Abstract
The present study was performed to investigate whether the markedly 2-deoxy-2-(fluorine-18) fluoro-D-glucose (18F-FDG) uptake in the bone marrow (BM) is a presentation of malignant infiltration (MI).
Super bone marrow uptake (super BMU) was used to name the markedly 18F-FDG uptake on BM, which was similar to or higher than that of the brain. From April 2008 to December 2015, 31 patients with such presentation were retrospectively reviewed. The 18F-FDG uptake was semiquantified using SUVmax and BM to cerebellum (BM/C) ratio. The origin of super BMU was diagnosed by pathology. Some blood parameters, as well as fever, were also collected and analyzed. For comparison, 106 patients with mildly and moderately uptake in BM and 20 healthy subjects were selected as the control group.
Bone marrow MI was diagnosed in 93.5% (29/31) patients with super BMU, which mostly originated from acute leukemia and highly aggressive lymphoma. The super BMU group had markedly higher 18F-FDG uptake in the BM than those of mildly and moderately uptake, and the control subjects (all P = 0.000) and the BM/C ratio reached a high of 1.24 ± 0.36. The incidence of bone marrow MI in the super BMU group was markedly higher than that of mildly and moderately uptake (93.5% vs 36.8%, P = 0.000). Based on the receiver operating characteristic analysis, when cut-off values of BM/C and SUVmax were set at 0.835 and 6.560, the diagnostic specificity for bone marrow MI reached the high levels of 91.4% and 95.7%, respectively. In 15 patients with bone marrow MI, the extra-BM malignant lesions were simultaneously detected by 18F-FDG PET/CT. The liver and the nasal cavity involvements were only found in the patients with lymphoma, but not in those with leukemia. A decrease of leukocyte, hemoglobin, and platelet counts was noted in 48.4%, 86.2%, and 51.5% of patients with bone marrow MI, respectively.
The present study revealed that super BMU was a highly potent indicator for the bone marrow MI.
Keywords: 18F-fluorodeoxyglucose, leukemia, lymphoma, malignant infiltration, PET/CT, super BMU
1. Introduction
Positron emission tomography/computed tomography using 2-deoxy-2-(fluorine-18) fluoro-D-glucose (18F-FDG) has become a standard diagnostic modality for the diagnosis, staging, treatment response assessment, and recurrence detection of malignant diseases.[1–5]18F-FDG is a glucose analog, which can be trapped in the tumor cells through the glycolysis pathway due to the Warburg effect in the malignant cells and reflects the increased level of glucose consumption. The malignant lesions often have the high level of 18F-FDG uptake because of increased expression of glucose transporters, particularly GLUT-1 and GLUT-3, as well as hexokinase in these cells.[6–9] However, 18F-FDG is not a tumor-specific substance, which also can accumulate in a variety of benign and physiologic conditions and may lead to false-positive interpretation. In general, 18F-FDG uptake in the benign disease and physiologic conditions was low.[10–12]
Diffuse 18F-FDG bone marrow uptake (BMU) is often encountered in the PET/CT routine practice. This phenomenon is always observed in the patients with the malignancy, the unknown origin fever, infection and those treated with the granulocyte colony-stimulating factor (GCSF) or erythropoietin (EPO).[13–20] In most cases, the diffuse BMU was slight and moderate which often presented as an uptake level corresponding to or slightly higher than that in the liver. In these situations, it was reported to be difficult to determine whether it is caused by malignant BMI or bone marrow inflammatory.[21,22]
However, in the clinical practice, we could occasionally find some patients presented with the markedly increased uptake of 18F-FDG in the BM, similar to or super to that of the brain and significantly higher than that of the liver. In the present study, we named it “super BMU,” mimicking the “super scan” on the bone scintigraphy, which was used to define the strikingly increased uptake of 99mTc-methylene diphosphonate in the bone.[23,24] It is well known that the uptake of 18F-FDG in the healthy brain is very intense, which is much higher than that in the normal BM.[1–5,15,16] Therefore, the super BMU reflects an unusually high 18F-FDG uptake in the BM. Until now, to our knowledge, this sign has not previously been described, and it is not clear whether the super BMU represents a PET presentation of malignant BMI. Therefore, this retrospective study was performed to investigate its origin and the clinical significance.
2. Patients and methods
2.1. Patients
The Ethical Committee of our Institutional Review Board of Nanfang Hospital, Southern Medical University has approved the study. As a result of the retrospective nature of the survey, the requirements for informed consent were waived.
Thirty-one patients with super BMU were selected from a retrospective review of the PET center database between April 2008 and December 2015 (20 males, 11 females; age [χ2±SD], 35.7 ± 23.3 years; range, 8–71 years) at Nanfang Hospital, Southern Medical University. The patients were referred for 18F-FDG PET/CT due to the unknown origin of fever in 12 patients, pain in the chest and bone in 8 patients, enlarged lymph nodes in the neck and the abdominal cavity in 4 patients, and dizzy and feeble accompanied with the unknown caused by decrease peripheral blood cells in 7 patients. All the patients had not the definite diagnosis before the examination of 18F-FDG PET/CT. They had also not received any treatments against the malignancy or infections or receiving any treatments with GCSF or EPO. The final diagnosis of the disease was established by the pathology. The bone marrow biopsy (BMB) was performed to identify the origin of super BMU.
For comparison and the receiver operating characteristic (ROC) analysis, 106 patients (age [χ2±SD], 39.0 ± 17.8 years, range: 9–79 years, 64 females, 42 males) with mildly and moderately 18F-FDG uptake in bone marrow were also randomly collected. These patients included 61 patients with primary malignant diseases (33 lymphoma, 25 leukemia, 2 multiple myeloma, and 1 lung cancer) and 45 ones with benign diseases (8 necrotic lymphadenitis, 6 Crohn diseases, 5 ulcerative colitis, 5 adult still disease, 5 myelodysplastic syndromes, 5 active tuberculosis, 3 bacterial infection, 2 lung fungal infection, 2 virus infection, 2 myelofibrosis, 1 autoimmune pancreatitis, and 1 acute hemolytic anemia). In the present study, the patients with mildly and moderately bone marrow uptake were merged together as 1 group for analysis. None of these patients received treatment with GCSF or EPO, neither antineoplastic, anti-infectious nor other specific treatments. The diagnosis of the primary disease among these patients was established by the pathology, experimental treatment response, and other etiologic examinations. All the patients, who had the primary malignant disease, received BMB for further identifying the cause of bone marrow uptake.
Twenty normal subjects (11 males, 9 females; age [χ2±SD], 52.0 ± 5.8 years; range, 42–63 years) were selected as the normal control group. These subjects underwent 18F-FDG PET/CT to exclude the malignancy due to the slightly increased level of tumor marker of carcinoembryonic antigen, CA199, or CA247. The results of 18F-FDG PET/CT were negative, and no malignancy was found in these subjects after a 1-year long following-up.
None of the patients and the control subjects had diabetes mellitus. The results of the recent experiment examinations including peripheral blood cells (white blood cell [WBC], hemoglobin [HB] and platelet [PLT] count, and serum C-reactive protein [CRP] and lactate dehydrogenase [LDH]) were collected from the PACS data system/electronic medical recorded system. The time interval between the peripheral sampling for blood cells examination and the PET/CT scan was not more than 2 days. The body temperature in the axilla of the patients was checked and recorded on the day when PET/CT scan was performed.
2.2. 18F-FDG PET/CT examination
The patients and normal control subjects underwent 18F-FDG PET/CT scans using GE Discovery LS PET/CT scanner (GE Healthcare, Waukesha, WI) and Biograph mCTx scanner (Siemens, Erlangen, Germany). The patients and the control subjects were instructed to fast for at least 6 hours, and the blood glucose level was monitored by glucometer prior to 18F-FDG injection. All the blood glucose level of the patients and the control subjects were lower than 7.0 mmol/L. 18F-FDG was manufactured automatically using the tracer synthesis system of a Tracerlab FXF-N(GE Healthcare) and the radiochemical purity was greater than 95%. Approximately 60 minutes after the intravenous injection of 161 to 465 MBq (4.35–12.56 mCi, 150 μCi/kg) of 18F FDG, whole-body PET/CT was performed.
Image acquisition using whole-body 18F-FDG PET/CT included 6 to 8 bed positions for each unenhanced contrast CT and PET scan, covering the entire range from the vertex of the skull to the mid thigh. Each CT scan was performed with a scout view using 30 mA and 80 kVp, followed by a spiral CT scan with 80 mA, 140 kVp, 0.8-second rotation time, a 5-mm section thickness (Discovery LS) or 80 mA, 120 kVp, 1.0-second rotation time, a 3-mm section thickness (Biograph mCTx), in high-speed mode. After CT had been completed, PET scan was then acquired in the 2-dimensional acquisition mode at 3 to 4 minutes per bed position (Discovery LS) and in the 3-dimensional acquisition mode at 2 minutes per bed position (BiographmCTx).
The PET images were reconstructed using a standard iterative algorithm (ordered-subset expectation maximization), with the CT data used for attenuation correction.
2.3. Image analysis
The acquired PET and CT images were sent to the Xeleris workstation (GE Healthcare) and Syngo MI workplace (Siemens, Germany) for registration and fusion. PET/CT scans were reviewed separately by 2 experienced nuclear medicine physicians. Both 18F-FDG uptake in bone marrow and extra-BM organs on PET images and the morphologic change on CT images were assessed visually. Consensus resolved any primary difference of opinion. The uptake of 18F-FDG in the bone marrow was determined to be normal when it was lower than or corresponding to that in the liver (Fig. 1). Mild uptake and moderate uptake were defined by comparing the 18F-FDG uptake in BM with that in the liver and brain (mildly uptake: slightly higher than that in the liver; moderately uptake: much higher than that in the liver but lower than that in the brain) (Fig. 1). If the diffuse uptake of 18F-FDG in the BM was markedly high and similar to or superior to that of the brain, then it was considered to be super BMU (Fig. 1). A lesion in the extra-BM organs, which had 18F-FDG uptake exceeding that of the surrounding normal tissue was considered positive.
Figure 1.
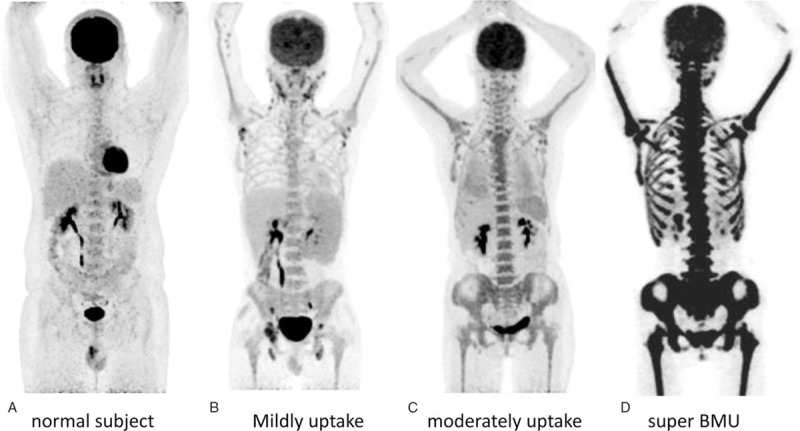
The MIP images of the normal uptake in BM (A), mildly uptake (B), moderately uptake (C), and a leukemia patient with super BMU (D). A, Normal uptake: 18F-FDG uptake in the normal BM is minimal, which was lower or corresponding to that in the liver. B, Mildly uptake: 18F-FDG uptake in BM was slightly higher than that in the liver. C, Moderately uptake: 18F-FDG uptake in BM was significantly higher than that in the liver, but lower than that in the brain. D, Super BMU: 18F-FDG uptake in the BM was markedly high, which was similar to that in the brain. 18F-FDG = fluorine-18 fluoro-D-glucose, BM = bone marrow, BMU = bone marrow uptake, MIP = maximum intensity projection.
A region of interest (ROI) with a circular 3 × 3 pixel was placed in the iliac crest bone on the transverse PET image to calculate the maximal SUV (SUVmax) for the BM. For determination of uptake in normal brain tissue, the same 3 × 3 pixel ROI was placed on the normal cerebellum. The bone marrow to cerebellum (BM/C) ratio was calculated by dividing the SUVmax of BM by that of the normal cerebellum. For calculation of the SUVmax for the extra-BM lesion, the ROIs were drawn along the margin of lesions, but not used the circular 3 × 3 pixels ROI.
2.4. Statistical analysis
Statistical analyses were executed using Statistical Package for the Social Sciences version 17.0. (SPSS Inc, Chicago, IL). The SUVmax, BM/C ratio, WBC count, HB, PLT, LDH, and CRP were expressed as the mean ± standard deviation. The Student t test was used to compare the difference between 2 groups and 1-way ANOVA for the multiple groups. The rates were compared using the crosstabs χ2 test. The ROC curve analysis was performed for determining the cut-off value for diagnosis. A P value of less than 0.05 was considered statistically significant.
3. Results
3.1. The causes of super BMU
Of 31 patients, the super BMU was confirmed to be caused by the bone marrow MI in 29 (93.5%) ones. The malignant diseases were diagnosed to be 14 lymphomas, 11 leukemia, 3 multiple myelomas, and 1 bone metastasis (Table 1). Among them, nearly all (96.5% [28/29]) of malignant diseases were the hematopoietic malignancies, particularly the lymphoma (45.2%) and leukemia (35.5%) (Table 1). All the leukemia were acute ones, and most of the lymphomas (71.4%) were the high aggressive ones, such as T-cell lymphoma, NK/T-cell lymphoma, diffuse large-B-cell lymphoma, and Burkitt lymphoma (Table 1). Infectious BM changes were identified by the BMB in the remaining 2 patients. The primary diseases in both patients were the adult Still disease and the severe infections of G bacillus and Candida albicans (Table 1).
Table 1.
The pathology of the patients with super bone marrow uptake (n = 31).
3.2. The findings of 18F-FDG PET/CT in the patients with super BMU
In 31 patients with super BMU, the 18F-FDG uptake in the bone marrow was markedly higher (Figs. 1–4), which was found to be superior to that of the cerebellum in 24 patients and slightly lower in 7 patients. It was approximately 5 to 6 times higher than that in the control subjects (Fig. 1 and Table 2). The BM/C ratios of super BMU patients reached a high level of 1.24 ± 0.36. When compared with the normal control group and mildly and moderately uptake group, both SUVmax in the BM and BM/C ratios showed significantly higher (all P <0.001) (Table 2 and Fig. 1). There was no significant difference in 18F-FDG uptake in the BM between the lymphoma and leukemia, neither the SUVmax (P = 0.406) nor the BM/C ratios (P = 0.942) (Table 3 and Fig. 2). The density of bone marrow was noted to be normal in nearly all of the patients except 2 patients with MM. In both MM patients, a considerable amount of osteolytic lesions was found in the BM on the images of CT.
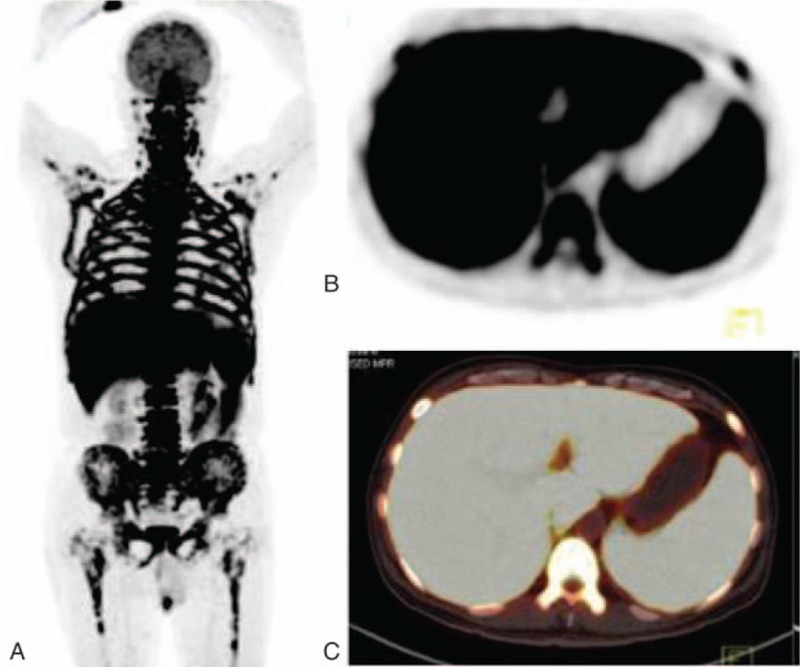
Figure 4.
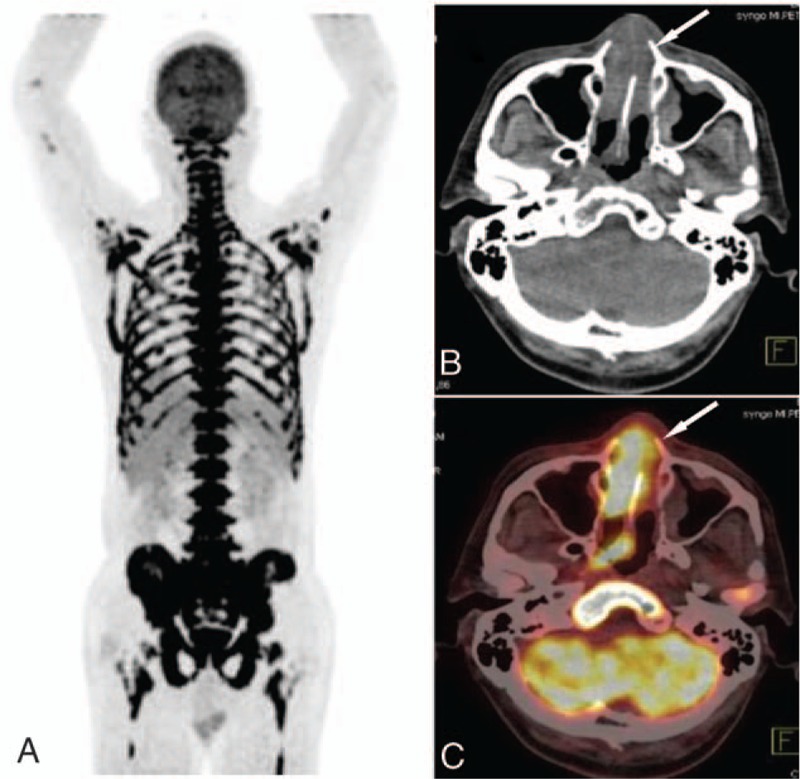
The images of a patient with lymphoma. A, The MIP image of super BMU with SUVmax 11.05. The CT image (B) and PET/CT fused image (C) showed the nasal cavity involvement (arrow) with an intense 18F-FDG uptake (SUVmax 10.6). 18F-FDG = fluorine-18 fluoro-D-glucose, BMU = bone marrow uptake, CT = computed tomography, MIP = maximum intensity projection, PET/CT = positron emission tomography/computed tomography, SUVmax = maximum standardized uptake value.
Table 2.
SUVmax and BM/C of BM in the patients with abnormal uptake and normal subjects.
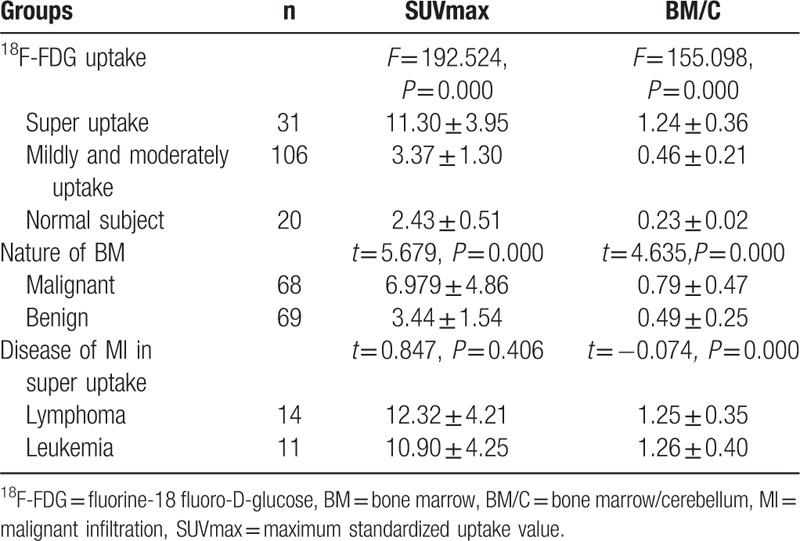
Table 3.
The specificity and sensitivity of BM/C and SUVmax for diagnosis of bone marrow MI.
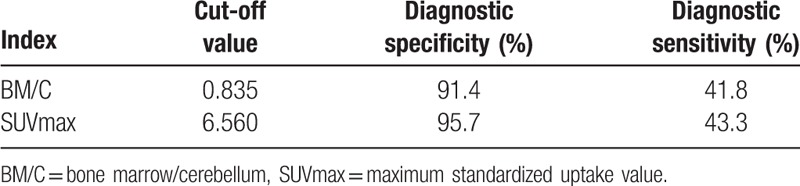
Figure 2.
The MIP images of patient with NK/T Lymphoma (A) and leukemia (B). A, 18F-FDG uptake in the BM was markedly increased, which was higher than that in the brain with SUVmax 11.05 and BM/C ratios 1.23. The patient also had the decrease in-the-blood hemogram (WBC, 1.10 × 106; HB, 6.0 g/d; PLT, 19 × 106). B, 18F-FDG uptake in the BM was markedly increased, which was similar to the brain with SUVmax 10.0 and BM/C ratios 1.02. The peripheral WBC count was increased (140.6 × 106), while the HB and PLT were decreased (HB, 13 1 g/dL; PLT, 114 × 106). 18F-FDG = fluorine-18 fluoro-D-glucose, BM = bone marrow, BM/C = bone marrow/cerebellum ratio, HB = hemoglobin, MIP = maximum intensity projection, PLT = platelate, SUVmax = maximum standardized uptake value, WBC = white blood cell.
Extra-BM malignant lesions were found in 15 patients with bone marrow MI, including 10 lymphomas and 5 leukemia. The lesions involved the spleen, lymph node, liver, nasal cavity, lung, parotid gland, pancreas, adrenal gland, kidney, and tonsil in 10, 7, 6, 5, 4, 2, 1, 1, 1, and 1 patients respectively. 18F-FDG uptake in the extra-BM malignant lesions was also very intense and the 18F-FDG uptake in the intro- and extra- bone marrow malignant lesions showed no significant difference (SUVmax, 11.3 ± 3.95 vs 10.3 ± 5.64, t = 0.862, P = 0.391).
The extra-BM involvements showed some differences between leukemia and lymphoma. More extra-BM organs were found to be involved by lymphoma, including the spleen, lymph node, liver, nasal cavity, lung, parotid gland, pancreas, adrenal gland, and tonsil. However, only the spleen, lymph node, and kidney were involved by leukemia. The liver and the nasal cavity involvements were only seen in the patients with lymphoma, but not leukemia. There were significant differences of liver and nasal cavity involvements between the lymphoma and leukemia groups (6/14 vs 0/11, P = 0.020; 5/14 vs 0/11, P = 0.046) (Figs. 3, 4).
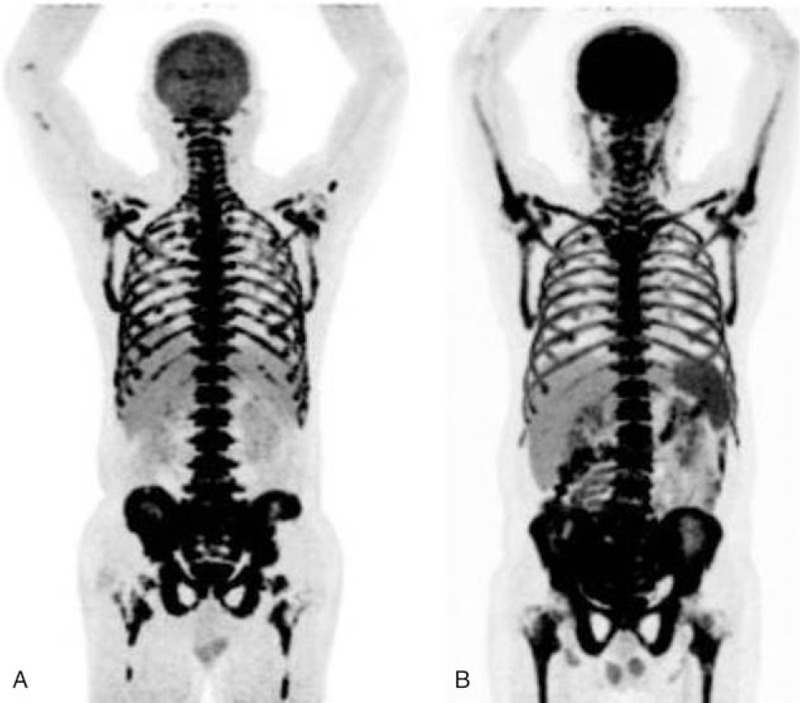
Figure 3.
The images of a patient with lymphoma. A, The MIP image of super BMU with SUVmax 16.6. PET image (B) and PET/CT fused image (C) showed 18F-FDG uptake was intense in the liver and spleen involvements with SUVmax of 13.7 and 10.2, respectively. 18F-FDG = fluorine-18 fluoro-D-glucose, BMU = bone marrow uptake, MIP = maximum intensity projection, PET/CT = positron emission tomography/computed tomography, SUVmax = maximum standardized uptake value.
3.3. Super BMU as an indicator for diagnosis of bone marrow MI
To determine whether the super BMU had a high specificity for diagnosis of bone marrow MI, 106 patients with mildly and moderately uptake of 18F-FDG in BM were also collected for analysis, which included 61 patients with primary malignant diseases and 45 ones with benign diseases. Of 61 patients with malignant diseases, 39 were finally confirmed to have bone marrow MI by BMB, including 12 lymphomas, 25 leukemias, and 2 multiple myelomas. Therefore, of the total 137 patients with abnormal bone marrow uptake, 68 were diagnosed to have bone marrow MI, including 29 ones of Super BMU and 39 ones of mild and moderate uptake groups, while other 69 patients had benign BM. The SUVmax and BM/C of the bone marrow MI were found to be significantly higher than those of benign ones (all P <0.000, Table 2).
Among patients with abnormal bone marrow uptake, the incidence of MI in super BMU group showed significantly higher than that of mildly and moderately uptake (93.5% vs 36.8%, P = 0.000, Fisher exact test). Two ROC curves of BM/C and SUVmax (Fig. 5A and B) were plotted to analyze the diagnostic efficiency for bone marrow MI in the patients with abnormal BM 18F-FDG uptake. From the curves, when cut-off values of BM/C and SUVmax were settled at 0.835 and 6.560, the diagnostic specificities reached the high level of 91.4% and 95.7%, respectively, although the sensitivities were low (41.8% and 43.3%, respectively) (Table 3).
Figure 5.
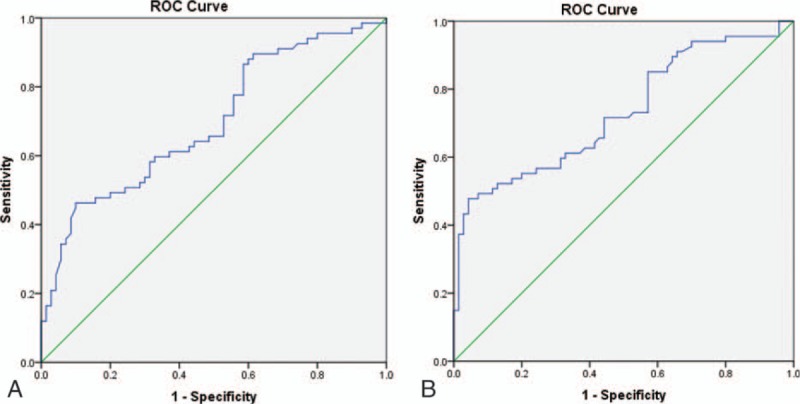
The ROC curve of BM/C (A) and SUVmax (B) for diagnosis of bone marrow MI. BM/C = bone marrow/cerebellum ratio, MI = malignant infiltration, ROC = receiver operating characteristic, SUVmax = maximum standardized uptake value.
3.4. The findings of blood parameters and body temperature in the patients with super BMU
In the patients with super BMU caused by MI, decreases of WBC, HB, and PLT counts were found in 48.4%, 86.2%, and 51.5% of patients, which included 71.4%, 78.6%, 64.3% decrease in lymphoma patients and 45.4%, 90.9%, 45.4% decrease in leukemia patients. An increase of WBC count was found in 22.6% patients including 36.4% (4/11) leukemia, 1 MM, and 2 benign diseases. In the super BMU group, an increase of LDH and CRP was found in 70.9% patients (68.9% of malignant BMI and 2/2 benign disease) and 83.8% patients (82.7% of malignant BMI and 2/2 benign disease), respectively. When compared with the normal subjects, both of the super BMU and mildly and moderately uptake groups showed significantly lower HB and PLT counts and significantly higher LDH, and CRP (all P <0.001) (Table 4), especially the super BMU. However, no significant difference was found in the WBC count between 3 groups (P = 0.432) (Table 4). Lower levels of HB and PLT were also found in the patients with bone marrow MI, compared with that of benign BM uptake (all P <0.01) (Table 4). On the other hand, no significant differences of LDH and CRP were observed between the malignant and benign bone marrow (all P >0.05) (Table 4), neither WBC (P >0.05) (Table 4).
Table 4.
The blood parameters in the patients with different level 18F-FDG uptake in BM and different nature of BM.

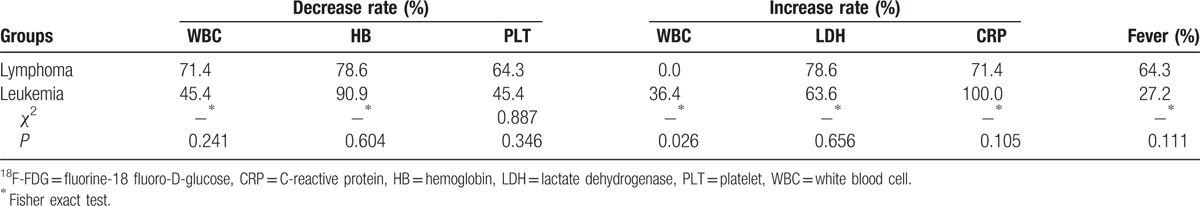
The levels of HB, PLT, LDH, CRP, and the fever incidence were not significantly different between the patients with lymphoma and those with leukemia (all P >0.05) (Table 5). However, the increase of WBC counts was only seen in the patients with leukemia, but none in lymphoma (P = 0.026) (Table 5).
Table 5.
The 18F-FDG metabolism in the patients with lymphoma and leukemia.
4. Discussion
“Super Scan” is a name, which was used to definite a phenomenon of strikingly increased uptake of 99mTc-methylene diphosphonate in the bone on the bone scintigraphy with diminished soft tissue activity, faint right kidney visualization, and bladder activity.[23] This phenomenon was described mostly in the diffuse bone metastases and sometimes in the discrete endocrine entities.[25,26] In the present study, we used the similar name of “super BMU” to define a similar status of markedly increased uptake of 18F-FDG in the BM on the PET/CT. To intuitively reflect the increased uptake level, we used the brain as the reference because the uptake of 18F-FDG in the brain is often very high and stable, particularly the cerebellum.[27] In the present study, it was found that SUVmax of super BMU was approximately 5 to 6 time higher than that in the normal BM and the BM/C ratios reached a high level of 1.24 ± 0.36.
Mildly and moderately 18F-FDG BMU is a common phenomenon in clinical PET/CT practice.[13,14,21] Nevertheless, super BMU is a rare one. Although mildly and moderately 18F-FDG BMU does not appear to be a useful tool to assess bone marrow MI, because it is associated with various conditions including not only the involvement of malignancy[13,14,28–30] but also the benign BM hyperplasia resulting from a variety of origins.[17–19,31–33] It was confirmed by the present study that only 36.8% of mildly and moderately uptake in bone marrow was caused by bone marrow MI. However, there was still a trend that low uptake often indicates the myeloid hyperplasia while the high uptake is often correlated with the malignant involvement.[28,34] Sach pekidis C reported that the bone marrow MI confirmed by BMB was positively correlated with SUVave and SUVmax (P <0.01).[28] Therefore, we put forward a reasonable assumption that a marked uptake of 18F-FDG in BM might indicate a higher possibility of bone marrow MI. In the present study, it was demonstrated that the incidence of bone marrow MI in the super BMU group was actually markedly higher than that of mildly and moderately uptake (93.5% vs 36.8%, P = 0.000, Fisher exact test). Based on the ROC analysis, when cut-off values of BM/C and SUVmax were settled at 0.835 and 6.560, the diagnostic specificity reached the high level of 91.4% and 95.7%, respectively, which indicated that super BMU was a highly specific indicator for diagnosing the marrow MI, although the sensitivities were low for all the patients with abnormal BM uptake (41.8% and 43.3%, respectively).
The present study also demonstrated that nearly all 96.5% (28/29) bone marrow MIs in the super BMU patients were originated from malignant hematological disease, particularly lymphoma and leukemia. Leukemia is a primary malignant disease of the BM.[35] With the rapid increase in the number of immature blood cells, it was reported that the18F-FDG uptake in BM in the leukemia was high and diffuse, especially in the patients with acute leukemia, which possibly reflects the leukemic cell activity.[13,36] Although primary BM lymphoma is rare,[37] the incidence of bone marrow involvement was reported to be relatively high in patients with lymphoma, and some of them can also present as diffuse BMU.[14,22,38] The strikingly high18F-FDG uptake may represent a markedly high activity of cell proliferation.[15,39] In our study, the super BMU actually mainly occurred in patients with acute leukemia and highly aggressive lymphoma, such as diffuse large-B-cell lymphoma, T-cell lymphoma, and NK/T lymphoma, but not in the patients with chronic leukemia and seldom in those with indolent lymphoma, which indicated that super BMU was correlated with highly aggressive property of malignancies in the bone marrow.
It is hard to differentiate whether the super BMU was caused by the lymphoma or leukemia without the BMB. In the present study, no significant difference of BM SUVmax and BM/C ratios was observed between leukemia and lymphoma (both P >0.05). Although the serum LDH was reported to be useful for evaluating the lymphoma,[40] the present study showed high LDH levels also occurred in the most of the patients with leukemia, which made it impossible for the differentiation. The limitation for the differentiation was also found in HB, PLT, CRP, and the fever incidence (all P >0.05). However, our study showed extra-BM involvements detected by PET/CT might provide some clues for the differentiation. More extra-BM involvements were often found in-patient with lymphoma. Meanwhile, the liver and nasal cavity involvements often prompt the lymphoma, which was only seen in the patients with lymphoma, but not leukemia.
In the present study, a deviated phenomenon was observed, which displayed with the markedly high 18F-FDG uptake in BM and a decrease of peripheral blood cells. In patients with lymphoma, the reduction of WBC, HB, and PLT was found in more than 70% of patients, especially HB. In about 50% patients with leukemia, the decrease of WBC, HB, and PLT was also noted. This phenomenon might be caused by the rapid and vast increase in the number of malignant cells in the bone marrow. As a result, the normal bone marrow becomes smaller and smaller, and the production of the healthy blood cells will be greatly impaired which leads to the decrease in the peripheral blood cells.[41] The present study indicated the decrease of peripheral blood cells in a patient with a super BMU might prompt the bone marrow MI, especially HB and PLT. In the present study, lower levels of HB and PLT were found in the patients with bone marrow MI, compared with that of benign ones. Meanwhile, they showed much lower in the super BMU than those in mildly and moderately uptake. In the present study, an increase of peripheral WBC was found in 4 of 11 patients with leukemia and 2 patients with adult-onset still's disease and severe infection. The former might be contributed by spilling over of the leukemia cells into the bloodstream from the BM, and the latter might be due to high degree myeloid hyperplasia, which was also reported by the previous report.[18]
There were some limitations in the present study. First, the sample size of super BMU patients was a little small, so in the future, more research with larger sample size is needed to confirm the finding of the present study. Second, due to the shortcoming of a retrospective study, the prospective study, particularly the multicenters study, is warranted.
5. Conclusions
The current study was the first time to give a name of 18F-FDG super BMU and illuminate its origins. It revealed that super BMU was a highly potent indicator for the bone marrow MI which mostly originated from lymphoma and leukemia. In most of the cases, it reflected the highly aggressive proliferation of malignant cells in BM. Super BMU accompanied by the decrease of peripheral blood cells might prompt the bone marrow MI. The liver and nasal cavity involvement detected by PET/CT could provide some clues for the differentiation between the lymphoma and leukemia patients with super BMU, although the definite diagnosis could not be established without the BM biopsy.
Acknowledgments
The authors thank their colleagues in Nanfang PET center, who manufactured the radiopharmaceutical and performed the PET/CT scans. The authors also give the appreciation to the colleagues in the Department of Hematology and Pathology in Nanfang Hospital, who provided the follow-up data and pathologic diagnosis.
Footnotes
Abbreviations: BM/C = bone marrow/cerebellum ratio, LDH = lactate dehydrogenase, MI = malignant infiltration, PET/CT = positron emission tomography/computed tomography, PLT = platelate, SBMU = super bone marrow uptake, SUVmax = maximum standardized uptake value.
MSA and LF contributed equally to this work.
Financial support: National Natural Science Foundation Project of China (81071175, 81371591).
The authors have no conflicts of interest to disclose.
References
- [1].Jeraj R, Bradshaw T, Simončič U. Molecular imaging to plan radiotherapy and evaluate its efficacy. J Nucl Med 2015;56:1752–65. [DOI] [PubMed] [Google Scholar]
- [2].Goense L, van Rossum PS, Reitsma JB, et al. Diagnostic performance of 18F-FDG PET and PET/CT for the detection of recurrent esophageal cancer after treatment with curative intent: a systematic review and meta-analysis. J Nucl Med 2015;56:995–1002. [DOI] [PubMed] [Google Scholar]
- [3].Konert T, Vogel W, MacManus MP, et al. PET/CT imaging for target volume delineation in curative intent radiotherapy of non-small cell lung cancer: IAEA consensus report 2014. Radiother Oncol 2015;116:27–34. [DOI] [PubMed] [Google Scholar]
- [4].Kaneko Y, Murray WK, Link E, et al. Improving patient selection for 18F-FDG PET scanning in the staging of gastric cancer. J Nucl Med 2015;56:523–9. [DOI] [PubMed] [Google Scholar]
- [5].Meignan M, Itti E, Gallamini A, et al. FDG PET/CT imaging as a biomarker in lymphoma. Eur J Nucl Med Mol Imaging 2015;42:623–33. [DOI] [PubMed] [Google Scholar]
- [6].Yoon SO, Jeon TJ, Park JS, et al. Analysis of the roles of glucose transporter 1 and hexokinase 2 in the metabolism of glucose by extrahepatic bile duct cancer cells. Clin Nucl Med 2015;40:e178–82. [DOI] [PubMed] [Google Scholar]
- [7].Alvarez JV, Belka GK, Pan TC, et al. Oncogene pathway activation in mammary tumors dictates FDG-PET uptake. Cancer Res 2014;74:7583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jo MS, Choi OH, Suh DS, et al. Correlation between expression of biological markers and [F]fluorodeoxyglucose uptake in endometrial cancer. Oncol Res Treat 2014;37:30–4. [DOI] [PubMed] [Google Scholar]
- [9].Kang F, Ma W, Ma X, et al. Propranolol inhibits glucose metabolism and 18F-FDG uptake of breast cancer through posttranscriptional downregulation of hexokinase-2. J Nucl Med 2014;55:439–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vineeth Kumar PM, Verma GR, Mittal BR, et al. FLT PET/CT is better than FDG PET/CT in differentiating benign from malignant pancreatobiliary lesions. Clin Nucl Med 2016;41:e244–50. [DOI] [PubMed] [Google Scholar]
- [11].Culverwell AD, Scarsbrook AF, Chowdhury FU. False-positive uptake on 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) positron-emission tomography/computed tomography (PET/CT) in oncological imaging. ClinRadiol 2011;66:366–82. [DOI] [PubMed] [Google Scholar]
- [12].Basu S, Saboury B, Werner T, et al. Clinical utility of FDG-PET and PET/CT in non-malignant thoracic disorders. Mol Imaging Biol 2011;13:1051–60. [DOI] [PubMed] [Google Scholar]
- [13].Arimoto MK, Nakamoto Y, Nakatani K, et al. Increased bone marrow uptake of 18F-FDG in leukemia patients: preliminary findings. Springerplus 2015;4:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adams HJ, Kwee TC, Fijnheer R, et al. Diffusely increased bone marrow FDG uptake in recently untreated lymphoma: incidence and relevance. Eur J Haematol 2015;95:83–9. [DOI] [PubMed] [Google Scholar]
- [15].Cerci JJ, Györke T, Fanti S, et al. Combined PET and biopsy evidence of marrow involvement improves prognostic prediction in diffuse large B-cell lymphoma. J Nucl Med 2014;55:1591–7. [DOI] [PubMed] [Google Scholar]
- [16].Djelbani-Ahmed S, Chandesris MO, Mekinian A, et al. FDG-PET/CT findings in systemic mastocytosis: a French multicentre study. Eur J Nucl Med Mol Imaging 2015;42:2013–20. [DOI] [PubMed] [Google Scholar]
- [17].Dong MJ, Wang CQ, Zhao K, et al. (18)F-FDG PET/CT in patients with adult-onset Still's disease. ClinRheumatol 2015;34:2047–56. [DOI] [PubMed] [Google Scholar]
- [18].Dong A, Wang Y, Gao L, et al. Increased FDG uptake of the bone marrow mimicking malignancy in a patient of eosinophilia secondary to Sparganum mansoni infection. Clin Nucl Med 2014;39:640–2. [DOI] [PubMed] [Google Scholar]
- [19].Blodgett TM, Ames JT, Torok FS, et al. Diffuse bone marrow uptake on whole-body F-18 fluorodeoxyglucose positron emission tomography in a patient taking recombinant erythropoietin. Clin Nucl Med 2004;29:161–3. [DOI] [PubMed] [Google Scholar]
- [20].Sugawara Y, Fisher SJ, Zasadny KR, et al. Preclinical and clinical studies of bone marrow uptake of fluorine-1-fluorodeoxyglucose with or without granulocyte colony-stimulating factor during chemotherapy. J Clin Oncol 1998;16:173–80. [DOI] [PubMed] [Google Scholar]
- [21].Inoue K, Goto R, Okada K, et al. A bone marrow F-18 FDG uptake exceeding the liver uptake may indicate bone marrow hyperactivity. Ann Nucl Med 2009;23:643–9. [DOI] [PubMed] [Google Scholar]
- [22].Salaun PY, Gastinne T, Bodet-Milin C, et al. Analysis of 18F-FDG PET diffuse bone marrow uptake and splenic uptake in staging of Hodgkin's lymphoma: a reflection of disease infiltration or just inflammation? Eur J Nucl Med Mol Imaging 2009;36:1813–21. [DOI] [PubMed] [Google Scholar]
- [23].Liu Y. Super-superscan on a bone scintigraphy. Clin Nucl Med 2011;36:227–8. [DOI] [PubMed] [Google Scholar]
- [24].Higginbotham EA. The “super” bone scan. J Natl Med Assoc 1980;72:291–4. [PMC free article] [PubMed] [Google Scholar]
- [25].Shikino K, Ikusaka M, Hirota Y, et al. Super bone scan: bone metastases of prostate cancer. BMJ Case Rep 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shinya T, Akaki S, Ogata T, et al. Super scan in a patient with diffuse bone metastases from intracranial glioma. Clin Nucl Med 2007;32:481–3. [DOI] [PubMed] [Google Scholar]
- [27].Britz-Cunningham SH, Millstine JW, Gerbaudo VH. Improved discrimination of benign and malignant lesions on FDG PET/CT, using comparative activity ratios to brain, basal ganglia, or cerebellum. Clin Nucl Med 2008;33:681–7. [DOI] [PubMed] [Google Scholar]
- [28].Sachpekidis C, Mai EK, Goldschmidt H, et al. (18)F-FDG dynamic PET/CT in patients with multiple myeloma: patterns of tracer uptake and correlation with bone marrow plasma cell infiltration rate. Clin Nucl Med 2015;40:e300–7. [DOI] [PubMed] [Google Scholar]
- [29].Balink H, Nabers H, Kibbelaar RE. High F-18 FDG uptake in bone marrow by cytokines secreting ectopic mucoepidermoid carcinoma. Clin Nucl Med 2009;34:823–4. [DOI] [PubMed] [Google Scholar]
- [30].Seshadri N, Wright P, Balan KK. Rhabdomyosarcoma with widespread bone marrow infiltration: beneficial management role of F-18 FDG PET. Clin Nucl Med 2007;32:787–9. [DOI] [PubMed] [Google Scholar]
- [31].Mostard RL, Prompers L, Weijers RE, et al. F-18 FDG PET/CT for detecting bone and bone marrow involvement in sarcoidosis patients. Clin Nucl Med 2012;37:21–5. [DOI] [PubMed] [Google Scholar]
- [32].Ruiz-Hernández G, Scaglione C, Delgado-Bolton RC, et al. Splenic and bone marrow increased 18F-FDG uptake in a PET scan performed following treatment with G-CSF/granulocyte colony-stimulating factor. Rev Esp Med Nucl 2004;23:124–6. [DOI] [PubMed] [Google Scholar]
- [33].Yi C, Shi X, Wang X, et al. The alteration of 18F-FDG uptake in bone marrow after treatment with interleukin 11. Clin Nucl Med 2014;39:934–5. [DOI] [PubMed] [Google Scholar]
- [34].Berthet L, Cochet A, Kanoun S, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med 2013;54:1244–50. [DOI] [PubMed] [Google Scholar]
- [35].Zhou WL, Wu HB, Wang LJ, et al. Usefulness and pitfalls of F-18-FDG PET/CT for diagnosing extramedullary acute leukemia. Eur J Radiol 2016;85:205–10. [DOI] [PubMed] [Google Scholar]
- [36].Su K, Nakamoto Y, Nakatani K, et al. Diffuse homogeneous bone marrow uptake of FDG in patients with acute lymphoblastic leukemia. Clin Nucl Med 2013;38:e33–4. [DOI] [PubMed] [Google Scholar]
- [37].Chang H, Hung YS, Lin TL, et al. Primary bone marrow diffuse large B cell lymphoma: a case series and review. Ann Hematol 2011;90:791–6. [DOI] [PubMed] [Google Scholar]
- [38].Lee Y, Hwang KH, Hong J, et al. Usefulness of (18)F-FDG PET/CT for the evaluation of bone marrow involvement in patients with high-grade non-Hodgkin's lymphoma. Nucl Med Mol Imaging 2012;46:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Paone G, Itti E, Haioun C, et al. Bone marrow involvement in diffuse large B-cell lymphoma: correlation between FDG-PET uptake and type of cellular infiltrate. Eur J Nucl Med Mol Imaging 2009;36:745–50. [DOI] [PubMed] [Google Scholar]
- [40].Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McKenna RW, Bloomfield CD, Brunning RD. Nodular lymphoma: bone marrow and blood manifestations. Cancer 1975;36:428. [DOI] [PubMed] [Google Scholar]


