Abstract
Diabetes mellitus (DM) is associated with a state of chronic hyperglycemia with a highly increased risk of vascular complications. The current study aimed to investigate microcirculation abnormalities in patients with type 2 DM and those with pre-DM using nailfold videocapillaroscopy (NVC) and evaluate the possible correlation with microvascular complications.
A total of 115 patients with type 2 DM, 41 patients with pre-DM, and 37 healthy subjects without diabetes were enrolled. All subjects underwent NVC to evaluate capillary density, length, morphology, distribution, presence of enlarged loops or hemorrhages, and blood flow. NVC score was used to quantitate the aforementioned characteristics.
Patients with type 2 DM showed significantly increased alterations including reduced capillary length (29.6%), irregular distribution (35.7%), and abnormal morphology (59.1%), while the corresponding NVC scores were comparable to those of control subjects. In addition, subjects with pre-DM had a significantly higher NVC score and greater alterations in distribution (26.8%) and morphology (48.8%) than control subjects. NVC score was positively correlated with diabetic peripheral neuropathy (DPN) and the number of microvascular complications.
NVC identified a high frequency of microcirculation abnormalities in subjects with pre-DM or type 2 DM compared to those in the control group. NVC score was also capable of detecting microvascular complications in patients with type 2 DM and was correlated with DPN and the number of microvascular complications.
Keywords: diabetic peripheral neuropathy, microcirculation, nailfold videocapillaroscopy, pre-diabetes, type 2 diabetes mellitus
1. Introduction
As population growth, aging, and the prevalence of obesity and inactivity have continued to increase rapidly, diabetes mellitus (DM) has become a major burden of adult public health.[1–3] DM increases the risk of adult disability and premature death and creates a tremendous socioeconomic burden due to micro- and macrovascular complications.[3] The long-term microvascular complications of diabetes include retinopathy, nephropathy, and neuropathy.[4,5] Several risk factors, such as duration of diabetes and degree of glycemic control, are involved in the clinical course of micro- and macroangiopathy. Thus, clinicians should focus closely on vascular complications in patients with diabetes.
Nailfold videocapillaroscopy (NVC) is a noninvasive diagnostic method that is used to evaluate the microcirculation and identify microvascular patterns in many rheumatic diseases, particularly systemic sclerosis and related disorders.[6–8] NVC is used to evaluate the morphology, distribution, density, and blood flow of nailfold dermal papillary capillaries. However, diabetic microangiopathy is a frequent and often early complication that mainly involves the retinal and renal microcirculations. Few previous studies demonstrated the presence of abnormalities in nailfold microcirculation and capillary morphology in patients with diabetes.[9–11] However, NVC has been shown to qualitatively and quantitatively identify microcirculation abnormalities in diabetic patients, and changes detected using NVC showed a positive correlation with diabetic retinopathy.[12,13] However, their microvascular characteristics and correlation with glycemic control and classical microvascular complications have not been systematically investigated. This study had 3 aims: 1st, to investigate the nailfold capillary abnormalities in patients with type 2 DM or pre-DM versus control subjects; 2nd, to compare the NVC characteristics in patients with type 2 DM according to disease duration and glycated hemoglobin (hemoglobin A1c [HbA1c]) level; and 3rd, to determine whether NVC score was correlated with diabetic microvascular complications.
2. Patients and method
2.1. Ethical approval
This was an observational cross-sectional and clinical pilot cohort study (protocol identification number NCT02564913 at http://www.clinicaltrials.gov). All candidates underwent a standardized interview process. The participants were introduced to the study purpose, procedures, potential risks, and benefits. Subsequently, they signed the informed consent form. The trial protocol was approved by the Institutional Review Board of Changhua Christian Hospital, Changhua, Taiwan (institutional review board reference number: 111106).
2.2. Patients
The participants in this prospective cross-sectional study were recruited from January 2012 to December 2013 from the outpatient clinic of endocrinology and Chinese medicine at Changhua Christian Hospital, Changhua, Taiwan. Participants in the type 2 DM group were diagnosed with DM based on criteria recommended by the American Diabetes Association and required to have a fasting plasma glucose of ≥7 mmol/L or an HbA1c of ≥6.5%, as measured on 2 separate occasions. People with pre-DM have a fasting glucose level of 5.6 to 7 mmol/L (impaired fasting glucose) or a 2-hour plasma glucose level of 7.8 to 11.1 mmol/L after an oral glucose tolerance test (impaired glucose tolerance) on 2 separate occasions.[14,15] Participants in the control group had a normal fasting glucose level (<5.6 mmol/L). Exclusion criteria were cancer, active liver disease, current pregnancy, active infection, and cerebrovascular disease.
All participants underwent a medical history collection, clinical examination including measurement of blood pressure (mmHg) and body mass index (kg/m2); and routine biochemistry testing including HbA1c (%), fasting blood glucose (mmol/L), triglycerides (mmol/L), total cholesterol (mmol/L), high-density lipoprotein cholesterol (mmol/L), and low-density lipoprotein cholesterol (mmol/L).
2.3. Nailfold videocapillaroscopy
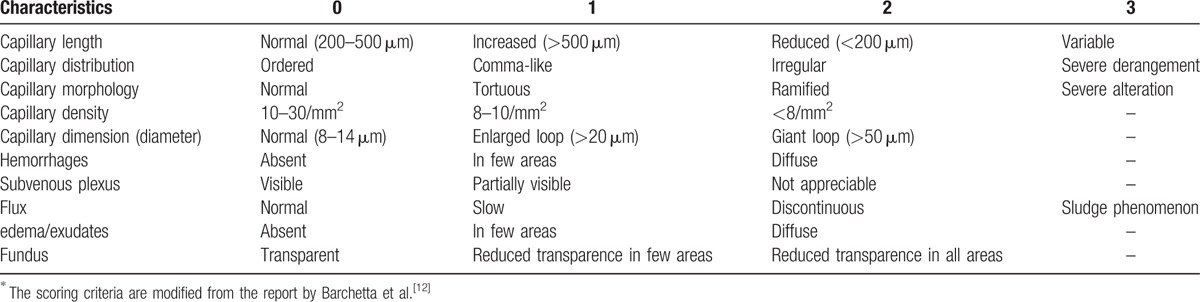
The NVC was performed on a microscope coupled with a digital video camera (DMX960, x320; Digilens Co., Taiwan). The same operator blinded to the patients’ clinical diagnoses performed the NVC examination according to a standardized and validated methodology on the 4th finger of each participant's left hand.[16] Each patient was acclimatized for 20 minutes at a room temperature of 24 °C prior to the NVC examination.[6] We examined 2 fields: capillaries in the distal row of the nailfold and hemorrhages near the distal row. The following morphological measurements were considered: alterations in capillary length (normal length [normal values are 200–500 μm], increased length, or reduced length), distribution (ordered, comma-like, irregular, or severely deranged), morphology (hairpin, tortuous, ramified, or bushy capillaries), capillary density (10–30/mm2, 8–10/mm2, or <8/mm2), diameter (normal, enlarged loop, or giant loop), hemorrhages (absent, in a few areas, or diffuse), and flux abnormalities (normal, slow, or discontinuous).[8,12] Representative images of the NVC alterations are shown in Fig. 1. NVC score was used to quantitate NVC characteristics.[12] The scoring criteria are shown in Table 1.
Figure 1.
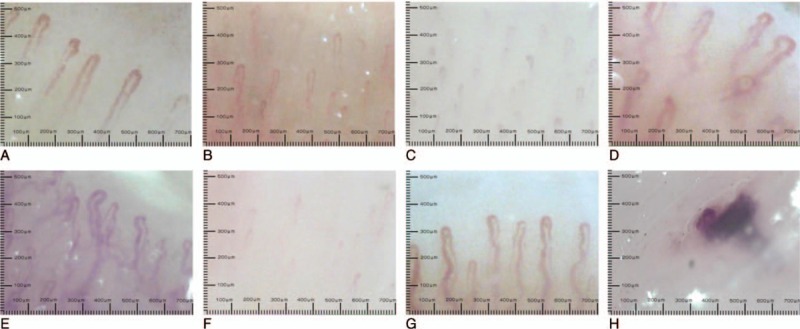
Representative characteristics of nailfold videocapillaroscopy alterations: (A) normal pattern of capillary vessels; (B) reduced capillary length; (C) irregular capillary distribution; (D) abnormal capillary morphology; (E) abnormal capillary morphology (severe alteration); (F) reduced capillary density; (G) enlarged capillary loop; and (H) hemorrhage.
Table 1.
Nailfold videocapillaroscopy (NVC) score was used to quantitate NVC characteristics according to several criteria∗.
2.4. Classification of diabetic microvascular complications
Common diabetic microvascular complications include retinopathy, neuropathy, and nephropathy. Diabetic retinopathy, a microvascular complication that can affect the retina as well as the macula, is a leading cause of visual impairment and blindness in patients with diabetes. A retinal examination was used to evaluate each patient for diabetic retinopathy according to the following diabetic retinopathy severity grading scale: no apparent retinopathy, nonproliferative diabetic retinopathy, and proliferative diabetic retinopathy.[17] Diabetic peripheral neuropathy (DPN), the most common complication of diabetes, is caused by impaired neural microvasculature.[4] Clinical examinations for DPN according to the Michigan diabetic neuropathy score include foot appearance, presence or absence of ulceration, and the evaluation of ankle reflexes and sensory responses to vibration, light touch, a pinprick, and the 10-g monofilament.[18,19] An early diagnosis of diabetic nephropathy can be made using a simple urine test to detect protein as an assessment of kidney function. Diabetic nephropathy has been categorized into 3 stages based on urinary albumin excretion values as follows: normoalbuminuria (albumin:creatinine ratio <30 μg/mg), microalbuminuria (30 μg/mg ≤ albumin:creatinine ratio ≤300 μg/mg), and macroalbuminuria (albumin:creatinine ratio ≥300 μg/mg).[20]
2.5. Data analysis
The statistical analysis of the data was performed using IBM SPSS Statistics 19 (IBM Co., NY). Tests included analysis of variance for continuous variables as well as Chi-square test or Fisher exact test for categorical variables as appropriate. Values of P < 0.05 were considered statistically significant. Alterations of NVC characteristics were quantified as NVC scores, while differences between the type 2 DM group and control subjects or individuals with pre-DM and control subjects were evaluated using the median values for the Mann–Whitney Utest. Spearman correlation was used to show the correlation among the NVC score, diabetic retinopathy severity grading scale, diabetic nephropathy stage, diabetic neuropathy examination score, and the number of microvascular complications.
3. Results
3.1. Study subject characteristics
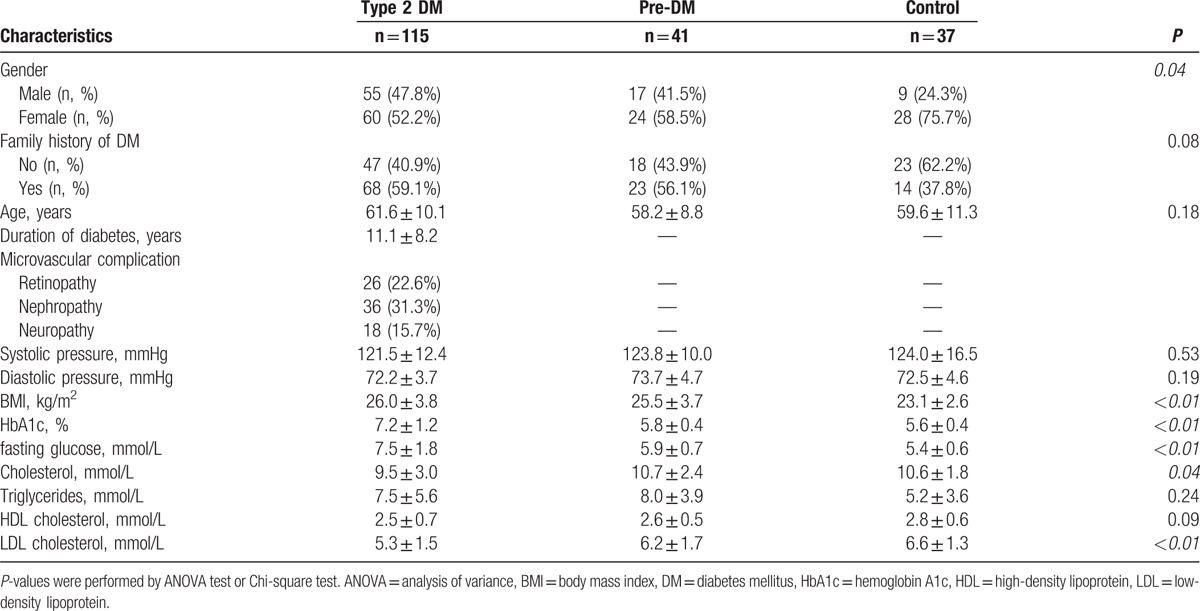
A total of 115 patients with a diagnosis of type 2 DM, 41 patients with impaired glucose tolerance, and 37 healthy controls without diabetes were enrolled in the study. The clinical characteristics of the patients with type 2 DM, patients with pre-DM, and healthy controls are shown in Table 2. The clinical characteristics of the patients with type 2 DM were as follows: mean age, 61.6 ± 10.1 years; mean diabetes duration, 11.1 ± 8.2 years; and mean body mass index, 26.0 ± 3.8. The patients with type 2 DM had significantly higher changes in HbA1c and fasting blood glucose levels but significantly lower changes in total cholesterol and low-density lipoprotein cholesterol levels than did those in the other 2 groups.
Table 2.
Clinical and biochemical characteristics of subjects with type 2 DM, subjects with pre-DM, and control subjects.
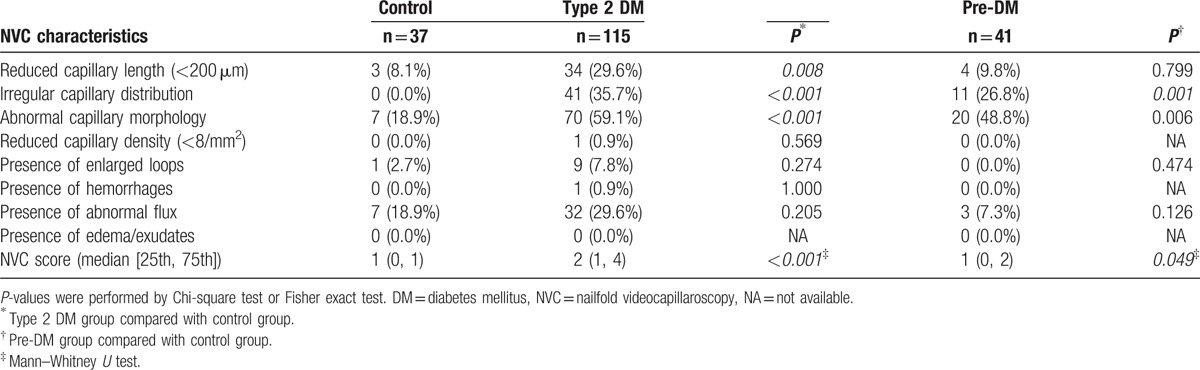
Results of the NVC measurements performed in the type 2 DM, pre-DM, and control groups are shown in Table 3. The patients with type 2 DM showed significantly higher frequencies of reduced capillary length (<200 μm) (29.6%), irregular capillary distribution (35.7%), abnormal capillary morphology (59.1%), and NVC score alteration compared to the control subjects. In addition, the pre-DM group had significantly higher frequencies of irregular capillary distribution (26.8%), abnormal capillary morphology (48.8%), and NVC score alteration than the control group.
Table 3.
NVC measurements in the total group with type 2 diabetes, prediabetes, and control subjects.
The NVC characteristics of patients with type 2 DM according to HbA1c level (HbA1c ≥ 7% and HbA1c < 7%) are shown in Table 4. The frequencies of reduced capillary length, irregular capillary distribution, abnormal capillary morphology, enlarged loop, and abnormal flux were increased in the subjects with HbA1c ≥ 7%. In addition, there was a significant increase in NVC score in the subjects with HbA1c ≥ 7%. However, NVC characteristics and scores did not vary significantly between patients with a type 2 DM disease duration ≥10 years and those with a disease duration <10 years (data not shown).
Table 4.
Comparison of NVC characteristics between the HbA1c <7% and HbA1c ≥7% groups in patients with type 2 diabetes mellitus.
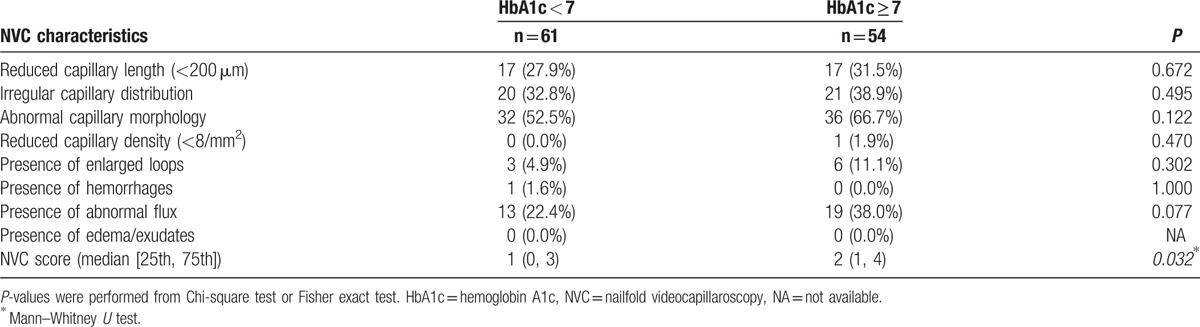
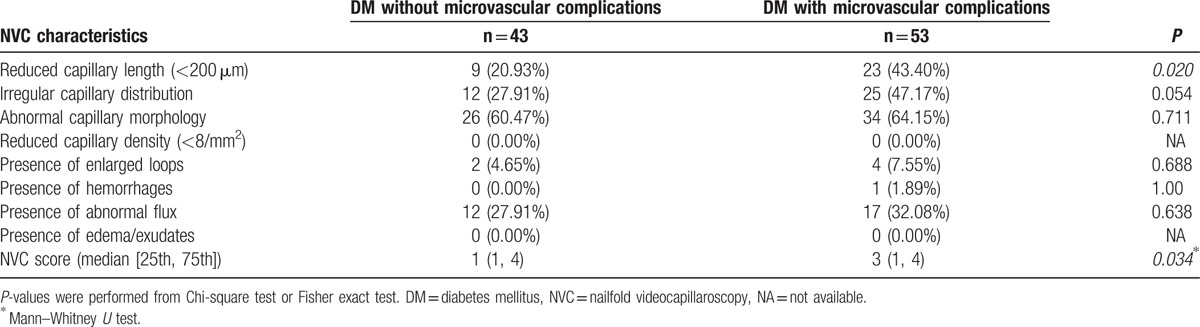
A comparison of NVC characteristics in type 2 DM patients without versus with microvascular complications is shown in Table 5. The frequencies of reduced capillary length, irregular capillary distribution, abnormal capillary morphology, enlarged loop, hemorrhage, and abnormal flux increased in type 2 DM patients with microvascular complications. Patients with type 2 DM and microvascular complications showed a significantly reduced capillary length (<200 μm) and increased NVC score.
Table 5.
Comparison of NVC characteristics between patients with type 2 diabetes mellitus without or with microvascular complications.
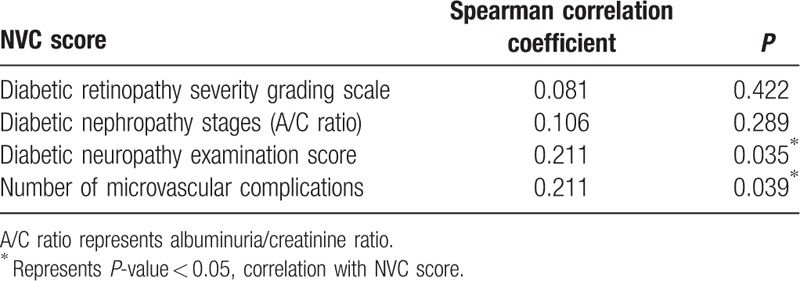
The correlations among NVC score, diabetic retinopathy severity grading scale, diabetic nephropathy stage, diabetic neuropathy examination score, and the number of microvascular complications are shown in Table 6. NVC score was significantly positively correlated with diabetic neuropathy examination score (Spearman correlation coefficient, 0.211; P = 0.035) and the number of microvascular complications (Spearman correlation coefficient, 0.211; P = 0.039).
Table 6.
Correlations among nailfold videocapillaroscopy (NVC) score, diabetic retinopathy severity grading scale, diabetic nephropathy stages, diabetic neuropathy examination score, and the number of microvascular complications.
4. Discussion
A typical diabetic pattern in the nailfold capillary has not yet been described, even if NVC detected increased density; reduced length and irregular distribution of the capillary loops; abnormal morphological features; and the presence of exudates, edema, and flux abnormalities variably associated with DM.[12,13] Our study showed that patients with type 2 DM had significantly greater alterations in reduced capillary length (<200 μm), an irregular capillary distribution, an abnormal capillary morphology, and increased NVC score compared with those of the control subjects. These previous studies found that patients with DM presented with a higher frequency of abnormal capillary morphology than control subjects.[10,11] Furthermore, we aimed to quantitatively and qualitatively study the nailfold capillary abnormalities of subjects with pre-DM. In particular, we found that NVC was able to detect microvascular abnormalities in prediabetic subjects compared with control subjects. The results revealed that subjects with pre-DM had significant alterations of irregular capillary distribution, abnormal capillary morphology, and NVC score. Other studies that used NVC to study red blood cell velocity at rest and at its peak after 1 minute of arterial occlusion revealed microcirculation dysfunction in patients with obesity and metabolic syndrome, but the same findings were not associated with impaired fasting glucose.[21,22] Thus, NVC could play a role in the characterization of diabetic microangiopathy and pre-DM among the NVC characteristics. We reported the nailfold capillary abnormalities based on a Taiwanese population, and these NVC characteristics were similar with those in patients of other ethnicities with diabetes.[12,13] Thus, we suggested that NVC abnormalities comprise a global phenomenon in patients with diabetes in different ethnic populations.
The challenge remains in identifying patients with DM who are at risk of developing microvascular complications.[23] Microvascular complications, including nephropathy, retinopathy, and neuropathy, are strongly related to both poor glycemic control and longer DM duration.[24] Here, we attempted to compare the NVC characteristics in patients with type 2 DM according to their diabetic duration and HbA1c level. The results revealed that the frequencies of capillary abnormalities were increased and NVC score was significantly increased in the subjects with HbA1c ≥7% compared to the subjects with HbA1c <7%. The results indicated that nailfold capillary abnormalities have been associated with vascular damage in patients with type 2 DM and poor glycemic control. On the other hand, there was no significant difference in NVC characteristics and scores according to disease duration. Therefore, we should consider other clinical and metabolic measurements to decrease bias. Capillary lengths were significantly reduced (<200 μm) and NVC score was increased in patients with type 2 DM and microvascular complications. Our study findings showed that type 2 DM with microvascular complications is indicative of significant microcirculation impairments. However, we did not compare each diabetic microvascular complication to determine severity in this study. Pseudo-3-dimensional vision-based nailfold morphological and hemodynamic analysis could potentially facilitate studies of microvascular complications and act as a significant index for a specific disease through the automatic analysis of a large number of microvascular images.[25] Therefore, NVC combined with pseudo-3-dimensional vision-based nailfold morphological and hemodynamic analysis could possibly be used to determine the severity of DM-related microvascular complications.
Our study demonstrated that NVC score was also capable of detecting microvascular abnormalities in patients with type 2 DM and was positively correlated with DPN and the number of microvascular complications. Microvascular abnormalities in the epineurial arteries and veins also revealed arteriosclerosis on the surface of the nerve and impaired blood flow in patients with DPN.[26,27] These microvascular changes were closely correlated with the presence of DPN. The other study surveyed nailfold capillary abnormalities in diabetic patients and revealed similar capillary characteristics and a correlation with diabetic retinopathy.[12] However, the previous study showed that more than half of the patients with DM suffered from one or more microvascular complications during >10 years of follow-up.[28] We tried to compare NVC characteristics of only 1 microvascular complication in patients with type 2 DM, but the sample size of patients with only 1 complication was too small to analyze. Future studies with a larger number of patients with a single microvascular complication would be valuable for evaluating NVC characteristics.
5. Conclusion
The current study revealed that NVC identified high frequencies of microcirculation abnormalities among subjects with pre-DM or type 2 DM. NVC score was positively correlated with DPN and the number of microvascular complications. The future application of NVC in patients with diabetes, pre-DM, and DM-related microvascular complications requires extensive investigation.
Acknowledgments
The authors thank all participants in the study. The authors also thank China Medical University Hospital, Taiwan (DMR-106-130), CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan, and also supported by Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), and Department of Chinese Medicine and Pharmacy (MOHW105-CMAP-M-114-133402) from Ministry of Health and Welfare, Taiwan for the support.
Footnotes
Abbreviations: DM = diabetes mellitus, DPN = diabetic peripheral neuropathy, HbA1c = hemoglobin A1c, NVC = nailfold videocapillaroscopy.
Funding/support: This study was supported in part by China Medical University Hospital, Taiwan (DMR-106-130), CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan, and also supported by Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), and Department of Chinese Medicine and Pharmacy (MOHW105-CMAP-M-114-133402) from Ministry of Health and Welfare, Taiwan.
The authors have no conflicts of interest to disclose.
References
- [1].Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. [DOI] [PubMed] [Google Scholar]
- [2].Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004;140:945–50. [DOI] [PubMed] [Google Scholar]
- [3].Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. [DOI] [PubMed] [Google Scholar]
- [4].Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther 2008;88:1322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kitada M, Zhang Z, Mima A, et al. Molecular mechanisms of diabetic vascular complications. J Diabetes Invest 2010;1:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gallucci F, Russo R, Buono R, et al. Indications and results of videocapillaroscopy in clinical practice. Adv Med Sci 2008;53:149–57. [DOI] [PubMed] [Google Scholar]
- [7].Ruaro B, Smith V, Sulli A, et al. Methods for the morphological and functional evaluation of microvascular damage in systemic sclerosis. Korean J Intern Med 2015;30:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chojnowski MM, Felis-Giemza A, Olesinska M. Capillaroscopy – a role in modern rheumatology. Reumatologia 2016;54:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Meyer MF, Pfohl M, Schatz H. Assessment of diabetic alterations of microcirculation by means of capillaroscopy and laser-Doppler anemometry. Medizinische Klinik 2001;96:71–7. [DOI] [PubMed] [Google Scholar]
- [10].Gasser P, Berger W. Nailfold videomicroscopy and local cold test in type I diabetics. Angiology 1992;43:395–400. [DOI] [PubMed] [Google Scholar]
- [11].Pazos-Moura CC, Moura EG, Bouskela E, et al. Nailfold capillaroscopy in diabetes mellitus: morphological abnormalities and relationship with microangiopathy. Braz J Med Biol Res 1987;20:777–80. [PubMed] [Google Scholar]
- [12].Barchetta I, Riccieri V, Vasile M, et al. High prevalence of capillary abnormalities in patients with diabetes and association with retinopathy. Diabetic Med 2011;28:1039–44. [DOI] [PubMed] [Google Scholar]
- [13].Rajaei A, Dehghan P, Farahani Z. Nailfold Capillaroscopy Findings in Diabetic Patients (A Pilot Cross-Sectional Study). Open J Pathol 2015;5:8. [Google Scholar]
- [14].American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(Suppl 1):S55–60. [DOI] [PubMed] [Google Scholar]
- [15].Nathan DM. Diabetes: advances in diagnosis and treatment. JAMA 2015;314:1052–62. [DOI] [PubMed] [Google Scholar]
- [16].Mark C Hou, Hui-Min Wang, Cheng-Lung Tseng, et al. A computerized system of nail-fold capillaroscopy for dry eye disease diagnosis. Multidim Syst Sign Process 2012;23:515–24. [Google Scholar]
- [17].Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes 2013;4:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Won JC, Park TS. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab 2016;31:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Basi S, Fesler P, Mimran A, et al. Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabetes Care 2008;31(Suppl 2):S194–201. [DOI] [PubMed] [Google Scholar]
- [21].Kraemer-Aguiar LG, Laflor CM, Bouskela E. Skin microcirculatory dysfunction is already present in normoglycemic subjects with metabolic syndrome. Metab: Clin Exp 2008;57:1740–6. [DOI] [PubMed] [Google Scholar]
- [22].Panazzolo DG, Sicuro FL, Clapauch R, et al. Obesity, metabolic syndrome, impaired fasting glucose, and microvascular dysfunction: a principal component analysis approach. BMC Cardiovasc Disord 2012;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med 2010;123(3 Suppl):S3–11. [DOI] [PubMed] [Google Scholar]
- [24].Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008;26:77–82. [Google Scholar]
- [25].Lo LC, Lin KC, Hsu YN, et al. Pseudo three-dimensional vision-based nail-fold morphological and hemodynamic analysis. Comput Biol Med 2012;42:873–84. [DOI] [PubMed] [Google Scholar]
- [26].Tesfaye S, Harris N, Jakubowski JJ, et al. Impaired blood flow and arterio-venous shunting in human diabetic neuropathy: a novel technique of nerve photography and fluorescein angiography. Diabetologia 1993;36:1266–74. [DOI] [PubMed] [Google Scholar]
- [27].Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes/Metab Res Rev 2012;28(Suppl 1):8–14. [DOI] [PubMed] [Google Scholar]
- [28].Huang CY, Tsai YT, Lai JN, et al. Prescription pattern of Chinese herbal products for diabetes mellitus in Taiwan: a population-based study. Evid Based Complement Alternat Med 2013;2013:201329. [DOI] [PMC free article] [PubMed] [Google Scholar]


