Supplemental Digital Content is available in the text
Keywords: colorectal cancer, cytoreductive surgery, hyperthermic intraperitoneal chemotherapy, peritoneal metastasis
Abstract
In Taiwan, colorectal cancer with peritoneal carcinomatosis is considered a terminal condition. We examined the clinical outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) treatment for colorectal cancer with peritoneal carcinomatosis in Taiwan.
We enrolled patients with colorectal cancer and peritoneal metastasis from Taipei Medical University, Wanfang Hospital between January 1999 and December 2014. Of the enrolled patients, 3 had mucinous-type tumors. In total, we enrolled 31 patients who underwent a total of 33 procedures. Of the 31 patients, 2 received the HIPEC procedure twice. Cytoreductive surgery was performed followed by HIPEC. The hazard ratios of death following cytoreductive surgery and HIPEC were calculated using the Cox proportional hazards model.
The 2- and 5-year overall survival rates of these patients following cytoreductive surgery and HIPEC were 57% and 38%, respectively. The completeness of cytoreduction (CC) scores were CC-0, CC-1, CC-2, and CC-3 in 18 (54.5%), 3 (9%), 7 (21.2%), and 5 (15.2%) patients, respectively. The mean peritoneal cancer index (PCI) was 16.20, and the mean postoperative PCI (PPCI) was 4.6. The major risk factors for death in these patients were a total PCI score > 20, total PPCI score > 0, and CC score ≥ 2 (P = 0.022, 0.031, and 0.0001, respectively; log-rank test). Multivariate analysis revealed that the total PPCI score was the strongest predictor of death following cytoreductive surgery and HIPEC in these patients.
In Taiwan, performing cytoreductive surgery and administering HIPEC for treating colorectal cancer with peritoneal metastasis are feasible and resulted in long-term survival. In addition, the total PPCI score was related to poor prognosis following cytoreductive surgery and HIPEC in patients with colorectal cancer and peritoneal metastasis.
1. Introduction
In Taiwan, patients with colorectal cancer and peritoneal carcinomatosis are considered to be in a terminal condition and thus are palliatively treated according to the guidelines recommended by Koppe et al.[1] A recent study of colorectal cancer cases reported that the prevalence of peritoneal carcinomatosis in patients with colorectal cancer was 10% to 15%.[1,2] Oncologists consider colorectal peritoneal carcinomatosis a form of systemic metastasis; thus, it is treated using systemic chemotherapy.[2] However, peritoneal carcinomatosis from colorectal cancer is associated with an extremely poor prognosis. Studies including systemic chemotherapy and symptom-directed surgery without cytoreduction have reported a median survival duration of 5.2 to 7 months when only fluorouracil was used for treatment.[1–3] A systematic review reported that the survival rate after cytoreductive surgery combined with perioperative intraperitoneal chemotherapy was higher than that after systemic chemotherapy in patients with peritoneal carcinomatosis from colorectal carcinoma.[4] If the peritoneal cavity is the only metastatic site (i.e., metastasis has not occurred in the lungs, liver, or other organs), aggressive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) might cure colorectal cancer with peritoneal carcinomatosis presenting as the first site of dissemination.[2,5]
In this study, we examined the clinical outcomes of cytoreductive surgery and HIPEC in patients with colorectal cancer and peritoneal carcinomatosis in Taiwan. The overall survival, toxicity, and factors related to poor prognosis identified in this study were compared with those reported in studies conducted in Western countries to determine whether the examined approach is suitable for Asian patients with colorectal cancer and peritoneal carcinomatosis.
2. Patients and methods
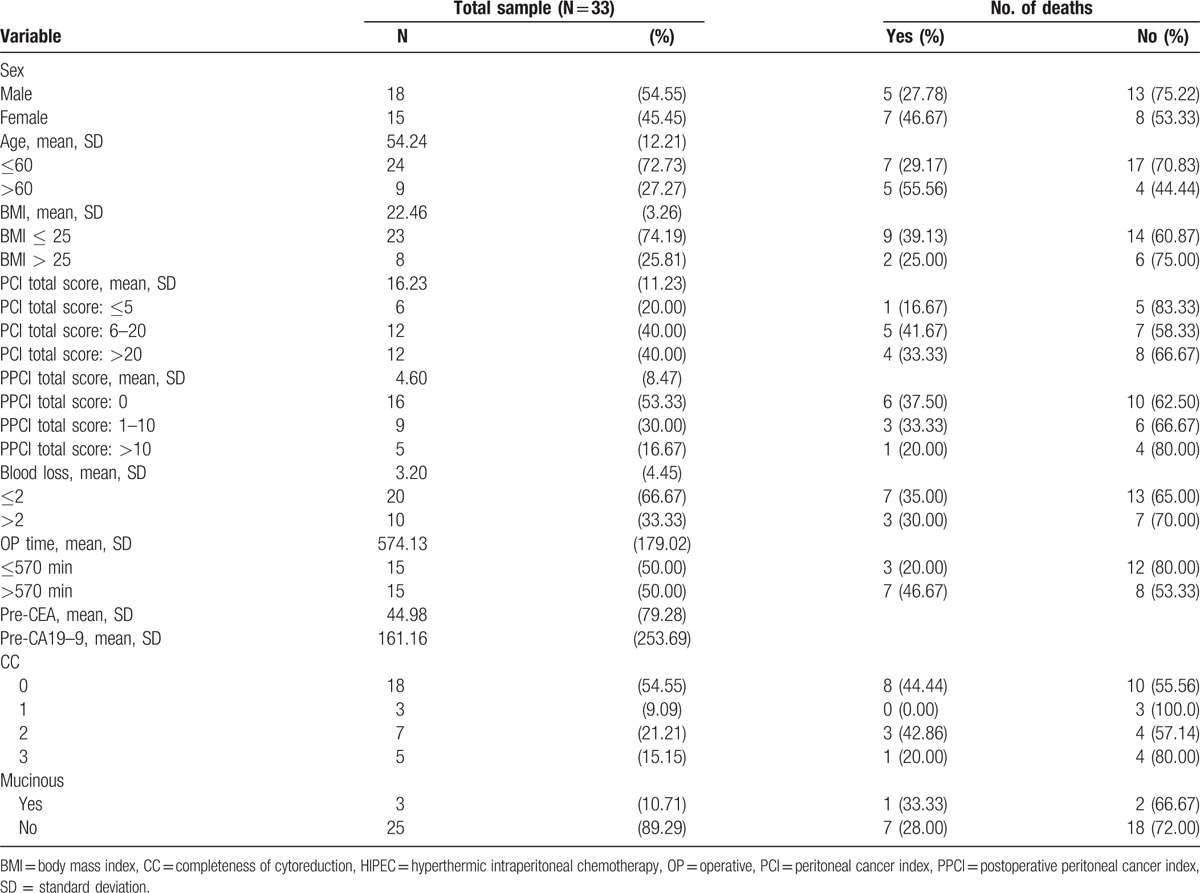
We enrolled patients with colorectal cancer and peritoneal metastasis from Taipei Medical University, Wanfang Hospital between January 1999 and December 2014. Of the enrolled patients, 3 had mucinous-type tumors. Our protocols were reviewed and approved by the institutional review board of our hospital (TMU-JIRB No. 201510056). In total, we enrolled 31 patients who underwent a total of 33 procedures. Of the 31 patients, 2 received the HIPEC procedure twice. The characteristics of the patients are listed in Table 1. Of the patients, 16 were women and 15 were men. The mean age and the body mass index (BMI) of the patients were 54.20 years and 22.5 kg/m2, respectively. Furthermore, 4, 23, and 4 patients had American Society of Anesthesiologists grades 1, 2, and 3, respectively. Of all the patients, 14, 17, and 21 received preoperative total parenteral nutrition, preoperative chemotherapy, and adjuvant chemotherapy, respectively. The mean values of the preoperative tumor markers carcinoembryonic antigen and cancer antigen 19–9 were 45 ng/dL and 161 U/mL, respectively.
Table 1.
Characteristics and follow-up status of patients who underwent HIPEC.
All procedures were performed by the same surgical team, led by a single surgeon (Mao-Chih Hsieh). A midline skin incision was made from the xiphoid process to the pubic tubercle. The peritoneal carcinomatosis index (PCI) was evaluated during laparotomy as described previously.[6] Cytoreductive surgery included several visceral resections such as those of the stomach, colon, ovary, uterus, spleen, gallbladder, and small bowel. In addition, parietal peritonectomy, greater omentectomy, and lesser omentectomy were performed. The residual tumors were intraoperatively classified using the completeness of cytoreduction (CC) score.[7] CC-0 indicates no residual macroscopical tumors, whereas CC-1, CC-2, and CC-3 indicate residual tumor nodules measuring < 2.5 mm, between 2.5 mm and 2.5cm, and >2.5 cm, respectively.
After cytoreductive surgery, HIPEC was administered. One outflow and 2 inflow drainage tubes were placed in the pelvic cavity and subphrenically, respectively. Before HIPEC, the abdominal cavity was lavaged 10 times by using 1L of normal saline. Heated normal saline was then circulated for 60 minutes by using a roller pump and heat exchanger. The chemotherapeutic agents used were 20 mg of mitomycin C and 100 mg of cisplatin. The intraperitoneal temperature was monitored by placing a thermometer in the abdominal cavity and maintaining its temperature at approximately 42°C to 43°C. After HIPEC, the abdominal cavity was lavaged 10 times by using 1L of normal saline. HIPEC was not administered if the risk of postoperative complications was high. Therefore, HIPEC was not administered in patients who had poor preoperative performance status, poor laboratory data, or excessive intraoperative bleeding or in those who underwent extremely aggressive surgical procedures.
Postoperative complications were assessed on the basis of the Common Terminology Criteria for Adverse Events, Version 4.0. The postoperative complications were determined by observing the first complication after cytoreductive surgery or by selecting a more severe complication when multiple complications occurred simultaneously. All analyses were performed using Statistical Analysis Software, Version. 9.3 (SAS, Cary, NC). A 2-tailed value of P < 0.05 was considered significant. The cumulative death incidence was calculated using the Kaplan–Meier method, and independent risk factors were compared using the log-rank test. The Cox proportional hazards model was used to calculate the hazard ratios (HRs) of death following cytoreductive surgery and HIPEC in patients with colorectal cancer and peritoneal metastasis. Age, sex, and variables with a univariate P value of ≤ 0.25 were selected for multivariate analysis by using the stepwise Cox proportional hazards model (with entry and retention criteria of P ≤ 0.25).
3. Results
The 2- and 5-year overall survival rates of the patients were 57% and 38%, respectively. Peritonectomy, bowel anastomosis, and diversion (colostomy/Hartmann's procedure/ileostomy) were performed in 23, 25, and 10 patients, respectively. Twenty patients (60.6%) required blood transfusion during the surgery and HIPEC procedure. The mean operative time was 9 hours and 30 minutes. CC-0, CC-1, CC-2, and CC-3 scores were obtained in 18 (54.5%), 3 (9%), 7 (21.2%), and 5 (15.2%) patients, respectively. The mean PCI and postoperative PCI (PPCI) scores were 16.20 and 4.6, respectively.
The HRs of death following cytoreductive surgery and HIPEC calculated using the Cox proportional hazards model are listed in Table 2. The major risk factors for death were a total PCI score > 20, total PPCI score > 0, and CC score ≥2 (P = 0.022, 0.031, and 0.0001, respectively; log-rank test; Fig. 1A–C). According to the results of the log-rank test, the P values of an age > 60 years, an operative time > 570 minutes, and mucinous-type tumors were 0.795, 0.235, and 0.683, respectively (Supplemental Figures 1–3). The multivariate analysis indicated that the total PPCI score was the highest risk factor for death following the cytoreductive surgery and HIPEC.
Table 2.
Cox regression analysis of the risk of death.
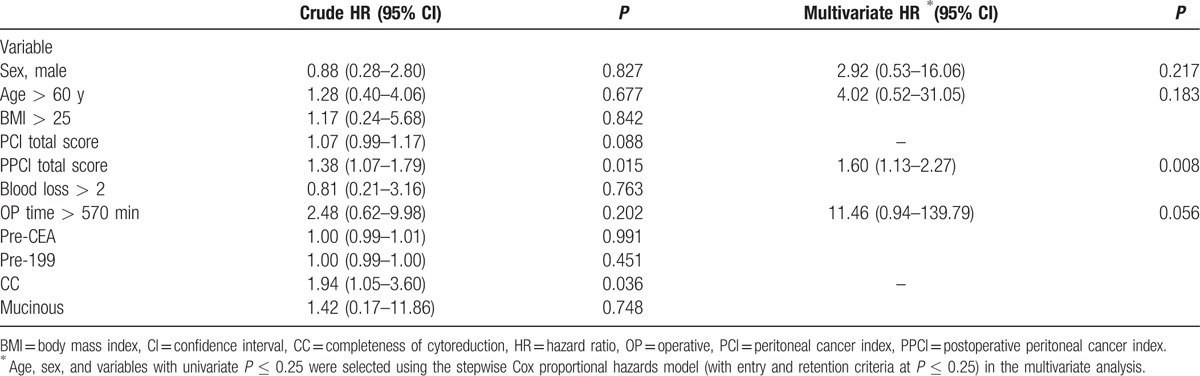
Figure 1.
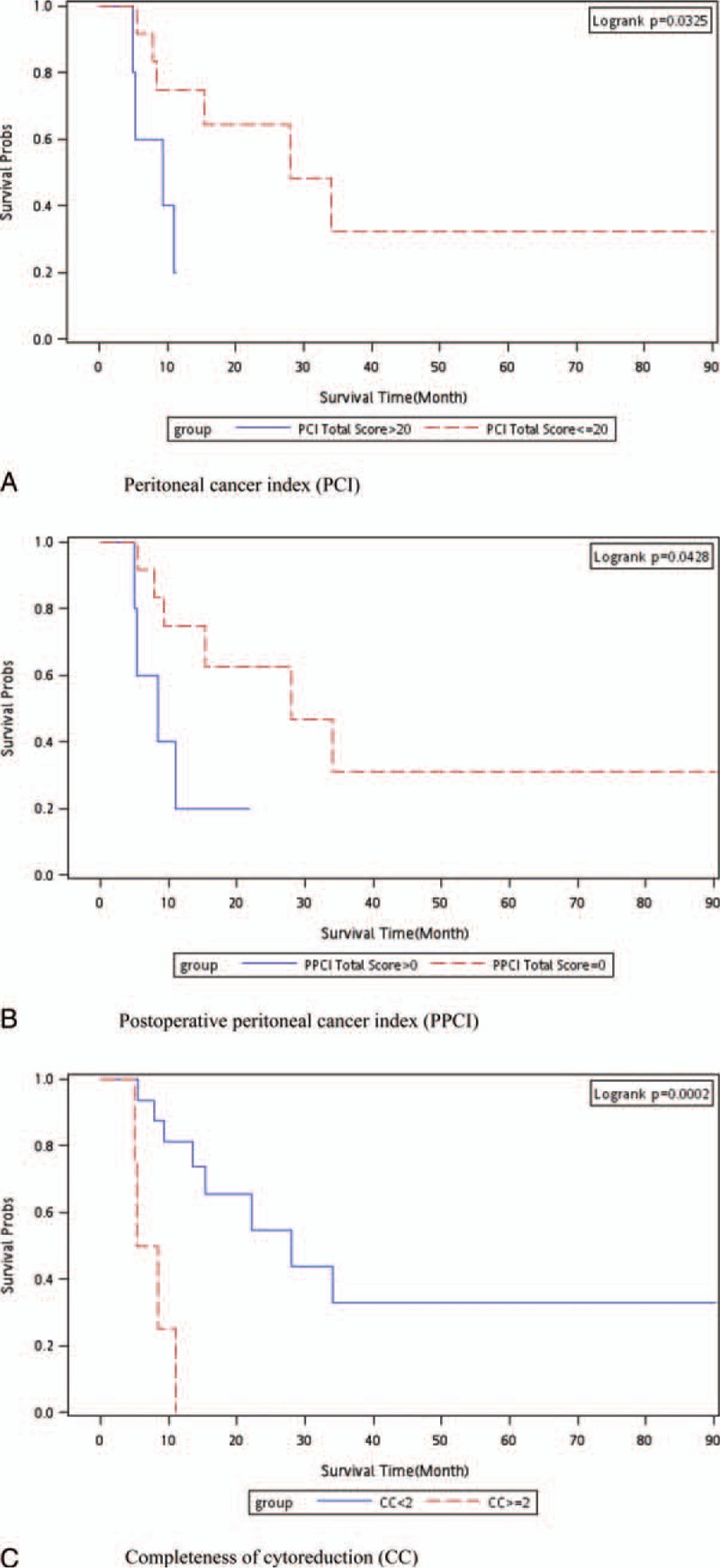
Kaplan–Meier survival curve for all-cause death. (A) Total PCI score > 20 vs total PCI score ≤ 20 m; (B) total PPCI score > 0 vs total PPCI score of 0; (C) CC score ≥ 2 vs CC score < 2. CC = completeness of cytoreduction, PCI = peritoneal carcinomatosis index, PPCI = postoperative peritoneal carcinomatosis index.
The morbidity rate for all the procedures was 21% (7/33). According to the Common Terminology Criteria for Adverse Events, 7 patients (21%) had grade I or II complications and no patients had grade III or IV complications after any procedure. The most frequent complication was surgical site infections including intraabdominal abscess, which accounted for 12% (4/33) of all postoperative complications. Other common complications following cytoreductive surgery and HIPEC were postoperative ileus, urinary disturbance, intestinal fistula, and postoperative bleeding.
4. Discussion
The optimal management of colorectal cancer patients with peritoneal carcinomatosis without distant metastasis after a rigorous diagnostic workup is controversial. HIPEC treats the residual microscopic disease following the surgery might be the theory using the cytoreductive surgery and HIPEC for treating patients with colorectal cancer and peritoneal metastasis.[8–10] Colorectal cancers could include transcoelomic metastasis within the peritoneal cavity, resulting in peritoneal carcinomatosis rather than lymphatic and hematogenous metastasis. Till now, peritoneal carcinomatosis are considered as a terminal condition by many oncologists and systemic chemotherapy with palliative-intent are used for these patients. However, the peritoneal cavity is identified to be the only site of metastasis in approximately 25% of cases, after a detailed workup of the lungs and liver. Therefore, some studies have hypothesized that in some cases, peritoneal carcinomatosis may represent the first site of dissemination and is thus not necessarily indicative of a generalized disease.[2,10,11] Therefore, in our institute, cytoreductive surgery with HIPEC is suggested for treating patients with peritoneal carcinomatosis from colorectal cancers.
Peritoneal carcinomatosis is frequently observed and is nearly always a lethal condition in patients with colorectal cancer. The 2-year survival rate is 10% with standard treatment (systemic chemotherapy and symptomatic surgery), and on the basis of a pooled analysis of North Central Cancer Treatment Group phase III trials N9741 and N9841, the median overall survival duration with systemic chemotherapy is 12.7 months.[12,13] To date, 2 randomized controlled trials have performed cytoreductive surgery followed by heated HIPEC in patients with peritoneal dissemination of colorectal cancer. In the first trial, 105 patients with established peritoneal carcinomatosis of colorectal (n = 87) or appendiceal (n = 18) origin were randomly assigned to cytoreductive surgery and HIPEC groups. Not all patients with colorectal cancers were enrolled in the first trial.[8] At the median follow-up of 8 years, 45% of the patients in the HIPEC group who underwent complete cytoreduction (no residual tumor nodules) were still alive.[14] These outcomes are consistent with those of our study, and all the patients in our study had colorectal cancers without an appendiceal origin. In this study, we examined the clinical outcomes of cytoreductive surgery and HIPEC in patients with colorectal cancer and peritoneal carcinomatosis in Taiwan. The overall survival, which was the primary endpoint, identified in this study was compared with that reported in studies conducted in Western countries to determine whether the examined approach is suitable for Asian patients with colorectal cancer and peritoneal carcinomatosis. Compared with North Central Cancer Treatment Group Phase III trials N9741 and N9841,[12,13] cytoreductive surgery and HIPEC prolonged survival in our patients with colorectal cancer and peritoneal carcinomatosis in Taiwan, irrespective of the sites of recurrence. In the second trial, which included cancers having an appendiceal origin, survival rates did not significantly differ between experimental and control groups. However, although patients with colorectal cancer undergo cytoreductive surgery with HIPEC in our institute, adjuvant systemic chemotherapy is still administered. Our outcomes and risk factors for death are valuable references for the effect, complications, and survival following cytoreductive surgery with HIPEC and adjuvant chemotherapy.
The following findings were reported in a comparative study, a multi-institutional registry series, and several case series[4,5,15]: Median survival varied from 13 to 29 months, and 5-year survival rates ranged from 11% to 19%. Patients who underwent complete surgical cytoreduction benefited the most, with the median survival duration and 5-year survival rate ranging from 28 to 60 months and from 22% to 49%, respectively. However, overall treatment-related morbidity and mortality rates were 23% to 44% and 0% to 12%, respectively. In our study, the median survival duration and 5-year survival rate were consistent with those reported in previous studies. The 5-year survival rate in our study was 38%. Furthermore, in our study, according to the Common Terminology Criteria for Adverse Events, 21% of patients had grade I or II complications, whereas no patients had grade III or IV complications. A study performed a retrospective analysis of the efficacy of modern systemic chemotherapy (oxaliplatin or irinotecan) in patients with peritoneal carcinomatosis.[16] They reported that in appropriately selected patients with peritoneal carcinomatosis, outcomes achieved using cytoreductive surgery and HIPEC were superior to those achieved using modern combination chemotherapy regimens. The overall 5-year survival rate was 51% in patients who underwent cytoreductive surgery and HIPEC for colorectal cancers with peritoneal carcinomatosis. Because of such promising outcomes, our institute has been performing cytoreductive surgery and HIPEC with a modern chemotherapy regimen to treat patients with colorectal cancers and peritoneal carcinomatosis. However, the retrospective nature of our study and the inherent bias in comparing nonrandomly assigned patients limit the confidence with which these conclusions can be drawn.
In the current study, the major risk factors for death in the patients with colorectal cancer and peritoneal metastasis were a total PCI score > 20, total PPCI score > 0, and CC score ≥ 2. These findings are consistent with those reported by Austin et al, Portilla et al, and Gonzalez-Moreno and Sugarbaker.[17–19] To the best of our knowledge, ours is the first study to report a total PPCI score > 0 as a major risk factor for death in this population. The multivariate analysis revealed that the total PPCI score was the highest risk factor for death following cytoreductive surgery and HIPEC. Few related studies have focused on peritoneal metastasis having a colorectal cancer origin. To the best of our knowledge, this is the first study reporting factors related to poor prognosis following HIPEC in patients with colorectal cancer and peritoneal metastasis.
The toxicity identified in this study was compared with that reported in studies conducted in Western countries to determine whether the examined approach is suitable for Asian patients with colorectal cancer and peritoneal carcinomatosis. The most frequent complication in our patients was surgical site infections including intra-abdominal abscess, which accounted for 12% (4/33) of all postoperative complications. The complications observed after surgical cytoreduction followed by HIPEC in our study are compatible with those reported in previous studies.[8,14,20] The morbidity rate after surgical cytoreduction followed by HIPEC for peritoneal carcinomatosis was extremely high (∼40%).[20] Cytoreductive surgery and HIPEC have a steep learning curve, requiring 140 procedures to acquire expertise.[21] Thus far, we have performed HIPEC in >300 patients with peritoneal carcinomatosis in our institute. Therefore, we believe that HIPEC can be performed with expertise in our institute in the north of Taiwan.
The therapeutic effect of HIPEC is associated with temperature and treatment time.[22] In clinical practice, the temperature might be 40°C to 44°C at different treatment times.[16,23,24] High-temperature (43°C–44°C) HIPEC could be performed in a short time interval (30 minutes),[22] whereas low-temperature HIPEC could be performed in a long time interval (60–120 minutes).[22] However, the final therapeutic effect was equal between high-temperature HIPEC with a short-time interval and low-temperature HIPEC with a long-time interval.[22] In our previous study,[22] we designed a protocol for patients with colorectal cancers and peritoneal carcinomatosis in Taiwan. This is the first report of our HIPEC protocol for patients with colorectal cancer and peritoneal carcinomatosis in Taiwan. The outcomes were promising, with some patients with colorectal cancer and peritoneal carcinomatosis exhibiting long-term survival.
This study has several strengths. First, this is the largest study conducted in Asia to examine the outcomes of cytoreductive surgery and HIPEC for treating colorectal cancer with peritoneal metastasis. The population was homogenous, and all patients had primary colorectal cancer with peritoneal metastasis. The extrapolation of cytoreductive surgery and HIPEC for treating colorectal cancer with peritoneal metastasis might be sufficient without other primary cancers with various responses to HIPEC. We observed favorable outcomes of HIPEC in Taiwan, which can be extrapolated to other Asian populations with colorectal cancer and peritoneal metastasis. In addition, the toxicity of this treatment approach was acceptable in these patients; moreover, long-term survival was achieved. The 2- and 5-year overall survival rates following cytoreductive surgery and HIPEC were 57% and 38%, respectively.
The limitations of our study are the small sample size and retrospective study design, which did not include a control group. When sample size is small, only very large effects can be statistically significant. In this case, it was assumed that the p value achieved statistical significance, and cytoreductive surgery and HIPEC showed marked therapeutic effects on colorectal cancer with peritoneal metastasis.[25,26] The scientific community should learn about our potentially large treatment benefits. The lower end of the effect size excludes a value of zero, but it probably cannot exclude a small effect size.[25,26] Although mounting data suggest that long-term survival can be achieved in a low number of patients by using aggressive surgical cytoreduction followed by HIPEC, it remains unclear whether these outcomes are more favorable compared with those achieved using modern oxaliplatin- and irinotecan-based systemic chemotherapy with or without biological agents.[10] Because median survival durations in contemporary reports were approximately 20 months, the use of a modern systemic oxaliplatin- or irinotecan-containing regimen could potentially have narrowed and even eliminated the survival difference between the groups.[10] Finally, the independent contribution of HIPEC to the favorable outcomes of this approach has not been proven. Randomized trials are warranted in the future.
5. Conclusions
Performing cytoreductive surgery and administering HIPEC for treating colorectal cancer with peritoneal metastasis are feasible and resulted in long-term survival in this study in Taiwan. Factors related to poor prognosis were a total PCI score > 20, total PPCI score > 0, and CC score ≥2 in patients with colorectal cancer and peritoneal metastasis. The multivariate analysis indicated that the total PPCI score was the highest risk factor for death following cytoreductive surgery and HIPEC in the patients with colorectal cancer with peritoneal metastasis.
Supplementary Material
Footnotes
Abbreviations: CC = completeness of cytoreduction, HIPEC = hyperthermic intraperitoneal chemotherapy, HRs = hazard ratios, PCI = peritoneal carcinomatosis index, PPCI = postoperative peritoneal carcinomatosis index.
E-KL and M-CH have contributed equally to this study.
Funding: Wan Fang Hospital 105swf05
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg 2006;243:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545–50. [DOI] [PubMed] [Google Scholar]
- [3].Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989;63:364–7. [DOI] [PubMed] [Google Scholar]
- [4].Yan TD, Black D, Savady R, et al. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol 2006;24:4011–9. [DOI] [PubMed] [Google Scholar]
- [5].Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284–92. [DOI] [PubMed] [Google Scholar]
- [6].Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 2010;36:456–62. [DOI] [PubMed] [Google Scholar]
- [7].Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359–74. [DOI] [PubMed] [Google Scholar]
- [8].Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43. [DOI] [PubMed] [Google Scholar]
- [9].Elias D, Delperro JR, Sideris L, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol 2004;11:518–21. [DOI] [PubMed] [Google Scholar]
- [10].Springer, Steele SR, Hull TL, Read TE, et al. The ASCRS Textbook of Colon and Rectal Surgery. 2nd ed2016. [Google Scholar]
- [11].Glehen O, Osinsky D, Beaujard AC, et al. Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surg Oncol Clin N Am 2003;12:729–39. xiii. [DOI] [PubMed] [Google Scholar]
- [12].Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 2012;30:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358–63. [DOI] [PubMed] [Google Scholar]
- [14].Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426–32. [DOI] [PubMed] [Google Scholar]
- [15].Mahteme H, Hansson J, Berglund A, et al. Improved survival in patients with peritoneal metastases from colorectal cancer: a preliminary study. Br J Cancer 2004;90:403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681–5. [DOI] [PubMed] [Google Scholar]
- [17].Austin F, Mavanur A, Sathaiah M, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann Surg Oncol 2012;19:1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Portilla AG, Shigeki K, Dario B, et al. The intraoperative staging systems in the management of peritoneal surface malignancy. J Surg Oncol 2008;98:228–31. [DOI] [PubMed] [Google Scholar]
- [19].Gonzalez-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg 2004;91:304–11. [DOI] [PubMed] [Google Scholar]
- [20].Elias D, Di Pietrantonio D, Boulet T, et al. Natural history of complete cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2009;35:434–8. [DOI] [PubMed] [Google Scholar]
- [21].Kusamura S, Baratti D, Deraco M. Multidimensional analysis of the learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies. Ann Surg 2012;255:348–56. [DOI] [PubMed] [Google Scholar]
- [22].Hsieh MC, Wu CW, Wu LH, et al. Heat shock and cytokines modulate the expression of adhesion molecules on different human gastric-cancer cell lines. Int J Cancer 1996;67:690–4. [DOI] [PubMed] [Google Scholar]
- [23].Kobayashi H, Kotake K, Sugihara K. Outcomes of surgery without HIPEC for synchronous peritoneal metastasis from colorectal cancer: data from a multi-center registry. Int J Clin Oncol 2014;19:98–105. [DOI] [PubMed] [Google Scholar]
- [24].Shimizu T, Sonoda H, Murata S, et al. Hyperthermic intraperitoneal chemotherapy using a combination of mitomycin C,5-fluorouracil, and oxaliplatin in patients at high risk of colorectal peritoneal metastasis: a Phase I clinical study. Eur J Surg Oncol 2014;40:521–8. [DOI] [PubMed] [Google Scholar]
- [25].Sullivan GM, Feinn R. Using effect size or why the P value is not enough. J Grad Med Educ 2012;4:279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sullivan GM. FAQs about effect size. J Grad Med Educ 2012;4:283–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


