Abstract
Introduction:
Hypertension is a major risk factor contributing to cardiovascular disease, which is the number one cause of deaths worldwide. Although antihypertensive medications are effective at controlling blood pressure, current first-line treatment for hypertension is nonpharmacological lifestyle modifications. Recent studies indicate that isometric resistance training (IRT) may also be effective for assisting with blood pressure management. The aim of this study was to determine the efficacy of IRT for blood pressure management and the suitability of a low-intensity working control group.
Methods:
Forty hypertensive individuals, aged between 36 and 65 years, conducted IRT for 8 weeks. Participants were randomized into 2 groups, working at an intensity of either 5% or 30% of their maximum voluntary contraction. Participants performed 4 × 2 minute isometric handgrip exercises with their nondominant hand, each separated by a 3-minute rest period, 3 days a week.
Results:
Blood pressure measurements were conducted at baseline and at the end of the protocol using a Finometer. Eight weeks of isometric resistance training resulted in a 7-mmHg reduction of resting systolic blood pressure (SBP) (136 ± 12 to 129 ± 15; P = 0.04) in the 30% group. Reductions of 4 mmHg were also seen in mean arterial pressure (MAP) (100 ± 8 to 96 ± 11; P = 0.04) in the 30% group. There were no statistically significant reductions in diastolic blood pressure for the 30% group, or any of the data for the 5% group.
Conclusion:
Isometric resistance training conducted using handgrip exercise at 30% of maximum voluntary contraction significantly reduced SBP and MAP. A lack of reduction in blood pressure in the 5% group indicates that a low-intensity group may be suitable as a working control for future studies.
Keywords: handgrip exercise, hypertension, isometric resistance training
1. Introduction
Approximately 40% of adults aged 25 years and older worldwide have been diagnosed with hypertension.[1] Hypertension is a major risk factor that contributes to cardiovascular disease, including coronary artery disease, stroke, and heart failure.[2,3] Cardiovascular diseases (CVDs) are the number one cause of death globally; according to World Health Organisation (2015), in 2012, 31% of global deaths (approximately 17.5 million people) were due to CVD.[4] Hypertension is responsible for 45% of cardiovascular deaths owing to heart disease and 51% owing to stroke worldwide.[1] Antihypertensive medications are effective at controlling blood pressure and have minimal side effects; however, only half the people with hypertension reach treatment goals.[5] Current first-line treatment for hypertension is nonpharmacological lifestyle modification including eating a healthy diet, cessation of smoking, and increasing physical activity.[2,3,6]
Currently, the recommended exercise programme for blood pressure management in adults is dynamic endurance aerobic exercise of at least 150-minute moderate intensity, 75-minute vigorous intensity, or an equivalent combination of both each week, as well as at least 2 days of muscle strengthening.[7] In 2011/12, only 44% of adults in the United States adhered to recommended exercise criteria.[7] Recent analyses suggest that isometric resistance training (IRT) may elicit blood pressure reductions greater than those seen with dynamic aerobic and resistance exercise.[2,8,9]
A recent systematic review and subsequent meta-analysis confirms previous findings that IRT reduces systolic blood pressure (SBP) by almost 7 mmHg, whereas diastolic blood pressure (DBP) and mean arterial pressure (MAP) were both lowered by almost 4 mmHg.[2] Low- to moderate-intensity isometric handgrip exercise can be performed anywhere, requires relatively inexpensive equipment, and does not elicit the same level of cardiovascular stress as aerobic exercise.[2] Recent work suggests that IRT may become a new tool in the nonpharmacological treatment of high blood pressure.[10–12] The 2015 systematic review by Inder et al [13] suggests certain demographic groups, males and individuals aged ≥45 years, may acquire greater blood pressure reductions from IRT.
Randomized controlled studies of IRT, for ≥4 weeks in duration, have predominately used a 30% maximum voluntary contraction (MVC) and a sedentary control.[9] Ray and Carrasco[14] utilized a sham group, which held a handgrip dynamometer, but did not generate any force. Previous studies have utilized a low intensity during isometric leg training.[15,16] We have found no reported studies, which have utilized an intensity <10% MVC handgrip exercise with prehypertensive and/or hypertensive participants. In addition, previous studies of 4 to 10 weeks duration have focused on people aged between 20 and 35 years or 60 and 80 years with a sedentary control. Peters et al[17] conducted an isometric handgrip study with 10 participants aged 52 ± 5 over 6 weeks. A larger, longer trial was needed to look at handgrip exercise in prehypertensive and hypertensive individuals aged between 35 and 70 years.
The primary aim of this study was to establish the size of reduction in blood pressure using handgrip exercise at 30% vs. 5% MVC in individuals aged 35 to 70 years. A 5% MVC was chosen to determine whether the low intensity would elicit reductions in BP, and therefore test suitability for control group allocation in future studies.
2. Methods
2.1. Participants
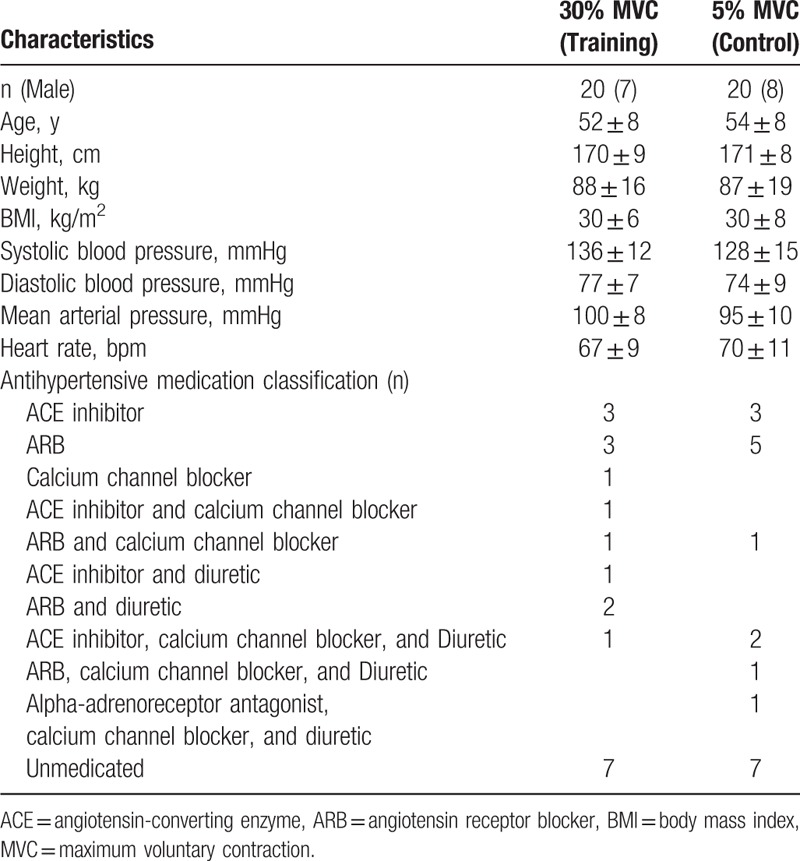
This study consisted of 40 white participants with mild or prehypertension, men (n = 15) and women (n = 25), aged between 36 and 65 years recruited from Armidale, NSW, Australia. Participants had a resting SBP ≥120 mmHg and/or a resting DBP ≥80 mmHg, or were receiving pharmacotherapy to treat their BP (65%). Participants were given a standardized health questionnaire and were excluded if they had known cardiovascular disease or multiple comorbidities, were unable to participate under their doctor's recommendation, smokers, and/or those with arthritis or carpal tunnel, which may have been aggravated with handgrip exercise. Participant baseline characteristics are displayed in Table 1.
Table 1.
Participant baseline characteristics.
The University of New England Human Ethics Committee approved the investigation, all participants provided written informed consent before participation, and all procedures were in accordance with the University's guidelines. This research project is registered with ClinicalTrials.gov, the identifier for the study is NCT02458456.
2.2. Study design
The blood pressure-lowering effects of IRT have been previously established; however, previous studies have used a nonexercising control group, and one study used a sham group.[9] The objectives of this study were to determine BP reductions utilizing handgrip exercise at 30% MVC as well as to test whether a 5% MVC training group would elicit blood pressure-lowering effects, or could be utilized as a working control. Eligible participants were familiarized to all testing equipments and randomized into either a 30% MVC (n = 20) or a 5% MVC (n = 20) training group. Participant enrolment and randomization were conducted by D.Carlson. Randomization was conducted before baseline blood pressure measurements using a computer-generated random number assignment, resulting in differences in SBP at baseline.
Participants were blinded as to which group they were randomized into; only the research team members who conducted the protocol were aware of group assignment. A numbered code system was used to identify participants, which allowed the researchers supervising the exercise protocol to ensure that participants worked at the correct intensity, while blinding participants to group assignment. A specifically designed light box was set up as a feedback mechanism for participants during IRT to prevent them knowing what intensity they were working at. All participants were exposed to the same conditions and equal attention to counteract concerns of disparities between groups owing to the Hawthorne effect.[18,19]
2.3. IRT training protocol
Participants trained 3 days per week for 8 weeks using a DHD-3 Digital Hand Dynamometer (Saehan Corporation, South Korea) with their nondominant hand. At the start of each training session, the participants conducted 3 contractions using maximum force, each separated by 30 seconds; these were then averaged to calculate the resistance at which they would perform either 30% MVC or 5% MVC on the day. Participants then completed 4 sets of 2-minute isometric handgrip contractions separated by 3-minute rest periods. Each training session was conducted in the Exercise Physiology Lab at the University of New England, Armidale, under direct supervision of a member of the research team.
2.4. Blood pressure measurements
Baseline and postintervention blood pressure was established using a beat-to-beat Finometer Midi Model-2 (Finapres Medical Systems B.V., Amsterdam, The Netherlands). Two minutes of continuous blood pressure measurement were recorded to assess resting SBP, DBP, heart rate (HR), and MAP. The Finometer utilizes an infrared photo-plethysmograph built into a finger cuff based on the Penaz volume-clamp method to enable continuous noninvasive BP measurements.[20,21] To address concerns of the accuracy of the Finometer, raised after commencement of the study, and to gauge consistency with brachial cuff BP, we compared the Finometer measurements with those of a sphygmomanometer in some participants. All baseline and post comparison data were performed with BP measurements, which were conducted using the Finometer. Upper arm cuff measurements were conducted on the last 16 participants at baseline, and 24 participants postintervention. The purpose of this was to assess the coefficient of variation between the Finometer and manual sphygmomanometer measurements.
All testing was conducted in a quiet, temperature-controlled room, following a 4-hour fast from food and caffeine, and a 12-hour abstinence from alcohol and vigorous exercise. All post-tests were conducted 24 hours after the final day of week 8 IRT and within 2 hours of the initial pretesting time of day, time of medication ingestion was standardized. Blood pressure was measured in the participants’ dominant arm (right n = 38, left n = 2) using the Finometer Midi and sphygmomanometer. Baseline and 24-hour post-IRT blood pressure measurements were conducted with the participant lying supine on a massage table, with their arm relaxed by their side.
An aneroid Heine Gamma G7 sphygmomanometer, which was calibrated by a technician before use, was utilized for brachial BP measurements. The auscultatory method was conducted by placing the cuff around the dominant arm of the individual and listening for Korotkoff sounds with a Littmann Classic IISE stethoscope, using the recommended blood pressure measurement guidelines.[22,23] Three blood pressure measurements were taken, each separated by a 5-minute rest period. After another 5 minutes of rest, the Finometer was used to record 2 minutes of continuous blood pressure measurements.
The Finometer was calibrated by a technician before commencement of the study, and extensive training and practice were provided to the observers to ensure accuracy of recordings. The finger cuff was placed on the middle finger of the dominant hand and the height correction unit used to correct hydrostatic blood pressure changes for the hand being away from heart level. Use of the height-adjusting component converts finger cuff pressure to brachial pressure, meeting the American Association for the Advancement of Medical Instrumentation (AAMI) criteria.[21]
2.5. Data analysis
BeatScope Easy software which records waveforms and beat-to-beat data were used to unpack the Finometer data into an excel spreadsheet. Microsoft Excel (Microsoft Corporation, Redmond, WA) was then used to calculate the mean and standard deviation for the last 15, 30, 60, and the entire 120 seconds of baseline and post-IRT recording. Shapiro-Wilk test of normality, repeated measures 2-way analysis of variance (ANOVA) (measure × time) with Tukey contrasts, and pair-wise comparisons were conducted to determine the best statistical model to use. Based on this, the entire 120 seconds of baseline and post-IRT data were used for all pre-post calculations. Paired t tests and repeated measures 2-way ANOVA (group × time) were conducted to evaluate the P value for differences in pre-post data. Multivariate analysis of variance (MANOVA) and independent t tests were conducted to compare the 5% and 30% MVC groups. All MANOVA and paired t tests were analyzed using SPSS (version 22); P ≤ 0.05 was considered statistically significant. Results are mean ± standard deviation, unless otherwise specified.
Shapiro-Wilk test of normality was conducted on all groups before analysis to confirm that ANOVA was suitable to analyze the data. There was one possible outlier in the 30% MVC group, which appeared consistently in the SBP, DBP, and MAP pre and post data. Cook's distance indicated that with ranges between 23% and 32%, although it may be influential, it is unlikely that it would be a major influence, so the data were retained during analysis.
In line with intention to treat, blood pressure was recorded on the last day of IRT and used as last outcome carried forward post measurements for participants unable to complete the entire eight week protocol.
3. Results
Of the 40 participants, 2 in the 30% MVC group were unable to complete the entire program due to work circumstances. The adherence to IRT was 100% in the 38 participants who completed the eight week study. Randomized groups were matched at baseline for age, gender, height, weight, and medication status as displayed in Table 1. There were no reported changes in exercise, diet, or medication throughout the study by any of the participants, and no difference between baseline and post weight for either group. Recruitment was conducted during 3 months, the trial ended when participants’ time of recruitment meant that completion of the 8-week protocol would be mid-December, and no follow-up was conducted. There was no harm or unintended effects reported in either group.
To establish the size of reduction in blood pressure in both groups, a 120-second resting baseline blood pressure recording was taken before and 24 hours post-IRT (Table 2). Table 3 exhibits comparisons between 15, 30, and 60-second sampling, against the 120-second blood pressure recording. Upper arm cuff measurements were taken in 16 participants at baseline and 24 participants post intervention to validate the measurements taken with the Finometer.
Table 2.
Comparison of 120-second blood pressure measurements.
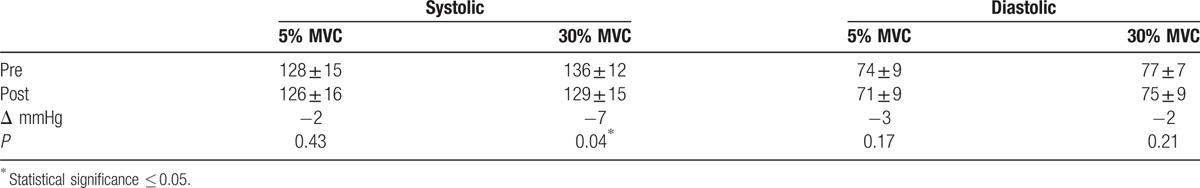
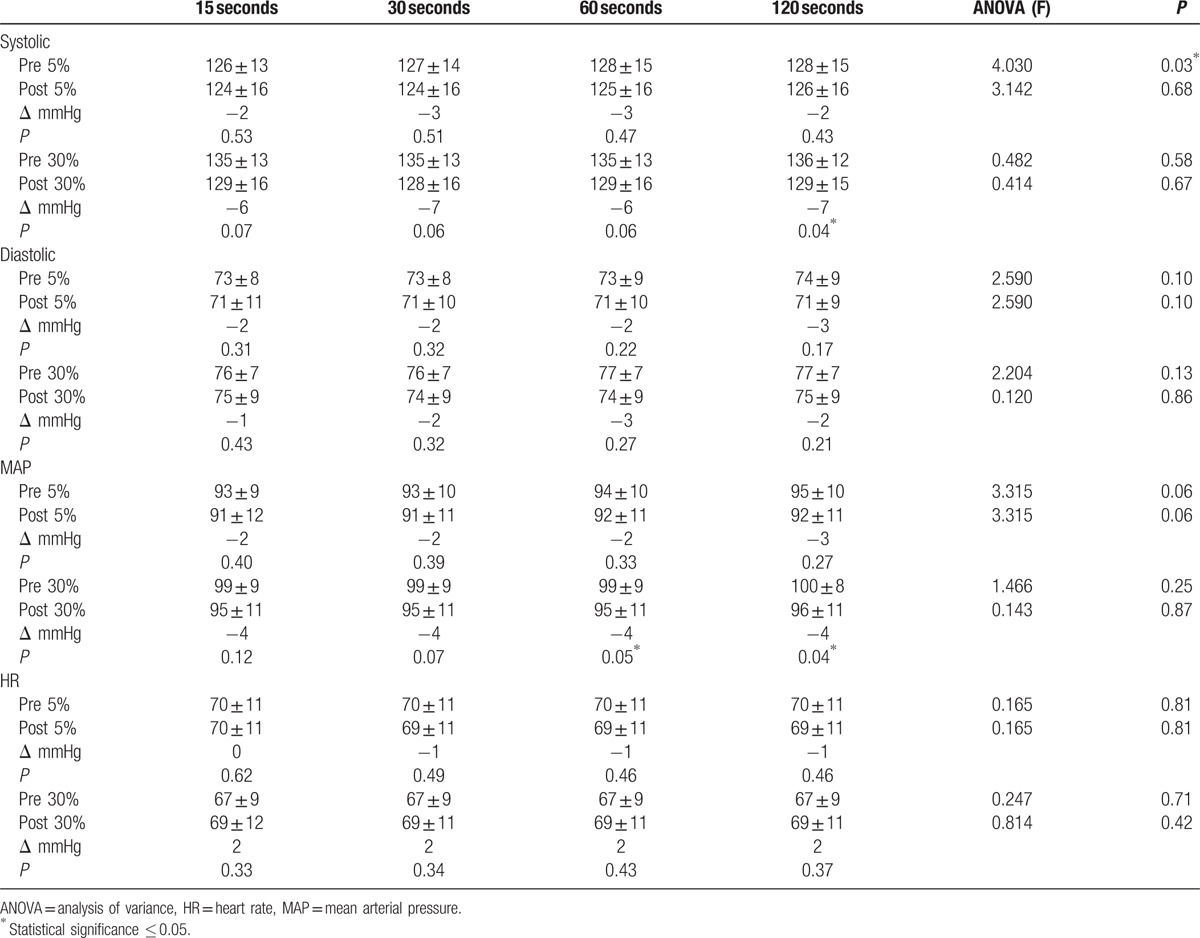
Table 3.
Comparison of effect of sampling duration.
3.1. ANOVA analysis of blood pressure, MAP, and HR
Eight weeks of isometric handgrip training resulted in a significant 7-mmHg reduction in baseline versus postintervention SBP in the 30% MVC group, with a nonsignificant 2-mmHg reduction in the 5% MVC group (Table 2). Individual variance in SBP was greater in participants in the 5% MVC group than that seen in the 30% group, as illustrated in Figure 1. Multivariate ANOVA with a Wilk Lambda of 0.86 (P = 0.24), indicate no statistically significant differences between the groups across the 4 sampling duration measurements at baseline. There were no significant differences for post-intervention SBP in both groups with 30% MVC at 129 ± 15 and 5% MVC at 126 ± 16 (95% confidence interval [CI] −13.14, 6.66; P = 0.51). The majority of participants with reductions in SBP had corresponding reductions in DBP as illustrated in Figure 2; however, there were no significant reductions in DBP in either the 30% or 5% MVC group. There was no difference between the 30% MVC and 5% MVC groups with baseline DBP 77 ± 7 and 74 ± 9 (95% CI −8.20, 1.64; P = 0.19) and also post-intervention 75 ± 9 and 71 ± 9 (95% CI −9.32, 2.21; P = 0.22), respectively. Significant reductions were observed in MAP from baseline to post-intervention of −4 mmHg (95% CI 0.14, 7.98; P = 0.04) in the 30% MVC group, but not in the 5% MVC group with −3 mmHg (95% CI −2.21, 7.56; P = 0.27) (Table 3). Analysis indicated an unchanged HR in the 30% MVC group (95% CI −6.13, 2.41; P = 0.37), and in the 5% MVC group (95% CI −2.04, 4.33; P = 0.46).
Figure 1.
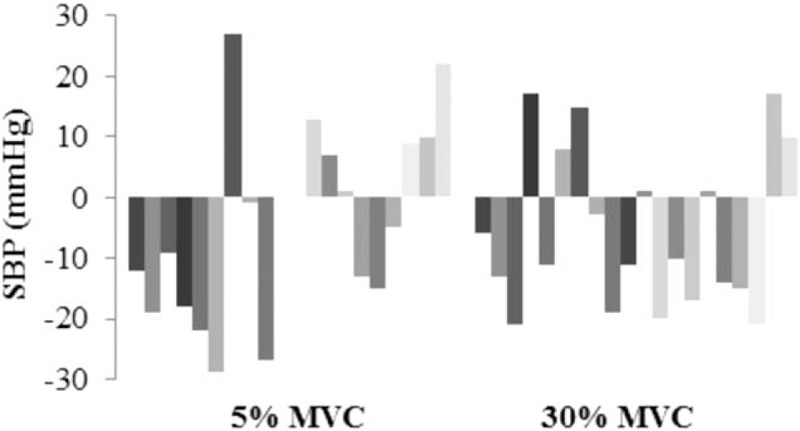
Individual participant changes in systolic blood pressure from baseline to post-intervention.
Figure 2.
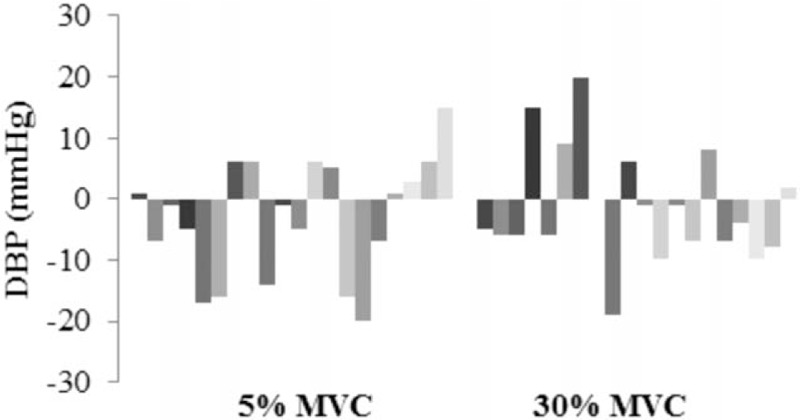
Individual participant changes in diastolic blood pressure from baseline to post-intervention.
3.2. Analysis of covariance analysis of blood pressure, MAP, and HR
Univariate analysis of covariance (ANCOVA) with baseline SBP as a covariate was conducted to verify possible outcome variances between the 5% and 30% groups. ANCOVA indicated significant reductions in SBP in both groups with 30% MVC (P = 0.03 and 5% MVC P = 0.02. There were no statistically significant differences in post-intervention SBP between the groups, when the analysis was conducted with baseline SBP as a covariate, with 30% MVC at 127 ± 3 (SEM) and 5% MVC at 128 ± 3 (SEM) (95% CI −8.19, 10.02, P = 0.84). Analysis of change in DBP with baseline DBP as a covariate indicated significant reductions in both groups of 30% MVC (P = 0.02) and 5% MVC (P < 0.01), with reductions of 2 and 3 mmHg, respectively. Analysis using baseline MAP as a covariate indicates significant reductions at 30% MVC (P < 0.01) and 5% MVC (P = 0.02). Analysis using baseline HR as a covariate shows significant differences between baseline and post-IRT in both groups (P < 0.01), despite HR reducing by 1 bpm in the 5% MVC group, and increasing by 2 bpm in the 30% MVC group. Post-intervention comparisons of HR indicated that both groups were identical at 69 ± 11 with no statistically significant differences (95% CI −6.46, 7.59; P = 0.87).
3.3. Effect of sampling duration
Repeated measures ANOVA for 15, 30, 60, and 120 seconds of pre- and post-resting SBP, DBP, MAP, and HR showed that the only data with statistically significant variation across the 4 measurements was the SBP in the 5% MVC group, as seen in Table 3. Population mean for SBP in the 5% group ranged from 126 mmHg at 15 seconds to 128 mmHg at both 60 and 120 seconds, (P = 0.03). Based on this analysis, it was determined that the 120-second data were more robust, so the consensus was for it to be used for our pre-post analyses.
3.4. Comparison of groups
Multivariate analysis of variance was conducted to compare SBP, DBP, MAP, and HR pre- and post-data for the 5% and 30% groups; all comparisons have a Wilk Lambda close to 1, all with a P value >0.05. No statistically significant group differences were indicated by the correlation between the dependent variables (measurement and time). Levene Test of Equality of error variances were not statistically significant for the 15, 30, 60, or 120 seconds of pre or post-data in any of the measures with all having a P value >0.05. As the assumption of homogeneity of variance has not been violated and Wilk Lambda shows correlation of the 5% and 30% groups, it is reasonable to say that both groups were the same, despite the 30% group having a larger baseline SBP than the 5% group.
3.5. Comparison of sphygmomanometer and Finometer averages
Owing to possible concerns regarding the accuracy of the Finometer, arm cuff measures using a sphygmomanometer were also taken at baseline and 24 hours post-intervention in some of the participants (baseline n = 16, post n = 24). As the following data are a comparison of measurement tools, all participants, regardless of which group they were in, were used for the analyses. Baseline SBPs using sphygmomanometer and Finometer was 132 ± 9 and 136 ± 17 (95% CI −11.69, 3.69; P = 0.29), whereas post measurements were 130 ± 9 and 131 ± 15 (95% CI −6.14, 4.39; P = 0.73), respectively. No significant difference was indicated between measurement tools for baseline and post-intervention SBP. There was a statistically significant difference in sphygmomanometer and Finometer DBP measurements with baseline 84 ± 7 and 77 ± 9 (95% CI 3.50, 11.00; P < 0.01), respectively. Post-DBP sphygmomanometer measurements of 81 ± 9 and Finometer 74 ± 10 (95% CI 2.54, 11.12; P < 0.01) were also significantly different.
There was a significant positive linear relationship between the sphygmomanometer versus Finometer measurements in both SBP and DBP at baseline and 24 hours post-intervention. Pearson correlation coefficients indicated positive correlation with baseline SBP 0.55 (P = 0.03) and DBP 0.64 (P < 0.01); 24-hour post-SBP 0.59 (P < 0.01) and DBP 0.44 (P = 0.05).
4. Discussion
The main finding of this study was that significant reductions were seen (with the primary analysis) in SBP and MAP in individuals conducting IRT for 8 weeks at 30% MVC. The reduction in SBP was clinically significant (>3 mmHg). It appears unlikely that 5% MVC elicits significant blood pressure reductions. Instead of a sedentary control, we might therefore consider utilizing a control group for future IRT studies at an intensity of 5% MVC.
4.1. Blood pressure, MAP, and HR
Our primary analysis showed that SBP reductions were seen in the 30% MVC group, but not the 5% MVC group. The 7-mmHg reduction in SBP is considered clinically meaningful (>3 mmHg).[24,25] The results seen in this study reflect those seen in previous IRT studies, which also demonstrated significant reductions in SBP over an 8-week period at 30% MVC.[2,6,8,13] When baseline blood pressure was added as a covariate, secondary analysis showed that SBP, DBP, MAP, and HR were all significantly reduced in both the 30% and the 5% MVC groups. Although it is unclear whether the size of these reductions is clinically meaningful, it has been previously found that the magnitude of blood pressure reductions following IRT is directly related to pre-training blood pressure levels,[26] which could perhaps be explained by regression to the mean.
Mean DBPs at baseline in both the 5% and 30% MVC groups in our study were within the normal range with both groups having population baseline mean <85 mmHg. Taking into account the limited potential for further reductions in DBP, we did not expect to see much of a reduction in DBP after IRT intervention in either group. We saw no significant reduction in DBP for both the 5% and 30% groups. Previous studies conducting isometric handgrip training at 30% MVC produced conflicting results. Some small studies have failed to show DBP reductions; Howden et al[27] who had 8 participants conducting 5 weeks of IRT and Taylor et al[28] with 9 participants after 10 weeks of IRT, saw no statistical reductions in DBP with baseline <85 mmHg. In contrast, both single studies[29] and pooled analyses from several studies[2,8,10] have shown significant reductions in DBP after IRT. Although baseline DBP may predict significant responses to IRT, again it is unclear whether the size of these reductions is clinically meaningful.
The significant reduction in MAP in the 30% group saw MAP lowered from 100 to 96 mmHg, which is clinically meaningful. Reductions in MAP at 30% MVC were also seen by Carlson et al[2] and Millar et al.[6] There were no statistically significant changes in HR for either the 5% or 30% MVC groups. The absence of change in resting HR indicates that IRT has a minimal effect on the parasympathetic nervous system. Other analyses have failed to show a reduction in HR with IRT when conducting an isometric handgrip protocol.[29–31]
4.2. Clinical significance
The risk of adverse health outcomes can be reduced if individuals with grade 1 hypertension can lower their blood pressure, there exists a dose-response.[32] Despite a 2-mmHg reduction in SBP in the 5% MVC group, this was only statistically significant when baseline blood pressure was used as a covariate. Reductions of 2 mmHg would be borderline for clinical significance.[33,34] One individual in the 5% group and 6 in the 30% group with SBP >120 mmHg at baseline were reduced to <120 mmHg post-intervention. The recent SPRINT trial demonstrated that lowering SBP to <120 mmHg resulted in significantly lower rates of cardiovascular events in adults with hypertension.[35] Although there is individual variation among participants, DBP population reductions of 3 mmHg in the 5% group and 2 mmHg in the 30% indicate IRT can have an impact on preventing adverse events.[9,36] According to the Framingham Heart Study, small reductions in DBP as low as 2 mmHg were shown to be associated with decreased risk of coronary heart disease and stroke.[9,34,36]
4.3. Limitations
The number of medicated (n = 26) versus nonmedicated participants (n = 14) prevented sub-analyses comparing the 2 groups. Although the 5% and 30% MVC groups were matched for equal numbers of participants, there were only 7 nonmedicated participants in each group. Parameters were matched at baseline for both groups; however, the 5% and 30% groups were not matched at baseline for SBP, resulting in the 30% SBP baseline being >5%. Two of the participants (30% MVC group) had to withdraw from the study because of work commitments, one after completing 4 weeks and the other at 5 weeks. As both had informed the researchers with enough notice, their blood pressure was measured on their last day of attending and their 2-minute rest period post-IRT was used as last outcome carried forward for analysis.
Controversy over the accuracy of the Finometer was mitigated to some extent by a comparative analysis with sphygmomanometer measures, which showed significant correlation. Previous studies looking at variation in the Finapres in relation to intra-arterial pressure indicated variability and bias in SBP and DBP measurements resulting in incorrect measurements.[37–39] We used a more recent Finometer, which supersedes the Finapres; although there was a significant variation in DBP, there was no significant variation in our SBP, and this was also seen by Schutte et al.[40]
4.4. Recommendations for future research
A larger cohort would enable subanalyses to look at individual variation in response to IRT, such as comparison of males and females and medicated versus nonmedicated participants. Future research would benefit from utilizing the current criterion standard for blood pressure measurement of 24-hour ambulatory monitoring; only 1 study to date has used ambulatory blood pressure. To date there has only been 2 randomized controlled studies of IRT for 10 weeks, the longest study duration.[9] Future studies should look at the effect of IRT over a period of ≥10 weeks, with a follow-up equivalent to the duration of IRT intervention. Investigation into the physiological mechanisms responsible for reductions in blood pressure will enable more understanding of the antihypertensive effects of IRT and aid in development of future research.
4.5. Novelty and significance
Previous isometric handgrip studies of ≥8 weeks have had small participant numbers; this is currently the largest cohort of participants in this field. This is the only study to utilize a low-intensity group for use as a control group; previous studies have either had a sedentary or sham control group.
5. Conclusion
A reduction in SBP was seen after 8 weeks of IRT, indicating that IRT may be an alternative exercise for people who are unable to reach the current recommendations of 2.5 hours of weekly aerobic exercise, to aid in their blood pressure management.
Acknowledgements
Exercise Physiology Department, School of Science and Technology, University of New England.
Footnotes
Abbreviations: ANCOVA = analysis of covariance, ANOVA = analysis of variance, CVD = cardiovascular disease, DBP = diastolic blood pressure, HR = heart rate, IRT = isometric resistance training, MANOVA = multivariate analysis of variance, MAP = mean arterial pressure, MVC = maximum voluntary contraction, SBP = systolic blood pressure, sec = second.
This research project is registered with ClinicalTrials.gov, the identifier for the study is Isometric handgrip exercise for blood pressure management, number NCT02458456.
Source of Funding: Australian Postgraduate Award (postgraduate student scholarship). University of New England, postgraduate research funds for equipment purchases. University of New England, Seed Grant to assist with paying research assistants.
The authors report no financial disclosures or conflicts of interest.
References
- [1].WHO, World Health Organisation. A global brief on hypertension: Silent killer, global public health crisis. Geneva Switzerland, World Health Organization, 2013:4. http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/. [Google Scholar]
- [2].Carlson DJ, Dieberg G, Hess NC, et al. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clin Proc 2014;89:327–34. [DOI] [PubMed] [Google Scholar]
- [3].Chobanian AV, Wright JJT, Roccella EJ, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- [4].WHO. Cardiovascular disease (CVDs). In:WHO, ed. Vol Fact sheet No.3172015. [Google Scholar]
- [5].Nuckols TK, Aledort JE, Adams J, et al. Cost implications of improving blood pressure management among U.S. adults. Health Serv Res 2011;46:1124–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Millar PJ, Levy AS, McGowan CL, et al. Isometric handgrip training lowers blood pressure and increases heart rate complexity in medicated hypertensive patients. Scand J Med Sci Sports 2013;23:620–6. [DOI] [PubMed] [Google Scholar]
- [7].Mozaffarian D, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics—2016 update: a report From the American Heart Association. Circulation 2015;133:e38–60. [DOI] [PubMed] [Google Scholar]
- [8].Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Millar PJ, McGowan CL, Cornelissen VA, et al. Evidence for the role of isometric exercise training in reducing blood pressure: potential mechanisms and future directions. Sports Med 2014;44:345–56. [DOI] [PubMed] [Google Scholar]
- [10].Kelley GA, Kelley KS. Isometric handgrip exercise and resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens 2010;28:411–8. [DOI] [PubMed] [Google Scholar]
- [11].Owen A, Wiles J, Swaine I. Effect of isometric exercise on resting blood pressure: a meta analysis (Provisional abstract). J Hum Hypertens 2010;24:796–800. [DOI] [PubMed] [Google Scholar]
- [12].Badrov MB, Horton S, Millar PJ, et al. Cardiovascular stress reactivity tasks successfully predict the hypotensive response of isometric handgrip training in hypertensives. Psychophysiology 2013;50:407–14. [DOI] [PubMed] [Google Scholar]
- [13].Inder JD, Carlson DJ, Dieberg G, et al. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res 2015;39:88–94. [DOI] [PubMed] [Google Scholar]
- [14].Ray CA, Carrasco DI. Isometric handgrip training reduces arterial pressure at rest without changes in sympathetic nerve activity. Am J Physiol Heart Circ Physiol 2000;279:H245–9. [DOI] [PubMed] [Google Scholar]
- [15].Baross AW, Wiles JD, Swaine IL. Effects of the intensity of leg isometric training on the vasculature of trained and untrained limbs and resting blood pressure in middle-aged men. Int J Vasc Med 2012;2012:964–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wiles JD, Coleman DA, Swaine IL. The effects of performing isometric training at two exercise intensities in healthy young males. Eur J Appl Physiol 2010;108:419–28. [DOI] [PubMed] [Google Scholar]
- [17].Peters PG, Alessio HM, Hagerman AE, et al. Short-term isometric exercise reduces systolic blood pressure in hypertensive adults: Possible role of reactive oxygen species. Int J Cardiol 2006;110:199–205. [DOI] [PubMed] [Google Scholar]
- [18].Parsons HM. What happened at Hawthorne? Science 1974;183:922–32. [DOI] [PubMed] [Google Scholar]
- [19].McCarney R, Warner J, Iliffe S, et al. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Truijen J, van Lieshout JJ, Wesselink WA, et al. Noninvasive continuous hemodynamic monitoring. J Clin Monit Comput 2012;26:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Experiment Physiol 2005;90:437–46. [DOI] [PubMed] [Google Scholar]
- [22].Ogedegbe G, Pickering T. Principles and techniques of blood pressure measurement. Cardiol Clin 2010;28:571–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Williams JS, Brown SM, Conlin PR. Blood-pressure measurement. N Engl J Med 2009;360:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hess N, Carlson D, Inder J, et al. Clinically meaningful blood pressure reductions with low intensity isometric handgrip exercise. A randomized trial. Physiol Res 2016;65:461–8. [DOI] [PubMed] [Google Scholar]
- [25].Wong GW, Wright JM. Blood pressure lowering efficacy of nonselective beta-blockers for primary hypertension. Cochrane Database Syst Rev 2014;CD007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Millar PJ, Bray SR, McGowan CL, et al. Effects of isometric handgrip training among people medicated for hypertension: a multilevel analysis. Blood Press Monit 2007;12:307–14. [DOI] [PubMed] [Google Scholar]
- [27].Howden R, Lightfoot JT, Brown SJ, et al. The effects of isometric exercise training on resting blood pressure and orthostatic tolerance in humans. Experiment Physiol 2002;87:507–15. [DOI] [PubMed] [Google Scholar]
- [28].Taylor AC, McCartney N, Kamath MV, et al. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc 2003;35:251–6. [DOI] [PubMed] [Google Scholar]
- [29].Millar PJ, Bray SR, MacDonald MJ, et al. The hypotensive effects of isometric handgrip training using an inexpensive spring handgrip training device. J Cardiopulm Rehabil Prev 2008;28:203–7. [DOI] [PubMed] [Google Scholar]
- [30].Stiller-Moldovan C, Kenno K, McGowan CL. Effects of isometric handgrip training on blood pressure (resting and 24 h ambulatory) and heart rate variability in medicated hypertensive patients. Blood Press Monit 2012;17:55–61. [DOI] [PubMed] [Google Scholar]
- [31].Millar PJ, Bray SR, MacDonald MJ, et al. Cardiovascular reactivity to psychophysiological stressors: association with hypotensive effects of isometric handgrip training. Blood Press Monit 2009;14:190–5. [DOI] [PubMed] [Google Scholar]
- [32].Sundstrom J, Arima H, Jackson R, et al. Effects of blood pressure reduction in mild hypertension A systematic review and meta-analysis. Ann Intern Med 2015;162:184–91. [DOI] [PubMed] [Google Scholar]
- [33].Devereux GR, Wiles JD, Howden R. Immediate post-isometric exercise cardiovascular responses are associated with training-induced resting systolic blood pressure reductions. Eur J Appl Physiol 2015;115:327–33. [DOI] [PubMed] [Google Scholar]
- [34].Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension. J Am Med Assoc 2002;288:1882–8. [DOI] [PubMed] [Google Scholar]
- [35].Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive vs standard blood-pressure control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cook NR, Cohen J, Hebert PR, et al. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 1995;155:701–9. [PubMed] [Google Scholar]
- [37].Dorlas JC, Nijboer JA, Butijn WT, et al. Effects of peripheral vasoconstriction on the blood pressure in the finger, measured continuously by a new noninvasive method (The Finapres(R)). Anesthesiology 1985;62:342–5. [DOI] [PubMed] [Google Scholar]
- [38].Epstein RH, Bartkowski RR, Huffnagle S. Continuous noninvasive finger blood pressure during controlled hypotension. A comparison with intraarterial pressure. Anesthesiology 1991;75:796–803. [DOI] [PubMed] [Google Scholar]
- [39].Silke B, McAuley D. Accuracy and precision of blood pressure determination with the Finapres: an overview using re-sampling statistics. J Hum Hypertens 1998;12:403–9. [DOI] [PubMed] [Google Scholar]
- [40].Schutte AE, Huisman HW, van Rooyen JM, et al. Validation of the Finometer device for measurement of blood pressure in black women. J Hum Hypertens 2004;18:79–84. [DOI] [PubMed] [Google Scholar]


