Abstract
Rationale and Patients concerns:
Despite the introduction of varied disease-modifying antirheumatic drugs and biological agents, a substantial proportion of patients remain untreatable. We report a 56-year-old Chinese female patient with a case of refractory rheumatoid arthritis (RA) complicated with multiple myeloma (MM) who was treated successfully with Bortezomib followed by autologous stem cell transplantation (ASCT).
Diagnosis and Interventions:
We report a 56-year-old Chinese female patient who was diagnosed as RA complicating with MM. She received 4 cycles of Bortezomib-based chemotherapy followed by ASCT. The response of her RA and MM were evaluated after every cycle of Bortezomib-based chemotherapy.
Interventions and Outcomes:
After the first Bortezomib-based chemotherapy cycle, this patient's symptoms were significantly alleviated and thereafter the RA activity continued to improve. After the 4 courses of Bortezomib-based chemotherapy, the C-reactive protein was <0.5 mg/dL and the disease activity score 28-erythrocyte sedimentation rate was 2.0. No hematological or nonhematological side effects were observed during the treatment of Bortezomib.
Lessons:
Bortezomib might be a new safe and promising drug for refractory RA patients.
Keywords: autologous stem cell transplantation, Bortezomib, proteasome inhibitor, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) has a high morbidity of autoimmune disease characterized by chronic synovial-based inflammation and bone erosion. It affects approximately 1% of the world population. The involved joints were swollen with tenderness and progressive destruction. If inadequately treated, there is a significant consequence of joint ankylosis and a considerable disability.[1–3] Early and aggressive treatment, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents can contribute to control of disease progression. However, there is still an important proportion of patients who do not respond well to the traditional drugs, and RA is still an incurable disease. Bortezomib (Millenium Pharmaceuticals, Inc., and Johnson & Johnson Pharmaceutical Research & Development, L.L.C., Latina, Italy), known as a registered proteasome inhibitor (PI), is highly effective in multiple myeloma (MM) and mantle cell lymphoma. Moreover, there is an increasing amount of data showing that this agent may also have effects for autoimmune diseases, for example, RA and ulcerative colitis.[4–5] Here, we report a patient with refractory RA complicating with MM who has been successfully treated with Bortezomib followed by autologous stem cell transplantation (ASCT).
2. Case report
2.1. Presenting concerns
A 56-year-old Chinese female with a 10-month history of RA was admitted to our hospital. She first developed polyarthralgia in Jan 2012 and visited Department of Rheumatology in our hospital. She manifested with symmetrical polyarthritis involving the metacarpophalangeal (MCP) joints and proximal interphalangeal (PIP) joints. Serological examinations exhibited high levels of anticyclic citrullinated peptide antibody (ACPA), immunoglobulin (Ig)M-rheumatoid factor (RF) and C-reactive protein (CRP). X-ray of hands showed obvious osteoporosis and bone erosion. A diagnosis of RA was made according to the classification criteria proposed by the American College of Rheumatology in 1987.[6] Initially, she was administered with oral nonsteroidal anti-inflammatory drugs (meloxicam 15 mg daily) and prednisone (10 mg daily) combined with subcutaneous methotrexate (15 mg weekly). After 3 months, a medical assessment was performed and showed the disease activity remaining high: the CRP was 8.22 mg/dL, and the score of the disease activity score 28-erythrocyte sedimentation rate (DAS28-ESR) was 4.12. Treatment with subcutaneous etanercept was added in May 2012 at a dose of 50 mg weekly. Her RA disease activity temporarily subsided, but later flared up again.
2.2. Clinical findings
She was referred to our department in Nov 2012 because of paleness and fatigue. Her family history included no consanguinity or collagen diseases. Her medical history was unremarkable. On physical examination, her blood pressure was 120/62 mm Hg with a regular heart rate of 80 bpm and a temperature of 36.0 °C. Cardiac, lung, and abdominal examination revealed no abnormalities. There was bilateral symmetric polyarthritis in the MCP and PIP joints. The laboratory data were as follows: leukocyte count 13.39 × 109/L, neutrophil 8.73 × 109/L, lymphocyte 2.95 × 109/L, hemoglobin 91 g/L, and platelet count 343 × 109/L. Urinalysis revealed proteinuria (5.6 g/d in pooled urine) and hematuria. The serum creatinine level was elevated at 279 μmol/L. The serum Ig levels declined entirely (IgG 10.5 g/L, IgA 0.65 g/L, and Immunoglobulin M (IgM) 0.23 g/L). Her IgM-RF level was 60 U/mL and ACPA was 100 U/mL. The level of CRP and ESR were increased at 8.46 mg/dL and 37 mm/h, respectively. The profiles of antinuclear antibodies were all negative, and complement 3 was normal. Bone marrow smears showed 18% plasma cells, and their immunophenotype characteristic by flow cytometry showed CD38+, CD45−, CD19−, CD20−, CD138+, CD54+, CD56+, and cytoplasm kappa light chain 1.2%, lambda light chain 97.4% which indicated that plasma cells were clonal. Serum and urine immune fixed electrophoresis showed monoclonal lambda light chain. A total of 24-hour urinary lambda light chain quantity was 3840 mg.
2.3. Diagnostic focus and assessment
The patient was diagnosed as symptomatic MM according to the previously published criteria.[7] After diagnosis of MM, treatment for MM took priority because it is the fatal disease and drugs for RA were discontinued.
2.4. Therapeutic focus and assessment
She received 4 courses of of Bortezomib-based chemotherapy regimen Bortezomib was administered intravenously (IV) at a dose of 1.3 mg/m2 on day 1, 4, 8, and 11 of each cycle. Pegylated liposomal doxorubicine (40 mg/m2) was administered IV on day 4. Dexamethasone (20 mg/d) was administered IV on days 1, 2, 3 and 4 in Nov 2012, Dec 2012, Jan 2013, and Feb 2013, respectively. After induction chemotherapy, she received high-dose chemotherapy (melphalan 140 mg/m2) followed by ASCT in Apr 2013. γ-Interferon was given as maintenance therapy once her hematopoietic function recovered with 3 Million international units 3 times a week and changed to twice a week 6 months later.
2.5. Follow-up and outcome
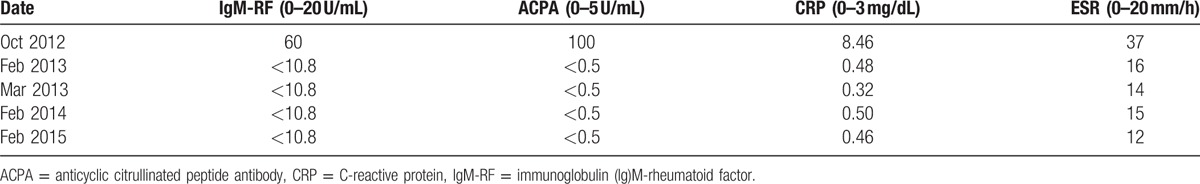
After the first Bortezomib-based chemotherapy cycle, her symptoms of RA alleviated significantly and thereafter the RA activity gradually improved (Table 1). After the 4 courses of Bortezomib-based chemotherapy, the CRP was <0.5 mg/dL, and the DAS28-ESR was 2.0 (the cutoff value representing remission defined as <2.6[8]). There was no hematological and nonhematological side effects during the treatment of Bortezomib. In addition, her MM achieved complete remission (CR) after 4 courses of Bortezomib-based chemotherapy regimen. The MM and RA conditions have been stabilized and remained at CR since Feb 15, 2015.
Table 1.
Changes of levels of autoantibodies and acute phase biomarkers in RA.
3. Discussion
We have described the successful clinical course of a RA patient with MM who has failed the treatment of methotrexate combined with biologic agents. This patient responded to Bortezomib treatment followed by ASCT. The RA activity decreased, the joint symptoms resolved, and the score of DAS28-ESR improved without any significant adverse effect.
RAs is a chronic inflammatory disease characterized by systemic inflammation, destruction of the joints, and production of autoantibodies recognizing dozens of putative autoantigens. Even though the shift in strategy toward the earlier treatment including DMARDs and the biological reagents have greatly improved the outcomes, 60% patients have been reported to fail to achieve remission or low disease activity.[9] Novel and promising therapeutic strategies need to be exploited.
Bortezomib, a PI targeting at the ubiquitin-proteasome pathway, is the first clinically approved PI for the treatment of MM.[10] The ubiquitin-proteasome system (UPS) is recognized as the major pathway for degradation of intracellular proteins, many of which play a key role in the regulation of proinflammatory cytokines. UPS deregulation is involved in several pathological conditions such as cancer, viral infections, and autoimmunities.[11–12] It is suggested that Bortezomib inhibits inducible NF-κB activity in MM. Hence, it was supposed that Bortezomib can inhibit NF-κB activation process and indirectly produces a potential anti-inflammatory response for treating autoimmune diseases.[13] Indeed, Bortezomib attenuated both the acute phase and chronic phase of the inflammatory response and the severity of collagen-induced arthritis and ameliorate histological features in RA rats.[14] In healthy volunteers and RA patients, Bortezomib was demonstrated to be a rapid and potent inhibitor of NF-κB-inducible cytokines, including tumor necrosis factor-α, interleukin (IL)-1β, IL-6, and IL-10, by activating T cells.[15] In addition, Bortezomib displayed clear therapeutic efficacy in animal models of systemic lupus erythematosus, inflammatory bowel disease, myasthenia gravis, and arthritis.[16–19]
From this refractory RA patient with MM who received the treatment of Bortezomib, we surprisingly found that her RA activity decreased, the symptoms of joint resolved and the score of DAS28-ESR improved except her MM was controlled well. She was followed for up to 2 years, her RA condition still maintained CR and indexes of RA activity were excellent. In addition, no side effects occurred during Bortezomib therapy. ASCT is increasingly used to control severe and refractory autoimmune diseases including RA. However, results from EBMT showed that the effect of ASCT on RA were not so good.[20] From our results, we suggest that ASCT may be only consolidated by the effects of Bortezomib.
Taken together, this study report indicated that Bortezomib might be a new safe and promising drug for refractory RA patients. However, more patient population need to be observed in clinic, and the mechanism needs to be investigated.
Footnotes
Abbreviations: ASCT = autologous stem cell transplantation, MM = multiple myeloma, RA = rheumatoid arthritis.
Ethical review and informed consent: This case report was approved by the Clinical Research Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University. The patient provided their written informed consent for the publication of this case report.
The authors have no conflicts of interest to disclose.
References
- [1].Tanaka Y, Yamaoka K. JAK inhibitor tofacitinib for treating rheumatoid arthritis: from basic to clinical. Mod Rheumatol 2013;23:415–24. [DOI] [PubMed] [Google Scholar]
- [2].Zerbini CA, Lomonte AB. Tofacitinib for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol 2012;8:319–31. [DOI] [PubMed] [Google Scholar]
- [3].Bannwarth B, Kostine M, Poursac N. A pharmacokinetic and clinical assessment of tofacitinib for the treatment of rheumatoid arthritis. Expert Opin Drug Metab Toxicol 2013;9:753–61. [DOI] [PubMed] [Google Scholar]
- [4].Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med 2003;81:235–45. [DOI] [PubMed] [Google Scholar]
- [5].Bennett MK, Kirk CJ. Development of proteasome inhibitors in oncology and autoimmune diseases. Curr Opin Drug Discov Dev 2008;11:616–25. [PubMed] [Google Scholar]
- [6].Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumtol 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [7].Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J 2003;4:379–98. [PubMed] [Google Scholar]
- [8].Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology 2004;43:1252–5. [DOI] [PubMed] [Google Scholar]
- [9].Mohammed RH, Farahat F, Kewan HH, et al. Predictors of European League Against Rheumatism (EULAR) good response, DAS-28 remission and sustained responses to TNF-inhibitors in rheumatoid arthritis: a prospective study in refractory disease. Springerplus 2015;4:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res 2008;14:1649–57. [DOI] [PubMed] [Google Scholar]
- [11].Finley D. Recognition and processing of ubiquitin-proteinconjugates by the proteasome. Ann Rev Biochem 2009;78:477–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].De Bettignies G, Coux O. Proteasome inhibitors: dozens of molecules and still counting. Biochimie 2010;92:1530–45. [DOI] [PubMed] [Google Scholar]
- [13].Verbrugge SE, Scheper RJ, Lems WF, et al. Proteasome inhibitors as experimental therapeutics of autoimmune diseases. Arthritis Res Ther 2015;28:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fierabracci A, Ayroldi E. Experimental strategies in autoimmunity: antagonists of cytokines and their receptors, nanocarriers, inhibitors of immunoproteasome, leukocyte migration and protein kinases. Curr Pharm Des 2011;17:3094–107. [DOI] [PubMed] [Google Scholar]
- [15].van der Heijden JW, Oerlemans R, Lems WF, et al. The proteasome inhibitor bortezomib inhibits the release of NFκB-inducible cytokines and induces apoptosis of activated T cells from rheumatoid arthritis patients. Clin Exp Rheumatol 2009;27:92–8. [PubMed] [Google Scholar]
- [16].Hainz N, Thomas S, Neuvert K, et al. The proteasome inhibitor bortezomib prevents lupus nephritis in the NZB/W F1 mouse model by preservation of glomerular and tubulointerstitial architecture. Nepron Exp Nephrol 2012;120:e47–58. [DOI] [PubMed] [Google Scholar]
- [17].Yanaba K, Asana Y, Sugaya M, et al. Proteasome inhibitor bortezomib ameliorates intestinal injury in mice. PLoS One 2012;7:e34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gomez AM, Vrolix K, Martínez- Martínez P, et al. Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol 2011;186:2503–13. [DOI] [PubMed] [Google Scholar]
- [19].Lee SW, Kim JH, Park YB, et al. Bortezomib attenuates murine collagen-induced arthritis. Ann Rheum Dis 2009;68:1761–7. [DOI] [PubMed] [Google Scholar]
- [20].Snarski E, Snowden JA, Oliveira MC, et al. Onset and outcome of pregnancy after autologous haematopoietic SCT (AHSCT) for autoimmune diseases: a retrospective study of the EBMT autoimmune diseases working party (ADWP). Bone Marrow Transplant 2015;50:216–20. [DOI] [PubMed] [Google Scholar]


