Supplemental Digital Content is available in the text
Keywords: biomarker, cardiac troponin, meta-analysis, mortality, survival
Abstract
Interest in the use of cardiac troponin T (cTnT) and cardiac troponin I (cTnI) has expanded from diagnosis of acute myocardial infarction to risk assessment for morbidity and mortality. Although cTnT and cTnI were shown to have equivalent diagnostic performance in the setting of suspected acute myocardial infarction, potential prognostic differences are largely unexplored.
The aim of this study is to quantify and compare the relationship between cTnT and cTnI, and cardiovascular and all-cause mortality in the general population.
Medline, Embase, and the Cochrane Library (from inception through October 2016) were searched for prospective observational cohort studies reporting on the prognostic value of basal high-sensitive cTnT and/or cTnI levels on cardiovascular and all-cause mortality in the general population. Data on study characteristics, participants’ characteristics, outcome parameters, and quality [according to the Effective Public Health Practice Project (EPHPP) “Quality Assessment Tool For Quantitative Studies] were retrieved. Hazard ratios per standard deviation increase in basal cardiac troponin level (HR per 1-SD; retrieved from the included articles or estimated) were pooled using a random-effects model.
On a total of 2585 reviewed citations, 11 studies, with data on 65,019 participants, were included in the meta-analysis. Random effects pooling showed significant associations between basal cardiac troponin levels and HR for cardiovascular and all-cause mortality [HR per 1-SD 1.29 (95% confidence interval, 95% CI, 1.20–1.38) and HR per 1-SD 1.18 (95% CI, 1.11–1.26), respectively]. Stratified analyses showed higher HRs for cTnT than cTnI [cardiovascular mortality: cTnT HR per 1-SD 1.37 (95% CI, 1.23–1.52); and cTnI HR per 1-SD 1.21 (95% CI, 1.16–1.26); all-cause mortality: cTnT HR per 1-SD 1.31 (955 CI, 1.13–1.53); and cTnI HR per 1-SD 1.14 (95% CI, 1.06–1.22)]. These differences were significant (P < 0.01) in meta-regression analyses for cardiovascular mortality but did not reach statistical significance for all-cause mortality.
Elevated, basal cTnT, and cTnI show robust associations with an increased risk of cardiovascular and all-cause mortality during follow-up in the general population.
Systematic review registration number PROSPERO CRD42014006964.
1. Introduction
Cardiac troponins are the preferred biomarkers in the diagnostic work up for non-ST elevation myocardial infarction (NSTEMI).[1,2] Troponin assays target either cardiac troponin T or cardiac troponin I, both sarcomere components of the heart.[3] The release of cardiac troponin in the peripheral blood is strongly associated with myocardial injury.[4] The recent introduction of high-sensitive cardiac troponin assays has not only expedited the early diagnosis of NSTEMI but also resulted in the detection of previously unnoticed cardiac troponin levels in various patient groups without acute cardiac injury such as chronic kidney disease, chronic heart failure, and even in apparently healthy subjects.[5,6] Subsequently, several studies have shown that even these minimal increases of cardiac troponin T and I are associated with unfavorable outcomes in various patient groups.[6–10]
The current guidelines recommend either cardiac troponin T and I for de diagnosis of acute myocardial infarction, suggesting equivalent diagnostic performance of troponin T and I in the acute situation.[11] In contrast, cardiac troponin T seems to have greater prognostic accuracy than cardiac troponin I in the acute setting.[12] Potential differences in prognostic value between basal cardiac troponin T and I concentrations in subjects from the general population are largely unexplored. In addition, the quantitative relationship between elevated cardiac troponin levels, also below the 99th percentile, and the magnitude of risk for adverse events has not been systematically assessed.
To address these knowledge gaps, we conducted a systematic review and meta-analysis of the available evidence from published prospective cohort studies. Specifically, we quantified the relationship between basal levels of high-sensitive cardiac troponins and cardiovascular and all-cause mortality during follow-up in the general population. In addition, the prognostic performance of high-sensitive cardiac troponin T and I assays was separately assessed and compared.
2. Methods
2.1. Data sources and searches
Medline, Embase, and the Cochrane Library were searched from inception through October 2016. Also, the reference lists of all relevant articles and Web of Science (for prospective citations of key publications) were checked for any additional articles. The PubMed search terms were (mortality OR death OR “Mortality”[Mesh]) AND (troponin OR “Troponin”[Mesh]) AND (predictive OR prediction OR prognostic OR prognosis OR “Prognosis”[Mesh] risk OR “Risk”[Mesh]) AND (follow-up OR prospective OR cohort). We adapted this search strategy for searches of Embase and the Cochrane Library. The search was restricted to English, Dutch, French, and German documents.
The protocol for this study was published on the International Prospective Register of Systematic Reviews, or PROSPERO. The registration number was CRD42014006964. Due to the design of the present study, ethical approval was not required. All included studies were approved by the notified ethics committees and institutional review boards.
2.2. Study selection
Study selection was performed by 2 independent investigators (NvdL and LK). We included all prospective cohort studies that evaluated the prognostic value of basal high-sensitive cardiac troponin T and I levels in subjects from the general population (without any suspected acute event or surgery at the time of sampling) for cardiovascular and all-cause mortality during follow-up.
Studies were excluded from the meta-analysis when the duration of follow-up was less than 1 year, hazard ratios (HRs) for cardiovascular or all-cause mortality were not provided [either as HR per stratum, or as HR per 1-standard deviation (SD) increase in logarithmic transformed cardiac troponin concentrations], or when HRs were not adjusted for conventional cardiovascular risk factors [at least for age, sex, smoking, hypertension (or systolic blood pressure), diabetes mellitus (or glucose levels), and dyslipidemia (or levels of total and high-density lipoprotein, HDL cholesterol)]. When duplicate publications of data were encountered, only results from the most recent publication were considered. Discrepancies between reviewers were resolved in the presence of a third reviewer (SM). All authors that performed search and selection were trained in systematic review and meta-analysis methods.
2.3. Data extraction and quality assessment
We extracted data using a customized and validated extraction form. Data on study characteristics (authors, publication year, journal, study design, sample size, country, and duration of follow-up), participants’ characteristics [age, sex, history of cardiovascular disease (CVD), hypertension, dyslipidemia, diabetes], cardiovascular and all-cause mortality rate, cardiac troponin assay, HRs for cardiovascular, and all-cause mortality, the factors for which the HRs had been adjusted for, and C-statistics and reclassification were retrieved. When multiple cardiac troponin assays of the same kind (cardiac troponin I or T) were presented, we used the data from the assay that was commercially available and most commonly used in clinical practice for our analyses. Two authors (NvdL and LK) performed the data-extraction separately. Discrepancies between reviewers were resolved in THE presence of a third reviewer (SM).
To assess study quality, each study was evaluated for selection bias, study design, confounders, blinding, data collection methods, and withdrawals/dropouts according to the Effective Public Health Practice Project (EPHPP) “Quality Assessment Tool For Quantitative Studies.”[13] Two authors (NvdL and LK) performed quality assessment separately, and disagreements were resolved by consensus in the presence of a third review author (SM)
2.4. Data synthesis and analysis
To quantitatively assess the relationship between basal cardiac troponin levels and cardiovascular and all-cause mortality during follow-up, we used HR per 1-SD increase in logarithmic transformed cardiac troponin levels. When HRs were provided for stratified cardiac troponin concentrations only, we mathematically derived HR per 1-SD increase in logarithmic transformed cardiac troponin concentration. This estimation was based on the assumption that logarithmic transformed cardiac troponin levels are normally distributed in the population,[14–16] and consisted of the following 3 steps: we extracted the cumulative distribution of the study population and calculated the associated z-scores on the basis of the cumulative standard normal distribution, we calculated the mean and SD based on the z-scores [z = (x-μ)/σ, where x is the log-transformed cardiac troponin concentration acting as the upper limit of the stratum (continuity correction was performed), μ is the mean, and σ is the SD], and we constructed a biomarker concentration-risk curve (x-axis: log-transformed medians for the strata, y-axis: associated HRs) for which we calculated the slope (HR/log cardiac troponin) and transformed this into HR per 1-SD increase in logarithmic transformed cardiac troponin level. This method was validated in 2 studies that reported HRs for both stratified data and log-transformed cardiac troponin as a continuous variable.[17,18] We found a minimal differences between the reported and the calculated HR per 1-SD in these 2 studies (2–8% difference).
For the meta-analysis, we used full-model adjusted HRs [at least adjusted for the conventional cardiovascular risk factors age, sex, smoking, hypertension (or systolic blood pressure), diabetes mellitus (or glucose levels), and dyslipidemia (or levels of total and HDL cholesterol]. As a result of this approach, the number of adjusting factors in the full-model differs between studies. Nevertheless, this method ensures that we correct for all significant confounders in the individual studies. As cardiac troponins are strongly associated with CVD, the set of adjusting factors mainly consists of cardiovascular risk factors. The adjusted HRs per 1-SD were pooled using a random-effects model and summarized using forest plots.
Examination of the impact of potential moderator variables on the study effect size was done by meta-regression analyses. Potential moderator variables included type of cardiac troponin assay, calculated versus reported HRs, study quality, geographic region, and mean age of the population. A sensitivity analysis was applied to examine the effect of the inclusion of studies, which did not adjust for the minimal set of 6 conventional risk factors, on the robustness of the pooled results.
The MOOSE guidelines[19] for meta-analysis of observational studies were followed, and PRISMA criteria were performed for the search methodology (Fig. 1). Publication bias was evaluated using Egger regression. Heterogeneity between studies was estimated using I2.[20]
Figure 1.
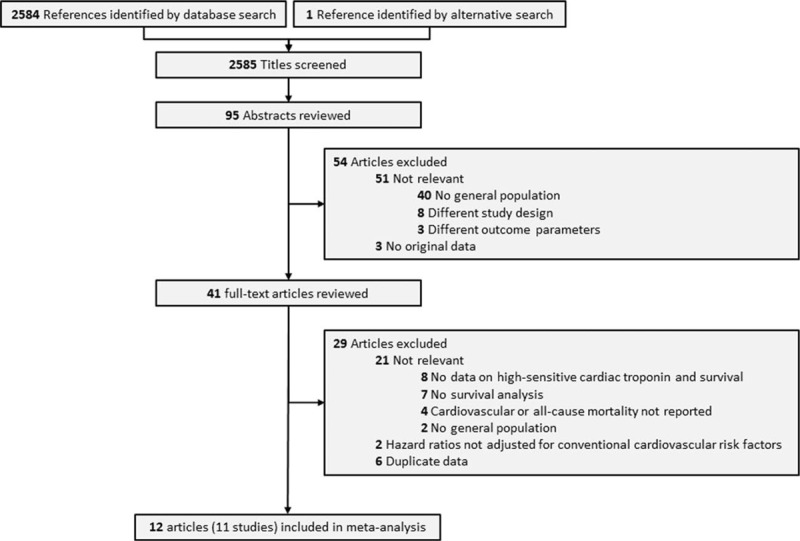
Flow chart of the study selection.
For all analyses, a 2-tailed P value less than 0.05 was considered statistically significant. All statistical analyses were performed with the Stata software package (Stata/IC version 13.1; Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
3. Results
3.1. Study characteristics
The literature search protocol is depicted in Fig. 1. Our initial search identified 2585 citations. On the basis of titles and abstracts, 41 articles were considered potentially eligible. After full text evaluation, 29 articles were excluded: 8 articles did not report HRs for high-sensitive cardiac troponin concentrations and survival, 7 did not perform a survival analysis, 4 did not report on cardiovascular or all-cause mortality as outcome measures, 2 were not performed in the general population, and 6 articles reported data that were also published in more recent articles. In 2 studies, the reported HRs were not adjusted for the minimal list of conventional risk factors. As the adjustment for these factors is one of the inclusion criteria, those studies were excluded. Hence, a total of 11 studies (12 articles) (65,019 participants) were eventually included in the meta-analysis; 7 studies (45,956 participants) for cardiovascular mortality[15,17,21–25] and 10 studies (48,679 participants) for all-cause mortality.[15,17,18,22–24,26–29] Five studies reported HRs related to cardiac troponin T levels,[17,18,21–23,26] and another 5 studies reported on cardiac troponin I levels.[15,24,25,27–29] Clinical and demographic characteristics are summarized in Table 1. The median follow-up duration of all studies was between 3.8 and 20 years. The percentage of individuals with detectable cardiac troponin levels varied from 27% to 96%.
Table 1.
Study characteristics.
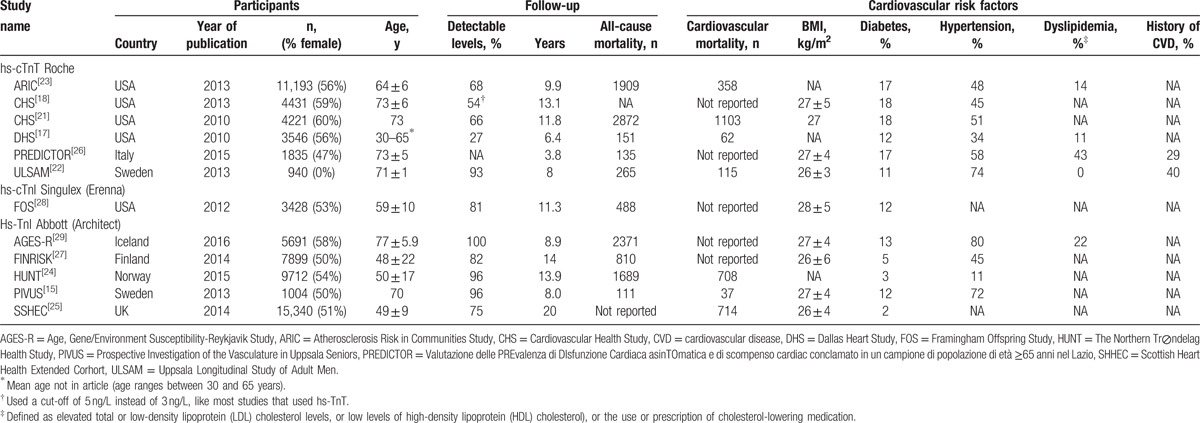
The study quality varied across studies. According to the EPHPP quality assessment tool,[13] 1 study was of high methodological quality,[27] 8 studies were of moderate quality,[17,18,21–25,28,29] and 2 studies were of low methodological quality.[15,26] The global rating “weak” for study quality was due to lack of information about subject inclusion, study withdrawals, and dropouts. To ascertain vital status, studies used death registries (n = 5),[17,22,24,26,29] medical records (n = 3),[18,21,27,28] the combination of medical records and death registries (n = 2),[15,25] and the combination of interviews, medical records, and a death register (n = 1).[23] All included studies provided HRs adjusted for at least 6 conventional cardiovascular risk factors, described in the Methods section. Quality assessment scores and additional adjustments made by the individual studies are presented in Supplemental Table S1.
3.2. Association between cardiac troponin concentration and mortality
We pooled adjusted HRs per 1-SD for all studies using a random-effects model. Pooling of the results from the 7 included studies for cardiovascular mortality showed a significant association between increased basal cardiac troponin levels and an elevated HR for cardiovascular mortality (HR per 1-SD 1.29, 95% confidence interval, 95% CI, 1.20–1.38). Cardiac troponin T was significantly stronger associated with cardiovascular mortality than with cardiac troponin I (meta-regression analysis P < 0.01; HR per 1-SD 1.37, 95% CI 1.23–1.52 and HR per 1-SD 1.21, 95% CI 1.16–1.26, for cardiac troponin T and I, respectively). The forest plots of the random-effects pooled data for all-cause mortality are shown in Fig. 2.
Figure 2.
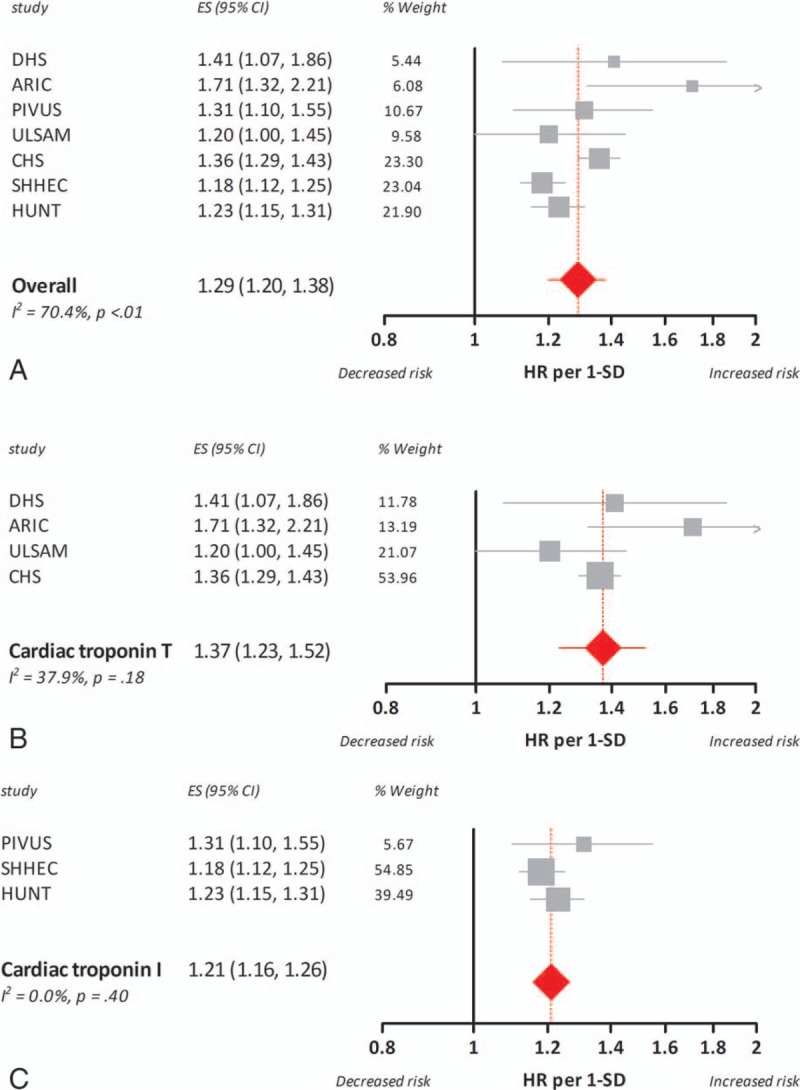
Association between 1 standard deviation increase in basal cardiac troponin concentrations and hazard ratio for cardiovascular mortality in the general population. The boxes and lines in the forest plots indicate the hazard ratio for cardiovascular mortality per standard deviation increase (HR per 1-SD) in cardiac troponin concentrations for individual studies and a pooled estimate (random-effects model) for all studies (panel A), studies on cardiac troponin T (panel B), and studies on cardiac troponin I (panel C).
Pooling of the results from the nine included studies for all-cause mortality showed a significant association between increased basal cardiac troponin levels and an elevated HR for all-cause mortality (HR per 1-SD 1.18, 95% CI 1.11–1.26). Considering the trend toward different effect sizes (HR per 1-SD) for cardiac troponin T and I shown by meta-regression analysis (P = 0.09), we performed stratified analyses for both isoforms of cardiac troponin. Basal cardiac troponin T levels translated to a higher HR for all-cause mortality [(HR per 1-SD 1.31, 95% CI 1.13–1.53) vs (HR per 1-SD 1.14, 95% CI 1.06–1.22)]. The forest plots of the random-effects pooled data for all-cause mortality are shown in Fig. 3.
Figure 3.
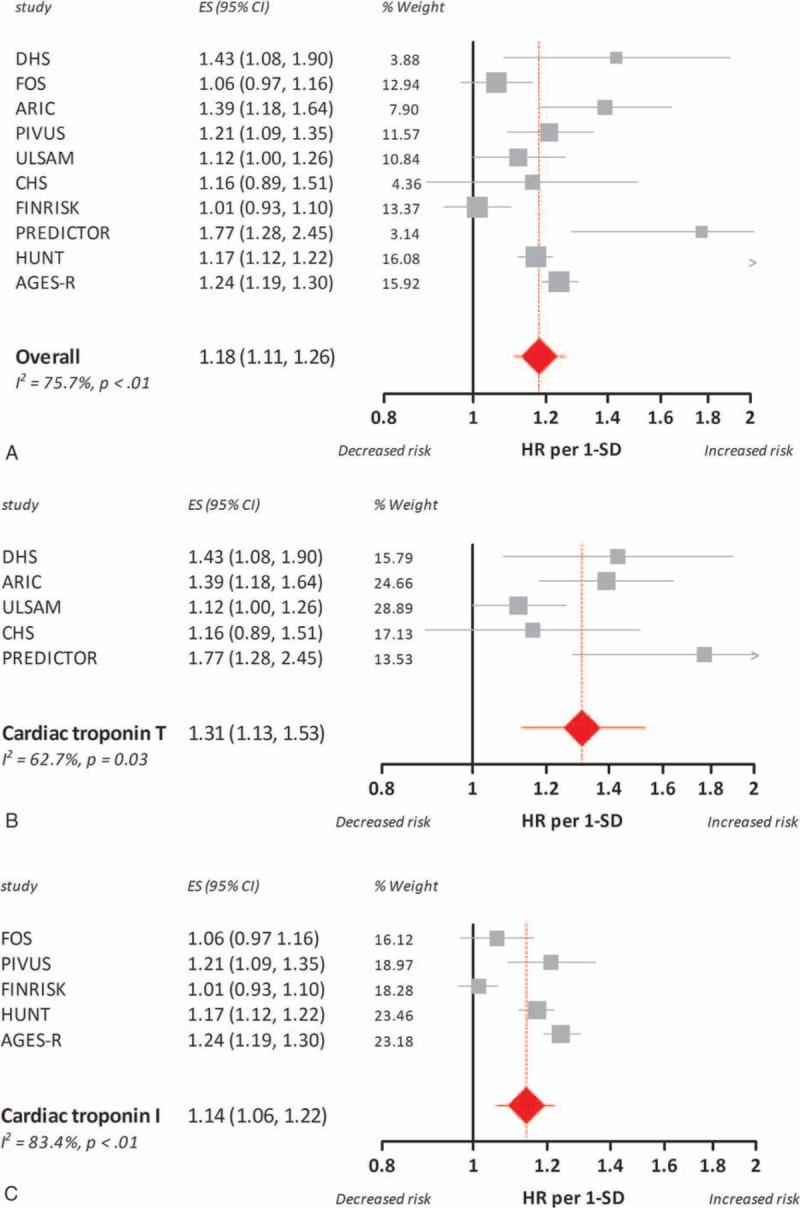
Association between 1 standard deviation increase in basal cardiac troponin concentrations and hazard ratio for all-cause mortality in the general population. The boxes and lines in the forest plots indicate the hazard ratio for all-cause mortality per standard deviation increase (HR per 1-SD) in cardiac troponin concentrations for individual studies and a pooled estimate (random-effects model) for all studies (panel A), studies on cardiac troponin T (panel B), and studies on cardiac troponin I (panel C).
3.3. Heterogeneity and publication bias assessment
Our meta-analysis revealed substantial heterogeneity across studies (Figs. 2–3). We used meta-regression analyses to identify the underlying factors. Univariable meta-regression analyses pointed out that estimated versus reported HR and study quality (strong/moderate vs weak global rating) were not statistically significant predictors, whereas the assay-type (high-sensitive cardiac troponin T or I) was statistically significant for cardiovascular mortality (P < 0.01) but did not reach significance for all-cause mortality (P = 0.09). Besides, age was a borderline significant factor in meta-regression analysis for cardiac troponin I and all-cause mortality (P = 0.05), indicating that older age is associated with a stronger correlation between troponin concentration and risk.
One SD increase in basal cardiac troponin T levels was associated with a higher HR for cardiovascular and all-cause mortality during follow-up than a similar increase in troponin I. Stratified analyses for cardiac troponin T and I decreased heterogeneity across troponin T studies (I2 = 37.9%, P = 0.18 and I2 = 62.7%, P = 0.03, for cardiovascular and all-cause mortality, respectively). Across studies with cardiac troponin I, heterogeneity for cardiovascular mortality decreased (I2 = 0.0%, P = 0.40), but remained high for all-cause mortality (I2 = 83.4%, P < 0.01).
Egger regression analysis was not indicative for publication bias (P = 0.50 and P = 0.68, for cardiovascular and all-cause mortality, respectively) (Supplemental Figure 1 and 2).
3.4. Sensitivity analysis
For all-cause mortality, 2 studies were excluded because they did not correct for at least the 6 conventional cardiovascular risk factors. To verify that pooled HRs were robust and independent, a sensitivity analysis including those 2 studies was performed. Random-effects pooling after the addition of 2 additional studies[30,31] revealed similar results regarding all-cause mortality and reduced heterogeneity for cardiac troponin T [HR per 1-SD 1.30, 95% CI 1.16–1.47; I2 = 52.9% (P = 0.060)], cardiac troponin I [HR per 1-SD 1.14, 95% CI 1.07–1.22; I2 = 79.3% (P < 0.01)], and the combined analysis [HR per 1-SD 1.19, 95% CI 1.12–1.26; I2 = 71.3% (P < 0.01)]. The meta-regression analysis that was performed after the inclusion of 2 additional studies[30,31] showed a significant effect of assay-type (cardiac troponin T vs I) on the observed heterogeneity (P = 0.05).
3.5. Translation to clinical practice
Together, the pooled data show that elevated basal cardiac troponin levels in the general population are associated with increased mortality during follow-up and suggest a slightly stronger association for cardiac troponin T than for cardiac troponin I. To relate the pooled summary measures per SD, to a clinically comprehensible risk estimation tool, we transformed HR per 1-SD to absolute cardiac troponin concentrations and corresponding HRs for all high-sensitive troponin assays that were included in the meta-analysis. Figure 4 depicts the relation between basal cardiac troponin T and I concentrations and mortality during follow-up.
Figure 4.
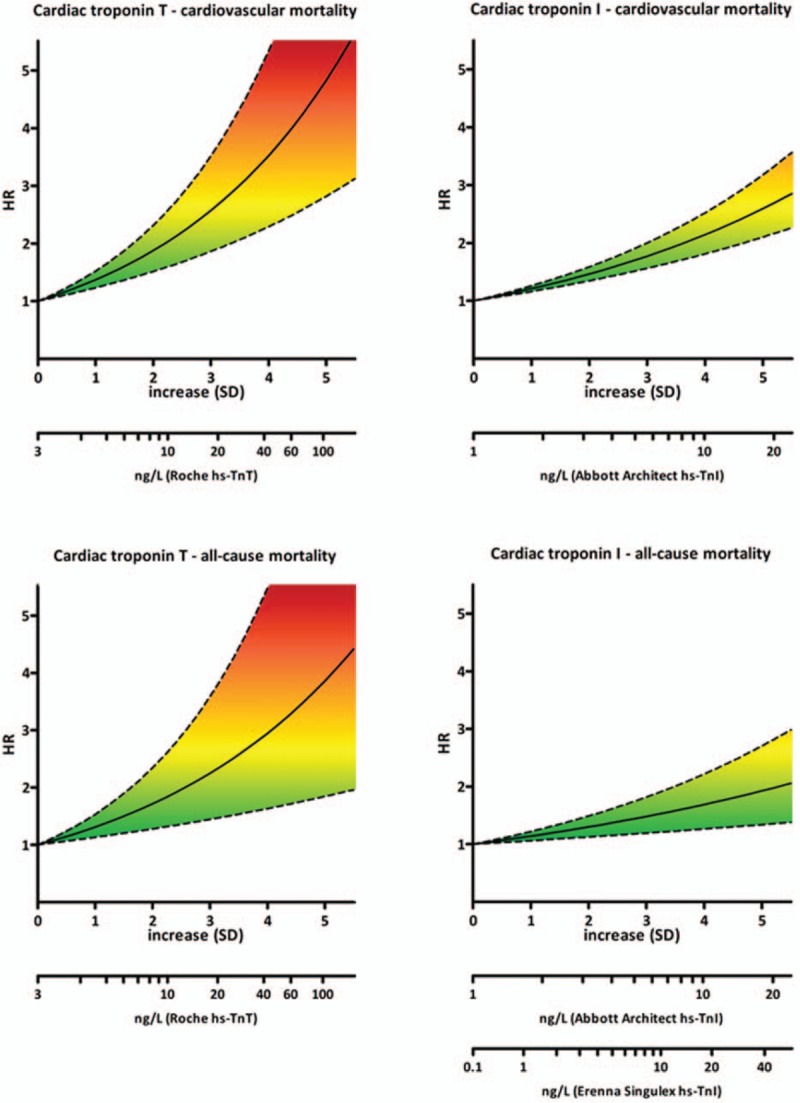
Cardiac troponin levels and corresponding hazard ratios. HR per 1-SD increase was transformed to absolute cardiac troponin concentrations and corresponding hazard ratios for all high-sensitive troponin assays that were included in the meta-analysis.
The majority of included studies performed discrimination and reclassification statistics (Supplemental Table S2).[15,17,21,22,24,25,27,29] The results from these analyses suggest small, but significant improvements in (established) prediction models based on established risk factors after the inclusion of high-sensitive cardiac troponin T or I.
4. Discussion
This meta-analysis with data on 65,019 participants from 11 prospective cohorts shows that basal elevated cardiac troponin concentrations, also below the 99th percentile, in the general population are associated with an increased risk for cardiovascular and all-cause mortality during follow-up. The pooled data analyses corroborate and extend the findings of previous studies.[7–9,32,33] Besides, our results suggest that the quantitative relationship between cardiac troponin concentrations is stronger for cardiovascular than for all-cause mortality, and may be slightly stronger for cardiac troponin T than for cardiac troponin I.
The observed association between elevated cardiac troponin concentrations and an increased risk for cardiovascular mortality and, to a lesser extent, for all-cause mortality, are reflected in the discrimination and reclassification statistics of the included studies. These analyses indicate that the addition of cardiac troponins may cause a small, but significant improvement in risk prediction models.[15,17,21,22,24,25,27,29] Some recent studies, but not all, suggest that the addition of cardiac troponin I to existing risk prediction models may be particularly useful among a subpopulation, especially in older and female subjects.[24,25,29,33] Before cardiac troponins can be actually implemented in clinical practice, it is necessary to identify the suitable target population and the best combination of biomarkers for risk prediction, also from a cost-effectiveness point of view.
The observed differences in the quantitative relationships of high-sensitive cardiac troponin T and I are clinically and scholarly interesting, and contrast with demonstrated equivalent performance of both assays in the acute setting, that is, for diagnosing acute myocardial infarction.[34] We can only speculate about the factors that might underlie these apparent prognostic differences. A first factor concerns the analytical aspects of the distinct cardiac troponin assays. It is conceivable that intrinsic assay differences, including the use of different antibodies,[35] may affect distinctive power of troponin assays with direct consequences for prognostic performance. A second factor involves the release of cardiac troponins. The mechanism of chronic cardiac troponin release is not elucidated, but recent observations suggest differences in release patterns of cardiac troponin T and I.[36] Third, postrelease modification such as fragmentation, complex formation, and elimination of cardiac troponins may differ between both cardiac troponins.[37,38]
This meta-analysis has several strengths, including its large patient populations, standardization of the outcome measure, and the comparison of cardiac troponin T and I. Limitations of our study merit consideration: First, despite the consistent adjustment for at least 6 established cardiovascular risk factors and the use of a standardized outcome measure (HR per 1-SD), heterogeneity was substantial, which persisted in assay-stratified analyses, in particular across all-cause mortality studies. Meta-regression analyses did not identify other suggestive factors that contributed to heterogeneity. A likely, remaining source of heterogeneity is the diversity in populations, caused by variable in- and exclusion criteria of the individual studies. Nonetheless, despite the detected heterogeneity, all studies revealed the same direction of effect, with increased mortality upon higher levels of circulating cardiac troponin. Second, due to the fact that we did not have access to the individual, data and that the percentage of included women showed minimal variation, we were unable to examine the effect of gender. Therefore, we cannot confirm that gender affects the relation between cardiac troponin concentrations and the risk on cardiovascular and all-cause mortality.[24,30,33] With respect to age, we could only examine the effect of the mean age across the included studies. In line with previous observations,[33] we found a borderline significant association between increasing age and a stronger correlation between troponin I and all-cause mortality. We cannot exclude that the variation in in- and exclusion criteria and adjusting factors might have masked a more explicit effect of age. Third, HR per 1-SD from stratified data has been mathematically derived. However, validation of this method in 2 studies revealed minimal discrepancies, and this approach to standardize risk-estimates enabled us to pool rather heterogeneously presented data, and to perform a meta-analysis with substantial statistical power and long-term follow-up. Fourth, the measurement and definition of mortality outcomes differs across studies. The definition of cardiovascular mortality varied slightly across studies, but in general, it concerned the ICD-10 diagnosis I00-I99.
5. Conclusion
Elevated, basal cardiac troponin T and I levels are significantly associated with an increased risk of cardiovascular and all-cause mortality during follow-up in the general population. The results of this meta-analysis suggest that this association is stronger for cardiovascular than for all-cause mortality. The observation that the quantitative relationship between cardiac troponin concentration and mortality risk may be stronger for cardiac troponin T than for cardiac troponin I is an interesting finding that requires further research. In addition, future studies should focus on further establishment and validation of cardiac troponins for the prediction of long-term outcomes.
Supplementary Material
Footnotes
Abbreviations: cTnI = cardiac troponin I, cTnT = cardiac troponin T, HR = hazard ratio, NSTEMI = non-ST elevated myocardial infarction, SD = standard deviation.
Funding/support: This study was supported by a research grant from Stichting De Weijerhorst to MPvDV.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–98. [DOI] [PubMed] [Google Scholar]
- [2].Al-Saleh A, Alazzoni A, Al Shalash S, et al. Performance of the high-sensitivity troponin assay in diagnosing acute myocardial infarction: systematic review and meta-analysis. CMAJ Open 2014;2:E199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Katrukha IA. Human cardiac troponin complex. Structure and functions. Biochemistry (Mosc) 2013;78:1447–65. [DOI] [PubMed] [Google Scholar]
- [4].de Lemos JA. Increasingly sensitive assays for cardiac troponins: a review. JAMA 2013;309:2262–9. [DOI] [PubMed] [Google Scholar]
- [5].Giannitsis E, Katus HA. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol 2013;10:623–34. [DOI] [PubMed] [Google Scholar]
- [6].Collinson P. The role of cardiac biomarkers in cardiovascular disease risk assessment. Curr Opin Cardiol 2014;29:366–71. [DOI] [PubMed] [Google Scholar]
- [7].Ahmed AN, Blonde K, Hackam D, et al. Prognostic significance of elevated troponin in non-cardiac hospitalized patients: a systematic review and meta-analysis. Ann Med 2014;46:653–63. [DOI] [PubMed] [Google Scholar]
- [8].Michos ED, Wilson LM, Yeh HC, et al. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta-analysis. Ann Intern Med 2014;161:491–501. [DOI] [PubMed] [Google Scholar]
- [9].Khan NA, Hemmelgarn BR, Tonelli M, et al. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation 2005;112:3088–96. [DOI] [PubMed] [Google Scholar]
- [10].Lipinski MJ, Baker NC, Escarcega RO, et al. Comparison of conventional and high-sensitivity troponin in patients with chest pain: a collaborative meta-analysis. Am Heart J 2015;169:6–16. e16. [DOI] [PubMed] [Google Scholar]
- [11].Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- [12].Haaf P, Reichlin T, Twerenbold R, et al. Risk stratification in patients with acute chest pain using three high-sensitivity cardiac troponin assays. Eur Heart J 2014;35:365–75. [DOI] [PubMed] [Google Scholar]
- [13].Thomas BH, Ciliska D, Dobbins M, et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176–84. [DOI] [PubMed] [Google Scholar]
- [14].Koerbin G, Potter JM, Abhayaratna WP, et al. The distribution of cardiac troponin I in a population of healthy children: lessons for adults. Clin Chim Acta 2013;417:54–6. [DOI] [PubMed] [Google Scholar]
- [15].Eggers KM, Venge P, Lindahl B, et al. Cardiac troponin I levels measured with a high-sensitive assay increase over time and are strong predictors of mortality in an elderly population. J Am Coll Cardiol 2013;61:1906–13. [DOI] [PubMed] [Google Scholar]
- [16].Venge P, Johnston N, Lindahl B, et al. Normal plasma levels of cardiac troponin I measured by the high-sensitivity cardiac troponin I access prototype assay and the impact on the diagnosis of myocardial ischemia. J Am Coll Cardiol 2009;54:1165–72. [DOI] [PubMed] [Google Scholar]
- [17].de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hussein AA, Gottdiener JS, Bartz TM, et al. Cardiomyocyte injury assessed by a highly sensitive troponin assay and sudden cardiac death in the community: the Cardiovascular Health Study. J Am Coll Cardiol 2013;62:2112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [21].deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eggers KM, Al-Shakarchi J, Berglund L, et al. High-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. Am Heart J 2013;166:541–8. [DOI] [PubMed] [Google Scholar]
- [23].Oluleye OW, Folsom AR, Nambi V, et al. Investigators AS Troponin T, B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Ann Epidemiol 2013;23:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Omland T, de Lemos JA, Holmen OL, et al. Impact of sex on the prognostic value of high-sensitivity cardiac troponin I in the general population: the HUNT study. Clin Chem 2015;61:646–56. [DOI] [PubMed] [Google Scholar]
- [25].Zeller T, Tunstall-Pedoe H, Saarela O, et al. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J 2014;35:271–81. [DOI] [PubMed] [Google Scholar]
- [26].Masson S, Agabiti N, Vago T, et al. The fibroblast growth factor-23 and Vitamin D emerge as nontraditional risk factors and may affect cardiovascular risk. J Intern Med 2015;277:318–30. [DOI] [PubMed] [Google Scholar]
- [27].Neumann JT, Havulinna AS, Zeller T, et al. Comparison of three troponins as predictors of future cardiovascular events: prospective results from the FINRISK and BiomaCaRE studies. PLoS One 2014;9:e90063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation 2012;126:1596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thorsteinsdottir I, Aspelund T, Gudmundsson E, et al. High-sensitivity cardiac troponin I is a strong predictor of cardiovascular events and mortality in the AGES-Reykjavik community-based cohort of older individuals. Clin Chem 2016;62:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dallmeier D, Denkinger M, Peter R, et al. Sex-specific associations of established and emerging cardiac biomarkers with all-cause mortality in older adults: the ActiFE study. Clin Chem 2015;61:389–99. [DOI] [PubMed] [Google Scholar]
- [31].McKie PM, AbouEzzeddine OF, Scott CG, et al. High-sensitivity troponin I and amino-terminal pro–B-type natriuretic peptide predict heart failure and mortality in the general population. Clin Chem 2014;60:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sze J, Mooney J, Barzi F, et al. Cardiac troponin and its relationship to cardiovascular outcomes in community populations: a systematic review and meta-analysis. Heart Lung Circ 2016;25:217–28. [DOI] [PubMed] [Google Scholar]
- [33].Blankenberg S, Salomaa V, Makarova N, et al. Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J 2016;37:2428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Irfan A, Reichlin T, Twerenbold R, et al. Early diagnosis of myocardial infarction using absolute and relative changes in cardiac troponin concentrations. Am J Med 2013;126:781–8. e782. [DOI] [PubMed] [Google Scholar]
- [35].Apple FS, Collinson PO, Biomarkers ITFoCAoC. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61. [DOI] [PubMed] [Google Scholar]
- [36].Klinkenberg LJ, Wildi K, van der Linden N, et al. Diurnal rhythm of cardiac troponin: consequences for the diagnosis of acute myocardial infarction. Clin Chem 2016;62:1602–11. [DOI] [PubMed] [Google Scholar]
- [37].Streng AS, de Boer D, van der Velden J, et al. Posttranslational modifications of cardiac troponin T: an overview. J Mol Cell Cardiol 2013;63:47–56. [DOI] [PubMed] [Google Scholar]
- [38].Martin AF. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem 1981;256:964–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


