR [63]. Streptococcus mitis B6 [64] was used as an outgroup to root the tree. First, the core genes of the 40 genomes were computed. In the next step, multiple alignments of the 1,388 core genes were generated using MUSCLE [65]. The alignments were than concatenated into a large multiple alignment, which was subsequently used as input for PHYLIP [66]. Here, a distance matrix based on the Jones-Taylor-Thornton model [67] was calculated from this alignment, and finally, based on this matrix, a phylogenetic tree was constructed using the Neighbour-Joining method. The tree was visualized by the program TreeGraph 2 [68]. The draft genomes of the 39 isolates were aligned with progressive Mauve [69] using standard parameters and a scoring matrix.
Statistical analysis
All statistics were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com.) The distributions of the genome sizes and the GC contents were analysed using the D'Agostino & Pearson omnibus normality test and the differences were determined using unpaired t tests with Welch's correction (normal distribution) or Mann-Whitney test (non-parametric). Significant differences were assumed as P-value of ≤ 0.05.
Ethical statement
Ethical approval was not required because the study did not involve human subjects, material or data.
Acknowledgments
We thank the CAPNETZ excellence network and the Robert Koch Institute for their cooperation and for providing the isolates and sample data.
Data Availability
All sequence files are available from the NCBI database (accession numbers are given in Table 1 in the manuscript).
Funding Statement
This study was funded by the Federal Ministry of Education and Research, grant numbers 01KI1204 (MWP), 01KI1501 (MWP) and 13GW0096D (OM) (https://www.bmbf.de/en/index.html).
References
- 1.Pletz MW, Welte T. Pneumococcal and influenza vaccination In: Chalmers J, Pletz MW, Aliberti S, editors. Community-Acquired Pneumonia. European Respiratory Monographs. 63: European Respiratory Society; 2014. p. 266–85. [Google Scholar]
- 2.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. The Lancet infectious diseases. 2004;4(3):144–54. 10.1016/S1473-3099(04)00938-7 [DOI] [PubMed] [Google Scholar]
- 3.Roca A, Hill PC, Townend J, Egere U, Antonio M, Bojang A, et al. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS medicine. 2011;8(10):e1001107 10.1371/journal.pmed.1001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putnam SD, Gray GC, Biedenbach DJ, Jones RN. Pharyngeal colonization prevalence rates for Streptococcus pyogenes and Streptococcus pneumoniae in a respiratory chemoprophylaxis intervention study using azithromycin. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2000;6(1):2–8. [DOI] [PubMed] [Google Scholar]
- 5.Conklin LM, Bigogo G, Jagero G, Hampton L, Junghae M, da Gloria Carvalho M, et al. High Streptococcus pneumoniae colonization prevalence among HIV-infected Kenyan parents in the year before pneumococcal conjugate vaccine introduction. BMC Infect Dis. 2016;16:18 10.1186/s12879-015-1312-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neufeld F. Über die Agglutination der Pneumokokken und über die Theorie der Agglutination. Z Hyg Infektionskr. 1902;40:54–72. [Google Scholar]
- 7.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. Epub 2009/12/02. 10.1086/648593 [DOI] [PubMed] [Google Scholar]
- 8.van der Linden M, Falkenhorst G, Perniciaro S, Imohl M. Effects of Infant Pneumococcal Conjugate Vaccination on Serotype Distribution in Invasive Pneumococcal Disease among Children and Adults in Germany. PloS one. 2015;10(7):e0131494 10.1371/journal.pone.0131494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Techasaensiri C, Messina AF, Katz K, Ahmad N, Huang R, McCracken GH Jr. Epidemiology and evolution of invasive pneumococcal disease caused by multidrug resistant serotypes of 19A in the 8 years after implementation of pneumococcal conjugate vaccine immunization in Dallas, Texas. Pediatr Infect Dis J. 2010;29(4):294–300. [DOI] [PubMed] [Google Scholar]
- 10.Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26(6):468–72. [DOI] [PubMed] [Google Scholar]
- 11.Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, Beall B, et al. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. The Journal of infectious diseases. 2005;192(11):1988–95. 10.1086/498043 [DOI] [PubMed] [Google Scholar]
- 12.Moore MR, Gertz RE Jr., Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. The Journal of infectious diseases. 2008;197(7):1016–27. 10.1086/528996 [DOI] [PubMed] [Google Scholar]
- 13.Del Grosso M, Camilli R, D'Ambrosio F, Petrucci G, Melchiorre S, Moschioni M, et al. Increase of pneumococcal serotype 19A in Italy is due to expansion of the piliated clone ST416/CC199. J Med Microbiol. 2013;62(Pt 8):1220–5. 10.1099/jmm.0.061242-0 [DOI] [PubMed] [Google Scholar]
- 14.Thomas JC, Figueira M, Fennie KP, Laufer AS, Kong Y, Pichichero ME, et al. Streptococcus pneumoniae clonal complex 199: genetic diversity and tissue-specific virulence. PLoS One. 2011;6(4):e18649 10.1371/journal.pone.0018649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Linden M, Reinert RR, Kern WV, Imohl M. Epidemiology of serotype 19A isolates from invasive pneumococcal disease in German children. BMC infectious diseases. 2013;13:70 10.1186/1471-2334-13-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell DJ, Klugman KP, Pichichero M. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr Infect Dis J. 2007;26(2):123–8. [DOI] [PubMed] [Google Scholar]
- 17.Imohl M, Reinert RR, van der Linden M. Temporal Variations among Invasive Pneumococcal Disease Serotypes in Children and Adults in Germany (1992–2008). International journal of microbiology. 2010;2010:874189 10.1155/2010/874189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramdani-Bouguessa N, Ziane H, Bekhoucha S, Guechi Z, Azzam A, Touati D, et al. Evolution of antimicrobial resistance and serotype distribution of Streptococcus pneumoniae isolated from children with invasive and noninvasive pneumococcal diseases in Algeria from 2005 to 2012. New microbes and new infections. 2015;6:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leal J, Vanderkooi OG, Church DL, Macdonald J, Tyrrell GJ, Kellner JD. Eradication of invasive pneumococcal disease due to the seven-valent pneumococcal conjugate vaccine serotypes in Calgary, Alberta. Pediatr Infect Dis J. 2012;31(9):e169–75. [DOI] [PubMed] [Google Scholar]
- 20.Liyanapathirana V, Nelson EA, Ang I, Subramanian R, Ma H, Ip M. Emergence of serogroup 15 Streptococcus pneumoniae of diverse genetic backgrounds following the introduction of pneumococcal conjugate vaccines in Hong Kong. Diagnostic microbiology and infectious disease. 2015;81(1):66–70. 10.1016/j.diagmicrobio.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 21.Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS genetics. 2006;2(3):e31 10.1371/journal.pgen.0020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elberse K, Witteveen S, van der Heide H, van de Pol I, Schot C, van der Ende A, et al. Sequence diversity within the capsular genes of Streptococcus pneumoniae serogroup 6 and 19. PLoS One. 2011;6(9):e25018 10.1371/journal.pone.0025018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beis K, Srikannathasan V, Liu H, Fullerton SW, Bamford VA, Sanders DA, et al. Crystal structures of Mycobacteria tuberculosis and Klebsiella pneumoniae UDP-galactopyranose mutase in the oxidised state and Klebsiella pneumoniae UDP-galactopyranose mutase in the (active) reduced state. Journal of molecular biology. 2005;348(4):971–82. 10.1016/j.jmb.2005.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassau PM, Martin SL, Brown RE, Weston A, Monsey D, McNeil MR, et al. Galactofuranose biosynthesis in Escherichia coli K-12: identification and cloning of UDP-galactopyranose mutase. Journal of bacteriology. 1996;178(4):1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levinson G, Gutman GA. High frequencies of short frameshifts in poly-CA/TG tandem repeats borne by bacteriophage M13 in Escherichia coli K-12. Nucleic Acids Res. 1987;15(13):5323–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambrose KD, Nisbet R, Stephens DS. Macrolide efflux in Streptococcus pneumoniae is mediated by a dual efflux pump (mel and mef) and is erythromycin inducible. Antimicrobial agents and chemotherapy. 2005;49(10):4203–9. 10.1128/AAC.49.10.4203-4209.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiology and molecular biology reviews: MMBR. 1997;61(3):377–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill MJ, Brenwald NP, Wise R. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrobial agents and chemotherapy. 1999;43(1):187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrer E, Schad K, Satoh AT, Page MG, Johnson MM, Piddock LJ. Involvement of the putative ATP-dependent efflux proteins PatA and PatB in fluoroquinolone resistance of a multidrug-resistant mutant of Streptococcus pneumoniae. Antimicrobial agents and chemotherapy. 2006;50(2):685–93. 10.1128/AAC.50.2.685-693.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pestova E, Beyer R, Cianciotto NP, Noskin GA, Peterson LR. Contribution of topoisomerase IV and DNA gyrase mutations in Streptococcus pneumoniae to resistance to novel fluoroquinolones. Antimicrobial agents and chemotherapy. 1999;43(8):2000–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baylay AJ, Ivens A, Piddock LJ. A novel gene amplification causes upregulation of the PatAB ABC transporter and fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrobial agents and chemotherapy. 2015;59(6):3098–108. 10.1128/AAC.04858-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenwald NP, Appelbaum P, Davies T, Gill MJ. Evidence for efflux pumps, other than PmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2003;9(2):140–3. [DOI] [PubMed] [Google Scholar]
- 33.Barcus VA, Ghanekar K, Yeo M, Coffey TJ, Dowson CG. Genetics of high level penicillin resistance in clinical isolates of Streptococcus pneumoniae. FEMS microbiology letters. 1995;126(3):299–303. [DOI] [PubMed] [Google Scholar]
- 34.Munoz R, Dowson CG, Daniels M, Coffey TJ, Martin C, Hakenbeck R, et al. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Molecular microbiology. 1992;6(17):2461–5. [DOI] [PubMed] [Google Scholar]
- 35.Hakenbeck R, Grebe T, Zahner D, Stock JB. beta-lactam resistance in Streptococcus pneumoniae: penicillin-binding proteins and non-penicillin-binding proteins. Molecular microbiology. 1999;33(4):673–8. [DOI] [PubMed] [Google Scholar]
- 36.Brueggemann AB, Pai R, Crook DW, Beall B. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 2007;3(11):e168 10.1371/journal.ppat.0030168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mouz N, Di Guilmi AM, Gordon E, Hakenbeck R, Dideberg O, Vernet T. Mutations in the active site of penicillin-binding protein PBP2x from Streptococcus pneumoniae. Role in the specificity for beta-lactam antibiotics. The Journal of biological chemistry. 1999;274(27):19175–80. [DOI] [PubMed] [Google Scholar]
- 38.Pares S, Mouz N, Petillot Y, Hakenbeck R, Dideberg O. X-ray structure of Streptococcus pneumoniae PBP2x, a primary penicillin target enzyme. Nature structural biology. 1996;3(3):284–9. [DOI] [PubMed] [Google Scholar]
- 39.Smith AM, Klugman KP. Amino acid mutations essential to production of an altered PBP 2X conferring high-level beta-lactam resistance in a clinical isolate of Streptococcus pneumoniae. Antimicrobial agents and chemotherapy. 2005;49(11):4622–7. 10.1128/AAC.49.11.4622-4627.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferroni A, Berche P. Alterations to penicillin-binding proteins 1A, 2B and 2X amongst penicillin-resistant clinical isolates of Streptococcus pneumoniae serotype 23F from the nasopharyngeal flora of children. J Med Microbiol. 2001;50(9):828–32. 10.1099/0022-1317-50-9-828 [DOI] [PubMed] [Google Scholar]
- 41.Granger D, Boily-Larouche G, Turgeon P, Weiss K, Roger M. Molecular characteristics of pbp1a and pbp2b in clinical Streptococcus pneumoniae isolates in Quebec, Canada. The Journal of antimicrobial chemotherapy. 2006;57(1):61–70. 10.1093/jac/dki401 [DOI] [PubMed] [Google Scholar]
- 42.Smith AM, Klugman KP. Site-specific mutagenesis analysis of PBP 1A from a penicillin-cephalosporin-resistant pneumococcal isolate. Antimicrobial agents and chemotherapy. 2003;47(1):387–9. 10.1128/AAC.47.1.387-389.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todorova K, Maurer P, Rieger M, Becker T, Bui NK, Gray J, et al. Transfer of penicillin resistance from Streptococcus oralis to Streptococcus pneumoniae identifies murE as resistance determinant. Molecular microbiology. 2015. [DOI] [PubMed] [Google Scholar]
- 44.Filipe SR, Severina E, Tomasz A. Distribution of the mosaic structured murM genes among natural populations of Streptococcus pneumoniae. Journal of bacteriology. 2000;182(23):6798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Guilmi AM, Dessen A, Dideberg O, Vernet T. Functional characterization of penicillin-binding protein 1b from Streptococcus pneumoniae. Journal of bacteriology. 2003;185(5):1650–8. 10.1128/JB.185.5.1650-1658.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardete S, Ludovice AM, Sobral RG, Filipe SR, de Lencastre H, Tomasz A. Role of murE in the Expression of beta-lactam antibiotic resistance in Staphylococcus aureus. Journal of bacteriology. 2004;186(6):1705–13. 10.1128/JB.186.6.1705-1713.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pletz MW, Shergill AP, McGee L, Beall B, Whitney CG, Klugman KP. Prevalence of first-step mutants among levofloxacin-susceptible invasive isolates of Streptococcus pneumoniae in the United States. Antimicrobial agents and chemotherapy. 2006;50(4):1561–3. Epub 2006/03/30. 10.1128/AAC.50.4.1561-1563.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pletz MW, McGee L, Jorgensen J, Beall B, Facklam RR, Whitney CG, et al. Levofloxacin-resistant invasive Streptococcus pneumoniae in the United States: evidence for clonal spread and the impact of conjugate pneumococcal vaccine. Antimicrobial agents and chemotherapy. 2004;48(9):3491–7. Epub 2004/08/26. 10.1128/AAC.48.9.3491-3497.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens DS, Zughaier SM, Whitney CG, Baughman WS, Barker L, Gay K, et al. Incidence of macrolide resistance in Streptococcus pneumoniae after introduction of the pneumococcal conjugate vaccine: population-based assessment. Lancet. 2005;365(9462):855–63. Epub 2005/03/09. 10.1016/S0140-6736(05)71043-6 [DOI] [PubMed] [Google Scholar]
- 50.Richter SS, Diekema DJ, Heilmann KP, Dohrn CL, Riahi F, Doern GV. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrobial agents and chemotherapy. 2014;58(11):6484–9. 10.1128/AAC.03344-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim L, McGee L, Tomczyk S, Beall B. Biological and Epidemiological Features of Antibiotic-Resistant Streptococcus pneumoniae in Pre- and Post-Conjugate Vaccine Eras: a United States Perspective. Clinical microbiology reviews. 2016;29(3):525–52. 10.1128/CMR.00058-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones ME, Blosser-Middleton RS, Critchley IA, Karlowsky JA, Thornsberry C, Sahm DF. In vitro susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis: a European multicenter study during 2000–2001. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2003;9(7):590–9. [DOI] [PubMed] [Google Scholar]
- 53.Beall BW, Gertz RE, Hulkower RL, Whitney CG, Moore MR, Brueggemann AB. Shifting genetic structure of invasive serotype 19A pneumococci in the United States. J Infect Dis. 2011;203(10):1360–8. 10.1093/infdis/jir052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyrrell GJ. The changing epidemiology of Streptococcus pneumoniae serotype 19A clonal complexes. J Infect Dis. 2011;203(10):1345–7. 10.1093/infdis/jir056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harboe ZB, Benfield TL, Valentiner-Branth P, Hjuler T, Lambertsen L, Kaltoft M, et al. Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50(3):329–37. [DOI] [PubMed] [Google Scholar]
- 56.van der Linden M, Perniciaro S, Imohl M. Increase of serotypes 15A and 23B in IPD in Germany in the PCV13 vaccination era. BMC Infect Dis. 2015;15(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delcher AL, Phillippy A, Carlton J, Salzberg SL. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res. 2002;30(11):2478–83. Epub 2002/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer F, Goesmann A, McHardy AC, Bartels D, Bekel T, Clausen J, et al. GenDB—an open source genome annotation system for prokaryote genomes. Nucleic Acids Res. 2003;31(8):2187–95. Epub 2003/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, et al. Multilocus sequence typing of total-genome-sequenced bacteria. Journal of clinical microbiology. 2012;50(4):1355–61. 10.1128/JCM.06094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucleic acids research. 2011;39(Web Server issue):W347–52. 10.1093/nar/gkr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. The Journal of antimicrobial chemotherapy. 2012;67(11):2640–4. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edgar RC. Quality measures for protein alignment benchmarks. Nucleic acids research. 2010;38(7):2145–53. 10.1093/nar/gkp1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blom J, Albaum SP, Doppmeier D, Puhler A, Vorholter FJ, Zakrzewski M, et al. EDGAR: a software framework for the comparative analysis of prokaryotic genomes. BMC Bioinformatics. 2009;10:154 Epub 2009/05/22. 10.1186/1471-2105-10-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Denapaite D, Bruckner R, Nuhn M, Reichmann P, Henrich B, Maurer P, et al. The genome of Streptococcus mitis B6—what is a commensal? PLoS One. 2010;5(2):e9426 Epub 2010/03/03. 10.1371/journal.pone.0009426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. Epub 2004/03/23. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felsenstein J. An alternating least squares approach to inferring phylogenies from pairwise distances. Syst Biol. 1997;46(1):101–11. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 67.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8(3):275–82. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 68.Stover BC, Muller KF. TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics. 2010;11:7 Epub 2010/01/07. 10.1186/1471-2105-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5(6):e11147 Epub 2010/07/02. 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequence files are available from the NCBI database (accession numbers are given in Table 1 in the manuscript).