Abstract
Background:
Lactate dehydrogenase (LDH) as a hypoxia-regulator plays a vital role in alternative metabolic pathways of cancer cells. Numerous studies have assessed the prognostic value of elevated pretreatment LDH in malignant mesothelioma (MM). However, the results have been largely inconsistent. Hence, the aim of current study was to investigate the prognostic value of pretreatment LDH levels in patients with MM by performing a meta-analysis of relevant studies.
Methods:
A literature search for English language studies, which investigated the association of LDH levels with overall survival (OS) in malignant mesothelioma, was performed in the electronic databases, PubMed, Medline, Embase, and Web of Science. Pooled hazard ratios (HRs) and their 95% confidence intervals (95% CIs) were calculated. Heterogeneity was assessed using Cochran Q and I2 statistics. Sensitivity analysis, meta-regression model, and subgroup analysis were performed to trace the source of heterogeneity, if applicable.
Results:
A total of 9 studies with a combined study population of 1977 patients came within the purview of this meta analysis. Pooled HR for OS in patients with high LDH level was 1.68 (95% CI = 1.36–2.00). Significant heterogeneity was observed in the included studies (I2 = 54.1%, P = 0.026). Sensitivity analysis after sequential exclusion of 1 study at a time, and meta-regression with inclusion of 6 confounding factors failed to identify the source of heterogeneity. However, in the subgroup analysis, it was found that the publication of Nojiri et al was the origin of heterogeneity. When omitted the publication of Nojiri et al, the pooled HR of the rest 8 studies was 1.83 (95% CI = 1.45–2.20, I2 = 0.0%, P = 0.723). Egger test and funnel plots excluded the possibility of publication bias affecting the results of the current meta-analysis.
Conclusion:
A negative association was observed between high LDH levels and poor overall survival in the current study. Our findings suggest that pretreatment LDH level could serve as a useful predictor of prognosis in patients with malignant mesothelioma.
Keywords: malignant mesothelioma, meta-analysis, prognostic value, serum lactate dehydrogenase, survival outcome
1. Introduction
Malignant mesothelioma (MM) is a rare but aggressive and fatal neoplasm that originates from the thoracic and abdominal serosal membranes.[1] Environmental and occupational exposure to asbestos is causally associated with its causation.[2] Due to delayed diagnosis and long latent interval between onset and clinical symptoms, the prognosis of MM remains extremely poor. The natural history of untreated patients was only 4 to 12 months.[3] Conversely, specialized mesothelioma centers employ multimodality approaches, including surgical resection, chemotherapy, and radiation, with survival in excess of 20 months depending on stage.[4] Therefore, early predictors of prognosis may help guide intensive treatment protocols to improve survival outcomes or provide best supportive care to improve quality of life for patients with short life expectancy.
Clinical practice guidelines are based on the International Mesothelioma Interest Group (IMIG) TNM staging system.[5] However, the current staging system relies on anatomical information as the main reference, and does not take the biological heterogeneity into account. Therefore, the staging system is not enough for prediction of survival outcomes. In addition, only pathological type and performance status have been shown to be relatively consistent prognostic factors during pretreatment assessment.[1] Thus, it is necessary to identify more prognostic factors to tailor the treatment to individual patients.
Unlike normal cells, cancer cells tend to employ alternate metabolic pathways.[6] They generate adenosine triphosphate (ATP) mainly through anaerobic glycolysis. Lactate dehydrogenase (LDH) as a hypoxia-regulator plays a vital role in anaerobic glycolysis.[7] The prognostic value of serum LDH has been demonstrated in several tumors, including nonsmall cell lung cancer,[8] colorectal cancer,[9] prostate cancer,[10] as well as other solid tumors.[11,12]
Several studies have assessed the prognostic value of elevated pretreatment LDH levels for the prediction of survival outcomes in MM.[13–21] However, the results have been largely inconsistent. Moreover, because of the low incidence of MM, relatively small sample size is a key limitation of the studies conducted thus far. The limitation of small sample size in individual studies, however, can be compensated to some extent by meta-analysis. Therefore, we conducted a meta-analysis of studies that investigated the prognostic value of pretreatment serum LDH level in MM.
2. Materials and methods
2.1. Search and filtration strategy
A systematic literature search was performed in the electronic databases, PubMed, Medline, Embase, and Web of Science, to retrieve relevant clinical studies published up to April 3, 2016. The key words used were: [“mesothelioma∗” or “celothelioma” or “mesothelial neoplasm∗”] and [“L-Lactate dehydrogenase” or “lactate dehydrogenase” or “LDH”]. Reference lists of the included studies were manually screened to increase the yield of potential studies. This meta-analysis is reported in accordance with the preferred reporting items for PRISMA statement.[22]
2.2. Inclusion and exclusion criteria
The eligibility criteria for inclusion were: studies that included patients with pathological confirmation of malignant mesothelioma; patients underwent surgical resection, chemotherapy, radiotherapy, immunotherapy, or combination therapies; study design: retrospective or prospective design; full text available in English; investigated the association between pretreatment serum LDH level and survival outcomes; data on hazard ratios (HRs) with 95% confidence intervals (CIs) or a P value for overall survival (OS) reported.
Exclusion criteria were: case reports, letters, review articles, or meeting records; non-English language publications; studies had duplicate data or overlapping patients; sufficient data not available to estimate HRs and their 95% CIs.
2.3. Data extraction
Data from eligible articles were independently extracted after a review of full texts by 2 investigators using a standardized form. Any doubts pertaining to eligibility of a study for inclusion were resolved in consultation with a third independent senior oncologist. Data on following variables of interest were extracted: first author's name, publication year, sample size, study design (prospective or retrospective), follow-up time, cut-off value of pretreatment LDH, survival analysis method (multivariate or univariate), and HRs associated with their 95% CIs for OS. In case both multivariate and univariate outcomes were provided, multivariate proportional hazards models that adjusted for major clinical factors were preferred in statistical analysis. If multivariate results were not available, the univariate results were used instead.
2.4. Quality assessment
Newcastle–Ottawa Scale (NOS) was used for quality assessment of the included studies performed by 2 independent reviewers.[23] The 3 aspects of NOS criteria were: selection of cases and controls 0 to 4, comparability of subjects 0 to 2, outcome or exposure 0 to 3. Quality scores ranged from 0 to 9, and previous studies with scores ≥6 were deemed to be of “good quality.”
2.5. Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
2.6. Statistical analysis
All statistical analyses were performed using STATA 12.0 software (StataCorp LP, College Station, TX). HRs and their 95% CIs were used to calculate overall effects [STATA command line: metan hr ll ul, label (namevar=author, yearvar=year) wgt (sample size)]. Heterogeneity among included studies was evaluated using Cochran Q and I2 statistics test. The value of I2 ranges from 0% to 100%. I2 > 50% or P <0.05 was considered indicative of significant heterogeneity, and random effects model was employed to calculate pooled HRs and their 95% CI. I2 ≤50% or P >0.05 was considered indicative of a lack of significant heterogeneity, and fixed effects model was employed for statistical analysis.[24] Sensitivity and meta-regression analyses were performed to identify the source of heterogeneity, if applicable [STATA command lines: metaninf hr ll ul, label(namevar=author, yearvar=year) random, and metareg lnhr covariate, wsse(selnhr) bsest(reml)]. Publication year, age of patients, disease site, LDH cut-off value, study type, and variable type were considered potential confounding factors. Publication bias was graphically assessed by visual inspection of Begg funnel plot and the possibility of publication bias was tested by Egger test[25] [STATA command line: metabias hr ll ul, ci graph(begg) gweight]. Conventionally, HR >1 and its 95% CI did not overlap 1 indicated that the elevated LDH level was an adverse prognostic factor for overall survival. A 2-sided P value less than 0.05 was considered statistically significant.
3. Results
3.1. Study characteristics
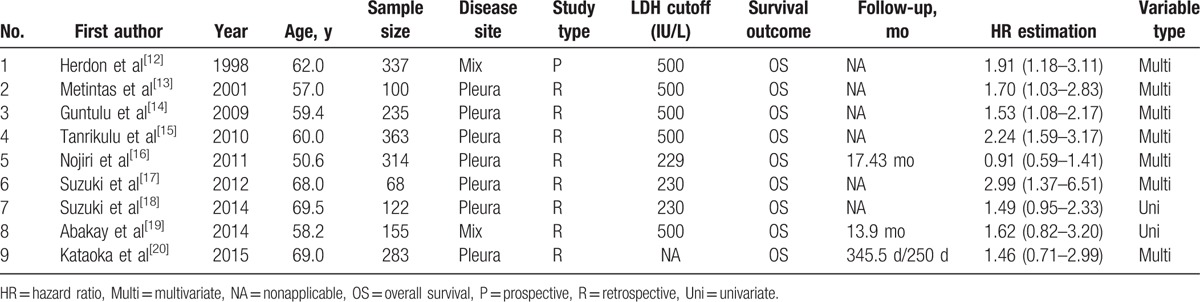
A total of 196 articles were identified on initial search in electronic databases. Titles and abstracts of all potentially relevant studies were screened for eligibility. One hundred seventy-eight articles were eliminated because of either being duplicate records, or not relevant or owing to nonavailability of full text. After review of original data and full texts of the remaining 18 studies, another 9 were also excluded because of lack of sufficient data to estimate the overall effect. Finally, 9 eligible studies with a combined study population of 1977 patients were included in the meta-analysis. A schematic illustration of the selection criteria is shown in Fig. 1. The summary of the main characteristics of eligible studies is presented in Table 1. The majority of the eligible studies were adjusted for major prognostic factors using the multivariate proportional hazard model. Univariate outcomes were acquired when no multivariate outcomes were available. Hereinto, 4 studies reported the negative results, while the rest 5 studies reported the positive results. In addition, the average quality scores for included studies, as assessed by 2 independent reviewers, were 6.7 and 6.8, which indicates a “good” quality of all studies included in the current meta-analysis.
Figure 1.
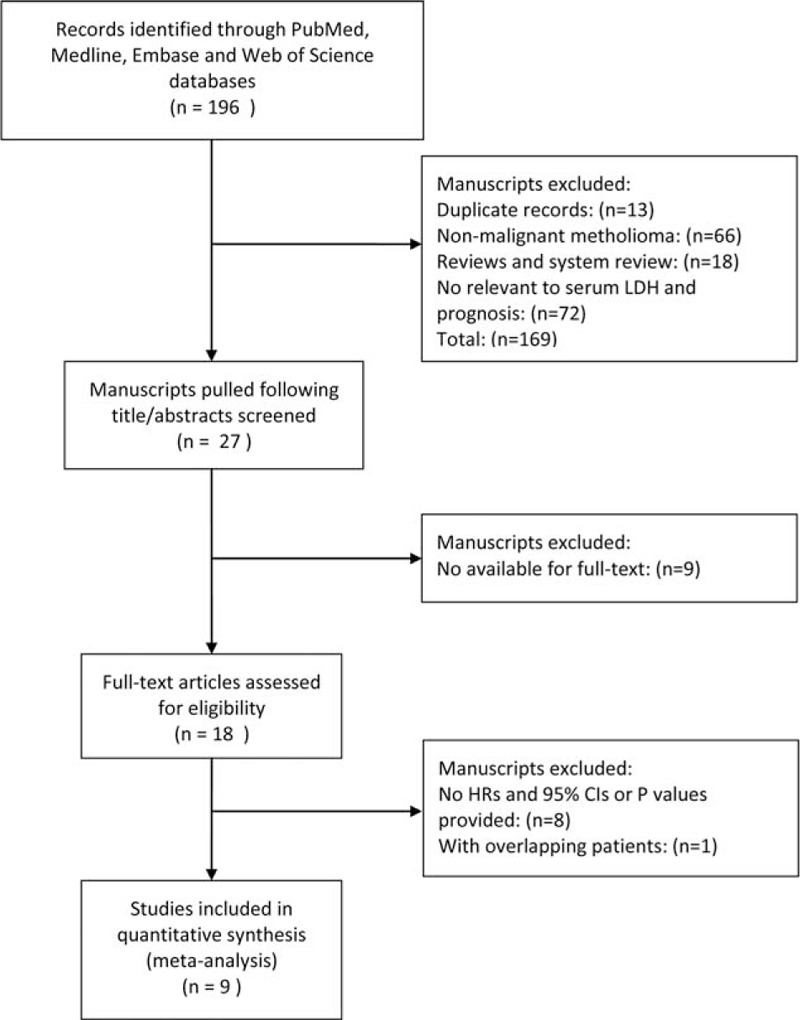
Schematic illustration of the procedure and study selection criteria for inclusion in current meta-analysis.
Table 1.
Baseline characteristics of eligible studies.
3.2. Overall survival
Elevated LDH levels predicted an adverse prognosis for OS with a pooled HR of 1.68 (95% CI = 1.36–2.00, I2 = 54.1%, P = 0.026) using the random-effect model (Fig. 2).
Figure 2.
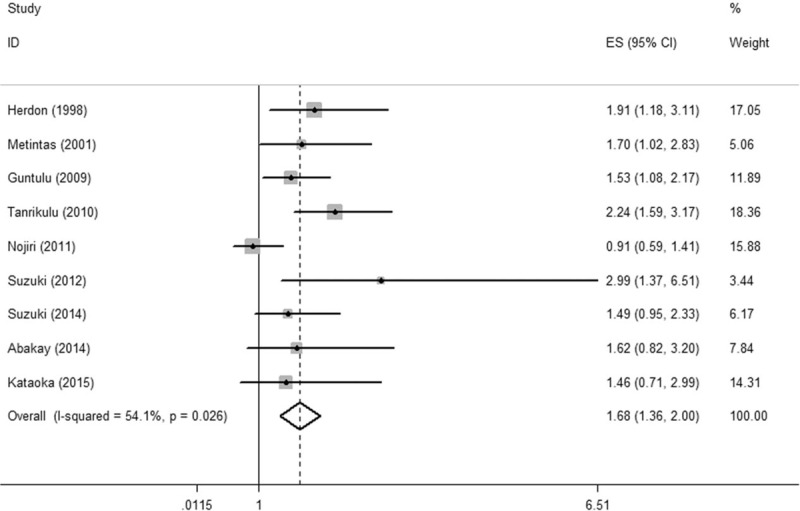
Forest plot illustrating HRs for OS associated with LDH levels greater than or less than the cut-off value. HRs for each study are represented by squares; the size of the square represents the weight of the study in the meta-analysis; the horizontal line crossing the square represents the 95% CI. CI = confidence interval, HR = hazards ratio, LDH = lactate dehydrogenase, OS = overall survival.
3.3. Heterogeneity analysis
As significant heterogeneity was observed, sensitivity analysis, meta-regression model, and subgroup analysis were applied to trace its origin. On sensitivity analysis, the pooled overall effects were found to be stable after sequential exclusion of studies one at a time (Fig. 3). Next, a univariate meta-regression analysis comprising 6 confounding factors was conducted. In aggregate, no significant association was observed among age, publication year, disease site, LDH cut-off value, type of study, variable type, and the HR for OS (P = 0.168, 0.327, 0.590, 0.282, 0.486, and 0.785 respectively). Subsequently, the subgroup analysis was conducted to trace the origin of heterogeneity. The results are summarized in Table 2. Intriguingly, in each subgroup of 5 confounding factors, there always exists a pooled hazard ratio with no heterogeneity (I2 = 0.0%). In the further analysis of the results in Table 2, it was found that the publication of Nojiri et al was the origin of heterogeneity. When omitted the publication of Nojiri et al, the pooled HR of the rest 8 studies was 1.83 (95% CI = 1.45–2.20, I2 = 0.0%, P = 0.723) using the fixed effects model (Fig. 4).
Figure 3.
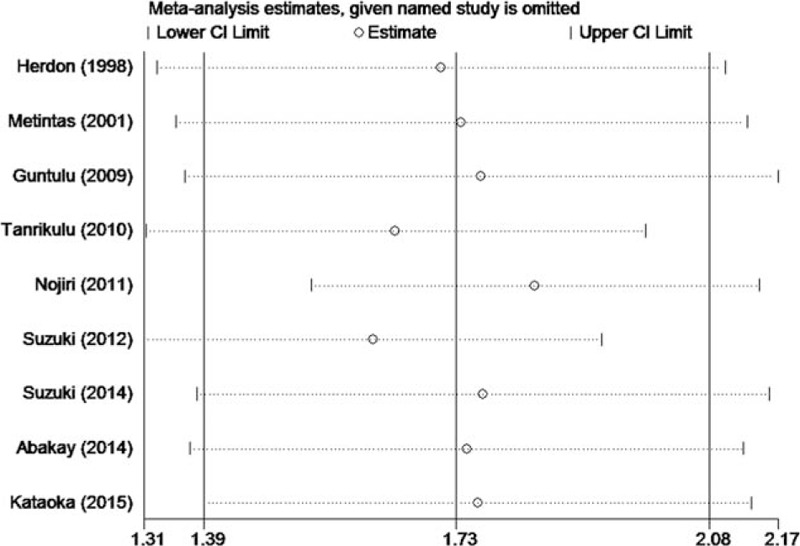
Forest plot for the sensitivity analysis in current meta-analysis. CI = confidence interval.
Table 2.
The results of the subgroup analyses in the included studies.
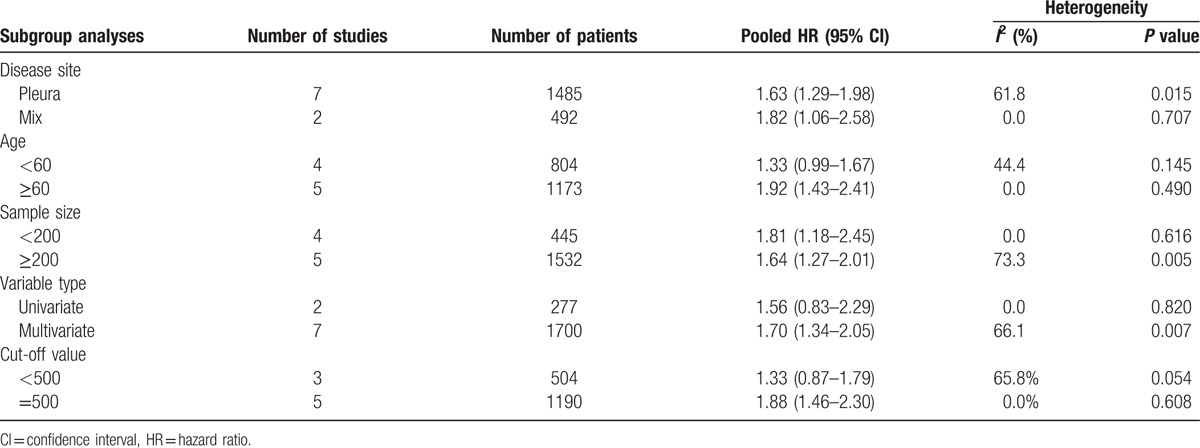
Figure 4.
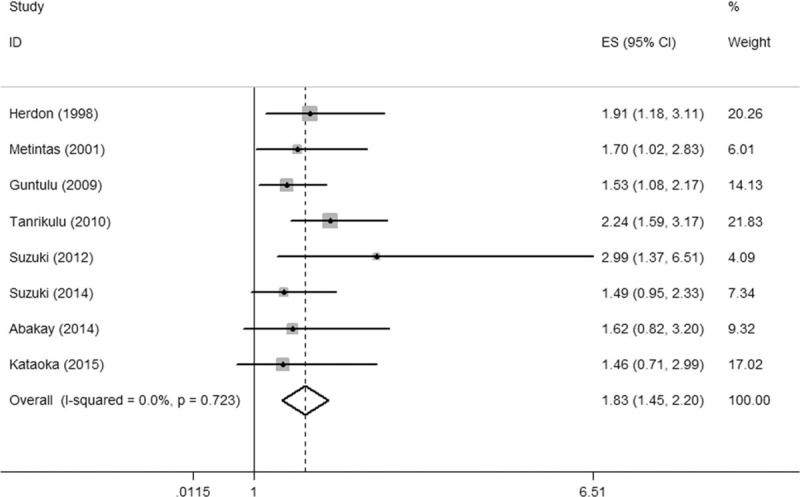
Forest plot illustrating HRs for OS when omitting the publication of Nojiri et al. CI = confidence interval, HR = hazards ratio, OS = overall survival.
3.4. Publication bias
No obvious asymmetry was observed in the Begg funnel plot (Fig. 5), and no significant publication bias was observed to affect the association of elevated LDH levels with OS on Egger test (P = 0.832).
Figure 5.
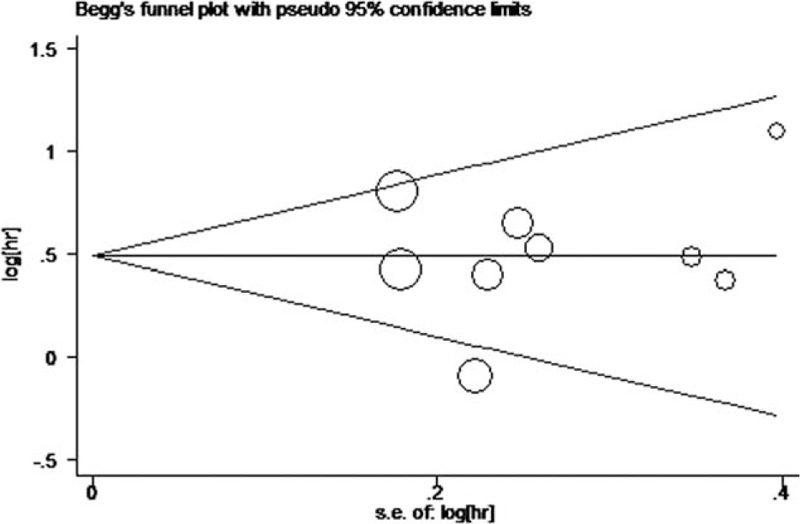
Visual assessment of publication bias on Begg funnel plot analysis. hr = hazard ratio, s.e. = standard error.
4. Discussion
To date, only TNM stage system, pathological subtype, and performance status have been consistently identified as prognostic factors of MM in clinical practice.[1] However, the above-mentioned factors are not enough to guide individualized treatment. Biological behavior of tumor essentially differs from that of normal cells, including the metabolic pathways.[6] Cancer cells generate ATP mainly through anaerobic glycolysis. LDH as a hypoxia-regulator plays a vital role in anaerobic glycolysis.[7] The relevance of serum LDH level as a cheap and convenient prognostic factor in patients with MM has been investigated. However, the results have largely been inconsistent. The conflict may result from the relatively small sample size and different follow-up periods. It is generally acknowledged that meta-analysis is a powerful statistic tool to overcome the limitation of different sample sizes by combining results from several individual studies to generate the best assessment. Therefore, we conducted a meta-analysis to quantify the prognostic value of LDH in MM. Finally,9 studies (N = 1977 patients) were included in the meta-analysis. High pretreatment LDH levels indicated a worse OS in patients with MM. Sensitivity analysis revealed that the pooled HR is stable when excluded any individual study each time. Furthermore, 6 confounding factors comprising “age,” “publication year,” “disease site,” “LDH cut-off value,” “type of study,” and “variable type” were performed in meta-regression analysis; however, no significant association was observed. Intriguingly, when omitted the publication of Nojiri et al, no heterogeneity was observed in the rest 8 studies.
A meta-analysis by Chen et al[26] also found elevated LDH to have an adverse impact on OS in patients with osteosarcoma (combined HR: 1.92 [95% CI: 1.53–2.40]). Similar findings were reported in the context of solid tumors from a systematic review by Zhang et al[27] (N = 29,620 patients). These findings suggest that serum LDH could be employed as a prognostic factor for overall survival. However, further investigations are needed to explore the mechanism of LDH and poor prognosis.
As compared with normal cells, cancer cells consume glucose avidly and produce abundant lactic acid rather than catabolise glucose via the tricarboxylic acid (TCA) cycle, which is the key pathway for the generation of ATP under normoxic conditions.[6] In the absence of oxygen, pyruvate is not preferentially metabolized by TCA cycle but is converted into lactic acid by lactate dehydrogenase.[7] The linkage between high LDH levels and poor prognosis could be explained by several hypotheses. First, the production of lactate acid could be up-regulated by the increased level of LDH which could result in the lower PH in the extracellular space.[28,29] Acidic extracellular pH has been shown to enhance activation of metalloproteases to facilitate the decomposition of extracellular matrix and enhance the activation of macrophage-mediated angiogenesis.[30,31] Furthermore, lower PH may serve to protect mitochondria from oxidative stress, which induces resistance to hypoxia-induced apoptosis of tumor cells.[32] Thus, up-regulation of LDH may facilitate the invasiveness and resistance of tumor cell to apoptosis. Second, a strong correlation between LDH-5, one of the main isoenzymes of LDH, with the expression of HIFαs and VEGF is well documented. Thus, elevated LDH levels may reflect HIF-dependent tumor angiogenesis and aggressiveness.[33,34] In addition, Kolev et al[35] reported a negative association between LDH-5 and lymphocytic infiltration at the tumor edge, indicating that elevated LDH levels may indicate an up-regulation of HIF-molecular pathway, attenuation of host immunologic function, and enhanced tumor angiogenesis, which have an adverse impact on prognosis in malignant tumors.
A strength of the current study is a relatively large combined study population (N = 1977) from a total of 9 studies used for the calculation of the overall effect. The adverse prognostic effect of elevated LDH levels on OS was unequivocal. Our findings build on the previous studies that have indicated LDH as a potential therapeutic target. Recently, Le et al[36] reported that inhibition of LDH-A inhibited tumor progression and induced apoptosis via accumulation of reactive oxygen species (ROS) in lymphoma cells. Zhai et al[37] also demonstrated that oxamate (LDH-inhibitor) reduced ATP levels and enhanced radiation sensitivity in nasopharyngeal carcinoma. Hence, inhibition of LDH appears to be a promising prospect for individualized treatment of cancers. Second, several studies have revealed that serum LDH level could be a reflection of tumor burden and invasiveness,[33,34] which is consistent with the use of LDH level as an appropriate indicator of survival outcomes. To our knowledge, no present study has employed dynamic LDH levels to monitor therapeutic effects or survival outcome in MM; hence, it is necessary to conduct several investigations to comprehensively explore the value of serum LDH level in prognosis.
Some limitations of the current meta-analysis need to be acknowledged. First, significant heterogeneity was observed in all included studies (I2 = 54.1%, P = 0.026). When utilizing sensitivity analysis and meta-regression analysis, the origin of heterogeneity could not be fully identified. However, in the subgroup analysis, the publication of Nojiri et al was found to be the origin of heterogeneity. When omitted the publication of Nojiri et al, the pooled HR of the rest 8 studies was 1.83 (95% CI = 1.45–2.20, I2 = 0.0%, P = 0.723), suggesting that the pooled HR of the rest 8 studies was more homogeneous and reliable. Second, there were only 9 publications with a total of 1977 MM patients in current meta-analysis. The relatively modest sample size may unavoidably increase the risk of bias and lower the statistic power in current meta-analysis. Hence, further investigations with larger sample size are needed to improve the statistic power and achieve more meaningful results. Third, the publication dates of studies included in our meta-analysis spanned from 1998 to 2015. The therapeutic strategy in recent years may differ from what they were 5 years ago. Fourth, selection bias may be caused by the language restriction of English. Fifth, univariate and multivariate types were both used in the calculation of HR for OS. Univariate analysis is known to overestimate the effect size. Sixth, the cut-off values of LDH used in the included studies were inconsistent; it is important to standardize the LDH cut-off value in future studies. Seventh, the reliability of the results of prospective and retrospective studies selected in our study is lower than that of prospective randomized trials.
In conclusion, a negative association between high serum LDH level and poor survival outcome in malignant mesothelioma was observed in current meta-analysis. As a convenient and cost-effective indicator, serum LDH may be useful in clinical practice. Further prospective randomized trials with standardized LDH cut-off values are warranted to improve statistical power of future analysis.
Footnotes
Abbreviations: ATP = adenosine triphosphate, CI = confident interval, HIF = hypoxia inducible factor, HR = hazard ratio, IMIG = International Mesothelioma Interest Group, LDH = lactate dehydrogenase, MM = malignant mesothelioma, MPM = malignant pleura mesothelioma, NOS = Newcastle–Ottawa Scale, OS = overall survival, ROS = reactive oxygen species, TCA = tricarboxylic acid, VEGF = vascular endothelial cell growth factor.
YZ and LL are co-first authors; they contributed equally to the work.
The authors declared no conflicts of interest.
References
- [1].van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roe OD, Stella GM. Malignant pleural mesothelioma: history, controversy and future of a manmade epidemic. Eur Respir Rev 2015;24:115–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jaklitsch MT, Grondin SC, Sugarbaker DJ. Treatment of malignant mesothelioma. World J Surg 2001;25:210–7. [DOI] [PubMed] [Google Scholar]
- [4].Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957–65. [DOI] [PubMed] [Google Scholar]
- [5].Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma from the International Mesothelioma Interest Group. Lung Cancer 1996;14:1–2. [DOI] [PubMed] [Google Scholar]
- [6].Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 2008;134:703–7. [DOI] [PubMed] [Google Scholar]
- [7].Serganova I, Rizwan A, Ni X, et al. Metabolic imaging: a link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin Cancer Res 2011;17:6250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Giroux Leprieur E, Lavole A, Ruppert AM, et al. Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology 2012;17:134–42. [DOI] [PubMed] [Google Scholar]
- [9].Giessen C, Fischer von Weikersthal L, Laubender RP, et al. Evaluation of prognostic factors in liver-limited metastatic colorectal cancer: a preplanned analysis of the FIRE-1 trial. Br J Cancer 2013;109:1428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2014;32:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pierga JY, Asselain B, Jouve M, et al. Effect of adjuvant chemotherapy on outcome in patients with metastatic breast carcinoma treated with first-line doxorubicin-containing chemotherapy. Cancer 2001;91:1079–89. [DOI] [PubMed] [Google Scholar]
- [12].Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori 2004;90:478–84. [DOI] [PubMed] [Google Scholar]
- [13].Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723–31. [DOI] [PubMed] [Google Scholar]
- [14].Metintas M, Metintas S, Ucgun I, et al. Prognostic factors in diffuse malignant pleural mesothelioma: effects of pretreatment clinical and laboratory characteristics. Respir Med 2001;95:829–35. [DOI] [PubMed] [Google Scholar]
- [15].Ak G, Metintas S, Metintas M, et al. Prognostic factors according to the treatment schedule in malignant pleural mesothelioma. J Thorac Oncol 2009;4:1425–30. [DOI] [PubMed] [Google Scholar]
- [16].Tanrikulu AC, Abakay A, Kaplan MA, et al. A clinical, radiographic and laboratory evaluation of prognostic factors in 363 patients with malignant pleural mesothelioma. Respiration 2010;80:480–7. [DOI] [PubMed] [Google Scholar]
- [17].Nojiri S, Gemba K, Aoe K, et al. Survival and prognostic factors in malignant pleural mesothelioma: a retrospective study of 314 patients in the west part of Japan. Jpn J Clin Oncol 2011;41:32–9. [DOI] [PubMed] [Google Scholar]
- [18].Suzuki H, Hirashima T, Kobayashi M, et al. Prognostic factors in malignant pleural mesothelioma: a retrospective study. Intern Med 2012;51:707–10. [DOI] [PubMed] [Google Scholar]
- [19].Suzuki H, Asami K, Hirashima T, et al. Stratification of malignant pleural mesothelioma prognosis using recursive partitioning analysis. Lung 2014;192:191–5. [DOI] [PubMed] [Google Scholar]
- [20].Abakay O, Tanrikulu AC, Palanci Y, et al. The value of inflammatory parameters in the prognosis of malignant mesothelioma. J Int Med Res 2014;42:554–65. [DOI] [PubMed] [Google Scholar]
- [21].Kataoka Y, Yamamoto Y, Otsuki T, et al. A new prognostic index for overall survival in malignant pleural mesothelioma: the rPHS (regimen, PS, histology or stage) index. Jpn J Clin Oncol 2015;45:562–8. [DOI] [PubMed] [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [23].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [24].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [25].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen J, Sun MX, Hua YQ, et al. Prognostic significance of serum lactate dehydrogenase level in osteosarcoma: a meta-analysis. J Cancer Res Clin Oncol 2014;140:1205–10. [DOI] [PubMed] [Google Scholar]
- [27].Zhang J, Yao YH, Li BG, et al. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Sci Rep 2015;5:9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 1989;49:6449–65. [PubMed] [Google Scholar]
- [29].Stubbs M, McSheehy PM, Griffiths JR, et al. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today 2000;6:15–9. [DOI] [PubMed] [Google Scholar]
- [30].Bonuccelli G, Tsirigos A, Whitaker-Menezes D, et al. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010;9:3506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Martinez-Outschoorn UE, Prisco M, Ertel A, et al. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell Cycle 2011;10:1271–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nemoto S, Takeda K, Yu ZX, et al. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol 2000;20:7311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Semenza GL, Jiang BH, Leung SW, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 1996;271:32529–37. [DOI] [PubMed] [Google Scholar]
- [34].Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway—a report of the Tumour Angiogenesis Research Group. J Clin Oncol 2006;24:4301–8. [DOI] [PubMed] [Google Scholar]
- [35].Kolev Y, Uetake H, Takagi Y, et al. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol 2008;15:2336–44. [DOI] [PubMed] [Google Scholar]
- [36].Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 2010;107:2037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhai X, Yang Y, Wan J, et al. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol Rep 2013;30:2983–91. [DOI] [PubMed] [Google Scholar]


