Abstract
Isoprene emitted from plants is made in chloroplasts from dimethylallyl pyrophosphate (DMAPP). Leaves of Populus nigra and Phragmites australis exposed to 13CO2 for 15 min emitted isoprene that was about 90% 13C, but DMAPP isolated from those leaves was only 28% and 36% 13C, respectively. The labeled DMAPP is likely to represent chloroplastic DMAPP contributing to isoprene emission. A substantial 13C labeling was also found in both emission and DMAPP pool of low-emitting, young leaves of Phragmites. This confirms that low emission of young leaves is not caused by absence of chloroplastic DMAPP but rather by enzyme characteristics. A very low 13C labeling was found in the DMAPP pool and in the residual isoprene emission of leaves previously fed with fosmidomycin to inhibit isoprene formation. This shows that fosmidomycin is a very effective inhibitor of the chloroplastic biosynthetic pathway of isoprene synthesis, that the residual isoprene is formed from extra-chloroplastic sources, and that chloroplastic and extrachloroplastic pathways are not cross-linked, at least following inhibition of the chloroplastic pathway. Refixation of unlabeled respiratory CO2 in the light may explain incomplete labeling of isoprene emission, as we found a good association between these two parameters.
Isoprene, the most important volatile organic compound in biosphere-atmosphere interaction (Fuentes et al., 2000), is emitted by many plants in a light and CO2-dependent manner (Loreto and Sharkey, 1990). These observations led to the conclusion that isoprene emitted by plants is formed from carbon freshly fixed by photosynthesis. This was also proved by labeling experiments first carried out by Sanadze et al. (1972). Labeling with 13C showed rapid incorporation of the isotope in isoprene molecule (Delwiche and Sharkey, 1993), consistent with the labeling time-course of photosynthesis intermediates. Since the discovery that isoprene is made from a chloroplastic biosynthetic pathway, not from the classic isoprenoid pathway of formation (Zeidler et al., 1997), other studies have been designed to dissect origin and contribution of different sources of carbon to isoprene formation through 13C labeling experiments. Measurements of 13C natural abundance (Affek and Yakir, 2003) and on-line measurements in 13C-enriched atmosphere (Karl et al., 2002; Schnitzler et al., 2004), or with 13C-enriched putative precursors of isoprene (Kreuzwieser et al., 2002; Schnitzler et al., 2004), essentially confirmed that the contribution of sources of carbon other than chloroplastic for isoprene formation is minor, generally forming less than 20% of the emitted isoprene. These studies, however, indicated that the extrachloroplastic carbon also used to form isoprene may have multiple origins and can make a proportionally larger portion of isoprene under stress conditions.
Dimethylallyl pyrophosphate (DMAPP), the last precursor of isoprene, is also formed by chloroplastic and extrachloroplastic sources of carbon, depending on its pathway of formation. To investigate the localization of DMAPP in isoprene-emitting leaves, the nonaqueous fractionation method (Sharkey and Vanderveer, 1989) has been used. Rosenstiel et al. (2002) found DMAPP prevalently in chloroplasts, but Wolfertz et al. (2003) noted that chloroplastic DMAPP is inversely dependent on the emission rate of isoprene.
In this paper we show the 13C labeling pattern of DMAPP from isoprene-emitting leaves and from leaves in which isoprene emission is naturally low because of their young age (Sharkey and Loreto, 1993) or has been artificially inhibited with fosmidomycin (Zeidler et al., 1998; Loreto and Velikova, 2001). These experiments allowed us to separate, in a very simple and effective way, the pool of DMAPP incorporating labeled carbon (presumably chloroplastic) from the pool that remained unlabeled (presumably cytosolic). By comparing the labeling patterns of DMAPP and emitted isoprene, we also gained insight on the contribution of different sources of DMAPP and on the possible limitations set by DMAPP levels to the emission of isoprene under different developmental stages. Finally, we show that the extrachloroplastic (nonlabeled) fraction of emitted isoprene is associated with the fraction of refixed carbon released by mitochondrial respiration, suggesting that this may explain why unlabeled carbon is found in isoprene emitted by nonstressed, mature leaves.
RESULTS
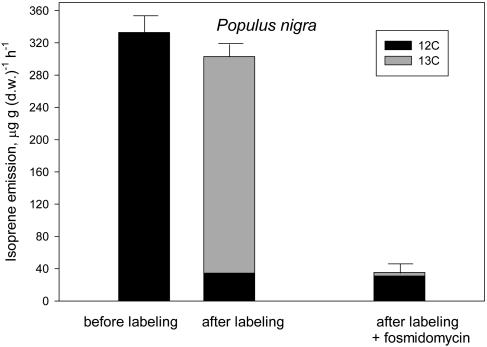
In Populus (Fig. 1) and Phragmites (Fig. 2) mature leaves, about 90% of the emitted isoprene was fully or partly labeled by 13C. Up to 20% 12C was still present in the labeled fraction of isoprene, as calculated from the percent distribution of labeling in partly labeled fragments (Fig. 5). In Populus, the residual emission after fosmidomycin feeding was virtually unlabeled by 13C, and this unlabeled fraction was not quantitatively different from the unlabeled fraction measured in isoprene-emitting leaves (compare second and third bars of Fig. 1).
Figure 1.
12C (black) and 13C (gray) in the emission of isoprene by P. nigra mature leaves, before and after exposure to a 15-min 13CO2 labeling (left and center bars) and by leaves labeled for 15 min with 13CO2 after inhibiting isoprene emission by fosmidomycin (right bar). Total isoprene emission is shown as mean ± se (error bars) of n = 4 measurements. Labeled and unlabeled fractions of isoprene are shown as mean values. se of these fractions was always <10% of the mean.
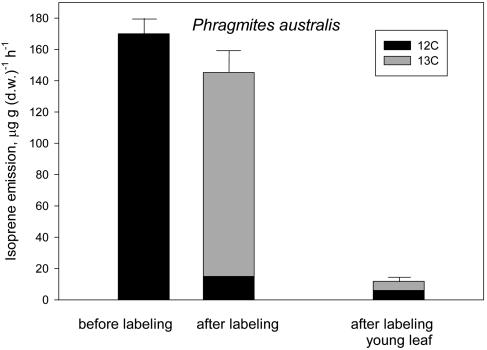
Figure 2.
12C (black) and 13C (gray) in the emission of isoprene by P. australis mature leaves, before and after 13CO2 labeling for 15 min (left and center bars) and by young leaves labeled for 15 min with 13CO2 (right bar). Total isoprene emission is shown as mean ± se (error bars) of n = 4 measurements. Labeled and unlabeled fractions of isoprene are shown as mean values. se of these fractions was always <10% of the mean.
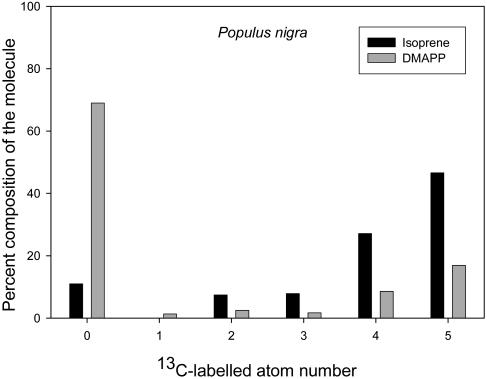
Figure 5.
Distribution of 13C within the five C atoms of the isoprene molecule determined in the emission (black) and in the acidic hydrolysis of DMAPP (gray) following a 15-min labeling with 13CO2 of P. nigra leaves.
In Phragmites, young leaves emitted a low amount of isoprene, and only 52% of this emission was labeled by 13C (Fig. 2).
Only 26% and 38% of the DMAPP content was labeled by 13C in the mature leaves of Populus (Fig. 3) and Phragmites (Fig. 4), respectively. The labeling pattern was similar to that observed in emitted isoprene; that is, there were no differences in the distribution of 13C atoms in the molecule of isoprene evolved from DMAPP after acidic hydrolysis and in that emitted by leaves (Fig. 5).
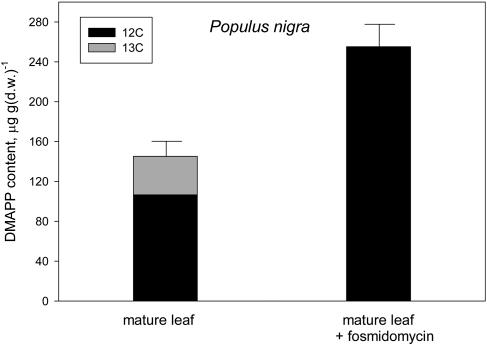
Figure 3.
12C (black) and 13C (gray) after a 15-min 13CO2 labeling in the DMAPP contained by mature leaves of P. nigra emitting isoprene (left bar) or after inhibiting isoprene emission by fosmidomycin feeding (right bar). Total DMAPP is shown as mean ± se (error bars) of n = 4 measurements. Labeled and unlabeled fractions of DMAPP are shown as mean values. se of these fractions was always <10% of the mean.
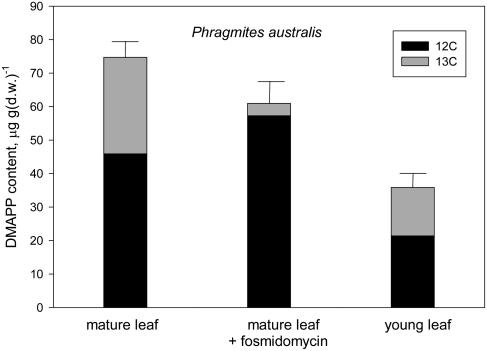
Figure 4.
12C (black) and 13C (gray) after a 15-min 13CO2 labeling in the DMAPP contained by mature leaves of P. australis emitting isoprene (left bar) or after inhibiting isoprene emission by fosmidomycin feeding (center bar) and by low-emitting young leaves (right bar). Total DMAPP is shown as mean ± se (error bars) of n = 4 measurements. Labeled and unlabeled fractions of DMAPP are shown as mean values. se of these fractions was always <10% of the mean.
The percentage of DMAPP labeling was not different in young leaves of Phragmites with respect to mature leaves (Fig. 4). In fosmidomycin-fed leaves, the 13C-labeled fraction of DMAPP became very low (Phragmites; Fig. 4) or absent (Populus; Fig. 3), while the unlabeled fraction quantitatively increased with respect to that measured in isoprene-emitting leaves. This increase was particularly relevant in Populus (Fig. 3).
The remaining 12C in the labeled fraction of isoprene, expressed as unlabeled percent, was associated with the estimated percentage of refixed respiratory carbon (Fig. 6). Mitochondrial respiration in the dark was similar in the two plants, averaging 1.2 ± 0.3 μmol m−2 s−1 (n = 8; data not shown).
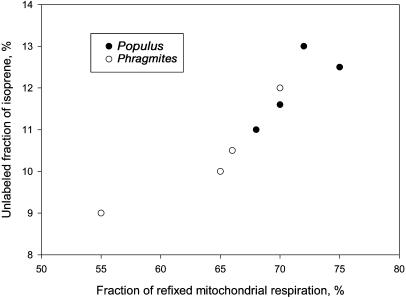
Figure 6.
Relationship between the unlabeled fraction of isoprene emitted by Populus and Phragmites leaves expressed as percentage of 12C remaining in the molecule that was involved in the labeling (see Fig. 5) and the calculated fraction of 12CO2 refixation from mitochondrial respiration in the light.
DISCUSSION
Rapid and quasitotal 13C labeling of isoprene emitted by Populus and Phragmites mature leaves (Figs. 1 and 2) confirmed that the largest part of the carbon incorporated in the molecule comes from photosynthetic metabolism, as already shown in many other plant species (Delwiche and Sharkey, 1993; Karl et al., 2002; Affek and Yakir, 2003; Schnitzler et al., 2004). The unlabeled fraction was suggested to be released by multiple sources either chloroplastic such as starch-breakdown (Karl et al., 2002) or extrachloroplastic such as xylem-transported carbohydrates (Kreuzwieser et al., 2002; Schnitzler et al., 2004) or by refixation of CO2 released by mitochondrial respiration (Anderson et al., 1998). Data of Figure 6 show that a relationship exists between the 12C carbons in labeled isoprene (Fig. 5) and the fraction of refixed respiratory CO2. Respiratory CO2 is not labeled fast by 13CO2 (Loreto et al., 2001). Thus, it may be an alternative source of carbon for isoprene formation influencing the completeness of isoprene labeling in nonstressed mature leaves. However, as the respiratory CO2 refixation is dependent on photosynthesis (Loreto et al., 2001), this source of carbon probably would not contribute significantly to isoprene formation in stressed leaves.
As expected, fosmidomycin reduced isoprene emission of mature leaves to about one-tenth of the original emission (Loreto and Velikova, 2001; Sharkey et al., 2001). Residual emission was not labeled, showing that (1) fosmidomycin totally inhibited only the chloroplastic pathway; and (2) the extrachloroplastic pathway cooperates in isoprene formation, as postulated by Lichtenthaler (1997). Moreover, the extrachloroplastic pathway feeds the same small amount of carbon to isoprene in isoprene-emitting and isoprene-inhibited leaves, indicating absence of substantial cross-talk between chloroplastic and extrachloroplastic pathways of isoprenoid formation, at least within 15 min from the inhibition of the chloroplastic pathway.
In young leaves of Phragmites, about 50% of the low isoprene emission remained unlabeled. Retrieval of a labeled fraction of isoprene indicates presence of chloroplastic sources, also confirmed by similar labeling of DMAPP (compare Figs. 2 and 4). The low emission of young leaves is therefore explained by the low activity (and/or concentration) of isoprene synthase in these leaf chloroplasts (Kuzma and Fall, 1993; Lehning et al., 1999) rather than to a substrate (chloroplastic DMAPP) limitation.
DMAPP labeling was fast, and 13C was distributed within the molecule of DMAPP similarly to labeling of emitted isoprene (Fig. 5). This suggests that labeled DMAPP originated isoprene and was located in the chloroplasts. Less than one-third of the total pool of DMAPP extracted from our leaves was labeled by 13C (Figs. 3 and 4). This indicates that the chloroplastic pool of DMAPP is also about one-third of the total pool. This ratio is low compared to the ratio between chloroplastic and extrachloroplastic DMAPP (about 70% and 30%) reported by Rosenstiel et al. (2002). However, the amount of chloroplastic DMAPP is inversely associated to isoprene emission capacity (Wolfertz et al., 2003). As our plants were both very strong emitters, it is conceivable that their chloroplastic pools of DMAPP were low.
Our experiment shows that 13C labeling of DMAPP is a very efficient, rapid, and elegant method to quantify the DMAPP chloroplastic pool, substantially less cumbersome than the only alternative of nonaqueous fractionation of leaf material. Extrachloroplastic (cytosolic, vacuolar) pools of DMAPP cannot be partitioned by 13C labeling, as they should remain unlabeled. However, if the vacuolar pool of DMAPP is absent or very low (Wolfertz et al., 2003), then about all nonlabeled DMAPP should belong to the cytosolic pool. Our results are further proof that whole leaf DMAPP content cannot be used to predict isoprene emission rates.
Data of Figure 3 show no 13C labeling of DMAPP in mature leaves previously fed with fosmidomycin, while the unlabeled fraction of DMAPP was quantitatively stimulated by the treatment, particularly in Populus. Suppression of the labeled pool of DMAPP by fosmidomycin indirectly confirmed that this pool is entirely of chloroplastic origin and feeds the chloroplastic pathway of isoprene formation. As already mentioned, Wolfertz et al. (2003) noted that the chloroplastic level of DMAPP is high when the emission of isoprene is low. This is apparently also the case for the cytosolic pool of DMAPP. An increase of cytosolic isoprenoids (sterols) was observed a few hours after the inhibition of the chloroplastic pathway (Laule et al., 2003). Our experiment indicates that this is due to the very rapid increase of cytosolic DMAPP, possibly caused, in turn, by increased availability of either chemical intermediates or ATP and NADPH.
The labeled and unlabeled pools of DMAPP were lower in young leaves than in mature leaves, and the fractions of labeled and unlabeled DMAPP of young leaves were similar to those measured for emitted isoprene in the same leaves (Figs. 2 and 4). This is different from what has been reported for mature leaves. It may indicate that in young leaves the chloroplastic and extrachloroplastic DMAPP contribute equally to isoprene formation.
CONCLUSION
In conclusion, 13C labeling of DMAPP allowed us to distinguish, as in the case of isoprene labeling, between labeled (chloroplastic) and unlabeled (presumably cytosolic) pools. Labeling of DMAPP in young leaves showed the presence of the chloroplastic pool and indicated that the low emission was probably due to low isoprene synthase activity and/or concentration. Labeling of leaves after fosmidomycin-feeding confirmed the complete inhibition of the chloroplastic pathway of isoprene formation while it revealed stimulation of the extrachloroplastic pool for a still unknown mechanism. This stimulation apparently does not affect the emission, suggesting no cross-talk between the chloroplastic and extrachloroplastic pathways. Finally, application of the 13C technique to detect refixed CO2 supplied by mitochondrial respiration showed that refixation was associated to the level of the incomplete labeling of isoprene emission, suggesting that respiratory CO2 may be a primary source of unlabeled carbon for isoprene formation.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Statistics
Two-year-old plants of Populus nigra and current year plants of Phragmites australis were used. Plants were grown in 10-L pots filled with commercial soil under optimal water and nutrient conditions in phytotrons with a light regime of 14 h/d at 1,000 μmol photons m−2 s−1, and a temperature regime of 30°C/27°C (day/night). Experiments were carried out on mature leaves of Populus. In Phragmites, both mature (fully expanded) and young (just unfolded) leaves were used. Total isoprene emission and DMAPP content are shown in Figures 1 to 4 as mean ± se of 4 measurements on different leaves of different plants. In the same figures, the labeled and unlabeled fractions of isoprene and DMAPP are shown as the mean, the se of each fraction being <10% of the mean. The typical 13C labeling distribution in the molecules of isoprene and DMAPP is shown in a single measurement (Fig. 5). Single measurements of 13C-labeled isoprene emission are also compared with the calculated mitochondrial 12CO2 refixation in the same leaves (Fig. 6).
Gas-Exchange Measurements
Single leaves were clamped in a 0.5-L gas-exchange plastic cuvette, entirely coated with Teflon, as previously explained (Loreto et al., 1996). All measurements were done exposing leaves at a light intensity of 1,000 μmol photons m−2 s−1 and maintaining the leaf temperature at 30°C with Peltier thermoelectric modules. Leaves were exposed to a flux of synthetic (isoprene- and contaminant-free) air composed by N2, O2, and CO2 in atmospheric concentrations (80%, 20%, and 370 μL L−1, respectively). All measurements were carried out when leaves reached a steady photosynthesis and conductance to CO2. Values of photosynthesis were higher in Populus (15.1 ± 2.3 μmol m−2 s−1, n = 4) than in Phragmites (11.3 ± 2.6 μmol m−2 s−1, n = 4). These gas-exchange parameters were measured with a LI-COR 6262 infrared gas analyzer (IRGA; LI-COR, Lincoln, NE).
Mitochondrial respiration in the light was measured by labeling all other sources of CO2 exchange (photosynthesis and photorespiration) in a 13CO2 atmosphere. Respiratory (12CO2) efflux was measured with a 13CO2-insensitive IRGA (Gashound; LI-COR). The fraction of respiratory CO2 refixed by leaves (Rdr) was estimated by
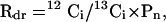 |
where 12Ci and 13Ci are the intercellular concentration of 12CO2 produced by mitochondrial respiration, and of 13CO2, respectively, and Pn is the photosynthetic rate, calculated by standard gas-exchange with the LI-COR 6262 IRGA immediately prior to the labeling. Details about this method are shown in Loreto et al. (2001).
Isoprene was trapped in cartridges containing three different graphitic carbons (Brancaleoni et al., 1999) set in series at the cuvette exit as shown by Loreto et al. (1996). This allowed us to completely recover isoprene in volumes of air as high as 5 L (Brancaleoni et al., 1999). One liter of air was collected into each cartridge at a flow rate of 250 mL min−1. Trapped compounds were then thermally desorbed and analyzed by gas chromatography-mass spectrometry as detailed in Brancaleoni et al. (1999).
Fosmidomicyn Feeding
After measuring gas-exchanges, some mature leaves of Populus and Phragmites were cut and placed in a vial filled with distilled water. When photosynthesis and isoprene emission returned to a steady state, comparable to those observed before cutting, fosmidomycin was added to the water. The resulting aqueous solution of fosmidomycin (5 μm) was taken up by leaves within 30 min (data not shown), as indicated by the strong and irreversible inhibition of isoprene emission, while photosynthesis was not affected.
13C Labeling of Isoprene and DMAPP
Labeling measurements of isoprene emission and DMAPP content were carried out in mature, isoprene-emitting leaves, in the same mature leaves but after inhibiting isoprene formation by fosmidomycin, and in young, low-isoprene emitting Phragmites leaves. When photosynthesis and isoprene emission were steady (about 30 min after inserting leaves in the cuvette, or 1 h after feeding fosmidomycin), the CO2 source (a flask containing synthetic air with a natural abundance of 13CO2) was replaced with a flask containing synthetic air with only 13C labeled CO2. The system used in this experiment is described in all details by Loreto et al. (1996). 13C labeling was carried out for 15 min. Preliminary experiments (not shown) indicated that this was the time necessary to complete (i.e. to reach the maximum) labeling of isoprene with our experimental system (cuvette volume and geometry, leaf area, and flow rate). Labeled isoprene was also analyzed by gas chromatography-mass spectrometry as described by Loreto et al. (1996) for monoterpenes. As in that study, labeling distribution was analyzed in the mass spectra obtained by electron impact at 70 eV, and the fractions of labeled (i.e. the sum of all fragments partially or completely labeled) and unlabeled (i.e. fragments showing the original isoprene m/z) are shown in Figures 1 to 4. The contribution to the labeling coming from individual carbon present in the isoprene molecule (Fig. 5) was derived by mathematically deconvolution of the percent composition of fully and partly labeled fragments, from m/z 63 to m/z 73.
DMAPP Measurements
At the end of each experiment, leaves were removed from the cuvette and rapidly frozen in liquid nitrogen and the amount of DMAPP contained in the leaf was derived by measuring the isoprene formed after acidic hydrolysis on leaf extract maintained with H2SO4 8 m for 1 h at 30°C (Fisher et al., 2001). However, to completely recover the evolved isoprene, we did not extract isoprene from headspace of sealed vials, but we performed the assay in a T-shaped glass tube. The tube was then connected to a carbon cartridge (as mentioned for isoprene emission analysis), and the evolved isoprene was trapped in the cartridge. We determined that complete recovery of the evolved isoprene required three cartridges. The yield of DMAPP was in fact linear with isoprene emission (data not shown) and similar to that reported by Fisher et al. (2001), confirming that only about 5% of the total DMAPP can be converted to isoprene with this chemical extraction and in absence of appropriate proton acceptors from the intermediate carbocation.
Acknowledgments
Giorgio Alessio, Domenico Tricoli, and Violeta Velikova helped with gas-exchange and labeling measurements, and Massimiliano Frattoni helped with mass-spectrometric measurements.
This work was supported by the European Commission project, Ecological and Physiological Functions of Biogenic Isoprenoids and Their Impact on the Environment (contract MC–RTN–CT–MC–RTN–CT–2003–504720, “ISONET”) and by the European Science Foundation program, Volatile Organic Compounds in the Biosphere-Atmosphere System (VOCBAS).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.039537.
References
- Affek HP, Yakir D (2003) Natural abundance carbon isotope composition of isoprene reflects incomplete coupling between isoprene synthesis and photosynthetic carbon flow. Plant Physiol 131: 1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MD, Che P, Song J, Nikolau BJ, Syrkin-Wurtele E (1998) 3-Methylcroronyl coenzyme A carboxylase is a component of the mitochondrial leucine catabolic pathway in plants. Plant Physiol 118: 1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaleoni E, Scovaventi M, Frattoni M, Mabilia R, Ciccioli P (1999) Novel family of multi-layer cartridges filled with a new carbon adsorbent for the quantitative determination of volatile organic compounds in the atmosphere. J Chromatogr A 845A: 317–328 [Google Scholar]
- Delwiche CD, Sharkey TD (1993) Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ 16: 587–591 [Google Scholar]
- Fisher AJ, Rosenstiel TN, Shirk MC, Fall R (2001) Nonradioactive assay for cellular dimethylallyl diphosphate. Anal Biochem 292: 272–279 [DOI] [PubMed] [Google Scholar]
- Fuentes JD, Lerdau M, Atkinson R, Baldocchi D, Botteneheim JW, Ciccioli P, Lamb B, Geron C, Gu L, Guenther A, et al (2000) Biogenic hydrocarbons in the atmosphere boundary layer: a review. Bull Am Meteorol Soc 81: 1537–1575 [Google Scholar]
- Karl T, Fall R, Rosenstiel TN, Prazeller P, Larsen B, Seufert G, Lindinger W (2002) On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta 215: 894–905 [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Graus M, Wisthaler A, Hansel A, Rennenberg H, Schnitzler J-P (2002) Xylem-transported glucose as additional carbon source for leaf isoprene formation in Quercus robur. New Phytol 156: 171–178 [DOI] [PubMed] [Google Scholar]
- Kuzma J, Fall R (1993) Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol 101: 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule O, Fuerholz A, Chang H-S, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange BM (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehning A, Zimmer I, Steinbrecher R, Bruegemann N, Schnitzler JP (1999) Isoprene synthase activity and its relation to isoprene emission in Quercus robur leaves. Plant Cell Environ 22: 495–504 [Google Scholar]
- Lichtenthaler HK (1997) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50: 47–65 [DOI] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Sharkey TD (1996) Different sources of reduced carbon contribute to form three classes of terpenoids emitted by Quercus ilex L. leaves Proc Natl Acad Sci USA 93: 9966–9969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD (1990) A gas exchange study of photosynthesis and isoprene emission in red oak (Quercus rubra L.). Planta 182: 523–531 [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127: 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Velikova V, Di Marco G (2001) Respiration in the light measured by 12CO2 emission in 13CO2 atmosphere in maize leaves. Aust J Plant Physiol 28: 1103–1108 [Google Scholar]
- Rosenstiel TN, Fisher AJ, Fall R, Monson RK (2002) Differential accumulation of dimethyallyl diphosphate in leaves and needles of isoprene- and methylbuthenol-emitting and non-emitting species. Plant Physiol 129: 1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanadze GA, Dzhaiani GI, Tevzadze IM (1972) Incorporation into the isoprene molecule of carbon from 13CO2 assimilated during photosynthesis. Fisiol Rast 19: 17–20 [Google Scholar]
- Schnitzler J-P, Graus M, Kreuzwieser J, Heizmann U, Rennenberg H, Wisthaler A, Hansel A (2004) Contribution of different carbon sources for isoprene emitted from poplar leaves. Plant Physiol 135: 152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Chen X, Yeh S (2001) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125: 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F (1993) Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 95: 328–333 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Vanderveer PJ (1989) Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol 91: 679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kuenhemann F, Yeh S, Weise SE (2003) Biochemical regulation of isoprene emission. Plant Cell Environ 26: 1357–1364 [Google Scholar]
- Zeidler JG, Lichtenthaler HK, May HU, Lichtenthaler FW (1997) Is isoprene emitted by plants synthesized via the novel isopentenyl pyrophosphate pathway? Z Naturforsch 52c: 15–23 [Google Scholar]
- Zeidler J, Schwender J, Müller C, Wiesner J, Weidemeyer C, Back E, Jomaa H, Lichtenthaler HK (1998) Inhibition of the non-mevalonate 1-deoxy-D-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z Naturforsch 53c: 980–986 [Google Scholar]