Abstract
Background:
Nonsmall cell lung cancer (NSCLC)-patients treated with standard chemotherapy experienced progression rapidly. A novel therapy based on programed death 1 (PD-1)/programed death ligand 1 (PD-L1) inhibitors showed an increasing potential in several malignancies including advanced NSCLC.
Objectives:
This article is a meta-analysis aiming to systematically evaluate the efficacy and safety profiles of PD-1/PD-L1 agents in patients with NSCLC.
Data sources:
Data were collected from eligible studies searched from PubMed, ScienceDirect, and Web of Science.
Synthesis methods:
Pooled hazard ratio (HR) for overall survival (OS) and progression-free survival (PFS) was estimated to assess the efficacy of PD-1/PD-L1 inhibitors versus docetaxel, pooled odds ratio (OR) was calculated for objective response rate (ORR). The overall frequency was estimated for 1-year OS, 1-year progression-free survival, and ORR. A subgroup analysis among NSCLC patients tested with different epidermal growth factor receptor (EGFR) status was also performed to figure out the relationship between EGFR status and efficacy of PD-1/PD-L1 therapies. OR for occurrence of any grade and grade 3 to 5 treatment-related adverse effect was calculated for evaluating the safety of PD-1/PD-L1 therapies.
Results:
Nine studies were included in this analysis. The pooled HRs for OS and PFS were 0.68 (95% confidence interval [CI] 0.61–0.75) and 0.83 (95% CI 0.75–0.91), respectively, the pooled OR for ORR was 1.83 (95% CI 1.41–2.36), indicating a significant improvement in OS, PFS, and ORR. In the results of subgroup analysis, the HR for OS in NSCLC patients was 1.05 (95% CI 0.69–1.59) in patients with mutant EGFR and 0.66 (95% CI 0.57–0.77) in patients with wild-type EGFR status. OR for occurrence was 0.36 (95% CI 0.28–0.46) in any grade treatment-related adverse effect and 0.18 (95% CI 0.14–0.22) in grade 3 to 5 treatment-related adverse effect, suggesting a superior safety profile of PD-1/PD-L1 inhibitors.
Conclusion:
The PD-1/PD-L1 therapy significantly prolonged the OS and improved the ORR, simultaneously lowering the treatment-related adverse effect events versus docetaxel.
Keywords: immunotherapy, meta-analysis, nonsmall cell lung cancer, PD-1, PD-L1
1. Introduction
Lung cancer remains to be one of the leading causes of cancer-related mortality around the world despite the development in treatment strategies of lung cancer.[1,2] In 2016, the number of patients suffering from lung cancer or bronchial cancer will increase by 224,390, including 117,920 in men and 106,470 in women in the United States.[3] In addition, most patients are generally diagnosed at an advanced and metastatic stage, often accompanied by poor prognosis and difficult-to-manage disease. Generally, lung cancer can be divided into 2 categories: small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC) which further includes 2 subdivisions (squamous and nonsquamous NSCLC).
For the early-stage lung cancer, local treatment strategies include surgical resection and definitive radiation. For the advanced cases, however, a multimodality strategy should be employed, and systemic therapy will be the principal treatment for metastatic disease. Cytotoxic and platinum doublet-based chemotherapy has been selected as the first-line treatment for patients with metastatic NSCLC, and a considerable median survival of 8 to 12 months was obtained.[4–6] Docetaxel was approved as the second-line treatment for patients based on 2 phase 3 trials.[7–9] Also, an improved response rate was observed in most NSCLC patients treated with first-line chemotherapy; however, the disease progressed rapidly during or after the treatment, and the clinical efficacy of second-line chemotherapy was unsatisfactory either.
Recently, the novel therapy based on the immune checkpoints exhibits significant potential in treatment of patients with advanced NSCLC and SCLC.[1,10,11] Programed death 1 (PD-1) is a vital immune checkpoint receptor which is expressed on activated T cells.[12] Normally, the interaction between PD-1 and programed death ligand 1 (PD-L1) will lead to the inhibition of immune response,[13] thus preventing the excessive inflammation. Otherwise, PD-L1 was also found to be expressed in some tumor cells including those of NSCLC.[14] Activated T cells that target the tumor cells will be inactivated by interaction of PD-1 and PD-L1, ultimately allowing tumor progress and metastasis. Therefore, blocking the PD-1 pathway by disrupting the binding of PD-1 to its ligand will provide an effective approach for recovering the antitumor immunity mediated by T cells. Up to now, several monoclonal antibodies targeting PD-1 or PD-L1 have already been developed. Nivolumab is a fully humanized Immunoglobulin G (IgG4) antagonist monoclonal antibody targeting PD-1 and is approved by the United States Food and Drug Administration for treatment of NSCLC. Findings of several single-arm and multiarm studies indicated an improved overall survival (OS) and response rate in advanced NSCLC patients when treated with nivolumab as monotherapy or combination with other chemotherapy.[15,16] Pembrolizumab is another humanized IgG4 antagonist antibody against PD-1.[17] A phase 2/3 study about pembrolizumab reported a better OS in patients treated with pembrolizumab than that of patients administrated with docetaxel.[18] Atezolizumab is an anti-PD-L1 antibody, an assessment concerning the efficacy and safety of atezolizumab versus docetaxel in patients with previously treated NSCLC was performed by Fehrenbacher et al,[19] the results exhibited a more considerable survival in atezolizumab-treated arm than docetaxel. Based on the study results of nivolumab, pembrolizumab, and atezolizumab, the anti-PD-1/PD-L1 therapy exerts as a highly promising treatment paradigm in patients with advanced NSCLC. However, the adverse effects potentially caused by PD-1/PD-L1 therapies cannot be ignored, which has been previously reported in several studies. This article is a meta-analysis aiming to further evaluate the efficacy and safety of anti-PD-1/PD-L1 agents in advanced NSCLC patients, subgroup analysis was also performed to figure out the efficacy among patients with different epidermal growth factor receptor (EGFR) status.
2. Method
2.1. Search strategy
A comprehensive search for studies published in English was performed in the PubMed, ScienceDirect, and Web of Science in order to collect all relevant citations. The date of the last search was May 20, 2016. Meeting abstracts from Major European and American oncology meetings were also evaluated. Keywords for studies search were as follows: “non small cell lung cancer” OR “NSCLC” AND “nivolumab” OR “pembrolizumab” OR “atezolizumab” OR “Opdivo” OR “BMS-936558” OR “MDX1106” OR “MK-3475” OR “lambrolizumab” OR “MPDL3280A”.
2.2. Selection criteria
Inclusion criteria are the followings: articles that evaluate anti-PD-1/PD-L1 agents in treatment of patients with NSCLC, articles with or without report of PD-L1 expression level will be included; studies including 1 or all of the following information: objective response rate (ORR), OS, and progression-free survival (PFS). Letters, editorials, expert opinions, case reports, duplicate publications, and reviews should be excluded as well as the studies without usable data.
2.3. Data extraction
Data were extracted independently by 3 authors from eligible studies. The following data were collected: authors, treatment strategy, number of patients, ORR, PFS, OS, adverse effect events, or frequency.
2.4. Outcome measures
The outcome measures were ORR, PFS, and OS. This systematic review follows the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Report (PRISMA statement).[20]
2.5. Data analysis
For ORR, odds ratio (OR) and corresponding 95% confidence intervals (CIs) are the principal summary measures, while for PFS and OS, hazard ratios (HRs) and corresponding 95% CIs are the principal measures. Relevant data were extracted from each study, and the pooled ORs and HRs were estimated through a meta-analysis. Fixed effects model will be used in the analyses if there is no substantial heterogeneity among different studies, or the random effects model will be applied. All analyses were conducted using the program RevMan5.3 (Nordic Cochrane center, Copenhagen, Denmark). For single-arm or noncontrolled studies, the pooled ORR, 1-year progression-free survival rate, and 1-year OS rate will be estimated by MetaAnalyst 3.13 (Boston, MA, USA). Heterogeneity will be assessed with the Chi2 testing and I2 statistic, P value less than 0.05 indicates significant heterogeneity, I2 value greater than 50% is considered significant heterogeneity. The publication bias will be assessed using funnel plots.
3. Results
3.1. Search results and characteristics of included studies
The PRISMA diagram for the study selection is summarized in Fig. 1. A total of 466 results were obtained from the searches in PubMed, 1563 from ScienceDirect, and 547 from web of science. A total of 2352 studies were excluded for duplication and 238 for not meeting the eligibility criteria in the initial selection. After the full-text search, 9 studies involving 2 phase 1 trials,[21,22] 3 phase 2 trials,[19,23,24] 4 phase 3 trials[18,25–27] were included in the following analysis. Six studies assessed nivolumab, 2 assessed pembrolizumab, and 1 assessed atezolizumab. Four studies compared the efficacy and safety of PD-1/PD-L1 agents with doectaxel, 5 studies evaluated efficacy and safety of PD-1/PD-L1 alone. A total of 3032 patients were included in this analysis. PD-1/PD-L1 agents were administrated as monotherapy in all included studies, different dose settings were found in 3 studies. Detailed treatment strategies are summarized in Table 1.
Figure 1.
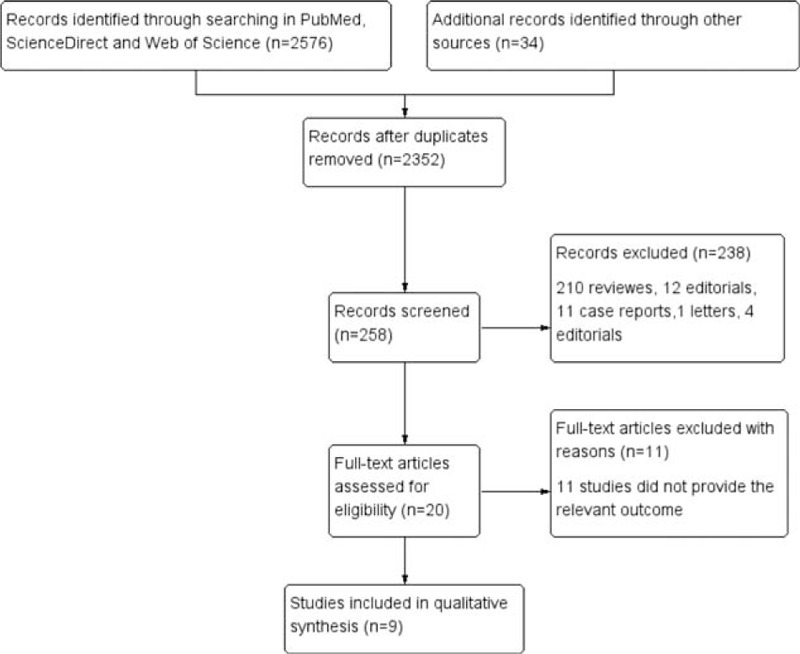
Flowchart of study selection procedure.
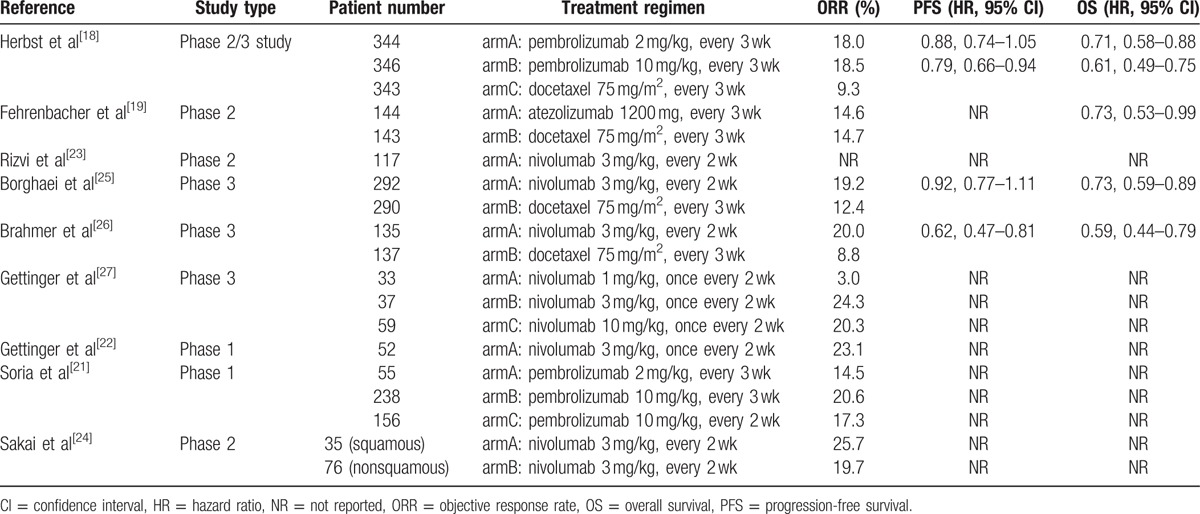
Table 1.
Efficacy outcomes in included studies.
3.2. Efficacy outcomes of PD-1/PD-L1 agents versus docetaxel
3.2.1. Overall survival, progression-free survival, and objective response rate
Four studies assessed the efficacy and safety of PD-1/PD-L1 agents versus docetaxel in patients with advanced NSCLC. Three studies were 2-arm trials,[19,25,26] the other one was a 3-arm trial including a different dose setting of pembrolizumab.[18] The pooled HR for OS was 0.68 (95% CI 0.61–0.75; P < 0.001) (Fig. 2A). The pooled OR for ORR was 1.83 (95% CI 1.41–2.36; P < 0.001) (Fig. 2C). Since the PFS was not available in 1 study, the pooled HR for PFS was estimated with 3 studies only. The pooled HR for PFS was 0.83 (95% CI 0.75–0.91; P < 0.001) (Fig. 2B). Thus, the analysis suggested a significant benefit from anti-PD-1/PD-L1 therapies in treatment of patients suffering from advanced NSCLC when compared with docetaxel.
Figure 2.
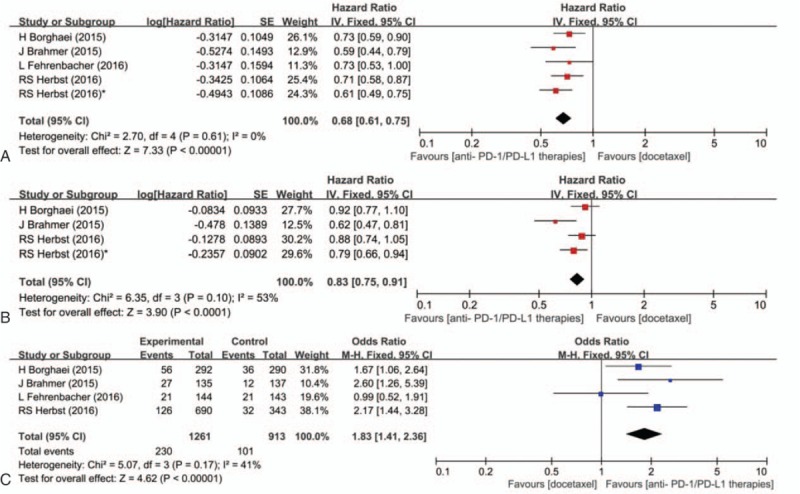
Pooled hazard ratio for overall survival (A), progression-free survival (B), and pooled odds ratio for objective response rate (C) in patients treated with programed death 1/programed death ligand 1 agents versus docetaxel. ∗ Represents an arm treated with pembrolizumab 10 mg/kg in Ref. [18].
3.2.2. EGFR affects the benefits from anti-PD-1/PD-L1 therapies
We observed that both Herbst and Borghaei reported the subgroup analysis of OS stratified by EGFR status. Here, we also analyzed the different efficacy of PD-1/PD-L1 agents among patients tested with different EGFR status. Finally, the HR values with 1.05 (95% CI 0.69–1.59; P = 0.81) and 0.66 (95% CI 0.57–0.77; P < 0.001) were obtained in patients with mutant and wild-type EGFR status, respectively (Fig. 3).
Figure 3.
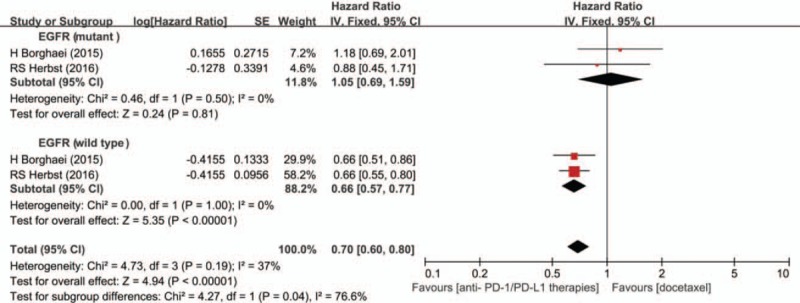
Subgroup analysis of overall survival in patients with different epidermal growth factor receptor status.
3.3. Efficacy outcomes of PD-1/PD-L1 agents when employed as monotherapy
Nine studies include available data of ORR, the overall ORR was 18.7% (95% CI 17.0–20.4). Four researches reported that the 1-year OS and 1-year program-free survival with the pooled value were 42.3% (95% CI 38.5–46.1) and 20.1% (95% CI 17.3–23.2), respectively (Fig. 4).
Figure 4.
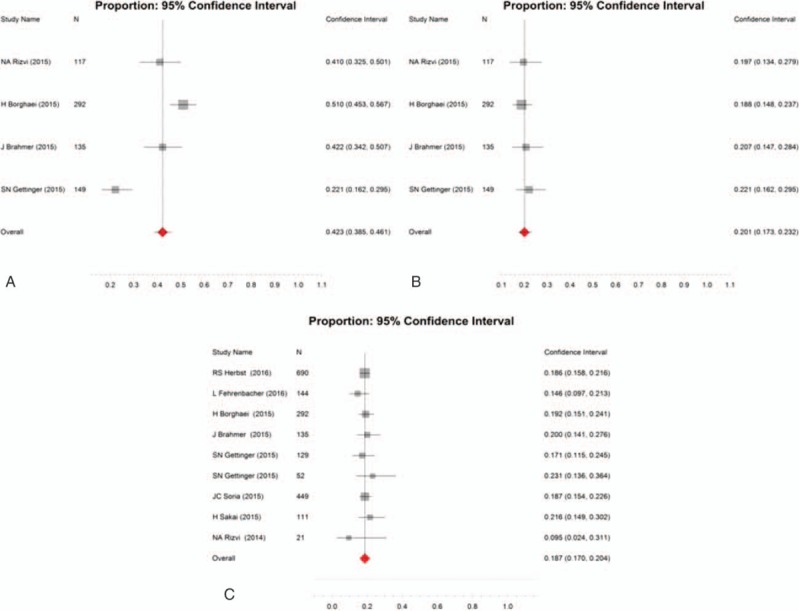
Overall 1-year overall survival (A), 1-year progression-free survival (B), and ORR (C) in patients administrated with programed death 1/programed death ligand 1 agents as monotherapy.
3.4. Safety assessment
Treatment-related adverse effect is an important evaluation index for any antitumor therapies. Many treatments have to be discounted for the severe adverse effects caused by the treatment agents. To evaluate the safety of PD-1/PD-L1 agents in advanced NSCLC patients, data of the total adverse effect events and grade 3 to 5 adverse effect events were collected and analyzed. The OR of the total adverse effect events for patients receiving PD-1/PD-L1 agents versus docetaxel was 0.36 (95% CI 0.28–0.46; P < 0.001), and the OR of grade 3 to 5 adverse effect events was 0.18 (95% CI 0.14–0.22; P < 0.001) (Fig. 5). On the basis of the observed results, it was indicated that the incidence of treatment-related adverse effect caused by PD-1/PD-L1 agents was significantly lower than that caused by docetaxel.
Figure 5.
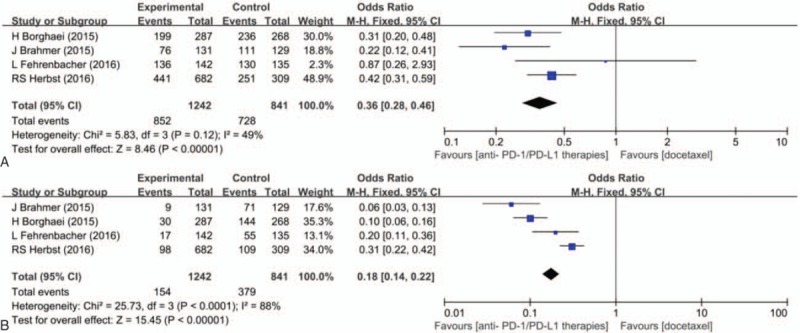
Pooled odds ratio for incidence of any grade treatment-related adverse effect (A) and grade 3 to 5 treatment-related adverse effect (B).
4. Discussion
Rapid progression during or after the standard chemotherapy in patients with NSCLC indicates that a new effective treatment diagram is in urgent need. PD-L1 has been demonstrated to be a tumor-related biomarker contributing to tumor advance. The binding of PD-1 expressed by activated T cells to PD-L1 induces the immune suppression, thus protecting the normal cells from being attacked by active T cells. Previous researches have found that some tumor cells can evade immune recognition via expressing PD-L1, which provided a potentially effective antitumor strategy. Monoclonal antibodies targeting PD-1 or PD-L1, like nivolumab, pembrolizumab, and atezolizumab, can restore the antitumor effect of T cells by blocking the PD-1 signal. An improvement in OS and PFS as well as lower incidence of treatment-related adverse effect was previously reported in several phase 2 and phase 3 trials focusing on PD-1/PD-L1 antibodies. To further validate this immune checkpoint therapy, the effect and safety of PD-1/PD-L1 agents in NSCLC patients were systematically analyzed in this article.
In this study, we first compared the efficacy of PD-1/PD-L1 agents with docetaxel in advanced NSCLC patients. OS and progression-free survival were selected as the primary endpoints, ORR was the second endpoint. According to the pooled HR values, patients administrated with nivolumab, pembrolizumab, or atezolizumab had a better OS and PFS than those who were treated with docetaxel. The pooled OR also suggested a higher ORR with 14.6% to 20.0% in PD-1/PD-L1 therapy group across the studies containing a docetaxel control, the reported median OS in patients with PD-1/PD-L1 agents administration was 9.2 to 12.7 months, which was longer than that in docetaxel-treated group (7.3–9.7 months), while the median progression-free survival was 2.3 to 4.0 months in PD-1/PD-L1 agents administration cohort and 2.8 to 4.2 in docetaxel-treated cohort. Hence, it was concluded that the PD-1/PD-L1 therapy significantly improved the OS and ORR rather than the progression-free survival when compared to docetaxel. It was reported that progression-free survival with pembrolizumab was superior to that of docetaxel in NSCLC patients with a tumor proportion score of >50%, but not in the total population, while OS with pembrolizumab was superior to that of docetaxel in total population. Here, the slight improvement of PD-1/PD-L1 agents on progression-free survival may be ascribed to the different tumor proportion score.[18]
Considering that the PD-L1 is the key target of the PD-1/PD-L1 therapy, the expression level of PD-L1 in tumor cells may presumably impact the efficacy of PD-1/PD-L1 therapy. Several recent trials demonstrated that PD-L1 expression had a significant correlation with OS, ORR, and PFS. A further meta-analysis pointed out that the benefit from PD-1/PD-L1 therapy versus docetaxel as second-line treatment in NSCLC patients was only limited to subpopulation with a PD-L1 expression level of >1%.[28] Not only the PD-L1 expression level but also the EGFR status and smoking history can affect the benefits from PD-1/PD-L1 therapy,[25] which has been reported in previous studies. Here, we conducted a subgroup analysis to clarify the different efficacy in NSCLC patients with different EGFR status. The results showed a significant improvement in OS of patients with wild-type EGFR; nevertheless, the same results were not observed in patients with mutant EGFR. An immunohistochemical analysis in 164 specimens of surgically resected NSCLC was conducted by Azuma et al,[29] the results of a multivariate analysis revealed that the presence of EGFR mutant was significantly associated with a higher PD-L1 expression level. In addition, patients with higher PD-L1 expression had a shorter OS, which was contrary to the results reported by Abdel-Rahman.[28] Taking into accounting of the above facts, the inherent relationship among PD-L1 expression, EGFR status, and benefits from PD-1/PD-L1 therapy was more complicated than we imaged. The role of EGFR status as well as PD-L1 expression level as a potential predictive biomarker for decision-making about treatment strategy in clinic need to be further discussed. As to the smoking history, patients who are current or former smoker experienced a significant benefit from PD-1/PD-L1 therapy. An HR value of 0.70 (95% CI 0.56–0.86) for OS was reported in a subpopulation with a smoking history in a phase 3 trial, while the HR value in patients never smoking was 1.02 (95% CI 0.64–1.61).[25] In another study, the author reported a numerically higher ORR among patients with smoking history.[22] A subgroup analysis was not performed here to systematically compare the efficacy of PD-1/PD-L1 therapy between smoking and nonsmoking patients, because the reported endpoint varied across the included studies. On account of limited trials toward the relationship between efficacy of PD-1/PD-L1 therapy and smoking history, the observed better efficacy in smoking patients needs to be further confirmed.
The safety profile of PD-1/PD-L1 therapy was also evaluated in this article. The reported treatment-related adverse effects include decreased appetite, fatigue, nausea, rash, diarrhea, asthenia, stomatitis, anemia, alopecia, and neutropenia.[18] The pooled OR was 0.36 for any grade adverse effects and 0.18 for grade 3 to 5 adverse effects, this indicated a protective role of PD-1/PD-L1 agents in NSCLC patients versus docetaxel. The superiority of PD-1/PD-L1 therapy versus docetaxel in the treatment of NSCLC patients was more notable when we turned our attention to neutropenia, febrile neutropenia, leukopenia, and alopecia of which a significantly lower incidence was observed in PD-1/PD-L1 agents treatment group. Fatigue is a common treatment-related adverse effect with a higher frequency than others, a meta-analysis performed by Abdel-Rahman et al[30] demonstrated that a lower risk of all grade fatigue, compared with control regimens, was possibly ascribed to PD-1 inhibitors. However, the high incidence of pneumonitis, hypothyroidism, and hyperthyroidism observed in pembrolizumab group cannot be ignored, whereas it was lower in docetaxel group. In a recently published research, the side effects caused by anti-PD-1 therapy were summarized.[31] Thus, a careful consideration against potential toxicities caused by PD-1/PD-L1 agents is necessary when PD-1/PD-L1 therapy is employed. In most studies included in this analysis, PD-1/PD-L1 inhibitors were employed as monotherapy, but the studies focusing on efficacy of its combination with chemotherapies or other target therapies were limited. The ORR between 33% and 47% were obtained in a study evaluating the effect of nivolumab in combination with platinum-based doublet chemotherapy,[15] it proved that PD-1/PD-L1 agents in combination with other therapies may exert as a promising therapy diagram, especially in NSCLC patients experiencing progression after several lines of therapy.
5. Conclusion
In conclusion, our analysis revealed that PD-1/PD-L1 agents significantly prolonged the OS and increased the ORR when compared to docetaxel, while more data are needed to confirm whether the PD-1/PD-L1 therapy is superior when challenging to other standard chemotherapies. The better benefits from PD-1/PD-L1 agents observed in patients with wild-type EGFR were controversial, more efforts are required to understand the real relationship between PD-1/PD-L1 efficacy and EGFR status. Based on the analysis of adverse effect, a lower risk was associated with the PD-1/PD-L1 therapy versus docetaxel, while the occurrence of related side effects like pneumonitis and endocrine dysfunction deserves more attention.
Footnotes
Abbreviations: CI = confidence interval, EGFR = epidermal growth factor receptor, HR = hazard ratio, NSCLC = nonsmall cell lung cancer, OR = odds ratio, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
CW, XY, and WW have contributed equally to the article.
Ethical approval: This article does not contain any studies with human participants performed by any of the authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Chae YK, Pan A, Davis AA, et al. Recent advances and future strategies for immune-checkpoint inhibition in small cell lung cancer (SCLC). Clinical Lung Cancer 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [2].Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines® insights: non-small cell lung cancer, Version 4.2016. J Natl Compr Cancer Netw 2016;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 2016;21:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Paramanathan A, Solomon B, Collins M, et al. Patients treated with platinum-doublet chemotherapy for advanced non-small-cell lung cancer have inferior outcomes if previously treated with platinum-based chemoradiation. Clin Lung Cancer 2013;14:508–12. [DOI] [PubMed] [Google Scholar]
- [6].Lu S, Cheng Y, Zhou CC, et al. Meta-analysis of first-line pemetrexed plus platinum treatment in compared to other platinum-based doublet regimens in elderly East Asian patients with advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer 2016;17:e103–12. [DOI] [PubMed] [Google Scholar]
- [7].He X, Wang J, Li Y. Efficacy and safety of docetaxel for advanced non-small-cell lung cancer: a meta-analysis of Phase III randomized controlled trials. Onco Targets Ther 2015;8:2023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fossella F, Devore R, Kerr R, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354–62. [DOI] [PubMed] [Google Scholar]
- [9].Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. Soc Clin Oncol 2000;18:8. [DOI] [PubMed] [Google Scholar]
- [10].Remon J, Chaput N, Planchard D. Predictive biomarkers for programmed death-1/programmed death ligand immune checkpoint inhibitors in nonsmall cell lung cancer. Curr Opin Oncol 2016;28:122–9. [DOI] [PubMed] [Google Scholar]
- [11].Bagley SJ, Kosteva JA, Evans TL, et al. Immune thrombocytopenia exacerbated by nivolumab in a patient with non-small-cell lung cancer. Cancer Treat Commun 2016;6:20–3. [Google Scholar]
- [12].Katsuya Y, Horinouchi H, Asao T, et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer 2016;99:4–10. [DOI] [PubMed] [Google Scholar]
- [13].La-Beck NM, Jean GW, Huynh C, et al. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy 2015;35:963–76. [DOI] [PubMed] [Google Scholar]
- [14].Shien K, Papadimitrakopoulou VA, Wistuba II. Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non-small cell lung cancer. Lung Cancer 2016;99:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Antonia SJ, Brahmer JR, Gettinger S, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC): metastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;90:S2. [Google Scholar]
- [16].Rizvi NA, Shepherd FA, Antonia SJ, et al. First-line monotherapy with nivolumab (anti-PD-1; BMS-936558, ONO-4538) in advanced non-small cell lung cancer (NSCLC): safety, efficacy, and correlation of outcomes with PD-L1 status. Int J Radiat Oncol Biol Phys 2014;90:S31. [Google Scholar]
- [17].Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- [19].Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46. [DOI] [PubMed] [Google Scholar]
- [20].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [21].Soria J, Flatten O, Horn L, et al. 33LBA efficacy and safety of pembrolizumab (Pembro; MK-3475) for patients (Pts) with previously treated advanced non-small cell lung cancer (NSCLC) enrolled in KEYNOTE-001. Eur J Cancer 2015;51:S726–7. [Google Scholar]
- [22].Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016;34:2980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;3:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sakai H, Nishio M, Hida T, et al. 521 Phase II studies of nivolumab in patients with advanced squamous (SQ) or non-squamous (NSQ) non-small cell lung cancer (NSCLC). Eur J Cancer 2015;51:S110–1. [Google Scholar]
- [25].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015;33:2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abdel-Rahman O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Crit Rev Oncol Hematol 2016;101:75–85. [DOI] [PubMed] [Google Scholar]
- [29].Azuma1 K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected non-small cell lung cancer. Ann Oncol 2014;25:1935–40. [DOI] [PubMed] [Google Scholar]
- [30].Abdel-Rahman O, Helbling D, Schmidt J, et al. Treatment-associated fatigue in cancer patients treated with immune checkpoint inhibitors; a systematic review and meta-analysis. Clin Oncol (R Coll Radiol) 2016;28:e127–38. [DOI] [PubMed] [Google Scholar]
- [31].Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210–25. [DOI] [PubMed] [Google Scholar]


