Abstract
Stereotactic radiotherapy (SRT) is a new approach to treat neovascular age-related macular degeneration (nAMD). The INTREPID trial suggested that SRT could reduce the frequency of regular intravitreal injections (IVIs) with antivascular endothelial growth factor drugs, which are necessary to control disease activity. However, the efficacy of SRT in nAMD and resulting morphological changes have not been validated under real-life circumstances, an issue, which we would like to address in this retrospective analysis.
Patients who met the INTREPID criteria for best responders were eligible for SRT. A total of 32 eyes of 32 patients were treated. Thereafter, patients were examined monthly for 12 months and received pro re nata IVI of aflibercept or ranibizumab. Outcome measures were: mean number of injections, best-corrected visual acuity, and morphological changes of the outer retina-choroid complex as well as patient safety.
Mean number of IVI decreased by almost 50% during the 12 months after SRT compared to the year before, whereas visual acuity increased by one line (logMAR). Morphological evaluation showed that most changes affect outer retinal layers.
Stereotactic radiotherapy significantly reduced IVI retreatment in nAMD patients under real-life circumstances. Therefore, SRT might be the first step to stop visual loss as a result of IVI undertreatment, which is a major risk.
Keywords: Intravitreal injection, neovascular age-related macular degeneration, optical coherence tomography, stereotactic radiotherapy, vascular endothelial growth factor
1. Introduction
Neovascular age-related macular degeneration (nAMD) is the leading cause of blindness in individuals over 50 years’ old in industrialized nations. In Germany alone, >30,000 new cases of nAMD are registered every year.[1] The current criterion standard of treating nAMD involves intravitreal injections (IVIs) of drugs like aflibercept, bevacizumab, and ranibizumab, which target the vascular endothelial growth factor (VEGF).[2] However, optimal treatment necessitates ongoing hospital review and repeated intravitreal injections, which, given the demographic development of an aging population and increasingly limited health care budget, will hardly be able to achieve in real-life.[1,3] Therefore, reduced injection frequency without compromising efficacy would be of major interest.
Ionizing radiation has been proposed for treatment of nAMD because of its anti-inflammatory, antifibrotic, and most notably antiangiogenic properties.[4] Radiation predominantly targets the DNA of cells that rapidly divide, which results in irreversible damage, preventing further replication and leading to apoptosis. Nondividing cells do have better repair mechanisms to keep their functional integrity.[5,6]
Radiotherapy can be divided into brachytherapy and teletherapy.[7,8] Brachytherapy involves a radiation source, which is directly placed next to the site of interest. Teletherapy is defined by radiation delivered from a distant source, from outside the patient's body and projected to the target tissue. Stereotactic radiotherapy (SRT), which is one form of teletherapy, involves focusing ≥2 narrow beams on a small area and by doing so delivering very high doses. This way, the ideal amount of radiation needed is brought directly to the lesion, whereas the amount of exposure to the surrounding area and the potential collateral damage is minimized.
Both types of radiotherapy have been clinically evaluated for nAMD.[7] The epimacular brachytherapy approach uses an intraocular probe containing a radioactive source that emits β radiation, which is positioned over the choroidal neovascular lesion next to the inner macular surface and held in position for 3 to 5 minutes to deliver a single dose of 24 Gy.[9] Although preliminary results were encouraging with regard to reduced injection frequency and improved vision, the CABERNET trial failed to replicate these results and did not reach the main end point of the study.[10–12]
The episcleral application of a strontium plaque (Sr90) was another brachytherapy method to treat nAMD.[13] The corresponding trial, which included 86 patients, however, only showed short-term changes of the clinical nAMD course. There was no treatment benefit apparent by 12 months.
Initial trials investigating the teletherapy approach in nAMD used high-energy radiation to target the macula. However, the results did not compare favorably to those achieved with anti-VEGF injections.[4,14,15] The lack of precise beam collimation resulted in severe collateral damage to the surrounding ocular tissue and vague targeting of the lesion site.
It was not until recently the INTREPID trial showed that a single dose of 16 Gy applied with the IRay® Radiotherapy System (formerly Oraya Therapeutics Inc., Newark, CA), which is a low-voltage, external-beam, SRT device, could reduce the frequency of intravitreal injections while maintaining visual acuity in nAMD patients.[16–18] The trial recruited 230 participants, who were previously treated for nAMD and already received at least 3 IVIs in the preceding year and had an ongoing need for anti-VEGF therapy at enrollment. Results showed a statistically significant, one-third reduction in anti-VEGF injections at 1 year after SRT. Participants whose lesions were ≤4 mm in greatest linear dimension, and who had significant fluid at the time of treatment responded best (55% reduction in anti-VEGF injections). No significant safety concerns were identified.[16,17] However, to date, there has been neither real-life efficacy nor data evaluating morphological changes published.
Herein, we report our experience with the application of SRT in nAMD patients. Our aim was to determine whether SRT maintains its efficacy under real-life circumstances and how it affects the outer retina-choroid complex from a morphological point of view.
2. Methods
2.1. Study design
Being the first medical center in Germany to use SRT in clinical routine, we retrospectively reviewed the medical records of 32 consecutive patients treated with SRT at our department from January 2014 through June 2015. The local institutional review board (University of Lübeck, Lübeck, Germany) approved data collection for this observational, noninterventional study. Everything complied with the tenets of the Declaration of Helsinki.
Patients received standard SRT (16 Gy) treatment if they had an active nAMD and the choroidal neovascularization (CNV) had a greatest linear dimension ≤4 mm in fluorescence angiography (FA). They needed to have received at least 3 IVIs (aflibercept or ranibizumab) during the previous 12 months and to present significant intraretinal or subretinal fluid in optical coherence tomography (OCT) with a continuing need for anti-VEGF treatment. These parameters corresponded to the criteria for best responders as determined in the INTREPID study.[16] Within 2 weeks after SRT, an IVI of aflibercept or ranibizumab, depending on the previous treatment regimen for each individual, was given. A switch of anti-VEGF agents was not performed during the whole observational period. Patients were assessed every 4 weeks with best-corrected visual acuity (BCVA) in Snellen, slit-lamp biomicroscopy, dilated fundus examination, enhanced depth imaging (EDI) spectral-domain (SD) OCT (Spectralis®; Heidelberg Engineering, Heidelberg, Germany). Patients received additional IVI based on a pro re nata (PRN) treatment regimen according to the guidelines of the German Ophthalmological Society, the German Retina Society, and the Professional Association of German Ophthalmologists.[19]
2.2. Outcome measures
The primary outcome measure was the number of IVI administered during the first year after SRT under real-life circumstances, which was compared to the number of IVI applied in the year before.
The secondary outcome measure was the BCVA at month 1, 3, 6, and 12 after SRT, which was compared to each patient's highest BCVA at any point since diagnosis of nAMD and treatment with anti-VEGF agents, as well as the BCVA at baseline right before SRT.
Investigated tertiary outcomes were morphological changes in the outer-retina-choroid complex. Therefore, EDI-SD-OCT scans at month 1, 3, 6, and 12 after SRT were compared to the one at baseline. In addition to the total macular thickness (TMT), emphasis was put on morphologic changes in the outer retina-choroid complex consisting of the photoreceptor cell bodies (ONL, outer nuclear layer), the photoreceptor inner segment/outer segment (IS/OS) junction as well as the outer photoreceptor segments (OPS), and the total choroidal thickness (TCT), with additional substructure analysis of the latter one in form of Sattler's layer (SL) and Haller's layer (HL) (Fig. 1). Segmentation of the layers was performed manually and measurements were done foveal and 1 mm as well as 2 mm nasal and temporal to the macular central fovea using Heidelberg Eye Explorer 1.9.10 (Heidelberg Engineering, Heidelberg, Germany).
Figure 1.
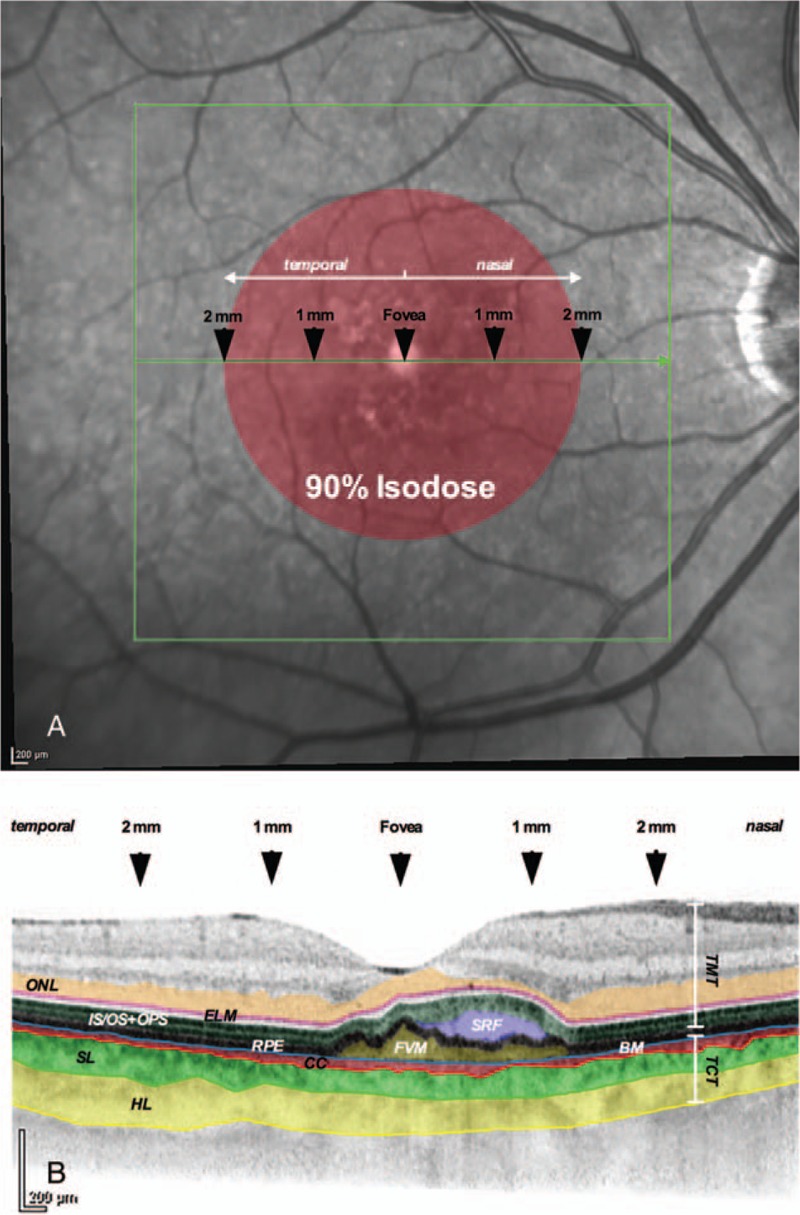
Red free photography and corresponding OCT scan. (A) The area of irradiation (90% isodose) is labeled schematically (red). Five locations within the most central OCT scan were evaluated for their morphologic characteristics. (B) The layers of interest are illustrated in color. BM = Bruch membrane, CC = choriocapillaris, ELM = external limiting membrane, FVM = fibrovascular membrane, HL = Haller's layer, IS/OS+OPS = junction of photoreceptor inner/outer segments plus outer photoreceptor segments, ONL = outer nuclear layer, RPE = retinal pigment epithelium, SL = Sattler's layer, SRF = subretinal fluid, TCT = total choroidal thickness TMT = total macular thickness.
Safety outcome measures included the incidence of adverse events and serious adverse events. Patients were evaluated by color fundus photography and indirect ophthalmoscopy in terms of changes possibly related to the radiation treatment.
2.3. Statistical methods
All values are expressed as mean ± standard deviation. Snellen BCVA was converted to logarithm of the minimum angle of resolution (logMAR) for statistical analysis. Statistical analysis was performed with Prism 6 (GraphPad, La Jolla, CA) using an analysis of variance and the Tukey multiple comparisons test. For all evaluations, a P value of <0.05 was considered statistically significant (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, ns: not significant).
3. Results
3.1. Patient characteristics
The medical records of 32 patients (32 eyes) with unilateral nAMD were reviewed. The mean age was 76.12 ± 7.33 years (range 59–89 years). Eighteen patients were female (79.44 ± 6.17 years), and 14 male (72.00 ± 6.69 years). Mean time from diagnosis was 35.4 ± 18.9 months. Patients had received 6.81 ± 1.77 IVIs (median 7; range 3–10) of aflibercept or ranibizumab during 12 months before SRT. The greatest linear dimension of the CNV lesions was 2.65 ± 0.78 mm (range 1.11–3.91 mm) and patients had a BCVA (logMAR) of 0.49 ± 0.29 (median 0.4; range 0.1–1.3) at baseline.
3.2. Primary outcome: number of anti-VEGF retreatments
At 12 months after SRT, significantly fewer IVIs (P < 0.0001) were given than in the year before radiation (Fig. 2).
Figure 2.
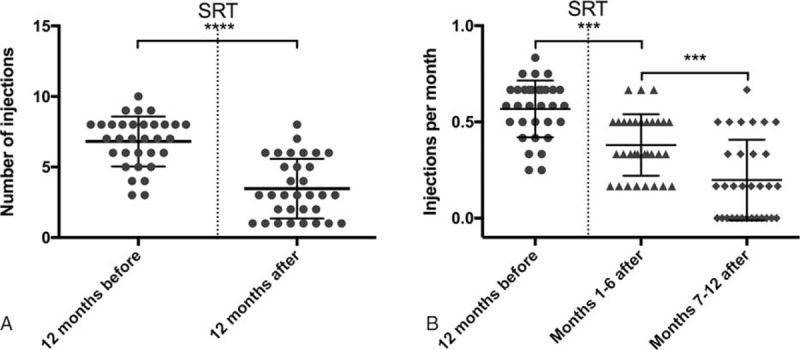
Number of injections and injection rate. (A) Mean total number of injections before and after SRT. (B) Mean number of injections per month (injection rate) before and after SRT. ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
The mean number of treatments was 3.47 ± 2.11 IVIs (median 3; range 1–6). The total number of injections was almost reduced by half (48.86%), and 8 patients only received that single mandatory injection after SRT. The injection rate (number of injections per month) was accordingly lowered by half (0.57 ± 0.15 to 0.29 ± 0.18; P < 0.0001).
Comparing the first half to the second half of the year after SRT, the effect on the injection rate was even more pronounced during the latter one (0.38 ± 0.16 to 0.20 ± 0.21; P < 0.001). Thirteen patients did not need any further IVI in the last 6 months of the observational period.
3.3. Secondary outcome: Visual acuity
The mean BCVA (logMAR) was significantly better at 1 (0.40 ± 0.28; median 0.3; P < 0.05), 3 (0.40 ± 0.32; median 0.3; P < 0.05), and 12 months (0.38 ± 0.30; median 0.3; P < 0.01) after SRT compared to the one at baseline (Fig. 3).
Figure 3.
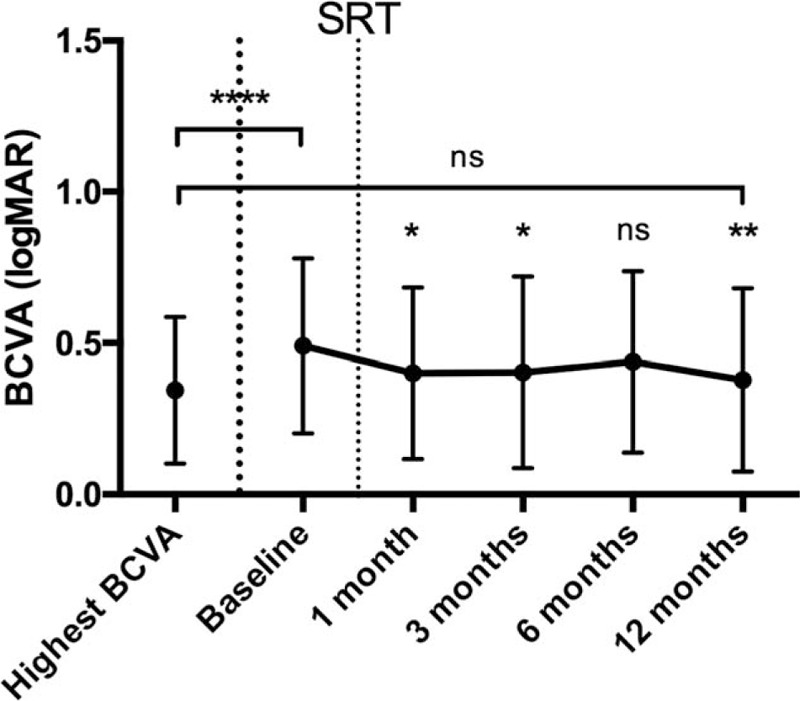
BCVA in logMAR before and after SRT. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001. BCVA = best-corrected visual acuity, ns = not significant, SRT = stereotactic radiotherapy.
Three of the 32 eyes showed a worse BCVA at 12 months compared to baseline. These 3 patients needed and received more than average injections (5.0 ± 1.0) after SRT.
Comparing the data at 12 months to each of the patients’ highest BCVA (0.34 ± 0.24) ever achieved since diagnosis of nAMD and treatment with IVI, there was no statistically significant difference evident. After SRT, 20 patients reached or even surpassed their highest BCVA of before.
3.4. Tertiary outcome: morphological changes of the outer retina-choroid complex
The mean TMT in the foveal region decreased from 271.64 ± 130.82 μm at baseline to 237.32 ± 101.23 μm at 1 month (P < 0.05), to 235.04 ± 79.49 μm at 3 months (P < 0.01), to 235.48 ± 100.62 μm at 6 months (P < 0.01), and to finally 200.60 ± 82.89 μm at 12 months (P < 0.0001) (Fig. 4A). In the adjacent area of 1 mm nasal to the central fovea, the mean TMT decreased from 339.40 ± 78.71 μm at baseline to 303.92 ± 40.96 μm at 12 months. At 2 mm, no statistically significant decline in TMT was measurable, neither nasal nor temporal to the central fovea. In addition, at 1 mm temporal to the central fovea, no statistically significant reduction in thickness was noticeable.
Figure 4.
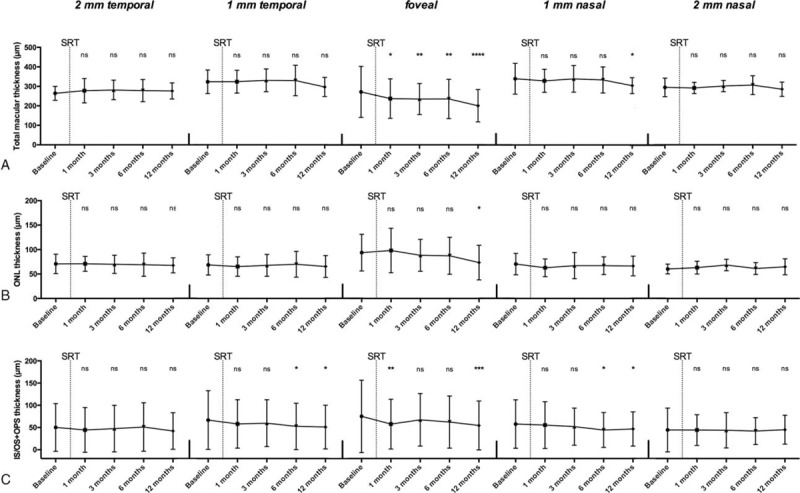
Morphological changes of retinal layers. (A) Total macular thickness, (B) ONL thickness, and (C) IS/OS and OPS thickness at the 5 locations are illustrated at baseline and after SRT. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. IS/OS = inner/outer segment junction, ONL = outer nuclear layer, OPS = outer photoreceptor segment, ns = not significant, SRT = stereotactic radiotherapy.
The mean ONL in the foveal region decreased from 93.72 ± 130.82 μm at baseline to 73.44 ± 35.33 μm at 12 months (P < 0.05) (Fig. 4B). In the bordering area of 1 mm and 2 mm, respectively, neither nasal nor temporal to the central fovea, no statistically significant decline in ONL thickness was detectable.
The mean thickness of the IS/OS junction and OPS layer in the foveal area shrunk from 75.04 ± 81.50 μm at baseline to 57.60 ± 56.02 μm at 1 month (P < 0.01) and to 54.52 ± 54.98 μm at 12 months (P < 0.001) (Fig. 4C). In the bordering area of 1 mm, either nasal or temporal to the central fovea the thickness declined from 55.40 ± 52.75 μm (nasal) and 57.96 ± 54.42 μm (temporal) at baseline to 46.64 ± 38.58 μm (nasal) and 50.96 ± 49.25 μm (temporal) at 12 months (P < 0.05). At 2 mm, neither nasal nor temporal to the central fovea, no significant change was measurable.
The mean thickness of the whole choroid, total choroidal thickness (TCT), did not show any statistical significant changes during 12 months after SRT, neither in the foveal region nor in the bordering locations measured (Fig. 5A). However, a tendency was evident with the subfoveal TCT decreasing from 203.83 ± 67.87 μm at baseline to 185.13 ± 46.68 μm at 12 months.
Figure 5.
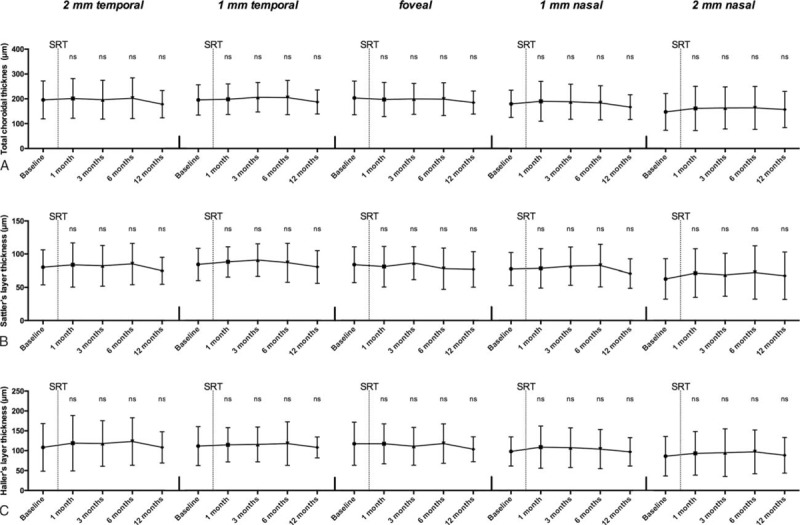
Morphological changes of choroidal layers. (A) Total choroidal thickness, (B) Sattler's layer, and (C) Haller's layer thickness at the 5 locations are illustrated at baseline and after SRT. ns = not significant, SRT = stereotactic radiotherapy.
Substructure analysis of the choroid did also not show any statistically significant changes, for neither Sattler's nor Haller's layer (Fig. 5B and C). The trend to a gradual decline in the subfoveal region, as described for the TCT, was more pronounced for Haller's layer (117.68 ± 54.18 μm at baseline to 103.77 ± 31.34 μm at 12 months, equivalent to 11.83%) than for Sattler's layer (83.86 ± 26.78 μm at baseline to 76.86 ± 26.51 μm at 12 months, equivalent to 8.35%), as there was a statistical significant difference in decrease (P < 0.05).
3.5. Safety outcome measures
No ocular or systemic adverse events or serious adverse events were observed during the study period. There were no signs of typical radiation-induced microangiopathy, such as microaneurysms, cotton wool spots, and hemorrhage on ophthalmoscopy or in the color photographs.
4. Discussion
The main objective of this study was to test the real-life effectiveness of SRT in nAMD patients and to capture potential morphological changes of the outer retina-choroid complex.
Analysis of the INTREPID data showed that, in patients previously treated with IVI and having lesions ≤4 mm in greatest linear dimension, SRT reduces the need for further injections (2.08) by half compared to anti-VEGF monotherapy (4.60).[16,18] At enrollment, the mean time from diagnosis of nAMD was 12.8 months in the SRT arm, and patients had already received a mean of 4.99 injections in the year before SRT. In patients of the control arm (anti-VEGF monotherapy), mean time from diagnosis was 16.2 months and they had already received 5.48 injections in the year before SRT.
It is particularly striking, that the patients in our study needed significantly more injections (6.81) in the 12 months before SRT than those in the INTREPID trial before SRT as well as those in the control arm after sham SRT treatment. Neovascular AMD is an extremely variable disease. Its activity, thus the need for IVI, changes through the years. This observation is outlined very nicely in a recently published study.[20] In this retrospective study, nAMD patients received a median number of 6 injections during the first year of onset. Thereafter, the frequency of injections declined to 4 to 5 in the following 4 years. Compared to these data, our patients, who had a mean time of 35.4 months (range 12–80 months) since diagnosis of nAMD, received above-average injections before SRT treatment, which might imply a lasting high activity of the disease. However, the SEVEN-UP study reported a mean of 6.8 injections for years 5–8 of the disease, which is much closer to our number of needed injections in the year before SRT.[21]
Differences in the number of injections applied could also be dependent on the treatment regimen applied. The other 3 studies as well as ours used a PRN regimen, which means that after the initial upload phase of 3 injections, monthly visits are necessary to determine disease activity by OCT. However, the follow-up treatment offers different protocols with the ones from the IVAN and CATT studies being the most commonly used.[22,23] Although the IVAN protocol suggests a set of 3 injections once retreatment criteria are fulfilled, the CATT protocol indicates only a single injection. Our as well as the treatment in the INTREPID trial was based on the CATT study's PRN protocol with a single injection, in contrast to Wecker et al's who used sets of three.[16,20] However, the patients included in the SEVEN-UP study received injections based on both treatment protocols.[21]
Another aspect, which might have influenced the number of injections applied, are the retreatment criteria. The INTREPID study protocol used a 100-μm increase in OCT thickness, new or increased macular hemorrhage, or a 1-line or more decrease in BCVA.[16] Especially the thickness criterion is less intensive when compared to others.[22,23] In contrast, we treated any persistent or increased intra- as well as subretinal fluid, irrespective of its impact on retinal thickness. This might imply that our number is closer to the actual number of needed injections to avoid undertreatment, which is the biggest risk to vision loss in nAMD patients.[1,3,24]
The INTREPID trial suggested that the benefits achieved through SRT do not come with the cost of loss in visual acuity. Looking at the whole study population, the mean change in BCVA at 12 months was −0.28 letters for the SRT arm and −1.57 letters in the sham arm compared to baseline, which is similar to the change in BCVA from year 1 to year 2 in the CATT study.[16,25] The best responder group had a statistically significant mean BCVA change (+2.18) that was 5.33 letters superior to sham (−3.15).[18] Our patients did also show that 1-line increase in BCVA at 12 months (from 0.49 logMAR to 0.38 logMAR). Only 3 patients (9.4%) showed a loss in BCVA after 12 months compared to baseline. However, their need for IVI was above average, which makes undertreatment unlikely and suggests a high disease activity being responsible for that observation.
Wecker et al[20] showed that the number of patients loosing >15 letters increases and those gaining the same amount of letters decreases from year 3 of the disease drastically. This fact becomes even more apparent when looking at the SEVEN-UP data.[21] From the therapeutic peak upon completion of 24 monthly treatments in the ANCHOR or MARINA trials, which corresponds to year 3 of the disease, mean visual acuity had extremely declined by almost 4 lines. The described vision loss derives from a variety of reasons including fibrosis, poor response to anti-VEGF treatment, and particularly geographic atrophy (GA). Recent clinical trials and laboratory studies suggest that the RPE may degenerate and perish through long-term anti-VEGF treatment, causing larger areas of geographic atrophy GA.[26–30] Hence, reducing the number of injections to control disease activity through SRT might have another advantage. However, to date there are no data on how SRT affects GA development and progression.
Because our study was not sham-controlled, we decided to add the highest BCVA ever achieved since diagnosis of nAMD as another parameter, which led to quite astonishing findings. At 12 months, we were not able to measure any statistically significant difference in BCVA (highest ever vs. 12 months) anymore and 20 patients reached or even surpassed their highest BCVA of before. Thus, our BCVA outcomes might indicate that the therapeutic effect of SRT, in conjunction with anti-VEGF treatment, differs substantially from anti-VEGF monotherapy. Furthermore, it supports the hypothesis that adjuvant low-voltage SRT produces a benefit.
The pathophysiology of nAMD lies in a functional and subsequent morphological impairment of the outer blood-retinal barrier, resulting in the accumulation of subretinal/intraretinal fluid and diffuse thickening of the affected central retina. That is why we decided not only to evaluate the total macular thickness, but also to perform a substructure analysis of the various layers in the outer retina-choroid complex. Evaluation was performed using the OCT data generated from each patient at the monthly examinations.
Looking at the total macular thickness in the central subfield, the INTREPID study showed a decrease of -85.90 ± 11.51 μm for SRT, whereas anti-VEGF monotherapy (sham) only resulted in a decline of -33.51 ± 12.65 μm. Since the morphologic outcome was favorable in the SRT arm compared to other studies like CATT, it was suggested that the therapeutic effects of SRT, in conjunction with anti-VEGF treatment, differ substantially from anti-VEGF monotherapy.[16,22,23] Our patients showed a mean reduction of -71.04 μm in total macular thickness, which is close to the results achieved in the INTREPID trial.
Different studies have shown that morphological recovery of the outer retinal layers is associated with recovery of visual functions.[31,32] The first retinal layer to be affected by breakdown of the outer blood-retinal barrier is the OPS layer as well as the neighboring ellipsoid zone, which is also referred as the photoreceptor inner segment/outer segment (IS/OS) layer. Accumulation of subretinal fluid leads to detachment of the neurosensory retina and functional impairment of the photoreceptors. Following that, the leakage fluid surrounds the cell bodies of the photoreceptors, indicated by swelling of the ONL.
Kashani et al[33] suggested that an increased total volume of the ONL and IS/OS is associated with decreased visual acuity in nAMD. Our data show that almost two-third of the total retinal thinning was contributed by decrease of the outer retinal layers in the foveal subfield. However, Sadda et al demonstrated similar results in patients treated with ranibizumab and bevacizumab monotherapy, as did Broadhead et al for aflibercept.[34–36] These results may suggest that IVI and SRT share a similar site of action and function synergistically. It could also provide an explanation, why the morphological results of the INTREPID study did not find any statistically significant difference between patients treated with SRT and IVI compared to those only receiving anti-VEGF monotherapy.[16,17]
During recent years, morphologic assessment of choroidal changes in health and disease has become a focus of ophthalmologic interest. The choroid supplies the outer retinal layers with nutrition and oxygen. Its vasculature is subdivided into choriocapillaris, SL and HL, of which the former has been more closely investigated owing to its proximity to Bruch's membrane and photoreceptors.[37] Choroidal thinning in AMD has been attributed to SL and HL.[38] Decreased vessel density and/or tissue shrinkage has been suggested. Interestingly, Shin et al[39] postulated the thinner the choroid the higher the prevalence of intra/subretinal fluid. Opposite to this, Razavi et al[40] associated a thicker choroid to hyperpermeability and enhanced exudation.
The results in the literature are controversial. Even though there is still some debate, most studies regarding changes of choroidal thickness following anti-VEGF treatment have demonstrated a decrease in choroidal thickness in nAMD eyes.[41,42] Razavi et al[40] interpreted this effect as a favorable influence of anti-VEGF treatment not only on retinal, but also on the underlying choroidal exudation by reduction of choroidal vascular hyperpermeability.
Ting et al[43] noted a significant reduction in subfoveal choroidal thickness from 213.7 μm to 190.3 μm (mean change −23.4 μm). So did Yamazaki et al, who demonstrated a significant choroidal thinning from 244 μm to 226 μm (mean change −18 μm) in eyes undergoing intravitreal ranibizumab treatment over 12 months.[42]
In contrast, our patients did not show a significant thinning of the choroid, but looking at the absolute values, a decreasing trend (mean change −18.7 μm) could be assumed, which probably would have become statistically significant if we had a bigger sample size. However, it remains unclear whether there is a threshold when choroidal thinning becomes disadvantageous for the course of the disease, since choroidal atrophy is a danger and associated with bad visual outcome.
Substructure analysis did not show any significant changes in SL and HL thickness. This is especially interesting when considering a strong association between activity of the neovascularization and the SL thickness.[38]
Although a potential weakness of our study may be the relatively small number of eyes from a single center, the retrospective study design and the absence of a control group, we did not aim at just repeating the INTREPID trial. Instead, this study relied on comparisons of individual patient's functional and morphological results before and after SRT. However, enough publications regarding the use and effects of anti-VEGF monotherapy in real-life are available, so that the literature provides control data to additionally compare ours to, as we did. Another important limitation of this study is the relatively short observational period of only 12 months follow-up. Especially in terms of safety this might be an issue, since effects and side effects of ionizing radiation do show a delayed onset. Despite having not found any adverse effect in our study population, a longer follow-up is needed to guarantee unconditional safety for patients treated with SRT. Moreover, given the retrospective nature of this study, visual acuity was measured using Snellen charts even though “early treatment diabetic retinopathy study” (ETDRS) charts would have been preferred.
A major strength of this study is its independence from any commercial intent and that it is not initiated by private industry. Our results are very promising, even under real-life circumstances, in which patient adherence and compliance are often a problem. In addition, we provide morphological data on the outer retina-choroid complex, which has not been done before in this context.
5. Conclusions
Demographic developments will only add to the clinical, financial, and social burden of nAMD in the future. Therefore, it is mandatory to explore novel treatment options for the mounting number of patients receiving regular IVI. SRT is a promising option, which not only showed its value within a large randomized, double-masked, sham-controlled clinical trial (INTREPID), but also under real-life circumstances. SRT may be the first step to stop visual loss through anti-VEGF undertreatment in the wake of diminishing patient adherence as well as compliance.[1] For the patients, responding well to SRT, there is the hope of less clinical attendance and reduced cumulative risk of potential adverse effects through repeated injections like endophthalmitis, increased intraocular pressure, and atrophic changes in the outer retina-choroid complex.
Footnotes
Abbreviations: ANOVA = analysis of variance, BCVA = best-corrected visual acuity, CNV = choroidal neovascularization, EDI = enhanced depth imaging, ETDRS = Early Treatment Diabetic Retinopathy Study, GA = geographic atrophy, HL = Haller's layer, IS/OS = photoreceptor inner segment/outer segment junction, IVI = intravitreal injection, logMAR = logarithm of the minimal angle of resolution, nAMD = neovascular age-related macular degeneration, OCT = optical coherence tomography, ONL = outer nuclear layer, OPS = outer photoreceptor segments, PRN = pro re nata, SD = spectral domain, SL = Sattler's layer, SRT = stereotactic radiotherapy, TCT = total choroidal thickness, TMT = total macular thickness, VEGF = vascular endothelial growth factor.
In particular, no commercial or proprietary interests exist. No funding was received. The procedures of the study were in accordance with the Declaration of Helsinki, and the ethical approval for this study was received from the institutional review board (vote reference number 16–104). All the participants gave an informed and written consent.
The authors have no conflicts of interest to disclose.
References
- [1].Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015;99:220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kaiser PK. Emerging therapies for neovascular age-related macular degeneration: drugs in the pipeline. Ophthalmology 2013;120:S11–15. [DOI] [PubMed] [Google Scholar]
- [3].Mantel I. Optimizing the anti-VEGF treatment strategy for neovascular age-related macular degeneration: from clinical trials to real-life requirements. Transl Vis Sci Technol 2015;4.DOI: 10.1167/tvst.4.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Holz FG, Engenhart R, Bellmann C, et al. Stereotactic radiation therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration. Front Radiat Ther Oncol 1997;30:238–46. [DOI] [PubMed] [Google Scholar]
- [5].Taddei PJ, Chell E, Hansen S, et al. Assessment of targeting accuracy of a low-energy stereotactic radiosurgery treatment for age-related macular degeneration. Phys Med Biol 2010;55:7037–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cantley JL, Hanlon J, Chell E, et al. Influence of eye size and beam entry angle on dose to non-targeted tissues of the eye during stereotactic x-ray radiosurgery of AMD. Phys Med Biol 2013;58:6887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Petrarca R, Jackson TL. Radiation therapy for neovascular age-related macular degeneration. Clin Ophthalmol 2011;5:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Neffendorf JE, Jackson TL. Stereotactic radiotherapy for wet age-related macular degeneration: current perspectives. Clin Ophthalmol 2015;9:1829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jackson TL, Dugel PU, Bebchuk JD, et al. Epimacular Brachytherapy for Neovascular Age-related Macular Degeneration (CABERNET): fluorescein angiography and optical coherence tomography. Ophthalmology 2013;120:1597–603. [DOI] [PubMed] [Google Scholar]
- [10].Dugel PU, Petrarca R, Bennett M, et al. Macular epiretinal brachytherapy in treated age-related macular degeneration: MERITAGE study: twelve-month safety and efficacy results. Ophthalmology 2012;119:1425–31. [DOI] [PubMed] [Google Scholar]
- [11].Dugel PU, Bebchuk JD, Nau J, et al. Epimacular brachytherapy for neovascular age-related macular degeneration: a randomized, controlled trial (CABERNET). Ophthalmology 2013;120:317–27. [DOI] [PubMed] [Google Scholar]
- [12].Jackson TL, Dugel PU, Bebchuk JD, et al. Epimacular brachytherapy for neovascular age-related macular degeneration (CABERNET): fluorescein angiography and optical coherence tomography. Ophthalmology 2013;120:1597–603. [DOI] [PubMed] [Google Scholar]
- [13].Jaakkola A, Heikkonen J, Tommila P, et al. Strontium plaque brachytherapy for exudative age-related macular degeneration: three-year results of a randomized study. Ophthalmology 2005;112:567–73. [DOI] [PubMed] [Google Scholar]
- [14].Evans JR, Sivagnanavel V, Chong V. Radiotherapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2010;CD004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sivagnanavel V, Evans JR, Ockrim Z, et al. Radiotherapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2004;CD004004. [DOI] [PubMed] [Google Scholar]
- [16].Jackson TL, Chakravarthy U, Kaiser PK, et al. Stereotactic Radiotherapy for Neovascular Age-related Macular Degeneration: 52-Week Safety and Efficacy Results of the INTREPID Study. Ophthalmology 2013;120:1893–900. [DOI] [PubMed] [Google Scholar]
- [17].Jackson TL, Chakravarthy U, Slakter JS, et al. Stereotactic Radiotherapy for Neovascular Age-Related Macular Degeneration: Year 2 Results of the INTREPID Study. Ophthalmology 2015;122:138–45. [DOI] [PubMed] [Google Scholar]
- [18].Jackson TL, Shusterman EM, Arnoldussen M, et al. Stereotactic radiotherapy for wet age-related macular degeneration (INTREPID): influence of baseline characteristics on clinical response. Retina (Philadelphia, Pa) 2015;35:194–204. [DOI] [PubMed] [Google Scholar]
- [19].Gesellschaft DO. Statement of the German Ophthalmological Society, the German Retina Society and the Professional Association of German Ophthalmologists: The anti-VEGF therapy in neovascular age-related macular degeneration: Therapeutic Strategies (November 2014). Klin Monbl Augenheilkd 2015;232:202–10. [DOI] [PubMed] [Google Scholar]
- [20].Wecker T, Ehlken C, Bühler A, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol 2016;DOI: 10.1136/bjophthalmol-2016-308668. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON. Ophthalmology 2013;120:2292–9. [DOI] [PubMed] [Google Scholar]
- [22].Chakravarthy U, Harding SP, Rogers CA, et al. IVAN Study Investigators Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119:1399–411. [DOI] [PubMed] [Google Scholar]
- [23].Martin DF, Maguire MG, Ying G, et al. CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond) 2015;29:721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 2012;119:1388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schütze C, Wedl M, Baumann B, et al. Progression of retinal pigment epithelial atrophy in antiangiogenic therapy of neovascular age-related macular degeneration. Am J Ophthalmol 2015;159:1100–14. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lois N, McBain V, Abdelkader E, et al. Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy. Retina (Philadelphia, Pa) 2013;33:13–22. [DOI] [PubMed] [Google Scholar]
- [29].Ranjbar M, Brinkmann MP, Tura A, et al. Ranibizumab interacts with the VEGF-A/VEGFR-2 signaling pathway in human RPE cells at different levels. Cytokine 2016;83:210–6. [DOI] [PubMed] [Google Scholar]
- [30].Ranjbar M, Brinkmann MP, Zapf D, et al. Fc Receptor inhibition reduces susceptibility to oxidative stress in human RPE cells treated with bevacizumab, but not aflibercept. Cell Physiol Biochem 2016;38:737–47. [DOI] [PubMed] [Google Scholar]
- [31].Simader C, Ritter M, Bolz M, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology 2014;121:1237–45. [DOI] [PubMed] [Google Scholar]
- [32].Casalino G, Bandello F, Chakravarthy U. Changes in neovascular lesion hyperreflectivity after anti-VEGF treatment in age-related macular degeneration: an integrated multimodal imaging analysis. Invest Ophthalmol Vis Sci 2016;57:OCT288–298. [DOI] [PubMed] [Google Scholar]
- [33].Kashani AH, Keane PA, Dustin L, et al. Quantitative subanalysis of cystoid spaces and outer nuclear layer using optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci 2009;50:3366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Keane PA, Liakopoulos S, Ongchin SC, et al. Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2008;49:3115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Joeres S, Kaplowitz K, Brubaker JW, et al. Quantitative comparison of optical coherence tomography after pegaptanib or bevacizumab in neovascular age-related macular degeneration. Ophthalmology 2008;115:347–54. e2. [DOI] [PubMed] [Google Scholar]
- [36].Broadhead G, Hong T, Li H, et al. Retinal morphology changes following intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2014;55:3924–13924. [DOI] [PubMed] [Google Scholar]
- [37].Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med 2012;33:295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Esmaeelpour M, Ansari-Shahrezaei S, Glittenberg C, et al. Choroid, Haller's, and Sattler's layer thickness in intermediate age-related macular degeneration with and without fellow neovascular eyes. Invest Ophthalmol Vis Sci 2014;55:5074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shin JY, Kwon KY, Byeon SH. Association between choroidal thickness and the response to intravitreal ranibizumab injection in age-related macular degeneration. Acta Ophthalmol 2015;93:524–32. [DOI] [PubMed] [Google Scholar]
- [40].Razavi S, Souied EH, Darvizeh F, et al. Assessment of choroidal topographic changes by swept-source optical coherence tomography after intravitreal ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol 2015;160:1006–13. [DOI] [PubMed] [Google Scholar]
- [41].Branchini L, Regatieri C, Adhi M, et al. EFfect of intravitreous anti–vascular endothelial growth factor therapy on choroidal thickness in neovascular age-related macular degeneration using spectral-domain optical coherence tomography. JAMA Ophthalmol 2013;131:693–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yamazaki T, Koizumi H, Yamagishi T, et al. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology 2012;119:1621–7. [DOI] [PubMed] [Google Scholar]
- [43].Ting DSW, Ng WY, Ng SR, et al. Choroidal thickness changes in age-related macular degeneration and polypoidal choroidal vasculopathy: a 12-month prospective Study. Am J Ophthalmol 2016;164:128–36. e1. [DOI] [PubMed] [Google Scholar]


